Abstract
Reduced function of the N-methyl-D-aspartate receptor (NMDAR) has been implicated in the pathophysiology of schizophrenia. The NMDAR contains a glycine binding site in its NR1 subunit that may be a useful target for the treatment of schizophrenia. In this study, we assessed the therapeutic potential of long-term increases in the brain levels of the endogenous NMDAR glycine site agonist D-serine, through the genetic inactivation of its catabolic enzyme D-amino acid oxidase (DAO) in mice. The effects of eliminating DAO function were investigated in mice that display schizophrenia-related behavioral deficits due to a mutation (Grin1D481N) in the NR1 subunit that results in a reduction in NMDAR glycine affinity. Grin1D481N mice show deficits in sociability, prolonged latent inhibition, enhanced startle reactivity, and impaired spatial memory. The hypofunctional Dao1G181R mutation elevated brain levels of D-serine, but alone it did not affect performance in the behavioral measures. Compared to animals with only the Grin1D481N mutation, mice with both the Dao1G181R and Grin1D481N mutations displayed an improvement in social approach and spatial memory retention, as well as a reversal of abnormally persistent latent inhibition and a partial normalization of startle responses. Thus, an increased level of D-serine resulting from decreased catalysis corrected the performance of mice with deficient NMDAR glycine site activation in behavioral tasks relevant to the negative and cognitive symptoms of schizophrenia. Diminished DAO activity and elevations in D-serine may serve as an effective therapeutic intervention for the treatment of psychiatric symptoms.
Keywords: NMDA receptor, D-serine, D-amino acid oxidase, genetic mouse model, schizophrenia, social behaviors, latent inhibition, spatial memory
Introduction
Schizophrenia is a chronic and severely debilitating mental illness that affects about 1% of the population worldwide. In addition to psychosis, schizophrenia is characterized by persistent negative symptoms of social withdrawal, flattened affect, and decreased motivation, and by profound cognitive deficits in attention, working memory, and executive functioning (Lewis & Gonzalez-Burgos 2006; Ross et al. 2006). Current antipsychotics have limited efficacy in reducing the negative and cognitive symptoms, and are often poorly tolerated (Lewis & Gonzalez-Burgos 2006; Ross et al. 2006). Consequently, there is a prevalent need for the development of improved therapeutic alternatives targeting the molecular alterations involved in schizophrenia.
Diminished glutamatergic neurotransmission mediated by the N-methyl-D-aspartate receptor (NMDAR) has been implicated in the biological mechanisms underlying schizophrenia (Coyle 2006). NMDAR inhibition with noncompetitive antagonists exacerbates schizophrenic symptoms in patients and elicits psychotomimetic effects in healthy individuals (Javitt & Zukin 1991; Krystal et al. 1994). Furthermore, genetic studies have identified several risk genes for schizophrenia influencing NMDAR activity (Coyle 2006; Ross et al. 2006). These include a number of genes that modulate the selective endogenous NMDAR glycine site agonist, D-serine (Chumakov et al. 2002; Morita et al. 2006; Schumacher et al. 2004). D-serine is an important contributor to NMDAR activation, as reduction of D-serine concentrations have been shown to impede NMDAR-mediated signaling (Mothet et al. 2000; Panatier et al. 2006; Yang et al. 2003). Reduced levels of D-serine have been reported in the serum and CSF of patients with schizophrenia (Bendikov et al. 2007; Hashimoto et al. 2003), and postmortem studies indicate an abnormal increase in D-serine degradation (Madeira et al. 2008). Thus, diminished NMDAR function in schizophrenia may be related to lower D-serine availability, and by corollary augmented D-serine levels may be a beneficial form of treatment.
Endogenous D-serine availability in the brain is determined by its catabolic enzyme, D-amino acid oxidase (DAO), its synthesis enzyme, serine racemase, and by various glial and neuronal transporters (Foltyn et al. 2005; Martineau et al. 2006). Compounds targeting these regulatory proteins may effectively enhance cerebral D-serine and occupancy of the NMDAR glycine site. Inhibition of DAO activity is of particular interest, as it would rectify the increase in DAO function reported in schizophrenia (Madeira et al. 2008) and avoid any nephrotoxic effects related to the metabolism of high levels of systemic D-serine (Maekawa et al. 2005a). DAO is a peroxisomal flavoprotein that at physiological pH is highly selective for D-serine (Mothet et al. 2000), consistent with the inverse correlation between the distribution of DAO and D-serine in the CNS (Moreno et al. 1999; Schell et al. 1995). In this study, we examined the efficacy of limited DAO activity in ameliorating behavioral phenotypes relevant to schizophrenia. We employed mice with a point mutation in the Dao1 gene (G181R) that produces a complete loss of DAO function and a subsequent elevation in brain D-serine (Labrie et al. 2008b; Sasaki et al. 1992). The Dao1G181R mutation is capable of augmenting NMDAR-mediated neurotransmission (Maekawa et al. 2005b; Wake et al. 2001), suggesting that diminished DAO function may benefit psychiatric conditions associated with reduced NMDAR activation. To evaluate the performance of Dao1G181R mice in a model pertinent to schizophrenia, the Dao1G181R mutation was combined with the Grin1D481N mutation. Grin1D481N mice have a five-fold decrease in NMDAR glycine affinity, and biochemical and electrophysiological studies have confirmed that reduced NMDAR activity in these mice can be reversed with exogenous D-serine or glycine application (Duffy et al. 2008; Kew et al. 2000). Moreover, these mice display behavioral abnormalities related to the negative and cognitive symptoms of schizophrenia, including deficient social approach, persistent latent inhibition, and impaired spatial recognition and memory (Labrie et al. 2008c). Here, we report that DAO inactivation improves behavioral deficits associated with NMDAR hypofunction.
Material and Methods
Animals
Mice carrying the Dao1G181R and Grin1D481N mutation were established through heterozygous intercrosses of the Dao1G181R and Grin1D481N lines (Kew et al. 2000; Konno & Yasumura 1983). Initially, Dao1G181R mice were obtained from the laboratory that identified a spontaneous missense mutation (glycine to arginine at amino acid 181) in the DAO gene of the ddY strain that results in a complete lack of DAO activity (Konno & Yasumura 1983; Sasaki et al. 1992). The Dao1G181R mutation was transferred onto a C57BL/6J genetic background, employing a marker-assisted speed congenic strategy (Wong 2002). After six generations of backcrossing, the resultant Dao1+/G181R mice contained >99% of the C57BL/6J genome. Grin1D481N transgenic mice contain a point mutation (aspartate to asparagine substitution at amino acid 481) in the NR1 glycine binding site that results in a five-fold decrease in NMDAR glycine affinity (Kew et al. 2000). These mice were generously provided by Dr. M. Pauly-Evers, Hoffman-La Roche Ltd. (Basel, Switzerland) and were backcrossed eleven generations onto the C57BL/6J strain. Heterozygous Dao1G181R and Grin1D481N mice were then crossed to produce Dao1+/G181R; Grin1+/D481N mice. All animals used in this study were bred from heterozygous intercrosses of Dao1+/G181R; Grin1+/D481N mice. Mice were genotyped using a PCR-amplicon restriction endonuclease protocol (Labrie et al. 2008a).
Groups of three to five same-sex littermates were housed in filtered polycarbonate cages, under controlled temperature (21°C ± 1°C), lighting (lights on: 0700–1900), and humidity (50–60%). The animals were given ad libitum sterile food (Purina mouse chow) and water, except in the latent inhibition and olfactory experiments. All animal procedures were approved by the Animal Management Committee of Mount Sinai Hospital and strictly followed the requirements of the Province of Ontario Animals for Research Act 1971 and the Canadian Council on Animal Care. Adequate measures according to these guidelines were taken to ensure minimal animal discomfort.
High-performance liquid chromatography (HPLC)
The HPLC procedure was adapted from a previously described protocol (Grant et al. 2006). Brain samples were homogenized in 5 volumes of ice-cold double distilled water. An aliquot was mixed with 100% methanol to give a final dilution of 60× and then centrifuged at 12000 × g for 4 min at 4°C. A 5 μl aliquot of the supernatant was mixed with 5 μl of the derivatizing reagent (2 mg N-isobutyryl-L-cysteine and 1 mg o-phthaldialdehyde dissolved in 0.1 ml methanol, followed by addition of 0.9 ml 0.1 M sodium borate buffer), and then was placed into a sample management system (Waters Alliance 2690XE, Waters, Milford, MA, USA). HPLC separation was achieved on a Symmetry C18 column (4.6 mm×150 mm; 3.5 μm particle diameter) coupled with a guard column of the same stationary phase (Waters, Milford, MA, USA). The column heater was set at 30°C and the sample cooler was held at 4°C. To separate the derivatized amino acids of interest, a gradient was established from equal parts of solvent A (850 ml of 0.04 M sodium phosphate buffer and 150 ml methanol, pH 6.2) and B (670 ml of 0.04 M sodium phosphate buffer, 555 ml methanol and 30 ml tetrahydrofuran, pH 6.2) to only solvent B by ~45 min, with a flow rate of 0.5 ml/min. The run time was 60 min for column washout and equilibrium, and 30 min to elute all compounds. A Waters 2475 fluorescence detector (Waters, Milford, MA, USA) was used to quantify the eluted compounds (excitation 344 nm; emission 433 nm).
Chemiluminescent assay
The procedure for the chemiluminescent assay was modified from a described protocol (Wolosker et al. 1999). Brain samples were homogenized in ice-cold buffer (50 mM Tris-HCl, pH 8.8, 10 mM KCl), and then centrifuged at 14,000 × g for 10 min at 4°C. From the supernatant, protein concentrations were measured and standardized. Samples were heated at 100°C for 20 min to eliminate endogenous DAO activity and then cooled to 4°C. Assay buffer (50 μl of 100 mM Tris, 50 mM NaCl, pH 8.8) with 0.1 U horseradish peroxidase (Sigma, St. Louis, MO, USA), 0.8 nmol luminol (Sigma, St. Louis, MO, USA), and 0.048 nmol flavin adenine dinucleotide (EMD Chemicals, Gibbstown, NJ, USA) was added to each sample (10 μl). A Veritas Microplate Luminometer (Turner Biosystems, Sunnyvale, CA, USA) was used to detect the production of H2O2 before and after the addition of 0.002 U R. gracilis DAO (courtesy of L. Pollegioni, U. Insubria, Varese, Italy). Triplicates of each sample were measured, and the content of D-serine was quantified using a calibration curve of D-serine standards.
Behavioral studies
Behavioral testing was conducted during the light phase between 0900 and 1600 h by an experimenter blind to genotype. Experiments were sex-balanced. Testing began when mice were approximately 8 weeks of age in the following order: physical examination, spatial and non-spatial object recognition, social affiliations, prepulse inhibition and startle reactivity, rotarod, latent inhibition, olfactory acuity, and the Morris water maze. To ensure the timely completion of the experiments, fewer animal subjects were used in latter tests. The initial cohort of mice was made smaller for the social affiliations and subsequent tasks, and again for the Morris water maze procedure. For the latent inhibition experiment, two additional cohorts of naive mice were assessed to attain sufficient experimental numbers, and no differences between the original and additional cohorts were detected (see Results section). Animals were given a minimum 1 week interval between tests. Prior to experiments, mice were left undisturbed in the room for at least 30 min to allow for acclimatization. Where possible, the testing equipment was cleaned with 70% ethanol between each subject.
Physical assessment and neurological reflexes
An evaluation of physical appearance, sensory functions, and reflexes was completed to detect any gross abnormalities. Fur condition, whisker presence, eye-blink, ear-twitch, whisker-twitch, and righting reflex were assessed, as described (Miyakawa et al. 2001). Vision was assessed using the visual placing test, where vision was considered to be normal in mice (>80% group criteria) that would reach for a passing table surface, after being lowered 15 cm above and 4 cm out from the table surface (Wersinger et al. 2002).
Olfactory acuity
Olfactory acuity was assessed as previously described (Wersinger et al. 2002). Mice were given a 24-h food deprivation period before testing. In the experiment, clean polycarbonate cages (30×17×12 cm) with fresh corncob bedding were used for each subject. One piece (approximately 1×1×1 cm) of Lab Diet rodent chow (PMI Nutrition, Brentwood, MO, USA) was buried in a random location beneath an evenly distributed layer of corncob bedding (2.5 cm in depth). The latency to find the buried food was recorded, with a maximum period of 10 min.
Accelerating rotarod
Performance in an accelerating rotarod task and motor learning was examined using a modification of a previously described protocol (Soleimani et al. 2008). The rotarod apparatus (Economex Rotarod; Columbus Instruments, Columbus, OH, USA) had a ribbed rotating axle (3 cm diameter) that was situated 30 cm above a plastic surface. A maximum of four mice could be tested simultaneously with each mouse being separated by an opaque wall (30 cm width×60 cm height). Mice were initially allowed to acclimatize to the stationary rod for 60 s. The axle was then set at a constant speed of 5 rpm for 90 s. Afterwards, the axle speed (starting at 5 rpm) was increased by 0.1 rpm/s and the latency to fall off the axle was recorded for each mouse, with a maximum of 360 s in the acceleration mode. On each of the three training days, 3 trials were completed with a 1-h intertrial interval. Motor coordination and balance was evaluated by comparing the mean latencies to fall from the rod (average of the 3 daily trials). Motor learning was determined by observing an improved latency on the last day compared to the first day (motor learning ratio = latency day 3/[latency day 1 + 3]).
Social affiliations
The social affiliations task was conducted similarly to previously described studies (Labrie et al. 2008c; Moy et al. 2004), using a clear Plexiglas box (61.5 cm length×46 cm width×23 cm height) divided into three interconnected chambers (outer chambers=19 cm length, central chamber=23.5 cm length). The outer chambers were identical to each other and divided from the central chamber by clear Plexiglas partitions (17.5 cm width×23 cm height) that each had a centrally placed opening (11 cm width×23 cm height) and a retractable chamber divider. Transparent Plexiglas cages with a cylindrical shape (13 cm height, 8 cm diameter) were used to contain the stranger mice and were perforated with evenly distributed holes (1 cm diameter). Throughout the experimental sessions, the cages were located at the center of each outer chamber and permitted auditory, visual, and olfactory investigation.
The stranger mice used were age- and sex-matched C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) that had never been in contact with the test subjects. At the beginning of each experimental session, the test mouse was placed in the central chamber and was allowed to freely explore for 10 min. Data were not recorded during this habituation period. Afterwards, the experimenter placed a stranger mouse (stranger 1) in one of the cages and handled the other empty cage similarly. The cage and outer chamber containing the stranger mouse was alternated across subjects. After the placement of the stranger mouse, the test subject explored the social apparatus for 10 min (sociability phase). The test mouse was considered to be in a chamber if its head and two front paws had entered the chamber. The amount of time spent investigating each chamber, the number of entries into each chamber, freezing, and grooming were scored using the Observer 5.0 software (Noldus, Wageningen, The Netherlands) and video recorded. Sociability was assessed by comparing the amount of time spent in the chamber with the caged stranger mouse to the amount of time spent in the opposite empty-cage chamber.
Preference for social novelty was also examined. Immediately after the sociability assessment detailed above, a second unfamiliar mouse (stranger 2) was placed beneath the previously empty cage (social novelty phase). The test mouse could now explore the central chamber, the chamber containing the initial stranger (stranger 1; now familiar), or the chamber containing a novel stranger (stranger 2) for a period limited to 5 min, to avoid acclimatization to stranger 2. All other parameters and measures were as described above in the sociability phase.
Latent Inhibition (LI)
LI experiments were conducted in 3 chambers (MED Associates Inc., VT, USA), as described previously (Labrie et al. 2008c; Lipina et al. 2005). Mice were weighed and water was removed from home cages 24 h prior to the start of the LI procedure. Throughout the experiment, weights were monitored to ensure animals did not lose more than 20% of their original body weight. During the 5-day pretraining phase mice were trained to drink from the sipper tube in the LI chamber. Animals were given 5 min to acclimatize to the chamber without access to water, followed by a 15-min period with free access. The number of licks was recorded.
The LI procedure was conducted on days 6–9 and was composed of preexposure, conditioning, baseline drinking, and testing stages. Preexposure (day 6) and conditioning (day 7) involved placing all mice in the testing chamber without access to water and began with a 5-min acclimatization period. During the preexposure phase, half the animals received 40 white-noise stimuli (85 dB, 10 s duration) interspaced 60 s apart (preexposed), and the other half received no tone stimulus (non-preexposed). In the conditioning stage, all animals received 2 or 4 noise-shock pairings interspaced by 5 min, where presentation of a 10-s white noise stimulus was immediately followed by a foot shock (0.37 mA intensity, 1 s duration). After both the preexposure and conditioning phases, water was administered in the home cages for 15 min. Day 8 was the baseline drinking stage, where animals received free access to the water for 15 min, as in the pretraining phase. The baseline drinking day was necessary to eliminate any context-shock association, and to ensure the animals would continue drinking from the sipper tube. The testing stage (day 9) involved a 5-min acclimatization period followed by access to the water. The chamber was silent from lick 0 to 75, but during lick 76 to 101 a continuous white noise stimulus (85dB) was presented. The time between lick 50–75 (before noise – A period), and the time between lick 76–101 (during noise – B period) was recorded. Suppression of the lick response was expressed as the suppression ratio A/(A+B). Latent inhibition is present when preexposed animals have a higher suppression ratio (lower tone response) than non-preexposed animals on testing day.
Prepulse inhibition (PPI) and startle reactivity
PPI and acoustic startle responses were tested using 4 sound-attenuating chambers (ENV-022s; MED Associates, St. Albans, VT, USA), as described previously (Lipina et al. 2005). Each chamber contained an acoustic stimulator (ANL-925) and an amplified load cell platform (PHM-255A and PHM-250B). Chambers were also equipped with a fan and a red light. Mice were placed into ventilated holders (ENV-263) that were attached to the platform of each chamber. Data acquisition and analysis employed the MED Associates software (Startle Reflex package).
PPI testing sessions began by individually placing mice into the startle chambers. Mice were given a 15-min acclimatization period to background noise (65 dB). Afterwards, subjects were presented with a series of five startle-pulse-alone (P) trials, each comprised of a single white noise burst (100 dB, 40 ms). This was followed by a series of trials consisting of no stimulus (background noise, 65 dB), a startle-pulse-alone, or one of three prepulse intensities (69, 73, and 81 dB, 20 ms) presented 100 ms before a startle-pulse (100 dB, 40 ms). This series of trials was given in 10 blocks each containing all five trial types (no stimulus, P, 69 dB + P, 73 dB + P, 81 dB + P) in pseudorandom order. Finally, an additional series of five startle-pulse-alone trials were presented. Intertrial intervals were between 12 to 30 s. The peak startle activity for each trial was recorded. PPI was expressed as the reduction in startle amplitude in prepulse + pulse trials compared to startle-pulse-alone trials. The % PPI for each prepulse intensity was calculated according to the following formula: %PPI = 100−(startle response on prepulse trials/startle response on startle-pulse-alone trials) × 100. It is common for PPI measurements taken with the MED Associates equipment employed to not augment with an increase in prepulse intensity (Labrie et al. 2009; Lipina et al. 2007; Shum et al. 2005).
To measure startle reactivity, mice were acclimatized to the testing chamber for 15 min prior to the presentation of startle stimuli of varying intensities (70, 75, 80, 85, 90, 95, 100, 105, 115, 120 dB), with a 25-ms duration and an inter-stimulus interval of 25–30 s. Startle stimuli were presented in three blocks each composed of two demonstrations of the 11 stimulus intensities given in pseudorandom order. The average startle amplitude for each stimulus intensity was calculated from the three blocks.
Spatial and non-spatial object recognition
The spatial and non-spatial discrimination task was performed in a transparent Plexiglas open field (41×41×31 cm) equipped with infrared beams to detect horizontal and vertical movements (model 7420/7430; Ugo Basile, Italy). The five objects used in this task differed in shape, color, and material (approximately 7×5×5 cm). A pilot experiment (n = 5 wild-type mice) was completed to ensure that no innate preference was exhibited for any single object. Animal behavior was scored and analyzed using The Observer 5.0 (Noldus, Wageningen, The Netherlands) and video recorded.
The testing procedure was based on previously described protocols (Labrie et al. 2008c; Mandillo et al. 2003; Roullet et al. 1996). On test day, each mouse was individually placed in the center of the empty arena for a 5-min session (S1), and locomotor activity (beam breaks) was recorded. The mouse was then placed in a holding cage for 2 min. Four objects were placed in specific positions near each corner of the arena (5 cm from corner walls). The mouse was returned to the center of the arena and allowed to explore the objects for four continuous 5-min sessions (habituation phase, S2–S5). Habituation to object exploration was measured by recording the time spent exploring the objects across sessions S2–S5. A mouse was considered to be exploring an object if its snout was in contact with the object. Duration of locomotor activity was also measured during these sessions (S2–S5) and in subsequent sessions. At the end of S5, the mouse was again placed in the holding cage for 2 min and the position of two objects (NW and SE or NE and SW) was switched to assess response to a spatial change. During the switch, all four objects were touched by the experimenter and objects that were moved were counterbalanced within groups. The mouse was returned to the arena, and the time spent exploring the displaced and non-displaced objects was recorded for 5 min (spatial change phase, S6). Reaction to a spatial change was assessed by comparing the mean time spent exploring the displaced and non-displaced object categories.
Reactivity to a non-spatial change was also examined. Directly after S6, the test subject was returned to the holding cage for 2 min, during which one of the familiar non-displaced objects in the arena was replaced by a novel object in the same location. The mouse was returned to the center of the arena for a 5-min period (non-spatial change phase, S7). Measurements were taken as described for S6, and the response to non-spatial change was evaluated by considering the mean time spent exploring the novel object and the three familiar objects.
Morris water maze (MWM)
The MWM consisted of a white Plexiglas, cylindrical pool (1.2 m diameter) that was filled with opaque water (26°C ± 1°C), as described (Duffy et al. 2008). The pool was arbitrarily divided into four equal quadrants: northeast, northwest, southeast, and southwest. The circular escape platform (10 cm diameter) was made of clear Plexiglas. Distal visual cues were fixed on each wall ~1 m from the pool edge. Activity in the water maze was recorded using a CCD camera on the ceiling above the center of the pool attached to an automated tracking system (HVS Image, Twickenham, Middlesex, UK). HVS Water 2020 software (HVS Image, Twickenham, Middlesex, UK) was used to establish experimental parameters and analyze performance. Behavioral measures in the MWM included path length to target (m), thigmotaxis (% time within 8 cm of the pool wall), swim speed (cm/s, excludes floating periods), floating (% time, swimming at less than 5cm/s), % time within target area, number of platform crosses, and distance from target area (cm).
Subjects were handled for 2 min/day during the 5 days prior to testing. The MWM procedure began with a 1-day stationary visible platform task. Mice were given 4 trials with a ~1-h intertrial interval. In each trial, mice were released facing the pool wall from one of four pseudo-randomized locations (N, S, W, E) at the pool periphery. The platform was at the center of the target quadrant (NE) and 25 cm from the pool wall. In the visible trials, the platform was raised 0.5 cm above the water surface and demarcated with a 10 cm vertical pole. The maximum duration for a platform search was 90 s. Animals that found the platform remained on it for an additional 15 s, whereas unsuccessful animals were assigned a 90-s latency and gently placed onto the platform for 15 s. The acquisition phase began 1 day later, and lasted for 4 consecutive days (4 trials/day, 1 h intertrial interval). Each day was performed similarly to the visible platform task, except the platform was now submerged ~1 cm below the surface of the water (hidden) in the NE quadrant. Retention of spatial memory was assessed in a 60-s probe trial that occurred 24 h after the last acquisition trial. In the probe trial, the platform was removed and mice were released from the point furthest (SW) from the former platform location. Performance in the probe trial was quantified by examining the % time, number of crosses, and distance from an area that was two times the platform diameter, centered over its former location.
Statistical analysis
Statistical analyses were completed using Statistica (Statsoft, Tulsa, OK, USA). Biochemical and behavioral data were analyzed using 3-way, 4-way, or repeated-measures ANOVA with Dao1G181R genotype, Grin1D481N genotype, and sex as the between-subjects factors. All statistical analyses included sex as a between-subjects factor, and main effects or interactions with sex are reported where significant. In the LI experiment, preexposure was also a between-subjects factor. Within-subjects factors were: apparatus side in the social affiliation task; prepulse and startle stimulus intensity in the PPI test; session, object position, and object category in the recognition task; day and platform location in the MWM experiment. Significant main effects or interactions were followed by Fisher’s least significant difference (LSD) post hoc comparisons. Significance was set at P < 0.05.
Results
D-serine measurements in the brains of mice carrying both the Dao1G181R and Grin1D481N mutations
To determine the effect of the Dao1G181R and Grin1D481N mutations on D-serine levels in mice, HPLC analysis was performed. In whole brain tissue, D-serine concentrations were significantly increased as a result of the Dao1G181R mutation (main effect of Dao1G181R genotype: F1,39 = 15.3, P < 0.001; Grin1D481N genotype: F1,39 = 0.08, P = 0.8; Dao1G181R × Grin1D481N genotype interaction: F1,39 = 0.03, P = 0.9; Table 1). D-serine was augmented in Dao1G181R/G181R; Grin1D481N/D481N (double mutant) mice compared to Dao1+/+; Grin1+/+ (wild-type) and Dao1+/+; Grin1D481N/D481N (+/Grin1) mice (P < 0.01), and the elevation in double mutant animals was comparable to that of Dao1G181R/G181R; Grin1+/+ (Dao1/+) mice (P > 0.05). In contrast, similar L-serine levels were observed in the whole brain (P > 0.05; Table 1), and no changes were detected in the concentrations of other amino acids, including glutamate, glutamine, glycine, arginine, alanine, and GABA (P > 0.05) (data not shown). A chemiluminescent assay measuring D-serine catabolism after exposure to the R. gracilis DAO enzyme was also used to quantify D-serine in whole brain tissue of double mutant mice. The concentration of D-serine was again higher in mice carrying the DaoG181R mutation (main effect of Dao1G181R genotype: F1,31 = 38.4, P < 0.001; Grin1D481N genotype: F1,31 = 2.7, P = 0.1; Dao1G181R × Grin1D481N genotype interaction: F1,31 = 1.8, P = 0.2; Table 1). Like Dao1/+ mice, double mutant animals displayed an enhancement in D-serine compared to wild-type and +/Grin1 animals (P < 0.001).
Table 1.
Analysis of amino acid concentrations in whole brain of mice with the Dao1G181R and Grin1D481N mutation
| Amino acid | Genotype | ||||
|---|---|---|---|---|---|
| +/+ | +/Grin1 | Dao1/+ | Double mutant | ||
| HPLC | D-serine | 21.7 ± 0.6 | 22.4 ± 0.4 | 25.4 ± 0.8* | 26.6 ± 1.5** |
| L-serine | 60.3 ± 2.3 | 63.1 ± 1.9 | 61.8 ± 2.2 | 67.0 ± 4.2 | |
| Chemiluminescent assay | D-serine | 4.1 ± 0.3 | 4.0 ± 0.3 | 7.3 ± 0.4*** | 6.1 ± 0.4 ** |
Data are expressed as mean amino acid concentrations (μg/g tissue for HPLC; nmol/mg protein for chemiluminescent assay) ± SEM.
Dao1+/+; Grin1+/+ (+/+), Dao1+/+; Grin1D481N/D481N (+/Grin1), Dao1G181R/G181R; Grin1+/+ (Dao1/+), Dao1G181R/G181R; Grin1D481N/D481N (Double mutant)
P < 0.05,
P < 0.001 compared to wild-type mice;
P < 0.01 compared to wild-type and +/Grin1 mice (post hoc LSD test, ANOVA).
Physical and neurological assessments
Mice carrying the Dao1G181R, Grin1D481N, or both these mutations were physically indistinguishable from each other and wild-type littermates. Examination of reflexes, vision, fur and whisker condition did not reveal any differences between the genotypes. Latencies to find a hidden food pellet were also similar, indicating normal olfactory acuity (wild-type = 109.8 ± 21.4 s; +/Grin1 = 107.7 ± 19.8 s; Dao1/+ = 99.6 ± 22.0 s; double mutant = 119.9 ± 17.0 s). Performance in a 3-day accelerating rotarod task, an indicator of motor coordination, balance, and motor learning, did not differ between the genotypes, as comparable latencies to fall from the rotating axle were observed throughout the task (wild-type = 128.3 ± 6.7 s; +/Grin1 = 127.4 ± 7.6 s; Dao1/+ = 108.7 ± 5.3 s; double mutant = 120.2 ± 7.0 s) and similar motor learning ratios were displayed (wild-type = 0.584 ± 0.020; +/Grin1 = 0.639 ± 0.021; Dao1/+ = 0.618 ± 0.013; double mutant = 0.608 ± 0.019).
Social affiliations in mice lacking DAO activity
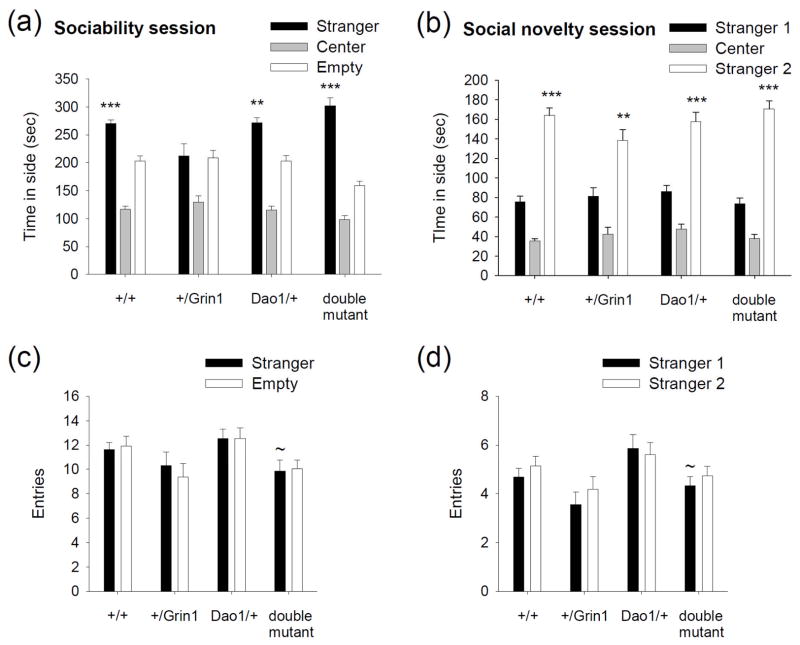
Social dysfunction is a prominent feature of the negative symptoms of schizophrenia that often appears in the prodromal stages and persists throughout the illness (Ellenbroek & Cools 2000; Ross et al. 2006). In rodents, measurements of social encounters serve as a heuristic model for the social deficits in schizophrenia (Ellenbroek & Cools 2000). Consequently, we examined the effects of Dao1G181R mutation on social behaviors in mice and in Grin1D481N mutant animals that exhibit endophenotypes related to schizophrenia (Labrie et al., 2008). In a test of sociability, the +/Grin1 mice displayed a social approach deficit that was normalized by the Dao1G181R mutation (main effect of Dao1G181R genotype: F1,60 = 3.3, P = 0.1; Grin1D481N genotype: F1,60 = 6.6, P < 0.05; apparatus side: F1,60 = 44.7, P < 0.001; Dao1G181R × Grin1D481N genotype × apparatus side interaction: F1,60 = 10.5, P < 0.01; Fig. 1a). Similar to wild-type and Dao1/+ mice, double mutant animals preferred the chamber containing an unfamiliar conspecific mouse over the empty cage chamber (P < 0.001), whereas +/Grin1 mice did not (P > 0.05). All genotypes demonstrated a comparable preference for social novelty (main effect of Dao1G181R genotype: F1,60 = 2.8, P = 0.1; Grin1D481N genotype: F1,60 = 1.6, P = 0.2; apparatus side: F1,60 = 138.5, P < 0.001; Dao1G181R × Grin1D481N genotype × apparatus side interaction: F1,60 = 3.9, P = 0.1), as demonstrated by greater time spent in the chamber containing a newly introduced mouse (stranger 2) than in the chamber containing a now familiar mouse (stranger 1) (P < 0.01; Fig. 1b). Examination of the number of chamber entries during the sociability and social novelty phases revealed significant main effects (sociability: main effect of Dao1G181R genotype: F1,60 = 0.5, P = 0.5; Grin1D481N genotype: F1,60 = 8.7, P < 0.01; apparatus side: F1,60 = 0.4, P = 0.8; Dao1G181R × Grin1D481N genotype interaction: F1,60 = 0.3, P = 0.6; social novelty: main effect of Dao1G181R genotype: F1,60 = 3.2, P = 0.1; Grin1D481N genotype: F1,60 = 8.1, P < 0.01; apparatus side: F1,60 = 1.7, P = 0.2; Dao1G181R × Grin1D481N genotype interaction: F1,60 = 0.04, P = 0.8). More entries were made by the Dao1/+ mice relative to the double mutant mice (P < 0.05; Fig. 1c and 1d). However, the number of chamber entries did not differ between wild-type, +/Grin1, and double mutant mice (P > 0.05), signifying that sociability deficit in +/Grin1 mice and its improvement in the double mutant animals during the sociability session was not related to an alteration in exploratory activity.
Figure 1. Deficits in sociability are rescued by the Dao1G181R mutation.
Wild-type, +/Grin1, Dao/+, and double mutant mice were assessed in a social affiliations task. (a) Mean time (sec) spent in a chamber containing a stranger mouse, a central chamber, and a chamber with an empty cage in the sociability phase. (b) Mean time (sec) spent in a chamber containing a newly introduced mouse (stranger 2), a central chamber, and a chamber with a familiar mouse (stranger 1) in the social novelty phase. Number of chamber entries during the sociability (c) and social novelty (d) phase. n = 22 wild-type, 16 +/Grin1, 15 Dao1/+, and 15 double mutant mice; **P < 0.01, ***P < 0.001 compared to the chamber with an empty cage or a familiar mouse, within genotype; ~P < 0.05 compared to Dao1/+ mice in the same chamber.
The effects of DAO inactivation on persistent latent inhibition
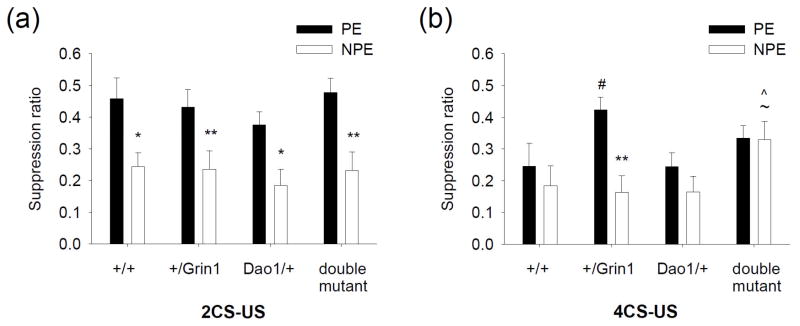
Latent inhibition (LI) is a well-established approach used to measure information-processing deficits in schizophrenia with a reasonable amount of face, predictive, and construct validity (Moser et al. 2000; Weiner 2003). LI is a cross-species phenomenon, occurring when previous exposure to a stimulus without consequence interferes with the ability to form subsequent associations with that stimulus (Moser et al. 2000; Weiner 2003). Recently, it has been shown that diminished NMDAR glycine site occupancy induces persistent LI (Labrie et al. 2008c), a form of cognitive inflexibility associated with the negative symptoms of schizophrenia (Cohen et al. 2004; Rascle et al. 2001; Weiner 2003). Here, we assessed the capacity of the Dao1G181R mutation to improve persistent LI in mice with reduced NMDAR glycine affinity. LI was measured after two or four conditioning trials (CS-US). Three cohorts of mice were used in this experiment and no significant differences were observed between cohorts in the pretraining phase (main effect of cohort for number of licks: F2,185 = 0.3, P = 0.7) or on test day (main effect of cohort for suppression ratio: F2,185 = 1.3, P = 0.3).
In the pretraining days, all genotypes displayed a similar drinking performance, as indicated by the number of sipper tube licks (wild-type = 702.8 ± 38.0; +/Grin1= 614.0 ± 38.3; Dao1/+ = 613.7 ± 33.2; double mutant = 682.0 ± 32.6). On test day, the time taken to complete lick 50–75 (A period) prior to CS onset did not differ between genotype or preexposure group (2CS-US group = 7.9 ± 1.0 s; 4CS-US group = 6.2 ± 1.3 s).
2CS-US
Animals were initially given a 2CS-US procedure to examine the effect of the Dao1G181R mutation under conditions that produce LI in wild-type mice. LI was present in all genotypes (main effect of Dao1G181R genotype: F1,80 = 0.8, P = 0.4; Grin1D481N genotype: F1,80 = 0.9, P = 0.3; preexposure: F1,80 = 27.6, P < 0.001; Dao1G181R × Grin1D481N genotype × preexposure interaction: F1,80 = 0.05, P = 0.8), as there was a significant difference between each preexposed (PE) and non-preexposed (NPE) group (P < 0.05; Fig. 2a).
Figure 2. Persistent latent inhibition is ameliorated by inactivation of DAO function.
Mean suppression ratios of pre-exposed (PE) and non-preexposed (NPE) wild-type, +/Grin1, Dao/+, and double mutant mice given 2 CS-US (a) or 4 CS-US (b) pairings. n = 8–18 per group; *P < 0.05, **P < 0.01 compared to PE score, within genotype; #P < 0.05 compared to PE score of wild-type mice; ~P < 0.05 compared to NPE score of Dao1/+ mice; ^P < 0.05 compared to NPE score of +/Grin1 mice.
4CS-US
An increase in conditioning trials in a 4CS-US protocol abolished LI in wild- type mice, whereas LI continued to be present in +/Grin1 mice (main effect of Dao1G181R genotype: F1,97 = 0.1, P = 0.7; Grin1D481N genotype: F1,97 = 6.6, P < 0.05; preexposure: F1,97 = 5.8, P < 0.05; Dao1G181R × Grin1D481N genotype × preexposure interaction: F1,97 = 2.2, P = 0.1; Fig. 2b). Persistent LI induced by the Grin1D481N mutation (P < 0.01) was reversed in double mutant animals (Fig. 2b). Double mutant animals also showed an increase in NPE ratio compared to +/Grin1 and Dao1/+ mice (P < 0.05).
Prepulse inhibition (PPI) and startle reactivity in double mutant animals
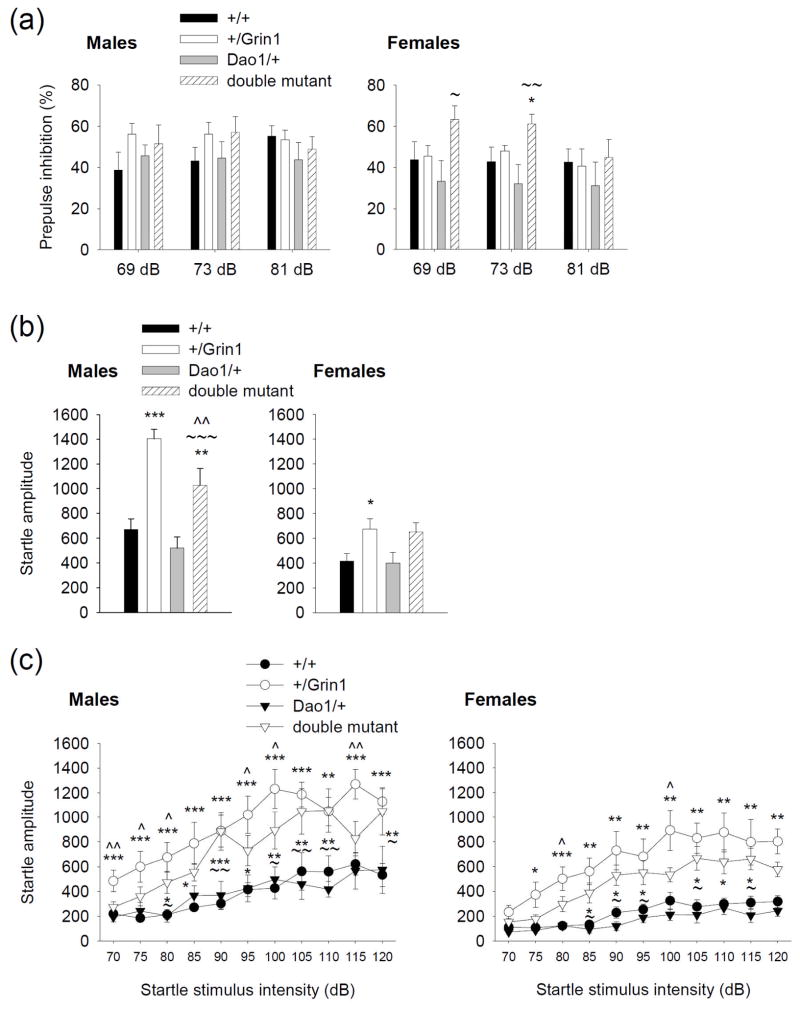
Prepulse inhibition (PPI) is another widely used method that quantifies impairments in the ability to filter or ‘gate out’ cognitive information in schizophrenia (Swerdlow et al. 1994). PPI was not altered in the +/Grin1 or Dao1/+ mice (Fig. 3a, left and right panel), however female double mutant mice showed an elevation in PPI at 69 and 73 dB compared to female wild-type and Dao/+ mice (P < 0.05; Fig. 3a, right panel). In the absence of a prepulse, responses to the startle stimulus differed between genotypes (main effect of Dao1G181R genotype: F1,71 = 4.9, P < 0.05; Grin1D481N genotype: F1,71 = 46.7, P < 0.001; Dao1G181R × Grin1D481N genotype interaction: F1,71 = 0.8, P = 0.4; Fig. 3b, left and right panel). Male and female +/Grin1 mice demonstrated an increase in startle reactivity to 100 dB compared to wild-type mice (P < 0.001), and this increase was displayed to a lesser extent in male double mutant animals (P < 0.01). Male animals demonstrated an increased startle reactivity in comparison to female animals in all genotypes (main effect of sex: F1,71 = 33.3, P < 0.001). Responses to startling stimuli ranging from 70–120 dB were also examined (Fig. 3c, left and right panel). +/Grin1 mice again demonstrated an increase in startle reactivity that was partially restored by the presence of the Dao1G181R mutation in double mutant animals (main effect of Dao1G181R genotype: F1,66 = 4.0, P ≤ 0.05; Grin1D481N genotype: F1,66 = 52.9, P < 0.001; sex: F1,66 = 17.5, P < 0.001; startle stimulus intensity: F10,660 = 38.6, P < 0.001; Grin1D481N genotype × startle stimulus interaction: F10,660 = 6.1, P < 0.001; Dao1G181R × Grin1D481N genotype × startle stimulus interaction: F10,660 = 0.7, P = 0.8), particularly at 70, 75, 80, 95, 100, and 115 dB in male mice and at 80 and 100 dB in female mice (P ≤ 0.05).
Figure 3. Prepulse inhibition and startle reactivity in mice with the Dao1G181R and Grin1D481N mutation.
(a, left and right panel) Mean % inhibition of the acoustic startle response at prepulse intensities of 69, 73, and 81 dB in male and female wild-type, +/Grin1, Dao/+, and double mutant mice. (b, left and right panel) Mean startle amplitude in response to a stimulus (100 dB) given in absence of a prepulse. (c, left and right panel) Acoustic startle response to varying stimulus intensities (70 – 120 dB) administered without a prepulse. n = 7–11 per group; *P < 0.05, **P < 0.01, ***P < 0.001 compared to wild-type mice, within the same prepulse or startle stimulus intensity; ~P < 0.05, ~~P < 0.01, ~~~P < 0.001 double mutant mice compared to Dao1/+ mice, within the same prepulse or startle stimulus intensity; ^P < 0.05, ^^P < 0.01 +/Grin1 mice compared to double mutant mice, within the same startle stimulus intensity.
Spatial and non-spatial object recognition in mice with a loss of DAO function
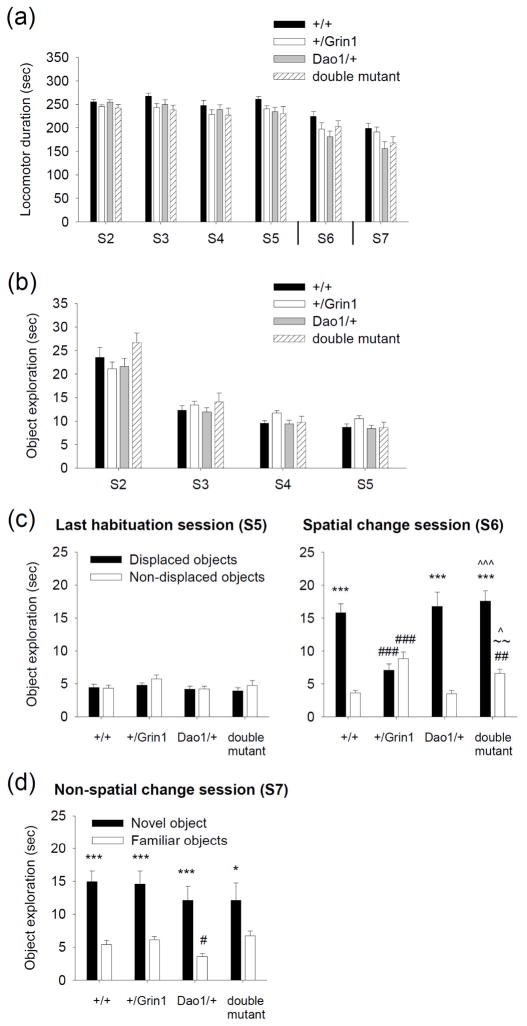
Cognitive disturbances are considered to be a core feature of schizophrenia and include deficits in visuospatial recognition (Barnett et al. 2007; O’Donnell et al. 1996). In rodents, ability to encode spatial information can be assessed in a non-associative spatial object discrimination task (Mandillo et al. 2003; Roullet et al. 1996). Mice were initially placed in an empty open field and locomotor function was measured (Table 2). In this session (S1), horizontal (main effect of Dao1G181R genotype: F1,64 = 0.5, P = 0.5; Grin1D481N genotype: F1,64 = 1.0, P = 0.3; Dao1G181R × Grin1D481N genotype interaction: F1,64 = 12.9, P < 0.001) and vertical locomotor activity (main effect of Dao1G181R genotype: F1,64 = 5.3, P < 0.05; Grin1D481N genotype: F1,64 = 11.5, P < 0.01; Dao1G181R × Grin1D481N genotype interaction: F1,64 = 6.0, P < 0.05) was lower in double mutant animals compared to Dao/+ mice (P < 0.01). Vertical locomotion was also greater in Dao1/+ mice than in wild-type animals (P < 0.05). Locomotor activity during the habituation sessions (S2–S5) and in the subsequent spatial (S6) and non-spatial change (S7) sessions did not differ between genotypes (Fig. 4a).
Table 2.
Locomotor activity during S1 of the spatial recognition experiment
| Horizontal | Vertical | |
|---|---|---|
| +/+ | 528.2 ± 20.2 | 50.6 ± 6.0 |
| +/Grin1 | 585.7 ± 24.2 | 44.5 ± 4.7 |
| Dao1/+ | 596.0 ± 32.4 | 81.6 ± 9.3** |
| Double mutant | 482.6 ± 18.4~~ | 43.1 ± 5.0~~~ |
Data are expressed as number of beam breaks ± SEM.
Dao1+/+; Grin1+/+ (+/+), Dao1+/+; Grin1D481N/D481N (+/Grin1), Dao1G181R/G181R; Grin1+/+ (Dao1/+), Dao1G181R/G181R; Grin1D481N/D481N (Double mutant)
P < 0.01 compared to wild-type mice;
P < 0.01 compared to +/Grin1 and Dao1/+ mice;
P < 0.001 compared to Dao1/+ mice (post hoc LSD test, ANOVA).
Figure 4. Impaired spatial recognition is improved by reduced DAO activity.
Spatial and non-spatial object discrimination was examined in wild-type, +/Grin1, Dao/+, and double mutant mice. (a) Mean duration (sec) of locomotor activity in the sessions of habituation (S2–S5), spatial change (S6), and non-spatial change (S7). (b) Mean duration (sec) of object exploration in the habituation phase. (c) Mean time (sec) spent exploring the displaced and non-displaced objects during the last habituation phase (S5; left panel) and the spatial change phase (S6; right panel). (d) Mean time (sec) spent exploring the novel item and three familiar objects in the non-spatial change phase (S7). n = 19 wild-type, 19 +/Grin1, 19 Dao1/+, 17 double mutant mice; *P < 0.05, ***P < 0.001 compared to the time spent exploring the non-displaced or familiar objects, within genotype; #P < 0.05, ##P < 0.01, ###P < 0.001 compared to wild-type mice exploring the same object category; ~~P < 0.01 compared to Dao1/+ exploring the non-displaced objects; ^P < 0.05, ^^^P < 0.001 compared to the +/Grin1 exploring the same object category.
Across the habituation sessions, a similar progressive decrease in the amount of time spent with all four objects was demonstrated in all genotypes (main effect of Dao1G181R genotype: F1,64 = 0, P = 1.0; Grin1D481N genotype: F1,64 = 2.4, P = 0.1; session: F3,192 = 130.6, P < 0.001; Dao1G181R × Grin1D481N genotype interaction: F1,64 = 0.4, P = 0.5; Fig. 4b). No preference was demonstrated for any individual object (Dao1G181R × Grin1D481N genotype × session × object position interaction: F9,576 = 0.6, P = 0.8). In the last habituation trial (S5), the genotypes did not favor either object category (to be displaced or non-displaced) (Fig. 4c). However, in the following session when two objects were repositioned (S6), +/Grin1 mice demonstrated an inability to selectively respond to the spatial change that was rescued by the Dao1G181R mutation in double mutant mice (main effect of Dao1G181R genotype: F1,66 = 5.7, P < 0.05; Grin1D481N genotype: F1,66 = 0.01, P = 0.9; object category: F1,66 = 125.8, P < 0.001; Dao1G181R genotype × object category interaction: F1,66 = 20.4, P < 0.001; Grin1D481N genotype × object category interaction: F1,66 = 28.4, P < 0.001; Dao1G181R × Grin1D481N genotype × object category interaction: F1,66 = 14.1, P < 0.001; Fig. 4c). Wild-type, Dao/+, and double mutant animals spent more time exploring the objects with a novel spatial configuration than the objects that remained stationary (P < 0.001), whereas +/Grin1 mice did not (P > 0.05). Comparisons between S5 and S6 revealed that all genotypes spent more time with the displaced objects in S6 (P < 0.05) and only the +/Grin1 mice showed a significant increase in time spent with the non-displaced objects during S6 (P < 0.05). This indicates that wild-type, Dao/+, and double mutant animals demonstrated a targeted exploration of the objects that underwent a spatial change, while +/Grin1 mice demonstrated a nonspecific increase in exploration for both object categories. In session 7 (S7) when a novel object was introduced, all genotypes spent more time investigating the novel object than the three familiar objects (main effect of Dao1G181R genotype: F1,66 = 2.2, P = 0.1; Grin1D481N genotype: F1,66 = 0.5, P = 0.5; object category: F1,66 = 69.7, P < 0.001; Dao1G181R × Grin1D481N genotype × object category interaction: F1,66 = 0.3, P = 0.6; Fig. 4d).
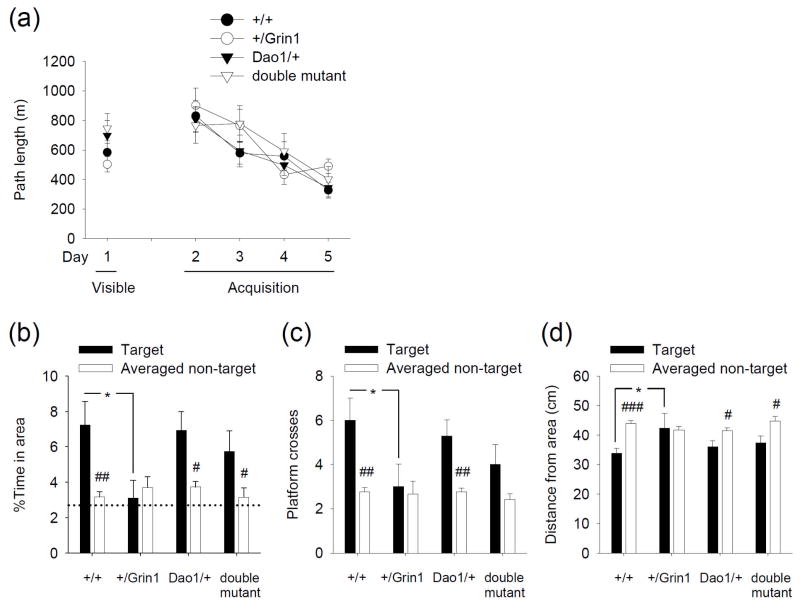
Long-term spatial memory assessed in the Morris water maze (MWM) in double mutant mice
The capacity of the Dao1G181R mutation to improve cognitive ability under conditions of limited NMDAR glycine site function was further investigated in the MWM task, a classic test of spatial and learning and memory (Morris et al. 1986). Performance in the visible platform session and the 4-day acquisition training phase involving a hidden platform was similar among all genotypes, as determined by the path length to the target platform (Fig. 5a). No differences in swim speed (main effect in acquisition trials: Dao1G181R genotype: F1,37 = 0.1, P = 0.8; Grin1D481N genotype: F1,37 = 2.8, P = 0.1), floating (F1,37 = 0.2, P = 0.7; F1,37 = 3.9, P = 0.1), or thigmotaxsis duration (F1,37 = 0.1, P = 0.8; F1,37 = 3.4, P = 0.1) was exhibited in any of the genotypes, indicating comparable sensorimotor abilities and search motivation. Assessment of spatial memory retention in the probe trial demonstrated deficient spatial memory in the +/Grin1 mice that was partially improved by DAO inactivation (Fig. 5b, 5c, and 5d). Compared to wild-type animals, +/Grin1 mice spent less time, made fewer crosses, and displayed a greater average distance from an area 2× the platform diameter centered over its former location (P < 0.05). Moreover, +/Grin1 mice did not display a greater amount of time, number of crosses, and proximity to the target platform compared to the averaged analogous non-target locations (P > 0.05). In contrast, double mutant animals spent more time and had a greater proximity to the target area than the averaged non-target areas, as seen in wild-type and Dao/+ mice (P < 0.05), signifying that spatial memory is partially restored in double mutant animals.
Figure 5. Partial amelioration of long-term spatial memory in double mutant animals.
Performance of wild-type, +/Grin1, Dao/+, and double mutant mice was evaluated in the Morris water maze (MWM) task. (a) Mean path length to reach a target platform in a visible platform session (day 1), and in a hidden-platform acquisition training phase (days 2–5). Spatial memory retention was assessed in a probe trial (b, c, and d) conducted 24 h after the last acquisition trial. During the probe trial, the time (sec) spent (a), frequency of platform crosses (b), and distance (cm) (c) from the correct target area and the averaged analogous non-target areas was determined. The dashed line represents chance level, corresponding to the ratio of the target area to the total pool area (2.8%). n = 14 wild-type, 9 +/Grin1, 14 Dao1/+, 8 double mutant mice. *P < 0.05 compared to wild-type mice, within the same platform location; #P < 0.05, ##P < 0.01, ###P < 0.001 compared to the target location, within genotype.
Discussion
Diminished DAO function enhanced D-serine levels in the brain and improved behavioral deficits associated with the negative and cognitive symptoms of schizophrenia. DAO inactivation effectively reversed sociability deficits, persistent LI, and spatial recognition impairments in mice with reduced NMDAR glycine occupancy. Additionally, loss of DAO function partially normalized the elevated startle reactivity and long-term spatial memory disruption. These findings indicate that reduced DAO activity may be beneficial for the treatment of psychiatric symptoms associated with diminished NMDAR activation, thereby supporting the therapeutic potential of this novel approach in the clinic.
DAO dysfunction may contribute to reduced NMDAR-mediated neurotransmission and D-serine availability implicated in the pathophysiology of schizophrenia. Several genetic association studies reveal that polymorphisms in the DAO gene confer an increased risk of schizophrenia (Chumakov et al. 2002; Corvin et al. 2007; Liu et al. 2004; Schumacher et al. 2004; Wood et al. 2007; but see Shinkai et al., 2007; Yamada et al. 2005). The DAO regulatory enzyme G72 has also been associated with schizophrenia susceptibility in numerous populations (Chumakov et al. 2002; Korostishevsky et al. 2004; Schumacher et al. 2004; Shinkai et al. 2007) and in a meta-analyses (Shi et al. 2008). Elevated DAO expression and/or activity has been detected in the cortex, hippocampus, and cerebellum of patients with schizophrenia (Bendikov et al. 2007; Kapoor et al. 2006; Madeira et al. 2008; Verrall et al. 2007), consistent with the decrease in central and peripheral D-serine levels (Bendikov et al. 2007; Hashimoto et al. 2003). Thus, through the elimination of D-serine, DAO could exacerbate NMDAR hypofunction in schizophrenia, signifying that models of reduced DAO activity such as the Dao1G181R mice can provide valuable therapeutic insight.
Limiting DAO function was an effective means of augmenting D-serine concentrations in the brain, even in subjects with reduced NMDAR activity. Although the rise in D-serine was relatively modest, its chronicity may be an important factor in its capacity to modulate the effects of NMDAR hypofunction. Previous studies have shown that DAO inhibitors produce similar modest increases in brain D-serine levels in rodents, and chronic treatments have greater success in reversing behavioral deficits induced by NMDAR antagonism than acute DAO blockade (Adage et al. 2008; Ferraris et al. 2008). Moreover, inhibition of D-serine catabolism can allow for smaller elevations in D-serine levels to be efficacious, while larger doses of exogenous D-serine are necessary to surmount the effects of DAO activity. The Dao1G181R mutation in mice with a C57BL/6J genetic background has previously been shown to increase D-serine in the cortex, hippocampus, and cerebellum (Labrie et al. 2008b). Changes in D-serine were most prevalent in caudal brain regions (Hashimoto et al. 1993; Labrie et al. 2008b), as DAO is highly abundant in glia of the hindbrain and cerebellum, while lower levels of DAO are present in neurons of the prefrontal cortex, hippocampus, and substantia nigra (Moreno et al. 1999; Verrall et al. 2007). Importantly, diminished DAO activity and the related rise in D-serine significantly potentiates NMDAR function. The NMDAR glycine site is not saturated at the synapses of many brain regions (Fuchs et al. 2005) and Dao1G181R mice display an in vitro enhancement of NMDAR-mediated synaptic transmission in the hippocampus and spinal cord (Maekawa et al. 2005b; Wake et al. 2001) and an in vivo increase in cerebellar cGMP levels, indicative of greater NMDAR activation (Almond et al. 2006). Furthermore, Dao1G181R mice do not exhibit obvious compensatory changes affecting [3H]-D-serine reuptake or the expression of proteins relevant to the NMDAR signaling, including NR1, serine racemase, glycine transporter 1, and alanine-serine-cysteine transporter 1 (Almond et al. 2006). This suggests that behavioral improvements induced by the Dao1G181R mutation are specifically related to decreased DAO function.
The advantage of using the Grin1D481N mice to test the ameliorative effects of the Dao1G181R mutation is that in contrast to paradigms involving challenge with NMDAR antagonists, the Grin1D481N mice model the chronic and presumably developmental nature of NMDAR hypofunction proposed to occur in schizophrenia. Additionally, decreased glycine site activation is relevant to the neural changes involved in schizophrenia, indicating that the Grin1D481N mice may be a more appropriate model for NMDAR hypofunction in schizophrenia than animals with limited expression of the NMDAR-NR1 subunit. The Grin1D481N mutation does not abrogate binding of agonists to the glycine site, but merely raises the Kd. Consequently, the requirement for a ligand such as D-serine is simply shifted towards higher concentration ranges, predicting the reversal of phenotypes under conditions of decreased D-serine catabolism.
Poor social functioning, a common and enduring aspect of the negative symptoms of schizophrenia (Ellenbroek & Cools 2000), was improved in Grin1D481N mice by the Dao1G181R mutation. Social deficits and their amelioration were specific to the test of sociability, which examines social approach- and avoidance-related motivation (Sankoorikal et al. 2006). Reversal of the sociability impairment could not be attributed to changes in olfactory acuity or to a nonspecific increase in exploratory activity. Previous studies with Dao1G181R mice or exogenous D-serine treatments do not reveal anxiolytic-like effects (Labrie et al. 2008a; Schmitt et al. 1995), indicating that reduced anxiety responses are not likely to account for the improvement in sociability.
Deficits in selective attention and information-processing are prevalent in schizophrenia and can be measured similarly in humans and rodents using an LI procedure (Moser et al. 2000). In accordance with previous findings, LI was abnormally persistent in Grin1D481N mice. Prolonged LI has similarly been observed in rodents treated with an inhibitor of the NMDAR glycine site (Labrie et al. 2008c) or the noncompetitive NMDAR antagonist MK-801 (Gaisler-Salomon & Weiner 2003; Lipina et al. 2005). LI perseveration indicates an impaired switching ability, as subjects are unable to switch from ignoring an irrelevant stimulus to responding in manner that demonstrates a CS-US association. Behavioral inflexibility is characteristic of the negative symptoms in schizophrenia (Berman et al. 1997) and clinical studies have correlated LI persistence to the severity of negative symptoms in patients (Cohen et al. 2004; Rascle et al. 2001). We found that decreased DAO function was capable of reversing persistent LI, indicating that activation of the NMDAR glycine site facilitates appropriate switching responses. Persistent LI was normalized, in part, by a decrease in conditional learning (NPE score), as seen in other studies examining the reversal of LI persistence by proglycinergic treatments (Gaisler-Salomon et al. 2008; Labrie et al. 2008c; Lipina et al. 2005). Also, the Dao1G181R mutation and exogenous D-serine application have previously been shown to improve spatial reversal memory and accelerate extinction learning (Duffy et al. 2008; Labrie et al. 2008b). Thus, D-serine and decreased DAO activity improve adaptive learning and may be useful for the treatment of psychiatric disorders characterized by cognitive inflexibility.
Loss of DAO function partly reversed the augmented startle reactivity in Grin1D481N mice, supporting its efficacy in ameliorating the effects of chronic NMDAR hypoactivity. Although Grin1D481N mice displayed normal PPI, chronic inhibition of DAO has been shown to improve PPI disruptions induced by phencyclidine, an NMDAR antagonist (Adage et al. 2008). Acute DAO blockade in combination with D-serine administration has also been shown to attenuate PPI deficits in mice treated with MK-801 (Hashimoto et al. 2009). The absence of PPI differences in the Grin1D481N mice may reflect the more subtle effects of this glycine site mutation compared to other genetic and pharmacological models of NMDAR deficiency (Fradley et al. 2005; Geyer et al. 2001; Moy et al. 2006). Indeed, MK-801 dosages used disrupt PPI in rodents are generally higher than those required to induce persistent LI and deficits in social interactions and spatial recognition (Gaisler-Salomon & Weiner 2003; Roullet et al. 1996; Rung et al. 2005). Consequently, like most animal models pertinent to schizophrenia, the Grin1D481N mice display several relevant endophenotypes, but do not recapitulate all aspects of the disease. It will therefore be necessary to examine the effects of DAO elimination in other schizophrenia models to fully explore the therapeutic utility of this approach.
Since cognitive deficits are a core feature of schizophrenia and a major contributor to functional disability (Lewis & Gonzalez-Burgos 2006), we evaluated the capacity of limited DAO function and elevated D-serine to ameliorate disturbances in a visuospatial object discrimination task and in the MWM. In the object recognition task, Grin1D481N mice displayed a selective inability to detect a spatial change that was rescued by DAO inactivation. The improvement in spatial recognition was not related to perturbations in locomotor activity or object exploration during the habituation phase. Additionally, all mice demonstrated a similar progressive acclimatization to the environment and object configuration in the habituation session. Loss of DAO function also partially restored spatial memory in Grin1D481N mice in the MWM. Double mutant animals favored the correct target location in the probe trial; however this preference did not reach significance compared to mice with only the Grin1D481N mutation. Previously, exogenous D-serine treatments have been shown to fully reverse spatial deficits in the object discrimination and MWM task (Duffy et al. 2008; Labrie et al. 2008c). The partial ameliorative effects of the Dao1G181R mutation in the MWM may be related to the less severe increases in D-serine induced by DAO inhibition (Smith et al. 2008). Also, spatial memory demand in the MWM is likely greater than in the object recognition task, due to the 24-h interval prior to the probe session. Nonetheless, these findings indicate that decreased DAO function may provide some benefit for the disturbances in perception and representation of spatial relationships in schizophrenia (Barnett et al. 2007; O’Donnell et al. 1996).
Clinical studies indicate that agonists of the NMDAR glycine site, including D-serine, produce symptomatic improvements in patients with schizophrenia when administered in conjunction with conventional antipsychotics (Coyle 2006; Heresco-Levy et al. 2005; Tsai et al. 1998). However, there are concerns with the administration of D-serine and similar compounds, as large doses are required in order to penetrate the blood-brain-barrier. In contrast, DAO inhibitors cross the blood-brain-barrier readily (Adage et al. 2008; Hashimoto et al. 2009) and may be a well-tolerated alternative for the modulation of D-serine levels and NMDAR glycine site occupancy in the clinical setting. Animals that chronically lack DAO activity exhibit normal development, longevity, and reproductive potential (Konno & Yasumura 1983). Furthermore, chronic administration of D-serine at therapeutic doses has not been found to produce adverse effects in humans (Tsai et al. 1998). However, under conditions promoting excitotoxicity and neuroinflammation, high levels of D-serine can potentially compromise neuronal survival through excessive NMDAR activation (Martineau et al. 2006; Wu et al. 2004), suggesting that modest rises in D-serine, as observed following DAO reduction, may be advantageous.
In conclusion, chronic DAO inactivation and elevated D-serine improved behavioral deficits caused by reduced NMDAR glycine site occupancy in mice. This suggests that inhibition of DAO function may effectively reverse NMDAR hypofunction implicated in schizophrenia pathogenesis and could serve as a valuable therapeutic approach for the treatment of negative and cognitive symptoms. Further research will be necessary to determine the extent to which DAO inhibition can ameliorate other neurochemical events and behavioral disturbances associated with schizophrenia.
Acknowledgments
V.L. was supported by a Natural Sciences and Engineering Research Council (NSERC, Canada) studentship. J.C.R. and G.B.B. are Canada Research Chairs. The research was supported by the CIHR (GMH-79044) and the United States National Institutes of Health (P01AG12411). The authors gratefully thank G. Rauw and E. Weiss for expert technical assistance.
References
- Adage T, Trillat AC, Quattropani A, et al. In vitro and in vivo pharmacological profile of AS057278, a selective d-amino acid oxidase inhibitor with potential anti-psychotic properties. Eur Neuropsychopharmacol. 2008;18:200–214. doi: 10.1016/j.euroneuro.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Almond SL, Fradley RL, Armstrong EJ, et al. Behavioral and biochemical characterization of a mutant mouse strain lacking D-amino acid oxidase activity and its implications for schizophrenia. Mol Cell Neurosci. 2006;32:324–334. doi: 10.1016/j.mcn.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Croudace TJ, Jaycock S, et al. Improvement and decline of cognitive function in schizophrenia over one year: a longitudinal investigation using latent growth modelling. BMC Psychiatry. 2007;7:1–10. doi: 10.1186/1471-244X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendikov I, Nadri C, Amar S, et al. A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. Schizophr Res. 2007;90:41–51. doi: 10.1016/j.schres.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Berman I, Viegner B, Merson A, Allan E, Pappas D, Green AI. Differential relationships between positive and negative symptoms and neuropsychological deficits in schizophrenia. Schizophr Res. 1997;25:1–10. doi: 10.1016/S0920-9964(96)00098-9. [DOI] [PubMed] [Google Scholar]
- Chumakov I, Blumenfeld M, Guerassimenko O, et al. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA. 2002;99:13675–13680. doi: 10.1073/pnas.182412499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Sereni N, Kaplan O, et al. The relation between latent inhibition and symptom-types in young schizophrenics. Behav Brain Res. 2004;2:113–122. doi: 10.1016/s0166-4328(03)00221-3. [DOI] [PubMed] [Google Scholar]
- Corvin A, McGhee KA, Murphy K, et al. Evidence for association and epistasis at the DAOA/G30 and D-amino acid oxidase loci in an Irish schizophrenia sample. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:949–953. doi: 10.1002/ajmg.b.30452. [DOI] [PubMed] [Google Scholar]
- Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- Duffy S, Labrie V, Roder JC. D-serine augments NMDA-NR2B receptor-dependent hippocampal long-term depression and spatial reversal learning. Neuropsychopharmacol. 2008;33:1004–1018. doi: 10.1038/sj.npp.1301486. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Cools AR. Animal models for the negative symptoms of schizophrenia. Behav Pharmacol. 2000;11:223–233. doi: 10.1097/00008877-200006000-00006. [DOI] [PubMed] [Google Scholar]
- Ferraris D, Duvall B, Ko YS, et al. Synthesis and biological evaluation of D-amino acid oxidase inhibitors. J Med Chem. 2008;51:3357–3359. doi: 10.1021/jm800200u. [DOI] [PubMed] [Google Scholar]
- Foltyn VN, Bendikov I, De Miranda J, et al. Serine racemase modulates intracellular D-serine levels through an alpha, beta-elimination activity. J Biol Chem. 2005;280:1754–1763. doi: 10.1074/jbc.M405726200. [DOI] [PubMed] [Google Scholar]
- Fradley RL, O’Meara GF, Newman RJ, Andrieux A, Job D, Reynolds DS. STOP knockout and NMDA NR1 hypomorphic mice exhibit deficits in sensorimotor gating. Behav Brain Res. 2005:257–264. doi: 10.1016/j.bbr.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Fuchs SA, Berger R, Klomp LW, de Koning TJ. D-amino acids in the central nervous system in health and disease. Mol Genet Metab. 2005;85:168–180. doi: 10.1016/j.ymgme.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Gaisler-Salomon I, Diamant L, Rubin C, Weiner I. Abnormally persistent latent inhibition induced by MK801 is reversed by risperidone and by positive modulators of NMDA receptor function: differential efficacy depending on the stage of the task at which they are administered. Psychopharmacol. 2008;196:255–267. doi: 10.1007/s00213-007-0960-3. [DOI] [PubMed] [Google Scholar]
- Gaisler-Salomon I, Weiner I. Systemic administration of MK-801 produces an abnormally persistent latent inhibition which is reversed by clozapine but not haloperidol. Psychopharmacol. 2003;166:333–342. doi: 10.1007/s00213-002-1311-z. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacol. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Grant SL, Shulman Y, Tibbo P, Hampson DR, Baker GB. Determination of D-serine and related neuroactive amino acids in human plasma by high-performance liquid chromatography with fluorimetric detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;844:278–282. doi: 10.1016/j.jchromb.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Nishikawa T, Konno R, et al. Free D-serine, D-aspartate and D-alanine in central nervous system and serum in mutant mice lacking D-amino acid oxidase. Neurosci Lett. 1993;152:33–36. doi: 10.1016/0304-3940(93)90476-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fujita Y, Horio M, et al. Co-administration of a D-amino acid oxidase inhibitor potentiates the efficacy of D-serine in attenuating prepulse inhibition deficits after administration of dizocilpine. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.1001.1002. in press. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fukushima T, Shimizu E, et al. Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry. 2003;60:572–576. doi: 10.1001/archpsyc.60.6.572. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Ebstein R, et al. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol Psychiatry. 2005;57:577–585. doi: 10.1016/j.biopsych.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Kapoor R, Lim KS, Cheng A, Garrick T, Kapoor V. Preliminary evidence for a link between schizophrenia and NMDA-glycine site receptor ligand metabolic enzymes, d-amino acid oxidase (DAAO) and kynurenine aminotransferase-1 (KAT-1) Brain Res. 2006;1106:205–210. doi: 10.1016/j.brainres.2006.05.082. [DOI] [PubMed] [Google Scholar]
- Kew JNC, Koester A, Moreau JL, et al. Functional consequences of reduction in NMDA receptor glycine affinity in mice carrying targeted point mutations in the glycine binding site. J Neurosci. 2000;20:4037–4049. doi: 10.1523/JNEUROSCI.20-11-04037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno R, Yasumura Y. Mouse mutant deficient in D-amino acid oxidase activity. Genetics. 1983;103:277–285. doi: 10.1093/genetics/103.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korostishevsky M, Kaganovich M, Cholostoy A, et al. Is the G72/G30 locus associated with schizophrenia? single nucleotide polymorphisms, haplotypes, and gene expression analysis. Biol Psychiatry. 2004;56:169–176. doi: 10.1016/j.biopsych.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Labrie V, Clapcote SJ, Roder JC. Mutant mice with reduced NMDA-NR1 glycine affinity or lack of D-amino acid oxidase function exhibit altered anxiety-like behaviors. Pharmacol Biochem Behav. 2008a;91:610–620. doi: 10.1016/j.pbb.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Labrie V, Duffy S, Wei W, Barger S, Baker GB, Roder JC. Genetic inactivation of D-amino acid oxidase enhances extinction and reversal learning in mice. Learn Mem. 2008b;16:28–37. doi: 10.1101/lm.1112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie V, Fukumura R, Rastogi A, et al. Genetic inactivation of serine racemase produces behavioral phenotypes related to schizophrenia in mice. Hum Mol Genet. 2009 doi: 10.1093/hmg/ddp1261. in press. [DOI] [Google Scholar]
- Labrie V, Lipina T, Roder JC. Mice with reduced NMDA receptor glycine affinity model some of the negative and cognitive symptoms of schizophrenia. Psychopharmacol. 2008c;200:217–230. doi: 10.1007/s00213-008-1196-6. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nature Med. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- Lipina T, Labrie V, Weiner I, Roder J. Modulators of the glycine site on NMDA receptors, D-serine and ALX-5407, display similar beneficial effects to clozapine in mouse models of schizophrenia. Psychopharmacol. 2005;179:54–67. doi: 10.1007/s00213-005-2210-x. [DOI] [PubMed] [Google Scholar]
- Lipina T, Weiss K, Roder J. The ampakine CX546 restores the prepulse inhibition and latent inhibition deficits in mGluR5-deficient mice. Neuropsychopharmacol. 2007;32:745–756. doi: 10.1038/sj.npp.1301191. [DOI] [PubMed] [Google Scholar]
- Liu X, He G, Wang X, et al. Association of DAAO with schizophrenia in the Chinese population. Neurosci Lett. 2004;369:228–233. doi: 10.1016/j.neulet.2004.07.078. [DOI] [PubMed] [Google Scholar]
- Madeira C, Freitas ME, Vargas-Lopes C, Wolosker H, Panizzutti R. Increased brain D-amino acid oxidase (DAAO) activity in schizophrenia. Schizophr Res. 2008;101:76–83. doi: 10.1016/j.schres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Okamura T, Kasai N, Hori Y, Summer KH, Konno R. D-aminoacid oxidase is involved in D-serine-induced nephrotoxicity. Chem Res Toxicol. 2005a;18:1678–1682. doi: 10.1021/tx0500326. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Watanabe M, Yamaguchi S, Konno R, Hori Y. Spatial learning and long-term potentiation of mutant mice lacking D-amino-acid oxidase. Neurosci Res. 2005b;53:34–38. doi: 10.1016/j.neures.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Mandillo S, Rinaldi A, Oliverio A, Mele A. Repeated administration of phencyclidine, amphetamine and MK-801 selectively impairs spatial learning in mice: a possible model of psychotomimetic drug-induced cognitive deficits. Behav Pharmacol. 2003;14:533–544. doi: 10.1097/00008877-200311000-00006. [DOI] [PubMed] [Google Scholar]
- Martineau M, Baux G, Mothet JP. D-serine signalling in the brain: friend and foe. Trends Neurosci. 2006;29:481–491. doi: 10.1016/j.tins.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yared E, Pak JH, Huang FL, Huang KP, Crawley JN. Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus. 2001;11:763–775. doi: 10.1002/hipo.1092. [DOI] [PubMed] [Google Scholar]
- Moreno S, Nardacci R, Cimini A, Ceru MP. Immunocytochemical localization of D-amino acid oxidase in rat brain. J Neurocytol. 1999;28:169–185. doi: 10.1023/a:1007064504007. [DOI] [PubMed] [Google Scholar]
- Morita Y, Ujike H, Tanaka Y, et al. A genetic variant of the serine racemase gene is associated with schizophrenia. Biol Psychiatry. 2006;61:1200–1203. doi: 10.1016/j.biopsych.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Moser PC, Hitchcock JM, Lister S, Moran PM. The pharmacology of latent inhibition as an animal model of schizophrenia. Brain Res Rev. 2000;33:275–307. doi: 10.1016/s0165-0173(00)00026-6. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Parent AT, Wolosker H, et al. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Perez A, Koller BH, Duncan GE. Amphetamine-induced disruption of prepulse inhibition in mice with reduced NMDA receptor function. Brain Res. 2006;1089:186–194. doi: 10.1016/j.brainres.2006.03.073. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, Swearer JM, Smith LT, Nestor PG, Shenton ME, McCarley RW. Selective deficits in visual perception and recognition in schizophrenia. Am J Psychiatry. 1996;153:687–692. doi: 10.1176/ajp.153.5.687. [DOI] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, et al. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Rascle C, Mazas O, Vaiva G, et al. Clinical features of latent inhibition in schizophrenia. Schizophr Res. 2001;51:149–161. doi: 10.1016/s0920-9964(00)00162-6. [DOI] [PubMed] [Google Scholar]
- Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Roullet P, Mele A, Ammassari-Teule M. Involvement of glutamatergic and dopaminergic systems in the reactivity of mice to spatial and non-spatial change. Psychopharmacol. 1996;126:55–61. doi: 10.1007/BF02246411. [DOI] [PubMed] [Google Scholar]
- Rung JP, Carlsson A, Markinhuhta KR, Carlsson ML. The dopaminergic stabilizers (-)-OSU6162 and ACR16 reverse (+)-MK-801-induced social withdrawal in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:833–839. doi: 10.1016/j.pnpbp.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Konno R, Nishio M, Niwa A, Yasumura Y, Enami J. A single-base-pair substitution abolishes D-amino-acid oxidase activity in the mouse. Biochim Biophys Acta. 1992;1139:315–318. doi: 10.1016/0925-4439(92)90107-x. [DOI] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci USA. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt ML, Coelho W, Lopes-de-Souza AS, Guimaraes FS, Carobrez AP. Anxiogenic-like effect of glycine and D-serine microinjected into dorsal periaqueductal gray matter of rats. Neurosci Lett. 1995;189:93–96. doi: 10.1016/0304-3940(95)11459-a. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Jamra RA, Freudenberg J, et al. Examination of G72 and D-amino acid oxidase as genetic risk factor for schizophrenia and bipolar affective disorder. Mol Psychiatry. 2004;9:203–207. doi: 10.1038/sj.mp.4001421. [DOI] [PubMed] [Google Scholar]
- Shi J, Badner JA, Gershon ES, Liu C. Allelic association of G72/G30 with schizophrenia and bipolar disorder: a comprehensive meta-analysis. Schizophr Res. 2008;98:89–97. doi: 10.1016/j.schres.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai T, De Luca V, Hwang R, et al. Association analyses of the DAOA/G30 and D-amino-acid oxidase genes in schizophrenia: further evidence for a role in schizophrenia. Neuromolecular Med. 2007;9:169–177. doi: 10.1007/BF02685890. [DOI] [PubMed] [Google Scholar]
- Shum FW, Ko SW, Lee YS, Kaang BK, Zhuo M. Genetic alteration of anxiety and stress-like behavior in mice lacking CaMKIV. Mol Pain. 2005;15:22. doi: 10.1186/1744-8069-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Uslaner J, Yao L, et al. The behavioral and neurochemical effects of a novel D-amino acid oxidase inhibitor 4H-thieno [3,2-b] pyrrole-5-carboxylic acid (compound 8) and D-serine. J Pharmacol Exp Ther. 2008;328:921–930. doi: 10.1124/jpet.108.147884. [DOI] [PubMed] [Google Scholar]
- Soleimani L, Roder JC, Dennis JW, Lipina T. Beta N-acetylglucosaminyltransferase V (Mgat5) deficiency reduces the depression-like phenotype in mice. Genes Brain Behav. 2008;7:334–343. doi: 10.1111/j.1601-183X.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficitent sensorimotor gaiting in schizophrenia patients. Arch Gen Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- Tsai G, Yang P, Chung LC, Lange N, Coyle JT. D-serine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 1998;44:1081–1089. doi: 10.1016/s0006-3223(98)00279-0. [DOI] [PubMed] [Google Scholar]
- Verrall L, Walker M, Rawlings N, et al. D-Amino acid oxidase and serine racemase in human brain: normal distribution and altered expression in schizophrenia. Eur J Neurosci. 2007;26:1657–1669. doi: 10.1111/j.1460-9568.2007.05769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake K, Yamazaki H, Hanzawa S, et al. Exaggerated responses to chronic nociceptive stimuli and enhancement of N-methyl-D-aspartate receptor-mediated synaptic transmission in mutant mice lacking D-amino-acid oxidase. Neurosci Lett. 2001;297:25–28. doi: 10.1016/s0304-3940(00)01658-x. [DOI] [PubMed] [Google Scholar]
- Weiner I. The “two-headed” latent inhibition model of schizophrenia: modeling positive and negative symptoms and their treatment. Psychopharmacol. 2003;169:257–297. doi: 10.1007/s00213-002-1313-x. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS., 3rd Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Sheth KN, Takahashi M, et al. Purification of serine racemase: Biosynthesis of the neuromodulator D-serine. Proc Natl Acad Sci USA. 1999;96:721–725. doi: 10.1073/pnas.96.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GT. Speed congenics: applications for transgenic and knock-out mouse strains. Neuropeptides. 2002;36:230–236. doi: 10.1054/npep.2002.0905. [DOI] [PubMed] [Google Scholar]
- Wood LS, Pickering EH, Dechairo BM. Significant support for DAO as a schizophrenia susceptibility locus: examination of five genes putatively associated with schizophrenia. Biol Psychiatry. 2007;61:1195–1199. doi: 10.1016/j.biopsych.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Wu SZ, Bodles AM, Porter MM, Griffin WS, Basile AS, Barger SW. Induction of serine racemase expression and D-serine release from microglia by amyloid beta-peptide. J Neuroinflammation. 2004;1:2. doi: 10.1186/1742-2094-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Ohnishi T, Hashimoto K, et al. Identification of multiple serine racemase (SRR) mRNA isoforms and genetic analyses of SRR and DAO in schizophrenia and D-serine levels. Biol Psychiatry. 2005;57:1493–1503. doi: 10.1016/j.biopsych.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ge W, Chen Y, et al. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci USA. 2003;100:15194–15199. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]