Abstract
Glucocorticoid administration is required for many inflammatory and autoimmune diseases, but use of these drugs is associated with skeletal side effects including bone loss, fractures, and osteonecrosis. Fractures often occur without a reduction in bone mineral density, strongly suggesting that glucocorticoid excess adversely affects other aspects of bone strength. Although the primary effects of glucocorticoid excess on the skeleton are directly on bone cells, a vascular connection between these cells and the loss of bone strength appears likely. This review examines this connection and how it may explain the greater decline in bone strength than loss of bone mass that occurs with glucocorticoid excess.
Keywords: glucocorticoid-induced osteoporosis, fractures, osteoblast and osteocyte apoptosis, bone formation, 11β-hydroxysteroid dehydrogenase, bone vasculature, bone hydration, angiogenesis, bone density, bone strength, osteonecrosis
Introduction
Glucocorticoid administration is the most frequent secondary form of osteoporosis and the most common iatrogenic reason for the disease [1,2]. Often the presenting manifestation is fracture, which occurs in 30 to 50% of patients receiving long-term glucocorticoid therapy. Many fractures are asymptomatic, possibly because of glucocorticoid-induced analgesia, but even asymptomatic fractures are important because they further reduce the vital capacity of patients with chronic lung disease receiving prednisone and increase the risk of subsequent fractures independently of the bone mineral density (BMD) [1,3,4]. In addition to glucocorticoid-induced osteoporosis (GIO), chronic glucocorticoid administration also causes osteonecrosis of the hip; a situation in which deformity and collapse occur in spite of increased radiographic sclerosis signifying a mismatch between the increased skeletal density and decreased bone strength [5]. The primary adverse effects of glucocorticoid excess on the skeleton are directly on bone cells: decreasing the production of both osteoblasts and osteoclasts, increasing the prevalence of osteoblast and osteocyte apoptosis, and prolonging the lifespan of osteoclasts (Figure 1) [6–10].
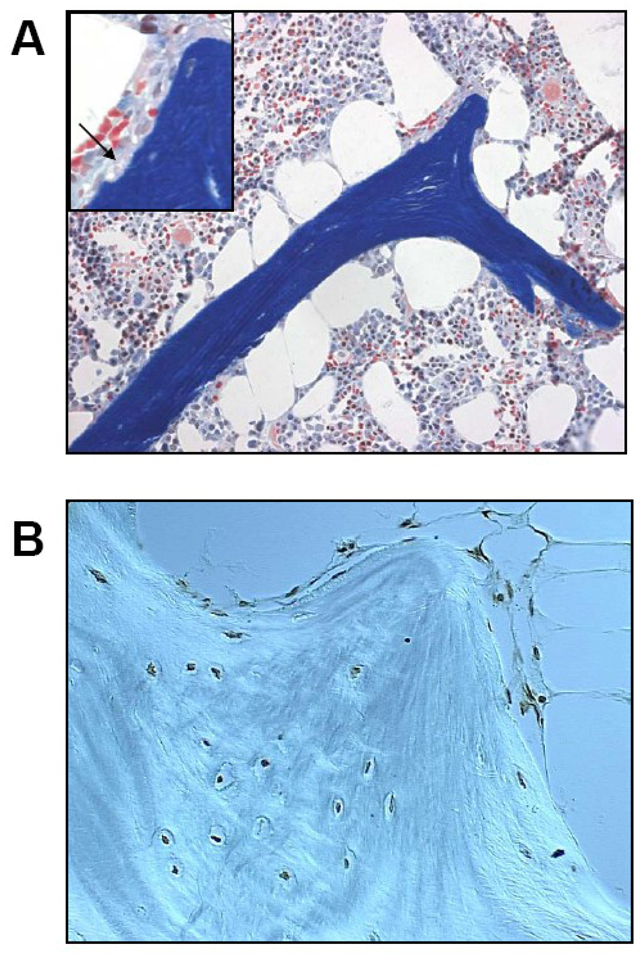
Figure 1. Glucocorticoid-induced osteoporosis.
(A) After long-term prednisone treatment in humans, cancellous bone tissue often shows an excessive number of enlarged adipocytes and atrophic, disconnected trabeculae without osteoblasts. The insert shows the accumulation of erosion cavities devoid of osteoclasts (arrow), measured as the reversal perimeter, which indicate delayed or defective bone formation rather than excessive bone resorption. The red cells in the bone marrow are hematopoietic cells and should not be mistaken for osteoclasts (Modified Masson staining, 200x with the insert at 400x). (B) In this specimen obtained from an osteonecrotic hip, virtually all cancellous osteocytes and lining cells are apoptotic as revealed by the dark brown stain (apoptosis staining was done by in-situ end-labeling or ISEL, 630x).
Determination of the bone impact of glucocorticoid therapy is complicated by the heterogeneity of the diseases that are treated with glucocorticoids and the wide variation in the dose, duration, and route of the administered treatment [1]. In general, bone loss in GIO is biphasic, with an early reduction in BMD of 6–12% within the first year, followed by a later continual loss of about 3% for each additional year of treatment [11]. However, there is more to consider than the reduction in BMD, as bone quality is also an issue [12]. The relative risk of fracture increases rapidly, escalating by as much as 75% within the first 3 months after initiation of glucocorticoid therapy, usually long before much change in BMD is detectable. This increase in fracture risk dissipates shortly after discontinuation of therapy [13]. These features strongly suggest that glucocorticoid-induced fractures are due to more than just decreasing bone mass. Advancing age is an another risk factor as shown by the 26-fold greater incidence of symptomatic vertebral fractures in glucocorticoid-treated patients aged 60 to 88 years as compared to patients aged 18–31 years [14]. Furthermore, the duration of treatment before the occurrence of a fracture decreased as patient age increased. Glucocorticoid-treated patients with vertebral fractures have higher BMD but suffer twice the risk of fracture than patients with vertebral fractures due to postmenopausal osteoporosis [13,15]. It is clear that BMD considerably underestimates the risk of fracture in GIO and accounts for, at least in part, the under-recognition and under-treatment of the disease. Additional evidence for a defect in bone quality in patients with GIO as compared to patients with postmenopausal osteoporosis is provided by the report of incapacitating adjacent vertebral fractures days after kyphoplasty in GIO [16]. Caution should be exercised before recommending the procedure in patients receiving glucocorticoids.
There is little doubt that BMD is a substantial contributor to bone strength but other important characteristics are also involved including bone size, cortical and cancellous architecture, bone turnover, and bone material properties, and these non-BMD characteristics may be even more important in GIO than they are in postmenopausal osteoporosis [12] (Table 1). In addition to these characteristics, the osteocyte-canalicular network may play an important role in the maintenance of bone strength [8]. Although these contributions to bone strength are often recognized [17,18], the role of skeletal water volume in the viscoelastic properties of bone is rarely appreciated [19,20]. This failure to realize the importance of the degree of hydration in the material properties of hard tissues is at least somewhat surprising with the widespread knowledge that it is much easier to break a dried wishbone than a hydrated one [21]. Finite element analysis of cortical bone indicates that water reduces stresses during dynamic loading and produces a 2.5-fold increase in ultimate strength [22].
Table 1.
Determinants of bone strength
| Mineral density |
| Size |
| Cortical and cancellous architecture |
| Turnover |
| Material properties |
| Osteocyte viability |
| Vascular volume/water content |
Direct effects on bone cells
Osteoblasts and osteocytes
Glucocorticoids affect every tissue in the body and thus, the negative impact of excess glucocorticoids on the skeleton could be through direct effects on bone cells or indirect effects on extraskeletal tissues or chemical mediators. However, there is a natural way to dissect these possibilities. In mineralocorticoid-sensitive tissues, such as the kidney, colon, sweat glands, and salivary glands, mineralocorticoid receptors have equal affinity for aldosterone and glucocorticoids. Therefore, to obtain the required aldosterone selectivity in the presence of the 1000-fold greater circulating concentration of glucocorticoids, rapid pre-receptor oxidative inactivation of glucocorticoids occurs via 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), an enzyme expressed in mineralocorticoid-sensitive tissues. 11β-HSD2 is a 42 kDa, high affinity, NAD-dependent enzyme, that converts biologically active glucocorticoid alcohols to their inert 11-keto metabolites [8] (Figure 2). Transgenic expression of 11β-HSD2 in mature osteoblasts and osteocytes renders them resistant to glucocorticoid action, and thus facilitates dissection of the portion of the adverse effects of glucocorticoid excess that results from direct action on these cells as opposed to actions on osteoclasts or tissues other than bone.
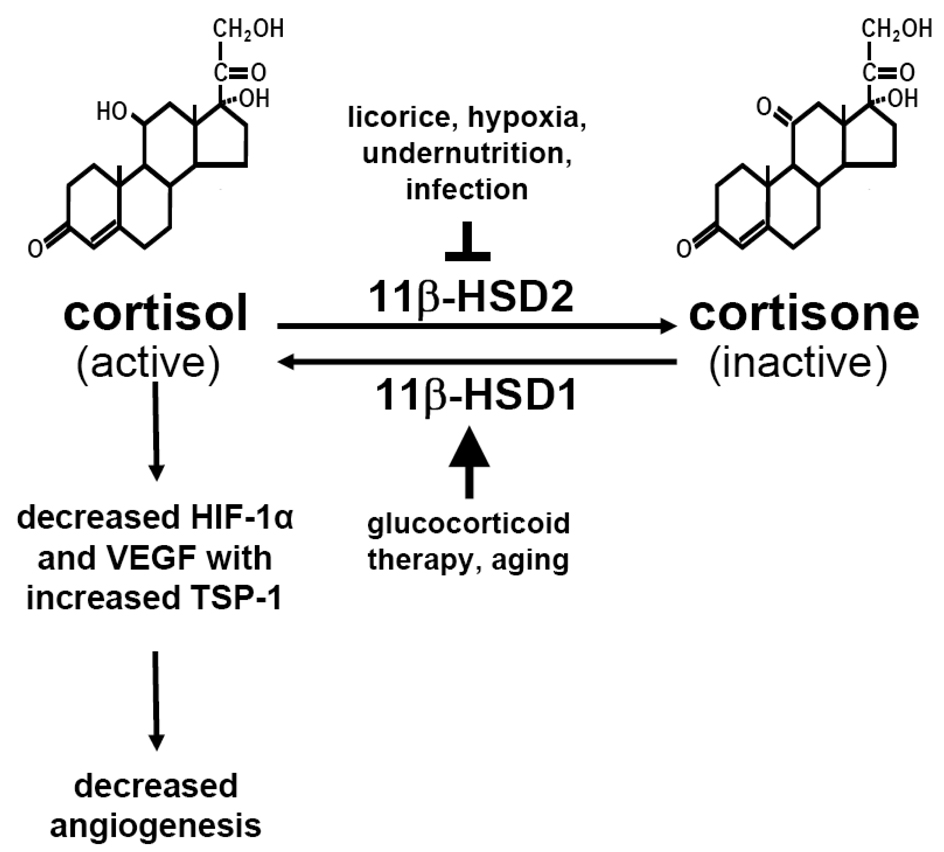
Figure 2. Cortisol, cortisone and angiogenesis.
Two isoenzymes of 11β-hydroxysteroid dehydrogenase, 11β-HSD1 and 11β-HSD2, catalyze the interconversion of hormonally active glucocorticoids (such as corticosterol or cortisol) and inactive glucocorticoids (such as corticosterone or cortisone). The 11β-HSD1 isoenzyme is an activating route, and the 11β-HSD2 isoenzyme is an inactivating route. The ability of any glucocorticoid to bind to glucocorticoid receptors depends on the presence of a hydroxyl group at C-11. Therefore, any tissue expressing 11β-HSDs can regulate the exposure of resident cells to active glucocorticoids. The 11β-HSD system also works on synthetic glucocorticoids (e.g., prednisolone or dexamethasone). Glucocorticoids decrease osteoblastic HIF-1α transcriptional activity and the message for the angiogenic factor VEGF while the drugs increase the message for the angiostatic factor TSP-1 increases. As a result, these hormones have powerful adverse effects on bone vascularity.
Overexpression of human 11β-HSD2 in mice was accomplished using the murine osteocalcin gene 2 promoter (OG2), which is active only in mature osteoblasts and osteocytes (OG2-11β-HSD2 mice) [8]. This strategy shields osteoblasts and osteocytes from glucocorticoids before the drugs reach the glucocorticoid receptor (GR). Under control of this promoter, 11β-HSD2 did not affect normal bone development or turnover as demonstrated by identical bone density, strength, and histomorphometry in adult transgenic and wild type animals. The absence of a baseline phenotype facilitated interpretation of a glucocorticoid challenge. Administration of excess glucocorticoids induced equivalent loss of BMD in wild type and transgenic mice, as expected because osteoclasts were unaffected by this particular transgenic strategy. However, the increase in osteoblast apoptosis that occurred in wild type mice was prevented in the transgenic mice. Consistent with this, osteoblasts, osteoid area, and bone formation rate were significantly higher in glucocorticoid-treated transgenic mice as compared to the similarly treated wild type mice. Glucocorticoid-induced osteocyte apoptosis was also prevented in the transgenic mice. Strikingly, the loss of vertebral compression strength observed in glucocorticoid-treated wild type mice was prevented in the transgenic mice, in spite of an equivalent loss of BMD. These results clearly demonstrate that excess glucocorticoids directly affect bone forming cells in vivo. Furthermore, these findings suggest that glucocorticoid-induced loss of bone strength results, at least in part, from increased death of osteocytes, independent of bone loss. The pathogenetic changes responsible for the disproportional loss of bone strength over bone mass with glucocorticoid excess may result from the negative effect of osteocyte apoptosis on bone quality.
A potential connection between the maintenance of osteoblast and osteocyte viability and the hydraulic stiffening of bone is revealed by the evidence that these cells respond to hypoxia by activating the hypoxia-inducible factor α (HIFα) pathway and thereby promote angiogenesis by producing vascular endothelial growth factor (VEGF) [23]. Administration of glucocorticoids decreases the angiogenic molecule VEGF [24–26] and increases the angiostatic glycopeptide thrombospondin-1 [27]. These actions may account for the reduction in bone water volume and skeletal blood flow that occurs with excess glucocorticoids [28,29]. In addition to decreased osteoblastogenesis and increased osteoblast apoptosis, the reduction in skeletal blood flow may be another way that glucocorticoids reduce bone formation as well as bone hydration. There is an intimate relationship between bone morphogenetic proteins (BMPs) and VEGF that is advantageous for delivery of bone cells to sites of new bone formation. BMP signalling activates endothelial cells via stimulation of VEGF expression [30]. In accord with this contention, inhibition of VEGF blocks BMP-induced bone formation [31]. Other angiogenic factors are involved in the vasculature of bone such as the fibroblast growth factor family, insulin growth factor-1, epidermal growth factor, platelet-derived growth factor, CCN1, the transforming growth factor family, and angiopoietin, but VEGF is arguably the most important [26,27,30,31]. An additional connection between these findings and glucocorticoid-induced osteoporosis is suggested by the evidence that low bone turnover and diminution of bone marrow blood vessels are both typical of glucocorticoid administration [6,24]. Each new basic remodeling unit of bone has a nearby blood vessel, necessary for the delivery of new osteoclasts and osteoblasts (Figure 3) [32,33], and skeletal blood flow is directly related to the mineral appositional rate [34].
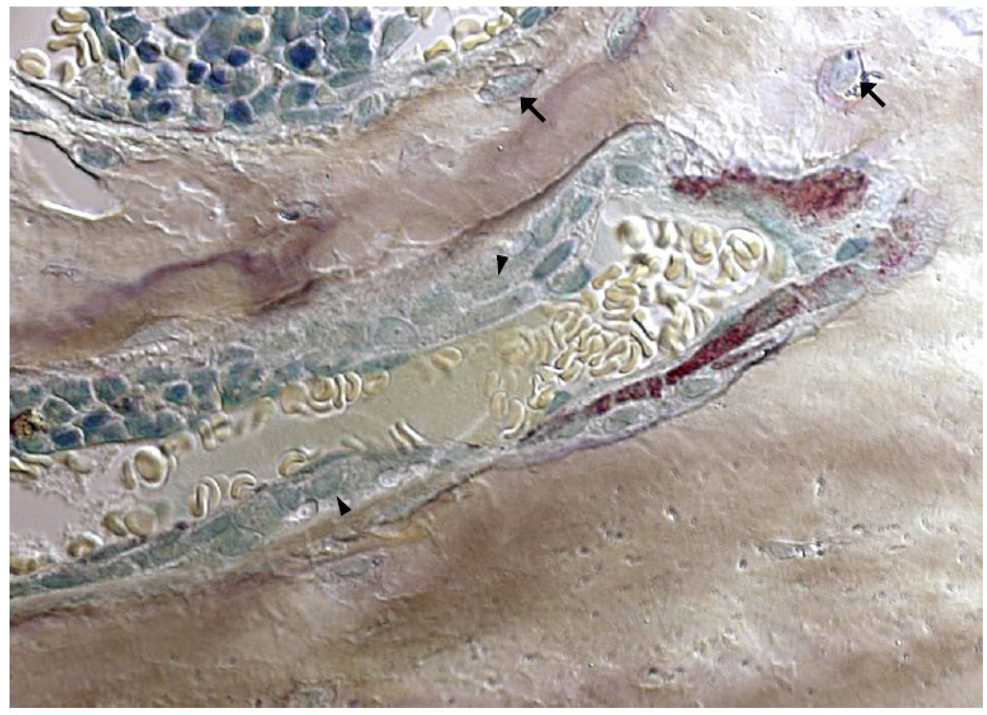
Figure 3. Each new basic remodeling unit of bone has a nearby blood vessel.
Murine osteoclasts with discrete tartrate-resistant acid phosphatase-positive red granules burrow deep into vertebral cancellous bone. Pale yellow erythrocytes are seen in the adjacent blood vessel that served as the conduit through which the osteoclast precursors were delivered to the remodeling site. Trailing behind the osteoclasts, teal-colored osteoblasts forming new bone are bringing up the rear (arrowheads). Osteocytes (arrows) are seen buried in the mineralized bone matrix. Methyl green and tartrate-resistant acid phosphatase-staining of undecalcified bone viewed with Nomarski differential interference contrast microscopy (x630).
The 11β-HSD shuttle system that determines glucocorticoid action also has an activating isoenzyme (Figure 2) [35]. 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) locally enhances glucocorticoid action by catalyzing the reactivation of inert 11-keto forms of glucocorticoids to their active 11-hydroxyl congeners. 11β-HSD1 is expressed in osteoblasts (probably also in osteocytes) and represents a “fast forward” mechanism for tissue-specific amplification of the ambient glucocorticoid level [36,37]. The expression of this activating enzyme is increased by glucocorticoids resulting in a vicious circle of hyperglucocorticoidism. Therefore, it is possible that inherited or acquired local changes in 11β-HSD1 activity could account for the variable sensitivity to glucocorticoids that is exhibited by some patients who become Cushingoid with relatively small doses of glucocorticoids while others seem to be remarkably resistant.
Osteoclasts
Glucocorticoid administration results in an early, rapid loss of BMD because of an imbalance in the number of osteoblasts and osteoclasts. While glucocorticoid administration dramatically reduces the production of both osteoblast and osteoclast precursors as measured in ex vivo bone marrow cell cultures [6,10], surprisingly, in vivo the cancellous osteoclast number does not decrease as does the number of osteoblasts, due to the ability of glucocorticoids to prolong osteoclast lifespan, an effect mediated by the GR as revealed by its blockade with RU 486, a potent GR antagonist [10]. This effect is powerful enough that it is not preventable by concurrent administration of bisphosphonates, possibly accounting for the reduced ability of bisphosphonates to protect BMD in GIO as compared with postmenopausal or male osteoporosis [38–41].
To determine whether glucocorticoids act directly on osteoclasts in vivo to promote their lifespan and whether this contributes to the rapid loss of bone seen with glucocorticoid administration, the 11β-HSD system was again used to over-express 11β-HSD2 in mice, but this time utilizing the murine tartrate-resistant acid phosphatase (TRAP) promoter (TRAP-11β-HSD2 mice) [7]. When challenged with prednisolone, equivalent increases in cancellous osteoblast apoptosis, and equivalent decreases in osteoblast number, osteoid perimeter, and rate of bone formation, occurred in wild-type and transgenic mice. In contrast, glucocorticoids stimulated expression of mRNA for the calcitonin receptor, an osteoclast product, in the wild-type but not in the transgenic mice. Consistent with the previous findings that glucocorticoids directly decrease osteoclast precursors and prolong the lifespan of mature osteoclasts, prednisolone administration maintained osteoclast number in the wild-type mice but osteoclast number fell in the transgenic animals. In accord with this decrease in osteoclast number, the loss of bone density observed in the wild-type mice was prevented in the transgenic animals. These results demonstrate that the early, rapid loss of bone caused by glucocorticoid excess results primarily from direct actions on osteoclasts. Furthermore, deflection of glucocorticoid action in osteoclasts did not prevent the expected glucocorticoid-induced decrease in osteoblast number or increase in the prevalence of osteoblast and osteocyte apoptosis. Even if osteoblasts and bone formation did not decrease with glucocorticoid administration, the glucocorticoid-induced prolongation of osteoclast lifespan would still result in an early transient increase in the remodeling space and loss of bone mass.
Kim and colleagues have confirmed that glucocorticoid excess prevents osteoclast apoptosis while promoting osteocyte apoptosis [42]. Based on studies in which the GR was deleted from murine osteoclasts, these workers proposed that all the adverse effects of glucocorticoids on bone formation were mediated by cells of the osteoclast lineage. However, glucocorticoid-induced bone loss did not occur in either the wild-type or GR−/− mice in these studies. The view that glucocorticoids decrease bone formation via the osteoclast is in contrast to the evidence provided by the TRAP-11β-HSD2 mice in which glucocorticoid-induced prolongation of osteoclast lifespan was prevented but decreased osteoblast number, osteoid perimeter, and bone formation and increased osteoblast apoptosis still occurred as expected [7]. Additional evidence against the suggestion that glucocorticoids decrease bone formation via the osteoclast is the abrogation of the glucocorticoid effects on bone formation by shielding the osteoblasts and osteocytes from glucocorticoid excess in the OG2-11β-HSD2 mice, as described above [8].
Changes in bone vasculature and strength
Glucocorticoid-induced disruption of bone vascularity and diminution of bone hydraulic support could be the mechanisms behind the greater decline in bone strength than in loss of bone mass that occurs with glucocorticoid excess. A link between the vascular system, canalicular processes, and osteocytes has been postulated from the evidence that canalicular fluid transport is directly connected to the vascular space as revealed by low molecular weight tracers that traverse the venous system into the lacunocanalicular fluid within minutes [43]. Preliminary data on the effects of glucocorticoids on bone density, strength, canalicular fluid, and vasculature in the mouse suggest that glucocorticoids do indeed disrupt the vascular system of bone [44,45]. After 28 days of administration of prednisolone to 8-month-old C57Bl/6 mice, a period equivalent in the mouse to about 3 to 4 years in humans, vertebral and femoral BMD decreased by 4.5–6.6% (p < 0.003), osteoblast and osteocyte apoptosis increased by 160–250% (p < 0.01), while vertebral compression strength and femoral 3-point bending decreased by 25% (p < 0.04). As has been noted clinically, glucocorticoid excess in these mice caused a four-fold greater decrease in bone strength than in BMD. Osteoblast and osteocyte apoptosis may reduce the expression of HIFα and the production of VEGF, thereby adversely affecting the vasculature [23]. In support of this contention, glucocorticoid excess was associated with a remarkable decrease in the interstitial fluid of cancellous bone, as determined by 2-dimensional fluorescent imaging of the osteocyte-lacunar-canalicular system using tail vein injections of procion red—a metabolically inert low molecular weight tracer. In preliminary experiments, mice were sacrificed at various intervals after the tail vein injection to determine the time required for maximal filling of the osteocyte-lacunar-canalicular system independently of the perfusion rate through bone. Filling of the canalicular system could be seen as early as 5 minutes after injection in ambulatory mice and fading of the procion signal occurred by 20 minutes so necropsy was done 15 minutes after injection. Quantification of the procion red in cancellous bone was done by image analysis using photomicrographs of lumbar vertebrae L1-4 taken with tomographic epifluorescent illumination. Further evidence of the glucocorticoid effect on bone blood vessels was obtained by 3-dimensional microCT imaging of decalcified bones following intravenous perfusion of a silicon slurry of lead chromate. Prednisolone administration dramatically decreased the vertebral and femoral vessel volume and surface area. These preliminary findings can be used to speculate that increased osteoblast and osteocyte apoptosis, decreased bone vasculature, and diminished osteocyte-lacunar-canalicular fluid may be at least some of the factors responsible for the disproportional loss of bone strength compared to bone mass with glucocorticoid excess.
Osteonecrosis
Glucocorticoid therapy causes osteonecrosis of the femoral neck or proximal humerus in as many as 25% of patients receiving exceptionally high short-term doses, long-term moderate doses, or only single intraarticular injections [5]. Unfortunately, the name osteonecrosis is misleading, since it has not been demonstrated that the bone cells in this condition die by necrosis. Cell swelling, white blood cell infiltration, and inflammation that characterize necrosis in soft tissues usually do not occur in glucocorticoid-induced osteonecrosis. The osteonecrosis has been attributed to fat emboli, microvascular tamponade of the blood vessels of the femoral head by marrow fat or fluid retention, and poorly mending fatigue fractures [5]. Occlusion of small bone blood vessels and a decrease in their number was observed weeks after calvarial radiation in rabbits, leading to radiation-induced osteonecrosis or osteoradionecrosis [46]. In osteoradionecrosis, loss of osteocyte viability, decreased intraosseous vasculature, and defective bone repair are pathogenetic features [46]. Similarly, loss of osteocyte viability was documented in patients with glucocorticoid-induced osteonecrosis [47,48]. Abundant apoptotic osteocytes were identified in sections of whole femoral heads obtained during total hip replacement for glucocorticoid-induced osteonecrosis, whereas apoptotic bone cells were absent from femoral specimens removed because of traumatic or sickle cell osteonecrosis, suggesting that glucocorticoid-induced osteonecrosis is actually osteocyte apoptosis [48]. In glucocorticoid-induced osteonecrosis, bone density is increased but collapse of the joint occurs anyway, in agreement with the previous contention that the apoptotic osteocytes adversely affect bone material properties regardless of bone density. Because of the glucocorticoid-induced decrease in the number of osteoblasts and rate of bone remodelling, glucocorticoid-induced osteocyte apoptosis becomes a cumulative and unrepairable defect. Osteocyte apoptosis may disrupt the mechanosensory function of the osteocyte-canalicular network, decrease bone water volume, and thus start the relentless sequence of events leading to collapse of the femoral head (Figure 1B). Glucocorticoid-induced osteocyte apoptosis also explains the correlation between total steroid dose and the incidence of osteonecrosis and its occurrence long after glucocorticoid administration has ceased [5,49,50]). In view of the proposed connection of osteoblasts and osteocytes with disruption of bone vascularity, it is ironic that an early name for glucocorticoid-induced osteonecrosis was avascular necrosis.
Conclusions
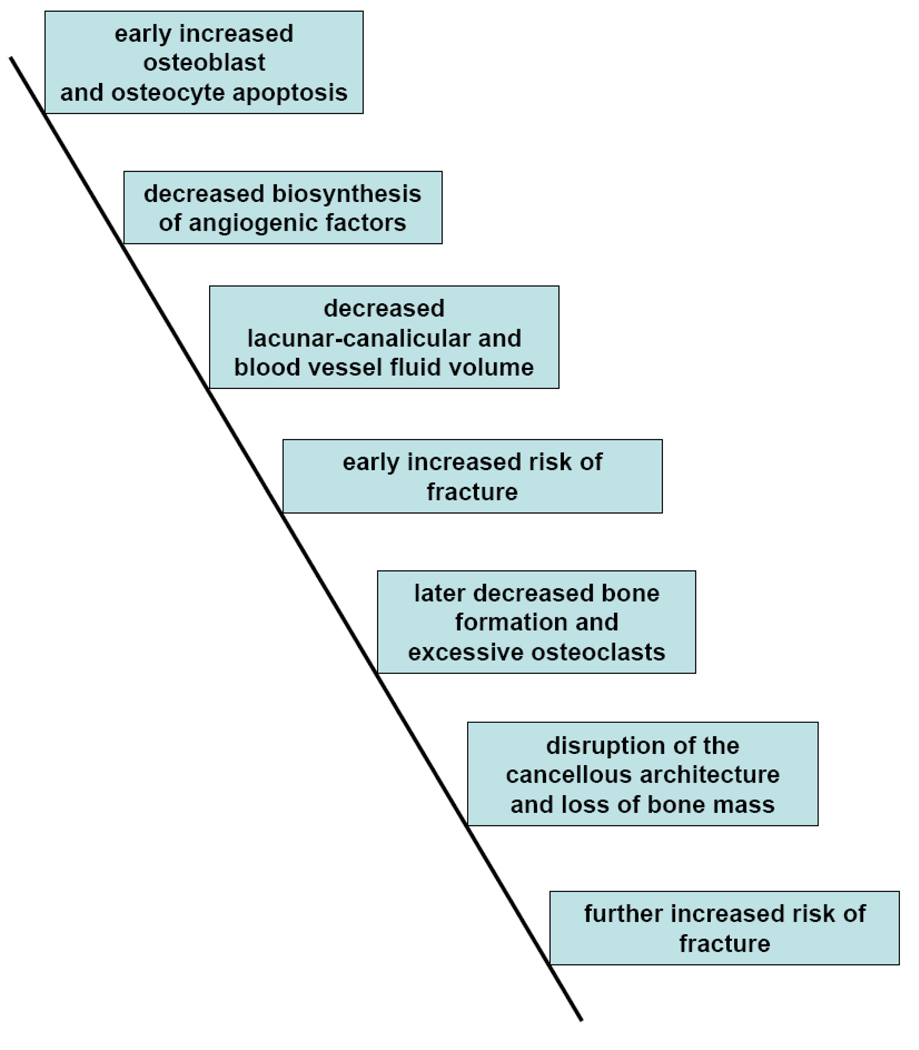
A provisional model to account for the adverse effects of glucocorticoid administration on bone strength could be constructed as follows (Figure 4): an early increase in osteoblast and osteocyte apoptosis causes decreased biosynthesis of angiogenic factors resulting in decreased osteocyte-lacunar-canalicular circulation and bone blood vessel volume that leads to decreased bone water volume, diminished bone strength, and an early increase in the risk of fractures. When the lifespan of the remaining osteoblasts is exhausted, the decrease in osteoblastogenesis causes a greater reduction in osteoblast number, bone formation rate and wall width, but osteoclast number remains normal or slightly elevated, resulting in disruption of the cancellous architecture, decreased bone mass, further loss of bone strength, and a further increase in the risk of fractures.
Figure 4. Pathogenesis of the loss of bone strength with glucocorticoid administration: a hypothesis.
Cover Illustration. Murine osteoclasts with discrete tartrate-resistant acid phosphatase-positive red granules burrow deep into vertebral cancellous bone. Pale yellow erythrocytes are seen in the adjacent blood vessel that served as the conduit through which the osteoclast precursors were delivered to the remodeling site. Trailing behind the osteoclasts, teal-colored osteoblasts forming new bone are bringing up the rear. Osteocytes are seen buried in the mineralized bone matrix. Methyl green and tartrate-resistant acid phosphatase-staining of undecalcified bone viewed with Nomarski differential interference contrast microscopy (x630).
Acknowledgements
RSW certifies that he has no affiliations or involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in the manuscript. Thanks to Charles O’Brien for designing the OG2-11β-HSD2 and TRAP-11β-HSD2 constructs and conceiving the transgenic strategy and to Maria Almeida, Charles O’Brien, Robert Jilka and Stavros Manolagas for sharing ideas.
Funding sources: This work was supported by a VA Merit Review Grant from the Office of Research and Development, Department of Veterans Affairs; the National Institutes of Health (P01-AG13918); and Tobacco Settlement Funds provided by the UAMS College of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinstein RS. Glucocorticoid-induced osteoporosis. Rev Endocr Metab Disorders. 2001;2:65–73. doi: 10.1023/a:1010007108155. [DOI] [PubMed] [Google Scholar]
- 2.Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporosis Int. 2007;18:1319–1328. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 3.Angeli A, Guglielmi G, Dovio A, Capelli G, de Feo D, Giannini S, Giorgino R, Moro L, Giustina A. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: A cross-sectional outpatient study. Bone. 2006;39:253–259. doi: 10.1016/j.bone.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Salerno A, Hermann R. Efficacy and safety of steroid use for postoperative pain relief. J Bone Joint Surg. 2006;88A:1361–1372. doi: 10.2106/JBJS.D.03018. [DOI] [PubMed] [Google Scholar]
- 5.Mankin HF. Nontraumatic necrosis of bone (osteonecrosis) N Engl J Med. 1992;326:1473–1479. doi: 10.1056/NEJM199205283262206. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of the deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia D, O’Brien CA, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoclasts to increase their lifespan and reduce bone density. Endocrinol. 2006;147:5592–5599. doi: 10.1210/en.2006-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinol. 2004;145:1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- 9.Sambrook PN, Hughes DR, Nelsen AE, Robinson BG, Mason RS. Osteocyte viability with glucocorticoid treatment: relation to histomorphometry. Ann Rheum Dis. 2003;62:1215–1217. doi: 10.1136/ard.2003.008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinstein RS, Chen JR, Powers CC, Stewart SA, Landes RD, Bellido T, Jilka RL, Parfitt AM, Manolagas SC. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J Clin Invest. 2002;109:1041–1048. doi: 10.1172/JCI14538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LoCascio V, Bonucci E, Imbimbo B, Ballanti P, Adami S, Milani S, Tartarotti D, DellaRocca C. Bone loss in response to long-term glucocorticoid therapy. Bone Miner. 1990;8:39–51. doi: 10.1016/0169-6009(91)90139-q. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein RS. Perspective: True strength. J Bone Miner Res. 2000;15:621–625. doi: 10.1359/jbmr.2000.15.4.621. [DOI] [PubMed] [Google Scholar]
- 13.van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arth Rheum. 2003;48:3224–3229. doi: 10.1002/art.11283. [DOI] [PubMed] [Google Scholar]
- 14.Tatsuno I, Sugiyama T, Suzuki S, Yoshida T, Tanaka T, Sueishi M, Saito Y. Age dependence of early symptomatic vertebral fracture with high-dose glucocorticoid treatment for collagen vascular diseases. J Clin Endocrinol Metab. 2009;94:1671–1677. doi: 10.1210/jc.2008-1578. [DOI] [PubMed] [Google Scholar]
- 15.Stockbrugger RW, Schoon EJ, Bollani S, Mills PR, Israeli E, Landgraf L, Felsenberg D, Ljunghall S, Nygard G, Persson T, Graffner H, Bianchi Porro G, Ferguson A. Discordance between the degree of osteopenia and the prevalence of spontaneous vertebral fractures in Crohn’s disease. Aliment Pharmacol Ther. 2002;16:1519–1527. doi: 10.1046/j.1365-2036.2002.01317.x. [DOI] [PubMed] [Google Scholar]
- 16.Donovan MA, Khandji AG, Siris E. Multiple adjacent vertebral fractures after kyphoplasty in a patient with steroid-induced osteoporosis. J Bone Miner Res. 2004;19:712–713. doi: 10.1359/JBMR.040207. [DOI] [PubMed] [Google Scholar]
- 17.Gennari L, Bilezikian JP. Glucocorticoid-induced osteoporosis: hope on the HORIZON. Lancet. 2009;373:1225–1226. doi: 10.1016/S0140-6736(09)60704-2. [DOI] [PubMed] [Google Scholar]
- 18.Seeman E. Bone quality – the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 19.Wilson EE, Awonusi A, Morris MD, Kohn DH, Tecklenburg MM, Beck LW. Three structural roles for water in bone observed by solid-state NMR. Biophys J. 2006;90:3722–3731. doi: 10.1529/biophysj.105.070243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nalla RK, Balooch M, Ager JW, Kruzic JJ, Kinney JH, Ritchie RO. Effects of polar solvents on the fracture resistance of dentin: role of water hydration. Acta Biomater. 2005;1:31–43. doi: 10.1016/j.actbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Miserez A, Schneberk T, Sun C, Zok FW, Waite JH. The transition from stiff to compliant material in squid beaks. Science. 2008;319:1816–1819. doi: 10.1126/science.1154117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liebschner MAK, Keller TS. Hydraulic strengthening affects the stiffness and strength of cortical bone. Ann Biomed Eng. 2005;33:26–38. doi: 10.1007/s10439-005-8960-0. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, Haase VH, Johnson RS, Schipani E, Clemens TL. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pufe T, Scholz-Ahrens KE, Franke ATM, Petersen W, Mentlein R, Varoga D, Tillmann B, Schrezenmeir J, Gluer CC. The role of vascular endothelial growth factor in glucocorticoid-induced bone loss: evaluation in a minipig model. Bone. 2003;33:869–876. doi: 10.1016/j.bone.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Harada S, Nagy JA, Sullivan KA, Thomas KA, Endo N, Rodan GA, Rodan SB. Induction of vascular endothelial growth factor expression by prostaglandin E2 and E1 in osteoblasts. J Clin Invest. 1994;93:2490–2496. doi: 10.1172/JCI117258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Athanasopoulos AN, Schneider D, Keiper T, Alt V, Pendurthi UR, Liegibel UM, Sommer U, Nawroth PP, Kasperk C, Chavakis T. Vascular endothelial growth factor (VEGF)-induced up-regulation of CCN1 in osteoblasts mediates proangiogenic activities in endothelial cells and promotes fracture healing. J Biol Chem. 2007;282:26746–26753. doi: 10.1074/jbc.M705200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rae M, Mohamad A, Price D, Hadoke PWF, Walker BR, Mason JI, Hillier SG, Critchley HOD. Cortisol inactivation by 11B-hydroxysteroid dehydrogenase-2 may enhance endometrial angiogenesis via reduced thrombospondin-1 in heavy menstruation. J Clin Endocrinol Metab. 2009;94:1443–1450. doi: 10.1210/jc.2008-1879. [DOI] [PubMed] [Google Scholar]
- 28.Goans RE, Weiss GH, Abrams SA, Perez MD, Yergey AL. Calcium tracer kinetics show decreased irreversible flow to bone in glucocorticoid treated patients. Calcif Tissue Int. 1995;56:533–535. doi: 10.1007/BF00298584. [DOI] [PubMed] [Google Scholar]
- 29.Drescher W, Li H, Qvesel D, Jensen SD, Flo C, Hansen ES, Bünger C. Vertebral blood flow and bone mineral density during long-term corticosteroid treatment: An experimental study in immature pigs. Spine. 2000;25:3021–3025. doi: 10.1097/00007632-200012010-00009. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki Y, Montagne K, Nishihara A, Watabe T, Miyazono BMPs promote proliferation and migration of endothelial cells via stimulation of VEGF-A/VEGFR2 and angiopoietin-1/Tie2 signalling. J Biochem. 2008;143:199–206. doi: 10.1093/jb/mvm215. [DOI] [PubMed] [Google Scholar]
- 31.Peng H, Wright V, Usas A, Gearhart B, Shen HC, Cummins J, Huard J. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751–759. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parfitt AM. Misconceptions V-activation of osteoclasts is the first step in the bone remodeling cycle. Bone. 2006;39:1170–1172. doi: 10.1016/j.bone.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 33.Burkhardt R, Kettner G, Böhm W, Schmidmeier M, Schlag R, Frisch B, Mallmann B, Eisenmenger W, Gilg T. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone. 1987;8:157–164. doi: 10.1016/8756-3282(87)90015-9. [DOI] [PubMed] [Google Scholar]
- 34.Reeve J, Arlot M, Wootton R, Edouard C, Tellez M, Hesp R, Green JR, Meunier PJ. Skeletal blood flow, iliac histomorphometry, and strontium kinetics in osteoporosis: a relationship between blood flow and corrected apposition rateq. J Clin Endocrinol Metab. 1988;66:1124–1131. doi: 10.1210/jcem-66-6-1124. [DOI] [PubMed] [Google Scholar]
- 35.Weinstein RS. 11β-HSD: Guardian or Gate Crasher? BoneKEy-OsteoVision. 2005;2:6–13. http://www.bonekey-ibms.org/cgi/content/full/ibmske;2/9/6?ct.
- 36.Cooper MS, Rabbitt EH, Goddard PE, Bartlett WA, Hewison M, Stewart PM. Osteoblastic 11β-hydroxysteroid dehydrogenase type 1 activity increases with age and glucocorticoid exposure. J Bone Miner Res. 2002;17:979–986. doi: 10.1359/jbmr.2002.17.6.979. [DOI] [PubMed] [Google Scholar]
- 37.Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, Hewison M, Stewart PM. 11β-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004;25:831–866. doi: 10.1210/er.2003-0031. [DOI] [PubMed] [Google Scholar]
- 38.Curtis JR, Westfall AO, Allison JJ, Becker A, Casebeer L, Freeman A, Spettell CM, Weissman NW, Wilke S, Saag KG. Longitudinal patterns in the prevention of osteoporosis in glucocorticoid-treated patients. Arth Rheum. 2005;52:2485–2494. doi: 10.1002/art.21194. [DOI] [PubMed] [Google Scholar]
- 39.Adachi JD, Saag KG, Delmas PD, Liberman UA, Emkey RD, Seeman E, Lane NE, Kaufman JM, Poubelle PE, Hawkins F, Correa-Rotter R, Menkes CJ, Rodriguez-Portales JA, Schnitzer TJ, Block JA, Wing J, McIlwain HH, Westhovens R, Brown J, Melo-Gomes JA, Gruber BL, Yanover MJ, Leite MO, Siminoski KG, Nevitt MC, Sharp JT, Malice MP, Dumortier T, Czachur M, Carofano W, Daifotis A. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arth Rheum. 2001;44:202–211. doi: 10.1002/1529-0131(200101)44:1<202::AID-ANR27>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 40.Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW, Dequeker J, Favus M The Alendronate Phase III Osteoporosis Treatment Study Group. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med. 1995;333:1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 41.Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, Adami S, Weber K, Lorenc R, Pietschmann P, Vandormael K, Lombardi A. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604–610. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 42.Kim HJ, Zhao H, Kitaura H, Bhattacharyya S, Brewer JA, Muglia LJ, Ross FP, Teitelbaum SL. Glucocorticoids suppress bone formation via the osteoclast. J Clin Invest. 2006;116:2152–2160. doi: 10.1172/JCI28084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knothe Tate ML, Niederer P, Knothe U. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone. 1998;22:107–117. doi: 10.1016/s8756-3282(97)00234-2. [DOI] [PubMed] [Google Scholar]
- 44.Weinstein RS, Goellner J, Chambers TM, Hogan EA, Berryhill SB, Shelton RS, Manolagas SC. Glucocorticoids, aging and bone hydration: new insights into qualitative aspects of bone strength. J Bone Miner Res. 2007;22:S25. [Google Scholar]
- 45.Weinstein RS, O’Brien CA, Roberson PK, Manolagas SC. Endogenous glucocorticoids are critical for the development of skeletal fragility with aging in mice. J Bone Miner Res. 2008;23:S70. [Google Scholar]
- 46.Desmons S, Heger M, Delfosse C, Falgayrac G, Sarrazin T, Delattre C, Catros S, Mordon S, Penel G. A preliminary investigation into the effects of X-ray radiation on superficial cranial vascularization. Calcif Tissue Int. 2009;84:379–387. doi: 10.1007/s00223-009-9217-y. [DOI] [PubMed] [Google Scholar]
- 47.Calder JDF, Buttery L, Revell PA, Pearse M, Polak JM. Apoptosis – a significant cause of bone cell death in osteonecrosis of the femoral head. J Bone Joint Surg. 2004;86B:1209–1213. doi: 10.1302/0301-620x.86b8.14834. [DOI] [PubMed] [Google Scholar]
- 48.Weinstein RS, Nicholas RW, Manolagas SC. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab. 2000;85:2907–2912. doi: 10.1210/jcem.85.8.6714. [DOI] [PubMed] [Google Scholar]
- 49.Zizic TM, Marcoux C, Hungerford DS, Dansereau J-V, Stevens MB. Corticosteroid therapy associated with ischemic necrosis of bone in systemic lupus erythematosus. Am J Med. 1985;79:596–604. doi: 10.1016/0002-9343(85)90057-9. [DOI] [PubMed] [Google Scholar]
- 50.Felson DT, Anderson JJ. Across-study evaluation of association between steroid dose and bolus steroids and avascular necrosis of bone. Lancet. 1987;I:902–905. doi: 10.1016/s0140-6736(87)92870-4. [DOI] [PubMed] [Google Scholar]