Abstract
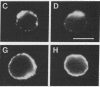
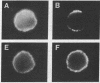
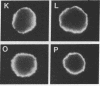
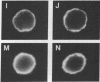
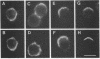
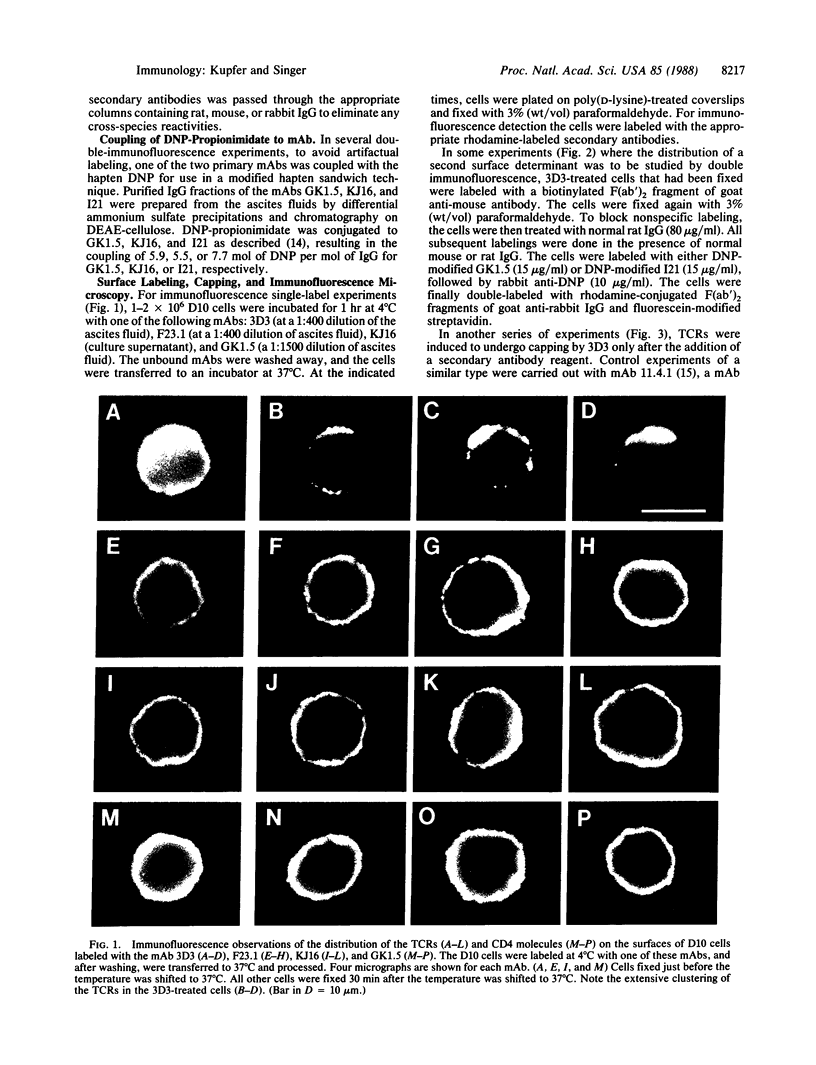
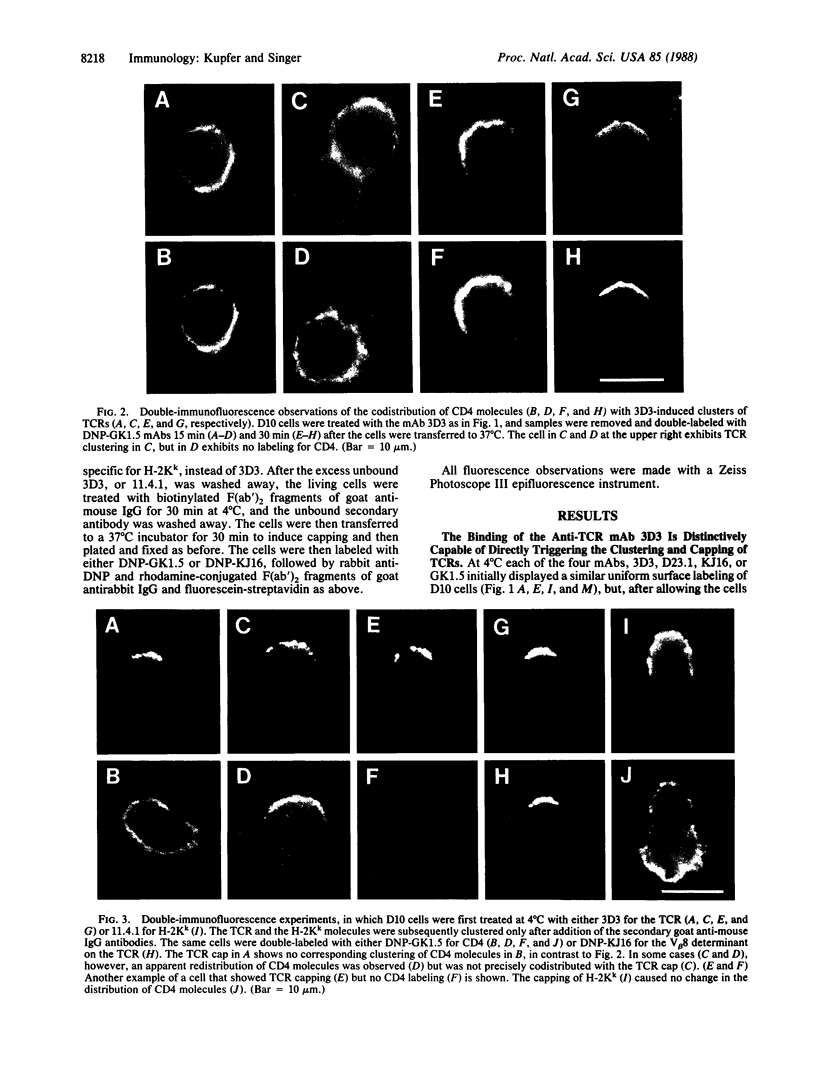
We provide evidence that redistributions and interactions of integral proteins in the fluid membranes of helper T (Th) cells may play important roles in Th-cell activation. A particular monoclonal antibody, 3D3, directed to a clonotypic determinant on the T-cell receptor (TCR) of the cloned Th-cell line D10, had previously been shown to be distinctively capable of directly activating D10 cells at low concentrations. We demonstrate here by immunofluorescence experiments that it is also distinctively able itself to produce a clustering (capping) of the TCRs on the D10 cell surface. Simultaneously, by means of double-immunofluorescence experiments, we find that the 3D3-induced clustering of the TCRs distinctively produces a co-clustering of the accessory molecule CD4 with the TCR clusters, although the CD4 and TCR molecules are normally independent of one another in the D10 cell membrane. These results, and related ones previously obtained from studies of the interactions of D10 Th cells with antigen-presenting cells, are analyzed to suggest that the membrane clustering of TCRs and the induced TCR-CD4 interactions are critical to the signaling events in Th-cell activation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Blue M. L., Schlossman S. F. Comodulation of CD3 and CD4. Evidence for a specific association between CD4 and approximately 5% of the CD3:T cell receptor complexes on helper T lymphocytes. J Immunol. 1988 Mar 15;140(6):1732–1737. [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Doyle C., Strominger J. L. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987 Nov 19;330(6145):256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- EISEN H. N., SISKIND G. W. VARIATIONS IN AFFINITIES OF ANTIBODIES DURING THE IMMUNE RESPONSE. Biochemistry. 1964 Jul;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Strittmatter U., Eichmann K. Synergism in the activation of human CD8 T cells by cross-linking the T-cell receptor complex with the CD8 differentiation antigen. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8298–8302. doi: 10.1073/pnas.83.21.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D., Maddon P., Sekaly R., Talle M. A., Godfrey M., Long E., Goldstein G., Chess L., Axel R., Kappler J. Functional interaction between human T-cell protein CD4 and the major histocompatibility complex HLA-DR antigen. Nature. 1987 Aug 13;328(6131):626–629. doi: 10.1038/328626a0. [DOI] [PubMed] [Google Scholar]
- Goldstein S. A., Mescher M. F. Cytotoxic T cell activation by class I protein on cell-size artificial membranes: antigen density and Lyt-2/3 function. J Immunol. 1987 Apr 1;138(7):2034–2043. [PubMed] [Google Scholar]
- Haskins K., Hannum C., White J., Roehm N., Kubo R., Kappler J., Marrack P. The antigen-specific, major histocompatibility complex-restricted receptor on T cells. VI. An antibody to a receptor allotype. J Exp Med. 1984 Aug 1;160(2):452–471. doi: 10.1084/jem.160.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Rammensee H. G., Ratcliffe M. J., Lamers M. C., Langhorne J., Köhler G. The molecular interactions with helper T cells which limit antigen-specific B cell differentiation. Eur J Immunol. 1988 Mar;18(3):381–386. doi: 10.1002/eji.1830180310. [DOI] [PubMed] [Google Scholar]
- Kaye J., Jones B., Janeway C. A., Jr The structure and function of T cell receptor complexes. Immunol Rev. 1984 Oct;81:39–63. doi: 10.1111/j.1600-065x.1984.tb01104.x. [DOI] [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Singer S. J., Janeway C. A., Jr, Swain S. L. Coclustering of CD4 (L3T4) molecule with the T-cell receptor is induced by specific direct interaction of helper T cells and antigen-presenting cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5888–5892. doi: 10.1073/pnas.84.16.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Swain S. L., Janeway C. A., Jr, Singer S. J. The specific direct interaction of helper T cells and antigen-presenting B cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6080–6083. doi: 10.1073/pnas.83.16.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Swain S. L., Singer S. J. The specific direct interaction of helper T cells and antigen-presenting B cells. II. Reorientation of the microtubule organizing center and reorganization of the membrane-associated cytoskeleton inside the bound helper T cells. J Exp Med. 1987 Jun 1;165(6):1565–1580. doi: 10.1084/jem.165.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., June C. H., Rabinovitch P. S., Grossmann A., Tsu T. T., Imboden J. B. Signal transduction through CD4 receptors: stimulatory vs. inhibitory activity is regulated by CD4 proximity to the CD3/T cell receptor. Eur J Immunol. 1988 Apr;18(4):525–532. doi: 10.1002/eji.1830180406. [DOI] [PubMed] [Google Scholar]
- Marrack P., Skidmore B., Kappler J. W. Binding of antigen-specific, H-2-restricted T cell hybridomas to antigen-pulsed adherent cell monolayers. J Immunol. 1983 May;130(5):2088–2092. [PubMed] [Google Scholar]
- O'Neill H. C., McGrath M. S., Allison J. P., Weissman I. L. A subset of T cell receptors associated with L3T4 molecules mediates C6VL leukemia cell binding of its cognate retrovirus. Cell. 1987 Apr 10;49(1):143–151. doi: 10.1016/0092-8674(87)90764-1. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Owens T., Fazekas de St Groth B., Miller J. F. Coaggregation of the T-cell receptor with CD4 and other T-cell surface molecules enhances T-cell activation. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9209–9213. doi: 10.1073/pnas.84.24.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas A., Takada S., Koide J., Sonderstrup-McDevitt G., Engleman E. G. CD4 molecules are associated with the antigen receptor complex on activated but not resting T cells. J Immunol. 1988 May 1;140(9):2912–2918. [PubMed] [Google Scholar]
- Rojo J. M., Janeway C. A., Jr The biologic activity of anti-T cell receptor V region monoclonal antibodies is determined by the epitope recognized. J Immunol. 1988 Feb 15;140(4):1081–1088. [PubMed] [Google Scholar]
- Saizawa K., Rojo J., Janeway C. A., Jr Evidence for a physical association of CD4 and the CD3:alpha:beta T-cell receptor. Nature. 1987 Jul 16;328(6127):260–263. doi: 10.1038/328260a0. [DOI] [PubMed] [Google Scholar]
- Sanders V. M., Snyder J. M., Uhr J. W., Vitetta E. S. Characterization of the physical interaction between antigen-specific B and T cells. J Immunol. 1986 Oct 15;137(8):2395–2404. [PubMed] [Google Scholar]
- Staerz U. D., Rammensee H. G., Benedetto J. D., Bevan M. J. Characterization of a murine monoclonal antibody specific for an allotypic determinant on T cell antigen receptor. J Immunol. 1985 Jun;134(6):3994–4000. [PubMed] [Google Scholar]
- Swain S. L. T cell subsets and the recognition of MHC class. Immunol Rev. 1983;74:129–142. doi: 10.1111/j.1600-065x.1983.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Takayama H., Sitkovsky M. V. Antigen receptor-regulated exocytosis in cytotoxic T lymphocytes. J Exp Med. 1987 Sep 1;166(3):725–743. doi: 10.1084/jem.166.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tite J. P., Kaye J., Saizawa K. M., Ming J., Katz M. E., Smith L. A., Janeway C. A., Jr Direct interactions between B and T lymphocytes bearing complementary receptors. J Exp Med. 1986 Jan 1;163(1):189–202. doi: 10.1084/jem.163.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Molecular complexity of leukocyte surface glycoproteins related to the macrophage differentiation antigen Mac-1. J Exp Med. 1981 Nov 1;154(5):1517–1524. doi: 10.1084/jem.154.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J., Fathman C. G. Modulation of CD4 by antigenic activation. J Immunol. 1987 Mar 1;138(5):1351–1354. [PubMed] [Google Scholar]