Abstract
In our prior studies, systemic administration of the opioid receptor antagonist naltrexone (NTX) did not block flavor preference conditioning by the sweet taste or post-oral actions of sugar despite reducing intake. Because opioid signaling in the nucleus accumbens (NAc) is implicated in food reward, this study determined if NTX administered into the NAc would block the expression of sugar-conditioned preferences. In Experiment 1, food-restricted rats with bilateral NAc shell or core cannulae were trained to drink a fructose (8%) + saccharin (0.2%) solution mixed with one flavor (CS+) and a less-preferred 0.2% saccharin solution mixed with another flavor (CS−) during one-bottle sessions. Two-bottle tests with the two flavors mixed in saccharin solutions occurred 10 min following total bilateral NAc shell or core doses of 0, 1, 25 and 50 μg of NTX. The rats preferred the CS+ over CS− following vehicle (80%) and all NTX doses in the shell and core. The CS+ preference was reduced to 64% and 72% by 50 μg NTX in the shell and core, although only the core effect was significant. In Experiment 2, food-restricted rats were trained to drink one flavored saccharin solution (CS+) paired with an intragastic (IG) glucose (8%) infusion and a second flavored saccharin solution (CS−) paired with an IG water infusion. In subsequent two-bottle tests, the rats displayed significant preferences for the CS+ (81-91%) that were unaltered by any NTX dose in the shell or core. CS+ intake, however, was reduced by NTX in the shell, but not the core. These data indicate that accumbal opioid antagonism slightly attenuated, but did not block the expression of sugar-conditioned flavor preferences. Therefore, while opioid drugs can have potent effects on sugar intake they appear less effective in altering sugar-conditioned flavor preferences.
Keywords: naltrexone, sweet taste, fructose, glucose, saccharin, conditioning
1. Introduction
There is extensive evidence that central opioid systems are importantly involved in the ingestive response to palatable foods and fluids (see reviews: Bodnar, 2004; Cooper, 2007; Levine, 2006). In a variety of rodent studies, opioid receptor agonists selectively increased, whereas antagonists selectively reduced, the intake of preferred foods and fluids when administered systemically or directly into the brain. The opioid modulation of the hedonic, or more broadly the reward evaluation of sweet substances has been studied in particular detail. For example, general opioid receptor antagonism is reported to (a) suppress intake of sweet solutions more than plain water (Cooper, 1983; Le Magnen et al., 1980; Sclafani et al., 1982); (b) block the portion of feeding that appears driven by sweet taste in food-restricted animals (Levine et al., 1995); (c) reduce sucrose's positive hedonic qualities in a taste reactivity (TR) paradigm (Levine et al., 1995; Parker et al., 1992); (d) reduce sucrose intake in sham-feeding tests which minimize post-oral effects (Kirkham and Cooper, 1988; Rockwood and Reid, 1982), but (e) does not reduce sweet taste discrimination (O'Hare et al., 1994). Some human studies also report that opioid receptor antagonism reduces sweet taste pleasantness without reducing sweet taste discrimination (Arbisi et al., 1999; Fantino et al., 1986).
An early study also implicated the opioid system in flavor preference conditioning by sugar (Mehiel, 1996). Subsequent work in our laboratories, however, failed to support this view. In separate studies, we investigated the effects of systemic administration of the opioid receptor antagonist naltrexone (NTX) on flavor preferences conditioned by either the sweet taste (flavor-taste conditioning) or the post-oral nutritive effects (flavor-nutrient conditioning) of sugars (Azzara et al., 2000; Baker et al., 2004; Yu et al., 1999). Our studies revealed that NTX treatment during training trials failed to block preference conditioning, and that drug treatment during post-training choice tests failed to block the expression of previously learned flavor preferences. These findings would appear to conflict with the general idea that central opioid signaling modulates the avidity for preferred foods and fluids (see review: Cooper, 2007). It may be that opioid antagonism influences primarily unlearned rather than learned preferences. It is also possible, however, that the systemic drug treatments used in our conditioning experiments obscured an important contribution of specific central opioid circuits on conditioned food preferences. Systemically administered drugs will act on opioid circuits in various brain regions that may have different functions, such as energy homeostasis as compared to food hedonics (Glass et al., 1999; Gosnell and Levine, 2009).
A recent study by Wooley et al. (2006) indicates that the route of drug administration can influence the effect of NTX on food preference. Rats were given choice tests with two differently-flavored (chocolate and banana) but nutritionally-identical food pellets. Most animals displayed a mild preference (presumably unlearned) for the chocolate-flavored food. Systemic NTX treatment did not alter this preference but rather reduced the intakes of both flavored foods. However, NTX microinfusions into the nucleus accumbens (NAc) selectively reduced the intake of the preferred chocolate-flavored food. The NAc is recognized as a critical site of opioid action on food intake and reward (see reviews: Kelley et al., 2002; Peciña, 2008). Thus, it is not surprising that NAc and systemic administration of opioid drugs might have different effects on food preferences.
In light of the food preference findings of Wooley et al. (2006), the present study determined if NAc microinfusions of NTX would substantially attenuate or block the expression of sugar-conditioned flavor preferences. Different groups of rats received drug microinfusions in the NAc shell and core regions since opioid activation of both regions alters food intake (Zhang and Kelley, 2000). As in our prior work, separate experiments focused on flavor-taste learning (flavor conditioning by the sweet taste of sugar) and flavor-nutrient learning (flavor conditioning by the post-oral nutritive effects of sugar) (Azzara et al., 2000; Baker et al., 2004; Bernal et al., 2008; Touzani et al., 2008; Yu et al., 1999). Flavor-nutrient learning was investigated by training rats to associate one flavored (e.g., grape) saccharin solution with intragastric (IG) infusions of glucose and a different flavored (e.g., cherry) saccharin solution with IG water infusions. Preference conditioning was then evaluated in two-bottle tests with both flavored saccharin solutions presented without IG infusions. Flavor-taste learning was investigated by training rats to associate one flavor mixed into a preferred fructose solution and another flavor (e.g., cherry) mixed into a less preferred saccharin solution. Preference conditioning was then evaluated in subsequent two-bottle tests with the two flavors presented in saccharin solutions. A preference for the fructose-paired flavor is considered to be reinforced by the sweet taste rather than a post-oral nutrient action of fructose. This interpretation is based on the finding that IG fructose infusions, unlike IG glucose infusions, do not condition flavor preferences using the training procedures of the present study (Sclafani et al., 1999).
2. Methods
2.1. Subjects
Adult male Sprague–Dawley rats obtained from Charles River Laboratories (Wilmington, MA, USA), or bred in the laboratory from Charles River stock, weighed between 260-300 g (Experiment 1) and 390–530 g (Experiment 2) at the time of brain surgery. In Experiment 1, rats were housed individually in wire mesh cages in the Queens College vivarium, while in Experiment 2 they were individually housed in plastic cages in the Brooklyn College vivarium. Rats were maintained at 21°C under a 12:12 h light:dark cycle and maintained on Laboratory Rodent Diet 5001 (PMI Nutrition International, Brentwood, MO, USA) and tap water. The experimental protocols in Experiments 1 and 2 were approved by the Queens College and Brooklyn College Institutional Animal Care and Use Committees, respectively.
2.2. Surgery
In Experiment 1 the rats were pretreated with chlorpromazine (3 mg/kg, i.p.) and anesthetized with Ketamine HCl (120 mg/kg, i.m.); in Experiment 2 they were anaesthetized with Ketamine HCl (63 mg/kg, ip) and xylazine (9.4 mg/kg, ip). Stainless steel guide-cannulae (26-g, i.d. 0.24, o.d. 0.46 mm; Plastics One Inc., Roanoke, VA) were aimed stereotaxically at bilateral placements in the NAc using the following coordinates: shell: incisor bar (+5 mm), 3.1-3.5 mm anterior to the bregma suture, 1.7-1.8 mm and angled 10° towards each side of the sagittal suture and 6.5-6.8 mm from the top of the skull; core: 3.1-3.5 mm anterior to the bregma suture, 2.7-2.8 mm and angled 10° towards each side of the sagittal suture and 6.5-6.8 mm from the top of the skull. The cannulae were secured to the skull by four anchor screws with dental acrylic. In Experiment 2 during the same surgery session, the rats were fitted with a gastric catheter (silastic tubing, i.d. 1.02, o.d. 2.16 mm) that was inserted into the fundus of the stomach and secured with sutures and polypropylene mesh. The tubing was routed under the skin and connected to pedestal that was mounted and secured on the animal's neck with polypropylene mesh and sutures. The animals were allowed at least two weeks to recover from surgery before behavioral testing began.
2.3. Drug and injection procedures
NTX (Sigma-Aldrich, St Louis, MO, USA) was dissolved in sterile isotonic saline (vehicle) and administered at a volume of 0.5 μl/side. Injections of the drug or vehicle into the NAc were performed bilaterally using a 33-g stainless steel internal cannula (Plastics One) connected to a 2μl Hamilton microsyringe (Hamilton Company, Reno, NV, USA) by polyethylene tubing. At the start of the intracerebral injections, the rats were held gently, the stylus was removed, and the cannula was inserted. The tip of the injection cannula protruded 1 mm beyond that of the guide. The injections were made at a rate of 0.5 μl/min, and the cannulae were left in place for an additional minute before their removal.
2.4. Experiment 1: Oral Fructose-Conditioned Flavor Preferences (Flavor-Taste Learning)
2.4.1. Test Solutions
The training solutions consisted of an 8% fructose (Sigma Chemical Co., St. Louis, MO) and 0.2% sodium saccharin (Sigma) mixture or a 0.2% sodium saccharin solution; the solutions were flavored with 0.05% unsweetened grape or cherry Kool-Aid (Kraft Foods, White Plains, NY). Half of the rats in each group had the cherry flavor added to the fructose+saccharin solution, and the grape flavor added to the saccharin only solution; the flavors were reversed for the remaining rats. In the two-bottle preference tests, the cherry and grape flavors were each presented in a 0.2% saccharin solution. The fructose+saccharin-paired flavor is referred to as the CS+, and the saccharin-paired flavor as the CS− because 8% fructose is preferred to 0.2% saccharin (Sclafani and Ackroff, 1994). CS+/F refers to the fructose-containing solution used in training, and CS+ and CS− refer to the flavored solutions used during two-bottle testing. Solution intakes were measured to the nearest 0.1 g.
2.4.2. Procedures
All testing took place in the rats’ home cage during the mid-light phase of the light:dark cycle. Two weeks before testing began, the rats (n=28) were placed on a food restriction schedule that maintained their body weights at 85-90% of their ad libitum level. The rats were initially trained to drink an unflavored 0.2% saccharin solution during daily 1 h sessions. This training procedure was repeated daily until all rats approached the sipper tubes with short (< 1 min) latency, typically within three days. The limited food rations were given 1 h after each training session.
The rats were given ten one-bottle training sessions (30 min/day) with 16 ml of the CS+/F solution presented on odd-numbered days, and 16 ml of the CS− solution presented on even-numbered days. On days 9 and 10, the rats had access to a second sipper tube containing water. This familiarized them with the presence of two sipper tubes used during the choice tests; water intake was negligible in these training trials. The position of the CS and water sipper tubes varied across days using a left-right-right-left pattern. Following training, the rats were given eight two-bottle choice test sessions (30 min/day) with unlimited access to the CS+ and CS− solutions. All injections occurred 10 min prior to the two-bottle tests. For the first two test sessions, the rats were given a vehicle (0.9% saline) microinfusion. Over the next six sessions the rats were given microinfusions of NTX at total doses of 1, 25 and 50 μg (0.5, 12.5, 25 μg/side) into the NAc shell (Experiment 1A, n=14 rats) or core (Experiment 1B, n=14 rats). Half of the rats were tested with an ascending dose order, and the remaining rats were tested in a descending dose order. The rats were tested twice at each drug dose with the left-right position of the CS+ and CS− solutions counterbalanced across sessions. A one-day rest period separated each pair of drug doses for both groups.
2.5. Experiment 2: IG Glucose-Conditioned Flavor Preferences (Flavor-Nutrient Learning)
2.5.1. Apparatus
Training and testing occurred in plastic infusion cages that gave the rat access to one or two stainless steel drinking spouts (Touzani and Sclafani, 2001). The spouts were attached to drinking bottles mounted on motorized holders that positioned the spouts at the front of the cage at the start of the session and retracted them at the end of the session. Licking behavior was monitored by an electronic lickometer interfaced to a microcomputer that activated a syringe pump as the rat drank. Plastic tubing connected the syringe pump via a swivel to the rat's gastric catheter via the neck-mount connector pedestal. The gastric infusion rate was 1.3 ml/min and oral intakes and IG infusions were matched in volume.
2.5.2. Test solutions
The test solutions contained 0.2% saccharin flavored with 0.05% cherry or grape Kool-Aid. The CS+ flavor was paired with IG infusions of 8% glucose (Bio-Serv, Frenchtown, NJ) and the CS− flavor was paired with IG water infusions. The specific flavor–infusion pairs were counterbalanced across subjects.
2.5.3. Procedures
Prior to the surgery, the rats (n=25) were familiarized with unflavored 0.2% saccharin solution by giving them ad libitum access to the saccharin solution along with water and chow in their home cages for 3 days. Then the rats were housed in the test cages overnight with ad libitum access to 0.2% saccharin solution, water and food to adapt them to the test cages. The saccharin and water bottles were automatically positioned to the front of the cages for 30 min every h.
Two weeks after the surgery, the rats were food restricted and maintained at 85-90% of their ad libitum body weights. They were adapted to drink the saccharin solution in the test cages during 8–10 daily 30-min sessions. During the last four of these sessions, the rats were connected to the infusion system and were given IG water infusions as they drank the saccharin solution. Flavor training occurred over eight one-bottle training sessions (30 min/day). In sessions 1, 3, 5 and 7, intake of the CS+ solution was paired with concurrent IG infusions of glucose; in sessions 2, 4, 6 and 8, intake of the CS− solution was paired with concurrent IG infusion of water. Following training, the rats were given a series of CS+ vs. CS− two-bottle tests with NTX infusions into the NAc shell (Experiment 2A, n=16) or core (Experiment 2B, n=9) as described in Experiment 1; there were no IG infusions during these test sessions.
2.6. Statistical analyses
CS intakes during training were averaged over the 4 or 5 sessions with each CS and analyzed using t-tests. CS intakes during 2-bottle tests were averaged over the 2 sessions with each dose and evaluated with analysis of variance. Individual comparisons were evaluated using simple main effects. Two-bottle preferences were also expressed as percent CS+ intake [(CS+ intake / total intake) ×100]. The percent data were analyzed with ANOVA after an arcsine transformation as recommended by (Kirk, 1995).
2.7. Histological analysis
At the completion of experiments, the rats were deeply anaesthetized with sodium pentobarbital and perfused transcardially with physiological 0.9% saline followed by a 10% formalin solution. The brains were removed and soaked in a 10% formalin solution containing 20% sucrose for 3–5 days. The brains were coronally sectioned (40 μm) with a freezing microtome and the sections were mounted on gelatin-coated glass slides and stained with cresyl violet (Experiment 1) or thionin (Experiment 2). The cannula placements were examined by light microscopy by an observer unfamiliar with the behavioral data, and reconstructed on the appropriate frontal planes of the atlas of Paxinos and Watson (1998).
3. Results
3.1. Experiment 1: Oral Fructose-Conditioned Flavor Preferences (Flavor-Taste Learning)
3.1.1. Histology
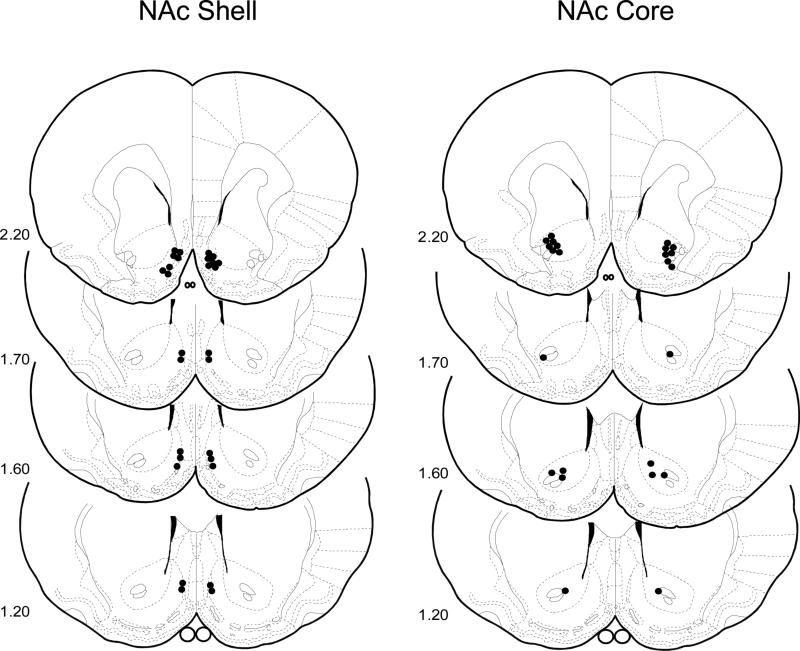
Cannula tip placements for all rats used in Experiment 1 are shown in Figure 1. Placements were deemed appropriate for fourteen rats in the NAc shell and twelve rats in the NAc core and were primarily restricted to the rostral portion between the Frontal Planes 2.20 and 1.20 mm of the Paxinos and Watson (1998) atlas. The remaining two rats with misplaced injection sites were discarded.
Figure 1.
Experiment 1. Bilateral representation of cannula sites of fourteen rats in the NAc shell and twelve rats in the NAc core which were primarily restricted to the rostral portion between the Frontal Planes 2.20 and 1.20 mm of the Paxinos and Watson (1998) atlas tested in the Fructose-CFP paradigm. The remaining two rats with misplaced injection sites were discarded.
3.1.2. Experiment 1A: NAc Shell
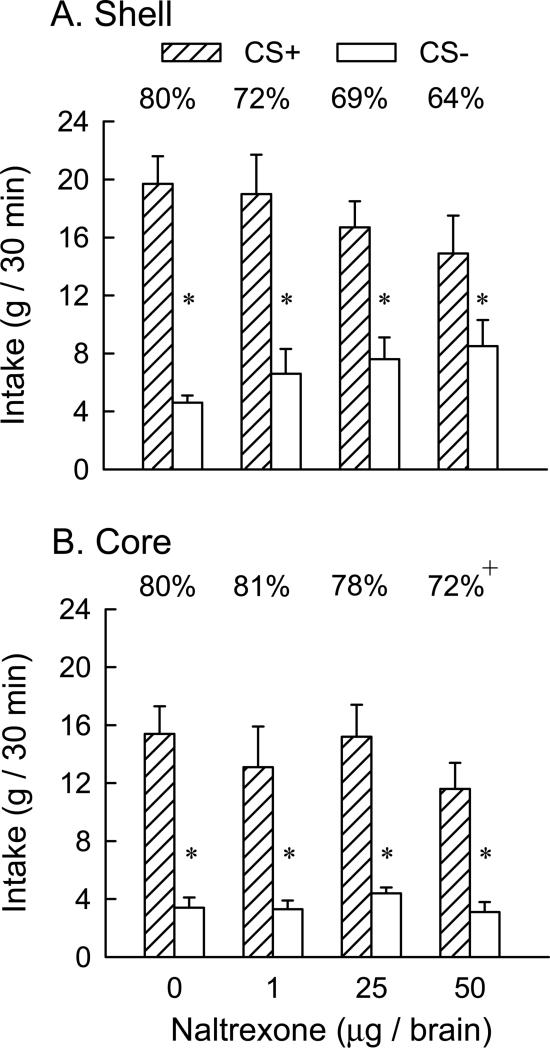
During one-bottle training, the rats consumed more CS+ than CS− (12.9 vs. 10.5 g/30 min, t(13) = 5.0, p < 0.001). In the two-bottle preference tests (Fig. 2A), overall, the rats consumed significantly more CS+ than CS− [F(1,13) = 41.4, p < 0.001] while the drug dose main effect was not significant. The interaction of CS × dose was not significant although the rats tended to consume less CS+ and more CS− as the NTX dose increased; individual tests indicated that the rats consumed significantly more ( p<0.05) CS+ than CS− at all NTX doses. Percent CS+ intakes (calculated as percent of total CS intake) ranged from 80% in the vehicle test to 72%, 69%, and 64% following the 1, 25 and 50 μg NTX doses respectively; these differences were not significant (Fig. 2A).
Figure 2.
Experiment 1: Oral fructose-conditioned flavor preferences. Intakes (+SEM, g/30 min) of CS+ and CS− solutions during two-bottle tests in animals receiving bilateral NAc shell (n=14, Panel A) or NAc core (n=12, Panel B) microinjections of naltrexone at total doses of 0, 1, 25 and 50 μg. A significant difference between CS+ and CS− intakes at a given dose is indicated by an asterisk (* P < 0.05). A significant difference in percent CS+ intakes at the dose of 50 μg as compared to the 0 dose is indicated by a plus (+ P < 0.05).The percentages of CS+ intake over total intake are indicated by the value above each CS+ bar.
3.1.3. Experiment 1B: NAc Core
The rats consumed more CS+ than CS− (13.2 vs. 9.3 g/30 min, t(11) = 7.8, p < 0.001) during one-bottle training. In the two-bottle preference tests (Fig. 2B), overall CS+ intakes exceeded CS− intakes [F(1,11) = 168.3, p < 0.001] while the drug dose main effect was not significant. There was a significant CS × dose [F(3,33) = 4.01, p< 0.05] although individual tests indicated that the rats consumed significantly more (p < 0.05) CS+ than CS− at all NTX doses. CS+ intakes declined and CS− intakes increased somewhat as drug dose increased but these changes were not significant. Significant differences in the percent CS+ preference occurred across doses [F(3,33) = 4.09, p < 0.014] (Figure 2B) with the CS+ preference at the 50 μg NTX dose significantly lower (p<0.05) than that following vehicle (72% vs. 80%, p<0.05). It should be noted that the 50 μg dose in the NAc core produced a smaller decline in CS+ preference (8%) than that observed in the NAc shell (16%) yet the later difference was not significant because of the greater variability in the percent CS+ preferences obtained in the Shell experiment.
3.2. Experiment 2: IG Glucose-Conditioned Flavor Preferences (Flavor-Nutrient Learning)
3.2.1. Histology
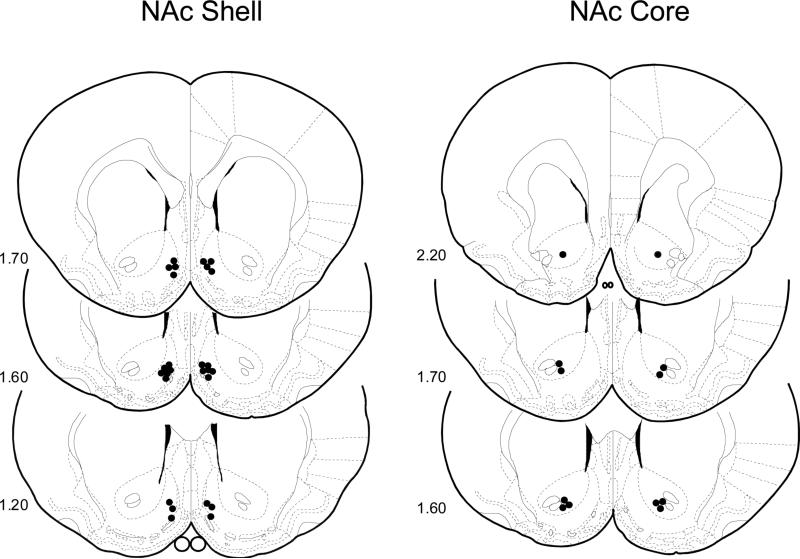
Cannula tip placements for all rats used in Experiments 2A and 2B are shown in Figure 3. Placements were deemed appropriate for thirteen rats in the NAc shell and six rats in the NAc core and were primarily restricted to the rostromedial portion between the Frontal Planes 2.20 and 1.20 mm of the Paxinos and Watson (1998) atlas. The remaining six rats with misplaced injection sites were discarded.
Figure 3.
Experiment 2. Bilateral representation of cannula sites of thirteen rats in the NAc shell and six rats in the NAc core which were primarily restricted to the rostral portion between the Frontal Planes 2.20 and 1.20 mm of the Paxinos and Watson (1998) atlas tested in the IG Glucose-CFP paradigm. The remaining six rats with misplaced injection sites were discarded.
3.2.2. Experiment 2A: NAc Shell
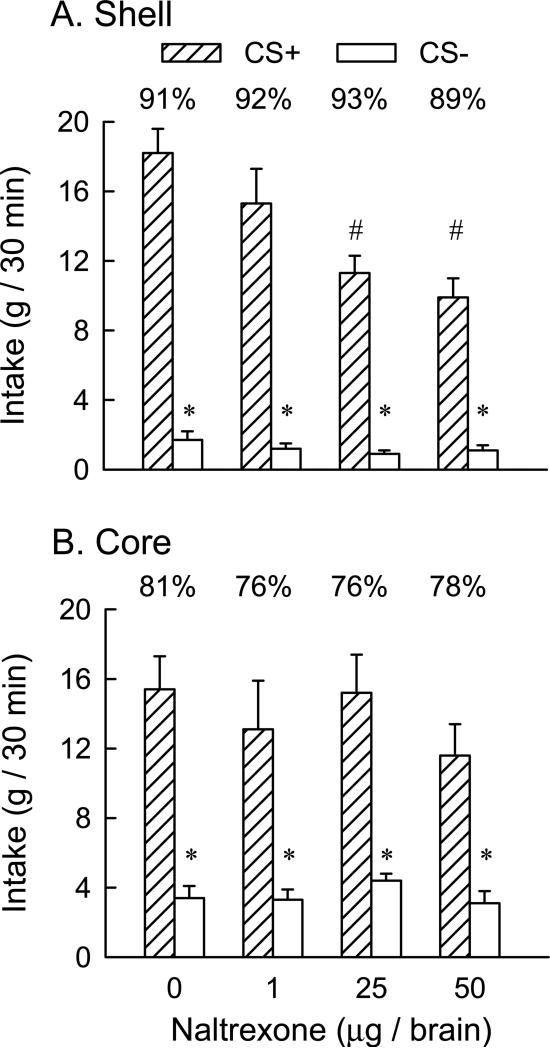
CS+ and CS− intakes did not differ during one-bottle training (12.6 vs. 12.9 g/30 min). In the two-bottle preference tests (Fig. 4A), overall the rats consumed significantly more CS+ than CS− [F(1,12) = 140.5, p < 0.001], and there was a significant dose effect [F(3,36) = 10.46, p< 0.001] and CS × dose interaction [F(3,36) = 7.70, p < 0.001]. A simple main effects test revealed that CS+ intakes declined (p < 0.001) as drug dose increased from 0 to 50 μg (18 to 10 g/30 min); CS− intakes declined slightly but not significantly over this dose range (1.8 to 1.1, g/30 min). Nevertheless, the rats consumed significantly more (p < 0.05) CS+ than CS− at all NTX doses. Percent CS+ intakes, which ranged from 91% in the vehicle test to 89% to 93% following NTX treatment, also did not significantly differ.
Figure 4.
Experiment 2: IG Glucose-conditioned flavor preferences. Intakes (+SEM, g/30 min) of CS+ and CS− solutions during two-bottle tests in animals receiving bilateral NAc shell (n=13, Panel A) or NAc core (n=6, Panel B) microinjections of naltrexone at total doses of 0, 1, 25 and 50 μg. A significant difference between CS+ and CS− intakes at a given dose is indicated by an asterisk (* P < 0.05). A significant difference in CS+ intakes at the doses of 25 and 50 μg as compared to the 0 and 1 μg doses is indicated by a number (# P < 0.05). The percentages of CS+ intake over total intake are indicated by the value above each CS+ bar.
3.2.3. Experiment 2B: NAc Core
During one-bottle training, the rats consumed less CS+ than CS− (10.9 vs. 13.4 g, t(5) = 3.6, p < 0.05). In the two-bottle preference tests (Figure 4B), overall, the rats consumed more CS+ than CS− [F(1,5) = 21.6, p < 0.01]. The drug dose main effect just failed to be significant [F(3,15) = 3.10, p = 0.058], and there was no significant CS × dose interaction. Percent CS+ intakes, which ranged from 81% in the vehicle test to 76% to 78% following NTX treatment, did not significantly differ.
4. Discussion
The present study evaluated the effect of opioid receptor antagonism in the NAc on the expression of sugar-conditioned flavor preferences. Confirming prior work, rats developed strong preferences (80-91%) for flavors paired with the sweet taste of fructose or the post-oral nutrient effects of glucose (Baker et al., 2004; Bernal et al., 2008; Touzani et al., 2008). The expression of these conditioned preferences was not substantially attenuated by NTX microinfusions into the NAc shell or core, regions where opioid agonists stimulate feeding (Zhang and Kelley, 2000). In the flavor-taste conditioning studies, the CS+ flavor preferences were reduced by the highest NTX dose in the NAc shell (from 80% to 64%) and core (from 80% to 72%), although only the core effect was significant. In the flavor-nutrient conditioning studies NTX has very little effects on CS+ preferences; at most, CS+ preferences declined by 2% and 5% in the shell and core, respectively. With respect to absolute intakes, NTX microinfusions into the NAc shell selectively reduced CS+ intakes in the glucose experiment (Experiment 2A). This might be taken as evidence that opioid receptor antagonism in the shell attenuated the flavor preference conditioned by the post-oral reinforcing properties of glucose but the small reduction in percent CS+ intakes (from 91% to 89%) argues against this interpretation. Instead, NTX may have reduced only CS+ intake because CS− intake was already very low in the vehicle test. The pattern of results obtained in Experiment 1A (reduced CS+ and increased CS− intakes) is more suggestive that shell opioid receptors contribute to the expression of learned flavor preferences, but NTX did not significantly alter CS intakes in Experiment 1A.
Overall, the relatively limited effects of NAc NTX on CS+ flavor preferences and intakes are comparable to the results obtained in our prior sugar-conditioning studies using systemic NTX treatment (Azzara et al., 2000; Baker et al., 2004; Yu et al., 1999). Systemic NTX selectively reduced CS+ intake but not percent preference in our fructose conditioning study (Baker et al., 2004) and did not significantly alter CS+ intake or preference in our IG sugar conditioning study conducted with food-restricted animals (Azzara et al., 2000). Furthermore, at all NTX doses administered in the NAc or systemically, the rats consumed more CS+ than CS− in the two-bottle tests.
The impetus for the present study was the report that NAc NTX microinfusions selectively reduced the intake of a preferred flavored food (chocolate), whereas systemic naltrexone injection decreased the intake of both the preferred (chocolate) and less-preferred (banana) foods (Woolley et al., 2006). Based on these results, we predicted that NAc NTX administration would have a much greater effect on the expression of sugar-conditioned flavor preferences than we previously observed with systemic administration. However, this prediction was not confirmed: the reductions in the sugar-conditioned flavor preferences following NAc drug treatment were not much different from those observed in our prior studies with systemic NTX treatment. Our findings do not contradict the Woolley et al. results given the many differences between the two studies. Whereas our present and prior studies investigated relatively strong conditioned flavor preferences, the food preferences in the Wooley et al. study were relatively weak, and based presumably on unlearned palatability differences between the flavored foods. In addition, the choice tests in the Wooley et al. study were conducted with non-deprived rats fed nutritionally-identical diets, whereas the choice tests of the present experiment involved food-restricted rats given non-nutritive fluids. The type of test diet or fluid and deprivation state can influence the response to opioid drugs (see reviews: Bodnar, 2004; Cooper, 2007; Levine, 2006).
Relevant to the issue of the selectivity of systemic versus NAc naltrexone treatments on flavor preferences is a recent study by Taha and co-workers (2006) which used an anticipatory contrast design. In this study, non-deprived rats were trained to drink a 4% sucrose solution only (4-0 group), or a 4% sucrose solution followed by a 20% sucrose solution (4-20 group). The 4-20 rats consumed less 4% sucrose than did the 4-0 group, indicating the reward value of 4% sucrose was reduced in those rats expecting the more preferred 20% sucrose solution. Systemic NTX injections (1 mg/kg) suppressed 4% sucrose intake in the 4-0 rats, but not in the 4-20 rats; the drug suppressed 20% sucrose intake in the latter group, however. In contrast to these findings, NTX microinfusions failed to suppress 4% and/or 20% sucrose intakes in the 4-0 and 4-20 groups. Note that other studies not involving a contrast design reported that NAc NTX infusions minimally reduced 10% or 20% sucrose intake in rats (Bodnar et al., 1995; Kelley et al., 1996). Thus, the effectiveness of NAc NTX infusions to alter flavor preferences or sweet solution intakes varies as a function of the specific test paradigm used.
The present study investigated the effects of NTX on the expression of previously learned sugar-conditioned flavor preferences. It is possible that infusing NTX into the NAc during initial training trials might have greater effects on the acquisition of sugar-conditioned preferences. This seems unlikely, however, in view of the failure of systemic NTX injections to block flavor-sugar conditioning (Azzara et al., 2000; Baker et al., 2004; Yu et al., 1999). On the other hand, systemic or NAc treatment with dopamine receptor antagonists attenuate, at least to some degree, flavor preference conditioning by the sweet taste and post-oral nutrient effects of sugars (Azzara et al., 2001; Baker et al., 2003; Bernal et al., 2008; Touzani et al., 2008; Yu et al. 2000). Other systemic studies also implicate endocannabinoid and glutamate receptor signaling in preference conditioning by the sweet taste of fructose (Golden and Houpt, 2007; Miner et al., 2008). Our studies do not argue against the involvement of opioid systems in food palatability and reward processing, but rather indicate that these systems have a limited role in sugar-conditioned changes in flavor preferences (Cooper, 2007). Other types of food-related learning, such as place preference conditioning, appear to be more dependent upon opioid signaling (Ågmo et al., 1993; Delamater et al., 2000; Jarosz et al., 2006).
Acknowledgements
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK071761. The first two authors contributed equally to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ågmo A, Federman I, Navarro V, Padua M, Velazquez G. Reward and reinforcement produced by drinking water: role of opioids and dopamine receptor subtypes. Pharmacol Biochem Behav. 1993;46:183–194. doi: 10.1016/0091-3057(93)90339-u. [DOI] [PubMed] [Google Scholar]
- Arbisi PA, Billington CJ, Levine AS. The effect of naltrexone on taste detection and recognition threshold. Appetite. 1999;32:241–249. doi: 10.1006/appe.1998.0217. [DOI] [PubMed] [Google Scholar]
- Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. Naltrexone fails to block the acquisition or expression of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacol Biochem Behav. 2000;67:545–557. doi: 10.1016/s0091-3057(00)00395-6. [DOI] [PubMed] [Google Scholar]
- Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. D1 but not D2 dopamine receptor antagonism blocks the acquisition of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacol Biochem Behav. 2001;68:709–720. doi: 10.1016/s0091-3057(01)00484-1. [DOI] [PubMed] [Google Scholar]
- Baker RM, Li Y, Lee M, Sclafani A, Bodnar RJ. Naltrexone does not prevent acquisition or expression of flavor preferences conditioned by fructose in rats. Pharmacol Biochem Behav. 2004;78:239–246. doi: 10.1016/j.pbb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Baker RM, Shah MJ, Sclafani A, Bodnar RJ. Dopamine D1 and D2 antagonists reduce the acquisition and expression of flavor-preferences conditioned by fructose in rats. Pharmacol Biochem Behav. 2003;75:55–65. doi: 10.1016/s0091-3057(03)00039-x. [DOI] [PubMed] [Google Scholar]
- Bernal SY, Dostova I, Kest A, Abayev Y, Kandova E, Touzani K, Sclafani A, Bodnar RJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens shell on the acquisition and expression of fructose-conditioned flavor-flavor preferences in rats. Behav Brain Res. 2008;190:59–66. doi: 10.1016/j.bbr.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ. Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides. 2004;25:697–725. doi: 10.1016/j.peptides.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Glass MJ, Ragnauth A, Cooper ML. General, μ and k opioid antagonists in the nucleus accumbens alter food intake under deprivation, glucoprivic and palatable conditions. Brain Res. 1995;700:205–212. doi: 10.1016/0006-8993(95)00957-r. [DOI] [PubMed] [Google Scholar]
- Cooper SJ. Effects of opiate agonists and antagonists on fluid intake and saccharin choice in the rat. Neuropharmacology. 1983;22:323–328. doi: 10.1016/0028-3908(83)90247-2. [DOI] [PubMed] [Google Scholar]
- Cooper SJ. Pharmacology of food, taste and learned flavor preference. In: Kirkham TC, Cooper SJ, editors. Appetite and Body Weight: Integrative Systems and the Development of Anit-Obesity Drugs. Elsevier Academic Press; Amsterdam, Boston: 2007. pp. 217–245. [Google Scholar]
- Delamater AR, Sclafani A, Bodnar RJ. Pharmacology of sucrose-conditioned place preference: Effects of naltrexone. Pharmacol Biochem Behav. 2000;65:697–704. doi: 10.1016/s0091-3057(99)00251-8. [DOI] [PubMed] [Google Scholar]
- Fantino M, Hosotte J, Apfelbaum M. An opioid antagonist, naltrexone, reduces preference for sucrose in humans. Am J Physiol. 1986;251:R91–R96. doi: 10.1152/ajpregu.1986.251.1.R91. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Billington CJ, Levine AS. Opioids and food intake: distributed functional neural pathways? Neuropeptides. 1999;33:360–368. doi: 10.1054/npep.1999.0050. [DOI] [PubMed] [Google Scholar]
- Golden GJ, Houpt TA. NMDA receptor in conditioned flavor-taste preference learning: Blockade by MK-801 and enhancement by D-cycloserine. Pharmacol Biochem Behav. 2007;86:587–596. doi: 10.1016/j.pbb.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosnell BA, Levine AS. Reward systems and food intake: role of opioids. Int J Obes. 2009;33(Suppl 2):S54–S58. doi: 10.1038/ijo.2009.73. [DOI] [PubMed] [Google Scholar]
- Jarosz PA, Sekhon P, Coscina DV. Effect of opioid antagonism on conditioned place preferences to snack foods. Pharmacol Biochem Behav. 2006;83:257–264. doi: 10.1016/j.pbb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bless EP, Swanson CJ. Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. J Pharmacol Exp Ther. 1996;278:1499–1507. [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. Brooks/Cole; Pacific Grove, CA: 1995. [Google Scholar]
- Kirkham TC, Cooper SJ. Naloxone attenuation of sham feeding is modified by manipulation of sucrose concentration. Physiol Behav. 1988;44:491–494. doi: 10.1016/0031-9384(88)90310-1. [DOI] [PubMed] [Google Scholar]
- Le Magnen J, Marfaing-Jallat P, Micelli D, Devos M. Pain modulating and reward systems: a single brain mechanism? Pharmacol Biochem Behav. 1980;12:729–733. doi: 10.1016/0091-3057(80)90157-4. [DOI] [PubMed] [Google Scholar]
- Levine AS. The animal model in food intake regulation: Examples from the opioid literature. Physiol Behav. 2006;89:92–96. doi: 10.1016/j.physbeh.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Levine AS, Weldon DT, Grace M, Cleary JP, Billington CJ. Naloxone blocks that portion of feeding driven by sweet taste in food-restricted rats. Am J Physiol. 1995;268:R248–R252. doi: 10.1152/ajpregu.1995.268.1.R248. [DOI] [PubMed] [Google Scholar]
- Mehiel R. The effects of naloxone on flavor-calorie preference learning indicate involvement of opioid reward systems. Psychol Rec. 1996;46:435–450. [Google Scholar]
- Miner P, Abayev Y, Kandova E, Gerges M, Styler E, Wapniak R, Touzani K, Sclafani A, Bodnar RJ. Role of systemic endocannabinoid CB-1 receptor antagonism in the acquisition and expression of fructose-conditioned flavor-flavor preferences in rats. Pharmacol Biochem Behav. 2008;90:318–324. doi: 10.1016/j.pbb.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare E, Cleary J, Bartz PJ, Weldon DT, Billington CJ, Levine AS. Naloxone administration following operant training of sucrose/water discrimination in the rat. Psychopharmacologia (Berlin) 1994;129:289–294. doi: 10.1007/s002130050193. [DOI] [PubMed] [Google Scholar]
- Parker LA, Maier S, Rennie M, Crebolder J. Morphine- and naltrexone-induced modification of palatability: analysis by the Taste Reactivity Test. Behav Neurosci. 1992;106:999–1010. doi: 10.1037//0735-7044.106.6.999. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Peciña S. Opioid reward ‘liking’ and ‘wanting’ in the nucleus accumbens. Physiol Behav. 2008;94:675–680. doi: 10.1016/j.physbeh.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Rockwood GA, Reid LD. Naloxone modifies sugar-water intake in rats drinking with open gastric fistulas. Physiol Behav. 1982;29:1175–1178. doi: 10.1016/0031-9384(82)90316-x. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Glucose- and fructose-conditioned flavor preferences in rats: Taste versus postingestive conditioning. Physiol Behav. 1994;56:399–405. doi: 10.1016/0031-9384(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Aravich PF, Xenakis S. Dopaminergic and endorphinergic mediation of a sweet reward. In: Hoebel B, Novin D, editors. The Neural Basis of Feeding and Reward. Haer Institute; Brunswick, Maine: 1982. pp. 507–515. [Google Scholar]
- Sclafani A, Fanizza LJ, Azzara AV. Conditioned flavor avoidance, preference and indifference produced by intragastric infusions of galactose, glucose and fructose in rats. Physiol Behav. 1999;67:227–234. doi: 10.1016/s0031-9384(99)00053-0. [DOI] [PubMed] [Google Scholar]
- Taha SA, Norsted E, Lee LS, Lang PD, Lee BS, Woolley JD, Fields HL. Endogenous opioids encode relative taste preference. Eur J Neurosci. 2006;24:1220–1226. doi: 10.1111/j.1460-9568.2006.04987.x. [DOI] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. Activation of dopamine D1 receptors in the nucleus accumbens is critical for the acquisition, but not the expression, of flavor preference conditioned by intragastric glucose in rats. Eur J Neurosci. 2008;27:1525–1533. doi: 10.1111/j.1460-9568.2008.06127.x. [DOI] [PubMed] [Google Scholar]
- Touzani K, Sclafani A. Conditioned flavor preference and aversion: Role of the lateral hypothalamus. Behav Neurosci. 2001;115:84–93. doi: 10.1037/0735-7044.115.1.84. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Lee BS, Fields HL. Nucleus accumbens opioids regulate flavor-based preferences in food consumption. Neuroscience. 2006;143:309–317. doi: 10.1016/j.neuroscience.2006.06.067. [DOI] [PubMed] [Google Scholar]
- Yu W-Z, Sclafani A, Delamater AR, Bodnar RJ. Pharmacology of flavor preference conditioning in sham-feeding rats: Effects of naltrexone. Pharmacol Biochem Behav. 1999;64:573–584. doi: 10.1016/s0091-3057(99)00124-0. [DOI] [PubMed] [Google Scholar]
- Yu W-Z, Silva RM, Sclafani A, Delamater AR, Bodnar RJ. Role of D1 and D2 dopamine receptors in the acquisition and expression of flavor-preference conditioning in sham-feeding rats. Pharmacol Biochem Behav. 2000;67:537–544. doi: 10.1016/s0091-3057(00)00396-8. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and Fos expression. Neuroscience. 2000;99:267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]