Abstract
The laminar sheet of epithelium (e.g., skin and mucous membrane) enclosing our bodies is represented in the dorsal horns of the medulla and spinal cord. The eyeball however indents this laminar sheet and is shrouded by different layers: the cornea/sclera, the conjunctiva, and hairy skin. This involution of the orb confounds defining the central representation of the cornea and its surrounding mucosa and skin. We used herein the transganglionic transport of a cocktail of HRP conjugated to cholera toxin and wheat germ agglutinin to determine the central representation of these epithelia in the dorsal horns of the rat. The HRP cocktail was injected either into the stroma of the cornea, the mucosa of the conjunctiva, or the supraorbital and infraorbital nerves. Injections of the cornea produced dense label in the interstitial islands in the ventral medullary dorsal horn (MDH), probably lamina I, and in neuropil in the ventromedial tip of the MDH, probably lamina II. There sometimes was variable, diffuse label in the C1 dorsal horn after corneal injections but more rostral parts of the trigeminal sensory complex were never labeled. Injections of the conjunctiva densely labeled laminae I–III in the C1 dorsal horn, while laminae IV–V were diffusely labeled. Sparser reaction product also was seen in lamina I in positions similar to the cornea projection. Label was seen ventrally in subnuclei interpolaris and oralis, as well as the principal trigeminal nucleus. Projections of the infraorbital nerve included all laminae in the trigeminocervical complex as well as large portions of the rostral subnuclei in the spinal trigeminal nucleus. The projections of the supraorbital nerve were similar, but were restricted to ventral parts of the trigeminal sensory complex.
In other cases the cornea was injected either after cutting the supraorbital and infraorbital nerves or the conjunctiva was injected after enucleating the eyeball. Any reaction product from corneal injections was reduced dramatically in the C1 dorsal horn after transection of the infraorbital and supraorbital nerves. Injecting the conjunctiva after enucleating the eyeball densely labeled the C1 projection to the dorsal horn, a small patch in lamina I in the MDH, as well as the rostral trigeminal complex. We propose that the cornea has but a single representation in the trigeminocervical complex in its ventral part near the caudal end of the medulla. We also propose the palpebral conjunctiva mucosa is represented in the C1 dorsal horn, and speculate that the bulbar conjunctiva overlaps with that of the cornea in lamina I. We discuss these projections in relation to the circuitry for the supraorbital-evoked and corneal-evoked blink reflexes. The relationship of the cornea and conjunctiva is intimate, and investigators must be very careful when attempting to stimulate them in isolation.
Keywords: trigeminal, primary afferent fibers, palpebrae, blink reflex, somatotopy, dorsal horn, transganglionic
Introduction
The eyeball is enclosed partially within a mucous membrane, the conjunctiva, which is itself surrounded by the skin of our eyelids; all three structures represent parts of our somatosensory surface. The eyeball itself is marked by the translucent cornea, a thick epithelium underlain by a stoma. The conjunctiva is a blind pocket of mucosa enveloping the eyeball and has two portions: 1) a palpebral conjunctiva subjacent to both the upper and lower eyelids which continues as, 2) the bulbar conjunctiva which lies over the sclera and fuses with the corneal epithelium at the limbus. However, the involution of the orb into the bony orbit produces overlap of these structures which makes deciphering the distinct somatotopy of the cornea, conjunctiva, and skin of the eyelids difficult. These facts also emphasize the difficulty in stimulating the cornea in isolation, and the interpretation of the sensations and reflex activity elicited from its stimulation complex.
The trigeminal ganglion supplies sensory neurons that innervate the whole head region, including periorbital structures. These ganglion neurons are derived from both neural crest and head placode cells, yet only neurons form neural crest origin innervate the cornea while those innervating the eyelids are derived from both neural crest and placodes (Lwigale, 2001). The ophthalmic division of the trigeminal nerve innervates the cornea via its long ciliary nerves. The cornea is one of the most densely innervated parts of the body (Rozsa et al., 1982; Beuerman et al., 1996; Müller et al., 2003) yet is only innervated by fibers with free nerve endings of very small diameter (Lele et al., 1959; Belmonte, 1996; Belmonte et al., 1997). Moreover, stimulation of the cornea induces only sensations of irritation and pain (Belmonte, 1996; Belmonte et al., 1997; Carstens et al., 1998). The bulbar conjunctiva also is innervated by the long ciliary branches of the nasociliary nerve from the ophthalmic division of the trigeminal nerve (Snell et. al., ‘97). However, the superior palpebral conjunctiva is innervated by the frontal and lacrimal branches of the ophthalmic nerve of the trigeminal, while the inferior palpebral conjunctiva is innervated by the infraorbital branch of the maxillary nerve of the trigeminal and by the lacrimal branch of the ophthalmic nerve (Williams et al., 1980). The conjunctiva is innervated by both myelinated and non-myelinated fibers (Oppenheimer et al., 1958; Weddell et al., 1962). The subjective sensations elicited by stimulating either the conjunctiva or cornea are similar except for cold thermal and the perception of irritation or burning (Acosta et al., 2001b). The supraorbital and infraorbital nerves also innervate the overlying cuticle on both the superior and inferior eyelids (Williams et al., 1980).
Accurate knowledge of the termination of the primary afferent fibers innervating the cornea is important since it is innervated only by small diameter fibers and most corneal stimulation is perceived as either irritating or painful by humans (Beuerman et al., 1979; Chen et al., 1995; Belmonte et al., 1997; Acosta et al., 2001a). Study of sensory perception induced by corneal stimulation thus may provide insight into the neural mechanisms underlying pain. Moreover, irritation or dehydration of either the cornea or conjunctiva induces blinking behavior. The neuronal circuits underlying the orbicularis oculi reflexes, commonly used clinically in diagnosis (Esteban, 1999; Aramideh et al., 2002), by necessity must be known accurately to best serve their utility as diagnostic tools.
Peripheral receptor fields should have but one representation in the dorsal horns (Brown et al., 1992), mimicking a single representation in the ventrobasal thalamic nucleus and primary somatosensory cortex. Peripheral receptive fields also are represented in secondary and tertiary cortical areas, and in the trigeminal system, the principal sensory nucleus and subnuclei oralis and interpolaris. All these areas have a single representation of a body part but are specialized regarding particular functions relating either to reflex behavior, different aspects of sensory discrimination, or of higher learning. We thus compared the projections of the cornea versus those from the conjunctiva in the rat either after cutting the supraorbital and infraorbital nerves or enucleating the eyeball. Our results confirm this theory of a single representation. The corneal representation is confined to laminae I and II in the ventral MDH while the conjunctiva is represented further caudally, mostly in lamina I–III of the spinal dorsal horn approximately 2.5– 4.0 mm caudal to the obex.
This hypothesis differs from that described by Panneton and Burton (1981) and Marfurt (1981) on the central projections of primary afferent fibers innervating the cornea in the cat. These early studies, as well as others in several species (Shigenaga et al., 1986a; Marfurt et al., 1987; Marfurt et al., 1988; Takemura et al., 1991; van Ham et al., 1996; May et al., 1998; Gong et al., 2003) demonstrated primary afferent projections from the cornea to both the medullary and spinal dorsal horns. However, if the cornea commands two representations, it would be unique in the body and would demand alteration of the accepted plan for neural organization of somatosensory pathways (Kandel et. al., ‘2000). Panneton and Burton (1981) discussed this dual representation of the cornea in the dorsal horns, and suggested these were projections to lamina I and II, respectively, separated by millimeters. This supposed dual representation has been exploited by some for numerous studies (e.g., see (Bereiter et al., 2000; Hirata et al., 2003). Nevertheless, the question has always remained as to why the cornea, versus all other body parts, would be represented in both the medullary and spinal dorsal horns.
Moreover, lesser projections also were noted from the cornea to the subnuclei interpolaris and oralis as well as to the principle trigeminal nucleus in cats (Panneton et al., 1981; Marfurt, 1981; Shigenaga et al., 1986b; Takemura et al., 1991), monkeys (Marfurt et al., 1988; May et al., 1998), and rabbits (van Ham et al., 1996), but not in the rat (Marfurt et al., 1987). More recent studies (van Ham et al., 1996; May et al., 1998) investigated the central termination of primary afferent fibers innervating periocular structures, including the conjunctiva. Both of these studies emphasized transganglionic label in the rostral spinal dorsal horn after injections into the conjunctiva, similar in position to that seen after injections of the cornea made in other species. A potential problem with most of these neuroanatomical studies however is that injections of tracers into either the cornea or conjunctiva will contaminate the other structure when the eye closes, since the eyeball is enveloped by the conjunctival mucosa. Thus it is important that controversies regarding the central representation of the cornea and conjunctiva be resolved.
It is the purpose of this study to offer another theory on the representation of periocular structures in the dorsal horns. Preliminary data of this study has appeared in abstract form (Panneton et al., 2006).
Materials and Methods
All experiments were done on male Sprague-Dawley rats (~275–340g). All protocols were approved by the Animal Care Committee of Saint Louis University and followed the guidelines published in the National Institutes of Health Guide for Care and Handling of Laboratory Animals.
Thirty-eight rats were anesthetized with intraperitoneal injections (0.1ml/100g) of a mixture of ketamine (60 mg/kg) and xylazine (40mg/kg) and then prepared for aseptic surgery. The tracing cocktail contained a 2% solution of horseradish peroxidase (HRP) conjugated to wheat germ agglutinin (WGA-HRP), 1% HRP conjugated to cholera toxin (BHRP), 1% saponin and 0.25% poly-L-ornithine and was colored with the vital dye Fast Green. Injections (0.4–3μL) of the HRP mixture were made into the substantia proprius of the cornea (20 rats), the submucosa of the palpebral conjunctiva (11 rats), the supraorbital nerve (4 rats), or the infraorbital nerve (3 rats) via a glass micropipette cemented to a 1 μL Hamilton syringe (see Panneton et al. 2005, for details). The corneal injections were all unilateral but two of the early conjunctival cases were bilateral. Eight of the rats with corneal injections had their infraorbital and supraorbital nerves transected prior to the injections in an effort to denervate the palpebral conjunctiva. The infraorbital nerve was transected in the infraorbital canal via an orbital approach while the supraorbital nerve was dissected and cut as it emerged under the orbital ridge of the frontal bone. The conjunctiva was injected immediately following the neurectomies. While conjunctival injections usually were placed in both its superior and inferior portions, some rats had injections only in their superior or inferior parts. Three of the rats with conjunctival injections were done after the eyeball had been enucleated. Both the bulbar and palpebral conjunctiva remained in situ in theses cases and the wound closed. Any leakage of the solution from the injection site was seen easily due to the Fast Green and immediately cleaned. In many of these rats, a nerve innervating the lower limb also was injected with a similar solution for a separate experiment (Panneton et al., 2005). After a 0.5-hour observation, the area was washed with saline and the wound closed.
After 48–60 hours the rats again were anesthetized (Sleepaway; 0.1ml/100g) and perfused transcardially, first with phosphate-buffered saline containing 0.25% procaine, and then with a mixture of 0.5% paraformaldehyde and 2.0% glutaraldehyde in phosphate buffer (PB; pH 7.3). The fixative usually was cleared from the brain with 10% sucrose in PB after approximately 1 hour. The brainstems were removed and stored in the refrigerator overnight in a 20% sucrose-buffer solution. Transverse sections (40 μm) were cut frozen through the brainstem and saved in 0.1 M PB. The sections were mounted serially on gelled slides, air-dried, and processed histochemically for HRP using TMB as the chromogen (Mesulam, 1978). After drying, the slides then were stained quickly with Thionin, dehydrated in alcohols, defatted in xylenes, and coverslipped with Permount.
Sections were examined with a Nikon E800 microscope equipped with bright- and dark-field optics, photographed digitally (MicroImager II), and processed and saved in the computer with Northern Eclipse software (Empix, Inc.). Label was reconstructed using a Neurolucida System (MicroBrightField, Inc.) interfaced with a Nikon E600 microscope. Reaction product aligned linearly was considered in labeled fibers, while that dispersed in the neuropil was considered punctate and in terminals. Photomicrographs were adjusted in Adobe Photoshop software (version 7.0), and figures made with Adobe Illustrator CS (version 11.0).
The medullary dorsal horn
The continuum of the epithelial sheet enveloping our head and body is represented in the medullary and spinal (SDH) dorsal horns. However a line separating the representation of the head versus body is as impossible to demarcate as those representing individual spinal nerves in the spinal cord. Thus the term ‘trigeminocervical complex’ (TCC) is used to describe the continuum in the dorsal horns representing the trigeminal distribution within them. Nevertheless, we will define arbitrarily the caudal pole of the MDH at the caudal poles of the inferior olivary and lateral reticular nuclei for those who can better relate to this terminology. These are both recognizable medullary nuclei and their end marks the initial transformation of the medulla into spinal organization.
Somatosensory receptors in the skin project to neurons in specific laminae within the dorsal horns, and help induce its laminar structure. However the rostral portion of the MDH commonly has been called its ‘alaminar’ part, since the usual arrangement of five laminae demarcating the dorsal horn is disrupted. There are several justifications for this distortion. Firstly, the rostral MDH deals most particularly with anterior receptive fields. Laminae III and IV of the laminar dorsal horns are dominated by neurons receiving information from touch and hair receptors. However the rostral MDH, which deals mostly with oral, nasal and ocular structures not populated by hairs, has ill-formed laminae III–IV, obscuring laminar structure. Secondly, the interface of the interpolar and caudal subnuclei of the spinal trigeminal nucleus takes place just caudal to the obex and this transition is in an oblique plane. The caudal pole of subnucleus interpolaris wedges itself ventral to the rostral MDH and displaces laminae I and II dorsally and medially (the displaced substantia gelatinosa). Defining the laminae for the central projections of sensory fibers is thus difficult for this region.
Results
Injections of the HRP cocktail into the cornea spread beneath the corneal epithelium giving the appearance of a bluish-green lens. Injections into the IO and SO nerves filled the epineurium and made the nerve dark blue. The largest volume (up to 3μl) of the HRP cocktail infiltrated the conjunctiva. In the corneal cases in which the IO and SO nerves were transected, between 50–100% of the IO was severed while the entire SO was transected. However, in neither instance was the lacrimal nerve, innervating conjunctiva near the lateral canthus, nor the supratrochlear or infratrochlear nerves, innervating the medial canthus, found. In the cases where the conjunctiva was injected after enucleation, both bulbar and palpebral conjunctivas probably were included in the injection, since the wound was ‘closed’ with both conjunctiva’s sutured together in the base of the orbit. All cases showed similar data relative to the nerve/receptive field injected. The label also was somatotopically appropriate in the principal trigeminal nucleus, the subnuclei of the spinal trigeminal nucleus and the dorsal horns (i.e., the trigeminocervical complex) after all the injections.
Heterogeneity of Reaction Product
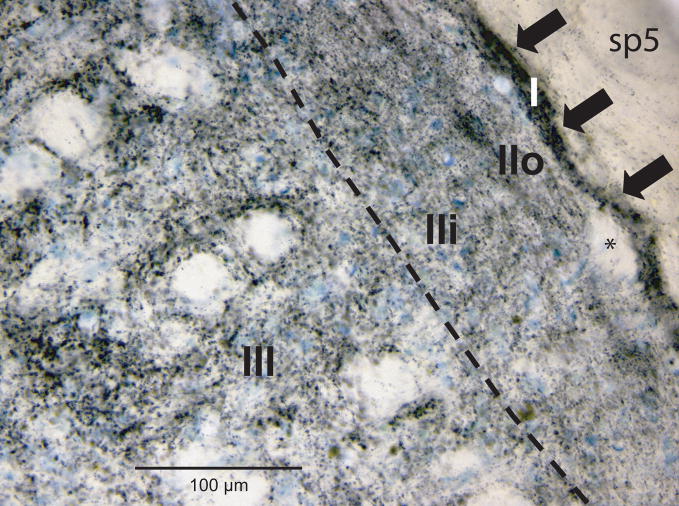
The dispersed reaction product found in the neuropil of the dorsal horns differed in appearance depending on its laminar location and the nerve/receptive field injected. First, the intensity of reaction product differed relative to the lamina in which it was found (Fig. 1). For example, label in lamina I was dense (arrows; Fig. 1) and found just superficial to the deep bundles (asterisk; Fig. 1). It outlined the superficial margins of the dorsal horn and its circumferential distribution often exceeded label seen subjacent in lamina II (Figs. 3B2, B4-D4). The small neurons representing lamina II were surrounded by reaction product consisting of very small grains (Fig. 1; but see descriptions for cornea below). Reaction product in outer parts of lamina II (IIo) usually presented as a ‘dusting’ of small aggregations in darkfield optics which generally was sparser and more homogeneous than that in inner parts of lamina II (IIi; Figs. 1, 3). The reaction product in lamina IIi sometimes showed larger aggregations that continued into deeper laminae (Fig. 1). The boundary between laminae II and III was the line of birefringence marking the myelinated fibers in lamina III seen with darkfield optics, and the bundles of longitudinal fibers and larger neurons seen in lamina III with brightfield optics. Reaction product in laminae III and IV of the dorsal horns consisted both of small grains and an abundance of larger clumps, particularly after injections of nerves innervating hairy skin (e.g., the IO and SO nerves). Label in lamina V was a mixture of small and large grains of reaction product, particularly after infraorbital and supraorbital nerve injections.
Figure 1.
Brightfield photomicrograph illustrating the distribution and heterogeneity of reaction product in the dorsal horn after an injection of an HRP-labeled cocktail of tracers into the infraorbital nerve. Note the differences in the size and density of reaction product in the different laminae. See text for details. Dashed line indicates separation between lamina II and lamina III. Abbreviation: sp5, spinal trigeminal tract
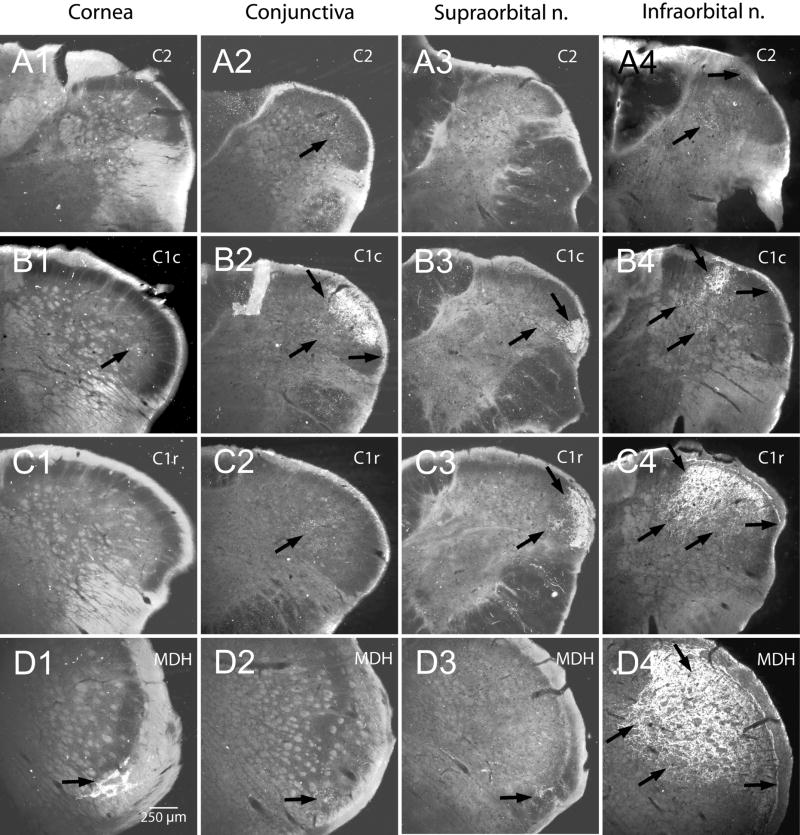
Figure 3.
Darkfield photomicrographs of sections cut through the trigeminocervical complex showing the transganglionic transport of a mixture of HRP-labeled molecules after its application to periorbital nerves/receptive fields. Note the cornea representation is shown almost exclusively in lamina I in the ventral MDH (D1; arrow) with only isolated fibers noted in the DH more caudally (B1; arrow). Both the infraorbital and supraorbital nerves were transected in this case. Injections into the conjunctival mucosa intensely labeled lamina I–III of the caudal C1 DH (B2, arrows), with only sparse label seen in lamina I where the corneal fibers terminate (D2, arrow). Note that the sparse label seen in lamina IV extended beyond that in lamina II and often ‘surrounded’ unlabeled neuropil (Figs A2–C2, arrows). The infraorbital and supraorbital nerves as well as the cornea remained intact in this case. All laminae were labeled somatotopically after injections of the supraorbital (A3–D3) and infraorbital (A4–D4) nerves. Note that the label from the conjunctiva (B2) and supraorbital nerve (B3, C3) ‘fill in’ areas in laminae II and III voided by the infraorbital nerve projections (B4, C4), but that projections to laminae I, IV, and V overlap.
Distribution of Sensory Fibers
Labeled fibers from all cases entered the brainstem at pontine levels and descended in the spinal trigeminal tract in a topographic fashion: SO and corneal fibers descended in the ventral fifth of the spinal trigeminal tract, conjunctival fibers in its ventral quarter and IO fibers in its middle two-thirds.
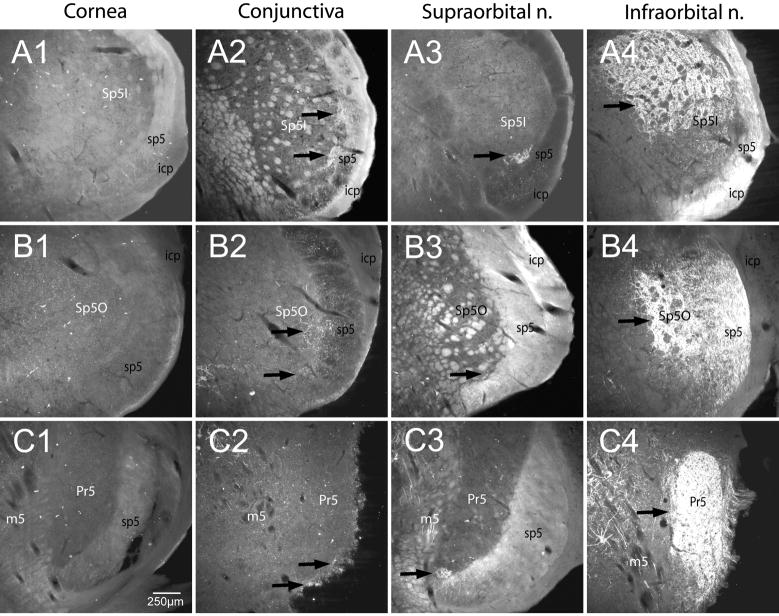
The rostral spinal trigeminal complex, including the principal trigeminal nucleus, was labeled in all cases except those where the cornea was injected. Dense terminal-like label was seen in middle parts of the principal trigeminal nucleus (Pr5) in IO cases (Fig. 2C4; arrow), in the ventral extreme of the Pr5 in SO cases (Fig. 2C3; arrow), and in small patches along its ventrolateral edge in conjunctival cases (Fig. 2C2; arrows). No such label was noted in the Pr5 in any of the corneal cases (Fig. 2C1). Subnucleus oralis (Sp5O) of the trigeminal sensory nucleus contained relatively sparse terminal-like label in conjunctival (Fig. 2B2; arrows) and SO cases (Fig. 2B3; arrow) but was considerably denser in IO cases, especially its dorsomedial subdivision (Fig. 2B4; arrow). No terminal-like label was noted in Sp5O after corneal injections (Fig. 2B1). Terminal-like label in the rostral nuclei was most dense in the subnucleus interpolaris (Sp5I) in these cases. Label was seen ventrolaterally in two separate patches after injections into the conjunctiva of both the upper and lower eyelids (Fig. 2A2; arrows), ventrally after SO injections (Fig. 2A3; arrow), and more dorsally after IO injections (Fig. 2A4; arrow), but was never seen in Sp5I near obex regions after corneal injections (Fig. 2A1).
Figure 2.
Darkfield photomicrographs of sections cut through the rostral trigeminal sensory complex showing the transganglionic transport of a mixture of HRP-labeled molecules after its application to periorbital nerves/receptive fields. Note that NO label was present in any part of the rostral trigeminal complex after corneal injections but double bursts of label were seen after injections of both superior and inferior parts of the conjunctiva (arrows). Also note that these partially overlap with projections from the supraorbital nerve and infraorbital nerve.
Abbreviations: Subnucleus interpolaris, Sp5I; subnucleus oralis, Sp5O; Principal trigeminal nucleus, Pr5; icp, inferior cerebellar peduncle; sp5, spinal trigeminal tract; m5, motor trigeminal root.
Nearly all cases resulted in some HRP reaction product in the trigeminocervical complex. The ventral interstitial islands in the caudal medulla (Panneton et al., 1981), the presumptive lamina I, were labeled robustly after corneal injections (Fig. 3D1; arrow). We considered the reaction product at the ventromedial tip of the MDH after corneal injections to be in the displaced substantia gelatinosa and be in lamina II (Figs. 4; cornea; −0.2, −0.6). Only sporadic fibers near lamina I and an occasional fiber in the neuropil of the SDH were seen more caudally after corneal injections (Figs. 3A1–C1; Fig. 4). Most reaction product after conjunctival injections was found in laminae I–III of the caudal C1 DH segment (Fig. 3B2; arrow), but sparser label was noted in lamina IV–V rostrally (Fig. 3C2; arrow) and caudally (Fig. 3A2; arrow). The ventral interstitial islands also showed some reaction product in all conjunctival cases (Fig. 3D2; arrow). Label in Sp5I after IO injections blended caudally with label in the TCC (Fig. 3D4; arrows). Terminal-like label appeared in laminae I and V at the level of the pyramidal decussation after SO injections, but became more robust in all laminae of the C1 DH (Figs. 3B3–C3, arrows; Fig. 4). Some label after SO injections also was seen in the ventral interstitial islands (Fig. 3D3; arrow). All laminae of the TCC (Figs. 3B4–D4; arrows) were labeled densely after IO injections. Label decreased in the rostral C2 DH (Fig. 3B4; arrows), and then continued only in laminae I and V (Fig. 3A4; arrows).
Figure 4.
Line drawings showing the distribution of reaction product in the trigeminocervical complex after the transganglionic transport of an HRP cocktail injected into the cornea, the conjunctiva, and the supraorbital and infraorbital nerves. Numbers above sections indicate distance in millimeters relative to obex. Arrows in the cornea series point to label in presumptive lamina II while those in the conjunctiva series point to the two bursts of label seen when the whole conjunctiva was injected.
We transected both the SO and IO nerves prior to eight corneal injections in an attempt to denervate the superior and inferior conjunctiva. Results basically were similar to that seen after our best corneal injections without transection, albeit the reaction product in the C1 DH was totally absent in two of these cases. Enucleations of the eyeball were done to eliminate contamination of the corneal receptive field after injections of the HRP cocktail into the conjunctiva. Reaction product was found in the C1 DH after such injections as well as rostral subnuclei of the trigeminal sensory complex (Fig. 5); only a small spot of label was found ventrally in the ventral interstitial islands. Qualitative observations after injections of either the superior or inferior conjunctiva suggested the superior conjunctiva is represented more ventrally than conjunctiva lining the inferior eyelid.
Figure 5.
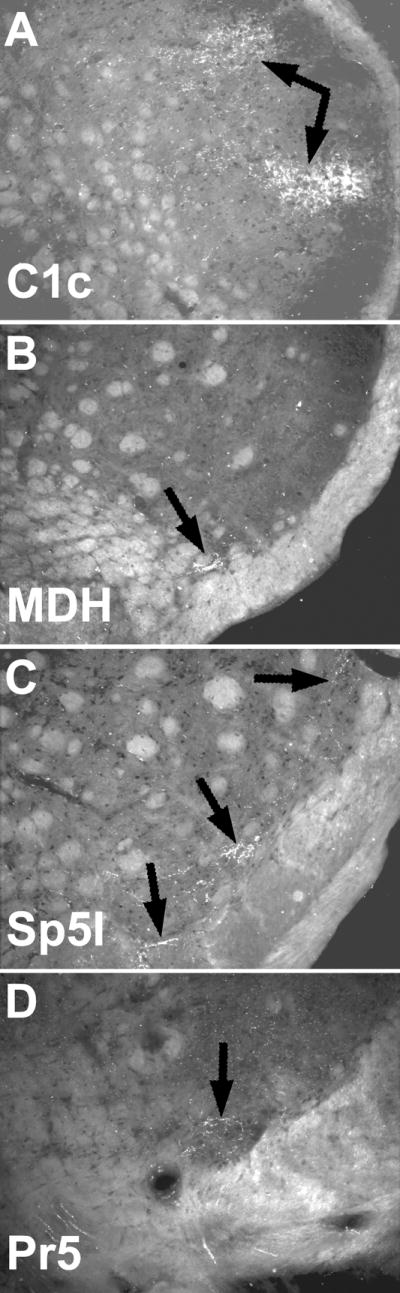
Darkfield photomicrographs of sections cut through the trigeminal sensory complex showing the transganglionic transport of a mixture of HRP-labeled molecules (arrows) after its application to the conjunctiva in an enucleated rat. This suggests that label noted in the caudal C1 DH (A) and rostral trigeminal sensory complex (C, D) is from the conjunctiva and that there is potential overlap with corneal projections only in lamina I in the ventral MDH (B).
Occasional lightly-labeled motor neurons were found along the dorsal and dorsolateral parts of the facial motor nucleus (7) after SO and conjunctival injections, and in intermediate subdivisions of 7 after IO injections, suggestive that some of the HRP cocktail spread into the orbicularis oculi and superior labial muscles, respectively. Some motor neurons of the trigeminal nerve and preganglionic neurons of the superior salivatory nucleus were labeled after IO injections.
Discussion
Technical Considerations
Our technique has improved immensely since our original presentation of projections from periorbital structures (Panneton et al., 1981). We now inject via a small diameter glass micropipette with a tip of between 20–30 microns glued to a Hamilton syringe; this tip penetrates both epineurium and corneal epithelium much easier than the 26 gauge needle used in our previous studies (Panneton et al., 1981). Moreover, the HRP cocktail marked with Fast Green spread throughout the lamina proprius of the cornea and the conjunctiva showed no recognizable green stain. Thus it was our impression that little of the nanoliter quantity of the HRP cocktail injected leaked from the small penetration holes made by the micropipette during the observation period. Marfurt and colleagues (Marfurt et al., 1987; Marfurt et al., 1988) bathed the cornea with up to 5μl of tracers and allowed them to spread into the orbit. They saw labeled ganglion cells in the rat but mentioned no transganglionic label in either species. We considered spread to be non-preventable in cases where the palpebral conjunctiva was injected, however; the micropipette was placed just beneath the epithelium and there were few barriers to contain the HRP cocktail. Moreover, either the bulbar conjunctiva or the cornea could be contaminated with every blink. The injections into the IO and SO nerves were reasonably straightforward, albeit the IO injection was fairly deep since it was made via the orbit as the nerve traversed the infraorbital canal.
We combined herein HRP conjugated to cholera toxin (BHRP), which preferentially labels larger diameter fibers, with HRP conjugated to wheat germ agglutinin (WGA-HRP), which preferentially labels smaller diameter fibers (Liu et al., 1995; Sugimoto et al., 1997; Panneton et al., 2005; Panneton et al., 2006). Thus the whole spectrum of fibers to all laminae of the dorsal horn were labeled, similar to our previous studies (Panneton, 1991; Panneton et al., 2005; Panneton et al., 2006). Our observations differ however with those of Liu et al. (Liu et al., 1995), who concluded that co-injection of these tracers somehow interfered with the other’s transport. While a few of our injections resulted in little label, the majority showed transganglionic transport of tracer to both superficial and deep laminae. Moreover, we saw large amounts of label yet the volume of tracers used herein were often ten times less than that used by other investigators. It may be due to our use of saponin in the injection cocktail; saponin breaks down membranes and theoretically promotes the uptake of the tracers. Several studies have documented changing patterns of labeling with different survival times (Shigenaga et al., 1986a; Liu et al., 1995), thus we maintained a tight window of 48–60 hrs for survival since this period is optimum for labeling (Liu et al., 1995). Moreover, the robust label illustrated in all laminae in Figures 1, 2, and 3 documents the validity of our technique.
It is well-known that fibers of different size project preferentially to different laminae of the dorsal horn, and that such projections correlate with both the functional fiber type and laminar neurons (Fyffe, 1992). Peripheral fibers innervating the cornea are of small diameter (Belmonte, 1996; Belmonte et al., 1997) and we show they projected only to lamina I and II of the MDH. Similar small diameter afferent fibers (Light et al., 1979; Sugiura et al., 1993; Ling et al., 2003) project to the superficial dorsal horn and may produce the ‘dust-like’ labeling we saw in lamina II. The conjunctiva is innervated mostly by small diameter fibers but some large diameter fibers also are present (Weddell et al., 1962; Snell et. al., ‘97). Reaction product seen after conjunctival cases herein was congruously dense in laminae I–III but sparser in lamina IV–V. The fibers contained within the IO and SO nerves have a wide spectrum of sizes and the projections from these nerves were distributed to all laminae within the dorsal horns. Moreover, the larger aggregates of reaction product seen in laminae III–V seen after injections of the these nerves correlate with terminals of larger fibers found in these laminae (Ralston, 1979).
Projections to Rostral Trigeminal Nuclei
The projections to the Pr5, Sp5O, and Sp5I subnuclei from the SO, IO and conjunctiva were somatopically appropriate and confirmed data from other transganglionic studies (Panneton et al., 1981; Marfurt, 1981; Shigenaga et al., 1986a; Marfurt et al., 1987; Marfurt et al., 1988; Takemura et al., 1991; van Ham et al., 1996; May et al., 1998; Gong et al., 2003). It is of interest that projections to these nuclei seen after injections of both the superior and inferior conjunctiva in the present study commonly appeared as two separate bursts of reaction product. We speculate that the more ventral burst is from the conjunctiva lining the upper eyelid and is transported via the SO nerve while the ventrolateral burst is from the mucosa lining the lower eyelid and is transported via the IO nerve. Indeed, in the two cases where either the superior or inferior palpebral conjunctiva was injected alone, only one such burst was seen.
We previously questioned (Panneton et al., 1981) that the cornea, which is only innervated by Aδ and C fibers (Belmonte, 1996; Belmonte et al., 1997), projected to the Pr5 in the cat. Our present findings however support those of Marfurt and Del Toro (1987) in the rat; neither study showed projections from the corneas of rats to rostral trigeminal sensory nuclei. We also suggest that the lack of reaction product in rostral trigeminal nuclei in these two studies is NOT due to species differences, but rather to overcoming the technical problems of injecting a structure lubricated by tears and surrounded by a mucosa.
Projections to the Dorsal Horns
A place on the epithelial sheet covering the head and body should be represented in the dorsal horn somatotpically with its size dependant on innervation density, and theoretically should be represented but once. This is the basis for ‘homunculi’ drawn for humans and similar cartoons drawn for many other species. Nevertheless our earlier data (Panneton et al., 1981) showed two representations in the dorsal horns for the cornea of the cat. Numerous other studies (Marfurt, 1981; Shigenaga et al., 1986a; Marfurt et al., 1987; Marfurt et al., 1988; Takemura et al., 1991; van Ham et al., 1996; May et al., 1998; Gong et al., 2003) showed similar results, a dense projection into the ventral part of the MDH as well as a lesser projection into the C1 DH. Moreover, similar projections also were noted for the conjunctiva (van Ham et al., 1996; May et al., 1998). Thus while the projections from the SO and IO nerves in the present study were similar to others (Panneton et al., 1981; Marfurt, 1981; Shigenaga et al., 1986a; Shigenaga et al., 1986b; Takemura et al., 1991; Gong et al., 2003), projections from the cornea and conjunctiva were different.
Our present results suggest that fibers innervating the cornea project to the ventral TCC in the caudal medulla while those innervating the conjunctiva project further caudally (e.g., the caudal C1 DH). We show however that while the projections to lamina II from these different receptive fields are separate (vide infra), they may overlap in lamina I in the ventral MDH. We have shown previously (Panneton et al., 1981; Panneton, 1991; Panneton et al., 2005; Panneton et al., 2006) that primary afferent fibers contained in nerves with juxtaposed receptive fields are found in similar parts of lamina I, suggesting a wide degree of convergence of fibers to this lamina (Pozo et al., 1993; Carstens et al., 1998; Henriquez et al., 2007). Since both the cornea and the bulbar conjunctiva are innervated by the long ciliary nerves (Snell et. al., ‘97), it also is possible fibers traveling in the bulbar conjunctiva in route to the cornea were included in conjunctiva injections in cases where the eyeball was enucleated.
It is difficult to determine if the central projections of the cornea to the TCC were confined to lamina I, also included lamina II, or also included the most caudal pole of the subnucleus interpolaris (see Fig. 4, cornea:−0.6, −0.2). It is of interest however that the anterior ethmoidal nerve (AEN), which innervates the nasal mucosa, projects to the TCC near to the corneal projection (Panneton et al., 2006). In the laminar caudal MDH, primary afferent fibers from the AEN project to laminae I and II in a superficial position (see Fig. 1E in Panneton et al., 2006). More rostrally in the alaminar MDH, the substantia gelatinosa is displaced by the caudal pole of Sp5I (see Fig. 1F in Panneton et al., 2006) and fibers from the AEN project ventrally around Sp5I into a more dorsally placed lamina II. The TCC projection of the cornea apparently infringes on that of the AEN in laminae I and II near this transition. Although it is possible that reaction product also impinged on the ventromedial tip of Sp5I, we suggest however that this was NOT the case. Labeling in Sp5I from the conjunctiva and other nerves continued rostrally to the junction with Sp5O, but that from the cornea did not. Nevertheless, corneal projections to Sp5I may still be considered moot however.
Stimulating either the cornea or conjunctiva yields the same sensations in humans differing only in perception of intensity (Acosta et al., 2001b; Feng et al., 2005) while paired-pulsed stimuli show that the receptive fields interact (Feng et al., 2005) and perception is altered. Paired-pulse stimuli to both the cornea and the C1 DH also modify the corneal-evoked blink reflex (Henriquez et al., 2005; Henriquez et al., 2007). Although a ‘dual’ projection for the cornea has been exploited by some in their neuroanatomical and physiological studies, we suggest great care be taken to eliminate contamination of the conjunctiva when attempting to stimulate the cornea alone, especially with liquids (Carstens et al., 1998). Nevertheless, the observations presented herein support the conclusions of several neurophysiological examinations of the cornea in the MDH (Pozo et al., 1993; Carstens et al., 1998; Henriquez et al., 2005; Henriquez et al., 2007).
Neural Substrate for Blink Reflexes
Recording brainstem reflexes allows for the assessment of pathways in brain pathologies (Esteban, 1999; Aramideh et al., 2002) and are commonly used in diagnosis of brainstem disease in humans. Orbicularis oculi reflexes, including blink reflexes, are the most widely-used electrophysiological test of brainstem function (Marx et al., 2001). The projections described herein provide the afferent link of the neural circuits mediating both the supraorbital and corneal-evoked blink reflexes, but there still are questions as to which central neurons are involved in these clinically-relevant behaviors. The SO-evoked blink reflex is commonly elicited by transcutaneous electrical stimulation of the SO nerve and usually elicits two responses. The R1 response is an early (10–13ms) but brief unilateral response ipsilateral to the stimulus in humans while the R2 response is slower (30–41ms) and bilateral (Esteban, 1999; Aramideh et al., 2002). It is of interest that the SO-evoked blink reflex also can be elicited by electrical stimulation of the IO nerve or by air puffs to the cornea, but such stimulation seldom evokes an R1 response but always an R2 response (Esteban, 1999; Aramideh et al., 2002; VanderWerf et al., 2003). The corneal-evoked blink reflex on the other hand is considered a nociceptive reflex and is induced by stimulating the cornea either mechanically or electrically (Esteban, 1999; Aramideh et al., 2002). Its activation induces bilateral closure of the eyes with latencies of 36–64ms after mechanical stimulation and 35–50ms after electrical stimulation (Aramideh et al., 2002), but is reduced to 24ms after air puff stimulation (VanderWerf et al., 2003). Thus the corneal-evoked blink reflex resembles the R2 response of the SO-evoked blink reflex but is thought to be relayed through different and fewer interneurons (Esteban, 1999; Aramideh et al., 2002).
It has been argued that the R1 response is mediated by rostral trigeminal neurons, particularly those in the principal trigeminal nucleus (Esteban, 1999; Aramideh et al., 2002). The fast response time for R1 thus would require large diameter fibers in the SO nerve projecting to the Pr5. Indeed we show such a projection herein and speculate that it is via large fibers. However, the R1 response is seldom seen after stimulation of the IO nerve or either air puff or electrical stimulation of the cornea (Aramideh et al., 2002; VanderWerf et al., 2003). The IO nerve also contains numerous large diameter fibers, but projections into the ventral, ophthalmic portions of the Pr5 are very sparse. Moreover, the cornea is innervated only by small diameter fibers and our results suggest that it has no projections to Pr5. Thus our data supports the hypothesis that neurons in the ventral Pr5 are important for the R1 component of the blink reflex.
The R2 response has a longer latency and is thought to be relayed through neurons in the cervical spinal cord by most investigators. Thus the distance traveled to the C1 DH is longer and probably uses smaller diameter fibers, thus making the latency longer. The R2 response also is activated by IO stimulation as well as corneal air puffs, which fits well with the data reported herein; the conjunctival representation in C1 DH is supplied by fibers carried in both the SO and IO nerves. Our neuroanatomical data also supports a circuit proposed for the corneal-evoked blink response which has been suggested to relay through neurons in the MDH nearer the obex (Henriquez et al., 2007). Experiments outlining circuits from neurons in the trigeminal sensory complex to facial motoneurons necessary to complete the reflex circuit is underway (Panneton et al., 2008).
Acknowledgments
The technical help of Rajko Juric was greatly appreciated. This study was supported in part by NIH grant R01 HL64772 and monies from the Saint Louis University School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Acosta MC, Belmonte C, Gallar J. Sensory experiences in humans and single-unit activity in cats evoked by polymodal stimulation of the cornea. J Physiol (Lond) 2001a;534:511–525. doi: 10.1111/j.1469-7793.2001.t01-1-00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta MC, Tan ME, Belmonte C, Gallar J. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Invest Ophthalmol Vis Sci. 2001b;42:2063–2067. [PubMed] [Google Scholar]
- Aramideh M, De Visser BWO. Brainstem reflexes: Electrodiagnostic techniques, physiology, normative data, and clinical applications. Muscle Nerve. 2002;26:14–30. doi: 10.1002/mus.10120. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Garcia-Hirshfeld J, Gallar J. Neurobiology of ocular pain. Prog Retinal Eye Res. 1997;16:117–156. [Google Scholar]
- Belmonte CGJ. Corneal nociceptors. In: Belmonte C, Cervero F, editors. Neurobiology of Nociceptors. Oxford University Press; New York: 1996. pp. 146–183. [Google Scholar]
- Bereiter DA, Hirata H, Hu JW. Trigeminal subnucleus caudalis: beyond homologies with the spinal dorsal horn. Pain. 2000;88:221–224. doi: 10.1016/S0304-3959(00)00434-6. [DOI] [PubMed] [Google Scholar]
- Beuerman RW, Pedroza L. Ultrastructure of the human cornea. Microsc Res Tech. 1996;33:320–335. doi: 10.1002/(SICI)1097-0029(19960301)33:4<320::AID-JEMT3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Beuerman RW, Tanelian DL. Corneal pain evoked by thermal stimulation. Pain. 1979;7:1–14. doi: 10.1016/0304-3959(79)90102-7. [DOI] [PubMed] [Google Scholar]
- Brown PB, Koerber R, Ritz LA. Somatotopic organization of primary afferent projections to the spinal cord. In: Scott SA, editor. Sensory neurons: diversity, development and plasticity. Oxford University Press; NY, NY: 1992. pp. 116–130. [Google Scholar]
- Carstens E, Kuenzler N, Handwerker HO. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J Neurophysiol. 1998;80:465–492. doi: 10.1152/jn.1998.80.2.465. [DOI] [PubMed] [Google Scholar]
- Chen X, Gallar J, Pozo MA, Baeza M, Belmonte C. CO2 stimulation of the cornea: a comparison between human sensation and nerve activity in polymodal nociceptive afferents of the cat. Eur J Neurosci. 1995;7:1154–1163. doi: 10.1111/j.1460-9568.1995.tb01105.x. [DOI] [PubMed] [Google Scholar]
- Esteban A. A neurophysiological approach to brainstem reflexes. Blink reflex Neurophysiol Clin. 1999;29:7–38. doi: 10.1016/S0987-7053(99)80039-2. [DOI] [PubMed] [Google Scholar]
- Feng Y, Simpson TL. The inhibitory interaction between human corneal and conjunctival sensory channels. Invest Ophthalmol Vis Sci. 2005;46:1251–1255. doi: 10.1167/iovs.04-1191. [DOI] [PubMed] [Google Scholar]
- Fyffe REW. Laminar Organization of primary afferent terminations in the mammalian spinal cord. In: Scott SA, editor. Sensory neurons: diversity, development, and plasticity. Oxford University Press; NY, NY: 1992. pp. 131–139. [Google Scholar]
- Gong S, Zhou Q, LeDoux MS. Blink-related sensorimotor anatomy in the rat. Anat Embryol (Berl) 2003;207:193–208. doi: 10.1007/s00429-003-0341-6. [DOI] [PubMed] [Google Scholar]
- Henriquez VM, Evinger C. Modification of cornea-evoked reflex blinks in rats. Exp Brain Res. 2005;163:445–456. doi: 10.1007/s00221-004-2200-y. [DOI] [PubMed] [Google Scholar]
- Henriquez VM, Evinger C. The three-neuron corneal reflex circuit and modulation of second-order corneal responsive neurons. Exp Brain Res. 2007;179:691–702. doi: 10.1007/s00221-006-0826-7. [DOI] [PubMed] [Google Scholar]
- Hirata H, Okamoto K, Bereiter DA. GABAA receptor activation modulates corneal unit activity in rostral and caudal portions of trigeminal subnucleus caudalis. J Neurophysiol. 2003;90:2837–2849. doi: 10.1152/jn.00544.2003. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neuroscience. St. Louis: McGraw-Hill; 2000. [Google Scholar]
- Lele PP, Weddell G. Sensory nerves of the cornea and cutaneous sensibility. Exp Neurol. 1959;1:334–359. doi: 10.1016/0014-4886(59)90025-1. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979;186:133–150. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- Ling LJ, Honda T, Shimada Y, Ozaki N, Shiraishi Y, Sugiura Y. Central projection of unmyelinated (C) primary afferent fibers from gastrocnemius muscle in the guinea pig. J Comp Neurol. 2003;461:140–150. doi: 10.1002/cne.10619. [DOI] [PubMed] [Google Scholar]
- Liu H, Llewellyn-Smith IJ, Basbaum AI. Co-injection of wheat germ agglutinin-HRP and choleragenoid-HRP into the sciatic nerve of the rat blocks transganglionic transport. J Histochem Cytochem. 1995;43:489–495. doi: 10.1177/43.5.7730587. [DOI] [PubMed] [Google Scholar]
- Lwigale PY. Embryonic origin of avian corneal sensory nerves. Dev Biol. 2001;239:323–337. doi: 10.1006/dbio.2001.0450. [DOI] [PubMed] [Google Scholar]
- Marfurt CF. The central projections of trigeminal primary afferent neurons in the cat as determined by the transganglionic transport of horseradish peroxidase. J Comp Neurol. 1981;203:785–798. doi: 10.1002/cne.902030414. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Del Toro DR. Corneal sensory pathway in the rat: a horseradish peroxidase tracing study. J Comp Neurol. 1987;261:450–459. doi: 10.1002/cne.902610309. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Echtenkamp SF. Central projections and trigeminal ganglion location of corneal afferent neurons in the monkey, Macaca fascicularis. J Comp Neurol. 1988;272:370–382. doi: 10.1002/cne.902720307. [DOI] [PubMed] [Google Scholar]
- Marx JJ, Thoemke F, Fitzek S, Vucurevic G, Fitzek C, Mika-Gruettner A, Urban PP, Stoeter S, Hopf HC. Top diagnostic value of blink reflex R1 changes: A digital postprocessing MRI correlation study. Muscle Nerve. 2001;24:1327–1331. doi: 10.1002/mus.1151. [DOI] [PubMed] [Google Scholar]
- May PJ, Porter JD. The distribution of primary afferent terminals from the eyelids of macaque monkeys. Exp Brain Res. 1998;123:368–381. doi: 10.1007/s002210050582. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem. 1978;26:106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Oppenheimer DR, Palmer E, Weddell G. Nerve endings in the conjunctiva. J Anat. 1958;92:321–352. [PMC free article] [PubMed] [Google Scholar]
- Panneton WM. Primary afferent projections from the upper respiratory tract in the muskrat. J Comp Neurol. 1991;308:51–65. doi: 10.1002/cne.903080106. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Burton H. Corneal and periocular representation within the trigeminal sensory complex in the cat studied with transganglionic transport of horseradish peroxidase. J Comp Neurol. 1981;199:327–344. doi: 10.1002/cne.901990303. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Gan Q. Another look at the central projections of sensory afferent fibers innervating the cornea. FASEB J. 2006:20. [Google Scholar]
- Panneton WM, Gan Q, Juric R. The central termination of sensory fibers from nerves to the gastrocnemius muscle of the rat. Neuroscience. 2005;134:175–187. doi: 10.1016/j.neuroscience.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Gan Q, Juric R. Brainstem projections from recipient zones of the anterior ethmoidal nerve in the medullary dorsal horn. Neuroscience. 2006;141:889–906. doi: 10.1016/j.neuroscience.2006.04.055. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Patel N, Gan Q. Trigeminal projections to the facial motor nucleus in rats: potential reflex circuits. Neurosci Abstr. 2008:33. [Google Scholar]
- Pozo MA, Cervero F. Neurons in the rat spinal trigeminal complex driven by corneal nociceptors: Receptive-field properties and effects of noxious stimulation of the cornea. J Neurophysiol. 1993;70:2370–2378. doi: 10.1152/jn.1993.70.6.2370. [DOI] [PubMed] [Google Scholar]
- Ralston HJ. The fine structure of laminae I, II, and III of the Macaque spinal cord. J Comp Neurol. 1979;184:619–642. doi: 10.1002/cne.901840403. [DOI] [PubMed] [Google Scholar]
- Rozsa AJ, Beuerman RW. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain. 1982;14:105–120. doi: 10.1016/0304-3959(82)90092-6. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Chen IC, Suemune S. Oral and facial representation within the medullary and upper cervical dorsal horns in the cat. J Comp Neurol. 1986a;243:388–408. doi: 10.1002/cne.902430309. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Okamoto T, Nishimori T, Suemune S, Nasution ID, Chen IC, Tsuru K, Yoshida A, Tabuchi K, Hosoi M, Tsuru H. Oral and facial representation in the trigeminal principal and rostral spinal nuclei of the cat. J Comp Neurol. 1986b;244:1–18. doi: 10.1002/cne.902440102. [DOI] [PubMed] [Google Scholar]
- Snell RS, Lemp MA. Clinical Anatomy of the Eye. Blackwell Science; 1997. [Google Scholar]
- Sugimoto T, Fujiyoshi Y, He YF, Xiao C, Ichikawa H. Trigeminal primary projection to the rat brain stem sensory trigeminal nuclear complex and surrounding structures revealed by anterograde transport of cholera toxin B subunit-conjugated and Bandeiraea simplicifolia isolectin B4-conjugated horseradish peroxidase. Neurosci Res. 1997;28:361–371. doi: 10.1016/s0168-0102(97)00064-3. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Terui N, Hosoya Y, Tonosaki Y, Nishiyama K, Honda T. Quantitative analysis of central terminal projections of visceral and somatic unmyelinated (C) primary afferent fibers in the guinea pig. J Comp Neurol. 1993;332:315–325. doi: 10.1002/cne.903320305. [DOI] [PubMed] [Google Scholar]
- Takemura M, Sugimoto T, Shigenaga Y. Difference in central projection of primary afferents innervating facial and intraoral structures in the rat. Exp Neurol. 1991;111:324–331. doi: 10.1016/0014-4886(91)90099-x. [DOI] [PubMed] [Google Scholar]
- van Ham JJ, Yeo CH. The central distribution of primary afferents from the external eyelids, conjunctiva, and cornea in the rabbit, studied using WGA-HRP and B-HRP as transganglionic tracers. Exp Neurol. 1996;142:217–225. doi: 10.1006/exnr.1996.0193. [DOI] [PubMed] [Google Scholar]
- VanderWerf F, Brassinga P, Reits D, Aramideh M, Ongerboer de Visser B. Eyelid movements: behavioral studies of blinking in humans under different stimulus conditions. J Neurophysiol. 2003;89:2784–2796. doi: 10.1152/jn.00557.2002. [DOI] [PubMed] [Google Scholar]
- Weddell G, Miller S. Cutaneous sensibility. Ann Reviews. 1962;206:199–222. doi: 10.1146/annurev.ph.24.030162.001215. [DOI] [PubMed] [Google Scholar]
- Williams PL, Warwick R. Gray’s Anatomy. Philadelphia: W. B. Saunders Co; 1980. [Google Scholar]