Abstract
The vanilloid receptor TRPV1 is a key nociceptive molecule located in primary afferent nociceptive neurons in dorsal root ganglia (DRG) for initiating neurogenic inflammation and pain. Our recent study demonstrates that up-regulation of TRPV1 receptors by intradermal injection of capsaicin is modulated by activation of the protein kinase C (PKC) cascade. Neurogenic inflammation and pain resulting from capsaicin injection are sympathetically dependent, responding to norepinephrine, adenosine 5′-triphosphate (ATP) and/or neuropeptide Y released from sympathetic efferents. In a rat model of acute neurogenic inflammatory pain produced by capsaicin injection, we used immunofluorescence and Western blots combined with pharmacology and surgical sympathectomies to analyze whether the capsaicin-evoked up-regulation of TRPV1 in DRG neurons is affected by sympathetic outflow by way of activating the PKC cascade. Sympathetic denervation reduced significantly the capsaicin-evoked expressions of TRPV1, calcitonin gene-related peptide and/or phosphorylated PKC and their co-expression. These reductions could be restored by exogenous pretreatment with an analog of ATP, α,β-methylene ATP. Inhibition of PKC with chelerythrine chloride prevented the ATP effect. Consistent results were obtained from experiments in which capsaicin-evoked changes in cutaneous inflammation (vasodilation and edema) were examined after sympathetic denervation, and the effects of the above pharmacological manipulations were evaluated. Our findings suggest that the capsaicin-evoked up-regulation of TRPV1 receptors in DRG neurons is modulated sympathetically by the action of ATP released from sympathetic efferents to activate the PKC cascade. Thus, this study proposes a potential new mechanism of sympathetic modulation of neurogenic inflammation.
Keywords: TRPV1 receptor, sympathetic efferents, PKC, P2X receptor, CGRP, neurogenic inflammation
Introduction
Postganglionic sympathetic efferents play a critical role in several types of pathological pain (Gibbs et al., 2008; Jänig and McLachlan, 1994; Jänig et al., 1996; Raja, 1995). For example, sympathetic stimulation may excite sensory neurons in animals with inflamed peripheral tissue or after peripheral nerve injury (Devor et al., 1994; Sato and Kumazawa, 1996; Sato and Perl, 1991). Sympathectomy relieves hyperalgesic and allodynic behaviors in several pathological pain models (Kim et al., 1993; Kinnman and Levine, 1995; Malmberg and Basbaum, 1998). Clinical observations and experimental studies suggest that a crosstalk between sympathetic efferents and sensitized primary afferent nociceptors is produced by the release of norepinephrine, adenosine 5′-triphosphate (ATP) and/or neuropeptide Y from sympathetic efferents onto dorsal root ganglion (DRG) neurons (Chung et al., 1996; Devor et al., 1994), at the site of nerve injury (Devor and Seltzer, 1999) and in the skin (Lin et al., 2003,2004; Sato and Perl, 1991).
Many studies reveal that neurogenic inflammation and the resulting pain induced by intradermal injection of capsaicin (CAP) are sympathetically dependent (Coderre et al., 1989; Lin et al., 2003,2004; Ren et al., 2005,2006). Activation of transient receptor potential vanilloid-1 (TRPV1) receptors in primary afferent nociceptive neurons and their axons evoked by CAP injection produces an efferent function that initiates neurogenic inflammation (Kessler et al., 1999; Szolcsanyi, 1996,2004) by the release of neuropeptides from the nociceptors (Garcia-Nicas et al., 2001; Li et al., 2008; Lin et al., 2007). We proposed in our previous studies that neurogenic inflammation is likely to be sympathetically-mediated by influencing the sensitivity of primary afferent nociceptive neurons and/or their terminals (Ren et al., 2005,2006). However, no direct evidence has so far been provided to show if this process is done by modulation of TRPV1 receptors.
A recent study by our group demonstrated that a rapid up-regulation of TRPV1 mRNA and protein levels evoked by CAP injection is modulated by activation of protein kinase C (PKC) in which increased level of calcitonin gene-related peptide (CGRP, an inflammatory neuropeptide) in DRG neurons may be related to the initiation of neurogenic inflammation (Xu et al., 2009). Preliminary studies have further shown that the presence of sympathetic efferents seems critical for the PKC modulation of TRPV1 receptors (Xu et al., 2008). These observations have prompted us to hypothesize that sympathetic modulation of neurogenic inflammation and resulting pain (Lin et al., 2003; Ren et al., 2005) is done partially by targeting TRPV1 receptors via activation of PKC. To test this hypothesis, the present study was performed in rats to examine if the PKC modulation of CAP-evoked up-regulation of TRPV1 receptors in DRG neurons is sympathetically dependent, and if this action is produced by the release of a neurotransmitter, ATP. Immunofluorescence was used for double and triple staining of TRPV1, phosphorylated PKC (p-PKC, an activated form of PKC) and CGRP in DRG neurons. Western blots were used for measuring TRPV1 receptors and p-PKC in DRG tissue. In addition, a possible role of the above process in the pathogenesis of neurogenic inflammation was analyzed by assessing how the CAP-evoked arteriolar vasodilation and edema in the hindpaw are affected by sympathetic outflow and if the PKC cascade is involved.
Materials and Methods
Experimental animals
Adult male Sprague-Dawley rats weighing 250–350 g were housed in groups of two to three in plastic cages with soft bedding under a 12-h light/dark cycle. The rats were kept 7–10 days under these conditions before surgery and up to 2 weeks after surgery. The experimental protocol was approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and was consistent with the ethical guidelines of the National Institutes of Health and of the International Association for the Study of Pain. Efforts were made to minimize the number of animals used and their suffering.
Lumbar sympathectomy
Postganglionic sympathetic denervation was done by a surgical sympathectomy at the L2–6 level as described previously by our group (Lin et al., 2003). Briefly, the sympathetic chains were identified through a transperitoneal approach. All ganglia and the chains from L2–6 were resected bilaterally. Animals were allowed to recover from surgery for at least 1 week before experiments were performed. At the termination of the experiment, the success of the sympathectomy was confirmed anatomically with the fluorescent glyoxylic acid method (Furness and Costa, 1975). Our previous studies have shown that a loss of noradrenergic axons on the femoral arteries on both sides was seen 7–10 days after surgical sympathectomy was performed (Lin et al., 2003; Zou et al., 2002a). Changes in pain behaviors, electrophysiology, immunohistochemistry and cutaneous blood flow have also shown that the effect of sympathetic denervation is obvious 7–10 days after surgical sympathectomy (Kim et al., 1993; Lin et al., 2003; Ren et al., 2005; Zou et al., 2002a). Therefore, experiments were performed 7–10 days after sympathectomy.
Induction of neurogenic inflammation
Rats were anesthetized with sodium pentobarbital at a dose of 50 mg Kg−1 i.p. One percent CAP (dissolved in 7% Tween 80 and 93% saline, pH=7.4) was injected intradermally into the glabrous skin of the hindpaw on one side in a volume of 20 μl (Lin et al., 1999,2003,2004). The level of anesthesia was kept sufficiently deep during the induction and development of inflammation produced by CAP injection (see criteria for judging the depth of anesthesia in the section on cutaneous inflammation measurements). For control purposes, vehicle (7% Tween 80 and 93% saline) was injected intradermally.
Immunohistochemistry analysis
Anesthetized rats were perfused through the left ventricle with 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.3, 4°C). DRG tissue at L4–5 on the side ipsilateral to CAP injection was sampled at 30, 60, and 90 min after injection of CAP or vehicle and processed for immunofluorescence staining. The samples were placed in cold fixative for 4 h and then placed in 30% sucrose solution overnight. Frozen sections were cut at 10 μm on a cryostat. Triple immunofluorescence staining for TRPV1, CGRP and p-PKCα or p-PKCε was performed using a procedure described in our recently published work (Xu et al., 2009). Briefly, primary antibodies used included 1) anti-TRPV1 (1:1,000, guinea-pig polyclonal; Chemicon, Temicula, CA); 2) anti-CGRP (1:1,000, mouse monoclonal; Chemicon, Temicula, CA); and 3) anti-p-PKCα or p-PKCε (1:500, Ser 657 or Ser 729, rabbit; Santa Cruz Biotechnology, Santa Cruz, CA). After blocking by 10% normal goat serum in PBS/0.3% Triton X-100 (PBS-TX) for 1 h at room temperature, sections were incubated with a mixture of the three primary antibodies for 24 h at 4°C. The sections were then transferred to a secondary antibody solution containing Alexa Fluor 405 goat anti-mouse IgG (1:200), Alexa Fluor 488 goat anti-guinea pig IgG (1:200), and Alexa Fluor 568 goat anti-rabbit IgG (1:200) for 1 h, respectively, at room temperature. Control experiments were done for specificity of each primary antibody and its appropriate secondary antibody and for the species specificity of the secondary antibody (Xu et al., 2009).
After staining, labeled sections were examined and processed by confocal laser scanning microscopy (Nikon EZ-C1; Nikon Instruments Inc., Lewisville, TX) for staining of TRPV1, CGRP, and p-PKCα or p-PKCε immunofluorescence. Three fluorescence filters (Fluor 405 filter for blue, Fluor 488 for green, and Fluor 568 for red) were used to separate individual wavelengths for staining. Thus, digitized images were obtained of three different colors for TRPV1, CGRP, and p-PKCα (or p-PKCε). Analysis of costaining was done by matching of corresponding regions. A neuronal profile was considered as positively double or triple labeled if it showed two or three color codes overlapping in space. Sections were selected from each animal for counting and averaging cell profile numbers. For quantification, the average number of single-, double-, or triple-labeled neuronal profiles with different labeling per section from each DRG in 10 sections (at least 50 μm between consecutive sections) per animal was determined.
Western blot analysis
The same procedure for Western blotting was used as in our recently published study (Xu et al., 2009). Briefly, each sample of DRG tissue at L4 or L5 on the side ipsilateral to CAP injection was collected from 3 rats and homogenized in ice-cold homogenization buffer containing protease inhibitors. The concentration of protein in the homogenate was measured using a bicinchoninic acid kit on a microplate reader. After assuring linearity of band density, equivalent amounts of protein for each sample were fractionated and then transferred to a polyvinylidene difluoride (PVDF) membrane. The PVDF membranes were incubated respectively with the TRPV1 (1:2,000) or the p-PKC (α and ε, 1:200) antibody followed by incubation with horseradish peroxidase linked goat anti-rabbit IgG (1:2,000) for 2 h. The blots were exposed to autoradiographic film and the intensity of immunoreactive bands of interest was quantified using densitometric-scanning analyses. β-actin immunoreactivity was used as a loading control. A single band for TRPV1, p-PKCα or p-PKCε in Western blots was expressed relative to the values for β-actin.
Measurements of cutaneous inflammation in the hindpaw
Anesthesia was initiated by i.p. injection of sodium pentobarbital (50 mg/kg) to perform surgery and then maintained by a continuous i.v. infusion of a saline solution containing sodium pentobarbital at the rate of 5–8 mg/kg/h depending upon the desired depth of anesthesia. The level of anesthesia was judged as being sufficiently deep when withdrawal responses to noxious limb stimulation and/or the eye-blink reflex to air-puffs were absent. Once a stable level of anesthesia was reached, the animals were paralyzed with pancuronium (0.3–0.4 mg/h, i.v.) and artificially ventilated. Expiratory CO2 was monitored via a tracheal cannula and kept between 3.5 and 4.5%. The adequacy of the depth of anesthesia during an experiment was evaluated by examination of pupillary reflexes and assessing the stability of the expired CO2. Core body temperature was monitored by a rectal probe and maintained near 37°C by a servo-controlled heating blanket.
Cutaneous blood flow was recorded as blood cell flux by a laser Doppler flowmeter (Moor Instruments, UK), processed by a computer analysis system (CED 1401 plus, with Spike-2 software, UK), and shown in millivolt units (Lin et al., 2003,2004,2007). Changes in cutaneous blood flow (vasodilation) due to CAP injected in the plantar skin of the foot were measured by attaching a laser Doppler flowmeter probe to the skin of the foot with adhesive tape 15–20 mm away from the site where CAP was administered (Lin et al., 1999). It has been reported that the depth of laser penetration can be 500–700 μm below the surface where the probe is placed (Silverman et al., 1994). Therefore, the laser Doppler flow probe presumably picked up the blood flow signal mainly from the microvasculature in the dermis.
Paw-thickness was measured to reflect edema due to plasma extravasation following CAP injection. This was done with a digital caliper placed near the site where the laser Doppler probe was placed. Care was taken to assure that the caliper was placed at the same site on the paw for each measurement. Each measurement was the mean value calculated from 3 trials (Lin et al., 1999,2007).
The vehicle (7% Tween 80 and 93% saline) has been shown in our previous study to produce no obvious changes in blood flow and paw-thickness when injected intradermally in the foot skin (Lin et al., 1999).
Peripheral administration of drugs
An agonist of P2X receptors, α,β-methylene adenosine 5′-triphosphate (α,β-meATP, 25 μg; from Sigma) dissolved in saline, or of P2Y receptors, uridine 5′-triphosphate (UTP, 50 μg; from Sigma) dissolved in saline, was administered by intra-arterial injection in a volume of 25 μl locally into the hindpaw. Saline used for dissolving these drugs was given in the same volume for control purposes. Phorbol 12,13-dibutyate (PDBu, from Sigma), a PKC activator, dissolved in DMSO/saline (1:10), or chelerythrine chloride (C.C.; from Tocris), a PKC inhibitor, dissolved in DMSO/saline (1:20), was topically applied to the DRG at a dose of 5 μg. After laminectomy to expose the L4–5 DRG (Lyu et al., 2000), a small piece of absorbable gelatin sponge (Gelfoam) that was soaked with PDBu or C.C. solution (5 μg in 5 μl) was placed on the surface of each DRG for 10 min before CAP injection. Doses chosen for P2X and P2Y receptor agonists and PKC activator and inhibitor have been demonstrated to be selective for the target receptors and PKC, respectively, in other studies (Lin et al., 1996; Ren et al.,2006; Xu et al., 2009; Zou et al., 2004). All drugs were freshly prepared on the day when the experiment was performed. For control purposes, vehicle used for making the drug solution was given using the same procedure as for the drug.
Experimental strategies and protocol
Immunofluorescence and Western blots
In our recent study (Xu et al., 2009), evoked expression and co-expression of TRPV1, p-PKC and CGRP following CAP injection were visualized using immunofluorescence single labeling and Western blots to analyze the involvement of PKC activation in modulation of TRPV1 receptors. Using the same approach in the present study, we wanted to investigate further if the PKC-mediated up-regulation of TRPV1 involved sympathetic modulation by the action of released ATP. The following manipulations were performed. 1) CAP-evoked cellular expressions (including single and co-immunostaining) of TRPV1, CGRP and p-PKC and protein expression of TRPV1 and p-PKC were observed after sympathectomy. This procedure would help examine the role of sympathetic efferents under inflammatory conditions; 2) the role of the sympathetically released transmitter, ATP, in the CAP-evoked expressions was evaluated with and without PKC inhibition by exogenously administering an ATP analog under sympathectomized conditions. This manipulation would mimic the release of ATP due to activation of sympathetic postganglionic neurons and their efferents to see if this process is critical for up-regulation of TRPV1 receptors through PKC activation under inflammatory conditions. 3) Under sympathectomized conditions, the effect of activation of PKC on the CAP-evoked expression was tested to determine if sympathetic outflow exerted its modulatory effect through activating PKC.
Blood flow and paw-thickness
Since our previous studies have already demonstrated in the same model that vasodilation and edema (increased paw-thickness) induced by CAP injection are mediated by activation of TRPV1 receptors (Lin et al., 2007) and are sympathetically dependent (Lin et al., 2003,2004), in the present study we examined the possible role of PKC in the sympathetically dependent inflammation, so that the anatomical data would be functionally linked to the possible mechanism of sympathetic modulation of neurogenic inflammation. Thus, the same pharmacological manipulations as used for Immunofluorescence and Western blots were performed.
Statistical analysis
Immunohistochemistry and Western blots
Five animals were included in each group. The numbers of immunoreactive positive neuronal profiles showing TRPV1 (single-staining), TRPV1 with p-PKCα or p-PKCε (double-staining) and TRPV1, p-PKCα or p-PKCε with CGRP (triple staining) were counted using Metamorph offline software (Molecular Devices, PA). The numbers of TRPV1 positive neurons, percentage of TRPV1 positive neurons with p-PKCα or p-PKCε and percentage of TRPV1-p-PKCα or -p-PKCε positive neurons with CGRP were analyzed. Densitometric analysis of TRPV1 receptors, p-PKCα or p-PKCε and β-actin in Western blot immunoreactivity results was conducted using Metamorph offline. β-actin immunoreactivity was used as a loading control and immunoreactivity of TRPV1 and p-PKC was normalized to β-actin. Statistical differences between groups having different treatments were determined by one-way ANOVA followed by Dunnett’s post hoc test.
Blood flow and paw-thickness measurements
Baseline blood flow level (pre-CAP) was recorded continuously for 30–40 min and expressed as 100% and percentage changes after CAP injection were compared for groups of animals that received different treatments. A change in paw-thickness following CAP injection was presented as the difference score before and after CAP injection and compared for the groups of animals that received different treatments. Statistical differences between groups were determined by one-way ANOVA followed by the Dunnett’s analysis. Data obtained before and different time points after CAP injection were compared using one-way repeated measures ANOVA followed by Student t-tests.
Values are expressed as means±SEM. In all tests, p<0.05 was considered significant.
Results
1. Changes in the CAP-evoked expressions of TRPV1, p-PKC and CGRP in DRG after sympathetic denervation
Immunofluorescence
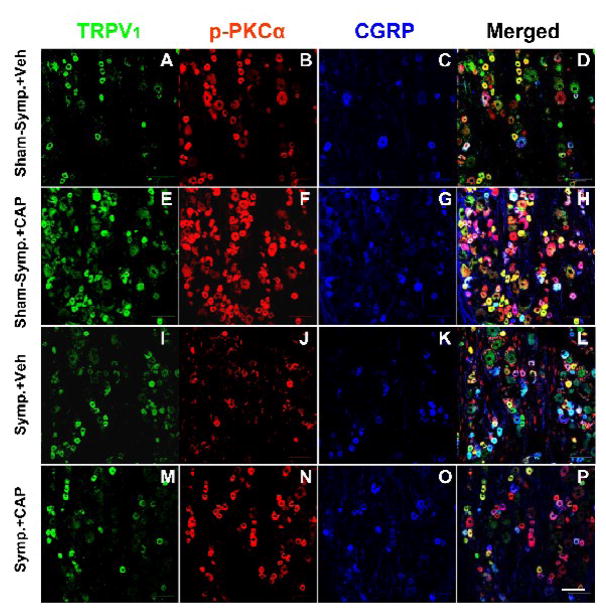
The effects of sympathetic denervation on the CAP-evoked staining of TRPV1, p-PKC and CGRP and their co-localizations in DRG neurons were examined in sham-sympathectomized and sympathectomized rats after intradermal CAP injection and the results were compared to the changes after intradermal vehicle injection. Confocal immunofluorescent images in panels E–H in Fig. 1 are examples showing that the numbers of both single and double/triple stained neurons in L4 DRG were increased 30 min after CAP injection in sham-sympathectomized rats, compared with vehicle injection (panels A–D). The evoked enhancement of both single and double/triple staining by CAP injection was remarkably reduced after sympathetic denervation (M–P vs. E–H in Fig. 1).
Figure 1.
Confocal immunofluorescence images showing single labeling of TRPV1, p-PKCα and CGRP, and double or triple labeling of these three molecules in L4 DRG on the side ipsilateral to intradermal injection of CAP at 30 min after intradermal injection of CAP or vehicle in sham-sympathectomized (Sham-symp.+Veh, Sham-symp.+CAP) and sympathectomized (Symp.+Veh, Symp.+CAP) rats. Bar=100 μm.
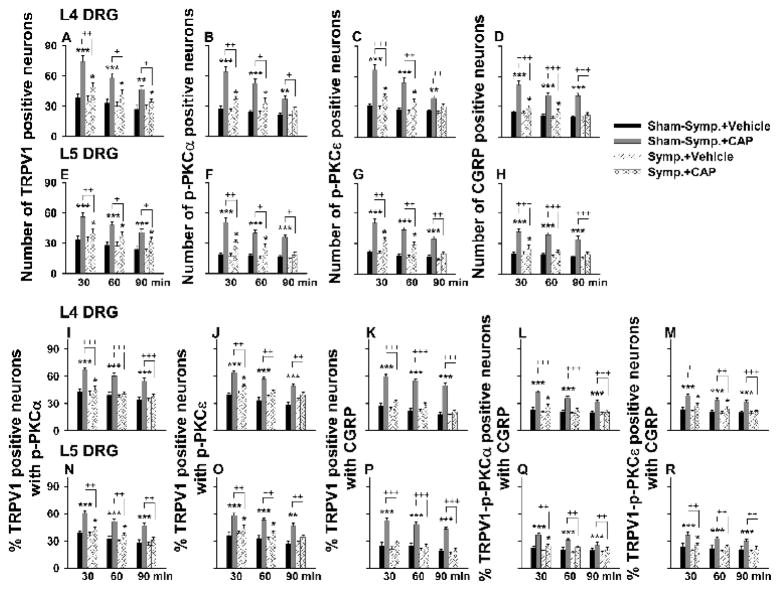
Grouped data in Fig. 2 summarize the numbers of single stained neurons, the percent of TRPV1 positive neurons double-stained for p-PKC or CGRP and the percent of TRPV1-p-PKC neurons triple-stained for CGRP in L4–5 DRGs at 30, 60 and 90 min following CAP injection under sham-sympathectomized and sympathectomized conditions. Consistent with the data obtained from naïve animals in our recent study (Xu et al., 2009), CAP-evoked staining and co-staining in sham-sympathectomized rats showed the largest increase when sampled at 30 min and this increase lasted up to 90 min after injection. These changes were statistically different compared to those evoked by vehicle injection at the same time point in sham-sympathectomized rats. After surgical sympathectomy, the evoked increase in staining and co-staining of all molecules was dramatically reduced at all time points (Fig. 2, +, ++ and +++, P<0.05, P<0.01 and 0.001, compared to the evoked increase in staining in sham-sympathectomized groups). In addition, there was no significant difference in single staining and co-staining evoked by intradermal vehicle injection between sham-sympathectomized and sympathectomized rats (I–L vs. A–D in Fig. 1 and Fig. 2). Thus, sympathetic denervation itself did not appear to affect significantly the baseline staining of these molecules.
Figure 2.
Grouped data summarizing changes in the number of TRPV1-, p-PKC (α and β subunits)-, and CGRP-positive neurons (A–H) and in percent TRPV1 positive neurons with p-PKC double staining, percent TRPV1 positive neurons with CGRP double staining, and percent TRPV1-p-PKC positive neurons with CGRP triple staining (I–R) in L4–5 DRGs after CAP injection in sympathetically intact (Sham-symp.+CAP) and sympathectomized (Symp.+CAP) groups. DRGs were sampled at 30, 60 and 90 min after vehicle or CAP injection. Statistical analysis (*, P<0.05, **, P<0.01; ***, P<0.001) was made of changes due to CAP injection vs. those due to vehicle injection at the same time point in the group having sham-sympathectomy or sympathectomy, and of the CAP-induced effects between the groups of sham-sympathectomy and sympathectomy at the same time point (+, P<0.05; ++, P<0.01; + ++, P<0.001).
Western blots
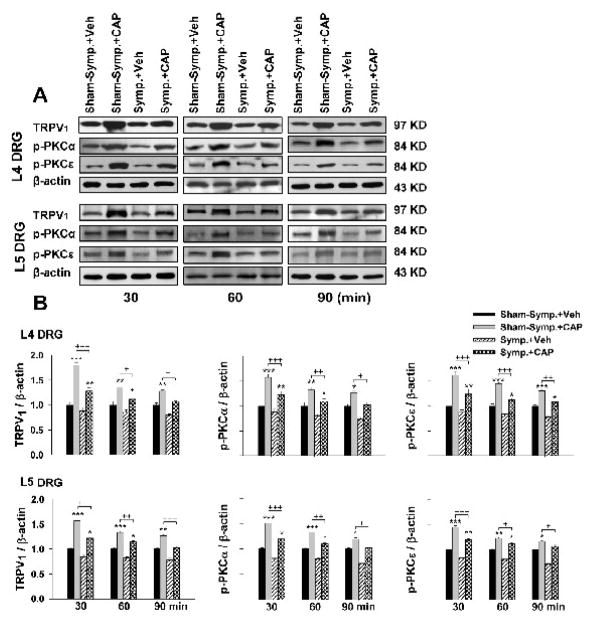
The relative density of immunoblots of TRPV1, p-PKCα, and p-PKCε proteins from DRG tissue after CAP or vehicle injection was compared and changes in evoked expression analyzed in groups having sham-sympathectomy and sympathectomy. Fig. 3 shows the results from L4–5 DRGs in which the time course in evoked expression was determined. Consistent with our recent report (Xu et al., 2009), the relative density of all three molecules was significantly increased at 30 min after CAP injection compared to vehicle injection under sham-sympathectomized (sympathetically intact) conditions. The enhanced expression lasted over 90 min. After sympathetic denervation, the expression of the three proteins evoked by the same dose of CAP was dramatically reduced (+, ++ and + ++: P<0.05, P<0.01 and P<0.001), compared to sham-sympathectomy at the same time point.
Figure 3.
Western blot analyses of the evoked changes in the relative density of TRPV1, p-PKCα and p-PKCε in L4 and L5 DRG tissue following CAP injection in sham-sympathectomized and sympathectomized rats at 30, 60 and 90 min after unilateral injection of CAP. Immunoreactivity of TRPV1, p-PKCα and p-PKCε was normalized to β-actin. A. Representative Western blots for TRPV1, p-PKCα and p-PKCε to CAP injection at 30, 60 and 90 min after injection in the sham-sympathectomized and sympathectomized rats. B. Grouped data of changes in these molecules. * P<0.05, ** P<0.01, *** P<0.001, compared with vehicle injection at the same time point in the group of sham-sympathectomy or sympathectomy. +, P<0.05, ++ P<0.01, +++ P<0.001, compared with the CAP-induced effects under sham-sympathectomized conditions.
2. The effects of exogenous ATP on the reduction in the CAP-evoked expression due to sympathetic denervation and the involvement of PKC
An attenuation in single stained neurons and in percentage of TRPV1 neurons double- and triple-stained for p-PKC and/or CGRP evoked by CAP injection was seen after sympathetic denervation in the first section, which indicates that the evoked up-regulation of TRPV1 receptors is sympathetically dependent. In this section, we wanted to further test pharmacologically if this sympathetic effect was due to the release of neurotransmitters, such as ATP, and if this process involved activation of the PKC cascade. Application of an ATP analog exogenously was presumed to mimic the release of ATP when postganglionic sympathetic efferents were activated.
Immunofluorescence
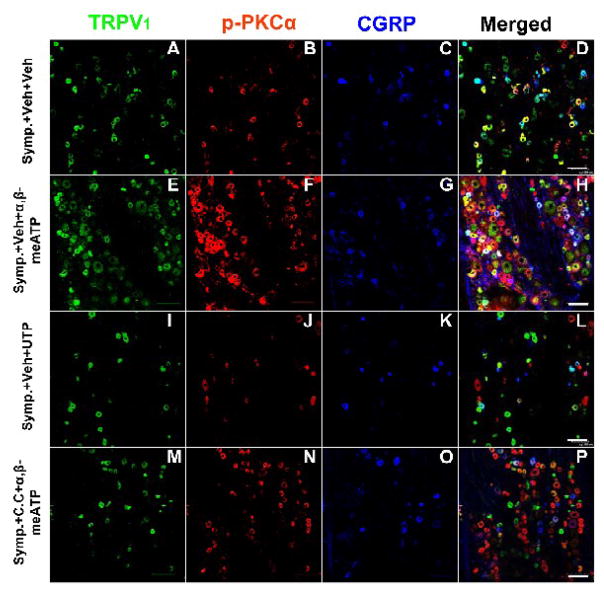
After sympathetic denervation, an ATP analog, α,β-meATP (a P2X receptor agonist) or UTP (a P2Y receptor agonist), was applied topically to the periphery prior to CAP injection. Pretreatment with α,β-meATP significantly restored the reduction in CAP-evoked single and co-staining of TRPV1, p-PKC and CGRP due to sympathetic denervation under conditions where PKC function was intact (vehicle pretreatment, E–H in Fig. 4). Single labeling and percent TRPV1 positive neurons doubly and triply labeled for p-PKC and/or CGRP after CAP injection were all significantly increased in the presence of α,β-meATP compared to vehicle (Fig. 5). In contrast, pretreatment with UPT did not significantly affect the reduction in CAP-evoked expression due to sympathetic denervation (I–L in Fig. 4, Fig. 5).
Figure 4.
Confocal immunofluorescence images showing the restoration by activation of peripheral P2X (not P2Y) receptors of the reduction in the CAP-evoked single staining for TRPV1, p-PKC, and CGRP and double or triple staining for these molecules in L4 DRG on the side ipsilateral to CAP injection after sympathectomy and the effects of PKC inhibition. Data were sampled at 30 min after CAP injection. Symp.+Veh+Veh: CAP-evoked expression in a sympathectomized rat with vehicle pretreatments (used for dissolving the P2X or P2Y agonist and C.C.); Symp.+Veh+α,β-meATP: CAP-evoked expression in a sympathectomized rat without C.C. and with α,β-meATP pretreatments; Symp.+Veh+UTP: CAP-evoked expression in a sympathectomized rat without C.C. and with UTP pretreatments; Symp.+C.C.+α,β-meATP: CAP-evoked expression with C.C. and α,β-meATP pretreatments. Scale bars=100 μm.
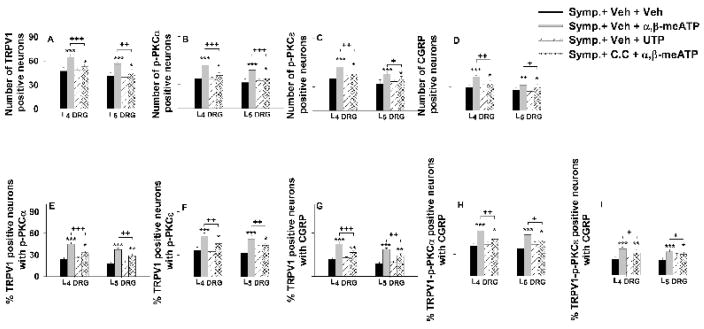
Figure 5.
Grouped data summarizing the restoration by activation of peripheral P2X (not P2Y) receptors on the reduction in the CAP-evoked single staining for TRPV1, p-PKC, and CGRP (A–D) and the reduction in percentage TRPV1 positive neurons with p-PKC double staining (E and F), percentage TRPV1 positive neurons with CGRP double staining (G), and percentage TRPV1-p-PKC positive neurons with CGRP triple staining (H and I) in L4 and L5 DRGs on the side ipsilateral to CAP injection after sympathectomy and the effects of PKC inhibition. *, **, and ***: P<0.05, P<0.01 and P<0.001, compared to the group of sympathectomy with vehicle pretreatment (Symp.+Veh+Veh). +, ++, and +++: P<0.05, P<0.01 and P<0.001, comparison between the groups of sympathectomy without C.C. and with α,β-meATP pretreatments (Symp.+Veh+α,β-meATP) and of sympathectomy with C.C. and α,β-meATP pretreatments (Symp.+C.C.+α,β-meATP).
In order to examine whether the restoration by α,β-meATP was possibly due to a direct effect on the baseline expression of these three molecules, changes in single staining after topical application of α,β-meATP to DRGs were observed in the absence of CAP injection under either sham-sympathectomized or sympathectomized conditions. Results show that α,β-meATP produced no significant effect on baseline expression compared to that in naïve rats (Table 1).
Table 1.
Effects of administration of a P2X purinergic receptor agonist or PKC activator on the baseline staining of TRPV1, p-PKC, and CGRP in DRG neurons in the absence of capsaicin injection in naïve, sham-sympathectomized and sympathectomized rats
| Number of stained neurons a | Naïve | P2X receptor agonist b (α,β-meATP) | PKC activator c (PDBu) | ||||
|---|---|---|---|---|---|---|---|
| Sham-Symp. | Symp. | Sham-Symp. | Symp. | ||||
| L4 DRG | TRPV1 | 33.4±5.7 | 33.1±6.1 | 33.6±5.4 | 35.1±6.2 | 34.7±4.3 | |
| p-PKCα | 25.2±5.2 | 25.7±4.1 | 26.2±3.9 | 27.4±5.5 | 27.1±4.8 | ||
| p-PKCε | 31.7±5.7 | 32.1±4.9 | 32.9±3.4 | 33.3±5.4 | 33.5±4.4 | ||
| CGRP | 24.6±3.3 | 25.5±4.3 | 24.9±3.6 | 25.8±4.2 | 25.1±3.9 | ||
| L5 DRG | TRPV1 | 30.6±5.0 | 32.0±5.8 | 31.5±4.1 | 32.9±6.7 | 32.6±4.3 | |
| p-PKCα | 18.4±2.8 | 20.1±3.8 | 19.2±2.5 | 19.8±3.6 | 19.4±2.1 | ||
| p-PKCε | 20.7±3.6 | 22.8±4.5 | 22.1±2.3 | 23.2±4.1 | 22.8±2.4 | ||
| CGRP | 18.5±2.8 | 20.6±3.9 | 18.8±2.7 | 19.3±3.4 | 19.2±2.2 | ||
PDBu: Phorbol 12,13-dibutyate; α,β-meATP: α,β-Methylene ATP.
Average number of single-stained neurons.
Effect of local application of a P2X receptor agonist (α,β-meATP).
Effect of local application of a PKC activator (PDBu).
The effect of drug was compared with the naïve group.
We then determined whether inhibition of PKC (by pretreatment with chelerythrine chloride) altered the restoration induced by α,β-meATP. A recent report by our group has shown that topical application of chelerythrine chloride did not produce a significant effect on baseline expression of TRPV1, p-PKC and CGRP in the absence of CAP injection but could remarkably block the expression evoked by CAP injection (Xu et al., 2009). In the current study, PKC inhibition prevented the restorative effect of α,β-meATP on the reduction in CAP-evoked expression due to sympathetic denervation (M–P in Fig. 4). After inhibition of PKC, the level of evoked expression and co-expression (Symp.+C.C.+α,β-meATP) was comparable to that evoked under sympathectomized conditions without activation of P2X receptors (Symp.+Veh+Veh, Fig. 5).
Western Blot
Consistent with the immunofluorescence results, a restoration of the reduction in CAP-evoked expression of TRPV1 and p-PKC proteins due to sympathetic denervation was evident after α,β-meATP, but not UTP, pretreatment (vehicle pretreatment) (Fig. 6). However, this restorative effect was significantly blocked when PKC was inhibited by pretreatment with chelerythrine chloride (Fig. 6).
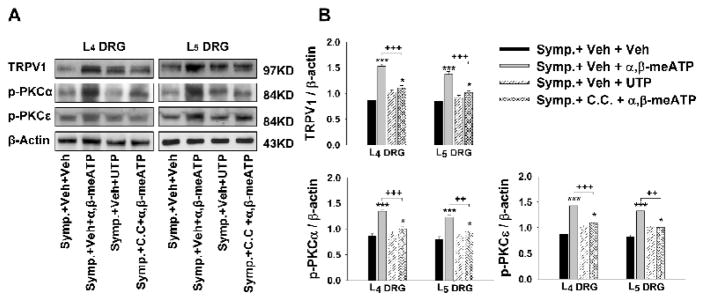
Figure 6.
Western blot analyses of the restoration by activation of peripheral P2X (not P2Y) receptors on the reduction in the CAP-evoked expressions of TRPV1, p-PKCα and p-PKCε proteins in L4 and L5 DRGs after sympathectomy and the effects of PKC inhibition. A: Representative Western blots for TRPV1, p-PKCα and p-PKCε in DRGs ipsilateral to CAP injection at 30 min after CAP injection in the sympathectomized rats pretreated with and without α,β-meATP or UTP when PKC was and was not inhibited by chelerythrine chloride. B: Grouped data of showing changes in these molecules. * and ***: P<0.05 and P<0.001, compared to the group with symapthectomy and vehicle pretreatments (Symp.+Veh+Veh). ++ and +++: P<0.01 and P<0.001, comparison between the groups of sympathectomy without C.C. and with α,β-meATP pretreatments (Symp.+Veh+α,β-meATP) and sympathectomy with C.C. and α,β-meATP pretreatments (Symp.+C.C.+ α,β-meATP).
3. The effects of PKC activation on the reduction in the CAP-evoked expressions of TRPV1, p-PKC and CGRP in DRG due to sympathetic denervation
Results described in section two support the view that the sympathetic effect on evoked up-regulation of TRPV1 receptors is through release of ATP and dependent upon activation of the PKC cascade. In this section, we tested whether the reduction in CAP-evoked expression following sympathectomy can be restored by activation of the PKC cascade.
Immunofluorescence
In sympathectomized rats, a PKC activator PDBu was applied topically to DRGs prior to CAP injection. For control purposes, DRGs were also topically pretreated with the vehicle used for dissolving PDBu in separate groups of sham-sympathectomized and sympathectomized rats (A–D and E–H in Fig. 7). In sympathectomized rats with vehicle pretreatment (Symp.+Veh), CAP-evoked single staining and the percent of TRPV1 positive neurons double-stained for p-PKC or CGRP and percent of TRPV1-p-PKC triple-stained for CGRP 30 min after CAP injection were inhibited (E–H in Fig. 7 and Fig. 8) compared to that in sham-sympathectomized rats (A–D in Fig. 7 and Fig. 8). However, the reduction in CAP-evoked staining and co-staining due to sympathectomy (E–H in Fig. 7 and Fig. 8) were partially restored when PKC was activated by PDBu (I–L in Fig. 7 and Fig. 8; +, ++ and +++: P<0.05, P<0.01 and P<0.001, compared with animals with Symp.+Veh). This was more obvious in L4 DRG neurons.
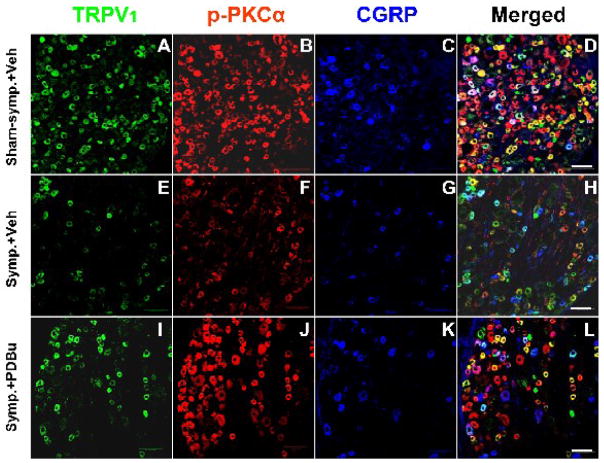
Figure 7.
Confocal immunofluorescence images showing changes in the CAP-evoked single labeling of TRPV1, p-PKCα, and CGRP and double or triple labeling of these molecules in neurons of the L4 DRG on the side ipsilateral to CAP injection after sympathectomy (E–H, Symp.+Veh) compared to the CAP-evoked changes in the sham-sympathectomized (A–D, Sham-symp.+Veh) and the effects of PKC activation by pretreatment with PDBu (I–L, Symp.+PDBu). Data were sampled at 30 min after CAP injection. Scale bar=100 μm.
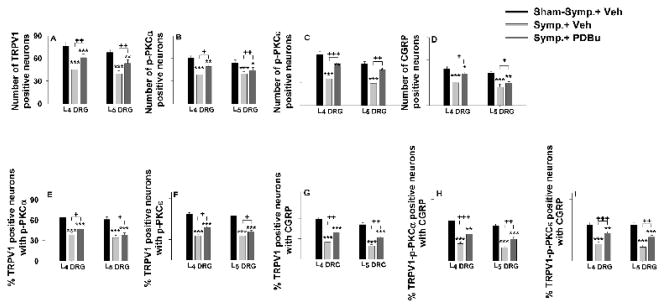
Figure 8.
Grouped data summarizing changes in the CAP-evoked single labeling for TRPV1, p-PKC (α and ε subunits), and CGRP (A–D) and changes in the CAP-evoked percentage of TRPV1 positive neurons with p-PKC double staining (E and F), percentage of TRPV1 positive neurons with CGRP double staining (G), and percentage of TRPV1-p-PKC positive neurons with CGRP triple staining (H and I) in L4 and L5 DRGs on the side ipsilateral to CAP injection after sympathectomy and the effects of PKC activation by pretreatment with PDBu (Symp.+PDBu). Data were sampled at 30 min after CAP injection. *, **, and ***, P<0.05, P<0.01, and P<0.001, compared with the group having sham-sympathectomy with vehicle pretreatment (Sham-symp.+Veh). +, ++, and +++: P<0.05, P<0.01, and P<0.001, comparison between the groups of sympathectomy with vehicle pretreatment (Symp.+Veh) and of sympathectomy with PDBu pretreatment (Symp.+PDBu).
The possibility that the restoration by PDBu was due to a direct effect on the baseline expression of these three molecules was excluded by the observations that pretreatment with PDBu produced no significant changes in baseline expression in the absence of CAP injection under either sham-sympathectomized or sympathectomized conditions (Table 1).
Western blots
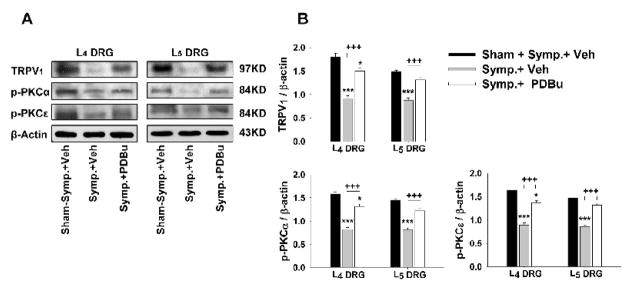
Changes in the CAP-evoked immunoblots of TRPV1 and p-PKC when DRGs were pretreated with PDBu were examined in the sympathectomized groups at 30 min after CAP injection. Pretreatment with PDBu partially restored the reduction in the CAP-evoked protein expression of all three molecules. The evoked immunoblots of TRPV1, p-PKCα and p-PKCε proteins were significantly higher than those in sympathectomized rats having vehicle pretreatment (+++, P<0.001, Fig. 9).
Figure 9.
Western blot analyses of the CAP-evoked changes in the relative density of TRPV1, p-PKCα and p-PKCε in L4 and L5 DRG tissue after sympathectomy and the effects of PKC activation by pretreatment with PDBu. Data were sampled at 30 min after CAP injection. A: Representative Western blots for TRPV1, p-PKCα and p-PKCε in DRG tissue ipsilateral to CAP injection at 30 min after CAP injection in the sham-sympathectomized and sympathectomized rats with and without PDBu pretreatments. B: Grouped data of changes in these molecules. * and ***: P<0.05 and P<0.001, compared to the group of sham-sympathectomy with vehicle pretreatment. +++: P<0.001, comparison between groups of sympathectomy with vehicle pretreatment and sympathectomy with PDBu pretreatment.
4. The effects of exogenous ATP on the reduction in the CAP-evoked inflammation due to sympathetic denervation and the involvement of PKC
Since our previous studies have demonstrated that CAP-evoked inflammation and pain are sympathetically dependent (Lin et al., 2003,2004; Ren et al., 2005,2006), we wanted to evaluate further how inflammation was modulated sympathetically by released ATP, and the role of PKC in this process. Tests were done under sympathectomized conditions to examine the possibility that release of ATP from sympathetic efferent terminals participates in CAP-evoked inflammation by activating peripheral P2X or P2Y receptors. The possible role of PKC in this process was also evaluated. The vehicle used for dissolving PKC inhibitor (chelerythrine chloride) was given 10 min prior to administration of the P2X (α,β-meATP) or P2Y (UTP) agonist, and CAP was injected intradermally 10 min after the α,β-meATP or UTP was given (Symp.+Vehicle+α,β-meATP or Symp.+Vehicle+UTP). Under the conditions where PKC function was intact (vehicle pretreatment), peripheral P2X or P2Y receptors were activated by intra-arterial injection of α,β-meATP or UTP 10 min prior to intradermal CAP injection. A slight decrease in blood flow level was seen immediately after injection of either α,β-meATP or UTP (the left panel in Fig. 10A). After this decrease, there was a large increase in blood flow immediately following CAP injection after α,β-meATP pretreatment, but not after UTP pretreatment. In rats pretreated with α,β-meATP followed by CAP injection, the peak increase reached 306±27% and the mean value recorded at 60 min was 223±20%. These changes were significantly different from responses measured in sympathectomized rats pretreated with vehicle (Symp.+Vehicle+Vehicle, P=0.0052 and P=0.007). This enhancement was also comparable to the increase in blood flow seen under sham-sympathectomized conditions. The enhanced blood flow persisted up to 1 h following CAP injection. In contrast, under the condition where PKC function was inhibited by pretreatment with chelerythrine chloride, the restoration by α,β-meATP was greatly reduced (Symp.+C.C.+ α,β-meATP, the left panel in Fig. 10A). The blood flow was comparable to that evoked under sympathectomized conditions without activation of P2X receptors. Similar results were seen in changes in paw-thickness produced by the same treatment (the right panel in Fig. 10A).
Figure 10.
A: Restorative effect of activation of peripheral P2X (not P2Y) receptors on the reduction in the CAP-induced enhancement of vasodilation (left panel) and edema (right panel) due to sympathectomy and the effect of PKC inhibition. Chelerythrine chloride (C.C., a PKC inhibitor) was given 20 min prior to CAP injection. α,β-meATP (a P2X agonist) or UTP (a P2Y agonist) was injected 10 min prior to CAP injection. **: P<0.01, compared to the group of sympathectomy with vehicle pretreatment/without C.C. pretreatment (Symp. +Vehicle+Vehicle). +: P<0.05, compared to the group of sympathectomy with α,β-meATP pretreatment/without C.C. pretreatment (Symp.+Vehicle+α,β-meATP). B: Restorative effect of activation of PKC by intra-arterial injection of PDBu (a PKC activator) on the reduction in the CAP-induced enhancement of vasodilation (left panel) and edema (right panel) due to sympathectomy (Symp.). PDBu was given 10 min prior to CAP injection. **: P<0.01, compared to the group of sympathectomy with vehicle pretreatment (Symp. +Vehicle). +: P<0.05, compared to the group of sham-sympathectomy with vehicle pretreatment (Sham-symp.+Vehicle).
5. The effects of PKC activation on the reduction in the CAP-evoked inflammation due to sympathetic denervation
Changes in blood flow and paw-thickness following CAP injection in sham-sympathectomized and sympathectomized rats were tested with vehicle pretreatment by intra-arterial injection to serve as controls for the following experiments using a PKC activator (PDBu). In agreement with our previous studies (Lin et al., 2003,2004), the increase in cutaneous blood flow in the hindpaw following intradermal injection of CAP was greatly reduced after the sympathetic efferents were removed surgically. The grouped data show that peak value and the value recorded at 60 min following CAP injection were significantly lower than those recorded from sham-sympathectomized rats (P=0.0032 and P=0.0053; the left panel in Fig. 10B). In the sham-operated group, the difference score of paw-thickness was 1.22±0.12. In the sympathectomized group of rats, the difference score of paw-thickness was significantly decreased to 0.66±0.11 (P=0.012, compared to the sham-operated group; the right panel in Fig. 10B). Pretreatment with vehicle did not prevent the reduction in CAP-induced inflammation following sympathectomy.
Pretreatment with PDBu 10 min prior to CAP injection produced no obvious change in blood flow level under sympathectomized conditions. However, the vasodilation evoked by CAP injection was largely restored in the presence of PDBu. The peak value and the value recorded at 60 min following CAP injection were 320±39% and 202±32%, respectively, without statistically significant differences from the values evoked in sham-sympathectomized rats pretreated with vehicle (the left panel in Fig. 10B). A similar change was seen in paw-thickness in which an increase in the difference score was largely restored by PDBu (the right panel in Fig. 10B).
The possibility that the local blood flow reaction was the result of a change in systemic blood pressure was excluded in our previous study in which intra-arterial drug injection did not significantly increase the blood flow level in the forepaw skin (Lin et al., 2004).
Discussion
The present study continues our series of studies investigating sympathetic modulation of neurogenic inflammation and resulting pain evoked by intradermal injection of CAP (Lin et al., 2003,2004; Ren et al., 2005,2006; Wang et al., 2004; Zou et al., 2002a) to examine whether and how TRPV1 receptors in DRG neurons are targeted by sympathetic outflow evoked by CAP injection. Our new findings are that 1) the evoked expression of TRPV1, p-PKC and CGRP in DRG neurons following CAP injection is sympathetically dependent; 2) in the presence of activated PKC, exogenously applied ATP restores the attenuation of CAP-evoked expression of TRPV1 receptors due to sympathectomy; 3) analysis of the potential contribution of the above process to the CAP-evoked vasodilation and edema suggests that this could be a mechanism by which neurogenic inflammation is sympathetically modulated.
Up-regulation of TRPV1 receptors evoked by CAP injection and modulated by PKC
TRPV1 in primary afferent nociceptive neurons and their axons frequently colocalizes with substance P (SP) and CGRP (Aoki et al., 2005; Guo et al., 1999; Price and Flores, 2007), the release of which is the pathophysiological basis by which neurogenic inflammation is induced when TRPV1 receptors are activated (Holzer, 1988; Kessler et al., 1999; Szolcsanyi, 1996). In cultured DRG neurons, CAP, acting on TRPV1 receptors, evokes a membrane current carried by influx of Ca2+ and/or Na+ to cause an increase in biosythesis and release of CGRP and SP (Jakab et al., 1994; Jung et al., 1999; Oh et al., 1996, Xing et al., 2006). Anatomical studies in vivo reveal that CAP administered in the periphery evokes an enhanced expression of TRPV1 receptors as well as enhanced co-expression with other nociceptive molecules and activated protein kinases, such as CGRP, p-PKC, p-tyrosine kinase A and p-PKB/Akt, in DRG neurons (Pezet et al., 2005; Sun et al., 2007; Xu et al., 2009). It is evident that activated PKC is involved in the nociception-evoked up-regulation of TRPV1 receptors (Ahern and Premkumar, 2002; Bhave et al., 2003; Harvey et al., 1995; Numazaki et al., 2002). TRPV1-mediated nociceptive neuronal and behavioral responses to CAP, tissue injury or inflammation are suggested to be modulated by PKC activation (Premkumar and Ahern, 2000; Vellani et al., 2001; Srinivassan et al., 2008; Zhou et al., 2001a,2003). In our recent and the present studies in vivo, we demonstrate that activation of PKC is critical for CAP-evoked up-regulation of TRPV1 receptors at mRNA and protein levels because blockade of TRPV1 receptors in the periphery or inhibition of PKC in DRG had no significant effect on the baseline (not evoked) expression of TRPV1, but reduced dramatically the CAP-evoked single- and double-labeled TRPV1 and CGRP DRG neurons (Xu et al., 2009).
It has been established that CAP injection in the periphery evokes a rapidly enhanced expression of receptor proteins and/or neuropeptide in DRG and spinal dorsal horn neurons (Fang et al., 2002,2003; Pezet et al., 2005; Sun et al., 2007, Xu et al., 2009). It is also evident that a fast activation of several signal transduction cascades is seen in phosphorylated forms, and some of them are co-expressed with TRPV1 receptors following CAP injection (Fang et al., 2002,2003; Sun et al., 2007; Xu et al., 2009; Zou et al., 2000,2002b). Therefore, the PKC-modulated ligand-receptor response accounts for one of the major mechanisms by which primary afferent nociceptors are sensitized and trigger neurogenic inflammation following CAP injection (Lin et al., 2007; Ren et al., 2005).
Sympathetic outflow modulates TRPV1 receptors and the role of PKC activation
It is hypothesized that interactions between sympathetic efferents and sensory afferent neurons or their terminals underlies several types of sympathetically maintained pathological pain (Dowd et al., 1998; Drummond, 1995,1998; Lee et al., 1999; Lin et al., 2004,2004; Ren et al., 2005,2006). There is ample evidence that plastic changes in primary afferent neurons and their axons that occur after peripheral nerve injury (neuropathy) or persistent nociceptive stimulation (CAP injection) are associated with the effects of the sympathetic outflow. These changes include development of adrenergic/purinergic sensitivity of nociceptors (Moon et al., 1999; Zhou et al., 2001b) and up-regulation of adrenergic/purinergic receptors in the periphery (Birder and Perl, 1999; Drummond et al., 1996; Eriksson et al., 1998; Novakovic et al., 1999; Xie et al., 2001). Moreover, our previous studies show that CAP-evoked sensitization of primary afferent nociceptors and inflammation can be reduced after sympathetic denervation and restored by exogenously applied adrenergic (norepinephrine) or non-adrenergic (ATP or neuropeptide Y) transmitter in the periphery (Lin et al., 2003,2004; Ren et al., 2005,2006) so we proposed that the sympathetic outflow is likely to be increased after peripheral injury or persistent nociceptive stimulation by the action of released transmitters. However, it is not known whether these plastic changes are due to the sympathetic effect on TRPV1 receptors.
In the present study, we found that sympathetic denervation could dramatically inhibit enhanced expression of TRPV1, CGRP and p-PKC and their co-expression in DRG neurons evoked by CAP injection. Pharmacology for testing the role of ATP in CAP-evoked events by exogenously applying α,β-meATP in sympathectomized rats showed that activation of peripheral P2X (not P2Y) receptors restored the reduction in the CAP-evoked expression and co-expression of TRPV1, CGRP and p-PKC. There have been some reports that sympathectomy and/or nerve injury induces an increase in adrenergic or purinergic sensitivity in afferents due to a proposed up-regulation of adrenergic or purinergic receptors (Chen et al., 2000; Moon et al., 1999). However, this phenomenon did not occur in the current study because sympathectomy reduced the CAP-evoked expression, and α,β-meATP restored partially (but did not potentiate) the reduction in evoked expression. Moreover, control experiments showed that neither sympathetic denervation nor exogenous ATP produced significant effects on baseline expression and co-expression in the absence of CAP injection. This is consistent with our previous studies in which sympathectomy or exogenous transmitter did not produce a significant effect on baseline responses of nociceptive afferents to mechanical stimuli in the absence of CAP injection (Ren et al., 2005,2006). Therefore, these findings provide new evidence to support the view that the presence of sympathetic efferents and released ATP are critical for sensitization of primary afferent nociceptors (Ren et al., 2006), which is done by modulating the up-regulation of TRPV1 receptors induced by CAP injection.
It is proposed that one of the ways that signal transduction cascades modulate TRPV1 receptors could be via the release of neurotransmitters or inflammatory mediators that would trigger intracellular cascades and result in up-regulation of TRPV1 receptors (Cortright and Szallasi, 2004; Huang et al., 2006). This mechanism can be linked to inflammatory disease-related changes in TRPV1 expression, such as inflammatory bowel disease and pancreatitis (Messeguer et al., 2006; Xu et al., 2007). ATP, acting as an inflammatory mediator and/or key nociceptive signaling molecule, participates in several types of pain by acting on P2X or P2Y receptors (Burnstock 2001; Dunn et al., 2001; Lazarowski et al., 2000; Premkumar 2001; Sawynok and Sweeney, 1989). Our previous study demonstrates that ATP can potentiate the CAP-induced sensitization of primary afferent nociceptors and restore CAP-induced sensitization following sympathetic denervation (Ren et al., 2006). Anatomically, P2X and P2Y receptors are predominantly expressed in small-sized DRG neurons that are usually TRPV1-positive (Chen et al., 1995; Guo et al., 1999; Moriyama et al., 2003; Ueno et al., 1999). Evidence has indicated that ATP modulates TRPV1-mediated nociceptive behaviors and neuronal responses preferentially through P2Y receptors in a PKC-dependent pathway (Moriyama et al., 2003; Tominaga et al., 2001). Our present study shows that the attenuation of CAP-evoked expression of TRPV1 and CGRP following sympathectomy can be restored by exogenous ATP acting on P2X (not P2Y) receptors in a PKC dependent manner. This phenomenon was further confirmed by the observation that activation of PKC by application of PDBu could restore the attenuation of CAP-evoked expression of TRPV1 and CGRP due to sympathetic denervation. Control experiments reveal that neither local application of α,β-meATP nor PDBu to DRG produce significant effects on baseline expression in either sympathetically intact or sympathetic denervated rats, and that chelerythrine chloride administered in DRG also did not obviously affect the baseline expression of these molecules in naïve rats (Xu et al., 2009). Therefore, activated PKC seems critical for the sympathetic modulation of TRPV1 receptors following CAP injection. However, at this time, little is known about how activation of purinergic (either P2X or P2Y) receptors links to up-regulation of TRPV1 receptors although an in vitro study indicates that ATP can phosphorylate TRPV1 receptors via PKC (Numazaki et al., 2002).
Pathophysiological significance of sympathetic effects on TRPV1 receptors in the pathogenesis of neurogenic inflammation
In the assessment of vasodilation and edema evoked by CAP injection under sympathectomized conditions, we have provided further evidence to support that the release of neurotransmitters, norepinephrine (Lin et al., 2003), neuropeptide Y (Lin et al., 2004) and/or ATP (reported in the present study), from sympathetic efferents is critical for acute neurogenic inflammation induced by CAP injection, which is done through activation of the PKC cascade. The results are consistent in time-course and effects with the data on immunochemistry and Western blot of those nociceptive molecules in DRG neurons modulated sympathetically. There is evidence that neurogenic inflammation and resulting sensitization of primary afferent nociceptors induced by CAP injection in the periphery is initiated by activation of TRPV1 receptors (Li et al., 2008; Lin et al., 2007). The development and maintenance of these phenomena are associated with an increased production of inflammatory neuropeptides, such as CGRP, in DRG neurons (Li et al., 2008; Xu et al., 2009). The release of CGRP by primary afferent nociceptive terminals is suggested to be driven antidromically by the way of dorsal root reflexes (DRRs), because interruption of DRRs (Li et al., 2008; Lin et al., 2003,2004), blockade of peripheral TRPV1 receptors or sectioning of afferent nerves (Garcia-Nicas et al., 2001; Lin et al., 1999,2007) can alleviate the evoked inflammation and sensitization of nociceptors. Many peptidergic DRG neurons co-express P2X and P2Y receptors (Ruan et al., 2003,2005), providing the anatomical basis by which ATP augments CAP-evoked release of neuropeptides from sensory neurons (Huang et al., 2003). Therefore, it is plausible that sympathetic effects on CAP-evoked expression of these nociceptive molecules in DRG neurons are a major mechanism by which neurogenic inflammation is modulated.
Conclusion
The present data suggest strongly that the CAP-evoked up-regulation of TRPV1 receptors in primary afferent nociceptive neurons is modulated by ATP, one of the sympathetically released neurotransmitters. Activation of the PKC cascade is critical in this process. Thus, we propose that this is potentially a new way by which sympathetic outflow modulates neurogenic inflammation and the resulting pain. Future studies are needed to further analyze the role of endogenous ATP by using purinergic receptor antagonists and to determine whether purinergic receptors on nociceptors are up-regulated due to the release of ATP from sympathetic efferents and whether this is linked to PKC activation.
Acknowledgments
The authors would like to thank Drs. Susan Carlton, Richard Coggeshall and William Willis for their valuable comments and advice on the preparation of this manuscript. The work was supported by NIH grants NS 040723 (Q Lin) and NS 011255 (JM Chung).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahern GP, Premkumar LS. Voltage-dependent priming of rat vanilloid receptor: effects of agonist and protein kinase C activation. J Physiol (Lond) 2002;545:441–451. doi: 10.1113/jphysiol.2002.029561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Ohtori S, Takahashi K, Ino H, Douya H, Ozawa T, Saito T, Moriya H. Expression and Co-expression of VR1, CGRP, and IB-4-binding glycoprotein in dorsal root ganglion neurons in rats: differences between the disc afferents and the cutaneous afferents. Spine. 2005;30:1496–1500. doi: 10.1097/01.brs.0000167532.96540.31. [DOI] [PubMed] [Google Scholar]
- Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Perl ER. Expression of alpha2-adrenergic receptors in rat primary afferent neurones after peripheral nerve injury or inflammation. J Physiol (Lond) 1999;515:533–542. doi: 10.1111/j.1469-7793.1999.533ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purine-mediated signaling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- Chen CC, Akoplan AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang YH, Bie BH, Zhao ZQ. Sympathectomy induces novel purinergic sensitivity in sciatic afferents. Acta Pharmacol Sin. 2000;21:1002–1004. [PubMed] [Google Scholar]
- Chung K, Lee BH, Yoon YW, Chung JM. Sympathetic sprouting in the dorsal root ganglia of the injured peripheral nerve in a rat neuropathic pain model. J Comp Neurol. 1996;376:241–252. doi: 10.1002/(SICI)1096-9861(19961209)376:2<241::AID-CNE6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Basbaum AI, Levine JD. Neural control of vascular permeability: interactions between primary afferents, mast cells, and sympathetic efferents. J Neurophysiol. 1989;62:48–58. doi: 10.1152/jn.1989.62.1.48. [DOI] [PubMed] [Google Scholar]
- Cortright DN, Szallasi A. Biochemical pharmacology of the vanilloid receptor TRPV1. Eur J Biochem. 2004;271:1814–1819. doi: 10.1111/j.1432-1033.2004.04082.x. [DOI] [PubMed] [Google Scholar]
- Devor M, Jänig W, Michaelis M. Modulation of activity in dorsal root ganglion neurons by sympathetic activation in nerve-injured rats. J Neurophysiol. 1994;71:38–47. doi: 10.1152/jn.1994.71.1.38. [DOI] [PubMed] [Google Scholar]
- Devor M, Seltzer Z. Pathophysiology of damaged nerves in relation to chronic pain. In: Wall PD, Melzack R, editors. Textbook of Pain. 4. Churchill Livingstone; London: 1999. pp. 129–164. [Google Scholar]
- Dowd E, McQueen DS, Cheesell IP, Humphrey PP. P2X receptor-mediated excitation of nociceptive afferents in the normal and arthritic rat knee joint. Br J Pharmacol. 1998;125:341–346. doi: 10.1038/sj.bjp.0702080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond PD. Noradrenaline increases hyperalgesia to heat in skin sensitized by capsaicin. Pain. 1995;60:311–315. doi: 10.1016/0304-3959(94)00130-7. [DOI] [PubMed] [Google Scholar]
- Drummond PD. Enhancement of thermal hyperalgesia by a-adrenoceptors in capsaicin-treated skin. J Auton Nerv Syst. 1998;69:96–102. doi: 10.1016/s0165-1838(98)00017-4. [DOI] [PubMed] [Google Scholar]
- Drummond PD, Skipworth S, Finch PM. α1-Adrenoceptors in normal and hyperalgesic human skin. Clin Sci. 1996;91:73–77. doi: 10.1042/cs0910073. [DOI] [PubMed] [Google Scholar]
- Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Prog Neurobiol. 2001;65:107–134. doi: 10.1016/s0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Eriksson J, Bongenhielm U, Kidd E, Matthews B, Fried K. Distribution of P2X3 receptors in the rat trigeminal ganglion after inferior alveolar nerve injury. Neurosci Lett. 1998;254:37–40. doi: 10.1016/s0304-3940(98)00656-9. [DOI] [PubMed] [Google Scholar]
- Fang L, Wu J, Lin Q, Willis WD. Calcium-calmodulin-dependent protein kinase II contributes to spinal cord central sensitization. J Neurosci. 2002;22:4196–4204. doi: 10.1523/JNEUROSCI.22-10-04196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Wu J, Zhang X, Lin Q, Willis WD. Increased phosphorylation of the GluR1 subunit of spinal cord α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor in rats following intradermal injection of capsaicin. Neuroscience. 2003;122:237–245. doi: 10.1016/s0306-4522(03)00526-8. [DOI] [PubMed] [Google Scholar]
- Furness JB, Costa M. The use of glyoxylic acid for the fluorescence histochemical demonstration of peripheral stores of noradrenaline and 5-Hydroxytryptamine in whole mounts. Histochemistry. 1975;41:335–352. doi: 10.1007/BF00490076. [DOI] [PubMed] [Google Scholar]
- Garcia-Nicas E, Laird J, Cervero F. Vasodilatation in hyperalgesic rat skin evoked by stimulation of afferent Aβ-fibers: further evidence for a role of dorsal root reflexes in allodynia. Pain. 2001;94:283–291. doi: 10.1016/S0304-3959(01)00365-7. [DOI] [PubMed] [Google Scholar]
- Gibbs GF, Drummond PD, Finch PM, Phillips JK. Unraveling the pathophysiology of complex regional pain syndrome: focus on sympathetically maintained pain. Clin Exp Pharmacol Physiol. 2008;35:71–724. doi: 10.1111/j.1440-1681.2007.04862.x. [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Harvey JS, Davis C, James IF, Burgess GM. Activation of protein kinase C by the capsaicin analogue resiniferatoxin in sensory neurons. J Neurochem. 1995;65:1309–1317. doi: 10.1046/j.1471-4159.1995.65031309.x. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Huang H, Wu X, Nicol GD, Meller S, Vasko MR. ATP augments peptide release from rat sensory neurons in culture through activation of P2Y receptors. J Pharmacol Exp Ther. 2003;306:1137–1144. doi: 10.1124/jpet.103.052951. [DOI] [PubMed] [Google Scholar]
- Huang J, Zhang X, McNaughton PA. Inflammatory pain: the cellular basis of heat hyperalgesia. Curr Neuropharm. 2006;4:197–206. doi: 10.2174/157015906778019554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G, Szallasi A, Agoston D. The calcitonin gene-related peptide (CGRP) phenotype is expressed early and up-regulated by resiniferatoxin (RTX) in mouse sensory neurons. Brain Res Dev Brain Res. 1994;80:290–294. doi: 10.1016/0165-3806(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Jänig W, Levine JD, Michaelis M. Interactions of sympathetic and primary afferent neurons following nerve injury and tissue trauma. In: Kumazawa L, Kruger L, Mizumura K, editors. Progress in Brain Research. Vol. 113. Elsevier Science; 1996. pp. 161–184. [DOI] [PubMed] [Google Scholar]
- Jänig W, McLachlan EM. The role of modifications in noradrenergic peripheral pathways after nerve lesions in the generation of pain. In: Fields HL, Liebeskind JC, editors. Progress in Pain research and Management. Vol. 1. IASP Press; Seattle: 1994. pp. 101–128. [Google Scholar]
- Jung J, Hwang SW, Kwak J, Lee SY, Kang CJ, Kim WB, Kim D, Oh U. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler F, Habelt C, Averbeck B, Reeh PW, Kress M. Heat-induced release of CGRP from isolated rat skin and effects of bradykinin and the protein kinase C activator PMA. Pain. 1999;83:289–295. doi: 10.1016/s0304-3959(99)00108-6. [DOI] [PubMed] [Google Scholar]
- Kim SH, Na HS, Sheen K, Chung JM. Effects of sympathectomy on a rat model of peripheral neuropathy. Pain. 1993;55:85–92. doi: 10.1016/0304-3959(93)90187-T. [DOI] [PubMed] [Google Scholar]
- Kinnman E, Levine JD. Sensory and sympathetic contributions to nerve injury-induced sensory abnormalities in the rat. Neuroscience. 1995;64:751–767. doi: 10.1016/0306-4522(94)00435-8. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- Lee DH, Liu X, Kim HT, Chung K, Chung JM. Receptor subtype mediating the adrenergic sensitivity of pain behavior and ectopic discharges in neuropathic Lewis rats. J Neurophysiol. 1999;81:2226–2233. doi: 10.1152/jn.1999.81.5.2226. [DOI] [PubMed] [Google Scholar]
- Li D, Ren Y, Xu X, Zou X, Fang L, Lin Q. Sensitization of primary afferent nociceptors induced by intradermal capsaicin involves the peripheral release of calcitonin gene-related peptide driven by dorsal root reflexes. J Pain. 2008;9:1155–1168. doi: 10.1016/j.jpain.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Li D, Xu X, Zou X, Fang L. Roles of TRPV1 and neuropeptidergic receptors in dorsal root reflex-mediated neurogenic inflammation induced by intradermal injection of capsaicin. Mol Pain. 2007;3:30. doi: 10.1186/1744-8069-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Peng YB, Willis WD. Inhibition of primate spinothalamic tract neurons by spinal glycine and GABA is reduced during central sensitization. J Neurophysiol. 1996;76:1005–1014. doi: 10.1152/jn.1996.76.2.1005. [DOI] [PubMed] [Google Scholar]
- Lin Q, Wu J, Willis WD. Dorsal root reflexes and cutaneous neurogenic inflammation following intradermal injection of capsaicin in rats. J Neurophysiol. 1999;82:2602–2611. doi: 10.1152/jn.1999.82.5.2602. [DOI] [PubMed] [Google Scholar]
- Lin Q, Zou X, Fang L, Willis WD. Sympathetic modulation of acute cutaneous flare induced by intradermal injection of capsaicin in anesthetized rats. J Neurophysiol. 2003;89:853–861. doi: 10.1152/jn.00568.2002. [DOI] [PubMed] [Google Scholar]
- Lin Q, Zou X, Ren Y, Wang J, Fang L, Willis WD. Involvement of peripheral neuropeptide Y receptors in sympathetic modulation of acute cutaneous flare induced by intradermal capsaicin. Neuroscience. 2004;123:337–347. doi: 10.1016/j.neuroscience.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Lyu YS, Park SK, Chung K, Chung JM. Low dose of tetrodotoxinreduces neuropathic pain behaviors in an animal model. Brain Res. 2000;871:98–103. doi: 10.1016/s0006-8993(00)02451-3. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Basbaum AI. Partial sciatic nerve injury in the mouse as a model of neuropathic pain: behavioral and neuroanatomical correlates. Pain. 1998;76:215–222. doi: 10.1016/s0304-3959(98)00045-1. [DOI] [PubMed] [Google Scholar]
- Messeguer A, Planells-Cases R, Ferrer-Montiel A. Physiology and pharmacology of the vanilloid receptor. Curr Neuropharm. 2006;4:1–15. doi: 10.2174/157015906775202995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon DE, Lee DH, Han HC, Xie J, Coggeshall RE, Chung JM. Adrenergic sensitivity of the sensory receptors modulating mechanical allodynia in a rat neuropathic pain model. Pain. 1999;80:589–595. doi: 10.1016/S0304-3959(98)00252-8. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, Noguchi K, Tominaga M. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23:6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakovic SD, Kassotakis LC, Oglesby IB, Smith JA, Eglen RM, Ford AP, Hunter JC. Immunocytochemical localization of P2X3 purinoceptors in sensory neurons in naive rats and following neuropathic injury. Pain. 1999;80:273–282. doi: 10.1016/s0304-3959(98)00225-5. [DOI] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Ce and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- Oh U, Hwang SW, Kim D. Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons. J Neurosci. 1996;16:1659–1667. doi: 10.1523/JNEUROSCI.16-05-01659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet S, Spyropoulos A, Williams RJ, McMahon SB. Activity-dependent phosphorylation of Akt/PKB in adult DRG neurons. Eur J Neurosci. 2005;21:1785–1797. doi: 10.1111/j.1460-9568.2005.04011.x. [DOI] [PubMed] [Google Scholar]
- Premkumar LS. Interaction between vanilloid receptors and purinergic metabotropic receptors: pain perception and beyond. PNAS. 2001;98:6537–6539. doi: 10.1073/pnas.121190798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- Price T, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 2007;8:263–272. doi: 10.1016/j.jpain.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja SN. Role of the sympathetic nervous system in acute pain and inflammation. Ann Med. 1995;27:241–246. doi: 10.3109/07853899509031966. [DOI] [PubMed] [Google Scholar]
- Ren Y, Zou X, Fang L, Lin Q. Sympathetic modulation of activity in Aδ- and C-primary nociceptive afferents after intradermal injection of capsaicin in rats. J Neurophysiol. 2005;93:365–377. doi: 10.1152/jn.00804.2004. [DOI] [PubMed] [Google Scholar]
- Ren Y, Zou X, Fang L, Lin Q. Involvement of peripheral purinoceptors in sympathetic modulation of capsaicin-induced sensitization of primary afferent fibers. J Neurophysiol. 2006;96:2207–2216. doi: 10.1152/jn.00502.2006. [DOI] [PubMed] [Google Scholar]
- Ruan HZ, Birder LA, de Groat WC, Tai C, Roppolo J, Buffington CA, Burnstock G. Localization of P2X and P2Y receptors in dorsal root ganglia of the cat. J Histochem Cytochem. 2005;53:1273–1282. doi: 10.1369/jhc.4A6556.2005. [DOI] [PubMed] [Google Scholar]
- Ruan HZ, Burnstock G. Localisation of P2Y1 and P2Y4 receptors in dorsal root, nodose and trigeminal ganglia of the cat. Histochem Cell Biol. 2003;120:415–426. doi: 10.1007/s00418-003-0579-3. [DOI] [PubMed] [Google Scholar]
- Sato J, Kumazawa T. Sympathetic modulation of cutaneous polymodal receptors in chronically inflamed and diabetic rats. In: Kumazawa T, Kruger L, Mizumura K, editors. Progress in brain research. Vol. 113. Elsevier Science BV; Amsterdam: 1996. pp. 153–159. [DOI] [PubMed] [Google Scholar]
- Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science. 1991;251:1608–1610. doi: 10.1126/science.2011742. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Sweeney MI. The role of purines in nociception. Neuroscience. 1989;32:557–569. doi: 10.1016/0306-4522(89)90278-9. [DOI] [PubMed] [Google Scholar]
- Silverman DG, Jotkowitz AB, Freemer M, Gutter V, O’Connor TZ, Braverman IM. Peripheral assessment of phenylephrineinduced vasoconstriction by laser Doppler flowmetry and its potential relevance to homeostatic mechanisms. Circulation. 1994;90:23–26. doi: 10.1161/01.cir.90.1.23. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Wolf D, Goss J, Watkins S, de Groat WC, Sculptoreanu A. Protein kinase C epsilon contributes to basal and sensitizing responses of TRPV1 to capsaicin in rat dorsal root ganglion neurons. Eur J Neurosci. 2008;28:1241–1254. doi: 10.1111/j.1460-9568.2008.06438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R, Yan J, Willis WD. Activation of protein kinase B/Akt in the periphery contributes to pain behavior indunced by capsaicin in rats. Neuroscience. 2007;144:286–294. doi: 10.1016/j.neuroscience.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Szolcsanyi J. Neurogenic inflammation: reevaluation of axon reflex theory. In: Geppetti P, Holzer P, editors. Neurogenic inflammation. CRC Press; New York: 1996. pp. 33–42. [Google Scholar]
- Szolcsanyi J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides. 2004;38:377–384. doi: 10.1016/j.npep.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Tsuda M, Iwanaga T, Inoue K. Cell type-specific ATP-activated responses in rat dorsal root ganglion neurons. Br J Pharmacol. 1999;126:429–436. doi: 10.1038/sj.bjp.0702319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol (Lond) 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ren Y, Zou X, Fang L, Willis WD, Lin Q. Sympathetic influence on capsaicin-evoked enhancement of dorsal root reflexes in rats. J Neurophysiol. 2004;92:2017–2026. doi: 10.1152/jn.00145.2004. [DOI] [PubMed] [Google Scholar]
- Xie J, Lee HY, Wang C, Chung JM, Chung K. Differential expression of alpha1-adrenoceptor subtype mRNAs in the dorsal root ganglion after spinal nerve ligation. Mol Brain Res. 2001;93:164–172. doi: 10.1016/s0169-328x(01)00201-7. [DOI] [PubMed] [Google Scholar]
- Xing Y, Liu Z, Wang LH, Huang F, Wang HJ, Li ZZ. Butyrate sensitizes the release of substance P and calcitonin gene-related peptide evoked by capsaicin from primary cultured rat dorsal root ganglion neurons. Neuro Endocrinol Lett. 2006;27:695–701. [PubMed] [Google Scholar]
- Xu GY, Winston JH, Shenoy M, Yin H, Pendyala S, Pasricha PJ. Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology. 2007;133:1282–1292. doi: 10.1053/j.gastro.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Xu X, Wang P, Zou X, Li D, Fang L, Lin Q. Up-regulation of transient receptor potential vanilloid-1 in primary afferent neurons is mediated by protein kinase C and involved in neurogenic inflammation. Soc Neurosci. 2008 doi: 10.1002/jnr.21844. Program No. 265.11/EE12 (Abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Wang P, Zou X, Li D, Fang L, Lin Q. Increases in transient receptor potential vanilloid-1 mRNA and protein in primary afferent neurons stimulated by protein kinase C and their possible role in neurogenic inflammation. J Neurosci Res. 2009;87:482–494. doi: 10.1002/jnr.21844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Chung K, Chung JM. Development of purinergic sensitivity in sensory neurons after peripheral nerve injury in the rat. Brain Res. 2001b;915:161–169. doi: 10.1016/s0006-8993(01)02845-1. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Li G, Zhao Z. State-dependent phosphorylation of ε-isozyme of protein kinase C in adult rat dorsal root ganglia after inflammation and nerve injury. J Neurochem. 2003;85:571–580. doi: 10.1046/j.1471-4159.2003.01675.x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhou ZS, Zhao Z. PKC regulates capsaicin-induced currents of dorsal root ganglion neurons in rats. Neuropharmacol. 2001a;41:601–608. doi: 10.1016/s0028-3908(01)00106-x. [DOI] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Enhanced phosphorylation of NMDA NR1 subunit in spinal dorsal cord dorsal horn and spinothalamic tract neurons following intradermal injection of capsaicin in rats. J Neurosci. 2000;20:6989–6997. doi: 10.1523/JNEUROSCI.20-18-06989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. The effects of sympathectomy on capsaicin-evoked Fos expression of spinal dorsal horn GABAergic neurons. Brain Res. 2002a;958:322–329. doi: 10.1016/s0006-8993(02)03621-1. [DOI] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Role of protein kinase A in phosphorylation of NMDA receptor 1 subunits in dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. Neuroscience. 2002b;115:775–786. doi: 10.1016/s0306-4522(02)00490-6. [DOI] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Effect of protein kinase C blockade on phosphorylation of NR1 in dorsal horn and spinothalamic tract cells caused by intradermal capsaicin injection in rats. Brain Res. 2004;1020:95–105. doi: 10.1016/j.brainres.2004.06.017. [DOI] [PubMed] [Google Scholar]