Abstract
Chemotactic cytokines mediate the recruitment of leukocytes into infected tissues. This study investigated the profile of chemokines during experimental Candida albicans keratitis and determined the effects of chemokine inhibition on leukocyte infiltration and fungal growth during murine keratomycosis. Scarified corneas of BALB/c mice were topically inoculated with C. albicans and monitored daily over one week for fungal keratitis. After a gene microarray for murine chemokines compared infected corneas to controls, real-time reverse transcription polymerase chain reaction (RT-PCR) and immunostaining assessed chemokine expression in infected and mock-inoculated corneas. An anti-chemokine antibody was then administered subconjunctivally and evaluated for effects on clinical severity, corneal inflammation, fungal recovery, and cytokine expression. Of 33 chemokine genes examined by microarray, 6 CC chemokines and 6 CXC chemokines were significantly (P<0.05) upregulated more than two-fold. Chemokine (CC-motif) ligand 3 (CCL3) was upregulated 108-fold (P=0.03) by real-time RT-PCR within one day after fungal inoculation and remained increased 28-fold (P=0.02) at one week, and its in situ expression increased in the epithelium and stroma of infected corneas. Compared to the control antibody-treated group, eyes treated with anti-CCL3 antibody showed reduced clinical severity (P<0.05), less corneal neovascularization (P=0.02), and fewer inflammatory cells infiltrating corneal tissue, but the amount of recoverable fungi was not significantly (P=0.4) affected. Anti-CCL3 treatment significantly (P=0.01) reduced the expression of tumor necrosis factor and interleukin-1β in infected corneas. These results indicate that chemokines, especially the CC chemokine CCL3, play important roles in the acute inflammatory response to C. albicans corneal infection.
Keywords: cornea, fungi, innate immunity, keratitis, mycology, pathogenesis
1. Introduction
Candida albicans, a component of the normal flora, is a fungal opportunist for the eye (Kercher et al., 2001). Trauma and disease predispose to corneal infection (Sun et al., 2007). During C. albicans keratitis yeasts transition into filamentary forms that breach the eye’s defenses and penetrate the stroma (Jackson et al., 2007). Hyphal invasion elicits host responses that bring about corneal inflammation and ulceration (Yuan et al., 2009).
Innate immunity and acute inflammation actively participate in the pathophysiology of fungal keratitis. The cornea detects the presence of invasive C. albicans by toll-like receptors and other pathogen-recognition molecules (Yuan and Wilhelmus, 2010). Corneal epithelial cells, keratocytes, and phagocytes are involved in distinguishing pathogen-associated molecular patterns, and this interaction activates innate responses. Neutrophils, key effector cells for controlling fungal growth, afford a first line of defense during mucosal C. albicans infection.
Leukocytes are drawn into the cornea by the local production of chemotactic cytokines during the initial stages of fungal keratitis (Zhong et al., 2009). Chemokines are released at an early stage of fungal infection and bind to receptors that trigger the synthesis of interleukins and other cytokines that promote fungal clearance (Traynor and Huffnagle, 2001). Members of the CC chemokine subfamily such as CCL2 and CCL3 have potent chemotactic and activating properties for neutrophils and are rapidly induced in bacterial keratitis (Xue et al., 2007).
This study examined the profile of chemokines after the onset of experimental C. albicans keratitis and followed the relative expression of selected CC chemokines during the first week of fungal keratitis in mice. We also studied the effect of an anti-CCL3 antibody on inflammatory severity, fungal growth, and cytokine expression during C. albicans keratitis. Our findings suggest that chemokines such as CCL3 facilitate innate immune responses in the pathogenesis of fungal keratitis.
2. Methods
2.1. Fungi and media
C. albicans strain SC5314, a clinical isolate capable of producing experimental keratomycosis, was cultured on Sabouraud dextrose agar (Difco, Detroit, MI) for 3 days at 25°C. Colonies were harvested after 3 days of inoculation and diluted in sterile phosphate-buffered saline (PBS) to yield 2 × 105 colony-forming units (CFU)/μL based on the optical density (OD) at 600 nm, using an OD600 conversion factor of 3 × 107 CFU/mL.
2.2. Animal model
Animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research under protocols approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. Female BALB/c mice 6 to 8 weeks of age (Harlan Sprague-Dawley, Houston, TX) were anesthetized with an intraperitoneal injection of ketamine, xylazine, and acepromazine. The corneas of right eyes were superficially scarified with a 22-gauge needle. A 5-μL inoculum of either C. albicans containing 1 × 106 CFU or sterilized PBS was topically applied to eyes of infected and control groups, respectively. Mice were monitored daily for 7 days postinoculation (p.i.) using a dissecting microscope to categorize corneal inflammation and angiogenesis. The severity of keratitis was graded by a scoring system that consisted of the relative area of corneal infiltrate, density of corneal opacity, and surface regularity (Wu et al., 2003). The amount of corneal vascularization was assessed by a neovascularization scoring system that assigned grades of 0 to 4 for the number, density, and length of visible corneal blood vessels (Yuan and Wilhelmus, 2009). Corneal photographs with the eye positioned en face or in lateral profile were captured with a Zeiss photo slit-lamp and Nikon digital camera.
2.3. RNA extraction
Mice were sacrificed 1 day p.i. After enucleation corneas were excised and dissected from surrounding conjunctiva and uvea. Pools of 5 corneas were prepared in triplicate from C. albicans-infected and control groups at day 1 p.i. RNA was extracted by a previously reported procedure (Yuan et al., 2009) and was isolated with RNeasy MicroKit columns (Qiagen, Valencia, CA). Samples were treated with DNase (Qiagen, Valencia, CA) and stored at −80°C.
2.4. Gene microarray
Genetic analysis was performed at the Microarray Core Facility, Baylor College of Medicine, as reported (Yuan et al., 2009). After checking RNA samples for quality assurance, Genechip (Affymetrix, Santa Clara, CA) microarray protocols were applied to qualified samples of 3 five-cornea pools from each group for two cycles of amplification. Images and quality control metrics were recorded using Affymetrix GCOS software version 1.4, and signal-intensity data were adjusted and analyzed with BioConductor software. The criterion for significance of differentially regulated genes was established as > 2-fold change with adjusted P < 0.05.
2.5. Quantitative polymerase chain reaction
Total RNA isolated from corneas at 1, 3, and 7 days p.i. was quantified by absorbance at 260 nm. The first-strand cDNA was synthesized from 0.4 μg RNA with Ready-To-Go You-Prime First-Strand Beads (GE Healthcare, Princeton, NJ) and random hexamers (Applied Biosystems, Foster City, CA). Real-time reverse transcription polymerase chain reaction (RT-PCR) was performed using TaqMan Gene Expression Master Mix and Assays (Applied Biosystems). Primers specific for CCL3, CCL2, CXCL1, TNF, IL-1β, IL-6, VEGF-A, MMP8, MMP13, and CRAMP (Applied Biosystems) were used to quantify gene expression levels. The threshold cycle (CT) for each target mRNA was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA and averaged. Three five-cornea pools were processed for each group. Two-group comparisons were done using the Student t-test, and three-group comparisons used one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
2.6. Immunofluorescence
Eyes obtained 1 day p.i. were embedded in OCT compound (Sakura Finetek, Torrance, CA), snap-frozen in liquid nitrogen, and sectioned at 15-μm thickness. Sections were thawed, dehydrated, and fixed in 2% paraformaldehyde then blocked with 10% normal donkey serum (Jackson ImmunoResearch Laboratories, Philadelphia, PA). Immunofluorescent staining was performed as reported (Yuan et al., 2009). Goat serum antibody to the N-terminus of murine CCL3 (R&D Systems, Minneapolis, MN) was diluted 1:100, and applied to blocked sections that were incubated overnight at 4°C. Secondary Alexa-Fluor 488-conjugated donkey anti-goat immunoglobulin (Invitrogen, Carlsbad, CA) was applied to sections that were then incubated in a dark chamber for 1 hour and counterstained with propidium iodine (Invitrogen) in Gel/Mount (Biomeda, Foster City, CA). Sections were observed with a laser-scanning confocal microscope (LSM 510, Zeiss, Thornwood, NY) with 488- and 543-nm excitation and emission filters. Images were acquired with a 40× oil-immersion objective and processed using Zeiss LSM-PC software.
2.7. Anti-CCL3 treatment
Goat anti-mouse CCL3 and control goat immunoglobulin were obtained (R&D Systems). Five mice were allocated to treatment and control groups, respectively. Each mouse was administered 10 μg of antibodies subconjunctivally 30 minutes before superficial corneal scarification. Scarified corneas of the treated group were topically inoculated with C. albicans at a dosage of 1 × 106 CFU/5μL. Control mice received 5-μL of normal goat IgG antibody. Eyes were observed daily with a dissecting microscope to grade the severity of keratitis. Ten additional mice were treated subconjunctivally with either anti-CCL3 antibody or goat IgG 30 minutes before fungal inoculation and then sacrificed one day p.i. for fungal recovery.
2.8. Quantitative fungal recovery
Quantitative fungal cultures were performed on excised corneas by a previously reported method (Yuan and Wilhelmus, 2009). In brief, corneas were homogenized in a frosted-glass grinder with 500 μL PBS, and the homogenated aliquot was diluted 10-fold with PBS. The entire aliquot was then inoculated onto Sabouraud dextrose agar that was incubated for 4 days at 25°C. Visible colonies were counted, and the number of CFUs per cornea was compared between treated and control groups with the Student t-test.
3. Results
3.1. Experimental fungal keratitis
All eyes inoculated with C. albicans developed clinical signs of keratitis. Corneal inflammation began 1 day p.i. (mean keratitis score ± SD, 7.4 ± 0.5), peaked 3 days p.i. (8.0 ± 0.7), and then diminished by 7 days p.i. (6.2 ± 0.8). Capillary budding of limbal vessels began 3 days p.i (mean neovascularization score ± SD, 6.3 ± 0.6) and progressed until 7 days p.i (8.7 ± 0.6). Histopathological evaluation of infected eyes 1 day p.i. revealed partial to total loss of epithelial integrity, the infiltration of neutrophils and macrophages in the stroma, the presence of acute inflammatory cells within the anterior chamber, and the invasion of fungal pseudohyphae and hyphae within the anterior stroma (Wu et al., 2003). No corneal inflammation or neovascularization was found in eyes from mock-inoculated controls or normal mice.
3.2. Chemokine gene expression profile
Gene arrays of C. albicans-infected corneas and mock-inoculated control corneas were compared for chemokine expression. Average ratios of expression levels at 1 day p.i. (Table 2) showed that 12 chemokine genes increased more than 2-fold (P<0.05) and that 2 chemokines decreased more than 2-fold (P<0.05). Compared to mock-infected controls, CCL2, CCL3, CCL4, CCL6, CCL7, and CCL24 were upregulated among CC group chemokines. CXCL1, CXCL3, CXCL4, CXCL12, CXCL14, and CXCL16 were upregulated in the CXC group. CXCL13 and CX3CL1 were slightly downregulated.
Table 2.
Microarray analysis of chemokine gene expression ratios comparing C. albicans keratitis to mock-infected controls.
| Systematic name | Original name(s) | Relative regulation | P value |
|---|---|---|---|
| CC group | |||
| CCL1 | TCA3, I-390 | −1.0 | 0.74 |
| CCL2 | MCP-1 | 38.6 | 0.015 |
| CCL3 | MIP-1α | 374.3 | 0.04 |
| CCL4 | MIP-1β | 145.2 | 0.011 |
| CCL5 | RANTES | −2.1 | 0.36 |
| CCL6 | MPR-1 | 17.4 | 0.0011 |
| CCL7 | MCP-3 | 47.1 | 0.012 |
| CCL8 | MCP-2 | −1.5 | 0.37 |
| CCL12 | MCP-5 | 2.1 | 0.32 |
| CCL17 | TARC | −1.0 | 0.96 |
| CCL19 | MIP-3β, ELC, exodus-3 | −1.2 | 0.80 |
| CCL20 | MIP-3α, LARC, exodus-1 | −1.0 | 0.12 |
| CCL21 | 6Ckine, SLC, exodus-2 | −2.2 | 0.84 |
| CCL22 | ABCD-1, MOC | 1.1 | 0.31 |
| CCL24 | MPIF-1 | 3.8 | 0.024 |
| CCL25 | TECK | −1.0 | 0.88 |
| CCL27 | CTACK | 1.4 | 0.42 |
| CCL28 | MEC | 1.0 | 0.94 |
| CXC group | |||
| CXCL1 | KC | 83.6 | 0.037 |
| CXCL2 | MIP-2α | 122.7 | 0.093 |
| CXCL3 | MIP-2B | 291.7 | 0.044 |
| CXCL4 | PF4 | 5.4 | 0.016 |
| CXCL5 | LIX, ENA78 | 132.4 | 0.052 |
| CXCL9 | MIG | −1.1 | 0.039 |
| CXCL10 | IP-10, CRG-2 | −1.6 | 0.58 |
| CXCL11 | ITAC | −2.6 | 0.086 |
| CXCL12 | SDF-1 | 5.5 | 0.022 |
| CXCL13 | BLC, BCA-1 | −2.1 | 0.0095 |
| CXCL14 | BRAK | 68.3 | 0.0045 |
| CXCL15 | Lungkine | −1.0 | 0.82 |
| CXCL16 | SR-PSOX | 2.7 | 0.039 |
| C group | |||
| XCL1 | Lymphotactin-α | −1.0 | 0.62 |
| CXXXC group | |||
| CX3CL1 | Fractalkine | −2.7 | 0.0022 |
Transcript levels of CCL3 and CCL2 were detected by quantitative real-time RT-PCR on total RNA extracted from groups of five-cornea pools of infected or mock-infected corneas (Fig. 1). Differences of CCL3 transcript were found between C. albicans keratitis and scarified controls at day 1 (108.1-fold, P=0.03), day 3 (41.7-fold, P=0.03), and day 7 (27.6-fold, P=0.02) p.i. In infected corneas, CCL3 transcripts were upregulated highly on day 1 p.i. then declined toward a baseline level but remained significantly increased after 3 days p.i. CCL2 was upregulated at day 1 p.i. (11.0-fold, P=0.01) and remained relatively upregulated in infected corneas at day 3 p.i. (9.3-fold, P=0.03) but not at day 7 p.i. (9.0-fold, P=0.13).
Figure 1.
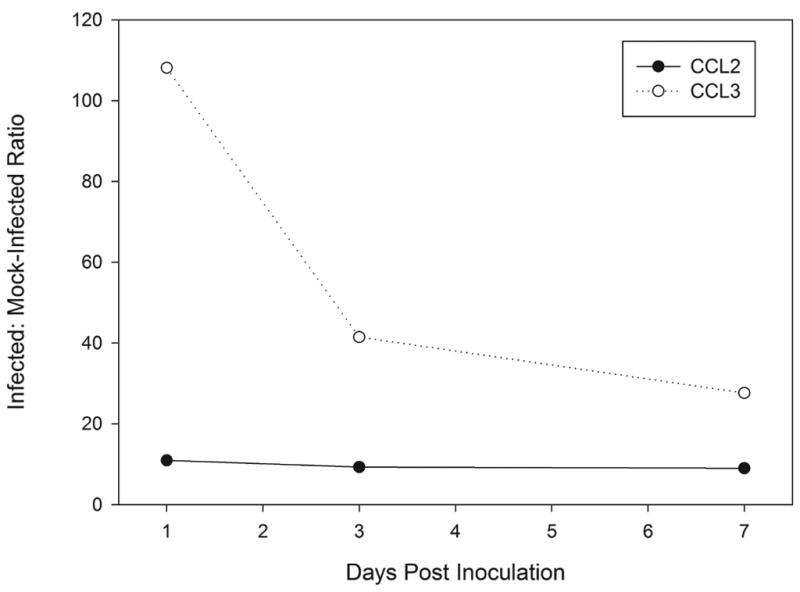
Differential gene expression ratios of CCL3 and CCL2 in C. albicans-infected corneas compared to mock-inoculated corneas.
3.3. CC chemokine protein expression pattern
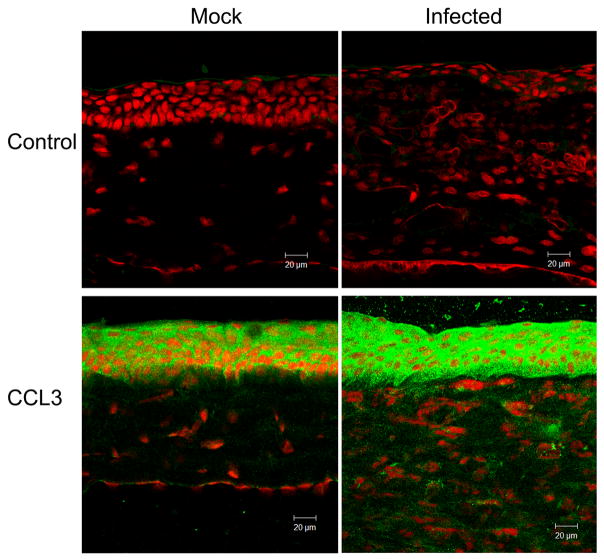
The in situ protein expression pattern determined by immunofluorescent staining at day 1 p.i. was consistent with transcript levels measured by real-time RT-PCR (Fig. 2). CCL3 was present in the epithelium of control corneas. Immunostaining for CCL3 was relatively more intense in the epithelium of infected corneas and was present throughout the stroma. CCL2 was present in mock-inoculated corneas, with moderate epithelial staining and minor stromal staining, and was increased in infected corneas, with enhanced staining of the epithelial and stromal layers. Negative controls in which no primary antibody was used demonstrated no detectable staining by immunofluorescence.
Figure 2.
Molecular expression patterns in situ in C. albicans keratitis (Infected) or mock-infected controls (Mock). Negative controls included no primary antibody (Control). Sections were processed one day after fungal inoculation for indirect immunofluorescence using a cyan-green-labeled secondary antibody and propidium iodine as a nuclear counterstain.
3.4. Treatment effect of CCL3 neutralizing antibody on C. albicans keratitis
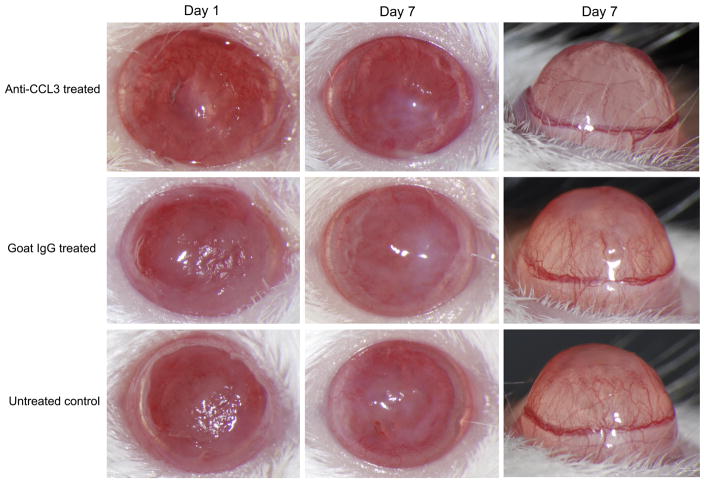
Mice treated with anti-CCL3 antibody showed significantly reduced severity (Fig. 3) at day 1 (mean keratitis score ± SD, 5.4 ± 1.1), day 3 (6.6 ± 0.5), and day 7 (4.0 ± 0.7), indicating reduced corneal inflammation during C. albicans corneal infection compared to the goat IgG control antibody-treated group at day 1 (7.2 ± 0.8, P=0.022), day 3 (7.4 ± 0.5, P=0.049), and day 7 (5.6 ± 0.9, P=0.014). Slit-lamp biomicroscopy also showed a substantially reduced corneal opacity in anti-CCL3 antibody-treated mice in comparison to the control antibody-treated group (Fig. 4). No difference was detected between the goat IgG control antibody-treated group and the untreated infected group during the clinical course (P>0.05). Corneal neovascularization was less in anti-CCL3-treated mice than in goat IgG antibody-treated controls (Fig. 4). An inhibitory effect was apparent by 3 days p.i., and treated animals continued to have a lower corneal neovascularization score on day 1 (4.0±1.0, P=0.64) and day 3 p.i. (5.3 ± 0.6, P=0.047). At 7 days p.i. the average vascularization score (5.33±1.52) in treated mice remained significantly less (P=0.024) than that (8.3 ± 0.6) of controls.
Figure 3.
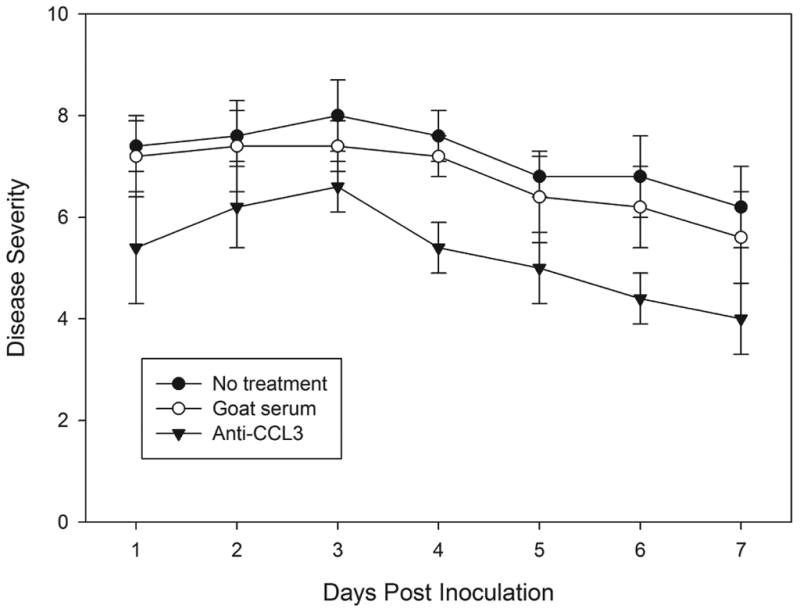
Clinical evaluation of C. albicans keratitis in anti-CCL3 treated, goat IgG antibody-treated, and untreated mice. Each point represents the mean severity score with standard deviation.
Figure 4.
Clinical appearance of C. albicans keratitis among anti-CCL3-treated, goat IgG antibody-treated, and untreated mice at day 1 and day 7 p.i., with corneal profile views showing the extent of corneal neovascularization at 7 days.
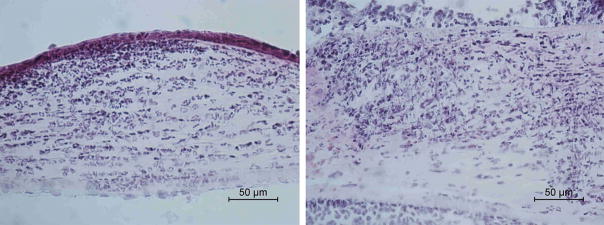
Histopathological examination demonstrated less inflammatory cell infiltration and less structural disruption in corneal tissue in the anti-CCL3-treated group compared to the goat IgG-treated group (Fig. 5). Cultures from excised corneas at 1 day p.i. showed no significant difference (P = 0.35) in the mean number (± SD) of viable fungi recovered from control corneas (13,750 ± 9,311 CFU/cornea) compared to those treated with anti-CCL3 antibody (9,250 ± 3,739 CFU/cornea). Treatment with anti-CCL3 antibody significantly reduced RNA expression of CCL3 (−4.30-fold, P=0.01), TNF (−2.81-fold, P=0.01), and IL-1β (−2.23-fold, P=0.01) compared to the goat IgG-treated group at day 1 p.i. (Table 3). The expression of CCL2, CXCL1, IL-6, VEGF-A, MMP-13, MMP-8, and CRAMP did not change significantly.
Figure 5.
Histological examination showed that infected corneas pretreated with anti-CCL3 antibody (A) had relatively mild stromal infiltration while control eyes (B) had marked infiltration by neutrophils in the stroma and anterior chamber.
Table 3.
Real-time RT-PCR comparison of relative gene expression for corneal inflammatory molecules in C. albicans keratitis, comparing anti-CCL3-treated group to controls.
| Gene | Treated* | Control* | Fold-change** | P value |
|---|---|---|---|---|
| CCL3 | 5.33±0.52 | 3.23±0.61 | −4.30 | 0.01 |
| CCL2 | 6.07±0.12 | 5.83±0.20 | −1.18 | 0.14 |
| CXCL1 | 4.37±0.32 | 3.76±0.31 | −1.53 | 0.08 |
| TNF | 6.70±0.28 | 5.21±0.43 | −2.81 | 0.01 |
| IL-1β | 3.94±0.41 | 2.78±0.17 | −2.23 | 0.01 |
| IL6 | 6.23±0.35 | 5.76±0.52 | −1.38 | 0.27 |
| VEGF-A | 5.50±0.06 | 5.10±0.06 | −1.32 | 0.001 |
| MMP13 | 4.69±0.29 | 4.46±0.17 | −1.17 | 0.31 |
| MMP8 | 9.90±0.37 | 9.30±0.31 | −1.51 | 0.10 |
| CRAMP | 9.14±0.21 | 10.21±1.96 | 2.10 | 0.40 |
Mean threshold cycle ± standard deviation for each target mRNA, normalized to GAPDH mRNA.
Relative fold-change of the comparison between normalized threshold cycles for each target mRNA in anti-CCL3-treated group compared to goat IgG antibody-treated group.
4. Discussion
4.1. Leukocyte recruitment during corneal infection
Innate immunity is a pivotal protective mechanism of the eye that involves corneal cells and leukocytes. Immunosuppression increases susceptibility to keratomycosis and aggravates fungal proliferation (Wu et al., 2003). Host immune responses are initiated by pathogen-recognition receptors such as toll-like receptors (TLRs) (Herring and Huffnagle, 2002). In C. albicans keratitis, TLRs recognize β-glucan of the C. albicans cell wall and lead to the expression of interleukins and chemotactic cytokines (Yuan and Wilhelmus, 2010). Synthesis of these soluble mediators actuates inflammatory reactions at the site of fungal infection (Traynor and Huffnagle, 2001).
Chemokines are secreted proteins that mediate cell migration, differentiation, and activation (Charo and Ransohoff, 2006). In a systematic nomenclature based on molecular structure (Zlotnik et al., 2006), chemokines are classed into subfamilies according to the arrangement of amino-terminal cysteine motifs. Members of the CC subfamily have two adjacent cysteine residues while those of the CXC subfamily have two cysteines separated by an amino acid. Produced by a variety of cells, CC and CXC chemokines actively participate in the immunopathological reactions contributing to the severity of microbial keratitis (Xue et al., 2003; Xue et al., 2007)
Certain chemokines influence the recruitment of leukocytes during fungal infection by enabling integrin-mediated diapedesis through local blood vessels and tissue infiltration (Romani, 2003). Neutrophils account for most of the leukocytes permeating the cornea during early fungal keratitis (Vemuganti et al., 2002). As in bacterial keratitis (Matsumoto et al., 2005), leukocytes are guided by chemokines that increase immediately after the onset of fungal corneal infection (Zhong et al., 2009). Since chemokines bind the glycosaminoglycan moiety of proteoglycans (Hamel et al., 2009), the extracellular matrix of the corneal stroma likely participates in the chemotactic gradient responsible for the directional movement of neutrophils and other inflammatory cells into the infected cornea (Yuan and Wilhelmus, 2010).
4.2. Chemokines in fungal keratitis
Chemokines mediate the recruitment of neutrophils and macrophages during experimental cryptococcosis, aspergillosis, and candidiasis (Saavedra et al., 1999; Herring and Huffnagle, 2002; Phadke and Mehrad, 2005) CCL3, formerly known as macrophage inflammatory protein-1 (MIP-1α), is a member of the CC chemokine subfamily that is made by the cornea during inflammatory stress (Menten et al., 2002; Yoon et al., 2007). Exposure to C. albicans induces the expression of CC chemokines (Inoue et al., 2007), and our studies demonstrate that CCL3 expression increases within one day after the onset of C. albicans keratitis. Immunofluorescent staining confirmed the presence of increased CCL3 in the corneal epithelium and stroma during early fungal infection. Leukocytes may augment the release of CCL3 (Sallusto and Baggiolini, 2008), but corneas inflamed by a perforating injury have minimally increased CCL3 levels (Spandau et al., 2003). Thus, corneal epithelial cells and fibroblasts appear to be directly involved in the substantial increase of selected chemokines that occurs in response to replicating microorganisms (Menten et al., 2002; Xue et al., 2007).
The profile of CC chemokines upregulated during early C. albicans keratitis is comparable to that of other corneal infections (Kernacki et al., 1998; Huang and Hazlett, 2003; Xue et al., 2007). In addition to CCL3, CCL2, also called monocyte chemoattractant protein-1 (MCP-1), is part of the inflammatory response during corneal infection (Melgarejo et al., 2009; Xue et al., 2007). Receptors for CCL3, such as CCR1 and CCR5, are expressed on tissue cells and leukocytes (Bacon et al., 2002). CC chemokines may also participate in corneal angiogenesis that can complicate fungal and bacterial keratitis. Corneal vessels form soon after the onset of C. albicans keratitis (Yuan and Wilhelmus, 2009), and both CCL3 and CCL2 promote neovascularization associated with corneal inflammation (Ogawa et al., 1999). Our studies with an experimental murine model indicate that CCL3 and other CC chemokines are involved in the pathogenesis of C. albicans keratitis.
CCL3 mediates acute corneal inflammation and chemoattracts leukocytes (Takano et al., 1999; Xue et al., 2007). Neutrophils predominate during early C. albicans keratitis and aid in fungal eradication (Hohl et al., 2006). Macrophages may also be important (Hu et al., 2009; Zhang et al., 2009). In addition to a role in directing innate responses, chemokines may assist in the development of adaptive immunity during fungal infection (Herring and Huffnagle, 2002). An interactive network of immune responses is likely responsible for the clinical manifestations of ocular inflammation caused by invasive fungi.
We hypothesize that the release of CCL3 leads to the recruitment of neutrophils and macrophages that may, in turn, produce additional CCL3 potentiating the inflammatory reaction and controlling fungal growth. The relative upregulation of chemokine expression in the cornea declines as innate immunity eradicates fungi. In the setting of antifungal therapy, CCL3 may result in undesirable inflammation contributing to stromal destruction and corneal opacification. Modifying the activity of CCL3 and other chemokines may offer a way to modify the outcome of fungal keratitis.
4.3. Chemokines as a therapeutic target in fungal keratitis
We studied how an anti-CCL3 antibody affected the progression of corneal inflammation and fungal infection during C. albicans keratitis. Inhibiting CCL3 activity modulated the severity of leukocytic infiltration, but a deleterious effect on fungal proliferation was not found. Our findings suggest that blocking components of the chemokine system may be an adjunctive therapeutic strategy to alter destructive inflammation during anti-infective treatment (Diab et al., 1999; Xue et al., 2007).
Several proinflammatory pathways are set into play by chemokines. Cytokines such as tumor necrosis factor-α and IL-1β increase during candidal infection (Kullberg et al., 1990; Murray et al., 2005; van der Graaf et al., 2005; Vonk et al., 2006). TNF-α, produced by neutrophils and macrophages, regulates leukocyte recruitment and promotes fungicidal activity during disseminated candidiasis (Romani, 2003). IL-1β also promotes neutrophil infiltration to the site of infection. The expression of these cytokines is downregulated after anti-CCL3 antibody is administered, suggesting that one or more CC chemokines help to direct the production of molecular intermediates during fungal keratitis. Our findings indicate that chemokines and cytokines have pivotal roles in intercellular signaling and that selective inhibition can modulate destructive responses during keratomycosis.
Table 1.
Primers Used for Real-time RT-PCR
| Gene name | Gene symbol | GenBank Accession No. | Assay ID | Amplicon length (bp) |
|---|---|---|---|---|
| CCL chemokine ligand 3 | CCL3 | NM_011337.2 | Mm99999057_m1 | 81 |
| CCL chemokine ligand 2 | CCL2 | NM_011333.3 | Mm99999056_m1 | 96 |
| CXCL chemokine ligand 1 | CXCL1 | NM_008176.2 | Mm00433859_m1 | 74 |
| Tumor necrosis factor | TNF | NM_013693.2 | Mm99999068_m1 | 63 |
| Interleukin 1-β | IL1β | NM_008361.3 | Mm00434228_m1 | 90 |
| Interleukin 6 | IL6 | NM_031168.1 | Mm00446191_m1 | 124 |
| Vascular endothelial growth factor A | VEGFA | NM_001025250.3 | Mm00437304_m1 | 77 |
| Matrix metalloproteinase 8 | MMP8 | NM_008611.4 | Mm00772335_m1 | 127 |
| Matrix metalloproteinase 13 | MMP13 | NM_008607.1 | Mm00439491_m1 | 65 |
| Cathelicidin | CAMP | NM_009921.1 | Mm00438285_m1 | 77 |
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | NM_008084.2 | Mm99999915_gl | 107 |
Assay ID is from Applied Biosystems.
Acknowledgments
This work was supported by the National Institutes of Health (EY02520), the Research to Prevent Blindness, Inc., and the Sid W. Richardson Foundation.
Abbreviations
- CCL2
chemokine (CC-motif) ligand 2
- CCL3
chemokine (CC-motif) ligand 3
- IL-1β
interleukin-1β
- TNF-α
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bacon K, Baggiolini M, Broxmeyer H, Horuk R, Lindley I, Mantovani A, Maysushima K, Murphy P, Nomiyama H, Oppenheim J, Rot A, Schall T, Tsang M, Thorpe R, Van Damme J, Wadhwa M, Yoshie O, Zlotnik A, Zoon K. Chemokine/chemokine receptor nomenclature. J Interferon Cytokine Res. 2002;22:1067–1068. doi: 10.1089/107999002760624305. [DOI] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Diab A, Abdalla H, Li HL, Shi FD, Zhu J, Höjberg B, Lindquist L, Wretlind B, Bakhiet M, Link H. Neutralization of macrophage inflammatory protein 2 (MIP-2) and MIP-1α attenuates neutrophil recruitment in the central nervous system during experimental bacterial meningitis. Infect Immun. 1999;67:2590–2601. doi: 10.1128/iai.67.5.2590-2601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel DJ, Sielaff I, Proudfoot AEI, Handel TM. Chapter 4. Interactions of chemokines with glycosaminoglycans. Methods Enzymol. 2009;461:71–102. doi: 10.1016/S0076-6879(09)05404-4. [DOI] [PubMed] [Google Scholar]
- Herring A, Huffnagle G. Innate immunity and fungal infections. In: Kaufmann SHE, Sher A, Ahmed R, editors. Immunology of Infectious Diseases. ASM Press; Washington, DC: 2002. pp. 127–137. [Google Scholar]
- Hohl TM, Rivera A, Pamer EG. Immunity to fungi. Curr Opin Immunol. 2006;18:465–472. doi: 10.1016/j.coi.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Hu J, Wang Y, Xie L. Potential role of macrophages in experimental keratomycosis. Invest Ophthalmol Vis Sci. 2009;50:2087–2094. doi: 10.1167/iovs.07-1237. [DOI] [PubMed] [Google Scholar]
- Huang X, Hazlett LD. Analysis of Pseudomonas aeruginosa corneal infection using an oligonucleotide microarray. Invest Ophthalmol Vis Sci. 2003;44:3409–3416. doi: 10.1167/iovs.03-0162. [DOI] [PubMed] [Google Scholar]
- Inoue K, Takano H, Oda T, Yanagisawa R, Tamura H, Ohno N, Adachi Y, Ishibashi K, Yoshikawa T. Candida soluble cell wall β-D-glucan induces lung inflammation in mice. Int J Immunopathol Pharmacol. 2007;20:499–508. doi: 10.1177/039463200702000308. [DOI] [PubMed] [Google Scholar]
- Jackson BE, Wilhelmus KR, Mitchell BM. Genetically regulated filamentation contributes to Candida albicans virulence during corneal infection. Microb Pathog. 2007;42:88–93. doi: 10.1016/j.micpath.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kercher L, Wardwell SA, Wilhelmus KR, Mitchell BM. Molecular screening of donor corneas for fungi before excision. Invest Ophthalmol Vis Sci. 2001;42:2578–2583. [PubMed] [Google Scholar]
- Kernacki KA, Goebel DJ, Poosch MS, Hazlett LD. Early cytokine and chemokine gene expression during Pseudomonas aeruginosa corneal infection in mice. Infect Immun. 1998;66:376–379. doi: 10.1128/iai.66.1.376-379.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg BJ, van ‘t Wout JW, van Furth R. Role of granulocytes in increased host resistance to Candida albicans induced by recombinant interleukin-1. Infect Immun. 1990;58:3319–3324. doi: 10.1128/iai.58.10.3319-3324.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Ikema K, Tanihara H. Role of cytokines and chemokines in pseudomonal keratitis. Cornea. 2005;24:S43–S49. doi: 10.1097/01.ico.0000178737.35297.d4. [DOI] [PubMed] [Google Scholar]
- Melgarejo E, Medina MA, Sanchez-Jimenez F, Urdiales JL. Monocyte chemoattractant protein-1: a key mediator in inflammatory processes. Int J Biochem Cell Biol. 2009;41:998–1001. doi: 10.1016/j.biocel.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002;13:455–481. doi: 10.1016/s1359-6101(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Murray RZ, Kay JG, Sangermani DG, Stow JL. A role for the phagosome in cytokine secretion. Science. 2005;310:1492–1495. doi: 10.1126/science.1120225. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Yoshida S, Ono M, Onoue H, Ito Y, Ishibashi T, Inomata H, Kuwano M. Induction of macrophage inflammatory protein-1α and vascular endothelial growth factor during inflammatory neovascularization in the mouse cornea. Angiogenesis. 1999;3:327–334. doi: 10.1023/a:1026554404941. [DOI] [PubMed] [Google Scholar]
- Phadke AP, Mehrad B. Cytokines in host defense against Aspergillus: recent advances. Med Mycol. 2005;43 (Suppl 1):S173–S176. doi: 10.1080/13693780500052099. [DOI] [PubMed] [Google Scholar]
- Romani L. Cytokines of innate and adaptive immunity to Candida albicans. In: Kotb M, Calandra T, editors. Cytokines and Chemokines in Infectious Diseases Handbook. Humana; Totowa, NJ: 2003. pp. 227–241. [Google Scholar]
- Saavedra M, Taylor B, Lukacs N, Fidel PLJ. Local production of chemokines during experimental vaginal candidiasis. Infect Immun. 1999;67:5820–5826. doi: 10.1128/iai.67.11.5820-5826.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Baggiolini M. Chemokines and leukocyte traffic. Nat Immunol. 2008;9:949–952. doi: 10.1038/ni.f.214. [DOI] [PubMed] [Google Scholar]
- Spandau UH, Toksoy A, Verhaart S, Gillitzer R, Kruse FE. High expression of chemokines Gro-α (CXCL-1), IL-8 (CXCL-8), and MCP-1 (CCL-2) in inflamed human corneas in vivo. Arch Ophthalmol. 2003;121:825–831. doi: 10.1001/archopht.121.6.825. [DOI] [PubMed] [Google Scholar]
- Sun RL, Jones DB, Wilhelmus KR. Clinical characteristics and outcome of Candida keratitis. Am J Ophthalmol. 2007;143:1043–1045. doi: 10.1016/j.ajo.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano K, Al-Mokdad M, Shibata F, Tsuchiya H, Nakagawa H. Rat macrophage inflammatory protein-1α, a CC chemokine, acts as a neutrophil chemoattractant in vitro and in vivo. Inflammation. 1999;23:411–424. doi: 10.1023/a:1021908908833. [DOI] [PubMed] [Google Scholar]
- Traynor TR, Huffnagle GB. Role of chemokines in fungal infections. Med Mycol. 2001;39:41–50. doi: 10.1080/mmy.39.1.41.50. [DOI] [PubMed] [Google Scholar]
- van der Graaf CA, Netea MG, Verschueren I, van der Meer JW, Kullberg BJ. Differential cytokine production and toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect Immun. 2005;73:7458–7464. doi: 10.1128/IAI.73.11.7458-7464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuganti GK, Garg P, Gopinathan U, Naduvilath TJ, John RK, Buddi R, Rao GN. Evaluation of agent and host factors in progression of mycotic keratitis: a histologic and microbiologic study of 167 corneal buttons. Ophthalmology. 2002;109:1538–1546. doi: 10.1016/s0161-6420(02)01088-6. [DOI] [PubMed] [Google Scholar]
- Vonk AG, Netea MG, van Krieken JH, Iwakura Y, van der Meer JWM, Kullberg BJ. Endogenous interleukin (IL)-1α and IL-1β are crucial for host defense against disseminated candidiasis. J Infect Dis. 2006;193:1419–1426. doi: 10.1086/503363. [DOI] [PubMed] [Google Scholar]
- Wu TG, Wilhelmus KR, Mitchell BM. Experimental keratomycosis in a mouse model. Invest Ophthalmol Vis Sci. 2003;44:210–216. doi: 10.1167/iovs.02-0446. [DOI] [PubMed] [Google Scholar]
- Xue ML, Thakur A, Cole N, Lloyd A, Stapleton F, Wakefield D, Willcox MD. A critical role for CCL2 and CCL3 chemokines in the regulation of polymorphonuclear neutrophils recruitment during corneal infection in mice. Immunol Cell Biol. 2007;85:525–531. doi: 10.1038/sj.icb.7100082. [DOI] [PubMed] [Google Scholar]
- Xue ML, Thakur A, Willcox MD, Zhu H, Lloyd AR, Wakefield D. Role and regulation of CXC-chemokines in acute experimental keratitis. Exp Eye Res. 2003;76:221–231. doi: 10.1016/s0014-4835(02)00270-1. [DOI] [PubMed] [Google Scholar]
- Yoon KC, De Paiva CS, Qi H, Chen Z, Farley WJ, Li DQ, Pflugfelder SC. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007;48:2561–2569. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- Yuan X, Mitchell BM, Wilhelmus KR. Expression of matrix metalloproteinases during experimental Candida albicans keratitis. Invest Ophthalmol Vis Sci. 2009;50:737–742. doi: 10.1167/iovs.08-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Wilhelmus KR. Corneal neovascularization and vascular endothelial growth factor during experimental Candida albicans keratitis. Mol Vis. 2009;15:1988–1996. [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Wilhelmus KR. Toll-like receptors involved in the pathogenesis of experimental Candida albicans keratitis. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.09-4330. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Chen H, Niu J, Wang Y, Xie L. Role of adaptive immunity in the pathogenesis of Candida albicans keratitis. Invest Ophthalmol Vis Sci. 2009;50:2653–2659. doi: 10.1167/iovs.08-3104. [DOI] [PubMed] [Google Scholar]
- Zhong W, Yin H, Xie L. Expression and potential role of major inflammatory cytokines in experimental keratomycosis. Mol Vis. 2009;15:1303–1311. [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7:243.1–243.11. doi: 10.1186/gb-2006-7-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]