Abstract
Astrocyte dysfunction may contribute to epileptogenesis and other neurological deficits in Tuberous Sclerosis Complex (TSC). In particular, decreased expression and function of astrocyte glutamate transporters have been implicated in causing elevated extracellular glutamate levels, neuronal death, and epilepsy in a mouse model of TSC (Tsc1GFAPCKO mice), involving inactivation of the Tsc1 gene primarily in astrocytes. Here, we tested whether pharmacological induction of astrocyte glutamate transporter expression can prevent the neurological phenotype of Tsc1GFAPCKO mice. Early treatment with ceftriaxone prior to the onset of epilepsy increased expression of astrocyte glutamate transporters, decreased extracellular glutamate levels, neuronal death, and seizure frequency, and improved survival in Tsc1GFAPCKO mice. In contrast, late treatment with ceftriaxone after onset of epilepsy increased glutamate transporter expression, but had no effect on seizures. These results indicate that astrocyte glutamate transporters contribute to epileptogenesis in Tsc1GFAPCKO mice and suggest novel therapeutic strategies for epilepsy in TSC directed at astrocytes.
Keywords: epilepsy, seizure, glia, GLT-1, ceftriaxone, epileptogenesis, mice
Introduction
Tuberous Sclerosis Complex (TSC) is an autosomal dominant genetic disorder, resulting from mutation of either the TSC1 or TSC2 genes and involving tumor or hamartoma formation in multiple organs (Kwiatkowski, 2003; Crino et al., 2006; Holmes et al., 2007). Neurological involvement, including epilepsy, cognitive deficits, and autism, often constitute the most disabling symptoms of the disease. TSC is one of the most common genetic causes of epilepsy, and epilepsy in TSC is usually severe and intractable to currently available treatments. Although hamartomas in the brain (tubers) may serve as the foci for seizures in TSC, this is controversial, and the specific cellular and molecular mechanisms of epileptogenesis in TSC are incompletely understood (Wong, 2008). Achieving a better understanding of the cellular and molecular basis of epileptogenesis should lead to more effective, rational therapies for epilepsy in TSC.
Recent studies in both human brain tissue and animal models of TSC suggest that astrocytes may play an important role in epileptogenesis and other neurological deficits in TSC (Uhlmann et al., 2002; Erbayat-Altay et al., 2007; Sosunov et al., 2008). In one putative astrocyte-related mechanism, astrocytes could potentially promote epileptogenesis and neuronal dysfunction through abnormal regulation of extracellular and synaptic glutamate homeostasis. In support of this hypothesis, a knock-out mouse model of TSC (Tsc1GFAPCKO mice) exhibits decreased expression and function of the astrocyte glutamate transporters, GLT-1 and GLAST, and an associated increase in extracellular glutamate levels and excitotoxic neuronal death (Wong et al., 2003; Zeng et al., 2007). Abnormal glutamate homeostasis and neuronal death, in turn, may result in neurological deficits and promote neuronal hyperexcitability and seizures.
Targeting astrocytic mechanisms could represent a novel therapeutic approach for epilepsy and TSC. Beta-lactam antibiotics, such as ceftriaxone, and other pharmacological compounds have recently been reported to increase the expression of astrocyte glutamate transporters and correspondingly protect against glutamate excitotoxicity and neuronal injury (Rothstein et al., 2005; Ganel et al., 2006; Chu et al., 2007; Lipski et al., 2007). Furthermore, modulation of astrocyte glutamate transporter expression improves survival and other neurological deficits in animal models of various neurological disorders, such as amyotrophic lateral sclerosis (Rothstein et al., 2005; Ganel et al., 2006; Lepore et al., 2008). In the present study, we have tested whether a similar therapeutic approach of modulating astrocyte glutamate transporter expression is effective in countering the abnormal glutamate homeostasis, neuronal death, and epilepsy phenotype in a mouse model of TSC.
Methods
Animals and drug protocols
Care and use of animals were conducted according to an animal protocol approved by the Washington University Animal Studies Committee. Tsc1flox/flox-GFAP-Cre knock-out (Tsc1GFAPCKO) mice with conditional inactivation of the Tsc1 gene in GFAP-positive cells starting around embryonic day 14.5 were generated as described previously (Bajenaru et al., 2002; Uhlmann et al., 2002). Tsc1flox/+-GFAP-Cre and Tsc1flox/flox littermates, which have been shown to have normal phenotypes, were used as control animals in these experiments. Two main drug treatment protocols were used, differing only in time of initiation of treatment. In “early treatment” studies, ceftriaxone or saline treatment was initiated at postnatal day 21, which precedes the onset of seizures and other neurological abnormalities in Tsc1GFAPCKO mice. In “late treatment” studies, drug treatment was initiated at six weeks of age, which is typically after the onset of seizures in these mice (Erbayat-Altay et al., 2007). Ceftriaxone (Sigma, St. Louis, MO) was dissolved in 0.9% NaCl. Tsc1GFAPCKO mice and control mice were administrated ceftriaxone (200 mg/kg, i.p.) or saline daily until death or the pre-defined endpoint of the experiment. In some studies, mice were monitored daily for survival and weekly for body weight without any other interventions. Other studies involved Western blotting, glutamate microdialysis, histological analysis, or video-EEG monitoring at defined time points, as described below. Both male and female mice were used for all studies, but the male:female ratio was similar for all groups within a study and no differences in seizure frequency or the effect of ceftriaxone was found between males and females. Littermates were used in an equal distribution between different groups.
Western blot analysis
After 1, 3 or 5 weeks of ceftriaxone or saline treatment, Western blotting was performed to assay expression GLT-1 using standard methods as described previously (Wong et al., 2003; Zeng et al., 2008). In brief, neocortex and hippocampus were dissected, sonicated, and centrifuged. Equal amounts of total protein extract were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. After incubating with primary antibodies specific to GLT-1 (1:1000, Alpha Diagnostics, San Antonio, TX) or actin (1:5000, Sigma), the membranes were reacted with a peroxidase-conjugated secondary antibody. Signals were detected by using enzyme chemiluminescence reagent (Pierce, Rockford, IL). Data were quantitatively analyzed with ImageJ software, calculating the intensity of each band using the Gel Analyzer method, dividing the test band intensity (e.g. Glt-1) by a protein-loading control (e.g. actin), and normalizing to the control group (e.g. saline-injected control mice).
In vivo microdialysis and glutamate concentration assay
Tsc1GFAPCKO mice receiving ceftriaxone or saline treatment for 3 weeks were implanted with microdialysis probes as previously described (Cirrito et al., 2003; Zeng et al., 2007). Briefly, after guide cannulae (BR-style, Bioanalytical Systems, Indianapolis, IN) and 2-mm microdialysis probes (BR-2, 38 kDa MWCO membrane, Bioanalytical Systems) were inserted into the hippocampus, mice were allowed to recover from anesthesia and were housed in a Raturn Cage system (Bioanalytical Systems), which permitted freedom of movement and ad libitum food and water for the remainder of the experiment. The microdialysis probe was connected to a Univentor syring pump (SciPro) and artificial cerebrospinal fluid [ACSF (in mM): 1.3 CaCl2, 1.2 MgSO4, 3 KCl, 0.4 KH2PO4, 25 NaHCO3, and 122 NaCl, pH 7.35] was perfused through the microdialysis probe. To ensure that brain extracellular fluid (ECF) glutamate levels reached a steady-state concentration after probe insertion, six 1-h samples were taken at a constant flow rate during an initial equilibration phase prior to starting the protocol below. After a stable baseline was obtained, an extrapolated zero flow protocol was used to calculate the in vivo concentration of glutamate within the brain ECF, by measuring glutamate concentrations from dialysate samples acquired at different flow rates and extrapolating back to zero flow rate, at which point the dialysate should reach equilibrium with and equal the in vivo ECF glutamate concentration. Based on a modification of previous methods (Menacherry et al., 1992; Cirrito et al., 2003; Zeng et al., 2007), the extrapolation involved a second order polynomial fit: y = a*x2 + b*x + E, where y = glutamate concentration, x = flow rate, and E = extrapolated in vivo ECF concentration at zero flow rate. To assess whether the microdialysis sampling technique and other biological factors were consistent between different conditions (e.g. control versus Tsc1GFAPCKO mice), the percentage recovery of glutamate at each flow rate was determined and compared by the following equation: (Cx/E) * 100, where Cx is the measured glutamate concentration at a given flow rate and E is the in vivo concentration calculated by extrapolation.
All dialysate samples were collected with a refrigerated fraction collector into polypropylene tubes for subsequent measurement of glutamate concentration. Dialysate glutamate concentrations were measured using an Amplex red glutamic acid/glutamate oxidase assay kit (Molecular Probes, Eugene, OR) on the same day microdialysis was performed. For each sample, a total volume of 100 μl per microplate well was obtained by mixing 50 μl of sample with 50 μl of working solution (100 μM Amplex Red, 0.25 U/ml horseradish peroxidase (HRP), 0.08 U/ml L-glutamate oxidase, 0.5 U/ml L-glutamate-pyruvate transaminase, and 200 μM L-alanine). Samples were then incubated at 37 °C for 30 min and analyzed with a FL600 microplate reader (BioTek, Winooski, VT) with 530 nm excitation and 590 nm emission wavelengths. Glutamate concentrations of samples were determined by interpolation from a standard curve derived by measurements of other samples with known, pre-measured concentrations of glutamate. Each point was corrected for background fluorescence by subtracting values derived from glutamate-free control samples.
Histology and immunohistochemistry
After 5 weeks of ceftriaxone or saline treatment, Tsc1GFAPCKO and control mice were transcardially perfused with PBS followed by 4% paraformaldehyde. The brains were removed immediately and post-fixed with 4% paraformaldehyde overnight at 4°C. After dehydrating in 30% sucrose for at least 24 hours, the brains were sectioned coronally at a thickness of 50 μm with a vibratome. GFAP staining was performed as described previously (Zeng et al., 2008). Sections were incubated with GFAP antibody (anti-rabbit, 1:500, Sigma, Saint Louis, MO) followed by rhodamine-conjugated anti-rabbit IgG (1:500, Sigma) and then cover-slipped with anti-fade mount solution (Molecular Probes). In separate sections, staining for Fluoro-Jade B (FJB; Histo-Chem Inc., Jefferson, AR) was performed, as described previously (Schumed and Hopkins, 2000; Zeng et al., 2007). In brief, the sections were mounted on gelatin-coated slides and dried at room temperature. After rehydration in 100% ethanol (EtOH; 5 min), 70% EtOH (2 min), and distilled water (dH2O; 2 min), the sections were oxidized in 0.06% potassium permanganate (KMnO4) for 10 min, washed with water, and then immersed in 0.0004% FJB solution for 20 min in the dark. Thereafter, slides were washed in dH2O, air dried, cleared and cover-slipped. TUNEL staining was performed using the kit obtained from Chemicon (Temcula, CA) according to the manufacturer's instructions and as done previously (Zeng et al., 2007).
Images were acquired with a Zeiss LSM PASCAL confocal microscope. GFAP-immunoreactive, FJB- and TUNEL-positive cells in hippocampus were counted in the respective sections by an investigator blinded to the treatment of the mice. The distribution of GFAP-, FJB-, and TUNEL-positive cells, and the effects of ceftriaxone, appeared to be relatively homogeneous throughout multiple regions of hippocampus. For quantitative analysis, images from the anterior, dorsal hippocampus in coronal sections at ∼2 mm posterior to bregma and ∼1 mm from midline were specifically analyzed. Standardized 200×200 μm2 regions of interest were identified in hippocampus within striatum radiatum and pyramidale of CA1, and positive cells were quantified in the regions of interest from 3 sections per animal.
Video-EEG monitoring
In early treatment studies, saline- and ceftriaxone-treated Tsc1GFAPCKO mice underwent weekly video-EEG monitoring starting at 4 weeks of age to assess seizure frequency, as described previously (Erbayat-Altay et al., 2007; Zeng et al., 2008). In late treatment studies, weekly video-EEG monitoring was started at 6 week of age, with the first monitoring session occurring immediately prior to initiation of ceftriaxone (or saline) treatment. Briefly, four epidural screw electrodes were surgically implanted in mice under isoflurane anesthesia. Mice were allowed to recover from surgery for at least 24 hours before recording. Continuous EEG data were saved digitally on personal computers using Grass P-100 AC amplifiers (Astro-Med, West Warwick, RI), Axon Digidata A-D converters, and Axoscope software (Molecular Devices, Sunnyvale, CA). To determine the behavioral correlate of electrographic seizures, simultaneous digital video was recorded using a Sanyo Day-Night camera and a Darim MG-100 MPEG video capture card (Darim Vision Corp., Pleasanton, CA). Forty-eight hour epochs of continuous video-EEG data were obtained once a week from each mouse, until the animal died or the electrodes malfunctioned. The clinical and electrographic characteristics of seizures of Tsc1GFAPCKO mice have been reported in detail previously and were analyzed quantitatively by identical methods as described in the previous studies (Erbayat-Altay et al., 2007; Zeng et al., 2008). Seizure frequency (# seizures/48 hr period, based on analysis of the entire EEG record) were calculated from each 48 hr epoch.
Statistics
Data are expressed as mean values ± SEM. Student's two-tailed t test was used for quantitative comparisons between two groups and ANOVA for comparisons for more than two groups, with Tukey multiple comparisons post-tests. Survival of ceftriaxone and saline-treated Tsc1GFAPCKO mice was analyzed by a Kaplan-Meier LogRank test. Statistical significance was defined as p<0.05.
Results
Early treatment with ceftriaxone increases GLT-1 expression in both control and Tsc1GFAPCKO mice
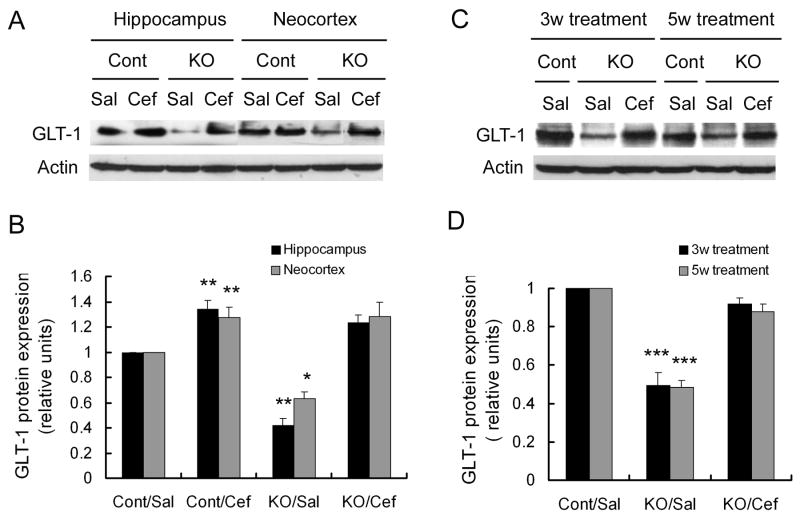
Previous studies have shown that daily injections of ceftriaxone at a dose of 200 mg/kg can induce increased expression of the astrocyte glutamate transporter, GLT-1 (Rothstein et al., 2005). In “early treatment” studies, we first initiated daily ceftriaxone (200 mg/kg, i.p., daily) or saline treatment at 3 weeks of age. Consistent with previous studies (Wong et al., 2003), saline-treated Tsc1GFAPCKO mice showed decreased GLT1 expression in both hippocampus and neocortex compared to saline-treated control mice. One week of ceftriaxone treatment increased GLT-1 protein expression in control mice by about 1.3 fold. Ceftriaxone increased GLT-1 expression in Tsc1GFAPCKO mice by 2-3 fold, correcting the deficient GLT-1 expression seen in these mice (Figure 1A,B). In other experiments, longer ceftriaxone treatment for 3 weeks or 5 weeks maintained this increase in GLT-1 expression in Tsc1GFAPCKO mice (Figure 1C,D). Dose-response studies showed that ceftriaxone reached a maximal effectiveness in inducing GLT-1 expression at 200 mg/kg (Supplementary Fig. 1A). In contrast, ceftriaxone did not affect phospho-S6 expression, indicating that any effects of ceftriaxone were not mediated by directly regulating the mTOR pathway (Supplementary Fig. 1B). While Tsc1GFAPCKO mice also exhibit decreased expression of the other major astrocyte glutamate transporter, GLAST (Wong et al., 2003), ceftriaxone did not alter the expression of GLAST in Tsc1GFAPCKO mice, demonstrating that ceftriaxone's effect was specific for GLT-1 (Supplementary Fig. 1C).
Figure 1.
Ceftriaxone increases astrocyte GLT-1 expression. (A) Three week old Tsc1GFAPCKO (KO) and control (Cont) mice received daily injections of ceftriaxone (Cef) or saline (Sal) for one week. A representative western blot of neocortical and hippocampal extracts shows a decrease in GLT-1 expression in saline-treated Tsc1GFAPCKO compared to control mice. Ceftriaxone increases GLT-1 expression in both control and Tsc1GFAPCKO mice. (B) Quantitative summary of all experiments confirms that ceftriaxone causes a significant increase in GLT-1 expression in both control and Tsc1GFAPCKO mice. The ratio of GLT-1/actin was normalized to the saline-treated control group. *p < 0.05, **p < 0.01 for both neocortex and hippocampus compared to the saline-treated control group by ANOVA (n = 6 mice per group). (C,D) Treatment of ceftriaxone for three and five weeks causes a sustained increased in GLT-1 expression in hippocampus of Tsc1GFAPCKO mice. ***p < 0.001 compared to the saline-treated control group by ANOVA (n = 6 mice per group).
Early treatment with ceftriaxone decreases extracellular glutamate levels and neuronal cell death in Tsc1GFAPCKO mice
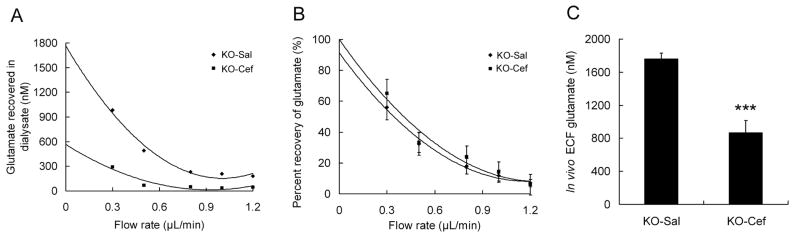
As GLT-1, a specific glutamate transporter in astrocytes, mediates synaptic glutamate uptake, a decrease in GLT-1 expression can lead to excessive extracellular glutamate levels and excitotoxic neuronal death. Related to the decreased GLT-1 expression of Tsc1GFAPCKO mice, we have previously demonstrated that extracellular glutamate levels are correspondingly elevated in the hippocampus of Tsc1GFAPCKO mice in vivo (Wong et al., 2003; Zeng et al., 2007). Thus, we performed additional microdialysis experiments to test whether ceftriaxone treatment could decrease extracellular glutamate levels in Tsc1GFAPCKO mice. After saline or ceftriaxone treatment for 3 weeks, the average hippocampal ECF glutamate concentration was significantly reduced in ceftriaxone-treated Tsc1GFAPCKO mice (Figure 2A,C; 867.6 ± 146.1 nM vs 1762.4 ± 70.1 for saline treated group; p<0.001, n=5 mice per group). By comparison, no difference in percentage recovery of glutamate was observed between Tsc1GFAPCKO mice treated with saline and ceftriaxone at each flow rate (Figure 2B), indicating that the microdialysis technique and other potentially confounding biological factors were consistent between the two groups. Although a direct comparison to glutamate levels in control mice was not repeated in the present studies, the extracellular glutamate concentration in ceftriaxone-treated Tsc1GFAPCKO mice was comparable to that of control mice in our previous study (Zeng et al., 2007).
Figure 2.
Ceftriaxone decreases extracellular glutamate levels in hippocampus in Tsc1GFAPCKO mice. (A) Extracellular glutamate concentrations were measured from saline- (KO-Sal) and ceftriaxone-treated (KO-Cef) Tsc1GFAPCKO mice with microdialysis at different flow rates. Representative examples from two mice are shown. A fitted polynomial curve shows the dependence of the measured dialysate glutamate concentration on flow rate for each mouse and allows extrapolation of the ECF glutamate concentration by the extrapolated zero flow method (See Methods). (B) Recovery percentage of glutamate at various flow rates was not significantly different between control and KO mice, indicating that the microdialysis technique and other biological factors were consistent between the two groups. (C) Average in vivo ECF glutamate concentration of saline- and ceftriaxone-treated Tsc1GFAPCKO mice was determined based on the extrapolated ECF glutamate concentration calculated individually for each mouse. Ceftriaxone-treated Tsc1GFAPCKO mice had significantly decreased ECF glutamate concentrations compared to saline-treated mice ***p<0.001 by t-test (n = 5 mice per group).
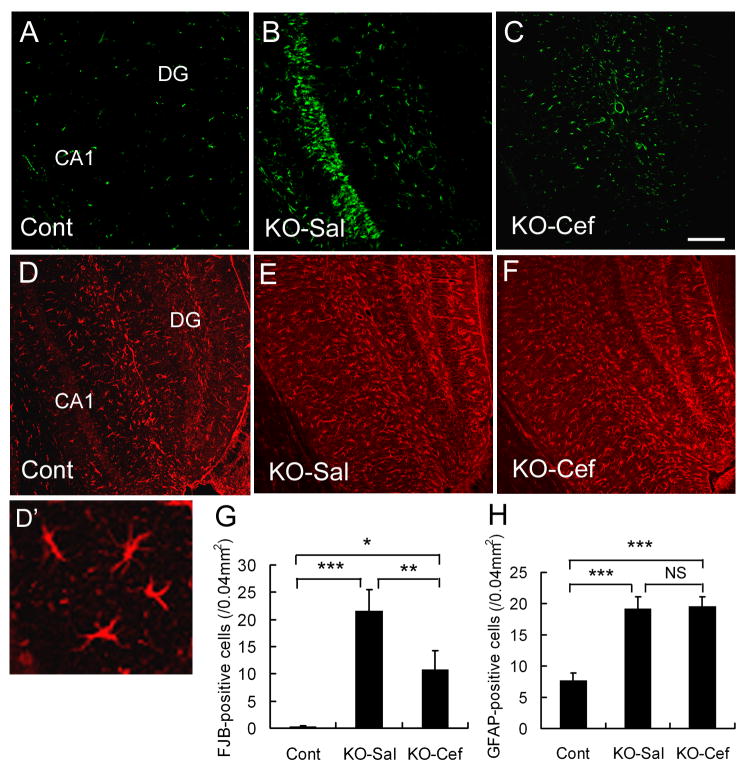
We have previously reported that Tsc1GFAPCKO mice exhibit excitotoxic neuronal death in hippocampus (Zeng et al., 2007). Since decreased extracellular glutamate may protect against neuronal excitotoxicity, we used multiple methods to assay neuronal cell death in control and Tsc1GFAPCKO mice treated with saline or ceftriaxone for 5 weeks. While control mice showed minimum neuronal death, saline-treated Tsc1GFAPCKO mice exhibited obvious pyramidal neuronal cell death in hippocampus, particularly in the CA1 region, as assayed by Fluoro-Jade B (Figure 3A,B) and TUNEL staining (not shown), and consistent with our previous studies (Zeng et al., 2007). Ceftriaxone treatment caused a significant decrease in neuronal death in hippocampus of Tsc1GFAPCKO mice (Figure 3C,G).
Figure 3.
Ceftriaxone reduces neuronal death in Tsc1GFAPCKO mice, but has no effect on astrocyte number. (A-C) Representative examples of Fluoro-Jade B positive cells in CA1 region of hippocampus in a control mouse (Cont) and Tsc1GFAPCKO mice treated with saline (KO-Sal) or ceftriaxone (KO-Cef). Saline-treated Tsc1GFAPCKO mice exhibit an increase in Fluro-Jade B (FJB) positive cells compared to control mice, and ceftriaxone treatment significantly reduces the amount of neuronal death in the CA1 region of Tsc1GFAPCKO mice (G). (D-F) Representative examples of GFAP staining in hippocampus of a control mouse and Tsc1GFAPCKO mice treated with saline or ceftriaxone (High power image showing astrocyte morphology in D'). Both saline- and ceftriaxone-treated Tsc1GFAPCKO mice exhibit an increase in GFAP-positive astrocytes compared to control mice, but ceftriaxone treatment has no effect on GFAP expression in Tsc1GFAPCKO mice (H). Calibration bars = 100 μm for all panels. *p < 0.05, **p < 0.01, ***p < 0.001 by ANOVA (n = 6 mice per group).
As Tsc1GFAPCKO mice exhibit increased proliferation of astrocytes (Uhlmann et al., 2002; Zeng et al., 2008) and a difference in astrocyte number may affect extracellular glutamate levels and neuronal death, we tested whether ceftriaxone treatment affected astrocyte number in Tsc1GFAPCKO mice. Consistent with the previous studies, Tsc1GFAPCKO mice showed a significant increase in GFAP-positive cells as compared to control mice (Figure 3D,E). However, ceftriaxone treatment did not affect astrocyte number (Figure 3F,H) in the Tsc1GFAPCKO mice. Correspondingly, Tsc1GFAPCKO mice exhibit megalencephaly and increased brain weight compared to control mice due to the glial proliferation (Zeng et al., 2008), but ceftriaxone had no effect on brain size or weight (control mice: 0.39 ± 0.03 g Tsc1GFAPCKO mice + saline: 0.50 ± 0.06 g; Tsc1GFAPCKO mice + ceftriaxone: 0.51 ± 0.05 g). These results indicate that the effects of ceftriaxone on GLT-1 expression and seizure frequency (below) were independent of changes in astrocyte proliferation.
Early treatment with ceftriaxone decreases seizures and improves survival in presymptomatic Tsc1GFAPCKO mice
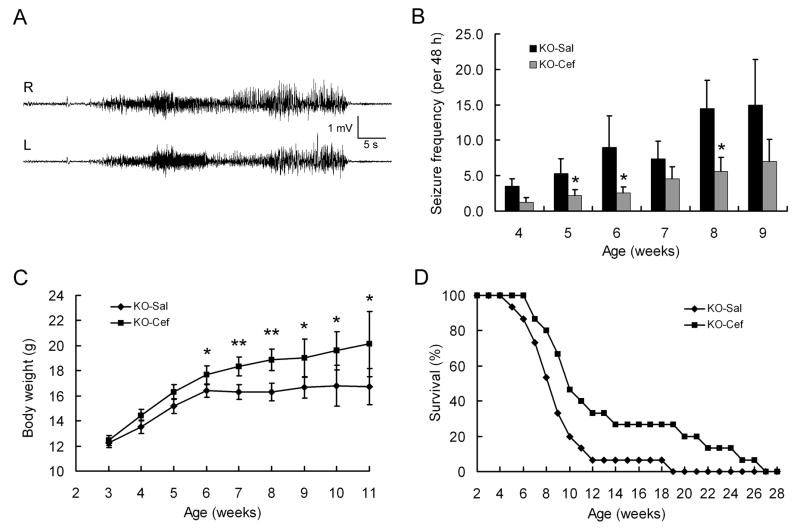
As elevated glutamate levels and excitotoxic neuronal death may promote epileptogenesis, we next examined the potential neuroprotective effect of ceftriaxone in decreasing or preventing epilepsy. In an “early treatment” paradigm, Tsc1GFAPCKO mice were treated with daily saline or ceftriaxone injections starting at 3 weeks of age, which precedes the typical age of onset of epilepsy in these mice (Erbayat-Altay et al., 2007), and underwent weekly video-EEG monitoring sessions to detect seizures. Consistent with previous studies (Erbayat-Altay et al., 2007; Zeng et al., 2008), seizures start to develop in saline-treated Tsc1GFAPCKO mice around 4-5 weeks of age and become progressively more frequent over the ensuing month (Figure 4A,B). By comparison, ceftriaxone-treated Tsc1GFAPCKO mice exhibited a similar onset and progression of seizures, but had a significantly decreased seizure frequency compared to saline-treated Tsc1GFAPCKO mice (Figure 4B). There was no significant difference in seizure frequency or the effect of ceftriaxone between male and female Tsc1GFAPCKO mice, except that the statistically-significant effects of ceftriaxone were observed at an earlier time point in female, compared to male mice (Supplementary Fig. 2A).
Figure 4.
Early treatment with ceftriaxone decreases seizures and improves survival in presymptomatic Tsc1GFAPCKO mice. (A) Representative EEG recording of a typical seizure in a Tsc1GFAPCKO mouse, recorded with right and left frontal epidural electrodes. Behaviorally, mice typically display rearing and repetitive forelimb clonus. (B) Saline-treated Tsc1GFAPCKO mice (KO-Sal) exhibit a progressive increase in seizures starting at 4 weeks of age. Tsc1GFAPCKO mice treated with daily ceftriaxone starting at 3 weeks of age (KO-Cef) exhibit a similar time course of epilepsy, but have a significant decrease in seizure-frequency. *p < 0.05 by ANOVA (n = 15 mice per group). (C) Ceftriaxone-treated Tsc1GFAPCKO mice have improved weight gain compared to saline-treated Tsc1GFAPCKO mice, which exhibit minimal weight gain after 6 weeks of age. *p < 0.05, **p < 0.01 by ANOVA (n = 15 mice per group). (D) Survival analysis shows that ∼50% of vehicle-treated Tsc1GFAPCKO mice died by 8 weeks of age, with all dead by about 4 months. In contrast, ceftriaxone causes a small but significant increase in survival of Tsc1GFAPCKO mice. p<0.05 by Kaplan-Meier LogRank test (n = 15 mice per group).
Tsc1GFAPCKO mice normally develop a wasting syndrome, with poor weight gain, and then die prematurely by 3-4 months of age (Uhlmann et al., 2002; Zeng et al., 2008). Compared to saline-treated Tsc1GFAPCKO mice, ceftriaxone-treated Tsc1GFAPCKO mice had improved weight gain and moderately, but significantly, prolonged survival, although all ceftriaxone-treated Tsc1GFAPCKO mice still died by about six months of age (Figure 4C,D). The beneficial effects of early ceftriaxone treatment on weight gain and survival were observed in both male and female mice (Supplementary Fig. 2C-E). Thus overall, early ceftriaxone treatment had some beneficial effects on seizures and survival of Tsc1GFAPCKO mice, but did not completely prevent epileptogenesis or neurological progression of these mice.
Late treatment with ceftriaxone fails to decrease seizures or improve survival in already symptomatic Tsc1GFAPCKO mice
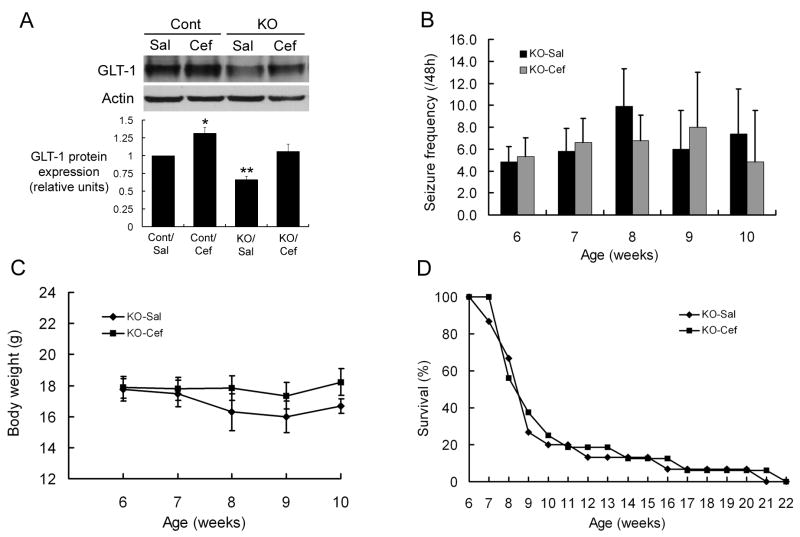
As early ceftriaxone treatment had significant effects on the neurological phenotype of Tsc1GFAPCKO mice, we next tested the effects of later treatment with ceftriaxone, starting at 6 weeks of age, after the typical onset of epilepsy in these mice. Although late ceftriaxone treatment increased GLT-1 expression in both control and Tsc1GFAPCKO mice (Figure 5A), there was no significant difference in seizure frequency between saline- and late ceftriaxone-treated Tsc1GFAPCKO mice (Figure 5B). When comparing seizure frequency between mice receiving early versus late treatment, there were trends toward lower seizure frequency in late saline-treated Tsc1GFAPCKO mice and higher seizure frequency in late ceftriaxone-treated Tsc1GFAPCKO mice compared to their respective counterparts in the early treatment groups, but these differences were not statistically significant. Similarly, late ceftriaxone treatment had no effect on body weight or survival (Figure 5C,D). Furthermore, the lack of effect of late ceftriaxone treatment on seizures (Supplementary Fig. 2B), body weight (data not shown), and survival (Supplementary Fig. 2F) was similarly observed in both male and female mice. Thus, in order to be effective, ceftriaxone had to be initiated prior to the onset of epilepsy and neurological progression in Tsc1GFAPCKO mice.
Figure 5.
Late treatment with ceftriaxone fails to decrease seizures or improve survival in already symptomatic Tsc1GFAPCKO mice. (A) Initiation of ceftriaxone at six weeks of age (after the typical onset of epilepsy in Tsc1GFAPCKO mice) for one week causes a significant increase in GLT-1 expression in hippocampus of both control and Tsc1GFAPCKO mice. *p < 0.05, **p < 0.01 compared to the saline-treated control group by ANOVA (n = 6 mice per group). (B-D) Daily late ceftriaxone treatment has no effect on seizure frequency, body weight, or survival of Tsc1GFAPCKO mice p > 0.05 by ANOVA (n = 15 mice per group).
Discussion
As seizures in TSC are often severe and intractable to current treatments, novel therapeutic approaches for epilepsy in TSC are definitely needed. Ideally, to increase the likelihood of effectiveness, new rational treatments for epilepsy would target and correct specific abnormal cellular or molecular mechanisms that mediate epileptogenesis. Although mechanisms of epileptogenesis in TSC are incompletely understood, there is some evidence in animal models and human tissue that abnormal glutamate homeostasis due to impaired astrocyte glutamate transport could be a contributing factor to epilepsy and other neurological deficits in TSC (Wong et al., 2003; Wu et al., 2005; Zeng et al., 2007). In the present study, we have shown that ceftriaxone, a drug that increases astrocyte glutamate transporter expression, restores normal extracellular glutamate levels and, when administered at an early age, correspondingly decreases excitotoxic neuronal death and severity of epilepsy in a mouse model of TSC. These results provide evidence to support the role of deficient astrocyte glutamate transporters in epileptogenesis in the Tsc1GFAPCKO mice and suggest a potential novel therapeutic option for epilepsy in TSC.
A deficiency in astrocyte glutamate transporters has been implicated in the pathophysiology of other neurological diseases, as well as other types of epilepsy. For example, expression of the astrocyte glutamate transporter, GLT-1, is dramatically decreased in human ALS and mouse models (Rothstein et al., 1995; Howland et al., 2002). Similarly, abnormalities in glutamate transporter expression have been reported in various animal models of epilepsy (Miller et al., 1997; Akbar et al., 1998; Samuelsson et al., 2000; Ingram et al., 2001; Dutuit et al., 2002; Harrington et al., 2007), as well as in human brain specimens resected from epilepsy patients (Mathern et al., 1999; Tessler et al., 1999; Crino et al., 2002; Proper et al., 2002). Furthermore, genetic or pharmacological manipulations that decrease astrocyte glutamate transporters can induce an epileptic phenotype in rodents (Tanaka et al., 1997; Watanabe et al., 1999; Milh et al., 2007). Conversely, beta-lactam antibiotics, such as ceftriaxone, and other drugs can cause potent upregulation of GLT-1 expression (Rothstein et al., 2005; Ganel et al., 2006), likely by a mechanism involving stimulation of GLT-1 expression through the nuclear factor-kappaB signaling pathway (Lee et al., 2008). Correspondingly, ceftriaxone has been shown to attenuate neurological deficits in animal models of ALS (Rothstein et al., 2005), Huntington's disease (Miller et al., 2008), and stroke (Thone-Reineke et al. 2008). Although it is always difficult to rule out the involvement of complementary or alternative mechanisms of a drug, the cumulative evidence of similar effects of ceftriaxone on GLT-1 expression and the behavioral phenotype in models of multiple neurological disorders makes GLT-1 regulation the most likely mechanism of action for its neuroprotective effects. Ceftriaxone has recently also been shown to reduce the severity of acute seizures induced in normal rats by the convulsant penetylenetetrazole (Jelenkovic et al, 2008), but, to our knowledge, the present study of Tsc1GFAPCKO mice represents the first reported evidence that upregulating astrocyte glutamate transporters can decrease seizures in a chronic epilepsy model.
This study provides some insights into the timing and mechanisms of epileptogenesis in Tsc1GFAPCKO mice. Consistent with previous reports (Rothstein et al., 2005), ceftriaxone administration was able to increase astrocyte GLT-1 expression within a week and this was associated with a corresponding decrease in extracellular glutamate levels in Tsc1GFAPCKO mice. While both early and late treatments with ceftriaxone were able to restore deficient GLT-1 expression of Tsc1GFAPCKO mice back to control levels, the effectiveness of ceftriaxone in subsequently decreasing excitotoxic neuronal death and epilepsy progression was dependent on the age of the mice, only being effective when starting treatment at 3 weeks, but not 6 weeks of age. In the first few weeks of life, Tsc1GFAPCKO mice have decreased astrocyte glutamate transporter expression, but otherwise appear normal, with no pathological abnormalities (Uhlmann et al. 2002; Wong et al., 2003). However, by 6 weeks of age, progressive neuropathological and neurological changes develop, including astrocyte proliferation, megalencephaly, and seizures (Uhlmann et al., 2002; Erbayat-Altay et al., 2007). Thus, it is not surprising that modulation of glutamate transporters by ceftriaxone would be more effective when administered at an earlier age before this progressive process occurs, as ceftriaxone did not reverse the pathological changes, as evident by the lack of effect on astrocyte number. Even with the earlier treatment, ceftriaxone was only partially effective in slowing the progression of epilepsy, indicating that modulation of astrocyte glutamate transporter only partially accounts for the neurological phenotype of this mouse model. Other relevant brain abnormalities in Tsc1GFAPCKO mice, such as glial proliferation and megalencephaly, were not affected by ceftriaxone and involve upstream signaling mechanisms, such as the mammalian target of rapamycin (mTOR). As the mTOR inhibitor, rapamycin, can completely prevent most of the neurological phenotype of Tsc1GFAPCKO mice, including deficient astrocyte glutamate transporters, glial proliferation, and epilepsy (Zeng et al., 2008), it is likely that mTOR is upstream from both astrocyte glutamate transporters and other parallel mechanisms.
The lack of effect of late ceftriaxone treatment could potentially also represent a false-negative result due to technical or experimental issues. The seizure frequency of the late saline-treated Tsc1GFAPCKO mice seemed lower than the early saline-treated mice (although the difference was not statistically significant) and our previously-published data on the natural history of seizures in the Tsc1GFAPCKO mice (Erbayat-Altay et al., 2007; Zeng et al., 2008), possibly obscuring an effect of late ceftriaxone. Natural variability or experimental factors, such as increased mortality of older Tsc1GFAPCKO mice related to EEG surgery or monitoring, could have contributed to an outlier effect in the late treatment groups. The sex of mice can also potentially influence a number of factors examined in this study, but a sub-analysis of the data in male and female mice found minimal influence of sex on the effects (or lack of effects) of ceftriaxone. There appeared to be a slight sex difference in the age at which early ceftriaxone treatment decreased seizure frequency, with female mice showing a beneficial response at an earlier age, relative to male mice. However, this could simply be due to low sample size of the single-sex analysis, as there was a trend toward decreased seizure frequency with early ceftriaxone in both sexes at all time points examined. Additional studies, with larger sample sizes for various subgroups, should improve the likelihood of finding other beneficial effects of late treatment of already symptomatic Tsc1GFAPCKO mice, which may have more immediate clinical relevance.
These findings have direct translational applications for developing better treatments for epilepsy in TSC based on rational targeting of specific mechanisms of epileptogenesis. Manipulation of upstream mechanisms most immediately regulated by the TSC genes, such as mTOR inhibition by rapamycin, may represent the most effective way of reversing the neurological phenotype of TSC (Zeng et al., 2008; Ehninger et al., 2008; Meikle et al., 2008). However, such an approach may affect numerous downstream pathways and thus also has the most potential for adverse side effects, including disruption of important developmental processes or learning mechanisms (Tang et al., 2002). More selectively targeting a downstream mechanism, such as deficient astrocyte glutamate transporters, may still maintain adequate efficacy for epilepsy, but may avoid other more widespread and unintended consequences. Furthermore, specifically targeting astrocytic mechanisms may also reduce common side effects, such as sedation and cognitive slowing, often seen in drugs that directly decrease neuronal excitability. As more is discovered about the mechanisms of epileptogenesis and other neurological deficits in TSC, the number of rational mechanistic-based treatments should increase, hopefully providing a range of therapeutic options for alleviating the neurological symptoms of TSC.
Supplementary Material
Supplementary Figure 1. (A) Dose-dependence of the effect of ceftriaxone on GLT-1 expression. GLT-1 expression is decreased in hippocampus of saline (Sal)-treated Tsc1GFAPCKO mice (KO) compared to control (Con) mice. Ceftriaxone treatment (number denotes daily ceftriaxone dose in mg/kg i.p. for one week) increases GLT-1 expression in a dose-dependent fashion, with maximal efficacy achieved at 200 mg/kg. (B) Ceftriaxone has no effect on mTOR pathway activation. Hippocampus of saline-treated Tsc1GFAPCKO mice has elevated mTOR activity, as reflected by phospho-S6 expression, compared to control mice, but ceftriaxone does not alter mTOR activation in control or Tsc1GFAPCKO mice. (C) In contrast to GLT-1 expression, ceftriaxone (200 mg/kg) has no effect on GLAST expression in hippocampus of Tsc1GFAPCKO mice. Representative blots are shown; experiments were repeated three times with similar results.
Supplementary Figure 2 Analyses of baseline characteristics and treatment effects on Tsc1GFAPCKO mice based on sex. (A, B) Analysis of seizure frequency. There were no significant differences in seizure frequency between male and female Tsc1GFAPCKO mice within all ages and treatment paradigms. (A) At specific ages, early ceftriaxone treatment decreased seizure frequency in female (4 weeks) and male (6 weeks) mice (n = 6 females, 9 males per group), although some significant effects of ceftriaxone in the combined sex data (5 weeks; Fig. 4B) were not detectable in either male or female subgroups due to the smaller sample size. *p<0.05 by ANOVA. (B) Late ceftriaxone had no effect on seizure frequency in both male and female Tsc1GFAPCKO mice (n = 10 females, 5 males per group). For some of the later time points in the combined sex analysis (Figs. 4B, 5B), the sample size in the individual sex groups was too small due to mortality to allow statistical analysis; thus, these later time points were not included in the single sex analysis. (C,D) Analysis of weight gain. Comparing male versus female mice (n = 5 females, 10 males per group), early ceftriaxone-treated and saline-treated male mice weighed significantly more than the respective ceftriaxone-treated and saline-treated female mice at some time points (8 or 9 weeks; p<0.05 by ANOVA) and thus were plotted separately. Early ceftriaxone treatment caused improvement in weight gain in both male (C) and female (D) Tsc1GFAPCKO mice. *p<0.05, **p<0.01 by ANOVA. Late ceftriaxone treatment had no effect on weight gain in both male and female Tsc1GFAPCKO mice (data not shown). For some of the later time points in the combined sex analysis (Figs. 4C, 5C), the sample size in the individual sex groups was too small due to mortality to allow statistical analysis; thus, these later time points (>9 weeks) were not included in the single sex analysis. (E,F) Survival analysis. There were no significant differences in survival between male and female mice within each of the treatment paradigms (early and late, saline and ceftriaxone treatment). (E) Early ceftriaxone treatment improved survival in both male and female Tsc1GFAPCKO mice. p<0.05 by Kaplan Meier LogRank test (n = 5 females, 10 males per group). (F) Late ceftriaxone had no effect on survival in both male and female Tsc1GFAPCKO mice (n = 10 females, 5 males per group).
Acknowledgments
This work was supported by the National Institutes of Health (K02NS045583 and R01NS056872 to MW; AG13956 to DH; P30 NS057105 to Washington University) and the Tuberous Sclerosis Alliance (MW). We thank the Division of Biostatistics at Washington University for assistance with statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbar MT, Rattray M, Williams RJ, Chong NW, Meldrum BS. Reduction of GABA and glutamate transporter messenger RNAs in the severe-seizure genetically epilepsy-prone rat. Neurosci. 1998;85:1235–1251. doi: 10.1016/s0306-4522(97)00684-2. [DOI] [PubMed] [Google Scholar]
- Bajenaru ML, Zhu Y, Hedrick NM, Donahoe J, Parada LF, Gutmann DH. Astrocyte-specific inactivation of the neurofibromatosis (NF1) gene is insufficient for astrocytoma formation. Mol Cell Biol. 2002;22:5100–5113. doi: 10.1128/MCB.22.14.5100-5113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Lee ST, Sinn DI, Ko SY, Kim EH, Kim JM, Kim SJ, Park DK, Jung KH, Song EC, Lee SK, Kim M, Roh JK. Pharmacological induction of ischemic tolerance by glutamate transporter-1 (EAAT2) upregulation. Stroke. 2007;38:177–82. doi: 10.1161/01.STR.0000252091.36912.65. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, May PC, O'Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMattos RB, Holtzman DM. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino PB, Shumate MD, Robinson MB, Coulter DA, Brooks-Kayal AR. Increased expression of the neuronal glutamate transporter (EAAT3/EAAC1) in hippocampal and neocortical epilepsy. Epilepsia. 2002;43:211–218. doi: 10.1046/j.1528-1157.2002.35001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- Dutuit M, Touret M, Szymocha R, Nehlig A, Belin MF, Didier-Bazes M. Decreased expression of glutamate transporters in genetic absence epilepsy rats before seizure occurrence. J Neurochem. 2002;80:1029–1038. doi: 10.1046/j.0022-3042.2002.00768.x. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2(+/-) mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbayat-Altay E, Zeng LH, Xu L, Gutmann D, Wong M. The natural history and treatment of epilepsy in a murine model of tuberous sclerosis. Epilepsia. 2007;48:1470–1476. doi: 10.1111/j.1528-1167.2007.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganel R, Ho T, Maragakis NJ, Jackson M, Steiner JP, Rothstein JD. Selective up-regulation of the glial Na+-dependent glutamate transporter GLT1 by a neuroimmunophilin ligand results in neuroprotection. Neurobiol Dis. 2006;21:556–567. doi: 10.1016/j.nbd.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Harrington EP, Moddel G, Najm IM, Baraban SC. Altered glutamate receptor-transporter expression and spontaneous seizures in rats exposed to methylaxoxymethanol in utero. Epilepsia. 2007;48:158–168. doi: 10.1111/j.1528-1167.2006.00838.x. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Stafstrom CE the Tuberous Sclerosis Study Group. Tuberous Sclerosis Complex and epilepsy: recent developments and future challenges. Epilepsia. 2007;48:617–630. doi: 10.1111/j.1528-1167.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc Natl Acad Sci USA. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram EM, Wiseman JW, Tessler S, Emson PC. Reduction of glial glutamate transporters in the parietal cortex and hippocampus of the EL mouse. J Neurochem. 2001;79:564–575. doi: 10.1046/j.1471-4159.2001.00612.x. [DOI] [PubMed] [Google Scholar]
- Jelenkovic AV, Jovanovic MD, Stanimirovic DD, Bokonjic DD, Ocic GG, Boskovic BS. Beneficial effects of ceftriaxone against pentylenetetrazole-evoked convulsions. Exp Biol Med. 2008;233:1389–1394. doi: 10.3181/0803-RM-83. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski DJ. Tuberous sclerosis: from tubers to mTOR. Ann Hum Genet. 2003;67:87–96. doi: 10.1046/j.1469-1809.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, Gupta P, Sarakar D, Borajabad A, Volsky DJ, Fisher PB. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem. 2008;283:13116–13123. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, Rauck B, Dejea C, Pardo AC, Rao MS, Rothstein JD, Maragakis NJ. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11:1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Wan CK, Bai JZ, Pi R, Li D, Donnelly D. Neuroprotective potential of ceftriaxone in in vitro models of stroke. Neuroscience. 2007;146:617–29. doi: 10.1016/j.neuroscience.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Mendoz D, Lozada BS, Pretorius JK, Dehnes Y, Danbolt NC, Nelson N, Leite JP, Chimelli L, Born DE, Sakamoto AC, Assirati JA, Fried I, Peacock WJ, Ojemann GA, Adelson PD. Hippocampal GABA and glutamate transporter immunoreactivity in patients with temporal lobe epilepsy. Neurology. 1999;52:453–472. doi: 10.1212/wnl.52.3.453. [DOI] [PubMed] [Google Scholar]
- Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski DJ. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menacherry S, Hubert W, Justice JB., Jr In vivo calibration of microdialysis probes for exogenous compounds. Anal Chem. 1992;64:577–583. doi: 10.1021/ac00030a003. [DOI] [PubMed] [Google Scholar]
- Milh M, Becq H, Villeneuve N, Ben-Ari Y, Aniksztejn L. Inhibition of glutamate transporters results in a “suppression-burst” pattern and partial seizures in the newborn rat. Epilepsia. 2007;48:169–174. doi: 10.1111/j.1528-1167.2006.00839.x. [DOI] [PubMed] [Google Scholar]
- Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, Kennedy RT, Rebec GV. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington's disease phenotype in the R6/2 mouse. Neurosci. 2008;153:329–337. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller HP, Levey AI, Rothstein JD, Tzingounis AV, Conn PJ. Alterations in glutamate transporter protein levels in kindling-induced epilepsy. J Neurochem. 1997;68:1564–1570. doi: 10.1046/j.1471-4159.1997.68041564.x. [DOI] [PubMed] [Google Scholar]
- Proper EA, Hoogland G, Kappen SM, Jansen GH, Rense GA, Schrama LH, van Veelen CWM, van Rijen PC, van Nieuwenhuizen O, Gispen WH, de Graan PNE. Distribution of glutamate transporters in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain. 2002;125:32–43. doi: 10.1093/brain/awf001. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Hoberg MD, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;43:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Samuelsson C, Kumlien E, Flink R, Lindholm D, Ronne-Engström E. Decreased cortical levels of astrocytic glutamate transport protein GLT-1 in a rat model of posttraumatic epilepsy. Neurosci Lett. 2000;289:185–188. doi: 10.1016/s0304-3940(00)01284-2. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Sosunov AA, Wu X, Weiner HL, Mikell CB, Goodman RR, Crino PD, McKhann GM., 2nd Tuberous sclerosis: a primary pathology of astrocytes? Epilepsia. 2008;49(Suppl2):53–62. doi: 10.1111/j.1528-1167.2008.01493.x. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci USA. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessler S, Danbolt NC, Faull RLM, Storm-Mathisen J, Emson PC. Expression of the glutamate transporters in human temporal lobe epilepsy. Neurosci. 1999;88:1083–091. doi: 10.1016/s0306-4522(98)00301-7. [DOI] [PubMed] [Google Scholar]
- Thone-Reineke C, Neumann C, Namsolleck P, Schmerbach K, Krikov M, Schefe JH, Lucht K, Hortnagl H, Godes M, Muller S, Rumschussel K, Funke-Kaiser H, Villringer A, Steckelings UM, Unger T. The beta-lactam antibiotic, ceftriaxone, dramatically improves survival, increases glutamate uptake and induce neurotrophins in stroke. J Hyperten. 2008;26:2426–2435. doi: 10.1097/HJH.0b013e328313e403. [DOI] [PubMed] [Google Scholar]
- Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, Yamada KA, Gutmann DH. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Morimoto K, Hirao T, Suwaki H, Watase K, Tanaka K. Amygdala-kindled and pentyleneterazole-induced seizures in glutamate transporter GLAST-deficient mice. Brain Res 36. 1999;845:92–96. doi: 10.1016/s0006-8993(99)01945-9. [DOI] [PubMed] [Google Scholar]
- Wong M. Mechanisms of epileptogenesis in tuberous sclerosis complex and related malformations of cortical development with abnormal glioneuronal proliferation. Epilepsia. 2008;49:8–21. doi: 10.1111/j.1528-1167.2007.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Ess KE, Uhlmann EJ, Jansen LA, Li W, Crino PB, Mennerick S, Yamada KA, Gutmann DH. Impaired astrocyte glutamate transport in a mouse epilepsy model of tuberous sclerosis complex. Ann Neurol. 2003;54:251–256. doi: 10.1002/ana.10648. [DOI] [PubMed] [Google Scholar]
- Wu X, Sosunov AA, Tikoo R, Weiner HL, Crino PB, McKhann GM., 2nd Glutamate transport is impaired in the human tuberous sclerosis tissue. Epilepsia. 2005;46(S8):279. [Google Scholar]
- Zeng LH, Ouyang Y, Gazit V, Cirrito JR, Jansen LA, Ess KC, Yamada KA, Wozniak DF, Holtzman DM, Gutmann DH, Wong M. Abnormal glutamate homeostasis and impaired synaptic plasticity and learning in a mouse model of tuberous sclerosis complex. Neurobiol Dis. 2007;28:184–196. doi: 10.1016/j.nbd.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. (A) Dose-dependence of the effect of ceftriaxone on GLT-1 expression. GLT-1 expression is decreased in hippocampus of saline (Sal)-treated Tsc1GFAPCKO mice (KO) compared to control (Con) mice. Ceftriaxone treatment (number denotes daily ceftriaxone dose in mg/kg i.p. for one week) increases GLT-1 expression in a dose-dependent fashion, with maximal efficacy achieved at 200 mg/kg. (B) Ceftriaxone has no effect on mTOR pathway activation. Hippocampus of saline-treated Tsc1GFAPCKO mice has elevated mTOR activity, as reflected by phospho-S6 expression, compared to control mice, but ceftriaxone does not alter mTOR activation in control or Tsc1GFAPCKO mice. (C) In contrast to GLT-1 expression, ceftriaxone (200 mg/kg) has no effect on GLAST expression in hippocampus of Tsc1GFAPCKO mice. Representative blots are shown; experiments were repeated three times with similar results.
Supplementary Figure 2 Analyses of baseline characteristics and treatment effects on Tsc1GFAPCKO mice based on sex. (A, B) Analysis of seizure frequency. There were no significant differences in seizure frequency between male and female Tsc1GFAPCKO mice within all ages and treatment paradigms. (A) At specific ages, early ceftriaxone treatment decreased seizure frequency in female (4 weeks) and male (6 weeks) mice (n = 6 females, 9 males per group), although some significant effects of ceftriaxone in the combined sex data (5 weeks; Fig. 4B) were not detectable in either male or female subgroups due to the smaller sample size. *p<0.05 by ANOVA. (B) Late ceftriaxone had no effect on seizure frequency in both male and female Tsc1GFAPCKO mice (n = 10 females, 5 males per group). For some of the later time points in the combined sex analysis (Figs. 4B, 5B), the sample size in the individual sex groups was too small due to mortality to allow statistical analysis; thus, these later time points were not included in the single sex analysis. (C,D) Analysis of weight gain. Comparing male versus female mice (n = 5 females, 10 males per group), early ceftriaxone-treated and saline-treated male mice weighed significantly more than the respective ceftriaxone-treated and saline-treated female mice at some time points (8 or 9 weeks; p<0.05 by ANOVA) and thus were plotted separately. Early ceftriaxone treatment caused improvement in weight gain in both male (C) and female (D) Tsc1GFAPCKO mice. *p<0.05, **p<0.01 by ANOVA. Late ceftriaxone treatment had no effect on weight gain in both male and female Tsc1GFAPCKO mice (data not shown). For some of the later time points in the combined sex analysis (Figs. 4C, 5C), the sample size in the individual sex groups was too small due to mortality to allow statistical analysis; thus, these later time points (>9 weeks) were not included in the single sex analysis. (E,F) Survival analysis. There were no significant differences in survival between male and female mice within each of the treatment paradigms (early and late, saline and ceftriaxone treatment). (E) Early ceftriaxone treatment improved survival in both male and female Tsc1GFAPCKO mice. p<0.05 by Kaplan Meier LogRank test (n = 5 females, 10 males per group). (F) Late ceftriaxone had no effect on survival in both male and female Tsc1GFAPCKO mice (n = 10 females, 5 males per group).