Abstract
Background
The performance characteristics of colonic sensation and motility measurements are unclear.
Aim
To compare left colon compliance, tone and sensation in males and females and to evaluate inter- and intra-individual coefficients of variation (COV) in these measurements.
Methods
Data were acquired using standard barostat methods, by one technologist, in 72 human volunteers (38 males, 18–65 y). We measured compliance, fasting tone and sensation during baseline and post-placebo; postprandial (PP) tone was measured only post-placebo. Compliance and thresholds for first sensation, gas and pain were measured using ascending method of limits; sensory ratings (0–100 mm VAS) using random phasic distensions at 8 to 36 mmHg above baseline operating pressure. Change in PP tone was measured by barostat balloon volume for the first 30 min after a 1000kcal meal. Inter-COV was calculated as 100 (SD/mean), and intra-COV as (100*SD delta/overall mean).
Results
There were no statistically significant associations with gender for most sensory or motor parameters at baseline. A modest association of fasting colonic tone and gender was observed. COV are lower (20–35%) for compliance, fasting tone, pain threshold, and sensation ratings than for PP tone and threshold for first or gas sensation (>45%). COV data are similar in males and females; sensation COVs appear smaller in females relative to males.
Conclusions
Testing of compliance, tone and pain and gas sensation in left colon performs adequately to assess these functions in humans. Lower COV for sensation tests among females is relevant to plan studies of drugs intended for functional GI disorders.
Keywords: sensory ratings, ascending method of limits, perception, visceral, postprandial
INTRODUCTION
Visceral hypersensitivity, or an excessive perception of visceral stimuli that are not usually painful, is considered a pathophysiological mechanism in functional bowel disorders such as the irritable bowel syndrome [IBS (1,2)] and functional dyspepsia (3). Visceral hypersensitivity is a target for the development of novel therapies modulating afferent neurotransmission (4). Standardized distensions of the rectum using balloon distension with a barostat device are currently used for the identification of hypersensitivity in IBS (2, 5–10). These same methods also provide assessment of sensorimotor function and are used in experimental therapy trials in health and disease (11). In a prior two-center study, we previously documented the performance characteristics of rectal compliance and sensation studies in healthy volunteers (12). Others have also documented the ability of the rectal barostat studies to measure rectal compliance, tone and the response to the meal (13,14). However, there are technical and anatomical reasons why evaluation of colonic function may be required instead of rectal function studies. These include the artifact introduced from weight of intra-abdominal organs or the presence of the uterus in close proximity to the rectum, which may impact on measurements of tone and compliance. There is also evidence that the rectum is associated with relatively blunted tone responses to agents, such as glucagon, which relax tone (14).
In the published literature, visceral sensitivity has been quantified by measuring the threshold, which is expressed as the pressure at which a sensation (such as urgency or pain or gas-like sensations) occurs, or the sensory rating using visual analog scales in response to graded distensions (11).
Our previous study evaluated the intra- and inter-individual variability of these measurements in the rectum (12). In contrast, the performance characteristics of measurements of colonic compliance, tone and sensation used in physiological and pharmacodynamic studies in healthy volunteers are unclear. Therefore, our aims were to compare these functions between males and females, and to evaluate intra- and inter-individual coefficient of variation (COV) of left colon compliance, tone and sensation in humans.
METHODS
Study Design and Participants
This study was based on data acquired in different pharmacodynamic studies using standard barostat methods using a rigid piston device and conducted by one technologist (I.B.), in 72 human volunteers (38 males, aged 18–65 years). All participants signed informed consent in the respective studies. In all of the studies, concurrent co-morbidities, abdominal symptoms, previous abdominal surgery and concomitant somatization or psychological disorders were excluded by means of a validated bowel symptom questionnaire, a somatic symptom checklist, and a physical exam (15). The current study, based on the combined database, was approved by the Mayo Clinic Institutional Review Board.
When the studies were conducted, treatment was double-blinded. Subsequently, the data from participants randomized to placebo were included in this study. All participants underwent examinations of compliance, fasting tone and sensation during baseline and post-placebo; postprandial tone was measured only post-placebo.
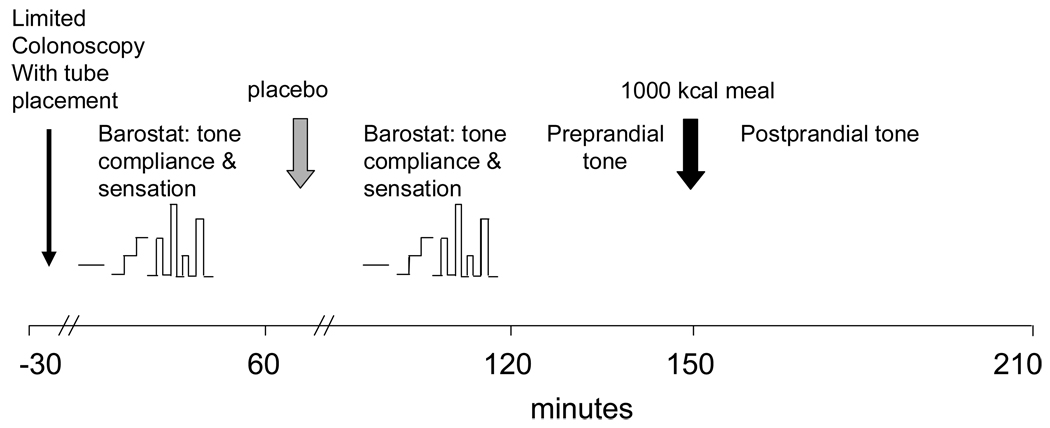
The experimental design is summarized in Figure 1. Intubation was achieved with unsedated, limited colonoscopy and fluoroscopy. All methods used in this study have been described in detail elsewhere and are summarized briefly here.
Figure 1.
Experimental Design
Establishing baseline operating pressure (BOP)
The pressure in the balloon was increased from 4 mmHg in steps of 1 mmHg for 1 minute per step until respiratory excursions were observed. The BOP was defined as 2 mmHg above the minimal distension pressure at which respiratory excursions were clearly recorded from the barostat tracing. If respiratory variations were not seen by 18 mmHg, BOP was set at 12 mmHg.
An initial “conditioning” distension of the colon was performed with pressure increased from 0 mmHg in steps of 4 mmHg for 15 seconds per step until 20 mmHg was reached. Previous studies have shown that this procedure renders subsequent assessments of compliance and perception more reproducible (16).
Ascending method of limits for compliance and sensory thresholds
Colonic compliance and sensory thresholds were measured by stepwise inflation, starting at 0 mmHg and increasing in steps of 4 mmHg for 1 minute per step to a maximum of 64 mmHg. Linear interpolation of the pressure volume curves were used to identify pressure at half maximum volume [Pr50 (17)]. Thresholds for first sensation, gas, and pain were indicated by pressing a button when respective sensations were perceived.
Random order phasic distensions: sensory ratings
Phasic distensions between 8 and 36 mmHg above BOP were applied once in random order provided by the study statistician (A.R.Z.), maintained for 60 seconds with an inter-stimulus interval of 2 minutes during which the balloon was deflated to BOP, a reliable method in which intensity ratings were generally proportional to the magnitude of the distension pressures (18–20). Subjects marked separate 100 mm visual analog scales (VAS) for the sensations of gas and pain 30 seconds after the onset of the distension. The ends of the scales were anchored by the terms ‘unnoticeable’ and ‘unbearable’. Pressure was immediately released if the subject reported greater than 80 mm of pain on the VAS scale and higher distensions were not subsequently administered. During the assessment of sensation, the interaction between the subject and the study investigator was kept to a minimum.
Fasting tone and postprandial change in colonic tone
Fasting tone for 30 minutes was measured as the baseline barostat balloon volume; measurement was obtained for two periods: during fasting at baseline and after ingestion of placebo (double-blinded treatment); and during 30 minutes after ingestion of a 1000 kcal liquid meal (milkshake). The change in barostat balloon baseline volume was an expression of the postprandial increase in tone.
Data Analysis
The following measurements were derived as the primary endpoints for analysis: (i) sensory thresholds for first sensation, gas, and pain during ascending method of limits, (ii) sensation ratings for gas and pain in response to the random phasic distensions, (iii) colonic compliance; and (iv) fasting tone and postprandial change in colonic tone.
A summary value for compliance was computed by linear interpolation on the pressure volume curve (17) to identify the pressure at one half of the maximum observed volume (Pr50), where a smaller Pr50 corresponds to higher compliance.
Statistical Analysis
The association of gender with response was assessed using analysis of covariance models (compliance, tone, VAS sensation ratings) and proportional hazards regression models (sensation thresholds) including age and BMI as covariates in each model.
Coefficients of variation
Inter-coefficient of variation (COV) was calculated as 100 (SD/mean), and intra-COV as (100*SD delta/overall mean), where the overall mean is the mean of the per subject means of the pair of values used to compute the within subject deltas.
RESULTS
Participant Demographics
A total of 38 males and 34 females were enrolled in the study and the participants were similar in regards to age, BMI and racial/ethnic background (Table 1).
Table 1.
Demographics of Healthy Volunteers Included in the Study
| Overall | Males (n=38) | Females (n=34) | |
|---|---|---|---|
| Race (% Caucasian) | 86 | 87 | 85 |
| Mean Age (SD), yr | 33 (12) | 33 (13) | 33 (11) |
| Mean BMI (SD) | 25.0 (4.0) | 25.3 (3.4) | 24.6 (4.6) |
Colonic Motor and Sensory Functions and Effect of Gender
Data are summarized in Table 2. Note that the intra-COV was not available for PP tone and the change (fasting vs. PP), since there was only comparable post (placebo) data routinely obtained for the study of meal effects in each participant. Mean and standard deviation of baseline measurements are provided, except where noted; there were no statistically significant associations with gender for any of the baseline measurement of colonic compliance, tone or sensation thresholds or ratings except for fasting tone.
Table 2.
Intra-and Inter-individual Coefficients of Variation in Colonic Motor and Sensory Testing (COV=coefficient of variation; SD=standard deviation; VAS=visual analog scale; NA=not available)
| Group | All participants, n=72 | Males, n=38 | Females, n=34 | |||
|---|---|---|---|---|---|---|
| Function | Mean± SD | % COV inter/intra |
Mean±SD | % COV inter/intra |
Mean±SD | % COV inter/intra |
| Compliance Pr50, mmHg |
19.7±4.5 | 22.8 / 17.5 | 20.6 ± 4.9 | 23.7 / 17.0 | 18.8 ± 3.9 | 20.8 / 18.2 |
| Fasting 30 min tone§, ml |
113.4±34.9 | 30.8 / 21.5 | 106 ± 31.9 | 30.2 / 19.5 | 122 ± 36.6 | 30.0 / 21.1 |
| PP 30min tone §, ml |
74.5±26.8 | 35.9 /NA | 70.7±23.4 | 33.2/NA | 78.8±29.8 | 37.9/NA |
| Δ30min PP tone§, ml |
−38.9±23.0 | 59.1/ NA | −35.1±20.9 | 59.6 /NA | −43.1±24.7 | 57.3 / NA |
| 1st threshold, mmHg |
15.6±14.4 | 91.9 / 90.2 | 17.1±15.6 | 91.4 / 84.9 | 14.0±12.9 | 92.0 / 98.1 |
| Gas threshold, mmHg |
24.0±17.5 | 72.8 / 75.9 | 24.9±18.5 | 74.1 / 72.7 | 22.9 ±16.5 | 71.7 / 71.9 |
| Pain threshold, mmHg |
42.4±13.2 | 31.0 / 30.6 | 43.6±14.4 | 33.1 / 35.4 | 41.2±11.7 | 28.4 / 27.2 |
| Sensory Ratings Gas (mm VAS) at 36mmHg distension |
53.3±22.3 | 41.9 / 36.6 | 53.8±18.5 | 34.5 / 43.1 | 52.8.±25.9 | 49.1 / 29.1 |
| Sensory Ratings Pain (mm VAS) at 36mmHg distension |
48.6±27.3 | 56.3/ 33.0 | 49.4±27.2 | 55.1 /38.3 | 47.9±28.1 | 58.6 / 27.1 |
Post (placebo) data
A modest association with gender was observed for baseline compliance (Pr50 values), p=0.080, however this was not significant after adjusting for maximum pressure level attained in the AML sequence (p=0.22). Significant associations of baseline compliance with age (p=0.043) and BMI (p=0.003) were observed even after adjusting for maximum pressure level. Similar results were observed for the post-placebo fasting data. No associations of gender with sensation VAS ratings or with sensation thresholds were detected, though age was associated with threshold levels.
A modest association of fasting tone levels and gender was observed (p=0.040), but not with PP change in tone. No associations with age or BMI and colonic tone were observed.
COVs are generally lower for compliance, fasting tone, pain threshold, and sensation ratings than for PP tone and threshold for first or gas sensation. COV data are similar in males and females; sensation COVs appear smaller in females relative to males.
Sample Size Estimates for Future Studies
Based on the interindividual COVs, we have calculated in Table 3 the sample size required to demonstrate an effect size of 25 to 35% for the most relevant endpoints. For first sensation and gas threshold, the sample size to detect a 25% effect is greater than 100 per treatment group. Therefore, this information is excluded from the table.
Table 3.
Number of Subjects (per treatment group or per disease/phenotype) Needed to Detect Listed Effect Size with ≥80% Power (2-sided α=0.05) Based on a Two-Sample t-Test.
| Response | CV inter (%) | Effect size (%) demonstrable | N per group required |
|---|---|---|---|
| Compliance Pr50 mmHg |
22.8 | 25 | 15 |
| 30 | 11 | ||
| 35 | 8 | ||
| Fasting Tone, ml | 30.8 | 25 | 25 |
| 30 | 18 | ||
| 35 | 14 | ||
| First 30 minute postprandial change in tone vs fasting, ml |
59.1 | 25 | 89 |
| 30 | 62 | ||
| 35 | 46 | ||
| Pain Threshold, mmHg |
31 | 25 | 26 |
| 30 | 18 | ||
| 35 | 14 | ||
| Gas sensation rating with 36 mmHg distension |
41.9 | 25 | 46 |
| 30 | 32 | ||
| 35 | 24 | ||
| Pain sensation rating with 36 mmHg distension |
56.3 | 25 | 81 |
| 30 | 57 | ||
| 35 | 42 |
DISCUSSION
We observed substantial reproducibility in the testing of compliance, tone and pain and gas sensation in left colon. The test performance is certainly adequate to assess these colonic functions in humans. The performance of sensation thresholds and postprandial responses are less reproducible, though the intra-individual COV is smaller than inter-individual COV, suggesting that crossover studies might be preferable for studies of sensation thresholds and postprandial tone. These data provide the basis for calculating sample sizes for future studies. Moreover, the lower COV for sensation tests among females is relevant to plan pharmacodynamic studies of drugs intended for patients with functional gastrointestinal disorders. Interestingly, there were no statistically significant gender differences in these sensory and motor endpoints.
The importance of this study is that it establishes the performance characteristics of different measurements of colonic motor and sensory functions in humans. These observations are relevant in research studies that evaluate the pharmacodynamic effects of novel medications. Moreover, measurements of colonic compliance and tone are also used in evaluating colonic motor function in adult patients, such as those with significant constipation, as reported in a recent consensus document on intraluminal motility measurements (21). Studies of colonic motor function have also demonstrated clinical utility in pediatric gastroenterology practice. For example, colonic motility tests facilitate the selection of medical and surgical treatment when conventional medical and behavioral treatments have failed (22, 23). Colonic motility tests may also be used to better understand the cause of persistent symptoms after removal of the aganglionic segment in children with Hirschsprung’s disease (24), to evaluate the function of the colon after diversion colostomy prior to closure of the stoma (25) and to predict the response to antegrade enemas via cecostomy (26).
Rectal sensation has been proposed as a biomarker for irritable bowel syndrome (11) and the performance of the studies has been tested in a two-center study conducted at University of Manchester, England and Mayo Clinic (12). The current paper provides the first documentation of the performance of sensory testing in the colon proximal to the rectum. While the study has a number of strengths including conduct by a single operator and the relatively large number of people evaluated, there are inherent weaknesses: first, results are not necessarily generalizable to other centers and second, they do not present inter-center variability data. The relevance of sensory testing to clinical practice may still be debatable since hypersensitivity is documented in a range of IBS patients from 21% to 94% in the published literature (2,5,8,9,27,28). However, if such sensation tests are to be used in diagnostic or research studies, it is clear that the performance, normal range and coefficient of variation are key properties of interest.
There are other techniques that are proposed to measure rectosigmoid colonic sensation. A multimodal method was tested in 12 healthy volunteers (six men mean age 32 years) on two subsequent days (29). Stimulation with electrical, thermal and mechanical modalities resulted in different sensory perceptions; however, the relationship between stimulus intensity and sensory response was linear for all modalities, and sensory response to different modalities did not differ between investigation days. It is interesting to note that, for sensory threshold, the cross-sectional area showed the best reproducibility (intra-class correlation, ICC ≥ 0.9) and, at pain detection threshold, stretch ratio, CSA and electrostimulation showed best reproducibility (ICC = 1.0; 0.9; 0.9 respectively). On the other hand, cross-sectional area in the rectosigmoid maybe constrained by the effect of the uterus, if it is retroverted or has unsuspected myomas. Further studies in the colon, using this interesting multimodal approach are awaited.
One of the constraints of the current study is that reproducibility was tested on the same day. While we cannot exclude the possibility that reproducibility may have differed if the tests were each conducted on separate days, the previous data for rectal sensitivity in a two-center study (12) and using multimodal rectal stimulation (29) suggests that the results on one day generally reflect the reproducibility observed when studies are repeated on different days.
Table 4 shows a review of papers in the literature (with far smaller sample sizes than documented in our current study) that provide information on coefficient of variation for a few of the sensation endpoints specifically, thresholds for sensation in the papers published in the literature; none of the studies actually provide information on colonic compliance and postprandial tone or sensation ratings. Note that the COVs for first sensation and gas thresholds are generally larger, as observed in our study (30–32). In the study by Tack et al., two measurements were obtained at least 2 weeks apart (32), and the COVs appear to be much lower than in the study from Simren et al. and the current study.
Table 4.
Literature Review of Studies Showing Coefficient of Variation and Effect Sizes for Estimating Effects on Threshold Sensations
| Thresholds | N | mean | SD | COV, % | Effect size demonstrable With >80% power † |
Reference No. |
|---|---|---|---|---|---|---|
| First Sensation | 13 | 26.4 | 9.01 | 34.14 | 35% | Simren (30) |
| Gas | 13 | 36.4 | 10.81 | 29.70 | 30% | |
| Discomfort | 13 | 43.3 | 8.29 | 19.15 | 20% | |
| Pain | 13 | 46.4 | 5.41 | 11.66 | 12% | |
| Pain | 34 | 36.61 | 10.61 | 28.99 | 19% | Grover (31) |
| First Perception (Day 1) |
10 | 7.1 | 3.48 | 48.99 | 56% | Tack (32) |
| First Perception (Day 2 - preinfusion) |
10 | 9.6 | 4.11 | 42.82 | 49% |
Based on a 2-sample t-test (2-sided alpha level of 0.05) assuming the listed sample size per group(N) . Effect size is the difference in group means divided by the overall mean (in % scale)
The observations in our study apply to healthy subjects and may not be generalizable to IBS patients, in whom anxiety or fear of pain levels may be higher and in whom increased perception of non-noxious sensations has been reported (33). It is also conceivable that variability may be significantly greater in IBS patients due to a variety of factors unrelated to the stimulus.
In conclusion, we have reported the largest set of reproducibility data on colonic sensation and motility in humans to date, and we find that the results in healthy volunteers are generally reproducible, particularly for measurements of fasting tone, compliance and sensation ratings and pain threshold. Though reproducibility of other thresholds and postprandial tone response is not as good, assessment of variation allows for the use of these endpoints in adequately sized studies of experimental therapies or in studies comparing health and diseases such as irritable bowel syndrome. The lower intra-COV for sensations in females supports the use of these endpoints and methods in pharmacodynamic proof-of-concept studies or in dose-response studies in healthy volunteers. The lower COV for sensation tests among females is relevant to plan studies of drugs intended for functional GI disorders. Such studies may be useful prior to pursuing similar pharmacodynamic studies or clinical trials in patients with functional gastrointestinal disorders. The availability of such biomarkers is identified as an item of priority interest for the development of novel treatments in patients with lower functional gastrointestinal disorders, like irritable bowel syndrome (34).
Acknowledgments
Dr. Camilleri’s work on colon function is supported by grants R01-DK67071 and K24-DK02638 from National Institutes of Health. Dr Papathanasopoulos is supported by an international grant of the Hellenic Society of Gastroenterology
Contributor Information
Suwebatu T. Odunsi, Clinical Enteric Neuroscience Translational and Epidemiological Research (C.E.N.T.E.R.), Mayo Clinic, Rochester, Minnesota
Michael Camilleri, Clinical Enteric Neuroscience Translational and Epidemiological Research (C.E.N.T.E.R.), Mayo Clinic, Rochester, Minnesota.
Adil E. Bharucha, Clinical Enteric Neuroscience Translational and Epidemiological Research (C.E.N.T.E.R.), Mayo Clinic, Rochester, Minnesota
Athanasios Papathanasopoulos, Clinical Enteric Neuroscience Translational and Epidemiological Research (C.E.N.T.E.R.), Mayo Clinic, Rochester, Minnesota.
Irene Busciglio, Clinical Enteric Neuroscience Translational and Epidemiological Research (C.E.N.T.E.R.), Mayo Clinic, Rochester, Minnesota.
Duane Burton, Clinical Enteric Neuroscience Translational and Epidemiological Research (C.E.N.T.E.R.), Mayo Clinic, Rochester, Minnesota.
Alan R. Zinsmeister, Department of Health Sciences Research, Division of Biostatistics, College of Medicine, Mayo Clinic, Rochester, Minnesota
REFERENCES
- 1.Lembo T, Munakata J, Mertz H, Niazi N, Kodner A, Nikas V, Mayer EA. Evidence for the hypersensitivity of lumbar splanchnic afferents in irritable bowel syndrome. Gastroenterology. 1994;107:1686–1696. doi: 10.1016/0016-5085(94)90809-5. [DOI] [PubMed] [Google Scholar]
- 2.Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 3.Mearin F, Cucala M, Azpiroz F, Malagelada J-R. The origin of symptoms on the brain-gut axis in functional dyspepsia. Gastroenterology. 1991;101:999–1006. doi: 10.1016/0016-5085(91)90726-2. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri M. Management of the irritable bowel syndrome. Gastroenterology. 2001;120:652–668. doi: 10.1053/gast.2001.21908. [DOI] [PubMed] [Google Scholar]
- 5.Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganiere M, Verrier P, Poitras P. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771–1777. doi: 10.1053/gast.2002.33601. [DOI] [PubMed] [Google Scholar]
- 6.Bouin M, Lupien F, Riberdy M, et al. Intolerance to visceral distension in functional dyspepsia or irritable bowel syndrome: an organ specific defect or a pan intestinal dysregulation? Neurogastroenterol Motil. 2004;16:311–314. doi: 10.1111/j.1365-2982.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- 7.Lembo T, Naliboff B, Munakata J, et al. Symptoms and visceral perception in patients with pain-predominant irritable bowel syndrome. Am J Gastroenterol. 1999;94:1320–1326. doi: 10.1111/j.1572-0241.1999.01009.x. [DOI] [PubMed] [Google Scholar]
- 8.Posserud I, Syrous A, Lindström L, et al. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology. 2007;133:1113–1123. doi: 10.1053/j.gastro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Dorn SD, Palsson OS, Thiwan SI, et al. Increased colonic pain sensitivity in irritable bowel syndrome is the result of an increased tendency to report pain rather than increased neurosensory sensitivity. Gut. 2007;56:1202–1209. doi: 10.1136/gut.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steens J, Van Der Schaar PJ, Penning C, Brussee J, Masclee AA. Compliance, tone and sensitivity of the rectum in different subtypes of irritable bowel syndrome. Neurogastroenterol Motil. 2002;14:241–247. doi: 10.1046/j.1365-2982.2002.00332.x. [DOI] [PubMed] [Google Scholar]
- 11.Whitehead WE, Delvaux M. Dig Dis Sci. Vol. 42. UK: The Working Team of Glaxo-Wellcome Research; 1997. Standardization of barostat procedures for testing smooth muscle tone and sensory thresholds in the gastrointestinal tract; pp. 223–241. [DOI] [PubMed] [Google Scholar]
- 12.Cremonini F, Houghton LA, Camilleri M, Ferber I, Fell C, Cox V, Castillo EJ, Alpers DH, Dewit OE, Gray E, Lea R, Zinsmeister AR, Whorwell PJ. Barostat testing of rectal sensation and compliance in humans: comparison of results across two centres and overall reproducibility. Neurogastroenterol Motil. 2005;17:810–820. doi: 10.1111/j.1365-2982.2005.00709.x. [DOI] [PubMed] [Google Scholar]
- 13.Grotz RL, Pemberton JH, Levin KE, Bell AM, Hanson RB. Rectal wall contractility in healthy subjects and in patients with chronic severe constipation. Ann Surg. 1993;218:761–768. doi: 10.1097/00000658-199312000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell AM, Pemberton JH, Hanson RB, Zinsmeister AR. Variations in muscle tone of the human rectum: recordings with an electromechanical barostat. Am J Physiol. 1991;260:G17–G25. doi: 10.1152/ajpgi.1991.260.1.G17. [DOI] [PubMed] [Google Scholar]
- 15.Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–674. doi: 10.7326/0003-4819-111-8-671. [DOI] [PubMed] [Google Scholar]
- 16.Hammer HF, Phillips SF, Camilleri M, Hanson RB. Rectal tone, distensibility, and perception: reproducibility and response to different distensions. Am J Physiol. 1998;274:G584–G590. doi: 10.1152/ajpgi.1998.274.3.G584. [DOI] [PubMed] [Google Scholar]
- 17.Floyd BNI, Camilleri M, Andresen V, Esfandyari T, Busciglio I, Zinsmeister AR. Comparison of mathematical methods for calculating colonic compliance in humans: power exponential, computer-based, and manual linear interpolation models. Neurogastroenterol Motil. 2008;20:330–335. doi: 10.1111/j.1365-2982.2007.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ford MJ, Camilleri M, Zinsmeister AR, Hanson RB. Psychosensory modulation of colonic sensation in the human transverse and sigmoid colon. Gastroenterology. 1995;109:1772–1780. doi: 10.1016/0016-5085(95)90743-2. [DOI] [PubMed] [Google Scholar]
- 19.Bharucha AE, Camilleri M, Zinsmeister AR, Hanson RB. Adrenergic modulation of human colonic motor and sensory function. Am J Physiol. 1997;273:G997–G1006. doi: 10.1152/ajpgi.1997.273.5.G997. [DOI] [PubMed] [Google Scholar]
- 20.Delgado-Aros S, Chial HJ, Camilleri M, Szarka LA, Weber FT, Jacob J, Ferber I, McKinzie S, Burton DD, Zinsmeister AR. Effects of a kappa-opioid agonist, asimadoline, on satiation and GI motor and sensory functions in humans. Am J Physiol. 2003;284:G558–G566. doi: 10.1152/ajpgi.00360.2002. [DOI] [PubMed] [Google Scholar]
- 21.Camilleri M, Bharucha AE, di Lorenzo C, Hasler WL, Prather CM, Rao SS, Wald A. American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterol Motil. 2008;20:1269–1282. doi: 10.1111/j.1365-2982.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- 22.Pensabene L, Youssef NN, Griffiths JM, Di Lorenzo C. Colonic manometry in children with defecatory disorders. Role in diagnosis and management. Am J Gastroenterol. 2003;98:1052–1057. doi: 10.1111/j.1572-0241.2003.07412.x. [DOI] [PubMed] [Google Scholar]
- 23.Martin MJ, Steele SR, Mullenix PS, Noel JM, Weichmann D, Azarow KS. A pilot study using total colonic manometry in the surgical evaluation of pediatric functional colonic obstruction. J Pediatr Surg. 2004;39:352–359. doi: 10.1016/j.jpedsurg.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 24.Di Lorenzo C, Solzi GF, Flores AF, Schwankovsky L, Hyman PE. Colonic motility after surgery for Hirschsprung’s disease. Am J Gastroenterol. 2000;95:1759–1764. doi: 10.1111/j.1572-0241.2000.02183.x. [DOI] [PubMed] [Google Scholar]
- 25.Villarreal J, Sood M, Zangen T, Flores A, Michel R, Reddy N, Di Lorenzo C, Hyman PE. Colonic diversion for intractable constipation in children: colonic manometry helps guide clinical decisions. J Pediatr Gastroenterol Nutr. 2001;33:588–591. doi: 10.1097/00005176-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 26.van den Berg MM, Hogan M, Caniano DA, Di Lorenzo C, Benninga MA, Mousa HM. Colonic manometry as predictor of cecostomy success in children with defecation disorders. J Pediatr Surg. 2006;41:730–736. doi: 10.1016/j.jpedsurg.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Camilleri M, McKinzie S, Busciglio I, Low PA, Sweetser S, Burton D, Baxter K, Ryks M, Zinsmeister AR. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–781. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Veek PP, Van Rood YR, Masclee AA. Symptom severity but not psychopathology predicts visceral hypersensitivity in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:321–328. doi: 10.1016/j.cgh.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Brock C, Nissen TD, Gravesen FH, Frøkjaer JB, Omar H, Gale J, Gregersen H, Svendsen O, Drewes AM. Multimodal sensory testing of the rectum and rectosigmoid: development and reproducibility of a new method. Neurogastroenterol Motil. 2008;20:908–918. doi: 10.1111/j.1365-2982.2008.01126.x. [DOI] [PubMed] [Google Scholar]
- 30.Simrén M, Abrahamsson H, Björnsson ES. An exaggerated sensory component of the gastrocolonic response in patients with irritable bowel syndrome. Gut. 2001;48:20–27. doi: 10.1136/gut.48.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grover M, Kanazawa M, Palsson OS, Chitkara DK, Gangarosa LM, Drossman DA, Whitehead WE. Small intestinal bacterial overgrowth in irritable bowel syndrome: association with colon motility, bowel symptoms, and psychological distress. Neurogastroenterol Motil. 2008;20:998–1008. doi: 10.1111/j.1365-2982.2008.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tack J, Broekaert D, Corsetti M, Fischler B, Janssens J. Influence of acute serotonin reuptake inhibition on colonic sensorimotor function in man. Aliment Pharmacol Ther. 2006;23:265–274. doi: 10.1111/j.1365-2036.2006.02724.x. [DOI] [PubMed] [Google Scholar]
- 33.Holtmann G, Goebell H, Talley NJ. Functional dyspepsia and irritable bowel syndrome: is there a common pathophysiological basis? Am J Gastroenterol. 1997;92:954–959. [PubMed] [Google Scholar]
- 34.Camilleri M, Chang L. Challenges to the therapeutic pipeline for irritable bowel syndrome: end points and regulatory hurdles. Gastroenterology. 2008;135:1877–1891. doi: 10.1053/j.gastro.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]