Abstract
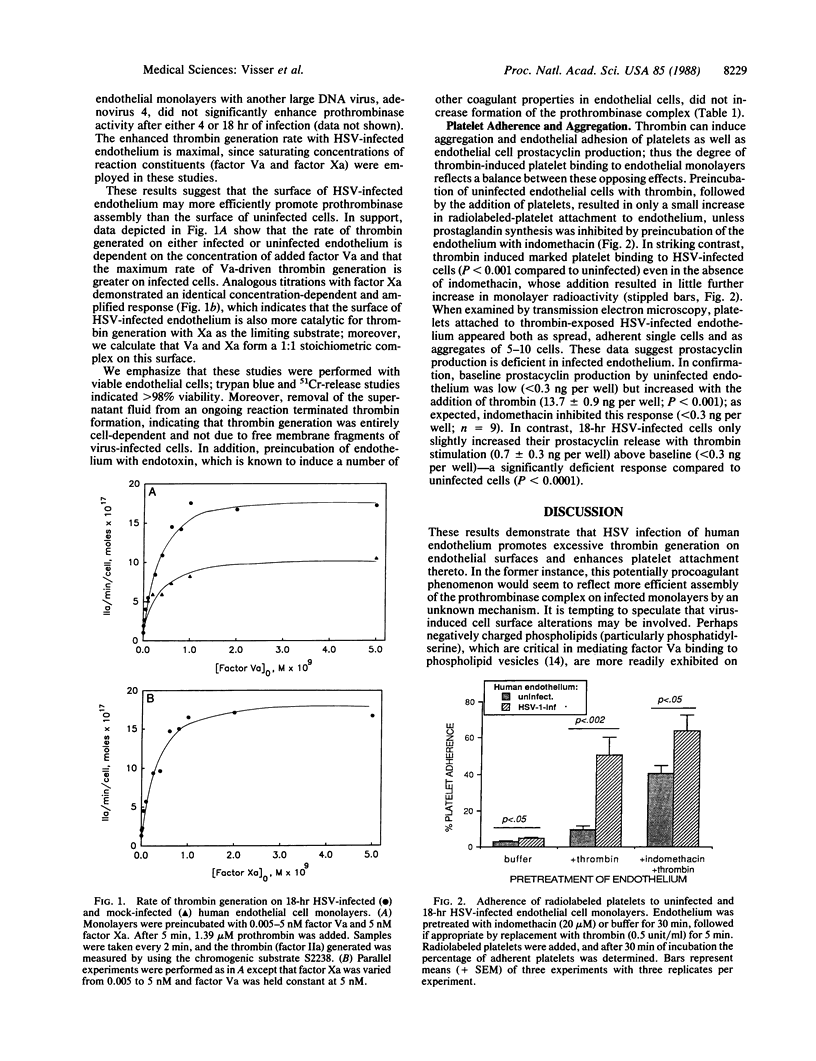
Atherosclerotic lesions have been reported to contain herpes simplex virus 1 (HSV-1) genomic material. This, and other previous evidence, suggests that latent viral infection may be an atherogenic trigger. Moreover, active HSV-1 lesions manifest marked fibrin deposition in microvessels. In this report we show that very early infection of human endothelial cells with HSV-1 appears to alter surface conformation as detected by merocyanine 540 staining. Concomitantly, the efficiency of prothrombinase complex assembly increases, resulting in a 2- to 3-fold accelerated rate of thrombin generation on the cell surface. Increased thrombin generation is probably doubly procoagulant, since we also demonstrate that thrombin-induced platelet accumulation on HSV-infected endothelium (50.7 +/- 9.3%) is increased compared to uninfected endothelium (9.5 +/- 2.1%; P less than 0.002). Associated with HSV infection, prostacyclin secretion in response to thrombin is diminished by a factor of 20, probably explaining the enhanced platelet attachment. We conclude that HSV infection shifts endothelial cell properties from anticoagulant to procoagulant, both by promoting prothrombinase complex formation and function and by increasing platelet binding, well before cell disruption takes place. Virus-induced changes in the endothelial plasma membrane and diminished prostacyclin secretion are suggested as the pathways for this pathophysiologic mechanism, which may be germane to atherosclerotic thrombosis as well as HSV-mediated tissue necrosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benditt E. P., Barrett T., McDougall J. K. Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6386–6389. doi: 10.1073/pnas.80.20.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cines D. B., Lyss A. P., Bina M., Corkey R., Kefalides N. A., Friedman H. M. Fc and C3 receptors induced by herpes simplex virus on cultured human endothelial cells. J Clin Invest. 1982 Jan;69(1):123–128. doi: 10.1172/JCI110422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czervionke R. L., Hoak J. C., Fry G. L. Effect of aspirin on thrombin-induced adherence of platelets to cultured cells from the blood vessel wall. J Clin Invest. 1978 Oct;62(4):847–856. doi: 10.1172/JCI109197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon C. T. The regulation of natural anticoagulant pathways. Science. 1987 Mar 13;235(4794):1348–1352. doi: 10.1126/science.3029867. [DOI] [PubMed] [Google Scholar]
- Fabricant C. G., Fabricant J., Litrenta M. M., Minick C. R. Virus-induced atherosclerosis. J Exp Med. 1978 Jul 1;148(1):335–340. doi: 10.1084/jem.148.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. M., Macarak E. J., MacGregor R. R., Wolfe J., Kefalides N. A. Virus infection of endothelial cells. J Infect Dis. 1981 Feb;143(2):266–273. doi: 10.1093/infdis/143.2.266. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Andrews C. A., Hirsch M. S. Replication of human cytomegalovirus in endothelial cells. J Infect Dis. 1984 Dec;150(6):956–957. doi: 10.1093/infdis/150.6.956. [DOI] [PubMed] [Google Scholar]
- Hoak J. C., Czervionke R. L., Fry G. L., Smith J. B. Interaction of thrombin and platelets with the vascular endothelium. Fed Proc. 1980 Jul;39(9):2606–2609. [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. J., Bültmann B. D., Gruler H. Interaction between enteroviruses and human endothelial cells in vitro. Alterations in the physical properties of endothelial cell plasma membrane and adhesion of human granulocytes. Am J Pathol. 1985 Jan;118(1):15–25. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macarak E. J., Friedman H. M., Kefalides N. A. Herpes simplex virus type 1 infection of endothelium reduces collagen and fibronectin synthesis. Lab Invest. 1985 Sep;53(3):280–286. [PubMed] [Google Scholar]
- Maruyama I., Salem H. H., Majerus P. W. Coagulation factor Va binds to human umbilical vein endothelial cells and accelerates protein C activation. J Clin Invest. 1984 Jul;74(1):224–230. doi: 10.1172/JCI111405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy L., Williamson P., Schlegel R. A. Membrane phospholipid asymmetry as a determinant of erythrocyte recognition by macrophages. Proc Natl Acad Sci U S A. 1986 May;83(10):3311–3315. doi: 10.1073/pnas.83.10.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSorley J., Shapiro L., Brownstein M. H., Hsu K. C. Herpes simplex and varicella-zoster: comparative histopathology of 77 cases. Int J Dermatol. 1974 Mar-Apr;13(2):69–75. doi: 10.1111/j.1365-4362.1974.tb01769.x. [DOI] [PubMed] [Google Scholar]
- Phinney P. R., Fligiel S., Bryson Y. J., Porter D. D. Necrotizing vasculitis in a case of disseminated neonatal herpes simplex infection. Arch Pathol Lab Med. 1982 Feb;106(2):64–67. [PubMed] [Google Scholar]
- Rosenberg R. D., Rosenberg J. S. Natural anticoagulant mechanisms. J Clin Invest. 1984 Jul;74(1):1–6. doi: 10.1172/JCI111389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal K. S., Leuther M. D., Barisas B. G. Herpes simplex virus binding and entry modulate cell surface protein mobility. J Virol. 1984 Mar;49(3):980–983. doi: 10.1128/jvi.49.3.980-983.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemerman M. B., Colton C., Morell E. Perturbations of the endothelium. Prog Hemost Thromb. 1984;7:289–324. [PubMed] [Google Scholar]
- Subak-Sharpe J. H., Brown S. M., Ritchie D. A., Timbury M. C., Macnab J. C., Marsden H. S., Hay J. Genetic and biochemical studies with herpesvirus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):717–730. doi: 10.1101/sqb.1974.039.01.085. [DOI] [PubMed] [Google Scholar]
- Tracy P. B., Eide L. L., Mann K. G. Human prothrombinase complex assembly and function on isolated peripheral blood cell populations. J Biol Chem. 1985 Feb 25;260(4):2119–2124. [PubMed] [Google Scholar]
- Van Dam-Mieras M. C., Bruggeman C. A., Muller A. D., Debie W. H., Zwaal R. F. Induction of endothelial cell procoagulant activity by cytomegalovirus infection. Thromb Res. 1987 Jul 1;47(1):69–75. doi: 10.1016/0049-3848(87)90241-6. [DOI] [PubMed] [Google Scholar]
- Vercellotti G. M., Lussenhop D., Peterson P. K., Furcht L. T., McCarthy J. B., Jacob H. S., Moldow C. F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984 Jan;103(1):34–43. [PubMed] [Google Scholar]
- Zwaal R. F., Comfurius P., van Deenen L. L. Membrane asymmetry and blood coagulation. Nature. 1977 Jul 28;268(5618):358–360. doi: 10.1038/268358a0. [DOI] [PubMed] [Google Scholar]