Abstract
Background
Amiodarone and ranolazine have been characterized as inactivated- and activated-state blockers of cardiac sodium channel current (INa), respectively, and shown to cause atrial-selective depression of INa-related parameters. This study tests the hypothesis that their combined actions synergistically depress INa–dependent parameters in atria but not ventricles.
Methods and Results
The effects of acute ranolazine (5–10 μM) were studied in coronary-perfused right atrial and left-ventricular wedge preparations and superfused left atrial pulmonary vein (PV) sleeves isolated from chronic amiodarone-treated (40 mg/kg daily for 6 weeks) and untreated dogs. Floating and standard microelectrode techniques were used to record transmembrane action potentials. When studied separately, acute ranolazine and chronic amiodarone caused atrial-predominant depression of INa-dependent parameters. Ranolazine produced a much greater reduction in Vmax and much greater increase in diastolic threshold of excitation and effective refractory period in atrial preparations isolated from amiodarone-treated vs untreated dogs, leading to a marked increase in post-repolarization refractoriness. The drug combination effectively suppressed triggered activity in PV sleeves, but produced relatively small changes in INa-dependent parameters in the ventricle. Acetylcholine (0.5 μM) and burst pacing induced atrial fibrillation (AF) in 100% of control atria, 75% of ranolazine (5 μM)-treated atria, 16% of atria from amiodarone-treated dogs, and in 0% of atria from amiodarone-treated dogs exposed to 5 μM ranolazine.
Conclusions
The combination of chronic amiodarone and acute ranolazine produces a synergistic use-dependent depression of INa–dependent parameters in isolated canine atria, leading to a potent effect of the drug combination to prevent the induction of AF.
Keywords: Atrial fibrillation, Antiarrhythmic drugs, Sodium channel blocker, Electrophysiology, Pharmacology
INTRODUCTION
Ranolazine is an antianginal agent recently shown to possess antiarrhythmic activity in ventricular and atrial myocytes, including pulmonary vein (PV) sleeve preparations. 1–6 Chronic amiodarone is commonly used for the treatment of ventricular and supraventricular arrhythmias, including atrial fibrillation (AF).7, 8
Recent studies have demonstrated that amiodarone is an atrial-selective, inactivated-state blocker of cardiac sodium channel activity and that ranolazine is an atrial-selective, activated-state blocker of sodium channel activity.4, 9 We hypothesized that the combination of ranolazine and chronic amiodarone would act synergistically to cause potent use-dependent depression of sodium channel current (INa)-dependent parameters in atrial but not ventricular tissues.
The present study was designed to determine the electrophysiologic and antiarrhythmic effects of ranolazine in coronary-perfused right atrial and left ventricular preparations and superfused PV sleeves isolated from untreated and chronic amiodarone-treated dogs.
METHODS
This investigation conforms to the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No 85–23, Revised 1996) and was approved by the animal care and use committee (ACUC) of the Masonic Medical Research Laboratory.
Adult mongrel dogs weighing 20–35 kg were anticoagulated with heparin (180 IU/kg) and anesthetized with sodium pentobarbital (35 mg/kg, IV). The chest was opened via a left-thoracotomy and the heart excised and placed in a cold cardioplegic solution ([K+]0 = 12 mmol/L, 4°C).
Arterially-perfused atrial and ventricular preparations
In vitro experiments were performed using isolated arterially-perfused canine right atrial (RA) and left ventricular (LV) coronary-perfused wedge preparations (≈3.0×1.2×1.2 cm). The methods used for isolation, perfusion of these preparations have been described in previous publications.1, 4, 10 Briefly, the preparations were dissected from hearts removed from anesthetized (sodium pentobarbital) adult mongrel dogs (20–25 kg), untreated or treated with chronic amiodarone (40 mg/kg/day for 6 weeks). Unfolded RA with a rim of the right ventricle attached was perfused through the ostium of the right coronary artery and the LV wedge was perfused through a diagonal branch of the left anterior descending coronary artery. Unperfused tissue was removed with a razor blade or scissors. The cut ventricular and atrial coronary artery branches were ligated using silk thread. After these procedures (performed in cold cardioplegic solution, 4–8°C), the preparations were transferred to a temperature-controlled bath and arterially-perfused with Tyrode’s solution by use of a roller pump at a rate of 8 to 10 ml/min. The composition of the Tyrode’s solution was (in mM): NaCl 129, KCl 4, NaH2PO4 0.9, NaHCO3 20, CaCl2 1.8, MgSO4 0.5, and D-glucose 5.5, buffered with 95% O2 and 5% CO2 (37±0.5 °C, pH=7.35).
Transmembrane action potential (AP) recordings were obtained using standard or floating glass microelectrodes. A pseudo-electrocardiogram (ECG) was recorded using two electrodes consisting of Ag/AgCl half cells placed in the Tyrode’s solution bathing the preparation, 1.0 to 1.2 cm from the two opposite sides of the atrial or ventricular coronary-perfused preparations. Diastolic threshold of excitation (DTE) was determined by increasing stimulus intensity in 0.01 mA steps. Effective refractory period (ERP) was measured by delivering premature stimuli at progressively shorter S1-S2 intervals after every 10th basic beats at a pacing cycle length (CL) of 500 ms (5 ms steps; 2 times the DTE). Post-repolarization refractoriness (PRR) was recognized when ERP exceeded action potential duration measured at 90% repolarization (APD90) in the ventricle and APD measured at 75% repolarization (APD75) in atria. Ventricular ERP was coincident with APD90, whereas atrial ERP was generally coincident with APD75.4 Stable action potential recordings could not be readily obtained in the vigorously contracting perfused preparations. Only action potentials having amplitudes of at least 100 mV were considered in the analysis. The largest recorded maximum rate of rise of the AP upstroke (Vmax) values per condition were taken for statistical comparison. The largest Vmax criterion was used because this was associated with the largest amplitude and the most negative resting membrane potential, depicting full or near full impalement. Due to a substantial inter-preparation Vmax values were normalized for each experiment and then averaged.
Experimental Protocols
The equilibration period for the preparations was 30–120 min. The electrical parameters described above were recorded at pacing CLs of 500 and 300 ms. To determine the anti-AF potential of ranolazine, we used acetylcholine (ACh, 0.5–1.0 μM) together with burst pacing in an attempt to induce persistent AF in coronary-perfused right atria from untreated and amiodarone-treated dogs.4, 11
Superfused pulmonary vein sleeve preparation
PV sleeve preparations (approximately 2.0 × 1.5 cm) were isolated from canine left atria. The thickness of the preparation was approximately 2 mm. Left superior pulmonary veins were used in most experiments. The preparations were placed in a small tissue bath and superfused with Tyrode’s solution. PV preparations were stimulated at a basic cycle length (BCL) of 1000 ms during the equilibration period (1h) using electrical stimuli of 1–3 ms duration and 2.5 times diastolic threshold intensity delivered through silver bipolar electrodes insulated except at the tips. Transmembrane potentials were recorded using glass microelectrodes filled with 2.7 M KCl (10–20 MΩ DC resistance) connected to a high input-impedance amplification system (World Precision Instruments, model KS-700, New Haven, CT). Transmembrane action potentials were recorded at a sampling rate of 41 kHz.
Drugs
Amiodarone (Cordarone®, 200 mg TAB) was obtained from Wyeth Pharmaceuticals, Vonore, TN, USA and was chronically administered orally at a dose of 40 mg/kg/day for a period of 6 weeks. Ranolazine (CV Therapeutics, now Gilead Sciences, Palo Alto) was used at concentrations of 5 and 10 μM).
Statistics
Statistical analysis was performed using one way analysis of variance (ANOVA) for multiple groups or repeated measures ANOVA followed by Bonferroni’s test as appropriate. The only exception was with comparison of changes in diastolic threshold of excitability (DTE), because, in contrast to all the other data sets in which we report an n of 1 for each dog/preparation/condition, DTE values in some cases had an n of 2 for each atria (i.e., DTE was measured in both crista terminalis and pectinate muscle regions of the right atrium). The following statistical approach was used for DTE comparison. In order to recognize that each preparation could have up to four measurements according to region and treatment with ranolazine, a mixed model including effects for region, chronic treatment with amiodarone (inter-preparation comparison), before or after treatment with ranolazine (intra-preparation comparison), and interaction of treatment with amiodarone and ranolazine, and with repeated measurements for region and treatment with ranolazine was performed. We have used a completely general covariance matrix (unstructured for both region and treatment with ranolazine) and adopted the “Kenward Roger” option for degrees of freedom. The hypothesis of synergy corresponds to an interaction between the pre-treatment of amiodarone versus placebo with before versus after treatment with ranolazine. The criterion for declaring statistically significant differences was p < 0.05 based on Bonferroni adjustment. All data are expressed as mean ± SD. The analysis of testing synergistic effect was conducted using software SAS 9.1.3 (SAS Institute Inc., Cary, NC, USA.), the Proc Mixed procedure.
RESULTS
Effects of ranolazine on APD, ERP and DTE
Under control conditions, coronary-perfused atrial ERP was coincident with the APD value at 75% repolarization (APD75), whereas ventricular ERP was coincident with APD90. APD and ERP were significantly longer in both atrial and ventricular preparations isolated from chronic amiodarone-treated dogs, compared to the respective tissues isolated from non-treated animals (Fig. 1). Amiodarone-induced APD and ERP prolongation was greater in atria vs. ventricles. ERP increased to a greater degree than APD in both atria and ventricles, particularly in the atria, due to development of post-repolarization refractoriness (PRR=ERP−APD).
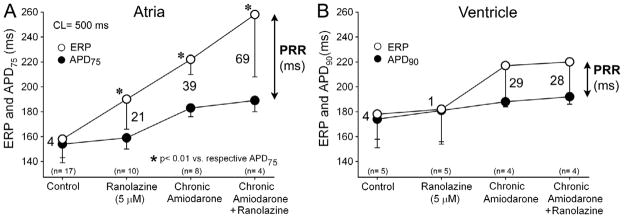
Figure 1.
Effects of acute ranolazine, chronic amiodarone, and its combination on action potential duration measured at 75 % and 90 % repolarization (APD75 and APD90) and effective refractory period (ERP) in coronary-perfused right atrial (A) and left ventricular wedge (B) preparations. Ranolazine significantly prolongs ERP but not APD in the atrium causing significant post repolarization refractoriness (PRR) in atrial, but not ventricular preparations isolated from chronic amiodarone-treated dogs. ERP-APD = PRR. n = 4–17. * p<0.01 vs respective APD75 controls: Chronic amiodarone + Ranolazine vs. Chronic amiodarone alone, Ranolazine (5 μM) vs. control, Chronic amiodarone vs. control. Atria: control n=17 dogs; ranolazine n=10 dogs; chronic amiodarone n =8 dogs; chronic amiodarone+ranolazine n=4 dogs. Ventricle: control n=5 dogs; ranolazine n=5 dogs; chronic amiodarone n =4 dogs; chronic amiodarone+ranolazine n=4 dogs.
In atrial preparations (Fig 1A), addition of ranolazine (5 μM) slightly prolonged APD75 in either untreated controls (from 154±11 to 159±9 ms; p=0.245) or chronic amiodarone atria (from 183±7 to 189±9 ms; p=0.156), but significantly prolonged ERP, in untreated (from 158±18 to 190±24 ms, p<0.05) and chronic amiodarone (from 217±9 to 258±50 ms, p<0.01). Ranolazine alone produced a 21 ms increase in PRR, whereas chronic amiodarone alone increased PRR by 39 ms. The combination of the two produced a synergistic effect, increasing PRR by 69 ms. In marked contrast, addition of ranolazine produced no significant change in either APD or ERP in ventricular wedge preparations isolated from untreated or chronic amiodarone-treated dogs (Fig. 1B). Ventricular PRR did not increase at all with ranolazine alone and only modestly with the combination of ranolazine and chronic amiodarone treatment (28 ms). Both treatments alone or combined caused produced an atrial-preferential prolongation of ERP and PRR.
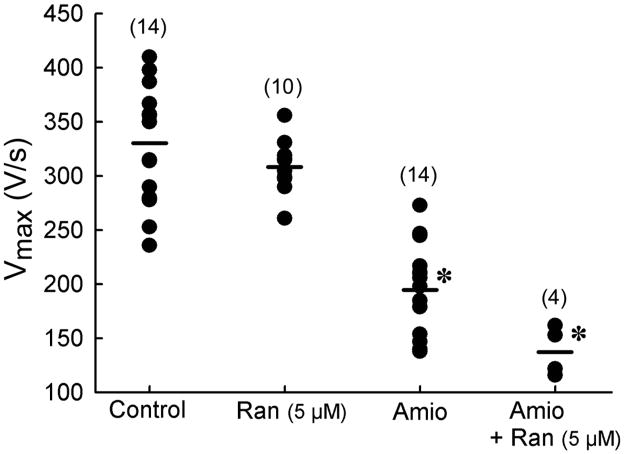
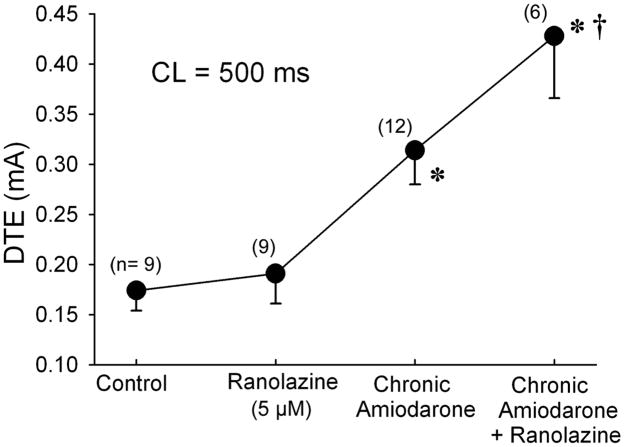
Ranolazine (5 μM) reduced Vmax of atrial AP to a much greater extent in atrial preparations isolated from chronic amiodarone-treated dogs, compared to untreated animals (Fig. 2). Ranolazine alone caused a small increase in DTE in endocardial pectinate muscle and crista terminalis of the coronary–perfused atrial preparation (0.17±0.02 to 0.19±0.03 mA, Δ=0.02 mA), chronic amiodarone increased DTE to 0.31±0.03 mA (Δ=0.14 mA) and the two treatments combined produced a synergistic effect increasing DTE to 0.42±0.06 mA (Δ=0.25 mA) (Fig 3) (p<0.05, chronic amiodarone + ranolazine vs chronic amiodarone).
Figure 2.
Synergistic reduction of the maximum rate of rise of the action potential upstroke (Vmax) by combination of chronic amiodarone and acute ranolazine in canine coronary perfused right atrial preparations. Shown are Vmax values from individual experiments n=4–14. Control (n=14), Ran = Ranolazine (n=10); Amio = Chronic amiodarone (n=14), Ran + Amio (n=4). * p<0.0.5 vs control and Ran 5 μM. Control n=14 dogs; ranolazine n=10 dogs; chronic amiodarone n =14 dogs; chronic amiodarone+ranolazine n=4 dogs.
Figure 3.
Synergistic effects of acute ranolazine and chronic amiodarone to significantly increase diastolic threshold of excitation (DTE) in coronary-perfused right atrial preparations. n=6–12. * p<0.001 vs control; † p<0.01 vs chronic Amiodarone alone and vs ranolazine. Control n=9 recordings (8 PM and 1 CT) from 8 dogs; ranolazine n=9 recordings(8 PM and 1 CT) from 8 dogs; chronic amiodarone n =12 recordings (7 PM and 5 CT) from 8 dogs; chronic amiodarone+ranolazine n=6 recordings (4 PM and 2 CT) from 4 dogs.
Effects of ranolazine on activation failure in atrial vs ventricular preparations
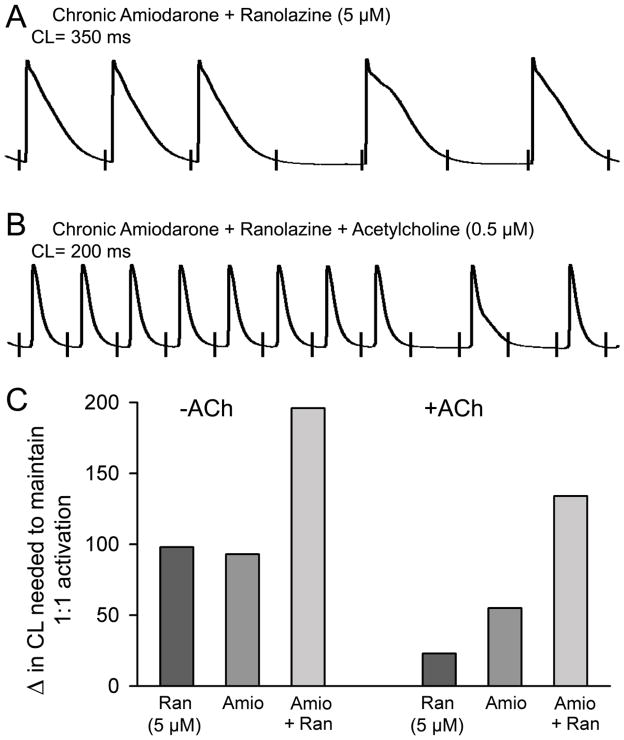
In coronary-perfused atrial preparations, the shortest pacing CL permitting a 1:1 response was 129±8 ms in untreated controls, 221±39 ms with chronic amiodarone treatment, 234±49 ms after acute ranolazine treatment alone (5 uM) and 325±34 ms after combined chronic amiodarone and ranolazine treatment (5 uM) (p<0.01 vs. either treatment alone) (Fig. 4 and Table 1), reflecting reduced excitability and accentuated PRR. In the presence of acetylcholine (ACh, 0.5 uM), the shortest pacing CL permitting a 1:1 response was 71±12 ms in untreated controls, 136±22 ms with chronic amiodarone treatment, 94±31 ms with acute ranolazine treatment alone, and 205±34 ms with chronic amiodarone + ranolazine treatment.
Figure 4.
Potent depression of excitability induced by a combination of chronic amiodarone and acute ranolazine (5 μM) leading to activation failure at rapid rates in coronary-perfused right atria. A: Failure of 1:1 activation at a pacing cycle length (CL) of 350 ms. B: Failure of 1:1 activation at a CL of 200 ms in the presence of acetylcholine (B). C: Increase in CL needed to maintain 1:1 activation (see Table 1 for actual numbers). Ran = Ranolazine (n=10); Amio = Chronic amiodarone (n=8). Ran + Amio (n=4). Ran n=10 dogs; Amio n =8 dogs; Amio+Ran n=4 dogs.
Table 1.
Shortest S1-S1 permitting 1:1 activation under control conditions, following chronic amiodarone, ranolazine and the combination of chronic amiodarone and acute ranolazine.
| Shortest S1-S1 permitting 1:1 activation (ms) | ||
|---|---|---|
| + Acetylcholine (0.5 μM) | ||
| Control (n= 11) | 129 ± 8 | 71 ± 12 |
| Chronic Amiodarone (n= 8) | 222 ± 39* | 136 ± 22* |
| Ranolazine (5 μM) (n= 10) | 227 ± 38* | 94 ± 31* |
| Chronic Amiodarone + Ranolazine (5 μM) (n= 4) | 325 ± 34*† | 205 ± 34*† |
p<0.05 vs. respective controls: chronic Amiodarone + Ranolazine vs. chronic Amiodarone, Ranolazine (5 μM) vs. control and chronic Amiodarone vs. control
p<0.05 vs. Ranolazine (5 μM), chronic Amiodarone and control
In ACh-pretreated preparations, burst pacing induced AF in 100% of controls from untreated animals (10/10), in 16% (1 of 6) of atria isolated from chronic amiodarone-treated dogs, in 75% (3 of 4) of atria treated with ranolazine (5 uM) only and in 0% (0 of 4) in preparations with combined chronic amiodarone and ranolazine (5 uM) treatments.
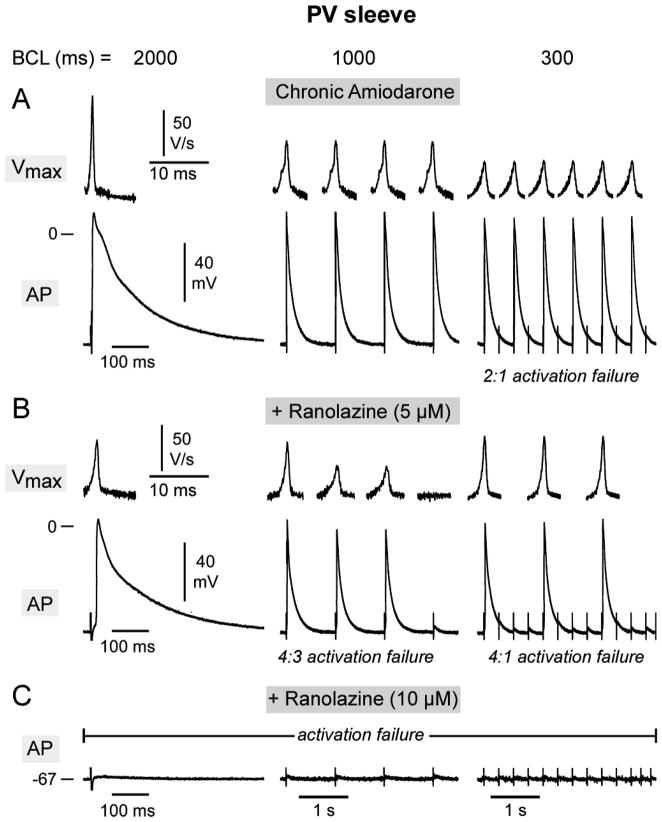
Similar depression of excitability was observed in PV sleeve preparations. Fig. 5 shows use-dependent depression of Vmax and action potential in PV sleeves. The addition of 5 uM ranolazine caused a marked reduction in Vmax and excitability resulting in 4:3 and 4:1 activation failure at CLs of 1000 and 300 ms. Addition of 10 μM ranolazine produced complete activation failure in the PV sleeve preparation.
Figure 5.
Rate-dependent effects of chronic amiodarone, alone and combined with acute ranolazine (5 and 10 uM), on maximum rate of rise of action potential upstroke (Vmax) and action potential characteristics in a pulmonary vein (PV) sleeve preparation. A–C: Action potentials (AP) recorded at different basic cycle lengths (BCLs) from a PV sleeve isolated from a chronic amiodarone-treated dog before (panel A) and after addition of ranolazine (5 and 10 μM) (panel B and C). In the absence of ranolazine, the chronic amiodarone-treated PV sleeve displayed 2:1 activation failure at BCL 300 ms. The addition of 5 μM ranolazine induced a marked decrease in Vmax and 4:3 and 4:1 activation failure at 1000 and 300 ms, respectively. The addition of 10 μM ranolazine rendered the preparation inexcitable.
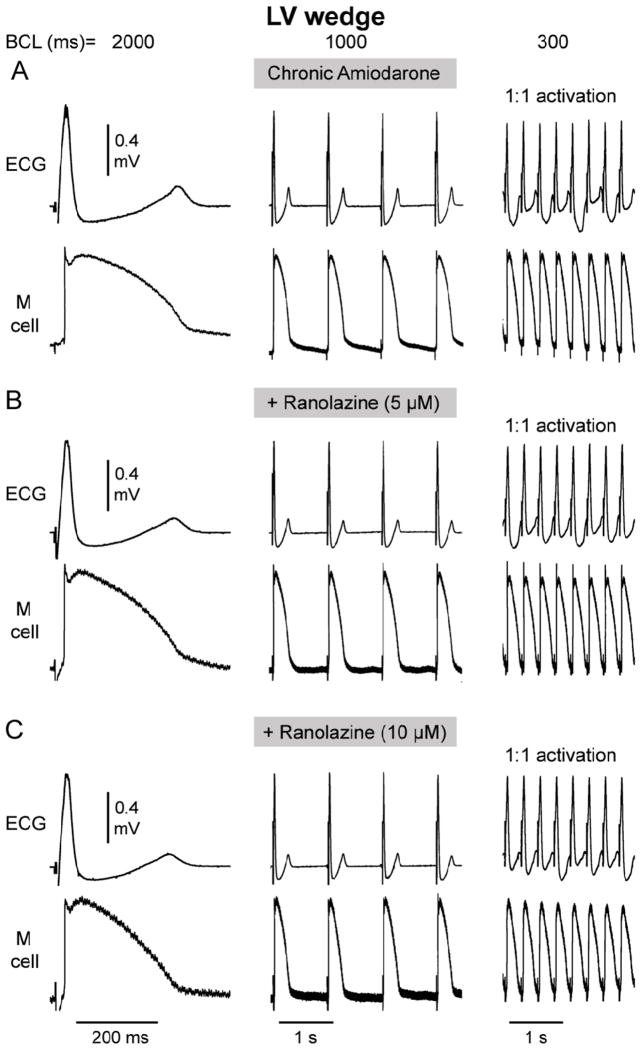
Acceleration-induced activation failure was not observed in ventricular wedge preparations isolated from LV wedge of chronic amiodarone-treated dogs either in the absence or presence of ranolazine (5 and 10 uM; Fig. 6).
Figure 6.
Rate-dependence of action potential and conduction characteristics in LV wedge preparation isolated from chronic amiodarone-treated dog in the absence and presence of acute ranolazine (5 and 10 uM). Each panel shows a pseudo-ECG (top trace) and action potentials recorded from the M cell region. 1:1 activation persisted following a decrease in basic cycle length (BCL) from 2000 to 1000 and 300 ms.
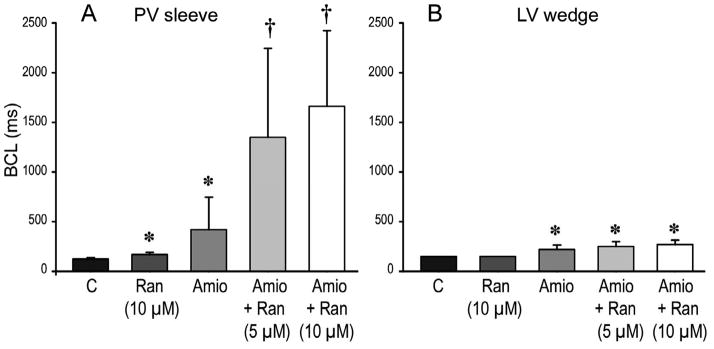
The marked rate-dependent depression of INa in PV sleeve preparations, as reflected by the use-dependent decrease in Vmax, led to activation failure at slower rates in chronic amiodarone-treated preparations. This effect was much more accentuated in the presence of ranolazine; Figure 7 shows composite data of the effect of ranolazine on the BCL at which 1:1 activation failure first occurred in pulmonary vein sleeve (Fig. 7A) and LV wedge (Fig 7B) preparations isolated from untreated and chronic amiodarone-treated dogs. In untreated preparations, ranolazine (10 μM) induced a significant increase in the BCL at which activation failure occurred in PV but not LV wedge preparations. Chronic amiodarone induced a significant increase in both PV sleeve and LV wedge preparations, but a much greater increase in PV sleeves. The combination of chronic amiodarone and ranolazine produced a remarkable synergistic increase in the BCL permitting 1:1 activation in PV sleeves, with little additional effect in the ventricular wedge preparations.
Figure 7.
Composite data of the effect of ranolazine (5 or 10 μM) on basic cycle length (BCL) at which 1:1 activation failure occurred in pulmonary vein (PV) sleeve (A, n=5) and left ventricular (LV) wedge (B, n=4) preparations isolated from untreated and chronic amiodarone-treated (Amio) dogs. In untreated dogs, ranolazine (10 μM) induced a significant increase of the BCL permitting 1:1 activation in PV but not LV wedge preparations. Chronic amiodarone treatment led to a significant increase in the basic cycle length (BCL) permitting 1:1 activation in both PV sleeve and LV, wedge, which was greatly accentuated in PV sleeves but not LV wedges following the addition of ranolazine (5 and 10 μM). *p<0.05 vs Control, † p<0.05 Amio+Ranolazine vs Amio alone. Ran = ranolazine; Amio = chronic amiodarone. PV sleeve: n=5 dogs for each condition; LV wedge: n =4 dogs for each condition.
Effects of acute ranolazine and chronic amiodarone on EAD and DAD-induced triggered activity
Previous studies have shown that ranolazine (10 μM) alone suppresses late phase 3 early afterdepolarizations (EADs), delayed afterdepolarizations (DADs) and triggered activity elicited by exposure of the PV sleeves to ACh + isoproterenol, or high [Ca2+]o + rapid pacing6 and that chronic amiodarone is capable of preventing the appearance of DADs, late phase 3 EADs, and triggered activity induced at fast rates in PV sleeves in 80% of PV sleeve preparations exposed to ACh, isoproterenol, high [Ca2+]o or their combination.12 In the present study, neither DADs nor late phase 3 EADs were observed in PV sleeves in the presence of combined ranolazine and chronic amiodarone (n=6).
DISCUSSION
The results of the present study indicate that the combination of chronic amiodarone and relatively low concentrations of acute ranolazine (therapeutic range = 2–8 μM) produces a synergistic use-dependent depression of sodium channel–dependent parameters including Vmax, DTE, PRR, 1:1 activation in isolated canine atria (coronary-perfused right atria and superfused left atrial PV sleeve preparations), leading to a much more potent effect of the drug combination to prevent the induction of AF. Importantly, left ventricular preparations were much less affected by the drug combination. Altogether the data show a marked atrial selectivity of combined acute ranolazine and chronic amiodarone to depress sodium channel-dependent parameters.
Pharmacologic and electrophysiological profile of chronic amiodarone and ranolazine
Amiodarone is widely used in the treatment of both supraventricular and ventricular arrhythmias. Its mechanism of action includes inhibition a number of cardiac ionic currents (IKr, IKs, INa, late INa, Ito, ICa-L, ICa-T, IKl, IK(ACh) IK(ATP)) as well as α- and β-adrenoceptor blocking activity.8,13 Acute amiodarone inhibits IKr, whereas chronic amiodarone also leads to a reduction of IKs.14 Chronic amiodarone, prolongs ventricular APD and QT interval.9,13,15 Both acute and chronic amiodarone have been shown to prolong the effective refractory period more than APD, resulting in post-repolarization refractoriness (PRR).16,17 While acute amiodarone reduces Vmax and blocks INa in practically all studies (for review see13), chronic amiodarone has been reported to either depress Vmax 9,18–20 or to cause little to no change in Vmax in superfused ventricular muscle and Purkinje fibers.13,15,21 Ventricular conduction velocity is slowed and QRS duration is prolonged in both acute and chronic amiodarone-treated humans and animals in vivo21–23
Ranolazine has been shown to have a pharmacologic profile similar to that of chronic amiodarone. Like amiodarone, ranolazine, although at different concentrations, causes inhibition of INa, IKr, and ICa.1, 24 Both agents have relatively rapid unbinding kinetics from the sodium channel (τ = 1.56 ± 56 and 0.3–1.6 sec, for ranolazine and chronic amiodarone, respectively).4, 13 Like amiodarone, ranolazine produces an atrial-selective depression of INa-dependent parameters. Unlike amiodarone, which is an inactivated-state blocker of cardiac sodium channels,13, 25 ranolazine is an activated-state blocker.26 We hypothesized that an atrial-selective activated state blocker such as ranolazine would potentiate the effects of atrial selective inactivated state blocker such as amiodarone in producing use-dependent depression of INa-mediated parameters and thus potentiate the effectiveness of amiodarone in the management of AF. Our results provide evidence in support of this hypothesis.
Atrial selective effects of ranolazine and chronic amiodarone on sodium channel activity
The combination of rapid dissociation of drug from the sodium channel and an effect to preferentially prolong atrial APD, secondary to IKr block, have been suggested to be key features for atrial-selective depression of sodium channel-dependent parameters and anti-AF efficacy.4, 20
Our data indicate the combination of ranolazine and chronic amiodarone treatment produce an atrial selective use-dependent depression of Vmax and excitability that is much greater than either treatment alone and greater than the algebraic sum of the individual treatments, thus pointing to a synergism of the effects of the two therapies.4, 6, 9, 20
Under control conditions, take-off potential (TOP) of PV sleeves was −81 ± 4, −80.6 ± 4, −79.8 ± 3,−77.6 ± 3 and −75.6 ± 3 mV at BCLs of 2000, 1000, 500, 300 and 200 ms, respectively. Thus, a change in BCL from 2000 to 200 ms results in a 5 mV depolarization of TOP. It is difficult to record a stable resting membrane potential (RMP) from vigorously contracting coronary-perfused LV wedge preparations. However when stable recordings were obtained during a change in rate, no changes in RMP were observed between BCL 2000 and 200 ms. Thus, average RMP in LV is more hyperpolarized at 85.3 ± 2 mV and does not depolarize appreciably at rapid rates. The difference in behavior of TOP between atrial and ventricular tissues at fast rates is attributable to the much slower action potential phase 3 in atrial vs. ventricular cells.4 A more positive TOP clearly contributes to the greater depression of atrial vs ventricular Vmax. We have previously shown that this contributes to the atrial selectivity of agents such as ranolazine and amiodarone.4, 9
The atrial-selective effects of both chronic amiodarone and ranolazine to depress peak INa and INa-dependent parameters are thought to result from the more negative steady-state inactivation relationship for INa as well as to the much slower action potential phase 3 of atrial compared to ventricular cells.4,9, 20 The slow phase 3 repolarization in atrial cells potentiates the effect of chronic amiodarone at rapid activation rates by abbreviating the diastolic interval and raising the take-off potential of the next beat, thus reducing the availability of sodium channels and the number of channels in the rested state, from which the drug unbinds. Another factor that contributes to the reduced availability of peak INa in atria is the more depolarized resting membrane potential of atrial compared to ventricular cells.4, 9, 20 Intrinsic atrioventricular differences in resting membrane potential is due principally to a smaller IK1 in atrial vs. ventricular cells.27
Effects on EAD and DAD-induced triggered activity in PV sleeves
Ectopic activity arising from the PV sleeves is thought to be an important source of triggers and in some cases substrate for the development of AF.28–30 Over the past decade, radiofrequency ablation has become the treatment of choice for drug-resistant AF. Segmental PV isolation and circumferential PV ablation are procedures now commonly used to suppress refractory atrial arrhythmias including AF.31
Our results suggest that combined ranolazine and chronic amiodarone prevent the appearance of DADs, late phase 3 EADs, and triggered activity induced in PV sleeves by exposure to ACh, isoproterenol, high [Ca2+]o or their combination. Previous studies have shown that 10 uM ranolazine can suppress EADs, DADs or triggered activity in 100 % (11 of 11) of PV sleeve preparations studied 6 and that chronic amiodarone alone is capable of preventing the appearance of DADs, late phase 3 EADs, and triggered activity induced in PV sleeves in 80 % of PV sleeve preparations. Additionally, chronic amiodarone was observed to prevent ACh-induced marked abbreviation of the action potential. The combination of ranolazine and chronic amiodarone was associated with a high degree of activation failure even at relatively slow rates. Neither EAD, DAD or reentrant activity is likely to develop under these conditions. This results in an apparent pharmacologic ablation of PV sleeve activity.
Study Limitations
The dose of amiodarone used in the present study (40 mg/kg/day) is larger than that typically used in the clinic, where the loading dose of amiodarone ranges from 800–1600 mg/day (approximately 20–25 mg/kg/day). Similar dosage regimens have been used in previous studies reflecting the relatively lower sensitivity of dogs to amiodarone. 32 It should be noted, however, that loading doses are not given to patients for 6 weeks duration, but typically for 1–2 weeks. Plasma or tissue concentrations of amiodarone were not measured in this study. However tissue concentrations were measured in a previous study using similar dose of oral amiodarone in a dog experimental model.19 The tissue concentration of amiodarone in ventricular epicardium was 19.1±8.1 ng/ml and remained largely unchanged over a 6 hours period period. It is noteworthy that this is lower that the tissue concentration of amiodarone found in humans following chronic amiodarone treatment (40 ng/ml) with a loading dose of 600 mg daily for one week, followed by 200 mg daily for a period of seven weeks.33
Clinical implications
Most antiarrhythmic agents shown to be effective in terminating and/or preventing clinical AF or atrial flutter act primarily by reducing sodium channel current, INa (e.g., propafenone or flecainide) or IKr (e.g., dofetilide) or by blocking multiple ion channels (amiodarone). Use of these agents is limited by their potential ventricular proarrhythmic actions and/or organ toxicity at therapeutically effective doses.2, 34, 35
Acute and chronic amiodarone are widely used in the clinical management of atrial and ventricular arrhythmias.8, 36, 37 Chronic amiodarone is the most effective pharmacological agent available for the maintenance of sinus rhythm following termination of AF.8 The anti-arrhythmic efficacy of chronic amiodarone has been attributed to the multiplicity of effects on ion channel activity (Class I, II, III and IV actions), reduction of transmural dispersion of repolarization, induction of post-repolarization refractoriness, prolongation of excitable gap, suppression of triggered activity, and inhibition of atrial remodeling.8,17,19,36,38,39
Ranolazine has a pharmacologic profile similar to that of chronic amiodarone.1, 24 Although clinical trials of the actions of ranolazine to suppress AF are not available as yet, results of the MERLIN trial indicate that the drug shows both ventricular and supraventricular antiarrhythmic activity. Treatment with ranolazine resulted in significantly lower incidence of ventricular tachycardia lasting ≥ 8 beats (5.3% vs 8.3%; p <0.001), supraventricular tachycardia (44.7% vs 55.0%; p < 0.001), or new-onset atrial fibrillation (1.7% vs 2.4%; p = 0.08).40
Both amiodarone and ranolazine are atrial-selective in their actions. The synergism of their combined electrophysiological and anti-AF effects may be due to their interaction with different states of the cardiac sodium channel. The potentiation by ranolazine of the anti-AF effects amiodarone may permit the use of a lower dose of amiodarone to obtain a similar outcome, thus reducing its adverse effects which limit its clinical use.8
Clinical and experimental studies have highlighted the role of PV in the triggering of atrial arrhythmias, AF in particular.41, 42 In the present study, PV-selective depression of INa-related parameters was potentiated by addition of ranolazine to chronic amiodarone, leading to activation failure at relatively long CLs and complete suppression of triggered activity. The combined effect of chronic amiodarone and acute ranolazine suggest that ranolazine may help suppress AF in patients in whom amiodarone was not effective.
The actions of chronic amiodarone plus ranolazine to produce potent block of the sodium channels in the atria are similar to that of Class IC antiarrhythmic agents such as propafenone and flecainide. However, unlike the IC agents, the electrophysiological effects of the drug combination is largely restricted to the atrial myocardium, rendering it atrial-selective.4, 9 The drug combination is thus effective in terminating and preventing the re-induction of AF in experimental models of AF, without exerting an arrhythmogenic effect on ventricular myocardium, as is the case with putative sodium channel blockers like propafenone.
We conclude that the combination of chronic amiodarone and relatively low concentrations of acute ranolazine produces a synergistic use-dependent depression of sodium channel–dependent parameters in isolated canine atria that lead to a potent effect of the drug combination to prevent the induction of AF.
Acknowledgments
We gratefully acknowledge the helpful advice of Jose M. Di Diego, MD and the expert technical assistance of Judith Hefferon and Robert Goodrow, Jr. We are grateful to Whedy Wang PhD for statistical advice.
Funding Sources: Supported by grant HL47678 from NHLBI (CA) and New York State and Florida Grand Lodges of Free and Accepted Masons.
Footnotes
Conflict of Interest Disclosures: Dr. Antzelevitch is a consultant to and received research support from CV Therapeutics (now Gilead Sciences, Inc). Dr. Luiz Belardinelli is an employee of Gilead Sciences, Inc., Palo Alto, CA.
Contributor Information
Serge Sicouri, Masonic Medical Research Laboratory, Utica, NY.
Alexander Burashnikov, Masonic Medical Research Laboratory, Utica, NY.
Luiz Belardinelli, Gilead Sciences, Inc., Palo Alto, CA.
Charles Antzelevitch, Masonic Medical Research Laboratory, Utica, NY.
References
- 1.Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas GP. Electrophysiologic effects of ranolazine: a novel anti-anginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antzelevitch C, Belardinelli L, Wu L, Fraser H, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Goodrow RJ, Scornik FS, Peréz GJ. Electrophysiologic properties and antiarrhythmic actions of a novel anti-anginal agent. J Cardiovasc Pharmacol Therapeut. 2004;9 (Suppl 1):S65–S83. doi: 10.1177/107424840400900106. [DOI] [PubMed] [Google Scholar]
- 3.Antzelevitch C. Ranolazine: a new antiarrhythmic agent for patients with non-ST-segment elevation acute coronary syndromes? Nat Clin Pract Cardiovasc Med. 2008;5:248–249. doi: 10.1038/ncpcardio1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–1457. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sicouri S, Timothy KW, Zygmunt AC, Glass A, Goodrow RJ, Belardinelli L, Antzelevitch C. Cellular basis for the electrocardiographic and arrhythmic manifestations of Timothy syndrome: effects of ranolazine. Heart Rhythm. 2007;4:638–647. doi: 10.1016/j.hrthm.2006.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sicouri S, Glass A, Belardinelli L, Antzelevitch C. Antiarrhythmic effects of ranolazine in canine pulmonary vein sleeve preparations. Heart Rhythm. 2008;5:1019–1026. doi: 10.1016/j.hrthm.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenbaum MB, Chiale PA, Halpern MS, Nau GJ, Przbylski J, Levi RJ, Lazzari JO, Elizari MV. Clinical efficacy of amiodarone as an antiarrhythmic agent. Am J Cardiol. 1976;38:934–944. doi: 10.1016/0002-9149(76)90807-9. [DOI] [PubMed] [Google Scholar]
- 8.Singh BN. Amiodarone: a multifaceted antiarrhythmic drug. Curr Cardiol Rep. 2006;8:349–355. doi: 10.1007/s11886-006-0074-2. [DOI] [PubMed] [Google Scholar]
- 9.Burashnikov A, Di Diego JM, Sicouri S, Ferreiro M, Carlsson L, Antzelevitch C. Atrial-selective effects of chronic amiodarone in the management of atrial fibrillation. Heart Rhythm. 2008;5:1735–1742. doi: 10.1016/j.hrthm.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burashnikov A, Mannava S, Antzelevitch C. Transmembrane action potential heterogeneity in the canine isolated arterially-perfused atrium: effect of IKr and Ito/IKur block. Am J Physiol. 2004;286:H2393–H2400. doi: 10.1152/ajpheart.01242.2003. [DOI] [PubMed] [Google Scholar]
- 11.Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107:2355–2360. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
- 12.Sicouri S, Belardinelli L, Carlsson L, Antzelevitch C. Potent antiarrhythmic effects of chronic amiodarone in canine pulmonary vein sleeve preparations. J Cardiovasc Electrophysiol. 2009;20:803–810. doi: 10.1111/j.1540-8167.2009.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kodama I, Kamiya K, Toyama J. Amiodarone: ionic and cellular mechanisms of action of the most promising class III agent. Am J Cardiol. 1999;84:20R–28R. doi: 10.1016/s0002-9149(99)00698-0. [DOI] [PubMed] [Google Scholar]
- 14.Kamiya K, Nishiyama A, Yasui K, Hojo M, Sanguinetti MC, Kodama I. Short- and long-term effects of amiodarone on the two components of cardiac delayed rectifier K+ current. Circulation. 2001;103:1317–1324. doi: 10.1161/01.cir.103.9.1317. [DOI] [PubMed] [Google Scholar]
- 15.Sosunov EA, Anyukhovsky EP, Rosen MR. Chronic in vivo and in vitro effects of amiodarone on guinea pig hearts. J Pharmacol Exp Ther. 1996;278:906–912. [PubMed] [Google Scholar]
- 16.Maruyama T, Richardson LC, Sun W, McCarthy JJ, Gettes LS. Acute effects of amiodarone on membrane properties, refractoriness, and conduction in guinea pig papillary muscles. Heart Vessels. 1995;10:78–86. doi: 10.1007/BF01744498. [DOI] [PubMed] [Google Scholar]
- 17.Kirchhof P, Degen H, Franz MR, Eckardt L, Fabritz L, Milberg P, Laer S, Neumann J, Breithardt G, Haverkamp W. Amiodarone-induced postrepolarization refractoriness suppresses induction of ventricular fibrillation. J Pharmacol Exp Ther. 2003;305:257–263. doi: 10.1124/jpet.102.046755. [DOI] [PubMed] [Google Scholar]
- 18.Kim YH, Lim HE, Kim SH, Pak HN, Ahn JC, Song WH, Kim YH. Brugada-like ST-segment abnormalities associated with myocardial involvement of hematologic diseases. Pacing Clin Electrophysiol. 2008;31:761–764. doi: 10.1111/j.1540-8159.2008.01082.x. [DOI] [PubMed] [Google Scholar]
- 19.Sicouri S, Moro S, Litovsky SH, Elizari MV, Antzelevitch C. Chronic amiodarone reduces transmural dispersion of repolarization in the canine heart. J Cardiovasc Electrophysiol. 1997;8:1269–1279. doi: 10.1111/j.1540-8167.1997.tb01018.x. [DOI] [PubMed] [Google Scholar]
- 20.Burashnikov A, Antzelevitch C. Atrial-selective sodium channel blockers: do they exist? J Cardiovasc Pharmacol. 2008;52:121–128. doi: 10.1097/FJC.0b013e31817618eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine JH, Moore EN, Kadish AH, Weisman HF, Balke CW, Hanich RF, Spear JF. Mechanisms of depressed conduction from long-term amiodarone theraphy in canine myocardium. Circulation. 1988;78:684–691. doi: 10.1161/01.cir.78.3.684. [DOI] [PubMed] [Google Scholar]
- 22.Nanas JN, Mason JW. Pharmacokinetics and regional electrophysiological effects of intracoronary amiodarone administration. Circulation. 1995;91:451–461. doi: 10.1161/01.cir.91.2.451. [DOI] [PubMed] [Google Scholar]
- 23.Morady F, Dicarlo LA, Krol RB, Baerman JM, De Buitleir M. Acute and chronic effects of amiodarone on ventricular refractoriness, intraventricular conduction and ventricular tachycardia induction. J Am Coll Cardiol. 1986;7:148–157. doi: 10.1016/s0735-1097(86)80273-x. [DOI] [PubMed] [Google Scholar]
- 24.Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006;17:S161–S177. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whalley DW, Wendt DJ, Grant AO. Basic concepts in cellular cardiac electrophysiology: Part II: Block of ion channels by antiarrhythmic drugs. PACE. 1995;18:1686–1704. doi: 10.1111/j.1540-8159.1995.tb06990.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang GK, Calderon J, Wang SY. State- and use-dependent block of muscle Nav1.4 and neuronal Nav1.7 noltage-gated Na+ channel isoforms by ranolazine. Mol Pharmacol. 2008;73:940–948. doi: 10.1124/mol.107.041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golod DA, Kumar R, Joyner RW. Determinants of action potential initiation in isolated rabbit atrial and ventricular myocytes. Am J Physiol. 1998;274:H1902–H1913. doi: 10.1152/ajpheart.1998.274.6.H1902. [DOI] [PubMed] [Google Scholar]
- 28.Haissaguerre M, Jais P, Shah DC, Garrigue S, Takahashi A, Lavergne T, Hocini M, Peng JT, Roudaut R, Clementy J. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation. 2000;101:1409–1417. doi: 10.1161/01.cir.101.12.1409. [DOI] [PubMed] [Google Scholar]
- 29.Nattel S, Allessie MA, Haissaguerre M. Spotlight on atrial fibrillation-the ‘complete arrhythmia’. Cardiovasc Res. 2002;54:197–203. doi: 10.1016/s0008-6363(02)00324-3. [DOI] [PubMed] [Google Scholar]
- 30.Nattel S. Combined parasympathetic-sympathetic nerve discharge and pulmonary vein afterdepolarizations: A new unifying concept with basic and clinical relevance. Heart Rhythm. 2005;2:632–633. doi: 10.1016/j.hrthm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 32.van Opstal JM, Schoenmakers M, Verduyn SC, De Groot SH, Leunissen JD, Der Hulst FF, Molenschot MM, Wellens HJ, Vos MA. Chronic amiodarone evokes no torsade de pointes arrhythmias despite QT lengthening in an animal model of acquired long-QT syndrome. Circulation. 2001;104:2722–2727. doi: 10.1161/hc4701.099579. [DOI] [PubMed] [Google Scholar]
- 33.Holt DW, Tucker GT, Jackson PR, McKenna WJ. Amiodarone pharmacokinetics. Br J Clin Pract Suppl. 1986;44:109–114. [PubMed] [Google Scholar]
- 34.CAST Investigators. Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406–412. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 35.Antzelevitch C, Shimizu W, Yan GX, Sicouri S, Weissenburger J, Nesterenko VV, Burashnikov A, Di Diego JM, Saffitz J, Thomas GP. The M cell: its contribution to the ECG and to normal and abnormal electrical function of the heart. J Cardiovasc Electrophysiol. 1999;10:1124–1152. doi: 10.1111/j.1540-8167.1999.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 36.Zimetbaum P. Amiodarone for atrial fibrillation. N Engl J Med. 2007;356:935–941. doi: 10.1056/NEJMct065916. [DOI] [PubMed] [Google Scholar]
- 37.Goldschlager N, Epstein AE, Naccarelli GV, Olshansky B, Singh B, Collard HR, Murphy E. A practical guide for clinicians who treat patients with amiodarone: 2007. Heart Rhythm. 2007;4:1250–1259. doi: 10.1016/j.hrthm.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 38.Shinagawa K, Shiroshita-Takeshita A, Schram G, Nattel S. Effects of antiarrhythmic drugs on fibrillation in the remodeled atrium: insights into the mechanism of the superior efficacy of amiodarone. Circulation. 2003;107:1440–1446. doi: 10.1161/01.cir.0000055316.35552.74. [DOI] [PubMed] [Google Scholar]
- 39.Maury P, Zimmermann M. Effect of chronic amiodarone therapy on excitable gap during typical human atrial flutter. J Cardiovasc Electrophysiol. 2004;15:1416–1423. doi: 10.1046/j.1540-8167.2004.04391.x. [DOI] [PubMed] [Google Scholar]
- 40.Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, Molhoek P, Verheugt FW, Gersh BJ, McCabe CH, Braunwald E. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116:1647–1652. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- 41.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 42.Chen YJ, Chen SA. Electrophysiology of pulmonary veins. J Cardiovasc Electrophysiol. 2006;17:220–224. doi: 10.1111/j.1540-8167.2005.00317.x. [DOI] [PubMed] [Google Scholar]