Abstract
Colorectal cancer is the third leading cause of cancer-related deaths in the United States. Novel prevention or therapeutic agents are needed to better manage this disease. American ginseng is a commonly used herb and is believed to have lots of health benefits, including anticancer activities. However there have been very few in-depth studies of the activities of this herb at the molecular level. In this report we showed that 4 hour-steamed American ginseng root extract (S4h) induced mitochondrial damage, increased reactive oxygen species (ROS), and apoptosis in colorectal cancer cells. We showed that the NF-κB pathway was activated by S4h and that removal of ROS inhibited S4h-induced NF-κB activation. We further showed that both antioxidants and a specific inhibitor of the NF-κB pathway enhanced S4h-induced cell death. Finally, we showed that protecting the mitochondria decreased both the level of ROS and apoptosis. Taken together, these results indicate that S4h-induced apoptosis in colorectal cancer cells is mediated by mitochondria damage and that damage to the mitochondria activates both the apoptosis pathway and the ROS/NF-κB mediated survival pathway. These results further suggest that the anticancer effect of steamed ginseng can be enhanced by antioxidants or inhibitors of the NF-κB pathway.
Keywords: American ginseng, apoptosis, ROS, NF-κB, antioxidant
1. Introduction
Colorectal cancer is the third leading cause of cancer-related deaths in the United States [1, 2]. Current treatment of this cancer generally employs surgical resection combined with chemotherapy using cytotoxic drugs and radiation therapy. Because this therapy is only moderately successful for late stage cancers, novel approaches to the treatment of colorectal cancer are required. Natural products have been a valuable source of new therapeutic candidate compounds. Plant-derived active principles and their semi-synthetic and synthetic analogs have served as one of the major sources for anti-cancer drugs [3, 4]. Therefore studies of plant-derived nature products can potentially provide new leads for novel anticancer agents.
Ginseng refers to several plants within Panax L., a genus of 12 species of slow-growing perennial plants with fleshy roots, in the family Araliaceae [5]. The commonly used ginseng principally includes Panax ginseng (Asian ginseng) and Panax quinquefolius (American ginseng) [6, 7]. Asian ginseng has a long history of being used as herbal medicine in oriental countries. A number of biological actions of Asian ginseng that are believed to be beneficial for health have been described [7-10]. In the 1990s, a case-control study on over a thousand Korean subjects showed that long-term ginseng consumption was associated with a decreased risk for many different malignancies compared with those who did not consume ginseng [11, 12], suggesting that Asian ginseng has anti-tumor activities.
Recently, we reported that extracts of steamed American ginseng root exerted potent anti-tumor effects in colorectal cancer cells and altered expression of genes in several pathways [13-15]. The increased anti-tumor effect of steamed ginseng was correlated with significant changes in the principal ginsenosides and mediated largely through the induction of apoptosis [14, 16, 17]. Apoptosis may occur via a death receptor-dependent extrinsic mechanism or a death receptor-independent mitochondrial mechanism [18, 19]. The mitochondrial pathway of apoptosis is mediated by the release of a number of factors from mitochondria, such as the cytochrome c, Smac/Diablo, and apoptosis-inducing factor, which will activate caspase cascades and promote apoptosis. Reactive oxygen species (ROS) is a natural byproduct of normal metabolism of oxygen in the mitochondria and can accumulate to high levels under certain conditions such as the disruption of normal mitochondrial function. ROS can function as an important signaling module to activate various pathways involved in the apoptotic or survival pathway [20]. One signaling pathway activated by ROS is the NF-κB pathway [21, 22]. NF-κB is a transcriptional factor, sequestered and inactivated in the cytoplasm by binding to IκB. Activation of the NF-κB pathway is mediated by the activation of the IκB kinase complex (IKK), which leads to the phosphorylation and degradation of IκB [23]. NF-κB is an anti-apoptotic transcription factor that regulates the expression of a number of genes whose products inhibit apoptosis [23].
In the present study, we showed that 4-hour-steamed American ginseng root extract (S4h) not only induced the apoptosis but also significantly increased intracellular ROS levels and caused mitochondria damage in colorectal cancer cells. Our results showed that S4h induced apoptosis and ROS induction was mediated, at least in part, by its effect on the mitochondria. Increased levels of ROS activated the NF-κB pathway and protected colorectal cancer cells from apoptosis induced by S4h. Importantly, antioxidants could decrease the level of ROS and enhanced S4h induced apoptosis of colorectal cancer cells.
2. Methods and Materials
2.1 Chemicals and reagents
N-Acetyl-L-cysteine (NAC), Vitamin C, and PS1145 were obtained from Sigma. NAC and vitamin C, which are antioxidants, were dissolved in the growth medium. PS1145, which is a specific inhibitor of NF-κB pathway, was dissolved in DMSO as a 20 mM stock buffer. Luciferase assay kits were obtained from Promega. Anti IκBα and anti β-actin antibodies were obtained from Cell Signaling Technology. Annexin V Apoptosis Kit was purchased from BD Biosciences. ROS dyes H2DCFDA (2′, 7′-dichlorodihydrofluorescein diacetate and JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide) were obtained from Invitrogen. Steamed American ginseng root extract (S4h) was prepared as previously described [24] with the following modifications. Briefly, fresh American ginseng roots were steamed at 120°C for 4 h, and then were lyophilized to obtain dried samples. The dried roots were ground and extracted with 70% ethanol. The solvent of the extract solution was evaporated under vacuum. The dried extract was dissolved in water; and then extracted with water-saturated n-butanol. The n-butanol phase was evaporated under vacuum and then lyophilized. The lyophilized sample was dissolved in DMSO as S4h for biological studies.
2.2 Ginsenoside analysis
Ginsenoside contents in S4h were determined by using high performance liquid chromatography (HPLC). The separation was carried out on an Alltech Ultrasphere C18 column (5 μm, 250 × 3.2 mm I.D) with a C18 guard column (5 μm, 7.5 × 3.2 mm I.D.). For the mobile phase, acetonitrile (solvent A) and water (solvent B) were used. Gradient elution solvent started with 18% solvent A and 82% solvent B. Eluting solvent was changed to 21% A for 20 min, then to 26% A for 3 min and held for 19 min. It was then changed to 36% A for 13 min, to 50% A for 9 min, to 95% A for 2 min, and held for 3 min, to 18% A for 3 min and held for 8 min. The detection wavelength was set to 202 nm. The linearity of this method was assayed by analyzing standard solutions in the range of 2-400 μg/ml for the 12 ginsenosides. The ginsenoside content in S4h was calculated using standard curves of ginsenosides.
2.3 Cell culture
Human colorectal cancer cells HCT116 and SW480 were obtained from the American Type Culture Collection. HCT116 cells were maintained in McCoy’s 5A medium supplemented with 10% fetal bovine serum (FBS, Hyclone Laboratories), 50 IU of penicillin/streptomycin (Gemini Bio-Products) and 2 mmol/L of L-glutamine (Invitrogen) in a humidified atmosphere with 5% CO2 at 37°C. SW480 cells were grown in DMEM containing 10% FBS, penicillin/streptomycin, and L-glutamine.
2.4 FACS analysis
For the apoptosis assay, 25×104 cells were seeded into each well of the 6-well plates. Apoptosis assays were carried out based on the instruction from the Annexin V Apoptosis Kit (BD Biosciences). Briefly, cells were collected and washed twice with binding buffer containing 10 mM HEPES, pH 7.4, 140 mM NaCl, 2.5 mM CaCl, and then resuspended at a concentration of 1×106 cells/ml in binding buffer. 100 μl of the cell suspension was mixed with 5 μl of Annexin V-FITC and 10 μl of propidium iodide (50 μg/ml stock) and incubated at room temperature for 15 min. 400 μl of binding buffer was added to each assay after the incubation and apoptotic cells were determined using a FACScan (BD Biosciences). Since S4h induced cell death is mostly apoptosis [16], total Annexin V-positive cells were used to determine the level of apoptosis.
Intracellular ROS production was monitored by the permeable fluorescence dye, H2DCFDA. H2DCFDA can readily react with ROS to form the fluorescent product 2,7-dichlorofluorescein (DCF) [25]. The intracellular fluorescence intensity of DCF is proportional to the amount of ROS generated by the cells [26]. After the indicated treatment, the cells were incubated with 10 μM of H2DCFDA dissolved in PBS for thirty minutes and then cells were harvested and resuspended in PBS (106 cells/mL). The fluorescence intensity of intracellular DCF (excitation 488 nm, emission 530nm) was measured using FACScan. For analysis of the correlation between apoptosis and ROS, cells were stained with H2DCFDA and annexin V-Cy5 (excitation 640 mm, emission 670 nm), and the samples were analyzed by Flow cytometry LSR II-blue (BD Biosciences).
The mitochondrial membrane potential (MMP) was monitored by the MMP probe JC-1 dye, which emits red fluorescence (590 nm, FL2-H, Red/Orange) under high MMP and green fluorescence (525 nm) under low MMP conditions [27]. The MMP assay was carried out according to the previous report [28] with a few modifications. Briefly, after desired treatments, cells were collected, resuspended in complete medium containing 2.5 μM of completely dissolved JC-1. The mixture was incubated at 37°C, 5% CO2 for 30 minutes. Cells were collected, resuspended in 0.5 ml of PBS for Flow assay. All the data analyses were performed using FlowJo analysis software, version 6.0 (Tree Star)
For the fluorescence microscope, cells are seeded in 8-well chamber for 24h. After the desired treatments, old medium was removed and fresh medium containing 2.5 μM of completely dissolved JC-1 was added. Cells were incubated for 30 min before processed for imaging under a Zeiss fluorescence microscope.
2.5 Western blot and Luciferase activity assay
After desired treatments as specified in the Results section, cells were washed twice with PBS, lysed in buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 m M EDTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM sodium vanadate, 1 μg/ml leupeptin, 1mM phenylmethylsulfonylfluoride). Equal amounts of protein were loaded. Western detection was carried out using a Li-Cor Odyssey image reader. The goat anti-mouse IgG (680 nm) and goat anti-rabbit IgG (800 nm) secondary antibodies were obtained from Li-Cor.
For Luciferase activity assay, plasmids containing luciferase reporter gene with or without a NF-κB response element and phRL-TK plasmid for the transfection control were gifts from Liao’s lab (Ben May Department for Cancer Research, University of Chicago). 104 cells were seeded into 48-well plates for 24h and were co-transfected with 0.5 μg of plasmid containing report construct and 10 ng of phRL-TK using transfection reagent Effectene (Qiagen). At 24h post-transfection, the cells were treated as desired. Luciferase activity was measured with a commercial kit (Promega Dual luciferase II) on a Monolight luminometer (Becton Dickinson).
2.6 Lentivirus generation and infection
Human catalase cDNA (from pC1-catalase, gift from Dr. Jian Wu, UC Davis Medical Center) and human Bcl-XL cDNA (from pBSSK-Bcl-XL, gift from Kay Macleod, University of Chicago) were subcloned into the lentiviral expressing vector pCDH-CMV-EF1-puro (System Biosciences). Viral packaging was done according to the previously described protocol [29]. Briefly, expressing plasmid pCDH-catalse-myc or pCDH-Bcl-XL-myc, pCMV-dR8.91, and pCMV-VSV-G were co-transfected into 293T cells using the Calcium Phosphate method at 20:10:10 μg (for a 10-cm dish). The transfection medium containing calcium phosphate and plasmid mixture was replaced with fresh complete medium after incubation for 5 hours. Media containing virus was collected 48 h after transfection and then concentrated using 20% sucrose buffer at 20000g for 4 hours. The virus pellet was re-dissolved in the proper amount of complete growth medium and stocked at −80°C. Colorectal cancer cells were infected with the viruses at the titer of 100% infection in the presence of Polybrene (5μg/ml) for 48 hours, and were treated as desired.
3. Results
3.1 HPLC analysis of ginsenoside composition in S4h
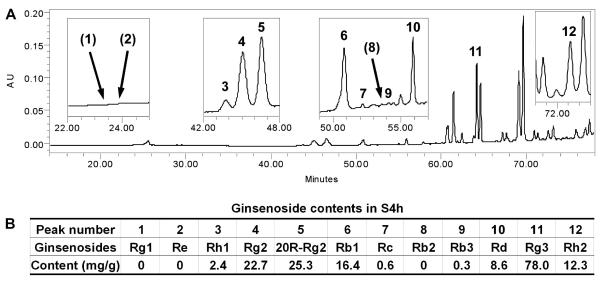
The main bioactive compounds in American ginseng are believed to be dammarane saponins, commonly referred to as ginsenosides [6, 7]. Ginsenosides from American ginseng have been proved to be effective for the treatment of many diseases, including cancer [8, 13, 15]. In addition to the ginsenosides Re, Rb1, Rc and Rd, which are major constituents in unsteamed American ginseng [14, 24], we also determined the ginsenosides Rg1, Rh1, Rg2, 20R-Rg2, Rb2, Rb3, Rg3 and Rh2. As shown in Fig. 1A, ginsenoside Rg3 is the major constituent in S4h, while the peak areas of ginsenosides Rb1, Rc and Rd were relatively small. Analytical data were shown in Fig. 1B. The content of three major constituents in S4h were ginsenosides Rg3 (78.0 mg/g), Rg2 (22.7 mg/g) and 20R-Rg2 (25.3 mg/g). Ginsenosides Rg1, Re and Rb2 were not detected.
Figure 1. Quantitative determination of ginsenosides in S4h using High-Performance Liquid Chromatography (HPLC).
(A) HPLC chromatogram of S4h recorded at 202 nm. Four group peaks are enlarged and the retention times indicated under the enlarged window (inset). (B) Analytical data of S4h. The major constituents in S4h were ginsenosides Rg3, Rg2 and 20R-Rg2. Ginsenosides Rg1, Re and Rb2 were not detected. Ginsenoside peak numbers in HPLC chromatogram (A) were shown in (B).
3.2 S4h-induced apoptosis in colorectal cancer cells is accompanied by decreased mitochondrial membrane potential and increased ROS generation
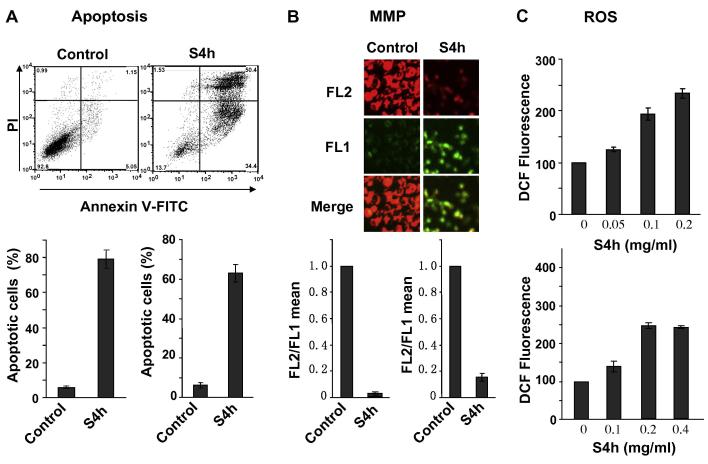
Consistent with previous reports that steamed American ginseng root extracts displayed potent anti-cancer effects and induced apoptosis, treatment of HCT116 and SW480 colorectal cancer cells with S4h induces significant levels of apoptosis [14-17](Fig. 2A). Since mitochondria permeability is often linked with the induction of apoptosis, we further characterized the effect of S4h on mitochondria membrane potential using the fluorescent cationic dye, JC-1. In non-apoptotic cells, the negative charge established by the intact mitochondrial membrane potential allows the JC-1 to accumulate in the mitochondria and forms “J-aggregates” that become fluorescent red. By contrast, in apoptotic cells the mitochondrial membrane potential collapses and JC-1 remains in the cytoplasm in a monomeric form that exhibits green fluorescence [28]. As shown in the top panel of Figure 2B, the red fluorescence was greatly decreased while the green fluorescence increased after HCT116 cells were treated with 0.2 mg/ml of S4h for 24h. Since several factors, such as mitochondrial size, shape, and density, may influence single-component fluorescence signals, we used the ratio of red-to-green JC-1 fluorescence to quantitatively describe the mitochondrial membrane potential. Quantification by flow cytometry (Figure 2B, bottom panel) showed the ratio of red to green (FL2/FL1) of JC-1 fluorescence was significantly decreased after either HCT116 or SW480 cells were treated with S4h, indicating that S4h treatment led to loss of mitochondrial membrane potential.
Figure 2. S4h induced apoptosis, ROS, and mitochondria damage in colorectal cancer cells.
(A) HCT116 (top and bottom left) and SW480 (bottom right) cells were treated with S4h for 24 hours, and apoptosis was quantified by FACS after staining with Annexin V-FITC and Propidium Iodide. The top are representative flow density plots for HCT116 cells. (B) Mitochondrial Membrane Potential (MMP) was determined by the JC-1 probe after colorectal cancer cells were treated under the conditions as in panel A. Red fluorescence (FL2) indicates high MMP, while green fluorescence (FL1) indicates low MMP. The top image is for HCT116, and the bottom graphs are for HCT116 (left) and SW480 (right). (C) ROS was determined by FACS based on DCF fluorescence. HCT116 (top) and SW480 (bottom) cells were treated with different concentrations of S4h for 24h. All the data were repeated at least three times. Unless otherwise indicated, S4h used for HCT116 and SW480 cells were 0.2 mg/ml and 0.4 mg/ml, respectively.
Increased apoptosis and mitochondria damage are also often associated with ROS accumulation, which can be detected by the ROS-sensitive fluorescence dye, H2DCFDA [25]. Indeed, treatment of HCT116 cells with S4h led to increased ROS accumulation in a concentration-dependent manner (Figure 2C top). After 24 hours of S4h treatment, an average increase of 2-3 fold in ROS was observed in both HCT116 and SW480 cancer cells (Figure 2C).
3.3 ROS level in S4h-treated colorectal cancer cells negatively correlated with apoptosis
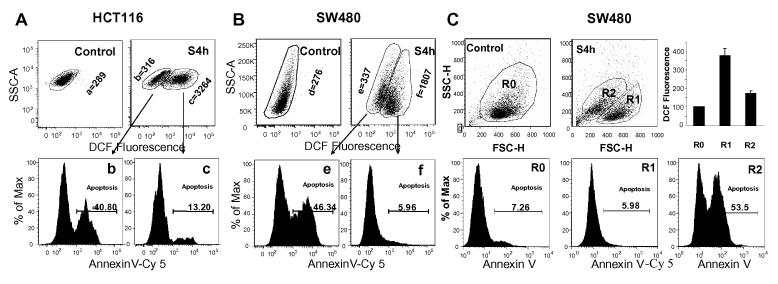
HCT116 and SW480 cells treated with S4h for 8 hours displayed two distinct populations of cells in terms of their intracellular ROS levels, as revealed by staining with the ROS dye H2DCFDA (top panels of Figures 3A and 3B). Since increased ROS can either induce apoptosis or activate survival pathways that protect cells from apoptosis in different cells [20], we analyzed how the level of ROS in S4h-treated colorectal cancer cells correlated with apoptosis as determined by Annexin V staining. Interestingly, while only low levels of apoptosis were observed in S4h-treated HCT116 and SW480 cells that have high ROS levels, much higher levels of apoptosis were observed in S4h-treated HCT116 and SW480 cells that have low ROS levels (Fig 3A and 3B, lower panels). These results indicate that ROS levels in S4h-treated colorectal cancer cells are negatively correlated with the level of apoptosis. To further confirm this observation, we analyzed different groups of cells based on cell size (FSC) and cell shape (SSC) for their levels of apoptosis and ROS because apoptosis often lead to changes in these parameters. As shown in Fig.3C top panels, SW480 cells treated S4h for 24 hours exhibited two different FSC/SSC groups, R1 and R2. Annexin V staining showed that R1 group cells exhibited little apoptosis as did untreated control R0 group cells. In contrast, R2 group cells exhibited very high levels of apoptosis (Fig. 3C bottom panels). Interestingly the ROS level in the non-apoptotic R1 group was about 2 fold higher than that in the highly apoptotic R2 group cells (Fig. 3C top right). These observations provide further support for the observation that ROS level in S4h-treated colorectal cancer cells negatively correlated with induction of apoptosis and suggest the possibility that ROS may protect S4h-treated colorectal cancer cells from apoptosis.
Figure 3. ROS level was negatively correlated with S4h-induced apoptosis.
HCT116 (A) and SW480 (B) were treated with S4h for 8 hours. S4h-treated cells were then collected and stained with H2DCFDA and Annexin V-Cy5 at the same time. Apoptosis was analyzed for both ROS-high and ROS-low cells based on DCF fluorescence. The geometrical mean of DCF fluorescence intensity for each group cells are indicated. (C) SW480 cells were treated with 0.3 mg/ml of S4h for 24 hours and were analyzed for ROS and apoptosis based on cell size (FSC) and cell shape (SSC) parameters.
3.4 Reducing ROS level in S4h-treated colorectal cancer cells increases apoptosis
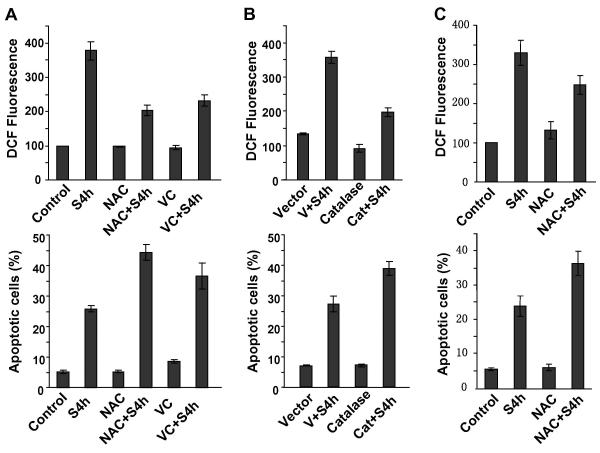
To test the possibility that ROS protected S4h-treated colorectal cancer cells from apoptosis, we tested the effect of two common antioxidants, N-acetyl cysteine (NAC) and Vitamin C, on the levels of ROS and apoptosis in S4h-treated colorectal cancer cells. As shown in Figure 4A, NAC and Vitamin C both decreased S4h-induced ROS in HCT116 cells and increased apoptosis. Furthermore overexpression of catalase, a ROS scavenging enzyme, also decreased S4h-induced ROS and increased apoptosis in HCT116 colorectal cancer cells (Figure 4B). Additionally, similar results were also obtained for SW480. As shown in Figure 4C, NAC blocked S4h-induced ROS and increased S4h-induced apoptosis. In conclusion, all these results showed that the high level of ROS in S4h-treated colorectal cancer cells protected these cells from undergoing apoptosis.
Figure 4. Decreasing the level of ROS increased S4h-induced apoptosis.
(A) HCT116 cells were treated with S4h with or without 5 mM NAC or 1 mM Vitamin C for 8 hours before analysis for apoptosis and ROS. (B) HCT116 cells were transiently infected with lentivirus expressing human catalase or with empty vector control (100% infection). 48 hour post-infection, cells were treated with S4h for 8 hours before determining the level of apoptosis and ROS by FACS. (C) SW480 cells were treated with S4h alone or in combination with 5 mM NAC before measuring the level of apoptosis and ROS by FACS. All the data are based on three independent experiments.
3.5 NF-κB pathway is activated by S4h-induced ROS and contributes to the survival of S4h-treated colorectal cancer cells
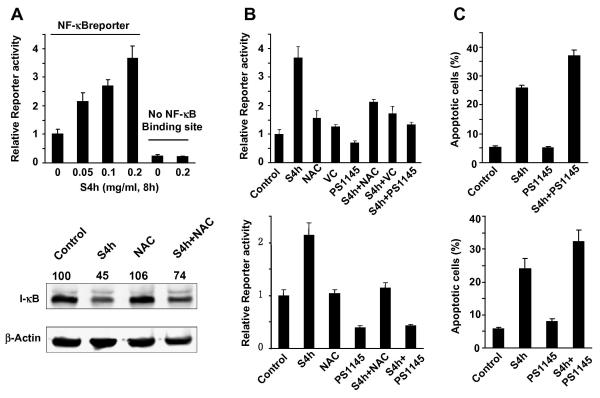
ROS has frequently been reported to activate the NF-κB pathway [21, 22], which exerts a strong anti-apoptotic function in diverse cell types. NF-κB is a potent transcription factor that regulates the expression of genes that control cell proliferation and cell survival. Therefore, we tested whether S4h activated the NF-κB pathway in HCT116 and SW480 colorectal cancer cells using an NF-κB luciferase reporter construct driven by a promoter containing an NF-κB response element. As shown in Figure 5A top, S4h induced expression from the NF-κB reporter in a concentration-dependent manner. In contrast, expression from the control luciferase reporter construct that did not contain the NF-κB response element was not affected by S4h treatment (Figure 5A top). To further demonstrate that S4h activates the NF-κB pathway, we determined the effect of PS1145, a small molecule inhibitor of the IκBα kinase [30], on the induction of NF-κB reporter expression by S4h. As shown in Fig. 5B, PS1145 strongly inhibited S4h-induced NF-κB reporter expression in both HCT116 and SW48 colorectal cancer cells. Additionally, the transcriptional activity of NF-κB is negatively regulated by IκB and ROS had been reported to decrease IκB level [31]. Therefore, we investigated the effect of S4h on IκB level by western blot. Indeed, S4h treatment significantly reduced the level of IκB protein (Fig. 5A, bottom panels). Taken together, these results showed that S4h treatment activates the NF-κB pathway in colorectal cancer cells.
Figure 5. S4h activated NF-κB pathway through ROS.
(A) 48 hours after transient transfection of NF-κB reporter plasmids, HCT116 cells were treated with different concentrations of S4h for 8 hours before the luciferase reporter activity was determined (top). After HCT116 cells were treated with S4h alone or in conjunction with 5 mM NAC for 8 hours, the level of IκB was determined by western blot (bottom). (B) and (C) After the indicated treatments for 8 hours, the level of apoptosis and NF-κB reporter activity in HCT116 (top) and SW480 (bottom) cells was measured. The following concentrations were used: 5 mM NAC, 1 mM Vitamin C and 50 μM PS1145. All the data are based on three independent experiments.
To determine if the observed activation of the NF-κB pathway involved S4h-induced ROS, the effects of the NAC and Vitamin C antioxidants on S4h-induced NF-κB mediated reporter expression were investigated. As shown in Figure 5B, NAC and Vitamin C significantly inhibited S4h-induced NF-κB reporter expression in HCT116 cells. Similarly, NAC also significantly inhibited S4h-induced NF-κB reporter expression in SW480 colorectal cancer cells. Furthermore, while NAC itself did not affect IκB levels, IκB level was higher in HCT116 cells treated with NAC+S4h than those treated with S4h alone (Figure 5A bottom). These results indicate that activation of the NF-κB pathway by S4h in colorectal cancer cells is mediated by its induction of ROS.
To further determine if activation of the NF-κB pathway contributes to the survival effect of S4h-induced ROS, the effect of inhibiting the NF-κB pathway on S4h-induced apoptosis was determined. As shown in Fig. 5C, inhibition of the NF-κB pathway using the small molecule inhibitor PS1145 increased S4h-induced apoptosis in both HCT116 and SW480 cells (Figure 5C top and bottom panels, respectively). Therefore activation of the NF-κB pathway in these colorectal cancer cells contributes to the protection against S4h-induced apoptosis.
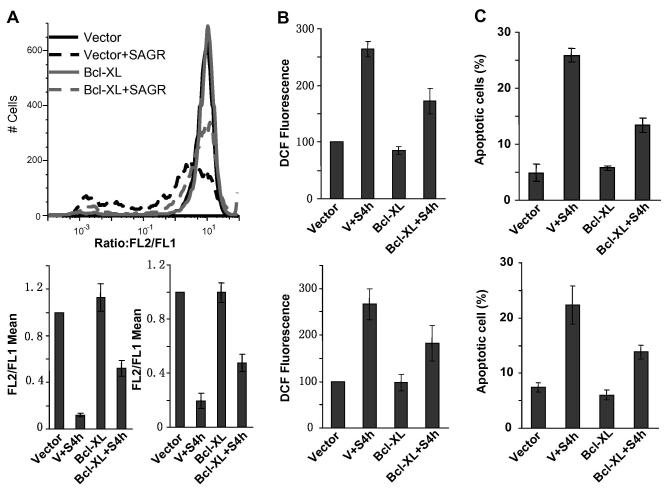
3.6 Overexpression of Bcl-xL decreased S4h-induced mitochondrial dysfunction, apoptosis, and ROS generation
Mitochondrial damage is often associated with induction of apoptosis and ROS generation [32, 33]. To determine whether S4h induced apoptosis via the mitochondrial pathway, we protected the mitochondria and measured the effect of S4h on apoptosis in colorectal cancer cells. Bcl-xL, a member of the Bcl-2 family proteins, is reported to protect mitochondrial function and thus exerted anti-apoptotic effect [19]. Cells were infected with virus expressing Bcl-xL, and apoptosis, mitochondrial membrane potential and ROS were measured after they were treated with S4h. As shown in Figure 6A, a large majority of the HCT116 cells infected with vector control had significantly decreased mitochondrial membrane potential after S4h treatment as determined by JC-1 staining. Interestingly, expression of Bcl-xL significantly increased mitochondria membrane potential, with a majority of the cells exhibiting normal mitochondrial membrane potential (Fig. 6A, top panel). These results indicate that S4h-induced dysfunction in the mitochondria was significantly inhibited by Bcl-xL (Figure 6A top and bottom). Similar results were also observed in SW480 cells (Figure 6A, bottom right). Coincident with the protection from mitochondrial damage, Bcl-xL expression significantly decreased the level of ROS and apoptosis induced by S4h in both HCT116 and SW480 cells (Fig. 6B and C). These results suggest that mitochondrial damage played an important role in S4h-induced apoptosis and ROS generation in colorectal caner cells.
Figure 6. Overexpression of Bcl-xL protected the mitochondria and attenuated S4h-induced apoptosis and ROS.
(A) MMP was measured by FACS using JC-1 dye. The top is a representative flow plot for HCT116 cells. The bottom plots were the means of FL2-H/FL1-H for HCT116 (left) and SW480 (right). (B) The ROS level was determined for HCT116 (top) and SW480 (bottom). (C) Apoptosis was measured for HCT116 (top) and SW480 (bottom). Colorectal cancer cells were infected with lentivirus expressing Bcl-xL. 48 hours post-infection, the cells were treated with S4h for 8 hours. All the data are based on three independent experiments.
4. Discussion
In this report, we showed that steamed ginseng extract (S4h) treatment induced mitochondrial damage, ROS accumulation, and apoptosis in colorectal cancer cells. We further characterized the relationship between the effects of S4h and summarized our results in a model (Fig. 7). Our results show that S4h-induced apoptosis in colorectal cancer cells is, at least in part, mediated through the mitochondrial pathway. In addition, S4h treatment also induced ROS accumulation in these colorectal cancer cells, which protected these cells from undergoing apoptosis, at least in part, by activation of the NF-κB pathway.
Fig. 7. A working model for American ginseng-induced apoptosis in colorectal cancer cells.
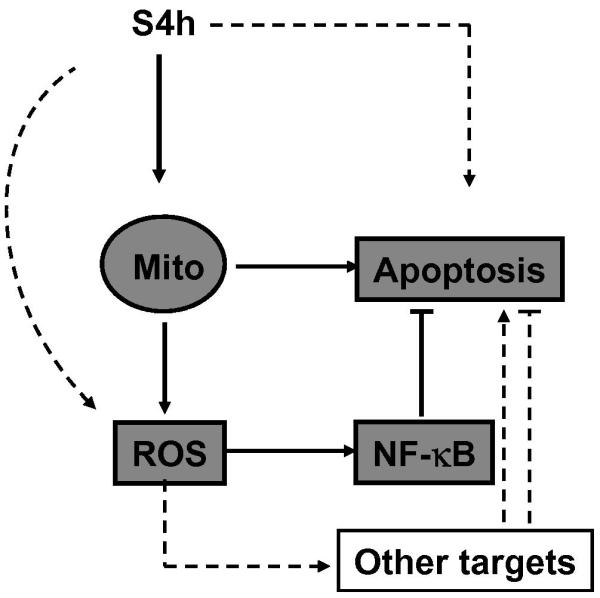
A key effect of American ginseng (S4h) in inducing apoptosis is through mitochondria damage, which releases factors promoting apoptosis and increases ROS. Although ROS has multiple targets, one of the key targets activated by ROS in colorectal cancer cells is the NF-κB pathway, which protects S4h-induced apoptosis. See discussion for more details.
This model is supported by the observations that S4h-induced NF-κB reporter activation in colorectal cancer cells was inhibited either by the NF-κB pathway inhibitor PS1145 or by antioxidants such as NAC and Vitamin C, or by the expression of the ROS-scavenging enzyme catalase. In contrast, inhibition of NF-κB activation by PS1145 did not affect S4h-induced ROS (data not shown). These observations support the idea that S4h-induced NF-κB activation is mediated by ROS generation. Furthermore, decreasing the level of ROS by treatment with NAC or expression of catalase had the effect of increasing S4h-induced apoptosis in colorectal cancer cells, similar to inhibition of NF-κB activation by the PS1145 inhibitor, indicating NF-κB activation mediates the key anti-apoptotic effect of ROS in these cells. Finally, protecting the mitochondria by expression of Bcl-XL decreased both S4h-induced ROS and apoptosis, indicating that S4h-induced ROS and apoptosis are at least partially caused by mitochondria damage.
ROS can be induced by a wide variety of extracellular stimuli and can potentially promote either cell survival or cell death. Why will ROS induced by S4h in colorectal cancer cells promote cell survival instead of cell death? While we do not yet know the precise mechanisms involved, previous studies on the distinct roles of NF-κB on cell death or cell survival in response to TNF-α or UV offered some clues [34]. It was shown that TNF-α activated both JNK signaling and NF-κB. Activation of NF-κB in response to TNF-α signaling blocked the prolonged JNK activation and promotes cell survival. In contrast, UV induced robust and prolonged JNK activation as well as delayed activation of NF-κB. Activation of NF-κB in this case promotes UV-induced cell death by cooperating with the JNK pathway to upregulate the proapoptotic Fas ligand. It should be pointed out that both TNF and UV can also induce ROS, which potentially contribute to the life or death outcome of these stimuli. Therefore whether NF-κB promoted cell survival or death in response to different stimuli is determined by the interactions of the network of pro-survival and pro-death signaling pathways activated by these stimuli. By analogy, it is likely that the interactions of the network of pro-survival and pro-death signaling pathways activated by ginseng and ROS determined the role of ROS in cell survival. Of course further studies will be needed to test this idea and to elucidate the detailed mechanism involved.
The observation that increased level of ROS is mostly caused by S4h-induced mitochondria damage in colorectal cancer cells also explains an apparent paradox: while it was reported that American ginseng contains antioxidants and steaming of American ginseng slightly increased its antioxidant content [35, 36], treatment of colorectal cancer cells with S4h led to increased cellular ROS due to mitochondrial damage. The observation that steamed ginseng simultaneously activates the apoptosis pathway and the ROS/NF-κB-mediated survival pathway in colorectal cancer cells could potentially provide us with new tools to increase the anti-tumor effect of steamed ginseng by the co-treatment of steamed ginseng with ROS scavengers, such as the commonly-used antioxidants NAC and Vitamin C, or with inhibitors of the NF-κB pathway. In this respect, the anti-tumor effect of steamed ginseng appears to be different from those of conventional cancer therapies such as radiation or doxorubicin. It is generally believed that ROS induced by these conventional anti-cancer treatment promotes cancer cell killing and contributes to the therapeutic effect [37, 38].
Both ginseng and antioxidants such as vitamin C are generally safe and commonly used as dietary supplements. Our in vitro observations that antioxidants such as vitamin C can enhance the anticancer activity of ginseng raise the possibility that combinations of ginseng and antioxidant can potentially be used as chemoprevention agents to prevent the development of colorectal cancers. Further studies will be needed to test such possibilities.
Acknowledgments
This work was supported in part by grants from the NIH/NCCAM AT004418, NIH GM074197, NIH/NCI CA106569, and from the American Cancer Society RSG-05-230-01DDC and RSG-05-254-01DDC.
We would like to thank Drs. Jian Wu for the Human catalase cDNA, Richard Hiipakka and John Kokontis for help with the Luciferase activity assay, Kay Macleod for the Bcl-XL plasmid. We also thank Gabe Gordon for carefully reading this manuscript.
Abbreviations
- D C F
2,7-dichlorofluorescein
- H2DCFDA
5-(and-6)-chloromethyl-2′7′-dichlorodihydrofluorescein diacetate acetyl ester
- MMP
mitochondrial membrane potential
- NAC
N-Acetyl-L-cysteine
- ROS
reactive oxygen species
- S4h
hour-steamed American ginseng root extract (S4h)
Footnotes
Conflicts of Interest Statement None Declared
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest.
References
- [1].Hawk ET, Levin B. Colorectal cancer prevention. J Clin Oncol. 2005;23:378–391. doi: 10.1200/JCO.2005.08.097. [DOI] [PubMed] [Google Scholar]
- [2].Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- [3].da Rocha AB, Lopes RM, Schwartsmann G. Natural products in anticancer therapy. Curr Opin Pharmacol. 2001;1:364–369. doi: 10.1016/s1471-4892(01)00063-7. [DOI] [PubMed] [Google Scholar]
- [4].Mann J. Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer. 2002;2:143–148. doi: 10.1038/nrc723. [DOI] [PubMed] [Google Scholar]
- [5].Wen J, Zimmer EA. Phylogeny and biogeography of Panax L. (the ginseng genus, araliaceae): inferences from ITS sequences of nuclear ribosomal DNA. Mol Phylogenet Evol. 1996;6:167–177. doi: 10.1006/mpev.1996.0069. [DOI] [PubMed] [Google Scholar]
- [6].Wang CZ, Mehendale SR, Yuan CS. Commonly used antioxidant botanicals: active constituents and their potential role in cardiovascular illness. Am J Chin Med. 2007;35:543–558. doi: 10.1142/S0192415X07005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- [8].Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–274. [PubMed] [Google Scholar]
- [9].Yuan CS, Wei G, Dey L, Karrison T, Nahlik L, Maleckar S, Kasza K, Ang-Lee M, Moss J. Brief communication: American ginseng reduces warfarin’s effect in healthy patients: a randomized, controlled Trial. Ann Intern Med. 2004;141:23–27. doi: 10.7326/0003-4819-141-1-200407060-00011. [DOI] [PubMed] [Google Scholar]
- [10].Koo HN, Jeong HJ, Choi IY, An HJ, Moon PD, Kim SJ, Jee SY, Um JY, Hong SH, Shin SS, Yang DC, Seo YS, Kim HM. Mountain grown ginseng induces apoptosis in HL-60 cells and its mechanism have little relation with TNF-alpha production. Am J Chin Med. 2007;35:169–182. doi: 10.1142/S0192415X07004710. [DOI] [PubMed] [Google Scholar]
- [11].Yun TK, Choi SY. Non-organ specific cancer prevention of ginseng: a prospective study in Korea. Int J Epidemiol. 1998;27:359–364. doi: 10.1093/ije/27.3.359. [DOI] [PubMed] [Google Scholar]
- [12].Yun TK, Choi SY. Preventive effect of ginseng intake against various human cancers: a case-control study on 1987 pairs. Cancer Epidemiol Biomarkers Prev. 1995;4:401–408. [PubMed] [Google Scholar]
- [13].Wang CZ, Yuan CS. Potential role of ginseng in the treatment of colorectal cancer. Am J Chin Med. 2008;36:1019–1028. doi: 10.1142/S0192415X08006545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang CZ, Aung HH, Ni M, Wu JA, Tong R, Wicks S, He TC, Yuan CS. Red American ginseng: ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007;73:669–674. doi: 10.1055/s-2007-981524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Luo X, Wang CZ, Chen J, Song WX, Luo J, Tang N, He BC, Kang Q, Wang Y, Du W, He TC, Yuan CS. Characterization of gene expression regulated by American ginseng and ginsenoside Rg3 in human colorectal cancer cells. Int J Oncol. 2008;32:975–983. [PMC free article] [PubMed] [Google Scholar]
- [16].Wang CZ, Li XL, Wang QF, Mehendale SR, Fishbein AB, Han AH, Sun S, Yuan CS. The mitochondrial pathway is involved in American ginseng-induced apoptosis of SW-480 colon cancer cells. Oncol Rep. 2009;21:577–584. doi: 10.3892/or_00000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang CZ, Zhang B, Song WX, Wang A, Ni M, Luo X, Aung HH, Xie JT, Tong R, He TC, Yuan CS. Steamed American ginseng berry: ginsenoside analyses and anticancer activities. J Agric Food Chem. 2006;54:9936–9942. doi: 10.1021/jf062467k. [DOI] [PubMed] [Google Scholar]
- [18].Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- [19].Wong WW, Puthalakath H. Bcl-2 family proteins: the sentinels of the mitochondrial apoptosis pathway. IUBMB Life. 2008;60:390–397. doi: 10.1002/iub.51. [DOI] [PubMed] [Google Scholar]
- [20].Azad MB, Chen Y, Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2270. [DOI] [PubMed] [Google Scholar]
- [21].Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. Embo J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Flohe L, Brigelius-Flohe R, Saliou C, Traber MG, Packer L. Redox regulation of NF-kappa B activation. Free Radic Biol Med. 1997;22:1115–1126. doi: 10.1016/s0891-5849(96)00501-1. [DOI] [PubMed] [Google Scholar]
- [23].Lin A, Karin M. NF-kappaB in cancer: a marked target. Semin Cancer Biol. 2003;13:107–114. doi: 10.1016/s1044-579x(02)00128-1. [DOI] [PubMed] [Google Scholar]
- [24].Wang CZ, Aung HH, Zhang B, Sun S, Li XL, He H, Xie JT, He TC, Du W, Yuan CS. Chemopreventive effects of heat-processed Panax quinquefolius root on human breast cancer cells. Anticancer Res. 2008;28:2545–2551. [PMC free article] [PubMed] [Google Scholar]
- [25].Robinson JP, Carter WO, Narayanan PK. Oxidative product formation analysis by flow cytometry. Methods Cell Biol. 1994;41:437–447. doi: 10.1016/s0091-679x(08)61733-1. [DOI] [PubMed] [Google Scholar]
- [26].Huang RF, Huang SM, Lin BS, Hung CY, Lu HT. N-Acetylcysteine, vitamin C and vitamin E diminish homocysteine thiolactone-induced apoptosis in human promyeloid HL-60 cells. J Nutr. 2002;132:2151–2156. doi: 10.1093/jn/132.8.2151. [DOI] [PubMed] [Google Scholar]
- [27].Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411:77–82. doi: 10.1016/s0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]
- [28].Cossarizza A, Salvioli S. Flow cytometric analysis of mitochondrial membrane potential using JC-1. Curr Protoc Cytom. 2001:14. doi: 10.1002/0471142956.cy0914s13. Chapter 9. Unit 9. [DOI] [PubMed] [Google Scholar]
- [29].al Yacoub N, Romanowska M, Haritonova N, Foerster J. Optimized production and concentration of lentiviral vectors containing large inserts. J Gene Med. 2007;9:579–584. doi: 10.1002/jgm.1052. [DOI] [PubMed] [Google Scholar]
- [30].Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, Munshi N, Dang L, Castro A, Palombella V, Adams J, Anderson KC. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277:16639–16647. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- [31].Pyo CW, Yang YL, Yoo NK, Choi SY. Reactive oxygen species activate HIV long terminal repeat via post-translational control of NF-kappaB. Biochem Biophys Res Commun. 2008;376:180–185. doi: 10.1016/j.bbrc.2008.08.114. [DOI] [PubMed] [Google Scholar]
- [32].Chalah A, Khosravi-Far R. The mitochondrial death pathway. Adv Exp Med Biol. 2008;615:25–45. doi: 10.1007/978-1-4020-6554-5_3. [DOI] [PubMed] [Google Scholar]
- [33].Nozoe M, Hirooka Y, Koga Y, Araki S, Konno S, Kishi T, Ide T, Sunagawa K. Mitochondria-derived reactive oxygen species mediate sympathoexcitation induced by angiotensin II in the rostral ventrolateral medulla. J Hypertens. 2008;26:2176–2184. doi: 10.1097/HJH.0b013e32830dd5d3. [DOI] [PubMed] [Google Scholar]
- [34].Liu J, Lin A. Wiring the cell signaling circuitry by the NF-kappa B and JNK1 crosstalk and its applications in human diseases. Oncogene. 2007;26:3267–3278. doi: 10.1038/sj.onc.1210417. [DOI] [PubMed] [Google Scholar]
- [35].Kim KT, Yoo KM, Lee JW, Eom SH, Hwang IK, Lee CY. Protective effect of steamed American ginseng (Panax quinquefolius L.) on V79-4 cells induced by oxidative stress. J Ethnopharmacol. 2007;111:443–450. doi: 10.1016/j.jep.2007.01.004. [DOI] [PubMed] [Google Scholar]
- [36].Kitts DD, Wijewickreme AN, Hu C. Antioxidant properties of a North American ginseng extract. Mol Cell Biochem. 2000;203:1–10. doi: 10.1023/a:1007078414639. [DOI] [PubMed] [Google Scholar]
- [37].Cossarizza A, Franceschi C, Monti D, Salvioli S, Bellesia E, Rivabene R, Biondo L, Rainaldi G, Tinari A, Malorni W. Protective effect of N-acetylcysteine in tumor necrosis factor-alpha-induced apoptosis in U937 cells: the role of mitochondria. Exp Cell Res. 1995;220:232–240. doi: 10.1006/excr.1995.1311. [DOI] [PubMed] [Google Scholar]
- [38].Lutzky J, Astor MB, Taub RN, Baker MA, Bhalla K, Gervasoni JE, Jr., Rosado M, Stewart V, Krishna S, Hindenburg AA. Role of glutathione and dependent enzymes in anthracycline-resistant HL60/AR cells. Cancer Res. 1989;49:4120–4125. [PubMed] [Google Scholar]