Abstract
We tested whether rosuvastatin (RST) protected against excitotoxic neuronal cell death in rat primary cortical neuronal cultures. L-glutamate (200µM, 1h) reduced neuronal viability (% of naive controls, mean±SEM, n=8–32, *p<0.05) from 100±2% to 60±1%*, but pretreatment with RST (0.5 µM, 3 days) increased survival to 88±2%*. RST-induced neuroprotection was not affected by coapplication with mevalonate (10µM), although the same dose of mevalonate fully prevented the neurotoxic effects of a high dose (20µM) of RST. RST (0.5 µM) pretreatment did not affect mitochondrial membrane potential or superoxide anion levels in quiescent neurons. However, RST pretreatment blunted elevations in free intracellular Ca2+ and reduced increases in superoxide anion levels following glutamate exposure. Manganese superoxide dismutase (SOD), copper-zinc SOD, catalase, and reduced glutathione levels were unaffected by RST pretreatment. In contrast, acute, one time RST application did not affect either baseline or L-glutamate-induced increases in superoxide levels. In summary, 3- day RST pretreatment induces resistance to the excitotoxic effect of L-glutamate in cultured neurons apparently by a mechanism that is independent of 3-hydroxy-3-methyl-glutaryl-coenzyme A- reductase inhibition. The delayed neuroprotection by RST against excitotoxicity does not involve sustained mitochondrial depolarization or superoxide anion production as initiating events, although it is associated with reduced Ca2+ influx and superoxide anion production upon L-glutamate challenge.
Keywords: neuroprotection, statins, cell culture, intracellular calcium, reactive oxygen species
Introduction
Statins are used to lower plasma cholesterol levels by blocking 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMGCoA) reductase which synthesizes mevalonate, the precursor molecule of cholesterol biosynthesis. In the central nervous system, however, cellular cholesterol homeostasis is independent of plasma cholesterol levels since blood to brain transport of cholesterol is virtually zero through the blood-brain barrier (Chobanian and Hollander, 1962). In the brain, steady state cholesterol homeostasis is achieved by a balance between exclusively local, de novo cholesterol biosynthesis and the removal of cholesterol and its metabolites from the brain to the blood plasma (Dietschy and Turley, 2004). Therefore, statins that cross the blood-brain barrier could disrupt normal cholesterol turnover and to induce neuronal cell death by depleting geranylgeranyl pyrophosphate levels, a non-sterol isoprenoid product also produced from mevalonate (Tanaka et al., 2000). In contrast to these concerns, a number of in vivo and cell culture studies have reported neuroprotective effect of statins (Chen et al., 2003; Domoki et al., 2009; Hayashi et al., 2005; Hong et al., 2006; Lim et al., 2006; Mayanagi et al., 2008; Roensch et al., 2007), for a recent review see (Orr, 2008).
L-glutamate is the most common excitatory neurotransmitter in the CNS. Excessive levels of L-glutamate elicit neuronal cell death (Kajta et al., 2009; Sen et al., 2008), and L-glutamate receptors may contribute to neutoxicity by other agents (Molz et al., 2009). Therefore, it is of interest to study whether statin treatments would reduce excitotoxic injury in cultured neurons (Hazell, 2007; Szenasi et al., 2008). Indeed, rosuvastatin (RST), atorvastatin, simvastatin, mevastatin, and pravastatin all attenuated N-methyl-D-aspartate (NMDA)-induced neuronal cell death in cultured murine cortical neurons (Zacco et al., 2003). The protective of effects of statins has been suggested to involve reduced neuronal cholesterol levels. A follow-up study found, however, that atorvastatin-induced neuroprotection against L-glutamate is independent of HMGCoA reductase inhibition in rat cultured cortical neurons (Bosel et al., 2005). Recently, simvastatin-induced neuroprotection against NMDA-induced neuronal cell death was again linked to cholesterol depletion, similar to the protection achieved by AY9944, an inhibitor of the final steps of cholesterol biosynthesis from lanosterol (Ponce et al., 2008).
We have recently reported that RST elicited protection against oxygen-glucose deprivation (OGD) in cultured cortical neurons via a delayed preconditioning-like mechanism involving HMG-CoA reductase inhibition, and depletion of geranylgeranyl pyrophosphate but not cholesterol (Domoki et al., 2009). The protective effect of RST against OGD was also simulated by inhibition of geranylgeranyl transferase activity by perillic acid. In this study, we sought to extend these findings and to determine whether RST would induce protection of rat primary cortical neuronal cell cultures against L-glutamate excitotoxicity via a similar mechanism.
Materials and Methods
Materials
Cell culture plastics were purchased from Becton-Dickinson (San Jose, CA, USA). Dulbecco’s modified Eagle medium (DMEM), F-12 HAM, Neurobasal medium, B27 Supplement, 2-mercaptoethanol, and horse serum were obtained from Gibco BRL (Grand Island, NY, USA). Dispase I was obtained from Roche (Mannheim, Germany), isoflurane from Baxter (Deerfield, IL, USA), and CellTiter 96 AQueous One Solution Assay were procured from Promega (Madison, WI, USA). Hydroethidine, tetramethylrhodamine ethyl ester (TMRE), monochlorobimane (MCB), Fluo-4 AM, and Pluronic F-127 were purchased from Molecular Probes (Eugene, OR, USA). All other chemicals were purchased from Sigma (St. Louis, MO, USA).
Antibodies were obtained from the following sources: anti-glial fibrillary acidic protein antibody from Chemicon (Temecula, CA, USA), anti-microtubule-associated protein-2 antibody and monoclonal anti-manganese-dependent superoxide dismutase (MnSOD) antibody from Becton-Dickinson, polyclonal anti-copper-zinc superoxide dismutase (Cu,ZnSOD) and anti-catalase antibody from Calbiochem (San Diego, CA, USA), and anti-rabbit IgG and anti-mouse IgG from Jackson Immuno-Research (West Grove, PA, USA).
Primary rat cortical neuronal culture
Timed pregnant Sprague-Dawley (SD) rats were obtained from Harlan (Indianapolis, IN, USA). The use of animals was approved by the Wake Forest University Health Sciences Animal Care and Ethics Committee. Animals were deeply anesthetized with 5% isoflurane in a 70:30 gas mixture of N2O and O2 and decapitated. Primary rat cortical neurons were isolated from E18 rat fetuses using a previously described method (Deadwyler et al., 1993; Kis et al., 2003). After digestion, and trituration, the isolated cells were plated onto poly-L-lysine coated glass coverslips at a density of 2×105 cells/cm2 for confocal microscopic analysis and onto poly-D-lysine coated plates or dishes at a density of 106 cells/cm2 for the other experiments. The plating medium consisted of 60% DMEM, 20% F-12 HAM, 20% horse serum, and L-glutamine (0.5 mM). The cultures were maintained in a humidified 5% CO2 incubator. After cell attachment, the plating medium was replaced with Neurobasal medium supplemented with B27 (2%), L-glutamine (0.5 mM), 2-mercaptoethanol (55 µM), and KCl (25 mM). Positive immunostaining for microtubule-associated protein-2 and negative immunostaining for glial fibrillary acidic protein verified that the cultures consisted of more than 99% of neurons on 7 days in vitro (DIV).
Study design
Primary cultures of cortical neurons on 5 DIV were treated for 3 days with RST (10nM–20µM RST) before exposing the cultures to L-glutamate excitoxicity. Previously, this treatment protocol for RST was found most effective to induce tolerance to OGD in cultured neurons (Domoki et al., 2009). Neuroprotective (0.5 µM) and neurotoxic (20 µM) doses of RST were coapplied with mevalonate (10 µM) to assess the role of HMG-CoA reductase inhibition in the mechanism of neuroprotection/toxicity, respectively. Cellular viability was determined 24h after completion of the experiments. We determined baseline and L-glutamate-induced levels for intracellular free calcium and superoxide anion in RST-preconditioned (0.5 µM, 3 days) and non-RST pretreated neurons. We also determined mitochondrial membrane potential (ΔΨm), GSH, and protein levels of MnSOD, Cu,ZnSOD and catalase in RST-preconditioned neurons (0.5 µM, 3 days) compared to paired, but untreated cultures.
L-glutamate excitotoxicity
The excitotoxic challenges were performed on 8 DIV. At this time, cultured neurons express functional glutamate receptors and are vulnerable to L-glutamate cytotoxicity (Mattson et al., 1991; Mattson et al., 1993). Neuronal cultures in 96-well plates were exposed to L-glutamate (200µM) dissolved in the culture medium for 1h at 37°C in the 5% CO2 incubator, after which the medium was replaced with regular culture medium. Control cell cultures were treated identically except that regular culture medium was used throughout the experiment.
Quantification of cellular viability
Viability was determined using the tetrazolium-based CellTiter 96 AQueous One Solution assay. Twenty µL of solution were added directly to culture wells, and then incubated for 1 hr at 37°C, followed by measurement of absorbance at λabs = 492 nm with a FLUOstar OPTIMA microplate reader (BMG Labtech GmbH, Offenburg, Germany). Results were compared to paired cultures exposed to the same neurotoxic stimulus on the same day, and cell viability was expressed as a percentage of the corresponding control culture (untreated, and not exposed to the lethal insult) using the following formula: % viabilitySAMPLE = (absorbanceSAMPLE − absorbanceBACKGROUND) × 100 / (absorbanceCONTROL − absorbanceBACKGROUND).
Analysis of mitochondrial membrane potential (ΔΨm) in neurons
The ΔΨm was analyzed using the ΔΨm-sensitive dye TMRE. Neuronal cultures in black 96-well plates were loaded in the dark with TMRE (0.5 µM) at 37°C in a 5% CO2 incubator. After 20 min loading, the cells were rinsed three times with phosphate-buffered saline (PBS). Experiments were performed in PBS containing glucose (1 mg/mL) at 37°C. TMRE-fluorescence was measured with a FLUOstar OPTIMA microplate reader (λex=510nm and λem=590nm). Data were expressed as a percentage of the intensity of the untreated control culture as follows: % ΔΨmSAMPLE = (TMRE-fluorescenceSAMPLE − TMRE-fluorescenceBACKGROUND) × 100/(TMRE-fluoresenceCONTROL − TMRE-fluorescenceBACKGROUND).
Measurement of intracellular free calcium levels
Changes of intracellular free calcium levels were monitored using the Ca-indicator dye Fluo-4 AM. Untreated and RST-pretreated (0.5 µM, 3 days) neuronal cultures on coverslips were loaded with 2 µM Fluo-4 AM and 1 µM pluronic F-127 in PBS containing 1mg/mL glucose in the dark for 1h at 21°C, and were then washed three times with PBS. Stained neurons were examined using an inverted confocal microscope connected to a Zeiss LSM-510 laser scanning confocal system with a Zeiss C-Apochromat 63×/NA 1.2 water-immersion objective (Carl Zeiss Inc., Jena, Germany). Vehicle (PBS w glucose) or L-glutamate (200 µM) were applied to untreated and RST-pretreated cultures - in the absence of RST - and confocal images of cellular Fluo-4 AM fluorescence (λex = 488 nm and λem = 520 nm) were acquired in a time series every 20 s for 10 min and the average pixel intensity of individual cell bodies was determined using the software supplied by the manufacturer (Zeiss). Imaging conditions such as gain levels, confocal aperture size, and laser power were held constant. Data were expressed as a percentage of the starting intensity of the untreated control culture: % change in calcium levelSAMPLE = (Fluo-4 fluorescenceSAMPLE − Fluo-4 fluorescenceBACKGROUND) × 100 / (Fluo-4 fluoresenceCONTROL − Fluo-4 fluorescenceBACKGROUND).
Analysis of neuronal superoxide anion levels
Superoxide anion levels in control or L-glutamate-treated neurons were assessed in black 96-well plates using the conversion of superoxide anion-sensitive fluorescent precursor hydroethidine to the fluorescent dye ethidium (Bindokas et al., 1996). Cultured neurons were washed and loaded with hydroethidine (5 µM) in glucose-containing (1 mg/ml) PBS 1 minute before the assay, and the fluorescence of ethidium was measured every minute for 30 min using the FLUOstar OPTIMA microplate reader (λex = 510, λem = 590 nm).
Measurement of neuronal glutathione (GSH) levels
The intracellular GSH level was measured with monochlorobimane (MCB), which is highly specific for GSH in rodent cells (Hedley and Chow, 1994). Cortical neurons cultured in black 96-well plates were incubated with MCB (50 µM) in DMEM for 30min at 37°C. After the incubation period, the cells were washed with PBS and the intensity of MCB-fluorescence was then determined with a FLUOstar OPTIMA microplate reader (λex=355nm and λem=460nm). Data were expressed as a percentage of the corresponding control culture as follows: % GSH levelsSAMPLE = (MCB-fluorescenceSAMPLE − MCB-fluorescenceBACKGROUND) × 100/ (MCB-fluoresenceCONTROL − MCB-fluorescenceBACKGROUND).
Western blotting for manganese-dependent superoxide dismutase (MnSOD), copper-zinc superoxide dismutase (Cu,ZnSOD), and catalase
Proteins from neurons cultured in 35mm dishes were harvested by scraping in ice-cold NP40 lysis buffer supplemented with proteinase inhibitors (1 µg/mL aprotinin, 50 µg/mL phenylmethylsulfonyl fluoride, and 1 µg/mL leupeptin), and a phosphatase inhibitor cocktail (1 mM EDTA, 1 mM sodium orthovanadate, 10 µg/mL benzamidine, 1 mM sodium pyrophosphate, and 1 mM sodium fluoride). For each sample, equal amounts of protein were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto a polyvinylidine difluoride sheet (Polyscreen PVDF; Perkin Elmer Life Sciences, Boston, MA, USA). Membranes were incubated in a blocking buffer (Tris-buffered saline, 0.1% Tween 20 and 5% skimmed milk powder) for 1 h at room temperature followed by incubation with monoclonal anti-MnSOD (1:3000), polyclonal anti-Cu,ZnSOD (1:1000), and polyclonal anti catalase (1:5000) antibodies overnight at 4 °C. The membranes were then washed three times in Tris-buffered saline with 0.1% Tween 20 and incubated for 1 h in the blocking buffer with anti-rabbit IgG (1:50 000) or anti-mouse IgG (1:5000) conjugated to horseradish peroxidase. The final reaction products were visualized using enhanced chemiluminescence (SuperSignal West Pico; Pierce, Rockford, IL, USA) and recorded on X-ray film. For quantitative analysis, the bands were scanned in a Foto/Analyst Investigator PC System using PC Image 5.0 software (Fotodyne Hartland, WI, USA) and the densities of the bands were quantified by using Image J 1.3.1 software (National Institutes of Health, Bethesda, MD, USA). The intensity of the bands was normalized to that of β-actin, and the normalized level of the examined protein in the untreated control group was considered 100%.
Statistical analysis
Statistical analysis was performed with SigmaStat (SPSS, Chicago, IL, USA). Data are presented as means ± SEM. Differences between groups were assessed by one-way analysis of variance (ANOVA) or two-way repeated measures ANOVA where appropriate. For pairwise comparisons the Tukey post hoc test was used. P< 0.05 was considered to be statistically significant.
Results
RST-preconditioning protects neurons against L-glutamate excitotoxicity independent of HMG-CoA reductase inhibition
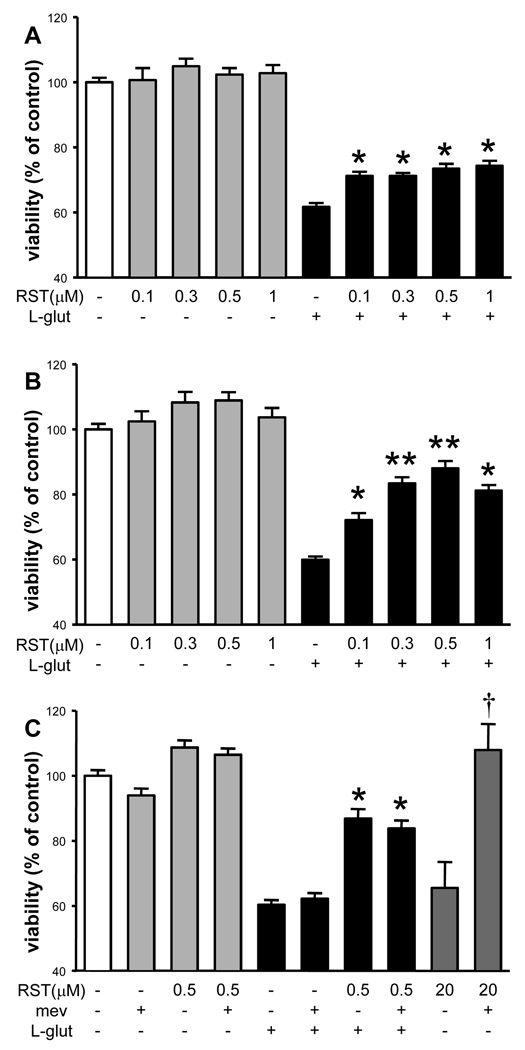
RST (<10 µM) did not significantly affect the viability of neuronal cultures (Figure 1A, B, C), whereas 3-day treatment with 20 µM RST significantly reduced neuronal viability (Figure 1C). L-glutamate reduced neuronal viability by ~40% (Figure 1A, B, C). One-day treatment with RST had only a minor beneficial effect on neuronal survival after L-glutamate challenge (Figure 1A). In contrast, three-day treatment with RST resulted in a more robust, dose-dependent increase in neuronal survival after excitotoxic stress (Figure 1B).
Figure 1. Rosuvastatin (RST) protects cultured neurons against L-glutamate toxicity independently of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibition.
A) One-day RST treatment did not affect neuronal viability (gray bars) compared to untreated control cultures (white bar). L-glutamate (L-glut, 200µM, 1h) significantly reduced neuronal viability in all groups (black bars) compared to both untreated and corresponding RST-treated cultures. However, all doses of RST elicited a small but significant neuroprotective effect: neuronal viability was increased compared to untreated controls exposed to L-glut (*p<0.05 versus untreated controls exposed to L-glut). B) Three-day RST treatment also had no effect on neuronal viability in cultures not exposed to L-glut. However, in neuronal cultures exposed to L-glut, RST induced a robust, dose-dependent neuroprotective effect (*p<0.05, versus untreated controls exposed to L-glut; **p<0.05 versus all groups treated with smaller RST doses and exposed to L-glut). C) Three-day treatment with mevalonate (mev, 10µM) did not affect neuronal viability and did not prevent neuronal cell death induced by L-glut. Co-application of mevalonate with 0.5µM RST did not reduce the neuroprotection afforded by RST. In contrast, neuronal cell death induced by 20µM RST was completely abolished by mevalonate co-application (dark gray bars; *p<0.05, versus untreated controls exposed to L-glut, †p<0.05 versus 20 µM RST treatment). Data are combined from 7 independent neuronal cultures, n≥16 for each group.
Mevalonate (10 µM) per se did not affect the viability of neuronal cultures and did not alter L-glutamate-induced cell death (Figure 1C). Coapplication of mevalonate with 0.5 µM RST did not abolish the neuroprotection afforded by RST against L-glutamate-induced excitotoxicity (Figure 1C). However, coapplication of mevalonate with 20 µM RST completely inhibited the neurotoxic effect of RST (Figure 1C).
RST-preconditioning abrogates L-glutamate-induced increases in intracellular levels of calcium and inhibits neuronal superoxide anion production upon L-glutamate exposure
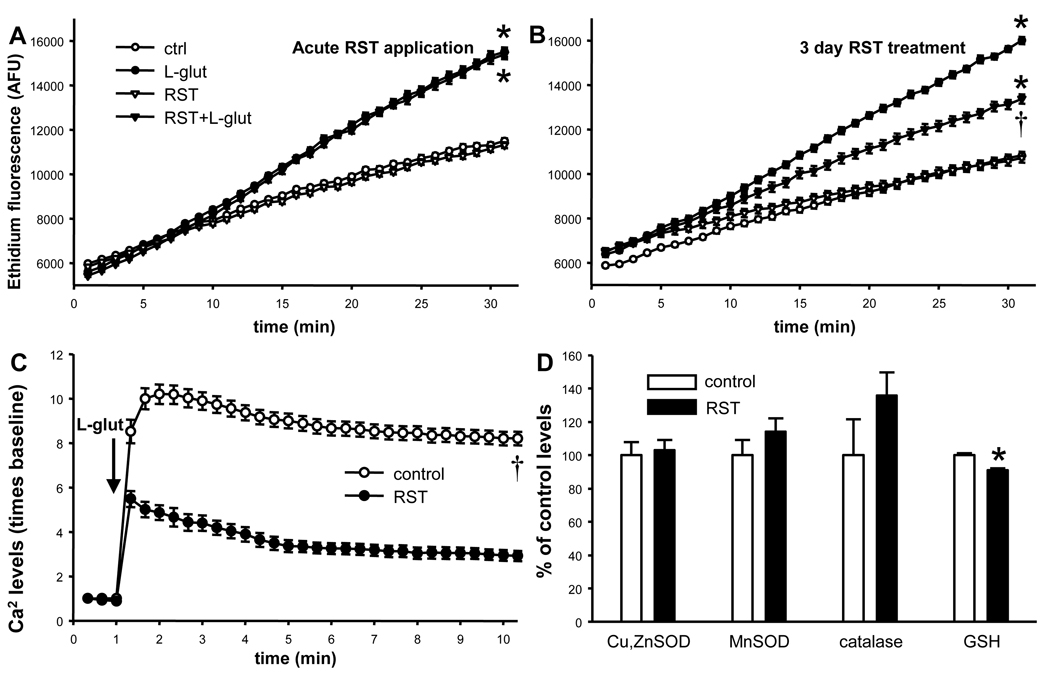
L-glutamate elicited a rapid increase in intracellular levels of free calcium and superoxide anion availability. Neither acute RST application nor RST-preconditioning affected baseline superoxide anion production (Figure 2A, B). Acute RST also did not modify L-glutamate-induced increases in superoxide anion levels (Figure 2A). However, RST-preconditioning did significantly reduce L-glutamate-induced increases in both neuronal intracellular levels of free calcium and superoxide anion levels (Figure 2B, C).
Figure 2. Rosuvastatin (RST) reduces L-glutamate-induced increases in intracellular Ca2+ and superoxide anion levels without increasing neuronal antioxidant capacity.
A–B) Neuronal superoxide anion production was assessed by determining ethidium fluorescence (in arbitrary fluorescence units, AFU) produced by the conversion of hydroethidine. Acute application of 0.5µM RST does not affect either baseline or L-glutamate-induced significant increases superoxide anion levels (Panel A). However, 3-day treatment with this RST dose significantly reduced L-glutamate-induced increases in superoxide anion levels, without affecting baseline superoxide anion production (Panel B). Data are from 2 independent cultures, n=32 wells; p<0.05, * versus respectively treated cultures not exposed to L-glutamate, † versus non-treated L-glutamate-exposed cells. C) L-glutamate significantly increased free calcium levels determined by Fluo4 AM fluorescence, in both control and RST-treated (0.5µM, 3 days) neurons at all time points after L-glutamate application. However, RST-pretreatment -in the absence of RST itself- significantly reduced L-glutamate-induced increases in free calcium levels. Data are from n=25 cells, p<0.05, † versus non-treated cells after L-glutamate-exposure at all time points. D) RST pretreatment (0.5µM, 3 days) did not significantly affect neuronal levels of copper-zinc superoxide dismutase (Cu,ZnSOD), manganese-dependent superoxide dismutase (MnSOD), and catalase (n=5–5, respectively). RST reduced neuronal glutathione (GSH) levels by ~10%. Data are from 2 independent cultures, n=32 wells; p<0.05, * versus untreated controls.
Three day RST-pretreatment did not depolarize neuronal mitochondria; ΔΨm in RST-preconditioned cells compared to untreated controls was 104±1% versus 100±1% (data from two independent cultures, n=32). Three day RST-pretreatment also had no significant effect on neuronal protein levels of MnSOD, Cu,ZnSOD and catalase, and elicited only a minor decrease in GSH levels compared to untreated controls (Figure 2D).
Discussion
The major findings of the present study are the following: 1) Three day RST-pretreatment protects against L-glutamate-induced excitotoxicity in cultured neurons, 2) RST-induced neuroprotection against L-glutamate excitotoxicity apparently is independent of HMGCoA reductase inhibition, 3) RST attenuates increases in intracellular levels of free calcium and superoxide anion during L-glutamate application, and 4) RST pretreatment does not substantially elevate GSH and antioxidant enzyme levels that could be responsible for the reduced superxoide anion levels upon L-glutamate exposure.
Statin-induced protection against OGD or excitotoxicity in neuronal cell cultures has been investigated previously but the results are conflicting. Thus, previous studies found either neuroprotection against excitotoxicity but not OGD (Bosel et al., 2005; Zacco et al., 2003), or neuroprotection against OGD but not excitotoxicity (Lim et al., 2006). In contrast, our present data extend our previous findings showing that RST can protect cortical neuronal cultures not only against OGD (Domoki et al., 2009), but also against excitoxicity using similar treatment protocols. Based on our present and previous data, we propose that the neuroprotective actions of RST against OGD and excitotoxicity and perhaps against other statins are mediated by distinct, independent mechanisms.
We have four important lines of evidence to support this claim. First, we demonstrated that in our primary cortical neuronal cultures, cell death induced by L-glutamate but not by OGD is prevented by the glutamate receptor antagonist MK-801 (Gaspar et al., 2008a). Second, RST-induced neuroprotection against OGD was absent below 1 µM, and the maximal effect was observed at 5 µM RST concentration (Domoki et al., 2009). In contrast, in the present study, RST-induced neuroprotection against excitoxicity was detected at 0.1 µM and the maximal effect appeared at 0.5 µM. Thus, RST is capable of inducing protection against excitotoxicity at a dose which is an order of magnitude smaller than its protective effect against OGD, thereby indicating a potentially different mechanism of action. Third, the protective effect of RST pretreatment against OGD coincided with significant mitochondrial depolarization, an important determining factor of neuronal survival (Misiti et al., 2008), but lower dose RST pretreatment effective against excitotoxicity had no sustained effect on ΔΨm. Fourth, in our previous study, co-application of 10 µM mevalonate with 5 µM RST fully prevented cholesterol depletion and abolished RST-induced protection against OGD (Domoki et al., 2009). In our present study, however, the same dose of mevalonate did not affect the neuroprotective effect of 0.5 µM RST against excitotoxicity, but completely prevented 20 µM RST-induced neurotoxicity induced by geranylgeranylpyrophosphate depletion (Tanaka et al., 2000). Mevalonate overcomes the inhibitory effect of statins on isoprenoid/cholesterol biosynthesis by providing the product of the HMGCoA reductase inhibited by statins. We conclude that the neuroprotective effect of RST against OGD is mediated by HMGCoA reductase inhibition, whereas the protective effect against excitotoxicity is independent of this effect.
This conclusion is in accordance with those of (Bosel et al., 2005), who found that 1 µM atorvastatin-induced protection against excitotoxicity is independent of HMGCoA reductase inhibition in cultured rat cortical neurons, since protection was not antagonized by co-application of mevalonate or isoprenoids. However, Zacco et al. found that 1mM mevalonate antagonized the protective effect of simvastatin in a murine neuronal-glial coculture system; the authors concluded that the cholesterol depleting effect of statins was responsible for the neuroprotective effect (Zacco et al., 2003). The cholesterol-depleting effect was also suggested as the mechanism of simvastatin-induced protection against excitotoxicity in a rat cortical neuronal culture in a recent paper. This conclusion was based on the efficacy of AY9944, an inhibitor of sterol Δ14-reductase and Δ 7- reductase - the last enzymes of the cholesterol biosynthetic pathway, and simvastatin to induce resistance against excitotoxic stress (Ponce et al., 2008). Unfortunately, the effect of mevalonate co-application on the simvastatin-induced protection against excitotoxicity was not tested in that study. However, both Zacco et al. and Ponce et al. found that co-application of simvastatin with cholesterol abolished the neuroprotective effect against excitotoxicity (Ponce et al., 2008; Zacco et al., 2003). This controversy may be resolved if simvastatin, but not atorvastatin or RST, can deplete membrane cholesterol levels independent of HMGCoA reductase inhibitors. Simvastatin was indeed found to provide acute protection against OGD in cultured neurons which Lim et al. attributed to direct inhibition of the production and cytoxicity of 4-hydroxy-2E-nonenal, the final product of of membrane lipid peroxidation (Lim et al., 2006). Thus, simvastatin, which is more lipid soluble than RST, may have specific membrane effects on cultured cortical neurons independent of HMGCoA reductase inhibition.
The exact mechanism of neuroprotection against excitotoxic stress yielded by either RST or other statins is unknown. Maximal statin-induced neuroprotection has been reported to develop after 3–4 days of treatment for all statins (Bosel et al., 2005; Ponce et al., 2008; Zacco et al., 2003), suggesting a delayed pharmacological preconditioning-like effect. Oxidative stress appears to play a central role in L-glutamate-induced necrotic cell death in cultured cortical neurons (Wang et al., 2003). L-glutamate elicits influx of extracellular Ca2+ that is coupled to superoxide anion production in cultured hippocampal neurons (Kahlert et al., 2005). In the present study, we demonstrated that RST reduced L-glutamate-induced increases in intracellular free calcium levels similar to results Bosel et al. obtained using atorvastatin (Bosel et al., 2005). Furthermore, we provide novel evidence that RST also blunts L-glutamate-induced increases in superoxide anion availability. Thus, we believe that the mechanism of RST-induced neuroprotection likely involves reduced oxidative stress upon L-glutamate exposure.
The mechanism of RST-induced protection is substantially different from that of other drugs which also protect against excitotoxicity. Under the same experimental conditions, the prototype mitochondrial ATP-sensitive K+-channel (mitoKATP) opener diazoxide, and the antianginal drug bepridil induced delayed preconditioning against excitotoxic stress by triggering an initial, relatively modest increase in neuronal superoxide anion levels (Gaspar et al., 2007; Kis et al., 2003). Neutralization of this increase in superoxide anion availability with scavengers prevented the development of the L-glutamate resistant neuronal phenotype. In contrast, RST in the present study did not elicit an acute increase in levels of superoxide anion, indicating that the development of the preconditioned phenotype is independent of superoxide anion. Superoxide anion-independent preconditioning against excitotoxicity has been demonstrated in this experimental model previously using the highly specific mitoKATP opener BMS-191095 (Gaspar et al., 2008b), however, the involvement of mitoKATP in the mechanism of RST-induced neuroprotection is unlikely since RST does not elicit mitochondrial depolarization. The mechanism of preconditioning may involve increased endogenous antioxidant defense capacity (Danielisova et al., 2005), but RST treatment did not affect the protein levels of any of the antioxidant enzymes studied. The minor inhibitory effect of RST on neuronal GSH levels probably indicate that reduced superoxide anion levels after L-glutamate exposure in RST treated cells are due to decreased production rather than increased scavenging.
In conclusion, RST elicits dose-dependent delayed protection against excitotoxic stress independently of its inhibitory effect on HMGCoA reductase inhibition. The mechanism of protection is markedly different from other pharmacological preconditioning drugs or even RST at much higher doses against OGD, but it likely involves reduced Ca2+ influx and subsequent decreased superoxide anion production allowing neuronal survival.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bindokas VP, et al. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. Journal of Neuroscience. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosel J, et al. Neuroprotective effects of atorvastatin against glutamate-induced excitotoxicity in primary cortical neurones. Journal of Neurochemistry. 2005;92:1386–1398. doi: 10.1111/j.1471-4159.2004.02980.x. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Annals of Neurology. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Hollander W. Body cholesterol metabolism in man. I. The equilibration of serum and tissue cholesterol. Journal of Clinical Investigations. 1962;41:1732–1737. doi: 10.1172/JCI104631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielisova V, et al. Changes of endogenous antioxidant enzymes during ischemic tolerance acquisition. Neurochemistry Research. 2005;30:559–565. doi: 10.1007/s11064-005-2690-4. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, et al. Cannabinoids modulate potassium current in cultured hippocampal neurons. Receptors Channels. 1993;1:121–134. [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. Journal of Lipid Research. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Domoki F, et al. Rosuvastatin induces delayed preconditioning against oxygen-glucose deprivation in cultured cortical neurons. American Journal of Physiology Cell Physiology. 2009;296:C97–C105. doi: 10.1152/ajpcell.00366.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar T, et al. Delayed neuronal preconditioning by NS1619 is independent of calcium activated potassium channels. Journal of Neurochemistry. 2008a;105:1115–1128. doi: 10.1111/j.1471-4159.2007.05210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar T, et al. Neuronal preconditioning with the antianginal drug, bepridil. Journal of Neurochemistry. 2007;102:595–608. doi: 10.1111/j.1471-4159.2007.04501.x. [DOI] [PubMed] [Google Scholar]
- Gaspar T, et al. ROS-independent preconditioning in neurons via activation of mitoK(ATP) channels by BMS-191095. Journal of Cerebral Blood Flow Metabolism. 2008b;28:1090–1103. doi: 10.1038/sj.jcbfm.9600611. [DOI] [PubMed] [Google Scholar]
- Hayashi T, et al. HMG CoA reductase inhibitors reduce ischemic brain injury of Wistar rats through decreasing oxidative stress on neurons. Brain Research. 2005;1037:52–58. doi: 10.1016/j.brainres.2004.12.051. [DOI] [PubMed] [Google Scholar]
- Hazell AS. Excitotoxic mechanisms in stroke: an update of concepts and treatment strategies. Neurochemistry International. 2007;50:941–953. doi: 10.1016/j.neuint.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Hedley DW, Chow S. Evaluation of methods for measuring cellular glutathione content using flow cytometry. Cytometry. 1994;15:349–358. doi: 10.1002/cyto.990150411. [DOI] [PubMed] [Google Scholar]
- Hong H, et al. Atorvastatin protects against cerebral infarction via inhibition of NADPH oxidase-derived superoxide in ischemic stroke. American Journal of Physiology Heart Circulatory Physiology. 2006;291:H2210–H2215. doi: 10.1152/ajpheart.01270.2005. [DOI] [PubMed] [Google Scholar]
- Kahlert S, et al. Glutamate-mediated influx of extracellular Ca2+ is coupled with reactive oxygen species generation in cultured hippocampal neurons but not in astrocytes. Journal of Neuroscience Research. 2005;79:262–271. doi: 10.1002/jnr.20322. [DOI] [PubMed] [Google Scholar]
- Kajta M, et al. Neuroprotection by co-treatment and post-treating with calcitriol following the ischemic and excitotoxic insult in vivo and in vitro. Neurochemistry International. 2009;55:265–274. doi: 10.1016/j.neuint.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Kis B, et al. Diazoxide induces delayed pre-conditioning in cultured rat cortical neurons. Journal of Neurochemistry. 2003;87:969–980. doi: 10.1046/j.1471-4159.2003.02072.x. [DOI] [PubMed] [Google Scholar]
- Lim JH, et al. Simvastatin prevents oxygen and glucose deprivation/reoxygenation-induced death of cortical neurons by reducing the production and toxicity of 4-hydroxy-2E-nonenal. Journal of Neurochemistry. 2006;97:140–150. doi: 10.1111/j.1471-4159.2006.03715.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, et al. Developmental expression, compartmentalization, and possible role in excitotoxicity of a putative NMDA receptor protein in cultured hippocampal neurons. Brain Research. 1991;565:94–108. doi: 10.1016/0006-8993(91)91740-r. [DOI] [PubMed] [Google Scholar]
- Mattson MP, et al. Growth factors prevent mitochondrial dysfunction, loss of calcium homeostasis, and cell injury, but not ATP depletion in hippocampal neurons deprived of glucose. Experimental Neurology. 1993;121:1–13. doi: 10.1006/exnr.1993.1066. [DOI] [PubMed] [Google Scholar]
- Mayanagi K, et al. Acute treatment with rosuvastatin protects insulin resistant (C57BL/6J ob/ob) mice against transient cerebral ischemia. Journal of Cerebral Blood Flow Metabolism. 2008;28:1927–1935. doi: 10.1038/jcbfm.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiti F, et al. Mitochondrial oxygen consumption inhibition importance for TMT-dependent cell death in undifferentiated PC12 cells. Neurochemistry International. 2008;52:1092–1099. doi: 10.1016/j.neuint.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Molz S, et al. Guanosine-5'-monophosphate induces cell death in rat hippocampal slices via ionotropic glutamate receptors activation and glutamate uptake inhibition. Neurochemistry International. 2009;55:703–709. doi: 10.1016/j.neuint.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Orr JD. Statins in the spectrum of neurologic disease. Current Atherosclerosis Reports. 2008;10:11–18. doi: 10.1007/s11883-008-0003-5. [DOI] [PubMed] [Google Scholar]
- Ponce J, et al. Simvastatin reduces the association of NMDA receptors to lipid rafts: a cholesterol-mediated effect in neuroprotection. Stroke. 2008;39:1269–1275. doi: 10.1161/STROKEAHA.107.498923. [DOI] [PubMed] [Google Scholar]
- Roensch J, et al. Effects of statins on alpha7 nicotinic receptor, cholinesterase and alpha-form of secreted amyloid precursor peptide in SH-SY5Y cells. Neurochemistry International. 2007;50:800–806. doi: 10.1016/j.neuint.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Sen I, et al. NMDA and non-NMDA receptor-mediated differential Ca2+ load and greater vulnerability of motor neurons in spinal cord cultures. Neurochemistry International. 2008;52:247–255. doi: 10.1016/j.neuint.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Szenasi G, et al. 2,3-benzodiazepine-type AMPA receptor antagonists and their neuroprotective effects. Neurochemistry International. 2008;52:166–183. doi: 10.1016/j.neuint.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Tanaka T, et al. Geranylgeranyl-pyrophosphate, an isoprenoid of mevalonate cascade, is a critical compound for rat primary cultured cortical neurons to protect the cell death induced by 3-hydroxy-3-methylglutaryl-CoA reductase inhibition. Journal of Neuroscience. 2000;20:2852–2859. doi: 10.1523/JNEUROSCI.20-08-02852.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, et al. Over-expression of antioxidant enzymes protects cultured hippocampal and cortical neurons from necrotic insults. Journal of Neurochemistry. 2003;87:1527–1534. doi: 10.1046/j.1471-4159.2003.02123.x. [DOI] [PubMed] [Google Scholar]
- Zacco A, et al. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors protect cortical neurons from excitotoxicity. Journal of Neuroscience. 2003;23:11104–11111. doi: 10.1523/JNEUROSCI.23-35-11104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]