Abstract
Kisspeptin, as well as insulin-like growth factor-1 (IGF-1), act centrally to stimulate luteinizing hormone-releasing hormone (LHRH) secretion at puberty. IGF-1 can induce KiSS-1 gene expression as an early pubertal event; however, the signaling pathway mediating this effect is not known. Since alcohol (ALC) blocks IGF-1 induced LHRH release acutely, we assessed whether this drug could affect IGF-1 stimulated prepubertal KiSS-1 gene expression following a binge type of exposure. Immature female rats were administered either ALC (3g/kg) or water via gastric gavage at 0730 h. At 0900 h the ALC and control groups were subdivided where half received either saline or IGF-1 (200ng) into the third ventricle. A second dose of ALC (1.5, 2 and 3 g/kg) or water was administered at 1130 h. These regimens produced moderate blood alcohol concentrations of 77, 89 and 117 mg/dl, respectively, over the time course of the experiment. Rats were sacrificed 6 h after the IGF-1 injection and tissues containing the anteroventral periventricular (AVPV) and arcuate (ARC) nuclei were collected. IGF-1 stimulated (p<0.01) KiSS-1 gene expression in the AVPV nucleus at 6 h, but did not affect expression of the kisspeptin receptor, GPR54. While ALC did not alter basal expression of either gene, it dose dependently blocked IGF-1-induced KiSS-1 gene expression in the AVPV nucleus. No changes were observed in the ARC nucleus. Assessment of IGF-1 signaling indicated that the acute administration of IGF-1, ALC, or both did not alter the basal expression of IGF-1 receptor protein. However, IGF-1 stimulated (P<0.05) phosphorylated Akt protein over basal levels, an action blocked by ALC. Our results indicate that the IGF-1 induction of KiSS-1 gene expression is mediated by Akt activation, and that ALC alters this important prepubertal action of IGF-1.
Keywords: kisspeptin, puberty, ALC, IGF-1
The preoptic area and hypothalamus are considered the reproductive control center in the brain. This region plays the pivotal role in synchronizing events leading to the onset of mammalian puberty. Thus, a drug capable of affecting this brain region could alter the timing of puberty. In this regard, ALC is known to cause delayed vaginal opening in rats (Bo et al., 1982; Dees and Skelley, 1990; Dees et al. 1990), delayed development of a normal pattern of menstruation in rhesus monkeys (Dees et al. 2000), and altered puberty related hormones in rats, as well as rhesus monkeys and human adolescents (Dees et al., 1990; Dees et al., 2000; Diamond et al., 1986). Studies using rhesus monkeys have shown that these effects are associated with an action within the reproductive control center of the brain to diminish LH-releasing hormone (LHRH) secretion (Dissen et al., 2004).
Insulin-like growth factor-1 (IGF-1) is produced in numerous body tissues including brain, but the largest quantities are mainly produced in the liver (Sara and Hall, 1990). IGF-1 gene expression increases in the female hypothalamus toward the end of juvenile development (Daftary and Gore, 2003) but does not further increase during the peripubertal period (Hiney et al., 1996). The circulating levels of the peptide rise prior to puberty in rodents (Handelsman et al., 1987; Hiney et al., 1996) as well as primates (Copeland et al., 1982), including humans (Anders et al., 1994; Tam et al., 2006). Thus, increasing levels of both centrally and peripherally derived peptide are available to the hypothalamus at the time of puberty. Importantly, we have shown that IGF-1 administration during late juvenile development is capable of acting centrally to induce LHRH secretion and advance the time of puberty in female rats (Hiney et al., 1996). Subsequently, it was shown that IGF-1 administration advanced first ovulation in rhesus monkeys (Wilson ME, 1998), and advanced puberty in growth hormone-receptor knockout mice expressing low levels of the peptide (Danilovich et al., 1999). Collectively, these results suggest IGF-1 is an important component contributing to early signaling processes controlling LHRH secretion at puberty. The fact that ALC is capable of suppressing circulating levels of IGF-1 in prepubertal rats and rhesus monkeys (Dees et al., 2000; Srivastava et al., 1995) and altering some of the central actions of the peptide in prepubertal rats (Dees et al., 2005; Hiney et al., 1998; 2004) indicates IGF-1 is a target for ALC actions in prepubertal animals.
Kisspeptins, which are products of the KiSS-1 metastasis suppressor gene and ligands of the G protein-coupled receptor 54 (GPR54), have been shown to play a key role in the timing of the pubertal process. The importance of the KiSS-1/GPR54 system to reproduction was revealed by the discovery that a mutation of GPR54 in human (de Roux et al., 2003; Seminara et al., 2003) and a deletion of GPR54 locus in mice (Seminara et al., 2003) caused hypogonadotropic hypogonadism and delayed puberty. Additionally, the expression of KiSS-1 increases in brain during puberty, and KiSS-1 derived products, kisspeptin-54 and kisspeptin-10, act at the hypothalamus to stimulate prepubertal LH secretion (Navarro et al. 2004a; Shahab et al., 2005; Thompson et al., 2004). Although these studies indicate that KiSS-1 plays an important role in the onset of puberty, only recently have studies been conducted to discern the precise signal(s) that controls the increase in KiSS-1 gene expression during prepubertal development. In this regard, we recently determined that both central and systemic administration of IGF-1 during late juvenile development stimulates an increase in KiSS-1 gene expression in the reproductive hypothalamus prior to its normal rise at puberty (Hiney et al., 2009). Interestingly, we showed that the IGF-1 induction of KiSS-1 gene expression is dependent on adequate circulating levels of estradiol (E2) (Hiney et al., 2009). Thus, the principal goals of this study were to determine if ALC, a drug capable of delaying puberty, interferes with the ability of IGF-1 to induce prepubertal KiSS-1 gene expression in the anteroventral periventricular (AVPV) nucleus, and to exclude the possibility of an ALC-induced suppression in circulating levels of E2. Furthermore, because IGF-1 has been shown to utilize the Akt signaling pathway in other physiological situations (Cardona-Gomez et al., 2003; Clemmons and Maile, 2003; Li et al., 2008), we assessed in prepubertal animals whether IGF-1 induced KiSS-1 expression is mediated by Akt, then addressed whether ALC could interfere with this action
EXPERIMENTAL PROCEDURES
Animals
Eighteen-day pregnant female rats of the Sprague-Dawley line were purchased from Charles River (Boston, MA) and allowed to deliver pups normally in the Texas A&M University lab animal facility. Female pups were weaned at twenty-one d of age and housed three per cage under controlled conditions of light (lights on, 0600h; lights off, 1800h) and temperature (23 °C), with ad libitum access to food and water. All procedures performed on the animals were approved by the University Animal Care and Use Committee and in accordance with the NAS-NRC Guidelines for the Care and Use of Laboratory Animals. Surgical anesthesia was an intraperitoneal injection of 2.5% Tribromoethanol (0.5ml/ 60g body weight).
Experimental Design
Twenty-two-day-old female rats were implanted with third ventricular (3V) cannulae as described previously (Hiney et al. 2004). After four days of recovery, half of the rats were administered water (control group) and the other half received a dose of ALC (3g/Kg; 1.5 ml 25% ALC/100 g rat) by gastric gavage at 0730 h. This dose of ALC was chosen because a single intragastric injection to immature female rats yields a moderate blood ALC level and is capable of consistently suppressing LH release (Dees et al., 2005; Hiney et al., 1998). The animals were left undisturbed for 90 min. to allow time for ALC absorption. The ALC and control groups were then subdivided such that half of the rats in each group were injected with IGF-1 (rat IGF-1, Novozymes Biopharma AU Limited, Adelaide Australia; 200ng/3μl) and the other half received an equal volume of saline into the 3V at 0900 h. A second dose of ALC (either 1.5, 2 or 3 g ALC/kg) or water was administered gastrically at 1130 h (4 hr after the first dose) in order to maintain a moderately elevated serum ALC level over the course of the 6 h after the IGF-1 injection. This overall protocol was repeated several times to complete the study, and each replica experiment always contained control and IGF-1 only groups, and one of the ALC doses with and without IGF-1. The three dosing regimens are referred to as ALC-1 (3g/1.5g ALC/kg), ALC-2 (3g/2g ALC/kg) and ALC-3 (3g/3g ALC/kg), respectively. All animals were killed at 1500 h, 6 h after the 3V injection of IGF-1 or saline. The IGF-1 dose and 6 h time point were chosen based on our recent dose response study demonstrating IGF-1 induction of KiSS-1 gene transcription (Hiney et al., 2009). The brains were removed, 3V placement verified and blocks of tissue containing the AVPV and ARC nuclei were dissected from the brain as described previously (Hiney et al.,. 2009), then frozen on dry ice for molecular assessments. Blood alcohol concentrations (BACs) were assessed from tail tip blood samples collected from each animal at 1.5 and 4 h after the first ALC injection, and from the trunk blood collected at the end of the experiment. These three time points were used to determine the average blood level of ALC from each animal during the course of the day, and the BACs reported represent the mean of the daily levels from all of the animals in each respective dosing group. The serum level of E2 was also measured from trunk blood.
Isolation of total RNA
Total cellular RNA was initially isolated from the tissue blocks by homogenizing in TRIZOL reagent (Invitrogen Lifetech, CA). Each homogenate was further extracted for RNA using the QIAGEN RNeasy kit and treated with RNase-free DNase I according to the manufacturer's instructions (Qiagen Inc., Valencia, CA). The integrity of the RNA was checked by the visualization of the ethidium bromide-stained 28S and 18S ribosomal RNA bands. The concentration of RNA was measured spectrophotometrically by absorbance at 260 nm in a Model Smartspec 3000 spectrophotometer (Bio-Rad Laboratories, Hercules, CA).
Reverse Transcription and real-time quantitative PCR
Total RNA (1μg) from each preparation was denatured at 65 °C for 5 min and then samples were used for reverse transcription with oligo (dT) using SuperScript III First - strand Synthesis System (Invitrogen life tech., CA). Real-time PCR was performed in 25 μl reactions containing 2 μl cDNA, 500 nM primer pairs and 1X SYBR green PCR master mix in 96-well plates on an ABI PRISM 7500 sequence detection system (Applied Biosystems, Foster City, CA). PCR primers for the analysis were designed according to the guidelines of Applied Biosystems with help of the Primer Express 2.0 software (Applied Biosystems, Foster City, CA) and each primer was checked by BLAST search for the absence of any crossreactivity. To check the integrity of the RNA and to normalize for the quantity of RNA in the initial reverse transcription reaction, the housekeeping gene, ß-actin, was included in all reactions separately under the same experimental conditions. A reaction without reverse transcriptase was also performed to rule out the possibility of any contamination of genomic DNA. The primers for the PCR reactions are rat KiSS-1 [GenBank accession AY196983], forward, 5'-GCTGCTGCTTCTCCTCTGTGT-3', reverse, 5'-CTGTTGGCCTGTGGGTTCA-3'(product size 88 bp), rat GPR54 [GeneBank accession AF115516], forward, 5'-GCGGCCACAGATGTCACTTT-3', reverse, 5'-AGGTGGGCAGCGGATAGAG-3'(product size 70 bp); and rat ß-actin [GenBank accession NM_031144], forward, 5'-TCTGTGTGGATTGGTGGCTCTA-3', reverse, 5'-CTGCTTGCTGATCCACATCTG-3'(product size 69 bp).The PCR cycling conditions were 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 sec and 60 °C for 1 min. Dissociation curve analysis was also done for each gene at the end of the PCR reaction. In this regard, each amplicon generated a single peak and did not show any peak when the template was not included in the PCR reaction. Additionally, each PCR product was electrophoresed onto a 2% agarose gel containing 0.5 μg/ml ethidium bromide, which showed a single band of the expected size. This confirms the specificity of the primers and shows that there was no formation of primer-dimers. The raw data from each experiment was used to determine the relative levels of expression for each gene by the delta-delta CT method as described by Hettinger et al (2001).
Immunoblotting
Brain tissues were homogenized in 1X PBS, 1% Igepal CA 630, 0.5% sodium deoxycholate, 0.1% SDS, 1mM PMSF, 10 μg/ml aprotinin, 10μg/ml leupeptin, 1 mM sodium orthovanadate at 4 °C. The homogenates were incubated on ice for 30 min and centrifuged at 12,000 × g for 15 min. The concentration of total protein in the resulting supernatants was then determined by the Bradford protein assay (Bio-Rad Laboratories, Richmond, CA) using bovine serum albumin as standard. Immunoblot analysis was performed by solubilizing the proteins (100μg) in a sample buffer containing 25 mM Tris HCl, pH 6.8, 1% SDS, 5% ß-mercaptoethanol, 1mM EDTA, 4% glycerol, and 0.01% bromophenol blue and electrophoresed through a 12% SDS-PAGE under reducing conditions. The separated proteins were electrophoretically transblotted onto polyvinylidene difluoride (PVDF) membranes. Membranes were blocked with 5% nonfat dried milk/0.05% Tween 20 in PBS (pH 7.4) for 3 h and subsequently incubated at 4 °C overnight with rabbit anti-IGF-1 receptor (IGF-1R; 1:300; Abcam Inc., Cambridge, MA), phospho-Akt (Ser 473, 1:300; Abcam Inc.) and total Akt (1μg/ml; Abcam Inc.). Following incubation, membranes were washed in PBS buffer containing 0.05% Tween-20 and then incubated with horseradish peroxidase-labeled goat anti-rabbit IgG (1:12000; Abcam Inc., Cambridge, MA) for IGF-1R, phospho-Akt and total Akt for 2 h at room temperature. After washing, the specific signals were detected with the enhanced chemiluminiscence method (Western Lightning Plus-ECL, PerkinElmer, Shelton, CT) and quantified with scanning densitometry using Quantity One software (Bio-Rad, Hercules, CA). Subsequently, membranes were stripped with 100mM ß-mercaptoethanol, 2% SDS, 62.5 mM Tris HCl, pH 6.7 and reprobed with a goat anti-mouse polyclonal antibody to ß-actin and anti-goat horseradish peroxidase-labeled secondary antibody, to normalize for the amount of sample loading. Following washing, the detection of proteins and quantitation was done as described above.
Enzyme-linked immunosorbent assay (ELISA)
Phosphorylated Akt and total Akt were measured in tissue lysates using the PathScan Elisa kit (Cell Signaling Technology, Inc. Danvers, MA) following the manufacturer's instructions. The concentration of total protein in tissue lysates was determined as described in the immunoblotting section. The color intensity was measured using ELx800™ (Bio-Tek Instrument, Highland Park, Vermont, USA) microplate reader at 450 nm. In this assay, the total Akt was used as an internal control to normalize for the amount of phosphorylated Akt and expressed as mean ± standard error.
Radioimmunoassays
The serum level of E2 was measured as described previously (Hiney et al., 1996). The E2 assay kit was purchased from Diagnostic Products Corp. (Los Angeles, CA) and the sensitivity of this assay was 5pg/ml and the intra-assay variation was 5%.
Statistical Analysis
Statistical comparisons shown in all the figures were performed using analysis of variance, with post hoc testing using the Student-Newman-Keuls multiple range test and the Bonferoni test. Both tests showed identical results. All statistical tests were conducted using INSTAT and Prism software for the IBM PC (GraphPad, Inc., San Diego, CA). Probability values <0.05 were considered to be statistically significant.
Serum ALC Analysis
Blood ALC concentrations (BACs) were assessed from tail tip blood samples collected from each animal at 1.5 and 4 h after the first ALC injection, and from the trunk blood collected at the end of the experiment. Serum was transferred to microcentrifuge tubes for analysis of serum ALC concentrations as described previously (Dees et al., 2005) by an enzymatic method using a diagnostic kit purchased from Genzyme, Oxford, CT.
RESULTS
Actions and interactions of IGF-1 and ALC on KiSS-1 and GPR54 genes
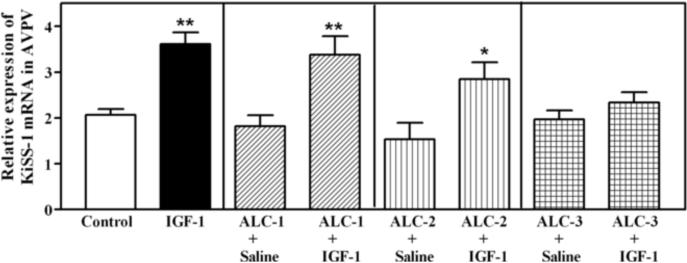
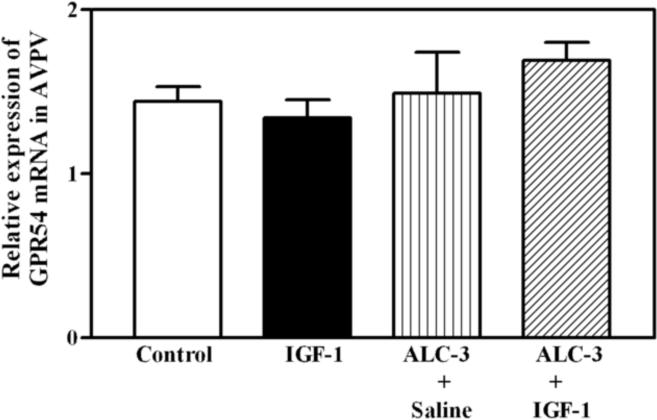
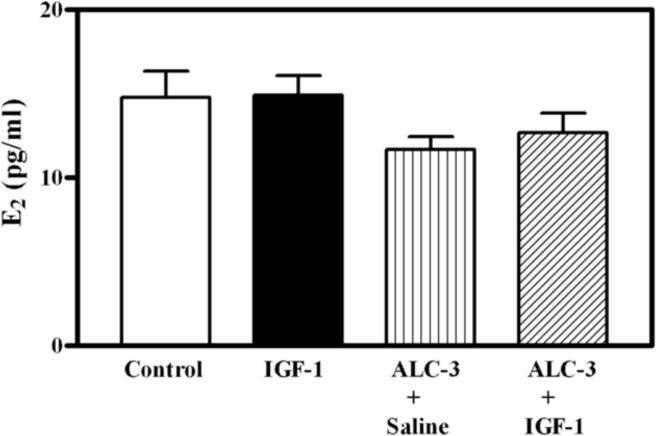
The benge type of ALC exposure method used in this study resulted in moderately elevated BACs. The three ALC administration regimens that were utilized produced BACs that averaged 77, 89 and 117 mg/dl, respectively, over the 6 hour time period that the animals were stimulated with the IGF-1. Figure 1 depicts the dose related effects of IGF-1 and ALC on KiSS-1 gene expression in the AVPV nucleus of prepubertal female rats. As expected, the level of KiSS-1 gene expression was markedly (p<0.01) increased in the animals 6 h after receiving a 3V injection of IGF-1 (200 ng), compared to the basal levels observed in control animals after receiving a 3V injection of saline. ALC did not acutely alter basal KiSS-1 gene expression at any of the doses assessed. The IGF-1 induced increase in KiSS-1 gene expression was not altered in animals with BACs averaging 77 mg/dl (ALC-1), and was only modestly affected in animals with BACs averaging 89 mg/dl (ALC-2); however, the IGF-1 stimulation of the KiSS-1 gene was blocked completely in animals with BACs averaging 117 mg/dl (ALC-3). This was shown to be a regional effect since neither IGF-1 nor ALC exposure altered expression of the KiSS-1 gene in the ARC nucleus (not shown). Figure 2 demonstrates that acute administration of IGF-1 did not induce gene expression of the kisspeptin receptor, GPR54, in the AVPV nucleus, and that the basal level of GPR54 was unaltered in rats with BACs averaging 117 mg/dl. Additionally, no changes in kisspeptin receptor gene expression were observed in the ARC nucleus (not shown). Since the circulating levels of E2 play an important role in regulating KiSS-1 gene expression, the serum levels of this steroid were assessed. Figure 3 shows that the basal levels of serum E2 were not altered by IGF-1 or ALC; thus, indicating that the ALC blockade of IGF-1 induced KiSS-1 gene expression shown in figure 1 was not due to changes in circulating E2.
Figure 1.
Effect of IGF-1 and ALC on KiSS-1 gene expression in the AVPV nucleus as assessed by real-time PCR. Animals that received saline intragastrically, plus a 3V injection of saline, showed basal (control; open bar) levels of KiSS-1 gene expression. The animals that received saline intragastrically, plus a 3V injection of IGF-1 (200 ng; black bar), showed an increased (p<0.01) level of KiSS-1 gene expression over controls. All of the animals that received ALC intragastrically by one of the three ALC dosing regimens (ALC 1–3), plus a 3V injection of saline, showed levels of KiSS-1 gene expression that were similar to the basal controls. These three regimens produced average BACs of 77 (ALC-1; hatched bars), 89 (ALC-2; horizontal bars) and 117 mg/dl (ALC-3; vertical bars), respectively. Note that ALC dose dependently altered the ability of IGF-1 to induce KiSS-1 gene expression, with the ALC-3 regimen completely blocking the IGF-1 effect. Each bar represents the mean (± SEM) relative expression of the KiSS-1 gene 6 h after the central infusion of IGF-1 or saline. The number of animals represented in each bar: Control = 30; IGF-1 = 30; ALC (1–3) + saline = 8/group; ALC (1–3) + IGF-1 = 8–19/group. **p <0.01; *p<0.05 by Student Neuman Keul's and Bonferroni multiple range tests.
Figure 2.
Effects of IGF-1 and ALC on kisspeptin receptor (GPR54) expression in the AVPV nucleus as assessed by real-time PCR. Animals that received saline intragastrically, plus a 3V injection of saline, showed basal (control; open bar) levels of GPR54 expression. Animals that received saline intragastrically, plus a 3V injection of IGF-1 (200 ng; black bar) or ALC intragastrically, plus a 3V injection of either saline (vertical line bar), or IGF-1 (hatched bar), showed that neither the acute administration of IGF-1 nor ALC altered GRP54 gene expression. The ALC was administered to animals using the ALC-3 regimen described in the methods. Each bar represents the mean (±SEM) relative expression of the GPR54 gene 6 h after the central infusion of IGF-1 or saline. The number of animals represented in each bar is 7–11.
Figure 3.
Effects of IGF-1 and ALC on the circulating levels of E2. Animals that received saline intragastrically, plus a 3V injection of saline showed basal (control; open bars) levels of E2. Animals that received saline intragastrically, plus a 3V injection of IGF-1 (200 ng; black bar) or ALC intragastrically, plus either a 3V injection of saline (vertical line bar) or IGF-1 (hatched bar), showed no changes in the serum level of E2, indicating that the suppressed KiSS-1 gene expression depicted in figure 1 was not due to altered circulating levels of the steroid. The ALC was administered using the ALC-3 regimen. Each bar represents the mean (±SEM) E2 serum levels 6 h after IGF-1 or saline. The number of animals represented in each bar is 16–22.
Effects of ALC on IGF Signaling in the AVPV nucleus
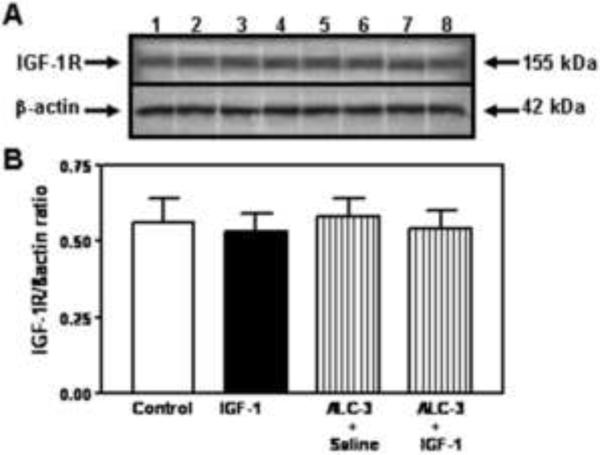
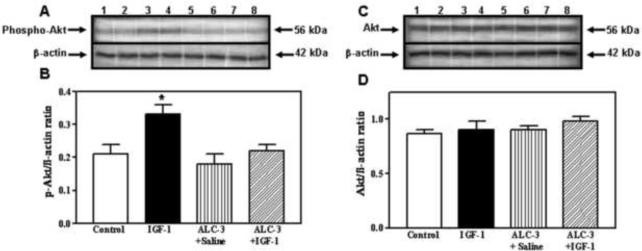
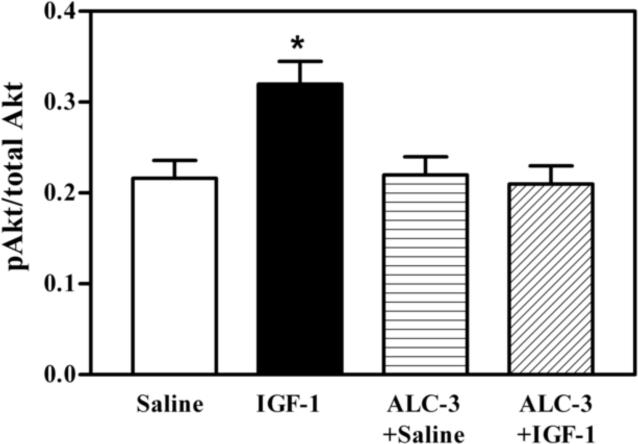
Because the interactions between IGF-1 and E2 are important for IGF-1 functions (Cardona-Gomez et al., 2003; Navarro et al., 2004b), and since the acute ALC exposure model that produced BACs of 117 mg/dl caused a suppression in IGF-1 induced KiSS-1 expression in the AVPV nucleus without altering serum E2 levels, we assessed the potential for this ALC exposure regimen to affect IGF-1 signaling in this brain region. The basal protein levels of IGF-1 receptor (IGF-1R) were not affected by the acute administration of either IGF-1 or ALC (Figure 4). Using immunoblotting, we demonstrate the effects of IGF-1 and ALC on Akt, a transduction signal that is synergistically activated by IGF-1 and E2. Figure 5A and B shows that the level of phosphorylated Akt protein expression in the AVPV nucleus was increased (p<0.05) in the animals 6 h after receiving a 3V injection of IGF-1 (200 ng), compared to the basal levels observed in control animals after receiving a 3V injection of saline. ALC did not acutely alter this basal level of protein expression; however, the noted IGF-1 stimulation in the expression of phosphorylated Akt protein was blocked completely by the presence of ALC. Importantly, Figure 5C and D show that total Akt protein expression in the AVPV nucleus was not altered by the acute administration of either IGF-1 or ALC. Additionally, figure 6 demonstrates that an ELISA assay produced identical results as shown with the immunoblotting method.
Figure 4.
Effects of IGF-1 and ALC on IGF-1R protein in the AVPV nucleus. The experimental protocol using the 4 groups (control, IGF-1, ALC + saline and ALC + IGF-1) is identical to that described in figure 2 which employed the ALC-3 regimen. A) Representative western blot of IGF-1R and ß- actin proteins from control (lanes 1–2), IGF-1 (lanes 3–4), ALC + saline (5–6) and ALC + IGF-1 (lanes 7–8) animals. B) Densitometric quantification of all bands assessing the IGF-1R protein. These data were normalized to the internal control β-actin protein. Note that neither IGF-1 (black bar), ALC (vertical bar), nor IGF-1 + ALC (hatched bar) altered the basal expression (open bar) of IGF-1R protein. Each bar represents the mean ± SEM of the IGF-1R/β-actin ratio. The number of animals represented by each bar is 4.
Figure 5.
Effects of IGF-1 and ALC on phosphorylated and total Akt protein expression in the AVPV nucleus. The experimental protocol using the 4 groups (control, IGF-1, ALC + saline, and ALC + IGF-1) is identical to that described in figure 2 which employed the ALC-3 regimen. A) Representative Western blot of phosphorylated (p) Akt and β-actin isolated from control (lanes 1–2), IGF-1 (lanes 3–4), ALC + saline (lanes 5–6) and ALC + IGF-1 (lanes 7–8) animals. B) Densitometric quantitation of all bands assessing the p-Akt protein. Note that IGF-1induced an increase in p-Akt protein levels (black bar) compared to basal protein levels (open bar). Importantly, while ALC alone (vertical bar) did not alter p-Akt levels compared to basal, it was capable of blocking the IGF-1-induced increase in p-Akt protein (hatched bar). C) Representative Western blot of the total, non-phosphorylated Akt and β-actin proteins isolated from control (lanes 1–2), IGF-1 (lanes (3–4), ALC + saline (lanes 5–6) and ALC + IGF-1 (lanes 7–8) animals. D) Densitometric quantitation of all bands assessing total, non-phosphorylated Akt protein. Note that neither IGF-1 nor ALC affected total Akt protein. All of the above data were normalized to the internal control β-actin. Each bar represents the mean ± SEM of the phosphorylated or total Akt/β-actin ratios. The number of animals represented in each bar is 4–6. *p<0.05 by Student Neuman Keul's and Bonferroni multiple range tests.
Figure 6.
Effects of IGF-1 and ALC on Akt protein expression in the AVPV nucleus using an enzyme-linked immunosorbent assay analysis of the ratio of phosphorylated to total Akt. The experimental protocol using the 4 groups (control, IGF-1, ALC + saline, and ALC + IGF-1) is identical to that described in figure 2 which employed the ALC-3 regimen. IGF-1 induced an increase in p-Akt protein levels (black bar) compared to basal protein levels (open bar). Importantly, while ALC alone (vertical bar) did not alter p-Akt levels compared to basal, it was capable of blocking the IGF-1-induced increase in p-Akt protein (hatched bar). Not that these results confirm the effect shown in figure 5 when ß-actin was used as the internal control. Each bar represents the mean ± SEM of the p-Akt/total Akt ratio. The number of animals represented by each bar is 5. *p<0.05 by Student Neuman Keul's and Bonferroni multiple range tests.
DISCUSSION
IGF-1 is a polypeptide hormone that plays an early role in the control of hypothalamic LHRH secretion and the timing of female puberty (Hiney et al., 1996; Wilson ME, 1998). Over the years we have shown that both acute and chronic ALC exposure alters IGF-1 signaling and causes suppressed LHRH/LH secretion and altered pubertal development in both rats and rhesus monkeys (Dees et al., 2000; Hiney et al, 1998; Srivastava et al., 1995). The KiSS-1/GPR54 system is also an important signaling component controlling LHRH secretion and the time of puberty in rats, rhesus monkeys and humans (Navarro et al., 2004a; 2004b; Seminara et al., 2003; Shahab et al., 2005; Thompson et al., 2004). Although these studies demonstrate that KiSS-1 and GPR54 play important roles in the onset of puberty, only recently have factors been investigated that may control or alter expression of the KiSS-1 gene during this important time of development. In this regard, we have shown that IGF-1 is capable of differential induction of prepubertal KiSS-1 expression in that the KiSS-1 gene increased in the AVPV nucleus, but not in the ARC (Hiney et al., 2009), suggesting that this peptide is an upstream regulator of this puberty-related gene. Furthermore, we have shown that chronic ALC administration suppresses basal prepubertal KiSS-1 gene expression in both the AVPV and ARC nuclei (Srivastava et al., 2009), and now describe the interrelationships between ALC, IGF-1 and the KiSS-1/GPR54 system.
In the present study, we have demonstrated that while acute ALC administration did not affect basal KiSS-1 gene expression in either the AVPV or ARC nuclei, it blocked the ability of IGF-1 to induce the expression of the KiSS-1 gene in the AVPV nucleus. The moderate BACs observed following acute exposure to the drug did not alter the circulating levels of E2. The fact that there was not a marked decrease in E2 is important, since it is known that IGF-1 induced KiSS-1 expression in the AVPV nucleus depends on the presence of E2 (Hiney et al., 2009), and that changes in the serum levels of E2 can differentially influence KiSS-1 expression in both AVPV and ARC nuclei (Roa et al., 2006; Smith et al., 2005; 2007); hence, the effect of acute ALC exposure to inhibit the IGF-1 induction of KiSS-1 gene expression in the AVPV nucleus appears to be a centrally mediated action of the drug and not due to E2.
ALC did not alter the gene expression of kisspeptin receptor, GPR54. Also, basal GPR54 expression was unaffected even though IGF-1 acutely stimulated the expression of KISS-1. Other studies have shown an increase in GPR54 gene expression when KiSS-1 gene expression had increased (Seminara et al., 2003; Shahab et al., 2005), however, those studies considered a much longer time frame than the present acute study in which there likely was not enough time for up-regulation of GPR54 to occur. Interestingly, Han et. al. (2005) showed that while the response of LHRH neurons to kisspeptins increased as puberty approached, expression of the GPR54 gene was not altered.
Because IGF-1 activates prepubertal KiSS-1 gene expression in the AVPV nucleus, and because ALC can block this event, we assessed the effect of ALC on hypothalamic IGF-1R protein expression. The basal expression of IGF-1R protein in the AVPV nucleus was not altered following the acute central delivery of IGF-1. Furthermore, the ALC did not alter IGF-1R protein synthesis, although we cannot rule out the possibility that it may have affected some pharmacological properties regulating IGF-1R function. Thus, these results suggest that ALC may target IGF-1 signaling components downstream from the IGF-1R.
IGF-1 and E2 work together to regulate hypothalamic neuronal development, plasticity and neuroendocrine function (Cardona-Gomez et al., 2002; 2003; Etgen et al., 2006; Hiney et al., 2004; 2009). Since both IGF-1 and E2 can independently and synergistically activate the Akt signaling pathway in the adult hypothalamus (Cardona-Gomez et al., 2002; 2003), and because serum E2 levels were not suppressed in the present study, we investigated the possibility that the central administration of IGF-1 at puberty can stimulate Akt, and that ALC may block this action resulting in suppressed KiSS-1 gene expression. Importantly, we demonstrated that IGF-1 induced the phosphorylation of Akt protein; hence, indicating that Akt is a major signaling component involved in IGF-1 stimulation of the KiSS-1 gene. Furthermore, while acute exposure to ALC did not affect the basal hypothalamic expression of phosphorylated Akt, it blocked the induction of phosphorylated Akt by IGF-1, an effect independent of circulating levels of E2. Although not related to IGF-1, ALC-induced suppressions in the phosphorylation of Akt have been observed previously in other brain regions (Li et al., 2004; Tsuji et al., 2008), as well as in the liver (He et al., 2006).
The fact that Akt is part of the IGF-1/KiSS-1 signaling pathway at the time of puberty is deserving of further discussion. A recent article has shown that the activation of the mammalian target of rapamycin (mTOR), a downstream serine/threonine protein kinase also results in an increase in KiSS-1 mRNA expression in prepubertal female rats (Roa et al., 2009). Therefore, based on the present results and the fact that mTOR is activated by Akt (Wullschleger et. al. 2006), we suggest that the IGF-1 induction of Akt results in the downstream activation of the mTOR signaling pathway and ultimately, the prepubertal stimulation of the KiSS-1 gene. With regard to the effect of ALC on this pathway, we propose that the disruption in IGF-1-induced Akt phosphorylation caused by the drug may lower mTOR signaling, resulting in a decrease in KiSS-1 gene expression.
While our study provides an insight regarding effects of ALC on IGF-1 signaling within the prepubertal hypothalamus, more work is needed to gain a better understanding of the mechanism by which ALC alters hypothalamic Akt phosphorylation. For example, it is possible that ALC may have interfered with translocation of Akt from the cytosol to the plasma membrane and binding to phospatidylinositol-3, 4, 5-triphosphate, (PIP3), a step critical for phosphorylation by phosphinositide-dependent kinase 1 (Andjelkovic et al., 1977; Hill and Hemmings, 2002). In this regard, Ling et al. (2006) showed that in liver cells, chronic ethanol exposure altered insulin signaling by preventing the association of Akt with plasma membrane bound PIP3, a prerequisite for 3-phosphoinositidede-dependent kinase-1 phosphorylation of Akt at Thr-308. Although the exact means by which ALC blocked IGF-1 induced Akt phosphorylation in the hypothalamus is not known, the fact that the drug can acutely block this transduction signal used by IGF-1 is important, because IGF-1 actions within the hypothalamus are critical for driving LHRH release during peripubertal maturation, with its stimulatory effect on KiSS-1/kisspeptin playing an important role in this regard (Hiney et al., 2009).
Results from the present study have extended our knowledge regarding the effects of ALC on IGF-1/KiSS-1 interactions. We clearly showed that the acute, single day administration of ALC did not affect basal KiSS-1 gene expression, but did block IGF-1 stimulated phosphorylation of Akt and the subsequent expression of KiSS-1. Interestingly, a recent study, in which ALC was administered to prepubertal female rats for 6 days using a specific liquid diet regimen, showed that this more chronic exposure to the drug caused suppression in basal KiSS-1 gene expression, an action that was associated with suppressed basal phosphorylated Akt protein expression (Srivastava et al., 2009). In that study, the ALC also caused suppressed levels of IGF-1 and E2, which is associated with ALC-induced delayed purberty and is common following extended exposure to the drug (Dees et al., 2000; Srivastava et al., 1995). It was not possible in that chronic study to discern whether the longer exposure to ALC altered Akt and KiSS-1 expressions by a direct hypothalamic action on the affected neurons, or acted indirectly, as a result of a low IGF-1 signal. In this regard, the lower IGF-1 signal, following the chronic ALC exposure, could have been due to the depressed circulating levels of the IGF-1 peptide available to the brain, as well as to the suppressed levels of the steroid resulting in a reduced facilitation of the IGF-1 effect. The present study does, however, show that the drug is capable of altering hypothalamic neuronal function directly by blocking the IGF-1 induction of Akt protein, and subsequently, causing suppressed KiSS-1 gene expression. In support of this neuronal action, ALC has also been shown to block insulin-stimulated Akt activation in cerebellar neurons (de la Monte and Wands, 2002), as well as inhibit brain derived neurotrophic factor-induced Akt phosphorylation in the cerebral cortex (Li et al., 2004). The noted hypothalamic effect of ALC described above is of potential importance since it suggests that the drug may contribute to altered pubertal development not only by causing suppressed circulating levels of IGF-1, but also by interfering with the ability of the peptide to act within the hypothalamus to induce a significant puberty-related gene.
The KiSS-1/kisspeptin system is a potent and critical regulator of LHRH secretion at puberty. Since we have recently shown that IGF-1 is an upstream regulator of the KiSS-1 gene, we assessed in this study whether Akt signaling was involved, and whether ALC, a drug known to alter prepubertal LHRH release, could interfere with this important action of the IGF-1 peptide. In this regard, we have shown for the first time that acute ALC exposure blocks IGF-1 stimulated KiSS-1 gene expression in the reproductive hypothalamus of prepubertal female rats. This action was associated with altered IGF-1 signaling since ALC inhibited the phosphorylation of Akt, an important transduction signal utilized by IGF-1. We suggest that this action of ALC contributes to the ability of this drug to cause diminished peripubertal LHRH/LH secretion at the time of puberty.
Acknowledgments
Grant Support: Supported by the National Institutes of Health Grant AA-07216 (to W.L.D.).
List of abbreviations
- 3V
third ventricle
- ALC
alcohol
- AVPV
anteroventral periventricular nuclei
- ARC
arcuate nuclei
- BAC
blood alcohol concentration
- E2
estradiol
- GPR54
G protein-coupled receptor 54
- IGF-1
insulin-like growth factor-1
- IGF-1R
insulin-like growth factor-1 receptor
- KiSS-1
kisspeptin gene
- LH
luteinizing hormone
- LHRH
luteinizing hormone releasing hormone
- mTOR
mammalian target of rapamycin
- PBS
phosphate buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anders J, Bang P, Hertel NT, Main K, Dalgaard P, Jorgensen K, Muller J, Hall K, Skakkebaek NE. Serum insulin-like growth factor-1 in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size and body mass index. J. Clin. Endocrinol. Metab. 1994;78:744–752. doi: 10.1210/jcem.78.3.8126152. [DOI] [PubMed] [Google Scholar]
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, French M, Cron P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1977;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- Bo WJ, Krueger WA, Rudeen PK, Symmes SK. Ethanol-induced alterations in the morphology and function of the rat ovary. Anat Rec. 1982;202:255–260. doi: 10.1002/ar.1092020210. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, Garcia-Segura LM. Synergistic interaction of estradiol and insulin-like growth factor-1 in the activation of P13K/Akt signaling in the adult rat hypothalamus. Mol Brain Res. 2002;107:80–88. doi: 10.1016/s0169-328x(02)00449-7. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogen and insulin-like growth factor-1 in the brain: molecular mechanisms and functional implications. J Steroid Biochem Mol Biol. 2003;83:211–217. doi: 10.1016/s0960-0760(02)00261-3. [DOI] [PubMed] [Google Scholar]
- Clemmons DR, Maile LA. Minireview: Integral membrane proteins that function coordinately with the insulin-like growth factor I receptor to regulate intracellular signaling. Endorcrinology. 2003;144:1664–1670. doi: 10.1210/en.2002-221102. [DOI] [PubMed] [Google Scholar]
- Copeland KC, Kuehl TJ, Castracane VD. Pubertal endocrinology of the baboon: elevated somatomedin-C/insulin-like growth factor 1 at puberty. J Clin Endocrinol Metab. 1982;55:1198–1201. doi: 10.1210/jcem-55-6-1198. [DOI] [PubMed] [Google Scholar]
- Daftary SS, Gore AC. Developmental changes in hypothalamic insulin-like growth factor-1: Relationship to gonadotropin-releasing hormone neurons. Endocrinology. 2003;144:2034–2045. doi: 10.1210/en.2002-221025. [DOI] [PubMed] [Google Scholar]
- Danilovich V, Wernsing D, Coschigano KT, Kopchick JJ, Bartke A. Deficits in female reproductive function in GH-R-KO mice; role for IGF-1. Endocrinology. 1999;140:2637–2640. doi: 10.1210/endo.140.6.6992. [DOI] [PubMed] [Google Scholar]
- Dees WL, Dissen G, Hiney JK, Lara F, Ojeda SR. Alcohol ingestion inhibits the increased secretion of puberty-related hormones in the developing female rhesus monkey. Endocrinology. 2000;141:1325–1331. doi: 10.1210/endo.141.4.7413. [DOI] [PubMed] [Google Scholar]
- Dees WL, Skelley CW. The effects of ethanol during the onset of female puberty. Neurondocrinology. 1990;51:64–69. doi: 10.1159/000125317. [DOI] [PubMed] [Google Scholar]
- Dees WL, Skelley CW, Hiney JK, Johnston CA. Actions of ethanol on hypothalamic and pituitary hormones in prepubertal female rats. Alcohol. 1990;7:21–25. doi: 10.1016/0741-8329(90)90055-h. [DOI] [PubMed] [Google Scholar]
- Dees WL, Srivastava VK, Hiney JK. Alcohol alters insulin-like growth factor-1 activated Oct-2 POU gene expression in the immature female hypothalamus. J Studies on Alcohol. 2005;66:35–45. doi: 10.15288/jsa.2005.66.35. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Wands JR. Chronic gestational exposure to ethanol impairs insulin-stimulated survival and mitochondrial function in cerebellar neurons. Cell Mol Life Sci. 2002;59:882–893. doi: 10.1007/s00018-002-8475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roux N, Genen E, Carel J, Matsuda F, Chaussin J, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS-1-derived peptide receptor GPR54. Proc Natl Acad Sci. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond F, Ringenberg L, MacDonald D, Barnes J, Shi Hu C, Ducket G, Sweetland M, Root A. Effects of drug and alcohol abuse upon pituitary-testicular function in adolescent males. J Alc Hlth. Care. 1986;7:28–33. doi: 10.1016/s0197-0070(86)80091-2. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Dearth RK, Scott HM, Ojeda SR, Dees WL. Alcohol alters prepubertal luteinizing hormone secretion in immature female rhesus monkeys by a hypothalamic action. Endocrinology. 2004;145:4558–4564. doi: 10.1210/en.2004-0517. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Gonzalez-Flores O, Todd BJ. The role of insulin-like growth factor-1 and growth factor-associated signal transduction pathways in estradiol and progesterone facilitation of female reproductive behaviors. Frontiers in Neuroendocrinol. 2006;27:363–375. doi: 10.1016/j.yfrne.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neuroscience. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelsman DJ, Spaliviero JA, Scott CD, Baxter RC. Hormonal regulation of the peripubertal stage of insulin-like growth factor-1 in the rat. Endocrinology. 1987;120:491–496. doi: 10.1210/endo-120-2-491. [DOI] [PubMed] [Google Scholar]
- He L, Simmen FA, Mehendale HM, Ronis MJJ, Badger TM. Chronic ethanol intake impairs insulin signaling in rats by disrupting Akt association with cell membrane. J. Biol. Chem. 2006;281:11126–11134. doi: 10.1074/jbc.M510724200. [DOI] [PubMed] [Google Scholar]
- Hettinger AM, Allen MR, Zhang BR, Goad DW, Malayer JR, Geisert RD. Presence of the acute phase protein, bikunin, in the endometrium of gilts during estrous cycle and early pregnancy. Biol Reprod. 2001;65:507–513. doi: 10.1095/biolreprod65.2.507. [DOI] [PubMed] [Google Scholar]
- Hill MM, Hemmings BA. Inhibition of protein kinase B/Akt. Implications for cancer therapy. Pharm. Ther. 2002;93:243–251. doi: 10.1016/s0163-7258(02)00193-6. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Dearth RK, Dees WL. Influence of estradiol on insulin-like growth factor-1-induced luteinizing hormone secretion. Brain Res. 2004;1012:91–97. doi: 10.1016/j.brainres.2004.03.054. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Lara F, Dees WL. Ethanol blocks the central action of IGF-1 to induce luteinizing hormone secretion in the prepubertal female rat. Life Sci. 1998;62:301–308. doi: 10.1016/s0024-3205(97)01111-9. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Nyberg CL, Ojeda SR, Dees WL. Insulin-like growth factor-1 (IGF-1) of peripheral origin acts centrally to accelerate the initiation of female puberty. Endocrinology. 1996;137:3717–3727. doi: 10.1210/endo.137.9.8756538. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Pine MD, Dees WL. Insulin-like growth factor-1 activates KiSS-1 gene expression in the brain of the prepubertal female rat. Endocrinology. 2009;150:376–384. doi: 10.1210/en.2008-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Chiu JF, Gagne J, Fukagawa NK. Age-related differences in insulin-like growth factor-1 receptor signaling regulates Akt/FOXO3a and ERK/Fos pathways in vascular smooth muscle cells. J Cell Physiol. 2008;217:377–387. doi: 10.1002/jcp.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Ding M, Thiele CJ, Luo J. Ethanol inhibits brain-derived neurotrophic factor-mediated intracellular signaling and activator protein-1 activation in cerebellar granule neurons. Neuroscience. 2004;126:149–162. doi: 10.1016/j.neuroscience.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Ling HE, Simmen FA, Mehendale HM, Ronis MJJ, Badger TM. Chronic ethanol intake impairs insulin signaling in rats by disrupting Akt association with the cell membrane: Role of TRB3 in inhibition of akt/protein kinase B activation. J Biol Chem. 2006;281:11126–11137. doi: 10.1074/jbc.M510724200. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barriero ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated mRNA expression of KiSS-1 and its putitive receptor, GPR54, in rat hypothalamus and potent LH-releasing activity of KiSS-1 peptide. Endocrinology. 2004a;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004b;561:379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa J, Vigo E, Castellano JM, Navarro VM, Fernandez-Fernandez R, Casanueva FF, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of the kisspeptin in different reproductive states of the female rat. Endocrinology. 2006;147:2864–2878. doi: 10.1210/en.2005-1463. [DOI] [PubMed] [Google Scholar]
- Roa J, Garcia-Galiano D, Varela L, Sanchez-Garrido MA, Pineda R, Castellano JM, Ruiz-Pino F, Romero M, Aguilar E, Lopez M, Gaytan F, Dieguez C, Pinilla L, Tena-Sempere M. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology. 2009;150 doi: 10.1210/en.2009-0096. doi:10.1210/en.2009-0096. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatizidaki E, Thresher A, Acierno J, Shagoury J, Bo-Abbas Y, Kuohung W, Schwinof K, Hendrick A, Zahn D, Dixon J, Kaiser U, Slaugenhaupt S, Gusella J, O'Rahilly S, Carlton M, Crowley W, Aparicio S, Colledge W. The GPR54 gene as a regulator of puberty. New Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Sara VR, Hall K. Insulin-like growth factors and their binding proteins. Physiol Rev. 1990;70:591–614. doi: 10.1152/physrev.1990.70.3.591. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara S, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- Srivastava VK, Hiney JK, Nyberg CL, Dees WL. Effect of ethanol on the synthesis of insulin-like growth factor-1 (IGF-1) and the IGF-1 receptor in late prepubertal female rats: A correlation with serum IGF-1. Alc Clin Exp Res. 1995;19:1467–1473. doi: 10.1111/j.1530-0277.1995.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Srivastava VK, Hiney JK, Dees WL. Short term alcohol administration alters KiSS-1 gene expression in the reproductive hypothalamus of prepubertal female rats. Alc Clin Exp Res. 2009;33:1605–1614. doi: 10.1111/j.1530-0277.2009.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam CS, de Zegher F, Garnett SP, Baur LA, Cowell CT. Opposing influences of prenatal and postnatal growth on the timing of menarche. J Clin Endocrinol Metab. 2006;91:4369–4373. doi: 10.1210/jc.2006-0953. [DOI] [PubMed] [Google Scholar]
- Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- Tsuji R, Fattori V, Abe S, Costa LG, Kobayashi K. Effects of postnatal ALC exposure at different developmental phases on neurotrophic factors and phosphorylated proteins on signal transductions in rat brain. Neurotoxicology and Teratology. 2008;30:228–236. doi: 10.1016/j.ntt.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Wilson ME. Premature elevation in serum insulin-like growth factor-1 advances first ovulation in monkeys. J Endocrinology. 1998;158:247–257. doi: 10.1677/joe.0.1580247. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]