Abstract
Regenerative healing is the process by which injured tissues are restored to their original structure and function. Many species are capable of healing in this manner. However, in mammals the healing response in most tissues is marked by fibroblast proliferation and scar tissue deposition. While scarring contributes to efficient resolution of mammalian wounds and restoration of at least partial structural and functional support, the final result of scar formation can be more deleterious than the initial insult. This is especially true in the heart, which is sensitive to electrical heterogeneities and altered mechanical properties produced by scarring. Several therapeutic modalities promoting regeneration in skin wounds have been developed that modulate various aspects of the healing process. Targets include cytokine stimulation, control of fibroblast activation, modulation of gap junctions, and stem cell differentiation. Here, we review and compare mechanisms of injury, repair, and scarring in the skin and heart and discuss the promise and caveats of future therapies that may translate to improving repair of myocardial tissues.
Keywords: regenerative healing, skin, heart, wound repair, myocardial infarction, TGF-β, connexin 43, stem cell, gap junction
Introduction
Tissue trauma initiates a cascade of events that requires the maintenance of a delicate balance between tissue repair and ongoing function. Early during cutaneous wound healing, the main priorities are hemostasis and prevention of infection, events that initiate reparative processes. The resulting clot serves to maintain the skin’s barrier function in addition to providing chemoattractants, cytokines, and a scaffold for infiltrating cells needed for repair. In the mammalian heart, however, the acute demands for maintenance of ongoing function are particularly critical. The competing priorities of the healing response can be likened to a mechanic who has to perform repairs on a fast-moving car without stopping. The cardiac healing response must proceed in a manner that does not result in compromised contractility, arrhythmia or rupture of heart muscle - a tall order for an organ that, in humans, must unfailingly maintain a mean arterial pressure of 90 mmHg.
Unfortunately, natural selection has not provided mammals the luxury of restorative repair of damaged organs and tissue de novo. In contrast to lower vertebrates such as the zebrafish, where regenerative healing is more the norm, opportunities for a degree of mammalian regeneration are only afforded in utero, where embryonic wounds in skin and developing appendages heal scarlessly [1]. In the adult mammal, the healing process is less restorative because of the ingress of inflammatory cells from the blood and differential expression of signaling molecules which orchestrate critical aspects of wound healing and produce a new permanent structure: the scar [2].
Healing and subsequent scarring does not occur to the same degree in all tissues. The discrepancy in the regenerative ability of human tissues is due to differences in the intrinsic properties of the cell types involved, the extracellular matrix (ECM) and cytokine signaling within the tissue, and the mechanical stress surrounding the injury. In general, less differentiated, mitotically active tissues heal with less scarring and fibrosis and preservation of structure compared to more highly differentiated tissues. This is certainly the case with the cells of the intestines and liver, where turnover is relatively rapid under normal conditions [3]. Damage to less mitotically active organs such as cardiac tissues results in profound fibrosis and scarring with minimal regeneration [4]. Mechanical stress is a critical factor for the healing response as well [5]. For example, skin wounds around joints, where frequent stretching occurs, develop larger scars and possess a slower healing response compared to wounds on less strained surfaces [5]. The high level of cyclic mechanical stress associated with the heartbeat may also be a contributing factor in the lower regenerative ability of the myocardium.
The process of healing in general has been extensively reviewed [2] and novel therapies are under development to improve the regenerative healing potential and reduce the scarring of skin wounds [6]. Anti-scarring therapies have focused primarily on skin regeneration, and it is unclear if they would reduce scarring in other tissues, such as the brain or heart, where the consequences of scar formation can be more deleterious than the original insult. The goal of this review is to compare skin wound healing and cardiac repair postinfarction, to characterize how disease disrupts the healing response in these tissues and discuss how lessons learned in cutaneous wound healing may be applied to reduce scar formation, as well as to promote regeneration of injured myocardial tissues.
Normal Skin Wound Healing and Cardiac Injury Repair
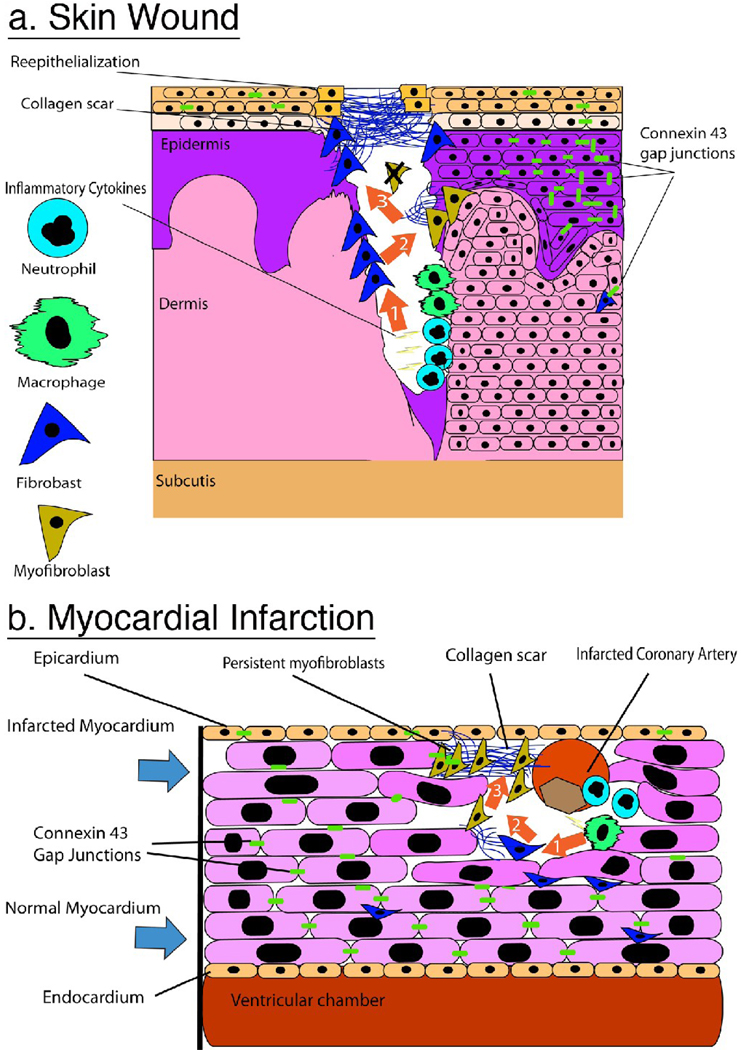
A similar progression of events characterizes repair following injury in most mammalian tissues. An excellent summary of this process in skin has been authored by Richard Clark [2]. Briefly, wound healing consists of three overlapping phases: inflammation, proliferation, and maturation. Inflammation begins with neutrophil accumulation in the wound area, followed by resident macrophages and circulating monocytes infiltrating the wound. Proliferation refers to propagation of a variety of cell types that have migrated into the wound including epithelial cells, fibroblasts, and endothelial cells. Epithelial cells contribute to re-epithelialization, and coordinated migration of macrophages, fibroblasts, and vasculature into the wound is dubbed granulation tissue and is responsible for the formation of the scar. Maturation is the accumulation and remodeling of collagen – largely by fibroblasts – in order to form a scar. A high rate of collagen synthesis generally lasts several months post-wounding, and matrix remodeling continues over a period of many months. The result of this process is that a breach of the dermal barrier is quickly patched, cleared of pathogens and cellular debris, and rebuilt as a functional collagenous matrix. Tissue specific examples of this process for the skin and the heart are provided in figure 1.
Figure 1.
A Comparison of scar formation in the skin and heart. Sequence of orange arrows in A and B convey sequential events in the healing process of each tissue. A) Skin wound, (Arrow 1) After hemostasis neutrophils and macrophages release proinflammatory cytokines for the destruction of dead tissue and invading organisms. (Arrow 2) Fibroblasts are recruited to the wound area and begin to lay down collagen to form a new dermal composite: granulation tissue. Myofibroblasts expressing α-SMA appear either from epithelial-mesenchymal transformation or circulating fibrocytes and continue to upregulate collagen expression. (Arrow 3) Collagen weave fully encloses the wound area and keratinocytes re-epithelialize from the periphery of the collagen rich scar. Myofibroblasts undergo apoptosis and are no longer detected. Notice Cx43 expression is down regulated in the epidermis proximal to the wound edge B) Heart Scarring post-MI (Arrow 1) Infarcted coronary artery results in death of local myocytes and recruitment of neutrophils and macrophages to the site of injury where they release pro-inflammatory cytokines. (Arrow 2) Fibroblasts and myofibroblasts are recruited from the surrounding myocardium and begin to lay down collagen. Note that Cx43 is down regulated in the infarct border zone and no longer localized at the end-to-end abutments of the myocytes. (Arrow 3) Myofibroblasts expressing Cx43 and forming intercellular junctions with adjacent myofibroblasts persist in the dense collagen scar.
There are multiple settings in which reduction or modulation of scarring in the myocardium is of interest to clinicians. Surgical repairs of the heart, such as during correction of a birth defect can result in scar tissue formation and lead to the generation of a pro-arrhythmic substrate. Conversely, ablative therapy is a frequently used tool in which targeted scar deposition is harnessed to eliminate myocardial tissues causing conduction disturbance or ectopic automaticity. An ability to control the extent and geometry of scarring in ablative therapies would reduce iatrogenic complications of these interventions.
The cardiac disease state in which a reduction in scar tissue would provide most benefit is following myocardial infarction (MI). MI is one the most frequent causes of death for both men and women throughout the world. Normally, it results from vascular occlusion of a coronary vessel and leads to ischemic death of myocardial tissue. Importantly, one of the most common causes of death post-MI is ventricular arrhythmia. Re-entrant arrhythmias following MI are caused by disorganized and electrically compromised myocardial tissues interspersed with fibrous tissues adjacent to the infarction scar (the infarct border zone - IBZ). The importance of maintaining stable electrophysiological properties of the IBZ cannot be overstated and must be considered when applying any treatment to the heart. This consideration is especially apropos for regenerative therapies, including stem cell treatments that, under some circumstances, might exacerbate electrical instability. Regenerative therapies for MI will probably also need to take into account the numerous comorbidities associated with the disease - examples of which would include heart failure, hypertension and diabetes. Care will likely have to be taken that interventions working in one MI patient don’t negatively impact the healing response in another presenting with a distinct clinical history.
Skin as a Model for Tissue Repair
Skin, while displaying unique responses to injury, in many aspects is a convenient general model for study of the wound response and regenerative healing. The type of injury imposed, the severity/depth of the wound, and the ability to sample multiple endpoints enables well-controlled study of the healing process in this tissue. The locale of an injury to the skin also provides the ability to study unique aspects of the response to the same injury, as different sites on the body display variance in underlying tissue architecture and are subject to distinct patterns of mechanical stress. Researchers also have a range of options in the type of animal used for skin healing studies, including transgenic mice which provide a model for large-scale investigations and manipulation of genes involved in injury response, and porcine skin which is said to provide a more human-like substrate [7]. By comparison to skin, wound healing research in the heart provides constraints of access, repeatability, surgical survival and limited ability to observe and sample during an ongoing experiment.
Initial Inflammatory Response
Similarities in the healing process may provide lessons to take from the skin to the myocardium. Beginning with inflammation, immediate infiltration of neutrophils is common to both types of injury response, and may be an important determinant in the rate of healing and size of injury. Indeed, studies in neutropenic animals have shown that neutrophil depletion results in an increased rate of re-epithelialization in skin [8] and decreased infarct size in the heart [9]. However, recent work with the leukocyte-specific, protein 1 null (Lsp1−/−) mouse challenges the mounting evidence that wound neutrophil reduction is beneficial. Lsp1 is a actin-binding cytoskeletal protein regulating cellular architecture and mobility of leukocytes [10]. Mice deficient in Lsp1 grow normally, but exhibit increased peritoneal macrophages compared to controls. Lsp1−/− mice show accelerated skin wound healing that is linked to increased infiltration of leukocytes (including neutrophils) and fibrocytes from the blood [10]. Conversely, recent work in the human heart has demonstrated that neutrophilia is a predictor of infarct severity and sudden cardiac death [11]. Further complicating the matter, are findings that markers of oxidative stress, but not inflammation are associated with persistent arrhythmias [12], a consideration that must be accounted for in the translation of therapies to the heart. Taken together these results suggest that either too few or too many neutrophils may be detrimental to the healing response, and caution should be taken when applying any technique that inhibits or promotes neutrophilia in humans.
Revascularization
Revascularization of the injured area is another common element in healing. As granulation tissue enters the wounded area, neovascularization provides nutrients and oxygen. In the case of the dermis, loss of normal patterns of vascularity may not compromise function seriously, although may have impacts on cosmetic scar appearance by changing skin tone and homogeneity [13]. However, in the heart, the potential for localized ischemia from vascular loss may be critical, possibly contributing to the generation of pro-arrhythmic substrates. Therefore, promoting neovascularization of the ischemic myocardium is thought by many to be an important therapeutic endpoint. A number of studies have examined the role of various growth factors in inducing vascular recruitment post-MI [14]. Growth factors that demonstrate potential in preclinical studies of therapeutic angiogenesis include vascular endothelial growth factor A (VEGF-A), fibroblast growth factor 2 (FGF-2), and platelet-derived growth factor (PDGF). Clinical trials of these factors however, have yielded disappointing results [15], possibly due to complications with factor delivery and maintenance of dosing [14]. Additionally, there is concern that promoting neovascularization, especially in a global setting, may lead to or encourage growth of neoplastic tissue or metastasis. It is also worth noting that the most prominent fibrous tissues in heart, the valves, are normally avascular with neovascularization occurring only in certain valve pathologies [16]. Thus, the benefits and costs of promoting neovascularization in the fibrous tissues of the cardiac scar may require further work and evaluation before it becomes part of any widely used clinical approach to treating cardiac injury.
TGF-β
TGF-β signaling has received much attention in the field of dermal wound healing. Work by Ferguson and coworkers has demonstrated that the relative abundance of TGF-β isoforms is critical to shifting the balance between scarring and scar-free healing of cutaneous wounds [17]. Specifically, in adult tissues there is a trend towards greater levels of TGF-β1, which is known to up-regulate collagen synthesis and reduce MMP expression in fibroblasts, while in embryonic tissues, which heal without scarring, the relative amount of TGF-β3 is increased. Based on this, the Ferguson group has shown that treatment of wounds in mice with either TGF-β1 and –β2 neutralizing antibodies or exogenous TGF-β3 reduces scarring [17]. The group has recently reported that TGF-β3 reduces scarring following incisional wounding of skin in humans [18]. Recent work using a TGF-β receptor 1 antagonist in a coronary artery ligation model indicates that the strategy of targeting this signaling pathway may also be of benefit in the heart [19].
It remains unclear whether reducing scar tissue formation is necessarily a desirable goal in repair of the injured heart. Clearly, the high pressures experienced in the ventricular walls necessitate maintenance of structural integrity. Therapies that simply reduce or disrupt scar tissue formation could increase the risk of rupture of the pumping chambers of the heart. Indeed, a case could be made that the development of therapeutic options for improving the long-term structural integrity, mechanical properties and electrical characteristics of cardiac scar tissue may be just as clinically important as therapies potentially leading to the regeneration of heart muscle.
The mode of action of TGF-β targeting in the heart and its anti-fibrotic effects appear to be via down regulation of the renin-angiotensin system, an intervention known to improve survival in patients post-MI [20] and improve ventricular function in diabetes [21]. Other commonly used cardiovascular drugs for hypertension and heart failure treatment act in a similar manner and have been shown to decrease fibroblast proliferation and prolong survival. Additionally, both neutralizing antibodies to TGF-β1 and blockers of the ACE cascade result in increased myocardial performance in a fibrillin-1 deficient mouse model of Marfan’s syndrome [22]. Other cytokine treatments targeted to cardiac fibroblasts have been tried with greater or lesser degrees of success in both the heart and skin, including ET-1 receptor antagonists, INF-therapy, Aldosterone antagonists, Matrix metalloproteinase (MMP) inhibitors, and anti-arrhythmogenic drugs [23, 24].
Signaling by Gap Junctions and Connexins
Gap junction-mediated signaling is a near universal feature of communal cellular function in mammalian tissues. During wound healing, connexins (the main protein subunit of gap junctions) play essential roles in leukocyte diapedesis, re-epithelialization, wound contraction, fibroblast function and collagen deposition and synthesis [25, 26, 27]. Connexin43 (Cx43) is the main connexin isoform expressed in ventricular myocardium; in this context, an interesting commonality between skin wounds and myocardial infarction is the downregulation/disruption of Cx43 expression at the leading edge of wounded epidermis and in IBZ myocytes, respectively [26, 28] (Figure 1). Pertinently, studies have shown that enhancement of Cx43 downregulation may improve the healing process in both skin and heart. For example, Cx43+/− mice display lesions of decreased size following coronary artery ligation [29], and application of a Cx43 antisense RNA to skin wounds has been reported to result in a smaller scar [26]. Some insight into mechanism comes from the finding that loss of Cx43 may result in dampened TGF-β signaling and also lead to decreased cardiac fibroblast to myofibroblast differentiation [30]. Additionally, the Gourdie lab has demonstrated that a peptide composed of the carboxyl–terminal ZO-1 binding domain of Cx43 reduces granulation tissue area and promotes regenerative healing in skin wounds [31]. Interestingly, it has been shown that Cx43 is a substrate for MMP-7, a metalloproteinase that is upregulated in the heart post-MI and contributes to ventricular remodeling [32]. In silico analysis indicates that MMP-7 cleavage of Cx43 may generate short peptides containing the Cx43 PDZ class II binding motif, which is bound by the PDZ-2 domain of zonula occludens-1 (ZO-1).
There are countervailing details that may have to be taken into account when considering downregulated Cx43 as a potential MI therapy. Cx43 coupling is a key mediator of the syncytial rhythmic contraction of the heart. Therefore, it is not unexpected that the aforementioned Cx43 heterozygous null mice appear to display an increased propensity for arrhythmia following MI [33]. Other work in mice with transgenic tissue-specific loss of Cx43 also support the concept that Cx43 downregulation tends to be pro-arrhythmic, particularly when the decrease is large [34]. Conversely, our group has demonstrated that application of the Cx43 carboxyl-terminal peptide reduces the incidence of paced ventricular fibrillation and increases left ventricular function in the mouse 1 week after a non-transmural cryo-injury [35]. These effects may be mediated by stabilization of gap junction plaques, as previous work in our lab has shown that disruption of Cx43/ZO-1 interaction in HeLa cells increases gap junction size [36]. Additionally, Cx43 is obligatory for cardiac preconditioning [37]. Preconditioning involves exposure to transient ischemia, which in turn provides protection from injury such as an MI. Hearts from Cx43−/+ mice display no preconditioning response. This phenomenon appears to be dependent on a non-junctional pool of Cx43 in sub-sarcolemmal mitochondria [37].
The full details of how different pools of cells expressing Cx43 contribute to physiologic disturbance remains to be established. In the heart, disruptions to conduction of electrical activation are attributed to aberrations in myocyte Cx43. However common cell types between the skin and heart such as leukocytes, fibroblasts, myofibroblasts and endothelial cells also express Cx43. Myocyte-fibroblast gap junction coupling in the heart is presently a topic of considerable interest [38]. The role of connexin-mediated interactions between different cell types during tissue repair is likely to be an area in which future research develops.
The Major Players in Scar Formation in the Heart and Skin
The primary factors that directly impinge on collagen deposition are fibroblast infiltration and the ability of these cells to produce, process, and deposit collagen. In the absence of stimuli, both cardiac and dermal fibroblasts are quiescent cells whose main function is maintenance of the ECM [38]. The normal turnover of ECM is accomplished by secretion of matrix molecules, such as collagen, and degradation of these proteins by MMPs and serine proteases. The list of MMPs in the heart that have been identified includes MMPs-1, 2, 3, 9, 13, and 14 which have overlapping specificities for a variety of collagen subtypes, basement membrane components, and fibronectin [39]. Following injury, during the proliferation phase, fibroblasts enter the wound, differentiate into myofibroblasts, begin to deposit collagen and contract the wound. Next, during maturation, synthesis and degradation of the collagen scar (accomplished by similar mechanisms to ECM homeostasis) begin to balance out, and myofibroblasts within the scar become apoptotic [2]. In the skin, the end result is a functional matrix that maintains the skin’s barrier function; however, in the heart, scarring post-MI is the source of several problems. Mechanically, the scar is thought to be a relatively passive structure and this may impair the normal contractile function of the myocardium. Perhaps more importantly, the boundary between the scar and myocardium, the IBZ, provides a substrate for arrhythmia, both as a passive barrier to conduction and as site of aberrant heterocellular fibroblast-myocyte interaction. Fibroblast-fibroblast and -myocyte intercellular communication may also exacerbate the spread of injury via the “bystander effect”, with consequences for infarct size [38].
An additional layer of complexity arises in the arrangement of the collagen fibrils in the scar. In the skin, the normal arrangement of collagen fibrils is a “basket-weave” pattern [2]. Similarly, in the heart, a weave of collagen fibers form the matrix that surrounds myocytes [38, 39]. In both tissues, scar collagen is generally aligned along axes of fibroblast migration into the wound, and has a disorganized appearance [40]. Modulation of collagen alignment patterns for improved and stable mechanical performance of cardiac scars may be another avenue for therapy.
Lastly, a newly identified cell type, the fibrocyte, provides a novel area of study for improving wound outcome. These bone marrow-derived cells have characteristics of inflammatory cells, but also express TGF-β1 and collagen. Furthermore, studies show that fibrocytes can enter the wound from the peripheral circulation, lose hematopoietic characteristics, and differentiate into fibroblasts and myofibroblasts with concomitant increases in collagen production [41]. The degree that these cells play a role in scarring remains to be determined, however bone marrow-derived fibroblasts and myofibroblasts have been detected in the scars of myocardial infarcts in mice [42]. Recent reports are conflicted regarding the role of these cells. Some indicate that, like neutrophils, reducing fibrocyte infiltration may improve the healing process [43], while another report from the Tredget group indicates otherwise [10]. Whether derived from the blood or already resident in the tissue, the fate of a myofibroblast in a healed cutaneous injury is distinct from that in the cardiac scar. In the skin wound, myofibroblasts undergo apoptosis and have been reported to be virtually absent in the mature scar [44]. In contrast, in the infarcted myocardium α-smooth muscle actin expressing myofibroblasts persist and continue to express fibrotic signals [4]. The persistence of these cells following healing may carry risks, as myofibroblasts have recently been identified as a potential source of arrhythmic activity in the heart [45].
Non-Mammalian Regeneration
While mammalian skin is a convenient setting to assess regeneration, promising evidence of the regenerative potential of the myocardium comes from lower vertebrates. The zebrafish heart has gained fame for its ability to grow back new cardiac muscle after apical resection of up to 20% of ventricular myocardium [46]. This regenerative ability has been proposed to arise from progenitor cells exhibiting epicardial markers that rapidly proliferate and differentiate into a functional myocardium in a process that is mediated by FGF signaling [47]. While this mechanism lends support to the potential of endogenous stem cell populations for regenerative therapy, it appears to be unique to the zebrafish heart. In contrast to the epicardial progenitor cell population of the heart, regeneration of the zebrafish fin occurs independent of endogenous progenitor cell populations. It has been suggested that fin regeneration in this species occurs via de-differentiation of endogenous somatic cells, a process more generally characteristic of other vertebrate regenerative models. For example, myocytes in the newt heart have been reported to dedifferentiate, disassemble sarcomeric structures and reenter the cell cycle after cardiac injury [48]. A similar process occurs in the regeneration of limbs and tails in amphibians [49]. Many investigators have focused on mechanisms to mimic the stem cell-dependent regrowth of the injured zebrafish heart. As momentum builds for approaches based on progenitor cells, it should be noted that there is a long history of research on regeneration indicating that many regeneratively permissive species rely on de-differentiation of somatic cells and formation of a blastema in their healing response [49].
The human heart poses special problems for renewal following injury, not the least of which is that it must maintain mean arterial pressure above 90 mmHg and that it is likely more susceptible to reentrant arrhythmias than the small hearts of fish and amphibians. The massive scarring response observed following injury to the mammal heart can perhaps be viewed as a conservative default that gave up cardiac regenerative potential for safety in maintaining the high arterial pressure required for terrestrial life. It is conceivable that the high-pressure requirements alone could functionally prohibit de-differentiation events of myocytes. This hurdle placed by evolution is the engineering problem that must be solved first if cardiac renewal is going to achieve practical application in man.
From Mammalian Skin to Heart
Many transgenic mouse models have been generated with interesting healing phenotypes. One of particular interest is the MRL mouse, a mouse with an insertion in the fas gene leading to a defect in the apoptotic pathway and unregulated lymphoproliferation with age. This mouse has the ability to scarlessly heal ear punch wounds within thirty days [50]. These results prompted a number of studies to examine the healing response of this mutant in skin wounding [51], retinal degeneration [52], and cardiac injuries, including cryo-injury and coronary artery ligation [50, 53]. There are conflicting results from this work, though most confirm that the MRL mouse exhibits scarless healing following punch wounding of the ear. However, skin wounds in MRL mice have been reported to show a similar scarring phenotype to those of wt controls [51]. One study of cardiac injury indicated decreased scar tissue formation and ventricular remodeling in MRL mice. However, other groups have demonstrated that the heart of the MRL mouse does indeed scar significantly after injury. The disparate results in skin, heart and ear regeneration in this model suggest that while certain interventions (in this case the MRL mutation) can lead to apparent improvement in the healing of one tissue, caution is required in anticipating regenerative potential in other tissues.
Pathologic Wound Healing and Cardiac Regeneration in Diabetes
Diabetes is well known for its debilitating chronic wounds that are refractory to treatment. More recently, it has been recognized that diabetes exhibits direct effects on the wound healing process itself [54]. Indeed, the diabetic state induces a vast array of physiologic changes, including decreases in growth factor production, fibrosis and cell proliferation [55, 56]. Several studies have demonstrated an increase in proinflammatory cytokines [57, 58] in diabetes resulting in a low grade chronic inflammatory state that hinders the acute wound healing process [59]. Ischemia in the diabetic state is thought to be due to decreased homing of endothelial progenitor cells (EPCs) due to down regulation of the cytokine SDF-1α, resulting in decreased neovascularization at sites of injury [60]. Like the skin, the diabetic heart exhibits a fibrotic phenotype and responds poorly to injury. Additionally, diabetic patients show increases in MMP expression and a decrease in TIMPs [61]. This shift in balance of ECM proteinases is known to contribute to the faulty healing phenotype of the chronic diabetic wound. Other mechanisims are known to contribute to the poor wound healing observed in diabetes. For example, Wang et al. demonstrated that Cx43 is up-regulated in the diabetic skin wound and application of an antisense Cx43 to diabetic wounds doubled the rate of re-epithelialization [62], implicating a role for intercellular communication in the diabetic injruy. The similarities in the response to glycemic injury of the skin and heart not only hold promise for the translation of dermal therapies to the regeneration of the myocardium, but also for the prediction of cardiac injury severity. Additionally, if future studies show a correlation between the severity of skin and heart injury in a diabetic model, then it may be possible to use dermal healing as a prognostic marker for cardiac disease in the diabetic population.
In the heart of diabetics there is evidence for a decreased healing response. It is known that streptozotocin (STZ)-induced diabetic rodents exhibit increased infarct size after coronary artery ligation [63] and that diabetic patients are at an increased risk of death after a MI. It is unclear if the increased infarct size is related to mechanisms involved in prolonged skin wound healing in diabetes, or alternative mechanisms [64]. However, it is apparent that the targets of certain pharmacologic therapies for skin wound healing are upregulated in the diabetic heart. For example, TGF-β1 is increased in the diabetic heart and as previously discussed, this increase may contribute to increased infarct size post-MI. Additionally, the effects noted by Wang et al. on reducing Cx43 in the diabetic skin wound may implicate a mechanism for the increased arrhythmic potential of diabetic patients involving increased cellular coupling. Lastly, a classic therapeutic intervention that has clinical promise for the healing of diabetic wounds is hyperbaric oxygen therapy [65]. Because of the skin’s natural exposure to the atmosphere hyperbaric oxygen is easily administered to skin wounds. A recent study has demonstrated that hyperbaric oxygen improves the survival of stem cells transplanted in the myocardium of rats post infarction [66]. Whether hyperbaric oxygen would benefit cardiac injury in humans and, if so, how it would be administered to patients efficiently, remain to be determined.
Bioengineering in the Healing Wound
Grafts
In treating skin wounds, the use of grafts and transplants of either synthetic or natural origin is sometimes applied to improve the organization and reduce scarring of the healed wound. In the heart there is little concern for cosmetics, but organization of the myocytes is vital to maintain both electrical and hemodynamic function. Because of this dependence on organization, many have tried to apply grafts to cardiovascular injury, some with encouraging results.
Few studies have applied grafts to the treatment of myocardial infarction in adult patients. Promising work by the Wagner group indicates that this may be a therapeutic option in the future. Wagner et al. implanted a biodegradable polyurethane patch directly into a rat infarcted left ventricular free wall. This patch was almost entirely absorbed by the surrounding tissue 8 weeks after implantation [67]. The infarcted myocardium receiving the patch exhibited increased compliance compared to controls and numerous smooth muscle bundles exhibiting a contractile phenotype, suggesting that the patch allowed for cell seeding, proliferation and differentiation. While it is unclear what role these cells have (if any) in maintenance of left ventricular function, it is clear that synthetic constructs such as these may have a role in future therapies for the treatment of MI.
Synthetic grafts allow for immediate structural stabilization and may provide needed support to the heart post-infarction, thereby preventing ventricular decompensation. Wagner et al. did not report an increase in myocyte regeneration. Thus, at this stage, the usefulness of their approach appears to be limited to prevention of cardiac decompensation, rather than regeneration. Investigations targeted at generating a natural platform for cardiac cell seeding are underway and these studies may prove to be complementary to synthetic grafts.
The work of the Taylor group [68] has laid a foundation for the use of natural scaffolds in artificial heart engineering. In one study, Ott et al. generated an acellular rat heart scaffold and subsequently seeded this scaffold with neonatal rat myocytes via direct injection into the left ventricular free wall. By 8 days, the heart construct exhibited electric and contractile responses to pacing. This work suggests not only the feasibility of generating transplantable cardiac tissue from cadaveric donors, but also the importance of the three-dimensional ECM structures in cardiac electrical and contractile function. Previous attempts to generate cell culture grafts of myocytes were limited in size because of the oxygen demands inherent to these cells [69]. By utilizing a scaffold with natural channels for delivery of oxygen and nutrients and maintaining perfusion pressures, cardiac tissue was assembled that was capable of performing stroke work [68].
In skin wound healing, recent studies have shown that natural decellularized constructs may be applied as grafts and these constructs result in more normal skin with less scarring when compared to synthetic grafts containing a similar protein complement [70]. This work indicates an underappreciated role of the physiologic extracellular matrix structure in cutaneous wound healing and scar formation. It can be assumed that the complex activities of the mammalian heart are even more dependent on the three-dimensional geometries of the surrounding cardiac ECM. Using a natural matrix for cardiac regenerative therapies may allow for improved myocyte organization and increases conductive anisotropy via Cx43 gap junction modification, a property recently found to enhance contractile function in bioengineered myocardial equivalents [71]. While the work of Taylor and others demonstrates the feasibility of generating a bio-artificial heart for either total or partial cardiac replacement, a major unresolved problem is the source of cells required to seed a human heart scaffold.
Stem Cell Based Therapies
In a study supporting earlier findings by Anversa, Leri and colleagues [72], it was recently reported that the human heart generates new muscle cells [73], with ~ 50% of myocytes undergoing replacement over an average lifetime. While this slow constitutive process may be insufficient to recover ventricular function after an ischemic insult, it may be possible to target therapies to stimulate this process or induce myocyte proliferation in vivo. The potential of mesenchymal stem cells (MSCs), embryonic stem cells (ESCs) and induced progenitor (Ip) cells and other cell-based therapies to augment regeneration is an active area of investigation [74, 75]. MSC-like cells have been detected in numerous tissues including adipose, muscle, tendon, liver, spleen, thymus, and the heart [76]. Studies using labeled bone marrow MSCs have demonstrated that these cells migrate to the myocardium, but whether they actually differentiate into myocytes in any significant number is controversial. Studies in skin wounds have shown that autologous MSC application improves healing [77]. As in the skin, it is clear in the heart that MSCs have positive impacts on post-MI cardiac repair, are involved in angiogenesis, and treatment with MSCs can reduce infarct size. One major advantage to MSCs is immunological tolerance, as in many experimental therapies these cells are harvested from the patients’ own bone marrow, and thus there is little chance of rejection. The fact that infrastructure and clinical expertise is already in place based on the longterm use of transplanted bone marrow as a therapy in blood disease and hematopoietic neoplasia is a further plus for future MSC-based therapies.
A disadvantage to the use of progenitor cell based therapies is the ability to obtain a sufficient number of cells for treatment. This may be eventually overcome by advanced tissue-matching or Ip cell-based technologies. However, caution is required in the use of stem cell therapies owing to the possibility of seeding neoplastic tissue or promoting neovascularization, an outcome that as previously discussed could be a double-edged sword. Additionally, there are concerns about increasing cell and tissue heterogeneity, particularly at the IBZ following myocardial injury that could increase the risk of arrhythmia. Despite these potential pitfalls there is promise and much active research on the use of progenitor cells to improve cardiac injury, either by direct cellular engraftment, or via modulation of endogenous stem cell populations. Great care is nonetheless required that public expectations are not raised too high when touting future prospects for stem cell-based therapies.
Summary
We have come a long way in understanding the regeneration of skin wounds. The focus in the skin has been primarily on the reduction and prevention of scarring. Because chronic scarring in the heart leads to dysfunction, therapies that have been shown to reduce scarring of wounded skin such as TGF-β antagonism and gap junction modulation may also prove useful in treating the infarcted heart in the future. Additionally, the use of stem cell therapy promoting a restorative healing similar to that observed in the heart of lower vertebrates may hold merit. In the long-term, it seems that further development of bioengineering techniques and myocardial equivalents may enable the production of bioartifical transplantable hearts or cardiac grafts. The three critical factors in either regenerating the myocardium, or repairs based on de novo cardiac constructs, is the maintenance of stable electrical properties, continuation of efficient pumping of blood at high pressure and the avoidance of cardiac rupture. The principle question that must be tackled to solve the problem of myocardial regeneration is how do we restore myocardial tissues when constrained by these three factors? Nature’s answer in the mammalian heart was simple: Do not regenerate – scar quickly and extensively. A summary of some of the major hurdles that must be overcome to achieve cardiac regeneration is presented in Table 1. By utilizing therapies developed for skin regeneration perhaps we can find an approach to promoting myocardial regeneration. Alternately, lessons learned in cutaneous healing may also teach us how to build a better cardiac scar, consistent with an extended and reasonable quality of life for patients with heart disease.
Table 1.
Future studies that will improve the field of cardiac regeneration
| Need | Current State | Future needs |
|---|---|---|
| Determination of myocardial regenerative potential in humans | Minimal regeneration of myocytes in humans contrasted to high regenerative/ proliferative potential in lower vertebrates | Establishment of methods to induce myocyte regeneration and/ or proliferation in humans |
| Assess safety of Regenerative interventions | Some exogenous cells shown to increase risk of arrhythmias and therapies targeting inflammation may increase risk of rupture | Establish methods of assessing the risk of specific regenerative therapies in patients with varying forms of heart disease |
| Generation of therapeutically effective numbers of cardiac stem cells | Current techniques for generating sufficient numbers of cardiogenic stem cells are limited | Efficient harvesting of massive quantities of cardiac stem cells for tissue repair or organ repopulation [78] |
| Replacement of cardiac sub- components e.g., pacemaker | Devices such as ventricular assist devices and electronic pacemakers work reasonably well, but can fail | Tissue engineered equivalents to replace cardiac sub-components (e.g., bio-pacemaker) or specialized cell types |
| Reduction of scarring and rejection of foreign materials and cells | Massive inflammatory and scarring response and rejection of foreign materials and cells | Increased ability for scarless and regenerative integration of foreign materials, tissue engineered devices and cells |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Longaker MT, Whitby DJ, Ferguson MW, Lorenz HP, Harrison MR, Adzick NS. Adult skin wounds in the fetal environment heal with scar formation. Ann Surg. 1994 Jan;219(l):65–72. doi: 10.1097/00000658-199401000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark R. Wound Repair Overview and General Considerations. In: Clark R, editor. The Molecular and Cellular Biology of Wound Repair. New York: Plenum Press; 1996. pp. 3–50. [Google Scholar]
- 3.Bertalanffy FD. Comparison of mitotic rates informal renewing and neoplastic cell populations. Proc Can Cancer Conf. 1967;7:65–83. [PubMed] [Google Scholar]
- 4.Sun Y, Weber KT. Infarct scar: a dynamic tissue. Cardiovasc Res. 2000 May;46(2):250–256. doi: 10.1016/s0008-6363(00)00032-8. [DOI] [PubMed] [Google Scholar]
- 5.Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, Loh SA, et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. Faseb J. 2007 Oct;21(12):3250–3261. doi: 10.1096/fj.07-8218com. [DOI] [PubMed] [Google Scholar]
- 6.Rhett JM, Ghatnekar GS, Palatinus JA, O'Quinn M, Yost MJ, Gourdie RG. Novel therapies for scar reduction and regenerative healing of skin wounds. Trends in biotechnology. 2008 Apr;26(4):173–180. doi: 10.1016/j.tibtech.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Prasertsung I, Kanokpanont S, Bunaprasert T, Thanakit V, Damrongsakkul S. Development of acellular dermis from porcine skin using periodic pressurized technique. J Biomed Mater Res B Appl Biomater. 2008 Apr;85(l):210–219. doi: 10.1002/jbm.b.30938. [DOI] [PubMed] [Google Scholar]
- 8.Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. 2003 Apr;73(4):448–455. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 9.Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983 May;67(5):1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Jiao H, Stewart T, Lyons MV, Shankosky HA, Scott PG, et al. Accelerated wound healing in leukocyte-specific, protein 1-deficient mouse is associated with increased infiltration of leukocytes and fibrocytes. J Leukoc Biol. 2007 Sep;4 doi: 10.1189/0507306. [DOI] [PubMed] [Google Scholar]
- 11.Bodi V, Sanchis J, Nunez J, Mainar L, Minana G, Benet I, et al. Uncontrolled immune response in acute myocardial infarction: unraveling the thread. Am Heart J. 2008 Dec;156(6):1065–1073. doi: 10.1016/j.ahj.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Neuman RB, Bloom HL, Shukrullah I, Darrow LA, Kleinbaum D, Jones DP, et al. Oxidative stress markers are associated with persistent atrial fibrillation. Clin Chem. 2007 Sep;53(9):1652–1657. doi: 10.1373/clinchem.2006.083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amadeu T, Braune A, Mandarim-de-Lacerda C, Porto LC, Desmouliere A, Costa A. Vascularization pattern in hypertrophic scars and keloids: a stereological analysis. Pathol Res Pract. 2003;199(7):469–473. doi: 10.1078/0344-0338-00447. [DOI] [PubMed] [Google Scholar]
- 14.Molin D, Post MJ. Therapeutic angiogenesis in the heart: protect and serve. Curr Opin Pharmacol. 2007 Apr;7(2):158–163. doi: 10.1016/j.coph.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Annex BH, Simons M. Growth factor-induced therapeutic angiogenesis in the heart: protein therapy. Cardiovasc Res. 2005 Feb 15;65(3):649–655. doi: 10.1016/j.cardiores.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Yoshioka J, Lee RT. Vascularization as a potential enemy in valvular heart disease. Circulation. 2008 Oct 21;118(17):1694–1696. doi: 10.1161/CIRCULATIONAHA.108.809475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995 Mar;108(Pt 3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson MW, Duncan J, Bond J, Bush J, Durani P, So K, et al. Prophylactic administration of avotermin for improvement of skin scarring: three double-blind, placebo-controlled, phase I/II studies. Lancet. 2009 Apr ll;373(9671):1264–1274. doi: 10.1016/S0140-6736(09)60322-6. [DOI] [PubMed] [Google Scholar]
- 19.Ellmers LJ, Scott NJ, Medicherla S, Pilbrow AP, Bridgman PG, Yandle TG, et al. Transforming growth factor-beta blockade down-regulates the renin-angiotensin system and modifies cardiac remodeling after myocardial infarction. Endocrinology. 2008 Nov;149(ll):5828–5834. doi: 10.1210/en.2008-0165. [DOI] [PubMed] [Google Scholar]
- 20.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Jr, Cuddy TE. Effect of captopril on mortality morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992 Sep 3;327(10):669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 21.Zhang CH, Lu J, Yu XJ, Sun L, Zang WJ. Ameliorative effect of Captopril and Valsartan on an animal model of diabetic cardiomyopathy. Biol Pharm Bull. 2008 Nov;31(ll):2045–2049. doi: 10.1248/bpb.31.2045. [DOI] [PubMed] [Google Scholar]
- 22.Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, et al. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007 Feb;13(2):204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007 Oct;87(4):1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 24.Wolk R, Cobbe SM, Hicks MN, Kane KA. Functional, structural, and dynamic basis of electrical heterogeneity in healthy and diseased cardiac muscle: implications for arrhythmogenesis and anti-arrhythmic drug therapy. Pharmacol Ther. 1999 Nov;84(2):207–231. doi: 10.1016/s0163-7258(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 25.Ehrlich HP, Diez T. Role for gap junctional intercellular communications in wound repair. Wound Repair Regen. 2003 Nov-Dec;l1(6):481–489. doi: 10.1046/j.1524-475x.2003.11616.x. [DOI] [PubMed] [Google Scholar]
- 26.Qiu C, Coutinho P, Frank S, Franke S, Law LY, Martin P, et al. Targeting connexin43 expression accelerates the rate of wound repair. Curr Biol. 2003 Sep 30;13(19):1697–1703. doi: 10.1016/j.cub.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Chanson M, Derouette JP, Roth I, Foglia B, Scerri I, Dudez T, et al. Gap junctional communication in tissue inflammation and repair. Biochim Biophys Acta. 2005 Jun 10;1711(2):197–207. doi: 10.1016/j.bbamem.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Kostin S, Rieger M, Dammer S, Hein S, Richter M, Klovekorn WP, et al. Gap junction remodeling and altered connexin43 expression in the failing human heart. Mol Cell Biochem. 2003 Jan;242(1–2):135–144. [PubMed] [Google Scholar]
- 29.Kanno S, Kovacs A, Yamada KA, Saffitz JE. Connexin43 as a determinant of myocardial infarct size following coronary occlusion in mice. J Am Coll Cardiol. 2003 Feb 19;41(4):681–686. doi: 10.1016/s0735-1097(02)02893-0. [DOI] [PubMed] [Google Scholar]
- 30.Asazuma-Nakamura Y, Dai P, Harada Y, Jiang Y, Hamaoka K, Takamatsu T. Cx43 contributes to TGF-beta signaling to regulate differentiation of cardiac fibroblasts into myofibroblasts. Exp Cell Res. 2009 Apr 15;315(7):1190–1199. doi: 10.1016/j.yexcr.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Ghatnekar GS, O'Quinn MP, Jourdan LJ, Gurjarpadhye AA, Draughn RL, Gourdie RG. Connexin43 carboxyl-terminal peptides reduce scar progenitor and promote regenerative healing following skin wounding. Regen Med. 2009 Mar;4(2):205–223. doi: 10.2217/17460751.4.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindsey ML, Escobar GP, Mukherjee R, Goshorn DK, Sheats NJ, Bruce JA, et al. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation. 2006 Jun 27;113(25):2919–2928. doi: 10.1161/CIRCULATIONAHA.106.612960. [DOI] [PubMed] [Google Scholar]
- 33.Lerner DL, Yamada KA, Schuessler RB, Saffitz JE. Accelerated onset and increased incidence of ventricular arrhythmias induced by ischemia in Cx43-deficient mice. Circulation. 2000;101(5):547–552. doi: 10.1161/01.cir.101.5.547. 2000/02/08/ [DOI] [PubMed] [Google Scholar]
- 34.Gutstein DE, Morley GE, Vaidya D, Liu F, Chen FL, Stuhlmann H, et al. Heterogeneous Expression of Gap Junction Channels in the Heart Leads to Conduction Defects and Ventricular Dysfunction. Circulation. 2001;104:1194–1199. doi: 10.1161/hc3601.093990. [DOI] [PubMed] [Google Scholar]
- 35.O’Quinn M, Harris B, Hewett K, Gourdie R. A Peptide Containing the Carboxy-Terminal Domain of Connexin43 Reduces Arrhythmias and Improves Cardiac Function after Myocardial Injury. Circulation. 2009:118. [Google Scholar]
- 36.Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005 Dec;16(12):5686–5698. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boengler K, Stahlhofen S, van de Sand A, Gres P, Ruiz-Meana M, Garcia-Dorado D, et al. Presence of connexin 43 in subsarcolemmal, but not in interfibrillar cardiomyocyte mitochondria. Basic Res Cardiol. 2009 Mar;104(2):141–147. doi: 10.1007/s00395-009-0007-5. [DOI] [PubMed] [Google Scholar]
- 38.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005 Jan 1;65(1):40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 39.Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res. 2002 Mar 22;90(5):520–530. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- 40.van Zuijlen PP, Ruurda JJ, van Veen HA, van Marie J, van Trier AJ, Groenevelt F, et al. Collagen morphology in human skin and scar tissue: no adaptations in response to mechanical loading at joints. Burns. 2003 Aug;29(5):423–431. doi: 10.1016/s0305-4179(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 41.Stramer BM, Mori R, Martin P. The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J Invest Dermatol. 2007 May;127(5):1009–1017. doi: 10.1038/sj.jid.5700811. [DOI] [PubMed] [Google Scholar]
- 42.Mollmann H, Nef HM, Kostin S, von Kalle C, Pilz I, Weber M, et al. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res. 2006 Sep 1;71(4):661–671. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Chen H, Shankowsky HA, Scott PG, Tredget EE. Improved scar in postburn patients following interferon-alpha2b treatment is associated with decreased angiogenesis mediated by vascular endothelial cell growth factor. J Interferon Cytokine Res. 2008 Jul;28(7):423–434. doi: 10.1089/jir.2007.0104. [DOI] [PubMed] [Google Scholar]
- 44.Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005 Jan-Feb;13(l):7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 45.Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res. 2007 Oct 12;101(8):755–778. doi: 10.1161/CIRCRESAHA.107.160549. [DOI] [PubMed] [Google Scholar]
- 46.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002 Dec 13;298(5601):2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 47.Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, et al. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006 Nov 3;127(3):607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 48.Ausoni S, Sartore S. From fish to amphibians to mammals: in search of novel strategies to optimize cardiac regeneration. J Cell Biol. 2009 Feb 9;184(3):357–364. doi: 10.1083/jcb.200810094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoick-Cooper CL, Moon RT, Weidinger G. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 2007 Jun 1;21(11):1292–1315. doi: 10.1101/gad.1540507. [DOI] [PubMed] [Google Scholar]
- 50.Heber-Katz E, Leferovich J, Bedelbaeva K, Gourevitch D, Clark L. The scarless heart and the MRL mouse. Philos Trans R Soc Lond B Biol Sci. 2004 May 29;359(1445):785–793. doi: 10.1098/rstb.2004.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colwell AS, Krummel TM, Kong W, Longaker MT, Lorenz HP. Skin wounds in the MRL/MPJ mouse heal with scar. Wound Repair Regen. 2006 Jan-Feb;14(l):81–90. doi: 10.1111/j.1524-475X.2005.00092.x. [DOI] [PubMed] [Google Scholar]
- 52.Tucker B, Klassen H, Yang L, Chen DF, Young MJ. Elevated MMP Expression in the MRL Mouse Retina Creates a Permissive Environment for Retinal Regeneration. Invest Ophthalmol Vis Sci. 2008 Apr;49(4):1686–1695. doi: 10.1167/iovs.07-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grisel P, Meinhardt A, Lehr HA, Kappenberger L, Barrandon Y, Vassalli G. The MRL mouse repairs both cryogenic and ischemic myocardial infarcts with scar. Cardiovasc Pathol. 2008 Jan-Feb;17(l):14–22. doi: 10.1016/j.carpath.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005 Nov 12;366(9498):1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 55.Galkowska H, Wojewodzka U, Olszewski WL. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regen. 2006 Sep-Oct;14(5):558–565. doi: 10.1111/j.1743-6109.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 56.Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol. 2006 Feb 21;47(4):693–700. doi: 10.1016/j.jacc.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 57.Acosta JB, del BarcoDG, Vera DC, Savigne W, Lopez-Saura P, Guillen NietoG, et al. The pro-inflammatory environment in recalcitrant diabetic foot wounds. Int Wound J. 2008 Oct;5(4):530–539. doi: 10.1111/j.1742-481X.2008.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hatanaka E, Monteagudo PT, Marrocos MS, Campa A. Neutrophils and monocytes as potentially important sources of proinflammatory cytokines in diabetes. Clin Exp Immunol. 2006 Dec;146(3):443–447. doi: 10.1111/j.1365-2249.2006.03229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pradhan L, Nabzdyk C, Andersen ND, LoGerfo FW, Veves A. Inflammation and neuropeptides: the connection in diabetic wound healing. Expert Rev Mol Med. 2009;ll:e2. doi: 10.1017/S1462399409000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu ZJ, Velazquez OC.Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing Antioxid Redox Signal 2008Nov; 10111869–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia. 2002 Jul;45(7):1011–1016. doi: 10.1007/s00125-002-0868-8. [DOI] [PubMed] [Google Scholar]
- 62.Wang CM, Lincoln J, Cook JE, Becker DL. Abnormal connexin expression underlies delayed wound healing in diabetic skin. Diabetes. 2007 Nov;56(l 1):2809–2817. doi: 10.2337/db07-0613. [DOI] [PubMed] [Google Scholar]
- 63.Marfella R, D'Amico M, Di Filippo C, Piegari E, Nappo F, Esposito K, et al. Myocardial infarction in diabetic rats: role of hyperglycaemia on infarct size and early expression of hypoxia-inducible factor 1. Diabetologia. 2002 Aug;45(8):1172–1181. doi: 10.1007/s00125-002-0882-x. [DOI] [PubMed] [Google Scholar]
- 64.McDonagh PF, Hokama JY.Microvascular perfusion and transport in the diabetic heart Microcirculation 2000Jun;73163–181. [PubMed] [Google Scholar]
- 65.Kranke P, Bennett M, Roeckl-Wiedmann I, Debus S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2004;(2) doi: 10.1002/14651858.CD004123.pub2. CD004123. [DOI] [PubMed] [Google Scholar]
- 66.Khan M, Meduru S, Mohan IK, Kuppusamy ML, Wisel S, Kulkarni A, et al. Hyperbaric oxygenation enhances transplanted cell graft and functional recovery in the infarct heart. J Mol Cell Cardiol. 2009 Apr 17; doi: 10.1016/j.yjmcc.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fujimoto KL, Tobita K, Merryman WD, Guan J, Momoi N, Stolz DB, et al. An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J Am Coll Cardiol. 2007 Jun 12;49(23):2292–2300. doi: 10.1016/j.jacc.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008 Jan 13; doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 69.Eschenhagen T, Zimmermann WH. Engineering myocardial tissue. Circ Res. 2005 Dec 9;97(12):1220–1231. doi: 10.1161/01.RES.0000196562.73231.7d. [DOI] [PubMed] [Google Scholar]
- 70.Seo YK, Song KY, Kim YJ, Park JK. Wound healing effect of acellular artificial dermis containing extracellular matrix secreted by human skin fibroblasts. Artif Organs. 2007 Jul;31(7):509–520. doi: 10.1111/j.1525-1594.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 71.Black LD, Meyers JD, Weinbaum JS, Shvelidze YA, Tranquillo RT. Cell-induced alignment augments twitch force in fibrin gel-based engineered myocardium via gap junction modification. Tissue Eng Part A. 2009 Apr;:1. doi: 10.1089/ten.tea.2008.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, et al. Chimerism of the transplanted heart. N Engl J Med. 2002 Jan 3;346(1):5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 73.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009 Apr 3;324(5923):98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kajstura J, Urbanek K, Rota M, Bearzi C, Hosoda T, Bolli R, et al. Cardiac stem cells and myocardial disease. J Mol Cell Cardiol. 2008 Oct;45(4):505–513. doi: 10.1016/j.yjmcc.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 75.Rubart M, Field LJ. Stem cell differentiation: cardiac repair. Cells Tissues Organs. 2008;188(1–2):202–211. doi: 10.1159/000112846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martin-Puig S, Wang Z, Chien KR. Lives of a heart cell: tracing the origins of cardiac progenitors. Cell Stem Cell. 2008 Apr 10;2(4):320–331. doi: 10.1016/j.stem.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 77.Liu P, Deng Z, Han S, Liu T, Wen N, Lu W, et al. Tissue-engineered skin containing mesenchymal stem cells improves burn wounds. Artif Organs. 2008 Dec;32(12):925–931. doi: 10.1111/j.1525-1594.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- 78.Akasha AA, Sotiriadou I, Doss MX, Halbach M, Winkler J, Baunach JJ, et al. Entrapment of embryonic stem cells-derived cardiomyocytes in macroporous biodegradable microspheres: preparation and characterization. Cell Physiol Biochem. 2008;22(5–6):665–672. doi: 10.1159/000185550. [DOI] [PubMed] [Google Scholar]