Abstract
Reciprocal interactions between tumor and stromal cells govern carcinoma growth and progression. Signaling functions between these cell types in the tumor microenvironment are largely carried out by secreted growth factors and cytokines. This review discusses how proteoglycans, which are abundantly present in normal and neoplastic tissues, modulate paracrine growth factor signaling events. General principles of proteoglycan involvement in paracrine signaling include stromal induction, core protein processing by proteases and growth factor binding via proteoglycan glycosaminoglycan chains or core protein domains.
Keywords: cancer, proteoglycans, extracellular matrix, paracrine, tumor microenvironment, stroma
1. Introduction
Tumor formation and progression are governed by reciprocal paracrine interactions between cancer and stromal cells [1]. This review will explore evidence that proteoglycans (PGs) play key roles directly as paracrine signaling molecules and indirectly as modulators of signaling events. PGs are ubiquitous molecules composed of highly diverse core proteins and covalently attached glycosaminoglycan (GAG) polysaccharide chains [2]. Principally via heparan sulfate GAGs, PGs bind extracellular matrix (ECM) constituents and a variety of growth factors with crucial roles in cancer cell – stroma communication. Binding of growth factors to ECM PGs creates mitogen reservoirs and gradients. Importantly, PG heparan sulfate also participates in ternary growth factor ligand – receptor complexes, thus stabilizing these complexes and amplifying the signal [3].
Space restrictions prevent a discussion of “part-time” proteoglycans such as CD44 or “protein-free” GAGs such as hyaluronan and heparin in malignancy. Cell-autonomous co-receptor activities of PGs in growth factor receptor signaling or integrin-mediated adhesion have been reviewed [3,4] and will generally not be considered here. Critical functions of PGs in angiogenesis have been described [5] but their discussion would exceed the scope of this paper. Instead, this review will focus on paracrine signaling functions of the “classical” cell surface PGs in the syndecan and glypican families and the secreted PGs perlecan, collagens XV and XVIII and decorin.
2. Cell Surface Proteoglycans
2.1 Syndecans
The syndecan family of PGs comprises four members (Sdc1–4), which are composed of transmembrane core proteins that are decorated with heparan sulfate (HS) and chondroitin sulfate (CS) GAG chains [4,6]. Sdc1 can be shed from the cell surface by proteolytic cleavage of the core protein [7]. Through its release from the cell surface, Sdc1 is converted from a cell surface receptor to a diffusible mediator, enabling this molecule to participate in paracrine signaling events. Sdc1 shedding occurs at a basal level in most cells but can be increased by a variety of stimuli, which include protein kinase C activators, epidermal growth factor family members, stromal cell derived factor-1 (SDF1, CXCL12), and thrombin [8,9]. A distinct proteolytic cleavage site is present between amino acids Ala-243 and Ser-244 in the juxtamembrane region of the Sdc1 ectodomain [10]. Proteolytic Sdc1 cleavage has been attributed to a variety of enzymes, which include matrix metalloprotease (MMP) 7, MMP9, and the membrane type MMPs MT1-MMP (MMP14) and MT3-MMP [8,10–13]. Interestingly, Sdc1 shedding is enhanced by the presence of the tumor promoting enzyme heparanase, which degrades heparan sulfate GAGs and clusters the Sdc1 protein cores [14,15].
In adult organisms, Sdc1 is found primarily on epithelial cells and plasma cells. In carcinomas, tumor cell Sdc1 can be either lost or upregulated [16–20]. Elevated tumor cell Sdc1 predicts poor clinical outcome in breast cancer [17,20,21]; an effect that might be related to cell autonomous growth factor receptor or integrin activation rather than paracrine signaling [22,23].
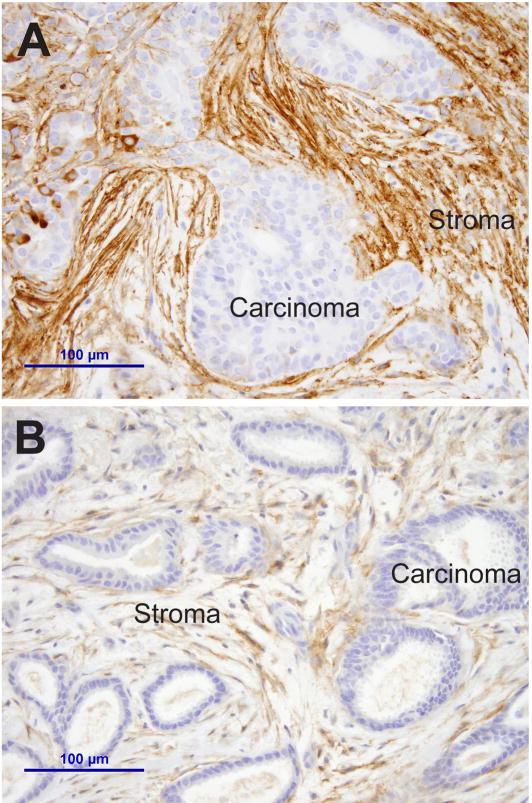
In addition to epithelial expression, Sdc1 is also transiently produced by mesenchymal cells at sites of epithelial induction during development, e.g. in the developing tooth and kidney [24,25]. This mesenchymal expression is recapitulated in cancer, when Sdc1 is induced in fibroblasts within the desmoplastic stroma in a variety of tumor types including breast cancer [26,27]. Sdc1 expression in stromal myofibroblasts is particularly well documented in breast cancer, where it is seen in the majority of breast carcinomas, independent of tumor subtype or prognostic markers (Fig. 1A) [26,27]. Initially, the biologic consequences of stromal Sdc1 expression were unclear but subsequent in vitro and in vivo work established stromal Sdc1 as a central mediator of paracrine growth stimulation [27–30]. Murine or human mammary fibroblasts stimulate breast carcinoma cell growth both in 2D and 3D co-culture, an activity that coincides with Sdc1 expression in the fibroblasts [27,29]. Importantly, the growth advantage attributable to the fibroblasts is abolished when Sdc1 induction is prevented either by genetic manipulation (using MEF from Sdc1 knock-out mice) [27] or by siRNA knock-down in human mammary fibroblasts [29]. In vivo, the growth of highly malignant MDA-MB-231 human breast carcinoma cells is accelerated when Sdc1-positive fibroblasts are admixed in the xenografts [28]. The paracrine growth promoting activity of Sdc1 depends on intact HS chains, since a core protein in which three serine GAG attachment domains are mutated to alanine fails to substitute for wild-type Sdc1 in the fibroblasts [29].
Figure 1.
Aberrant stromal expression of cell surface proteoglycans: A) Strong and diffuse syndecan-1 expression in stromal fibroblasts of a breast carcinoma. B) Glypican-1 expression in stromal fibroblasts of a breast carcinoma.
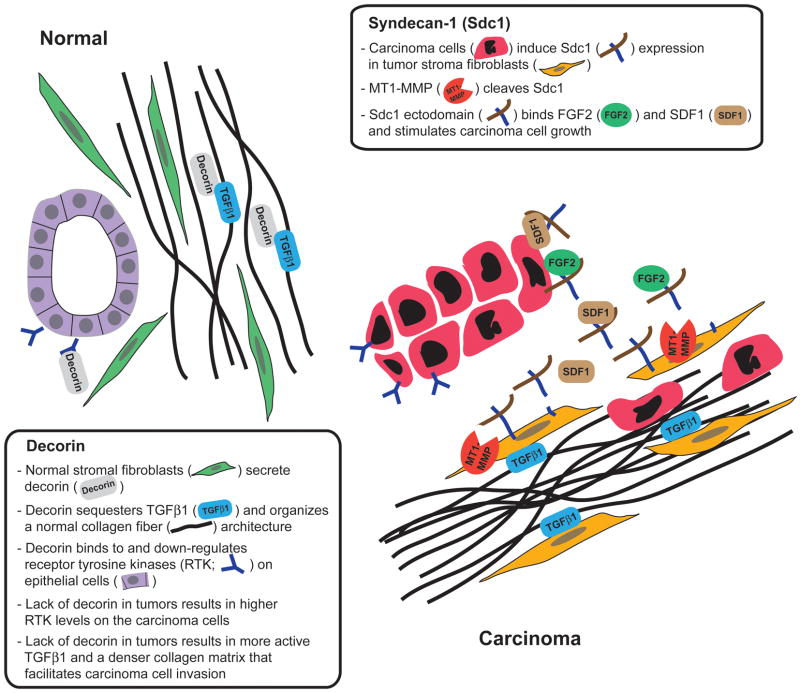
A further exploration of the underlying mechanism revealed that stromal Sdc1-mediated carcinoma cell growth stimulation requires Sdc1 shedding, because an “uncleavable” Sdc1 mutant was inactive [29]. In 3D co-cultures of immortalized human mammary fibroblasts and T47D breast carcinoma cells, Sdc1 release from the cell surface is mediated by fibroblast-derived membrane-type 1 matrix metalloprotease (MT1-MMP, aka MMP14), assigning another cancer-promoting function to this enzyme [30]. The soluble Sdc1 extracellular domain stimulates carcinoma cell growth in concert with fibroblast growth factor 2 (FGF2) and SDF1, as blocking of either one abolishes the growth advantage imparted on the carcinoma cells by the presence of fibroblasts [29]. Possibly, Sdc1 ectodomain shuttles SDF1 and/or FGF2 from the fibroblasts to the carcinoma cells and facilitates the formation of an active signaling complex. The apparent privileged role for Sdc1 is surprising, considering that human mammary fibroblasts produce abundant Sdc2 (also known as fibroglycan) and Sdc4, both of which can also be shed [8,31]. The specificity may be conveyed by a potential Sdc1 substrate selectivity of the MT1-MMP enzyme [10]. Notably, the Sdc2 and Sdc4 core proteins share little homology with Sdc1. Together, these findings are consistent with a complex, reciprocal paracrine signaling loop that involves Sdc1, an MMP and growth factors (schematically shown in Fig. 2).
Figure 2.
Schematic representation of the role of stromal syndecan-1 and decorin in normal mammary gland and breast cancer.
The role of the other syndecan family members in paracrine signaling events in cancer is less clear. The migration of fibroblasts into the fibrin-rich matrix of a wound site requires Sdc4 [32], which at least in part explains the wound healing defects seen in Sdc4 knockout mice [33]. It is therefore plausible that Sdc4 is also required for the recruitment of fibroblasts (from the local environment or the bone marrow) into the tumor stroma. In murine Lewis lung carcinomas, tumor cell Sdc2 expression and core protein phosphorylation lead to fibronectin binding followed by tumor stroma formation [34]. One can only speculate that the provisional ECM tethered to Sdc2 around the tumor cells facilitates the recruitment of stromal fibroblasts. A recently discovered unexpected activity of Sdc2 is the inhibition of MMP2, which results in the suppression of tumor invasion and metastasis [35]. Sdc2 and Sdc3 are found in human ovarian carcinoma stroma but their functions in this tumor type are unknown [18].
2.2 Glypicans
The glypicans constitute a six-member family of cell surface proteoglycans (Gpc1–6) [36]. Because glypicans are anchored in the cell membrane by a glycosylphosphatidylinositol (GPI)-linked lipid tail, they can be released into the pericellular environment by phosphoinositide-specific phospholipase-C; however, the regulation and physiologic relevance of glypican shedding are poorly understood. All glypicans share a globular domain, which regulates heparan sulfate synthesis [37]. Elevated Gpc1 expression has been documented in pancreas carcinoma, breast cancer and in glioma, and this proteoglycan appears to play a privileged role in promoting growth factor activity [38–40]. The involvement of Gpc3 in cancer is complex and context-dependent. Gpc3 is overexpressed in the majority of hepatocellular carcinomas and has in fact utility as a serum and tissue marker in the diagnosis of this malignancy [41–43]. In the majority of breast carcinomas, Gpc3 expression is silenced. Re-expression of Gpc3 suppresses invasion and promotes apoptosis [44,45]. Many of the cellular activities of Gpc3 are reportedly mediated by a modulation of paracrine canonical wnt signaling. Interestingly, binding interactions between wnt and the Gpc3 core protein rather than heparan sulfate chains appear to mediate this effect [44]. While glypicans may act on the tumor cells in a cell-autonomous fashion, they are also expressed by stromal fibroblasts (Fig. 1B) and are released from the tumor cell surfaces into the stromal microenvironment [18,38]. Regardless of the cell type of origin, glypicans modulate paracrine, stroma-derived signals and act primarily as pro-tumorigenic agents.
3. Secreted Extracellular Matrix Proteoglycans
3.1 Perlecan
Perlecan is a secreted PG, which consists of a large (~450 kDa) core protein, composed of modules homologous to growth factors and domains implicated in lipid metabolism or cell adhesion [46]. Heparan sulfate GAG chains are covalently attached at the N-terminus. This abundant and ubiquitous PG is incorporated into basement membranes and the pericellular space upon secretion. Its deposition at a strategic extracellular location makes perlecan a natural candidate for a paracrine mediator. Perlecan-derived HS was initially thought to carry a specific and privileged activity as a FGF2 co-factor [47,48] but later, other heparan sulfate PGs were implicated in FGF2 signaling as well [49,50]. Heparan sulfate sulfation pattern and growth factor binding affinities vary greatly between perlecan preparations isolated from different cell types [51]. This and other observations suggest that the cell source and context are more important determinants of heparan sulfate structure and function than the identity of the core protein.
The cumulative evidence indicates that perlecan is an important mediator of paracrine stroma-tumor cell interactions in a variety of cancers. Perlecan expression is greatly increased in metastatic human melanomas [52] and compared to normal pancreatic epithelial cells, perlecan is found in the secretome of pancreas carcinoma cells [53]. Tumor growth is retarded in mice genetically deficient in this PG [54]. In prostate cancer, perlecan production correlates with high Gleason grade and thus clinical outcome [55]. In vitro, silencing of perlecan expression diminishes carcinoma cell proliferation. Perlecan and the growth factor and morphogen Sonic hedgehog homolog (Shh) not only co-immunoprecipitate but mitogenesis is restored in perlecan siRNA-treated cells by simultaneous expression of the Shh downstream effector Gli1, indicating a critical role for perlecan in a growth promoting, paracrine Shh-mediated signaling pathway [55,56]. The importance of perlecan in prostate carcinoma may extend beyond Shh, as perlecan knock-down reduces the response to other growth factors [57].
The perlecan activities discussed so far are mediated by its heparan sulfate chains. Interestingly, binding interactions between the perlecan core protein and growth factors or other paracrine mediators have also been reported. The perlecan core protein not only binds FGF7 (also known as keratinocyte growth factor) [58] but is also required for the activation of FGFR2iiib, the cognate FGF7 receptor [59]. As a prototypic paracrine mediator, FGF7 is secreted by stromal fibroblasts and acts specifically on epithelia, including carcinoma cells [60]. Other perlecan core protein binding partners include FGF binding protein (FGF-BP), itself a modulator of FGF signaling [61], and progranulin [62], an autocrine, pro-tumorigenic growth factor [63,64].
3.2 Multiplexins (Collagens XVIII and XV)
The basement membrane constituents collagen XVIII and XV share characteristics of collagens and PGs [65]. These molecules are composed of interrupted triple-helical modules and non-collagenous domains at the N- and C-termini. Three Ser-Gly consensus sites are glycanated with heparan sulfate chains. Collagen XVIII prevents the invasion of squamous cell carcinoma cells by blocking the activation of MMPs 2, 9 and 13 [66]. A cryptic domain within collagen XVIII (but not the intact parent molecule) displays frizzled-like activity upon its proteolytic release and blocks canonical wnt/beta-catenin signaling [67]. Consequently, cyclin D1 and c-myc are down-regulated and tumor growth is abated. The forced overexpression of collagen XV in highly malignant human cervical carcinoma cells results in the deposition of this PG in the pericellular environment and a suppression of tumor cell growth in 3D cultures and of tumor growth in vivo [68]. The molecular mechanism of collagen XV-mediated tumor growth suppression is unclear.
Perlecan and collagens XVIII and XV, the three basement membrane PGs discussed here, are subject to proteolytic cleavage, which produces the diffusible peptide fragments endorepellin, endostatin, and restin, respectively. These fragments possess potent anti-angiogenic activities only after their release from the parent molecules. These intriguing activities have been reviewed elsewhere [65,69].
3.3 Decorin
Decorin belongs to a diverse family of extracellular small leucine-rich proteoglycans (SLRP) [reviewed in [70]]. Decorin participates in paracrine signaling events in normal and neoplastic tissues at multiple levels [71]. Decorin is produced primarily by fibroblasts (and carcinoma-associated myofibroblasts) and is essential for the proper assembly of collagen fibrils [72]. Genetically decorin-deficient mice develop spontaneous colon adenocarcinomas whereas de novo expression of decorin in human colon carcinoma cells suppresses the malignant phenotype [73,74]. These findings suggest a tumor suppressor role for this PG. Loss of decorin in the mouse model is accompanied by a decrease in E-cadherin, accompanied by elevated beta-catenin signaling and a reduction of the cyclin-dependent kinase inhibitor p21 [73].
The most extensively documented roles for decorin in stroma-carcinoma communication are mediated through its binding affinities for the epidermal growth factor receptor (EGFR) and transforming growth factor beta 1 (TGFβ1). Decorin binds to the EGFR, which results in transient phosphorylation followed by dephosphorylation, internalization and degradation of this receptor [75]. Findings by Goldoni and co-workers indicate that decorin also downregulates other Erb family members including ErbB2 [76]. In vivo, decorin effectively blocks the metastatic spread of ErbB2 overexpressing mammary carcinoma cells, whereas an ErbB2 receptor tyrosine kinase inhibitor lacks this activity [76]. More recently, the same group showed that decorin also binds to the Met receptor and acts as natural antagonist to its ligand, the pro-invasive and tumor promoting cytokine scatter factor/hepatocyte growth factor (SF/HGF) [77].
In the extracellular compartment, decorin binds and sequesters TGFβ1 and thus dampens the activity of this cytokine. TGFβ1 has complex roles in reciprocal paracrine signaling between carcinoma cells and stromal fibroblasts [reviewed in [78]]. Carcinoma cell-derived TGFβ1 contributes to the induction of a reactive, desmoplastic tumor stroma [79–81]. Conversely, fibroblast/myofibroblast-derived TGFβ1 either decelerates carcinoma cell proliferation or promotes tumorigenesis, dependent on the cellular context [82]. Based on its modulating activity on TGFβ1 signaling and direct effects on collagen fibril assembly, the absence of decorin in carcinomas leads to higher collagen density (schematically shown in Fig. 2) [83]. This finding is of great interest in the context of the well-established correlation between increased breast density and breast carcinoma risk [84] and the fact that in vitro, increased collagen density promotes tumorigenesis, invasion and metastasis [85–87].
4. Conclusions
Abundant evidence implicates PGs as crucial players in paracrine signaling between stromal fibroblasts/myofibroblasts and carcinoma cells. In general, this involves overexpression (example: Sdc1 induction in fibroblasts) or reduced secretion (example: decorin) in the stromal compartment (Fig. 2). Altered rates of PG processing by elevated activities of MMPs and heparanase enzymes likely contribute to generating abnormal levels of diffusible stromal PGs in tumors. The major mechanisms of action of the stromal PGs consist of the modulation of paracrine signals by shuttling cytokines from the stromal compartment to the carcinoma cell surface and forming an active complex with signaling receptors (example: Sdc1 ectodomain/FGF2/FGFR1), sequestering paracrine mediators (example: decorin/TGFβ1) or directly regulating carcinoma cell receptor tyrosine kinases (example: decorin/ErbB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Radisky DC, Bissell MJ. Cancer. Respect thy neighbor! Science. 2004;303(5659):775–7. doi: 10.1126/science.1094412. [DOI] [PubMed] [Google Scholar]
- 2.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446(7139):1030–7. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 3.Rapraeger AC. In the clutches of proteoglycans: how does heparan sulfate regulate FGF binding? Chem Biol. 1995;2(10):645–9. doi: 10.1016/1074-5521(95)90025-x. [DOI] [PubMed] [Google Scholar]
- 4.Beauvais DM, Rapraeger AC. Syndecans in tumor cell adhesion and signaling. Reprod Biol Endocrinol. 2004;2:3. doi: 10.1186/1477-7827-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J Clin Invest. 2001;108(3):349–55. doi: 10.1172/JCI13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, et al. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–93. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- 7.Subramanian SV, Fitzgerald ML, Bernfield M. Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor receptor activation. J Biol Chem. 1997;272(23):14713–20. doi: 10.1074/jbc.272.23.14713. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J Cell Biol. 2000;148(4):811–24. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Gotte M, Bernfield M, Reizes O. Constitutive and accelerated shedding of murine syndecan-1 is mediated by cleavage of its core protein at a specific juxtamembrane site. Biochemistry. 2005;44(37):12355–61. doi: 10.1021/bi050620i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, et al. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J Biol Chem. 2003;278(42):40764–70. doi: 10.1074/jbc.M306736200. [DOI] [PubMed] [Google Scholar]
- 11.Brule S, Charnaux N, Sutton A, Ledoux D, Chaigneau T, Saffar L, et al. The shedding of syndecan-4 and syndecan-1 from HeLa cells and human primary macrophages is accelerated by SDF-1/CXCL12 and mediated by the matrix metalloproteinase-9. Glycobiology. 2006;16(6):488–501. doi: 10.1093/glycob/cwj098. [DOI] [PubMed] [Google Scholar]
- 12.Chen P, Abacherli LE, Nadler ST, Wang Y, Li Q, Parks WC. MMP7 shedding of syndecan-1 facilitates re-epithelialization by affecting alpha(2)beta(1) integrin activation. PLoS One. 2009;4(8):e6565. doi: 10.1371/journal.pone.0006565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111(5):635–46. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 14.Levy-Adam F, Feld S, Suss-Toby E, Vlodavsky I, Ilan N. Heparanase facilitates cell adhesion and spreading by clustering of cell surface heparan sulfate proteoglycans. PLoS One. 2008;3(6):e2319. doi: 10.1371/journal.pone.0002319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Macleod V, Miao HQ, Theus A, Zhan F, Shaughnessy JD, Jr, et al. Heparanase enhances syndecan-1 shedding: a novel mechanism for stimulation of tumor growth and metastasis. J Biol Chem. 2007;282(18):13326–33. doi: 10.1074/jbc.M611259200. [DOI] [PubMed] [Google Scholar]
- 16.Anttonen A, Heikkila P, Kajanti M, Jalkanen M, Joensuu H. High syndecan-1 expression is associated with favourable outcome in squamous cell lung carcinoma treated with radical surgery. Lung Cancer. 2001;32(3):297–305. doi: 10.1016/s0169-5002(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 17.Barbareschi M, Maisonneuve P, Aldovini D, Cangi MG, Pecciarini L, Angelo Mauri F, et al. High syndecan-1 expression in breast carcinoma is related to an aggressive phenotype and to poorer prognosis. Cancer. 2003;98(3):474–83. doi: 10.1002/cncr.11515. [DOI] [PubMed] [Google Scholar]
- 18.Davies EJ, Blackhall FH, Shanks JH, David G, McGown AT, Swindell R, et al. Distribution and clinical significance of heparan sulfate proteoglycans in ovarian cancer. Clin Cancer Res. 2004;10(15):5178–86. doi: 10.1158/1078-0432.CCR-03-0103. [DOI] [PubMed] [Google Scholar]
- 19.Inki P, Joensuu H, Grenman R, Klemi P, Jalkanen M. Association between syndecan-1 expression and clinical outcome in squamous cell carcinoma of the head and neck. Br J Cancer. 1994;70(2):319–23. doi: 10.1038/bjc.1994.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baba F, Swartz K, van Buren R, Eickhoff J, Zhang Y, Wolberg W, et al. Syndecan-1 and syndecan-4 are overexpressed in an estrogen receptor-negative, highly proliferative breast carcinoma subtype. Breast Cancer Res Treat. 2006;98(1):91–8. doi: 10.1007/s10549-005-9135-2. [DOI] [PubMed] [Google Scholar]
- 21.Leivonen M, Lundin J, Nordling S, von Boguslawski K, Haglund C. Prognostic value of syndecan-1 expression in breast cancer. Oncology. 2004;67(1):11–8. doi: 10.1159/000080280. [DOI] [PubMed] [Google Scholar]
- 22.Mundhenke C, Meyer K, Drew S, Friedl A. Heparan sulfate proteoglycans as regulators of fibroblast growth factor-2 receptor binding in breast carcinomas. Am J Pathol. 2002;160(1):185–94. doi: 10.1016/S0002-9440(10)64362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beauvais DM, Burbach BJ, Rapraeger AC. The syndecan-1 ectodomain regulates alphavbeta3 integrin activity in human mammary carcinoma cells. J Cell Biol. 2004;167(1):171–81. doi: 10.1083/jcb.200404171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vainio S, Thesleff I. Sequential induction of syndecan, tenascin and cell proliferation associated with mesenchymal cell condensation during early tooth development. Differentiation. 1992;50(2):97–105. doi: 10.1111/j.1432-0436.1992.tb00490.x. [DOI] [PubMed] [Google Scholar]
- 25.Vainio S, Jalkanen M, Bernfield M, Saxen L. Transient expression of syndecan in mesenchymal cell aggregates of the embryonic kidney. Dev Biol. 1992;152(2):221–32. doi: 10.1016/0012-1606(92)90130-9. [DOI] [PubMed] [Google Scholar]
- 26.Stanley MJ, Stanley MW, Sanderson RD, Zera R. Syndecan-1 expression is induced in the stroma of infiltrating breast carcinoma. Am J Clin Pathol. 1999;112(3):377–83. doi: 10.1093/ajcp/112.3.377. [DOI] [PubMed] [Google Scholar]
- 27.Maeda T, Alexander CM, Friedl A. Induction of syndecan-1 expression in stromal fibroblasts promotes proliferation of human breast cancer cells. Cancer Res. 2004;64(2):612–21. doi: 10.1158/0008-5472.can-03-2439. [DOI] [PubMed] [Google Scholar]
- 28.Maeda T, Desouky J, Friedl A. Syndecan-1 expression by stromal fibroblasts promotes breast carcinoma growth in vivo and stimulates tumor angiogenesis. Oncogene. 2006;25(9):1408–12. doi: 10.1038/sj.onc.1209168. [DOI] [PubMed] [Google Scholar]
- 29.Su G, Blaine SA, Qiao D, Friedl A. Shedding of syndecan-1 by stromal fibroblasts stimulates human breast cancer cell proliferation via FGF2 activation. J Biol Chem. 2007;282(20):14906–15. doi: 10.1074/jbc.M611739200. [DOI] [PubMed] [Google Scholar]
- 30.Su G, Blaine SA, Qiao D, Friedl A. Membrane type 1 matrix metalloproteinase-mediated stromal syndecan-1 shedding stimulates breast carcinoma cell proliferation. Cancer Res. 2008;68(22):9558–65. doi: 10.1158/0008-5472.CAN-08-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fears CY, Gladson CL, Woods A. Syndecan-2 is expressed in the microvasculature of gliomas and regulates angiogenic processes in microvascular endothelial cells. J Biol Chem. 2006;281(21):14533–6. doi: 10.1074/jbc.C600075200. [DOI] [PubMed] [Google Scholar]
- 32.Lin F, Ren XD, Doris G, Clark RA. Three-dimensional migration of human adult dermal fibroblasts from collagen lattices into fibrin/fibronectin gels requires syndecan-4 proteoglycan. J Invest Dermatol. 2005;124(5):906–13. doi: 10.1111/j.0022-202X.2005.23740.x. [DOI] [PubMed] [Google Scholar]
- 33.Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, et al. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest. 2001;107(2):R9–R14. doi: 10.1172/JCI10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itano N, Oguri K, Nagayasu Y, Kusano Y, Nakanashi H, David G, et al. Phosphorylation of a membrane-intercalated proteoglycan, syndecan-2, expressed in a stroma-inducing clone from a mouse Lewis lung carcinoma. Biochem J. 1996;315 (Pt 3):925–30. doi: 10.1042/bj3150925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munesue S, Yoshitomi Y, Kusano Y, Koyama Y, Nishiyama A, Nakanishi H, et al. A novel function of syndecan-2, suppression of matrix metalloproteinase-2 activation, which causes suppression of metastasis. J Biol Chem. 2007;282(38):28164–74. doi: 10.1074/jbc.M609812200. [DOI] [PubMed] [Google Scholar]
- 36.Filmus J, Capurro M, Rast J. Glypicans. Genome Biol. 2008;9(5):224. doi: 10.1186/gb-2008-9-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen RL, Lander AD. Mechanisms underlying preferential assembly of heparan sulfate on glypican-1. J Biol Chem. 2001;276(10):7507–17. doi: 10.1074/jbc.M008283200. [DOI] [PubMed] [Google Scholar]
- 38.Kleeff J, Ishiwata T, Kumbasar A, Friess H, Buchler MW, Lander AD, et al. The cell-surface heparan sulfate proteoglycan glypican-1 regulates growth factor action in pancreatic carcinoma cells and is overexpressed in human pancreatic cancer. J Clin Invest. 1998;102(9):1662–73. doi: 10.1172/JCI4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su G, Meyer K, Nandini CD, Qiao D, Salamat S, Friedl A. Glypican-1 is frequently overexpressed in human gliomas and enhances FGF-2 signaling in glioma cells. Am J Pathol. 2006;168(6):2014–26. doi: 10.2353/ajpath.2006.050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuda K, Maruyama H, Guo F, Kleeff J, Itakura J, Matsumoto Y, et al. Glypican-1 is overexpressed in human breast cancer and modulates the mitogenic effects of multiple heparin-binding growth factors in breast cancer cells. Cancer Res. 2001;61(14):5562–9. [PubMed] [Google Scholar]
- 41.Baumhoer D, Tornillo L, Stadlmann S, Roncalli M, Diamantis EK, Terracciano LM. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am J Clin Pathol. 2008;129(6):899–906. doi: 10.1309/HCQWPWD50XHD2DW6. [DOI] [PubMed] [Google Scholar]
- 42.Capurro M, Filmus J. Glypican-3 as a serum marker for hepatocellular carcinoma. Cancer Res. 2005;65(1):372. author reply 372–3. [PubMed] [Google Scholar]
- 43.Zhu ZW, Friess H, Wang L, Abou-Shady M, Zimmermann A, Lander AD, et al. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut. 2001;48(4):558–64. doi: 10.1136/gut.48.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65(14):6245–54. doi: 10.1158/0008-5472.CAN-04-4244. [DOI] [PubMed] [Google Scholar]
- 45.Stigliano I, Puricelli L, Filmus J, Sogayar MC, Bal de Kier Joffe E, Peters MG. Glypican-3 regulates migration, adhesion and actin cytoskeleton organization in mammary tumor cells through Wnt signaling modulation. Breast Cancer Res Treat. 2009;114(2):251–62. doi: 10.1007/s10549-008-0009-2. [DOI] [PubMed] [Google Scholar]
- 46.Whitelock JM, Melrose J, Iozzo RV. Diverse cell signaling events modulated by perlecan. Biochemistry. 2008;47(43):11174–83. doi: 10.1021/bi8013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aviezer D, Hecht D, Safran M, Eisinger M, David G, Yayon A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. 1994;79(6):1005–13. doi: 10.1016/0092-8674(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 48.Aviezer D, Iozzo RV, Noonan DM, Yayon A. Suppression of autocrine and paracrine functions of basic fibroblast growth factor by stable expression of perlecan antisense cDNA. Mol Cell Biol. 1997;17(4):1938–46. doi: 10.1128/mcb.17.4.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinfeld R, Van Den Berghe H, David G. Stimulation of fibroblast growth factor receptor-1 occupancy and signaling by cell surface-associated syndecans and glypican. J Cell Biol. 1996;133(2):405–16. doi: 10.1083/jcb.133.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qiao D, Meyer K, Mundhenke C, Drew SA, Friedl A. Heparan sulfate proteoglycans as regulators of fibroblast growth factor-2 signaling in brain endothelial cells. Specific role for glypican-1 in glioma angiogenesis. J Biol Chem. 2003;278(18):16045–53. doi: 10.1074/jbc.M211259200. [DOI] [PubMed] [Google Scholar]
- 51.Knox S, Merry C, Stringer S, Melrose J, Whitelock J. Not all perlecans are created equal: interactions with fibroblast growth factor (FGF) 2 and FGF receptors. J Biol Chem. 2002;277(17):14657–65. doi: 10.1074/jbc.M111826200. [DOI] [PubMed] [Google Scholar]
- 52.Cohen IR, Murdoch AD, Naso MF, Marchetti D, Berd D, Iozzo RV. Abnormal expression of perlecan proteoglycan in metastatic melanomas. Cancer Res. 1994;54(22):5771–4. [PubMed] [Google Scholar]
- 53.Gronborg M, Kristiansen TZ, Iwahori A, Chang R, Reddy R, Sato N, et al. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol Cell Proteomics. 2006;5(1):157–71. doi: 10.1074/mcp.M500178-MCP200. [DOI] [PubMed] [Google Scholar]
- 54.Zhou Z, Wang J, Cao R, Morita H, Soininen R, Chan KM, et al. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Res. 2004;64(14):4699–702. doi: 10.1158/0008-5472.CAN-04-0810. [DOI] [PubMed] [Google Scholar]
- 55.Datta MW, Hernandez AM, Schlicht MJ, Kahler AJ, DeGueme AM, Dhir R, et al. Perlecan, a candidate gene for the CAPB locus, regulates prostate cancer cell growth via the Sonic Hedgehog pathway. Mol Cancer. 2006;5:9. doi: 10.1186/1476-4598-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Datta S, Pierce M, Datta MW. Perlecan signaling: helping hedgehog stimulate prostate cancer growth. Int J Biochem Cell Biol. 2006;38(11):1855–61. doi: 10.1016/j.biocel.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 57.Savore C, Zhang C, Muir C, Liu R, Wyrwa J, Shu J, et al. Perlecan knockdown in metastatic prostate cancer cells reduces heparin-binding growth factor responses in vitro and tumor growth in vivo. Clin Exp Metastasis. 2005;22(5):377–90. doi: 10.1007/s10585-005-2339-3. [DOI] [PubMed] [Google Scholar]
- 58.Mongiat M, Taylor K, Otto J, Aho S, Uitto J, Whitelock JM, et al. The protein core of the proteoglycan perlecan binds specifically to fibroblast growth factor-7. J Biol Chem. 2000;275(10):7095–100. doi: 10.1074/jbc.275.10.7095. [DOI] [PubMed] [Google Scholar]
- 59.Ghiselli G, Eichstetter I, Iozzo RV. A role for the perlecan protein core in the activation of the keratinocyte growth factor receptor. Biochem J. 2001;359(Pt 1):153–63. doi: 10.1042/0264-6021:3590153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu X, Jin C, Wang F, Yu C, McKeehan WL. Stromal cell heterogeneity in fibroblast growth factor-mediated stromal-epithelial cell cross-talk in premalignant prostate tumors. Cancer Res. 2003;63(16):4936–44. [PubMed] [Google Scholar]
- 61.Mongiat M, Otto J, Oldershaw R, Ferrer F, Sato JD, Iozzo RV. Fibroblast growth factor-binding protein is a novel partner for perlecan protein core. J Biol Chem. 2001;276(13):10263–71. doi: 10.1074/jbc.M011493200. [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez EM, Mongiat M, Slater SJ, Baffa R, Iozzo RV. A novel interaction between perlecan protein core and progranulin: potential effects on tumor growth. J Biol Chem. 2003;278(40):38113–6. doi: 10.1074/jbc.C300310200. [DOI] [PubMed] [Google Scholar]
- 63.He Z, Bateman A. Progranulin gene expression regulates epithelial cell growth and promotes tumor growth in vivo. Cancer Res. 1999;59(13):3222–9. [PubMed] [Google Scholar]
- 64.Lu R, Serrero G. Inhibition of PC cell-derived growth factor (PCDGF, epithelin/granulin precursor) expression by antisense PCDGF cDNA transfection inhibits tumorigenicity of the human breast carcinoma cell line MDA-MB-468. Proc Natl Acad Sci U S A. 2000;97(8):3993–8. doi: 10.1073/pnas.97.8.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iozzo RV, Zoeller JJ, Nystrom A. Basement membrane proteoglycans: modulators Par Excellence of cancer growth and angiogenesis. Mol Cells. 2009;27(5):503–13. doi: 10.1007/s10059-009-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nyberg P, Heikkila P, Sorsa T, Luostarinen J, Heljasvaara R, Stenman UH, et al. Endostatin inhibits human tongue carcinoma cell invasion and intravasation and blocks the activation of matrix metalloprotease-2, -9, and -13. J Biol Chem. 2003;278(25):22404–11. doi: 10.1074/jbc.M210325200. [DOI] [PubMed] [Google Scholar]
- 67.Quelard D, Lavergne E, Hendaoui I, Elamaa H, Tiirola U, Heljasvaara R, et al. A cryptic frizzled module in cell surface collagen 18 inhibits Wnt/beta-catenin signaling. PLoS One. 2008;3(4):e1878. doi: 10.1371/journal.pone.0001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harris A, Harris H, Hollingsworth MA. Complete suppression of tumor formation by high levels of basement membrane collagen. Mol Cancer Res. 2007;5(12):1241–5. doi: 10.1158/1541-7786.MCR-07-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colorado PC, Torre A, Kamphaus G, Maeshima Y, Hopfer H, Takahashi K, et al. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res. 2000;60(9):2520–6. [PubMed] [Google Scholar]
- 70.Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem. 1999;274(27):18843–6. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- 71.Goldoni S, Iozzo RV. Tumor microenvironment: Modulation by decorin and related molecules harboring leucine-rich tandem motifs. Int J Cancer. 2008;123(11):2473–9. doi: 10.1002/ijc.23930. [DOI] [PubMed] [Google Scholar]
- 72.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136(3):729–43. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bi X, Tong C, Dockendorff A, Bancroft L, Gallagher L, Guzman G, et al. Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis. 2008;29(7):1435–40. doi: 10.1093/carcin/bgn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Santra M, Skorski T, Calabretta B, Lattime EC, Iozzo RV. De novo decorin gene expression suppresses the malignant phenotype in human colon cancer cells. Proc Natl Acad Sci U S A. 1995;92(15):7016–20. doi: 10.1073/pnas.92.15.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Csordas G, Santra M, Reed CC, Eichstetter I, McQuillan DJ, Gross D, et al. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J Biol Chem. 2000;275(42):32879–87. doi: 10.1074/jbc.M005609200. [DOI] [PubMed] [Google Scholar]
- 76.Goldoni S, Seidler DG, Heath J, Fassan M, Baffa R, Thakur ML, et al. An antimetastatic role for decorin in breast cancer. Am J Pathol. 2008;173(3):844–55. doi: 10.2353/ajpath.2008.080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goldoni S, Humphries A, Nystrom A, Sattar S, Owens RT, McQuillan DJ, et al. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol. 2009;185(4):743–54. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6(7):506–20. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 79.Erickson AC, Barcellos-Hoff MH. The not-so innocent bystander: the microenvironment as a therapeutic target in cancer. Expert Opin Ther Targets. 2003;7(1):71–88. doi: 10.1517/14728222.7.1.71. [DOI] [PubMed] [Google Scholar]
- 80.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 81.Korc M. Pancreatic cancer-associated stroma production. Am J Surg. 2007;194(4 Suppl):S84–6. doi: 10.1016/j.amjsurg.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Massague J. TGFbeta in Cancer. Cell. 2008;134(2):215–30. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferdous Z, Wei VM, Iozzo R, Hook M, Grande-Allen KJ. Decorin-transforming growth factor- interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices. J Biol Chem. 2007;282(49):35887–98. doi: 10.1074/jbc.M705180200. [DOI] [PubMed] [Google Scholar]
- 84.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–69. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 85.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 86.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4(1):38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]