Abstract
The midbrain central gray (periaqueductal gray; PAG) mediates defensive behaviors and is implicated in the rewarding effects of opiate drugs. Projections from the PAG to the ventral tegmental area (VTA) suggest that this region might also regulate behaviors involving motivation and cognition. However, studies have not yet examined the morphological features of PAG axons in the VTA or whether they synapse onto dopamine (DA) or GABA neurons. In this study, we injected anterograde tracers into the rat PAG and used immunoperoxidase to visualize the projections to the VTA. Immunogold-silver labeling for tyrosine hydroxylase (TH) or GABA was then used to identify the phenotype of innervated cells. Electron microscopic examination of the VTA revealed axons labeled anterogradely from the PAG, including myelinated and unmyelinated fibers and axon varicosities, some of which formed identifiable synapses. Approximately 55% of these synaptic contacts were of the symmetric (presumably inhibitory) type; the rest were asymmetric (presumably excitatory). These findings are consistent with the presence of both GABA and glutamate projection neurons in the PAG. Some PAG axons contained dense-cored vesicles indicating the presence of neuropeptides in addition to classical neurotransmitters. PAG projections synapsed onto both DA and GABA cells with no obvious selectivity, providing the first anatomical evidence for these direct connections. The results suggest a diverse nature of PAG physiological actions on midbrain neurons. Moreover, as both the VTA and PAG are implicated in the reinforcing actions of opiates, our findings provide a potential substrate for some of the rewarding effects of these drugs.
Keywords: opiates, periaqueductal gray, reward, tract-tracing, ultrastructure
INTRODUCTION
Fluctuations in dopamine (DA) release within forebrain regions targeted by projections from the ventral tegmental area (VTA) have important significance for determining behavioral responses to salient environmental stimuli and for attention shifting (Overton and Clark 1997; Redgrave et al. 1999; Schultz 1997). Changes in DA cell activity and DA efflux in target areas also support performance of executive functions (Brozoski et al. 1979; Floresco and Magyar 2006; Stefani and Moghaddam 2006; Watanabe et al. 1997). Consequently, studies of afferent regulation of the VTA are essential for understanding DA modulation of cognitive and motivated behaviors.
The midbrain central gray, also called the periaqueductal gray (PAG), provides the third heaviest subcortical source of glutamate input to the VTA (Geisler et al. 2007). Based on its functions, the PAG is likely to supply VTA neurons with information important for processing nociceptive signals, defensive and stress behaviors, and rewarding responses to opiates (Bandler and Shipley 1994; Behbehani 1995; Brandao 1993; Carrive 1993; Keay and Bandler 2001; Lovick 1993a; Lovick 1993b; McBride et al. 1999; Wise 1989). The synaptology of PAG inputs to the VTA has not yet been explored, although the variety of functions mediated by PAG neurons suggests that synapses formed by these axons are likely to be complex in morphology and cellular targets. Nevertheless, a specific hypothesis regarding synaptology can be formulated by considering the possibility that the PAG may serve as a source of excitation to VTA DA cells in response to opiates.
It is well established that DA VTA cells play an important role in the reinforcing effects of many drugs of abuse, including opiates (Koob 1996; Spanagel and Weiss 1999). Systemic administration of opiates increases the firing rate of VTA DA neurons, promotes burst firing, and enhances forebrain DA efflux (Georges et al. 2006; Gysling and Wang 1983; Matthews and German 1984; Zhang et al. 2008). In contrast, in vitro analyses of VTA brain slices report that application of these drugs does not affect DA neurons directly (Johnson and North 1992a; Lacey et al. 1989). Opiates do inhibit local GABA cells (Gysling and Wang 1983; Johnson and North 1992a; Lacey et al. 1989), suggesting that DA neuron activation by these drugs may be mediated in part via the local collaterals of these secondary cells (Johnson and North 1992b; Nugent and Kauer 2008; Omelchenko and Sesack 2009; Phillipson 1979). Consequently, brain regions that are afferent to the VTA can excite DA cells in response to opiates by removing GABA tone (Johnson and North 1992a; Xi and Stein 2002b). An enhancement of glutamate tone, such as that which drives burst firing (Overton and Clark 1997), may also underlie opiate activation of DA neurons (Harris et al. 2004; Shabat-Simon et al. 2008; Xi and Stein 2002a).
It is important to note that acute lesions of the medial forebrain bundle do not alter the response of VTA DA neurons to systemic morphine (Gysling and Wang 1983), suggesting that an ascending afferent projection may be important. The PAG is known to play a key role in the circuits responsible for opiate responding (Brandao 1993; McBride et al. 1999; Wise 1989). For example, intracranial administration of morphine into the PAG is reinforcing and also produces conditioned place preference (David and Cazala 1994; Olmstead and Franklin 1997; van der Kooy et al. 1982). Furthermore, the region has a high density of μ-opioid receptors (Mansour et al. 1994). The supposition that the PAG projection to the VTA may contribute to the activation of DA cells in response to opiates leads to the specific hypothesis that PAG afferents will form predominantly excitatory synaptic inputs to VTA DA neurons and/or inhibitory inputs directed to GABA cells.
We tested this hypothesis by combining anterograde tract-tracing from the PAG with immunocytochemistry and electron microscopy as detailed in our prior publications (Carr and Sesack 2000; Omelchenko and Sesack 2005; Omelchenko and Sesack 2006). A preliminary report of a portion of this work has appeared in abstract form (Omelchenko and Sesack 2007a).
MATERIALS AND METHODS
Subjects and surgeries
Five adult male Sprague-Dawley rats (Hilltop Lab Animals Inc, 330–360 g) were used in accordance with the NIH Guide for the Care and Use of Laboratory Animals and with the approval of the Institutional Animal Care and Use Committee at the University of Pittsburgh. Animals were anesthetized with an i.m. injection of 34 mg/kg ketamine, 1 mg/kg acepromazine, and 7 mg/kg xylazine. A stereotaxic apparatus was used to fix the skull, and a hole was drilled over the midbrain. Iontophoretic injections (+5 μA pulsed 10 seconds on/off) of a 10% solution of biotinylated dextran amine BDA (10,000 MW; Molecular Probes, Eugene, Oregon) in 10 mM sodium phosphate buffer (4 animals) or Phaseolus vulgaris leucoagglutinin (PHAL, 1 animal) were conducting over 15 minutes using glass micropipettes with 75 μm tip diameters (15 μm for PHAL), lowered at a 5° angle. The coordinates for PAG injections were, according to the atlas of Paxinos and Watson (Paxinos and Watson 1998), 5.3 mm posterior to Bregma, 0.5 mm lateral to midline, and 5.3 mm ventral to the skull surface. The pipettes were left in place for 5 minutes before extracting them.
After 13–25 days post-surgery, the rats were injected with pentobarbital (60 mg/kg, i.p., with supplemental doses if necessary). Treatment for 15 min with 1 g/kg i.p. of the zinc chelator diethyldithiocarbamic acid (Sigma, St. Louis, MO) was used to prevent silver intensification of endogenous zinc (Veznedaroglu and Milner 1992). After this treatment, the rats were killed by a transcardial perfusion of heparin saline (Elkins-Sinn, NJ; 1000 U/ml), followed by 50 ml of 3.75% acrolein and 2% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4) and 200–400 ml of 2% paraformaldehyde in 0.1 M PB. Blocks of the brain containing the forebrain, diencephalon, PAG, VTA, or rostral brainstem were post-fixed in 2% paraformaldehyde for 0.5–1 hr. Coronal sections were sliced at 50 μm using a vibratome and collected in PB. The fixation process was stopped by placing sections in 1% sodium borohydride in PB for 30 min and then rinsing in PB.
Immunocytochemistry
The immunolabeling procedure was performed as previously described (Carr and Sesack 2000; Omelchenko and Sesack 2005; Omelchenko and Sesack 2006). The anterograde tracer in axons was detected with immunoperoxidase, whereas the dendrites of DA or GABA cells were identified using immunogold-silver labeling for the catecholamine synthetic enzyme tyrosine hydroxylase (TH) or for GABA.
Evidence for the specificity of the primary antibodies directed against PHAL, TH and GABA are provided in our prior publications (Carr and Sesack 2000; Omelchenko and Sesack 2005; Sesack et al. 1995) and outlined here. The rabbit antibody against PHAL (Vector, Burlingame, CA) does not label sections from naïve animals or brain regions that do not receive projections from the PAG. For the mouse monoclonal antibody directed against TH (Chemicon #MAB318), western blot analysis demonstrates recognition of a 59–63 kDa protein from the outside of the N-terminus of TH and not other monoamine synthetic enzymes (manufacturer’s information). Finally, a dot blot assay has confirmed that the mouse monoclonal antibody raised against GABA (Sigma #A-0310) does not recognize several closely related amino acids (manufacturer’s information).
Immunolabeling procedures were held at room temperature, included constant agitation of free-floating sections, and alternated exposure to different immunoreagents with extensive rinses in buffer. Following rinses in 0.1 M tris-buffered saline (TBS; pH 7.6), sections were incubated for 30 min in a blocking solution containing 1% BSA and 3% normal goat serum in 0.1 M TBS. The blocking solution also contained Triton X-100 (Sigma) at 0.04% for electron microscopy or at 0.2% for light microscopy for improvement of immunoreagent penetration.
Sections were incubated overnight in blocking solution with either mouse anti-TH (1:5000; Chemicon) or mouse anti-GABA (1:2000; Sigma), in one case in combination with rabbit anti-PHAL. After rinses, sections from the PHAL case were exposed for 30 min to the blocking solution containing donkey anti-rabbit biotinylated secondary antibodies (1:400; Jackson ImmunoResearch Laboratories, West Grove, PA). At this point, all cases had biotin incorporated, either as part of the tracer in the four cases with BDA transport or on the secondary antibody in the one case using PHAL. Subsequent processing steps were the same. The peroxidase enzyme was incorporated by incubation for 30 min in 1:100 avidin-biotin peroxidase complex (Vectastain Elite kit; Vector Laboratories). Peroxidase product was visualized by placing sections into 0.022% diaminobenzidine (Sigma) and 0.003% hydrogen peroxide in TBS for 3–3.5 min. The reaction was terminated by extensive rinses in TBS. A single labeling procedure was used for sections processed for light microscopic examination of injection sites and transport patterns.
For dual immunocytochemistry, immunogold-silver staining of TH or GABA was developed after immunoperoxidase labeling. First, sections were incubated in a washing buffer for 30 min containing 0.8% BSA, 0.1% fish gelatin, and 3% normal donkey serum in 10 mM phosphate buffered saline (PBS; pH 7.4). Then sections were placed overnight in this solution to which was added 1:50 donkey anti-mouse IgG conjugated to 1 nm gold particles (Electron Microscopy Sciences, Washington, PA). Following extensive rinsing, 2% glutaraldehyde in PBS was used for 10 min in order to postfix the sections. After several rinses in PBS and then 0.2 M sodium citrate buffer, pH 7.4, the size of the gold particles was enhanced by placing sections in silver solution (Amersham) for 4–6 min followed by rinsing in citrate buffer.
Tissue preparation for light and electron microscopy
Bright field microscopy was used to verify the correct position of the injections, absence of retrograde transport of BDA, and adequate anterograde transport to the ventral midbrain. Sections were dried onto glass slides, dehydrated in graded alcohol solutions, defatted in xylene, and coverslipped with DPX. Digital photomicrographs of the sections were matched for brightness and contrast in Adobe Photoshop.
After postfixation in 2% osmium tetroxide in PB for 1 hr, sections developed for electron microscopy were dehydrated in alcohol solutions, placed in propylene oxide, and then left overnight in equal volumes of propylene oxide and epoxy resin (EM bed 812; Electron Microscopy Sciences). The following day, sections were placed into straight epoxy resin for 2 hr and then embedded between sheets of commercial polymer film. The epoxy resin used for section embedding was hardened in an oven for 48 hr at 60°C.
Sections from the anterior to middle portions of the VTA were photographed to record the exact position of ultrastructural analysis. Ultrathin sections were cut from the interface of the plasticized tissue sections. Serials of 3–5 sections were placed onto copper mesh grids, and uranyl acetate and lead citrate were used to counterstained the tissue before examination on a transmission electron microscope: FEI Morgagni (Hillsboro, Oregon). Brightness, contrast and sharpness of the photomicrographs captured by an XR-60 digital camera from Advanced Microscopy Techniques (Danvers, MA) were adjusted in Adobe Photoshop.
Ultrastructural analysis
The ultrastructural analysis of the tissue was conducted at 14,000–22,000X magnification. Estimations of the area sampled were made by multiplying the approximate percentage of each grid square that contained tissue (versus embedding media), area of the grid square (3,025 μm2) supplied by the manufacturer, and the number of grid squares analyzed. In total, 2,939,544 μm2 of tissue was examined from TH-labeled sections and 4,788,575 μm2 from GABA-labeled sections. The neuronal and glial elements in the tissue were identified using criteria of Peters (Peters et al. 1991).
The analysis of the tissue was confined to the outmost surface of vibratome sections in order to overcome limited penetration of the immunoreagents. False negative findings due to this technical limitation were further addressed by including in the analysis only those images of immunoperoxidase labeled PAG profiles that had specific immunogold-silver labeling evident within the same photographic field (approximately 13.8 μm2). Specific gold-silver labeling was defined as profiles containing at least 3 particles in one section or 4 spatially distinct particles in two sections. Typically labeled dendrites contained many more than 3–4 particles. Serial section analysis was conducted in all cases when immunolabeling was weak or sparse, as well as in cases when synaptic type had to be verified. Occasionally gold-silver particles were observed in axons containing peroxidase product, and this was considered evidence of specific labeling if at least 3 such particles were counted in a single section. Although an affinity of silver for the diaminobenzidine reaction product has been reported (Smiley and Goldman-Rakic 1993), in our experience this artifact does not occur consistently and is easily discriminated by its markedly small size and dust-like, uniform distribution over peroxidase product. Furthermore, large, discretely-placed silver granules do not occur when the gold-conjugated secondary antibody is omitted.
RESULTS
Light microscopy
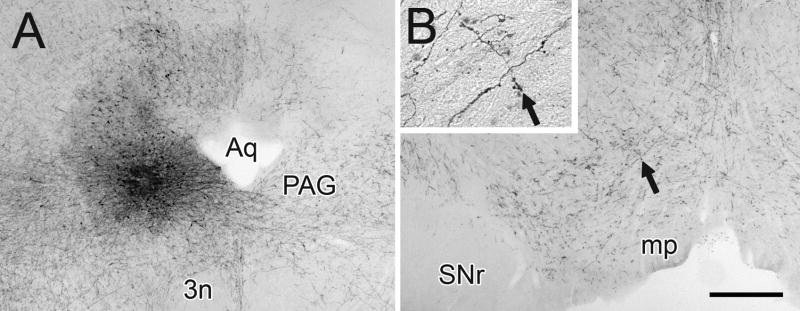
In this study, immunoperoxidase was used to visualize the injections of BDA or PHAL into the PAG and the anterograde transport to the VTA in the rat. The injection sites varied little in overall size and appeared as dense cores surround by more diffuse labeling (Fig. 1A). Injection sites were positioned near the center of the PAG along the dorsoventral axis (3 animals), within the dorsolateral quadrant (1 animal), or within the ventral portion of the PAG (1 animal; Fig. 1A). In the rostrocaudal dimension, injections were located between −5.2 and −5.6 posterior to Bregma. In one case, a slight diffusion of tracer was observed along the pipette track into the overlying medial superior colliculus, an area that projects only weakly to the VTA as compared to the substantia nigra (Comoli et al. 2003).
Figure 1.
Light micrographic images of rat coronal brain sections showing the injection of BDA into the PAG (A) and the anterograde transport of tracer to the VTA (B). Arrows in B and in the insert indicate the same axon cluster with multiple beaded varicosities. Abbreviations: 3n, oculomotor nucleus; Aq, cerebral aqueduct; mp, mammillary peduncle; SNr, substantia nigra reticulata. Scale bar represents 500 μm for A and B, 125 μm for the insert.
Following tracer injections into the PAG, we observed numerous anterogradely-labeled axons emanating from the injection sites. No retrogradely labeled cells were observed in regions of the forebrain, diencephalon, and rostral brainstem that are known afferents of the PAG. This finding indicates that artifactual labeling of divergent axon collaterals, which can occur with BDA (Sesack et al. 2006), did not compromise the outcomes of our study. There were no apparent differences in the appearance of the injection sites or anterograde labeling in the VTA with either BDA or PHAL tracers.
Many labeled axons were observed in the VTA and in the substantia nigra pars compacta (Fig. 1B), with the density of transport modestly higher on the ipsilateral than the contralateral side. Significantly fewer fibers were observed in the substantia nigra pars reticulata and in the interpeduncular nucleus. Within the VTA, the distribution of labeled axons was relatively uniform, and similar numbers of fibers were seen in paranigral, parabrachial and midline subregions. Many of these axons exhibited a beaded appearance (Fig. 1B, insert).
Ultrastructural examination of PAG axons in the rat VTA
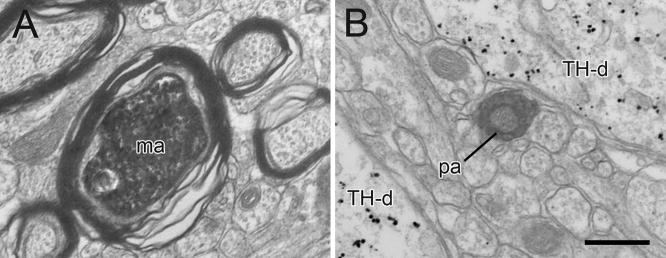
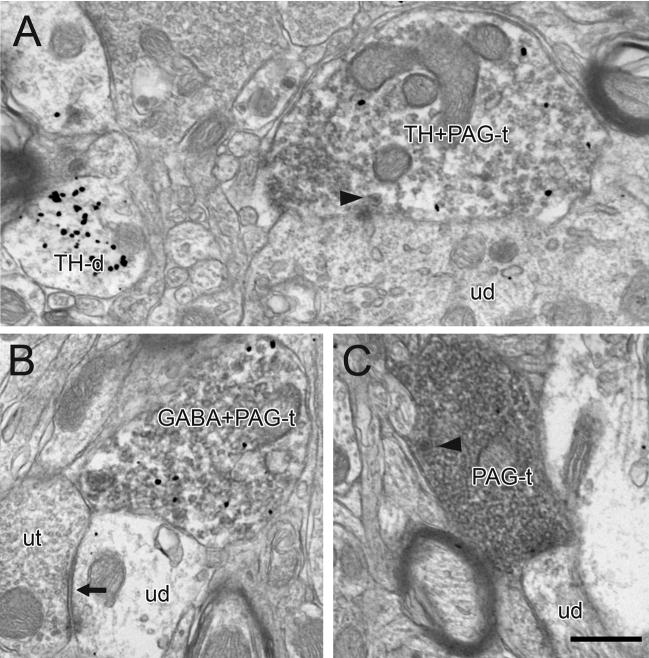
Immunoperoxidase labeling for BDA and PHAL transported from the PAG to the VTA was seen as a dark flocculent product within axons. The majority of immunoperoxidase-containing profiles were myelinated and unmyelinated passing fibers (Fig. 2). Immunoperoxidase was also detected in varicose axon profiles that contained small clear vesicles and occasional mitochondria (Figs. 3–6; Table 1). A few of these profiles also exhibited immunogold silver labeling for TH (3 out of 97, 3%; Fig. 3A) or GABA (10 out of 128, 8%; Figs. 3B, 6B), and some contained dense-cored vesicles (12 out of 227, 5%; Fig. 3A,C). There was no apparent difference in the morphology of identified PAG axons following anterograde transport of BDA or PHAL.
Figure 2.
Electron micrographs showing immunoperoxidase labeling in PAG axons within the rat VTA. The majority of immunoperoxidase labeled profiles are myelinated (ma) and unmyelinated passing axons (pa) located in bundles of unlabeled axons. In panel B, immunogold-silver labeling for TH in dendrites (TH-d) is evident in the adjacent neuropil. Scale bar, 0.5 μm.
Figure 3.
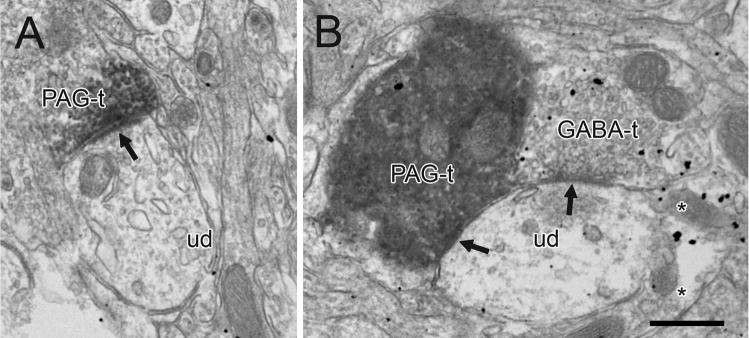
Electron micrographs showing immunoperoxidase labeling in PAG axon terminals in the rat VTA. Some PAG terminals also exhibit immunogold-silver labeling for TH (TH+PAG-t) or GABA (GABA+PAG-t), and some contain dense-cored vesicles (arrowheads in A and C). Many profiles are closely apposed to unlabeled dendrites (ud) which occasionally receive synaptic input (black arrow) from unlabeled terminals (ut in B). In panel A, a TH-labeled dendrite (TH-d) is evident in the adjacent neuropil. Scale bar, 0.5 μm.
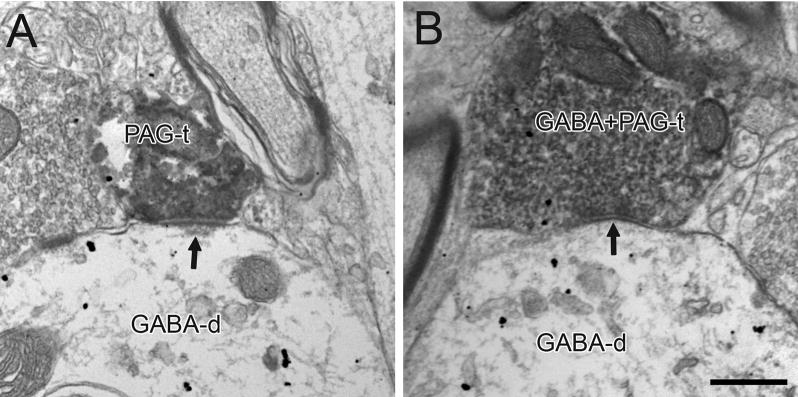
Figure 6.
Electron micrographs showing immunoperoxidase labeling in PAG axon terminals (PAG-t) in the VTA forming asymmetric and symmetric synapses (arrows in A and B, respectively) onto dendrites labeled for GABA (GABA-d). In B, the PAG terminal also contains sparse immunogold-silver labeling for GABA (GABA+PAG-t). Scale bar, 0.5 μm.
Table 1.
Targets of PAG Axons in the Rat VTA
| Dendrites labeled for: | ||||||
|---|---|---|---|---|---|---|
| Tissue Labeled for TH | Unlabeled | TH | ||||
| total axons | 97 | |||||
| total dendritic contacts* | 76 | 78% | 36 | 47% | 40 | 53% |
| total synapses | 24 | 25% | 12 | 50% | 12 | 50% |
| asymmetric synapses | 10 | 42% | 4 | 40% | 6 | 60% |
| symmetric synapses | 14 | 58% | 8 | 57% | 6 | 43% |
| Dendrites labeled for: | ||||||
| Tissue Labeled for GABA | Unlabeled | GABA | ||||
| total axons | 130 | |||||
| total dendritic contacts* | 94 | 72% | 51 | 54% | 43 | 46% |
| total synapses | 28 | 22% | 17 | 61% | 11 | 39% |
| asymmetric synapses | 14 | 50% | 10 | 71% | 4 | 29% |
| symmetric synapses | 14 | 50% | 7 | 50% | 7 | 50% |
dendritic contacts include synapses and appositions
The majority of PAG axon varicosities directly contacted dendrites without glial separation (170 out of 227, 75%, Table 1); the remaining terminals were completely surrounded by glial processes or were apposed to other axons. A significant number of PAG profiles were observed to form synapses (52 out of 227 axons, 23%). Of these junctional contacts, 46% had thickened postsynaptic densities characteristic of asymmetric synapses (24 out of 52, Fig. 4A, Table 1); the remaining junctions exhibited thin to absent densities featured by symmetric synapses (Fig. 4B). No difference in synaptic morphology was observed when comparing transport from dorsal, central, or ventral regions of the PAG. However, the density of varicosities innervating the VTA following injections in the dorsal PAG was low. Furthermore, anterograde transport of PHAL produced slightly fewer synapses than observed with BDA, which was therefore the preferred tracer for this pathway.
Figure 4.
Electron micrographs of the rat VTA showing immunoperoxidase labeling in PAG axon terminals (PAG-t) forming asymmetric or symmetric synaptic specializations (arrows in A and B, respectively) onto unlabeled dendrites (ud). In B, the unlabeled dendrite receives an additional symmetric synapse from a GABA-labeled terminal (GABA-t). GABA immunoreactivity is also evident in a glial process (asterisks). Scale bar, 0.5 μm.
Projection targets of PAG axons in the VTA
Immunogold-silver labeling for TH or GABA was seen as electron dense granules dispersed within labeled profiles throughout the VTA as previously described (Carr and Sesack 2000; Omelchenko and Sesack 2005; Omelchenko and Sesack 2007b). The majority of these profiles were defined as dendrites or soma. Axonal labeling was also observed for each marker, particularly near the tissue surface, and more commonly in GABA-labeled tissue. GABA labeling was also frequently detected in glial processes (Fig. 4B).
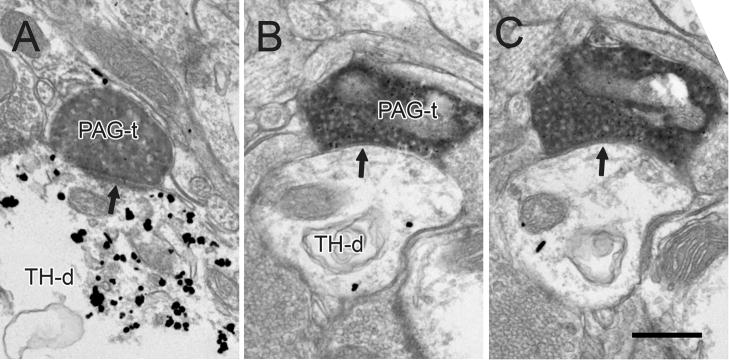
In tissue labeled for TH, 40 out of 76 PAG terminals contacted dendrites immunoreactive for TH (53%, Table 1). This number included 6 asymmetric (Fig. 5A) and 6 symmetric synapses (Fig. 5B,C) onto TH positive dendrites. The remaining terminals contacted TH-negative profiles.
Figure 5.
Electron micrographs showing immunoperoxidase labeling in PAG terminals (PAG-t) in the VTA forming asymmetric and symmetric synapses (arrows in A and B-C, respectively) onto dendrites containing immunogold-silver labeling for TH (TH-d). The sparse labeling for TH in panel B is confirmed by serial section analysis in panel C. Scale bar, 0.5 μm.
In tissue immunoreacted for GABA, 43 out of 94 terminals contacted GABA-labeled dendrites (46%, Table 1). These contacts included 4 asymmetric (Fig. 6A) and 7 symmetric synapses (Fig. 6B) onto GABA-immunoreactive dendrites. The remaining terminals contacted unlabeled profiles.
DISCUSSION
The present ultrastructural analysis provides the first anatomical data indicating that PAG neurons synapse directly onto DA and GABA cells in the rat VTA. The innervation is moderately dense, arises from diverse populations of PAG cells, and likely provides a variety of physiological actions on ventral midbrain neurons. In addition to the glutamate innervation previously reported in the literature (Geisler et al. 2007), we provide some evidence that GABA and DA cells located in the PAG also contribute to the VTA projection. Consistent with our initial hypothesis, we reveal presumably excitatory inputs coming onto VTA DA cells as well as the potential for disinhibitory circuitry via presumably inhibitory synapses directed to VTA GABA cells. However, evidence was also obtained for putative inhibitory synapses onto DA neurons and excitatory connections to GABA cells. Hence, further studies are needed to identify the precise role that PAG projections mediate in regulating the activity patterns of VTA neurons and their responses to drugs and environmental stimuli.
Methodological considerations
All studies that rely upon immunoelectron microscopy and tract-tracing have a common concern of false negative outcomes (Carr and Sesack 2000; Omelchenko and Sesack 2005; Sesack et al. 2006). Tracing studies cannot label all the neurons of a given pathway, so that our anterograde tracing only represented a portion of the projection from the PAG to the VTA. Nevertheless, we know of no evidence for differential uptake of tracer into PAG neuron classes, so that the whole of the projection population was likely to have been represented. Second, tissue profiles that actually contain the antigen of interest may remain unlabeled due to incomplete penetration of immunoreagents. In our experience, this second limitation particularly affects smaller profiles like axons, for which gold-silver penetration is often suboptimal compared to dendrites, particularly when axons also contain immunoperoxidase product (Omelchenko and Sesack 2009; Sesack et al. 2006). This factor is likely to have influenced estimates of dually-labeled PAG terminals, so that only the presence of GABA or TH in these profiles and not their exact contribution to the pathway can be reported here.
In order to minimize false negative counting, we limited analysis of the tissue to the section interface where antibody penetration is optimal. Moreover, as peroxidase labeling is more sensitive than immunogold-silver staining (Sesack et al. 2006), only PAG-labeled profiles that had immuno-gold silver labeling within the same photographic field were included in the analysis of projection targets of PAG axons (see Materials and Methods). Moreover, we analyzed serial sections to verify all cases with sparse labeling. Despite these precautions, we acknowledge that false negative results cannot be fully avoided. Nevertheless, the data are likely to represent with good accuracy the major characteristics of the PAG to VTA pathway.
PAG terminals within the VTA
The current observations confirm previous light microscopic evidence that the VTA receives substantial innervation from the PAG (Cameron et al. 1995; Eberhart et al. 1985; Geisler et al. 2007; Geisler and Zahm 2005). The fact that we found anterograde transport in many myelinated and unmyelinated fibers traveling in axon bundles suggests that a portion of the PAG projection passes through the VTA toward other structures. Of course these axons may synapse within more rostral or caudal levels of the VTA. The observation of both asymmetric and symmetric synapses formed by PAG axons indicates that this pathway arises from phenotypically diverse populations of cells. Electrophysiological analyses of VTA responses to PAG stimulation have not yet been conducted, but in other target areas, stimulation evokes mixed excitation and inhibition (Lovick 1992; Snowball et al. 1997; van der Plas et al. 1995). Given the functional distinction between dorsal and ventral divisions of the PAG (Lovick 1992) (see below), synaptic morphology might reflect the region of origin within the PAG, but this was not observed in the present study.
Analysis of synaptic morphology alone provides only a correlation to physiological action and not a direct indication of transmitter phenotype. Nevertheless, the detection of asymmetric synapses traditionally associated with an excitatory physiology (Carlin et al. 1980) is consistent with evidence that the PAG provides a source of glutamate innervation to the VTA (Geisler et al. 2007). Observation of symmetric synapses suggests that some of this innervation has a likely inhibitory (Carlin et al. 1980) or modulatory influence. This suggestion is supported by evidence for GABA immunolabeling in some PAG axons, keeping in mind that the extent of such labeling was probably underestimated as discussed above. This observation also agrees with a previous report of GABA neurons in the PAG (Barbaresi and Manfrini 1988). Although one paper has shown GABA neurons with extrinsic projections outside the PAG (Gervasoni et al. 2000), to our knowledge the present study is the first to report a GABA projection from the PAG to the VTA.
Other classical transmitters that can be present in PAG axons include DA and serotonin (Barbaresi and Manfrini 1988; Flores et al. 2006; Li et al. 1989; Lu et al. 2006; Zeng et al. 1991). The possible contribution of serotonin to the PAG projection to the VTA was not analyzed here, although it is likely to be minimal given that the injection sites did not include the ventromedial regions where serotonin neurons are densest. Our finding of a few PAG axons that also contained TH immunoreactivity represents the first report that DA PAG neurons contribute to the synaptic regulation of the VTA. A population of DA neurons with extrinsic projections has previously been demonstrated in the PAG (Arsenault et al. 1988; Flores et al. 2006; Freeman et al. 2001; Hasue and Shammah-Lagnado 2002; Lu et al. 2006; Takada et al. 1990; Yoshida et al. 1989). Further studies are needed to determine the true proportion of different neurotransmitters in PAG projections to the VTA.
The finding of dense-cored vesicles in some PAG terminals suggests that neuropeptides are also involved in regulating the activity of VTA neurons. The candidate peptides that have been localized to PAG cells include substance P, leucine-enkephalin, somatostatin, and neurotensin (Beitz et al. 1983b; Li et al. 1990b; Zeng et al. 1991). Of these peptides, only neurotensin has been specifically reported in a small number of PAG cells projecting to the VTA (Zahm et al. 2001). The identification of other neuropeptides contributing to the PAG to VTA projection will require further analysis.
Additional studies are also needed to identify whether PAG neurons projecting to the VTA issue collaterals to other brain regions. The projections of PAG cells to multiple brainstem nuclei reportedly arise from predominantly non-collateralized projections (Beitz et al. 1983a). On the other hand, PAG cells innervating the forebrain and diencephalon send branched projections to the medullary nucleus raphe magnus (Li et al. 1990a; Reichling and Basbaum 1991), and PAG neurons also provide collateralized axons to different subdivisions of the thalamus (Barbaresi et al. 1982). In the future, such analyses will be essential for determining whether PAG afferents to the VTA form part of a discrete circuit or contribute to a divergent functional output designed to coordinate activity in multiple targets.
VTA neuronal populations innervated by PAG terminals
Consistent with our initial prediction, we observed asymmetric, presumably excitatory synaptic inputs from the PAG onto VTA DA cells as well as symmetric, presumably inhibitory synapses onto GABA neurons. However, PAG synapses of both morphological types also provided innervation to the other cell populations identified in the study, suggesting an overall lack of selectivity in PAG target populations. A third population not identified in the current study includes a small set of glutamate-containing neurons that is most dense in medial portions of the VTA (Hur and Zaborszky 2005; Kawano et al. 2006; Nair-Roberts et al. 2008; Yamaguchi et al. 2007). Determining whether this population receives PAG synaptic input onto its dendrites will require development of markers for glutamate neurons that are suitable for electron microscopy. To date, these cells have only been identified by mRNA localization within soma.
Functional considerations
Our findings of putative excitatory innervation of DA cells and presumed inhibitory regulation of GABA cells is at least consistent with the possibility that PAG afferents could be involved in modulating VTA responses to opiates. The PAG consists of several subdivisions with differential connectivity and functions (Bandler and Shipley 1994; Behbehani 1995; Cameron et al. 1995; Carrive 1993; Lovick 1993b; Vianna and Brandao 2003). Although these were not well dissociated in the current study, it is unlikely that PAG cells involved in opiate responses are organized in a strictly topographical manner. Cells contributing to VTA projections (Geisler and Zahm 2005) and those expressing opioid receptors (Mansour et al. 1994) are essentially evenly spread across PAG subdivisions. Nevertheless, different subtypes of opioid receptors may be expressed by PAG neurons (Schurr et al. 1981; Schurr et al. 1982) with distinct neurotransmitter phenotypes (Flores et al. 2006), leading to dissimilar responses to systemic opiates. Future studies are needed to determine the protein expression patterns of PAG cells that respond to opiates and project to the VTA.
PAG afferents to the VTA may also be involved in other functions related to pain. For example, VTA neurons receive nociceptive information (Coizet et al. 2006; Ma et al. 1993; Ohtori et al. 2000; Smith et al. 1997; Ungless et al. 2004) and are involved in pain modulation (Altier and Stewart 1999; Morgan and Franklin 1990). The PAG also plays a key role in regulating pain responses (Bandler and Shipley 1994; Behbehani 1995; Clement et al. 2000; Lovick 1993a), making it plausible that the specific connection between these areas is important for processing and responding to pain signals. In this regard, putative inhibitory inputs from the PAG to VTA DA cells, or putative excitatory synapses to VTA GABA cells, may mediate the interruption of firing that occurs in response to aversive stimuli (Ungless et al. 2004). Aversive events trigger either active or passive defensive behaviors in animals, both of which are strongly regulated by the PAG (Bandler and Shipley 1994). Hence, projections of the PAG to VTA DA cells may act to facilitate the locomotor responses most appropriate to the situation (Redgrave et al. 1999).
PAG afferents may provide the VTA with information related to circadian rhythm, given that many PAG cells change activity across sleep-wake states, including GABA and DA neurons (Gervasoni et al. 1998; Gervasoni et al. 2000; Lu et al. 2006; Ni et al. 1990). Although most VTA DA cells are not affected by sleep/wake state (Miller et al. 1983; Steinfels et al. 1983; Trulson and Preussler 1984), but see (Dahan et al. 2007), the firing patterns of many secondary (presumably GABA) cells are altered (Lee et al. 2001; Miller et al. 1983), suggesting that PAG afferents may mediate some of this rhythmic drive. Alternatively, both the VTA and PAG may be similarly influenced by a common afferent that exhibits circadian rhythmicity.
Another potential function of projections from the PAG to the VTA is to establish a feedback loop. The VTA provides a GABA projection to the PAG that reportedly functions in cardiovascular depressor responses (Kirouac et al. 2004). It remains to be determined whether the GABA cells shown here to receive synaptic input from the PAG include those with reciprocal projections.
The PAG is an essential region coordinating the defensive behaviors, stress responses, and autonomic functions associated with aversive events (Bandler and Shipley 1994; Behbehani 1995; Carrive 1993; Keay and Bandler 2001; Lovick 1993a; Lovick 1993b). The PAG is also an important component of the neurobiological substrates for opiate responding (Brandao 1993; McBride et al. 1999; Wise 1989). The connections between the PAG and VTA that were explored in the current study are likely to be important for regulating the motivated behaviors that are controlled by VTA cells. They also elucidate pathways not fully appreciated in the literature that may be involved in opiate responses.
Acknowledgments
USPHS grant NIMH 067937
References
- Altier N, Stewart J. The role of dopamine in the nucleus accumbens in analgesia. Life Sci. 1999;65(22):2269–2287. doi: 10.1016/s0024-3205(99)00298-2. [DOI] [PubMed] [Google Scholar]
- Arsenault MY, Parent A, Seguela P, Descarries L. Distribution and morphological characteristics of dopamine-immunoreactive neurons in the midbrain of the squirrel monkey (Saimiri sciureus) J Comp Neurol. 1988;267(4):489–506. doi: 10.1002/cne.902670404. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17(9):379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Barbaresi P, Conti F, Manzoni T. Axonal branching in the periaqueductal gray projections to the thalamus: a fluorescent retrograde double-labeling study in the cat. Brain Res. 1982;252(1):137–141. doi: 10.1016/0006-8993(82)90986-6. [DOI] [PubMed] [Google Scholar]
- Barbaresi P, Manfrini E. Glutamate decarboxylase-immunoreactive neurons and terminals in the periaqueductal gray of the rat. Neuroscience. 1988;27(1):183–191. doi: 10.1016/0306-4522(88)90229-1. [DOI] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46(6):575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Beitz AJ, Mullett MA, Weiner LL. The periaqueductal gray projections to the rat spinal trigeminal, raphe magnus, gigantocellular pars alpha and paragigantocellular nuclei arise from separate neurons. Brain Res. 1983a;288(1–2):307–314. doi: 10.1016/0006-8993(83)90108-7. [DOI] [PubMed] [Google Scholar]
- Beitz AJ, Shepard RD, Wells WE. The periaqueductal gray-raphe magnus projection contains somatostatin, neurotensin and serotonin but not cholecystokinin. Brain Res. 1983b;261(1):132–137. doi: 10.1016/0006-8993(83)91292-1. [DOI] [PubMed] [Google Scholar]
- Brandao ML. Involvement of opioid mechanisms in the dorsal periaqueductal gray in drug abuse. Rev Neurosci. 1993;4(4):397–405. doi: 10.1515/revneuro.1993.4.4.397. [DOI] [PubMed] [Google Scholar]
- Brozoski T, Brown R, Rosvold H, Goldman P. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Khan IA, Westlund KN, Cliffer KD, Willis WD. The efferent projections of the periaqueductal gray in the rat: a Phaseolus vulgaris-leucoagglutinin study. I. Ascending projections. J Comp Neurol. 1995;351(4):568–584. doi: 10.1002/cne.903510407. [DOI] [PubMed] [Google Scholar]
- Carlin RK, Grab DJ, Cohen RS, Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980;86(3):831–843. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20(10):3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrive P. The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav Brain Res. 1993;58(1–2):27–47. doi: 10.1016/0166-4328(93)90088-8. [DOI] [PubMed] [Google Scholar]
- Clement CI, Keay KA, Podzebenko K, Gordon BD, Bandler R. Spinal sources of noxious visceral and noxious deep somatic afferent drive onto the ventrolateral periaqueductal gray of the rat. J Comp Neurol. 2000;425(3):323–344. doi: 10.1002/1096-9861(20000925)425:3<323::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Coizet V, Dommett EJ, Redgrave P, Overton PG. Nociceptive responses of midbrain dopaminergic neurones are modulated by the superior colliculus in the rat. Neuroscience. 2006;139(4):1479–1493. doi: 10.1016/j.neuroscience.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Comoli E, Coizet V, Boyes J, Bolam JP, Canteras NS, Quirk RH, Overton PG, Redgrave P. A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat Neurosci. 2003;6(9):974–980. doi: 10.1038/nn1113. [DOI] [PubMed] [Google Scholar]
- Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32(6):1232–1241. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- David V, Cazala P. Differentiation of intracranial morphine self-administration behavior among five brain regions in mice. Pharmacol Biochem Behav. 1994;48(3):625–633. doi: 10.1016/0091-3057(94)90324-7. [DOI] [PubMed] [Google Scholar]
- Eberhart JA, Morrell JI, Krieger MS, Pfaff DW. An autoradiographic study of projections ascending from the midbrain central gray, and from the region lateral to it, in the rat. J Comp Neurol. 1985;241(3):285–310. doi: 10.1002/cne.902410305. [DOI] [PubMed] [Google Scholar]
- Flores JA, Galan-Rodriguez B, Ramiro-Fuentes S, Fernandez-Espejo E. Role for dopamine neurons of the rostral linear nucleus and periaqueductal gray in the rewarding and sensitizing properties of heroin. Neuropsychopharmacology. 2006;31(7):1475–1488. doi: 10.1038/sj.npp.1300946. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 2006;188(4):567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Freeman A, Ciliax B, Bakay R, Daley J, Miller RD, Keating G, Levey A, Rye D. Nigrostriatal collaterals to thalamus degenerate in parkinsonian animal models. Ann Neurol. 2001;50(3):321–329. doi: 10.1002/ana.1119. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27(21):5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490(3):270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Georges F, Le Moine C, Aston-Jones G. No effect of morphine on ventral tegmental dopamine neurons during withdrawal. J Neurosci. 2006;26(21):5720–5726. doi: 10.1523/JNEUROSCI.5032-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasoni D, Darracq L, Fort P, Souliere F, Chouvet G, Luppi PH. Electrophysiological evidence that noradrenergic neurons of the rat locus coeruleus are tonically inhibited by GABA during sleep. Eur J Neurosci. 1998;10(3):964–970. doi: 10.1046/j.1460-9568.1998.00106.x. [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Peyron C, Rampon C, Barbagli B, Chouvet G, Urbain N, Fort P, Luppi PH. Role and origin of the GABAergic innervation of dorsal raphe serotonergic neurons. J Neurosci. 2000;20(11):4217–4225. doi: 10.1523/JNEUROSCI.20-11-04217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysling K, Wang RY. Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res. 1983;277:119–127. doi: 10.1016/0006-8993(83)90913-7. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Byrne R, Aston-Jones G. Glutamate-associated plasticity in the ventral tegmental area is necessary for conditioning environmental stimuli with morphine. Neuroscience. 2004;129(3):841–847. doi: 10.1016/j.neuroscience.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454(1):15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization. J Comp Neurol. 2005;483(3):351–373. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992a;12(2):483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992b;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H, Hisano S. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J Comp Neurol. 2006;498(5):581–592. doi: 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25(7–8):669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Li S, Mabrouk G. GABAergic projection from the ventral tegmental area and substantia nigra to the periaqueductal gray region and the dorsal raphe nucleus. J Comp Neurol. 2004;469(2):170–184. doi: 10.1002/cne.11005. [DOI] [PubMed] [Google Scholar]
- Koob GF. Hedonic valence, dopamine and motivation. Mol Psychiatry. 1996;1:186–189. [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J Neurosci. 1989;9(4):1233–1241. doi: 10.1523/JNEUROSCI.09-04-01233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RS, Steffensen SC, Henriksen SJ. Discharge profiles of ventral tegmental area GABA neurons during movement, anesthesia, and the sleep-wake cycle. J Neurosci. 2001;21:1757–1766. doi: 10.1523/JNEUROSCI.21-05-01757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Rao Z, Shi J. Collateral projections from the midbrain periaqueductal gray to the nucleus raphe magnus and nucleus accumbens in the rat. A fluorescent retrograde double-labelling study. Neurosci Lett. 1990a;117:285–288. doi: 10.1016/0304-3940(90)90678-3. [DOI] [PubMed] [Google Scholar]
- Li YQ, Jia HG, Rao ZR, Shi JW. Serotonin-, substance P- or leucine-enkephalin-containing neurons in the midbrain periaqueductal gray and nucleus raphe dorsalis send projection fibers to the central amygdaloid nucleus in the rat. Neurosci Lett. 1990b;120(1):124–127. doi: 10.1016/0304-3940(90)90184-b. [DOI] [PubMed] [Google Scholar]
- Li YQ, Rao ZR, Shi JW. Serotoninergic projections from the midbrain periaqueductal gray to the nucleus accumbens in the rat. Neuroscience Letters. 1989;98:276–279. doi: 10.1016/0304-3940(89)90413-8. [DOI] [PubMed] [Google Scholar]
- Lovick TA. Midbrain influences on ventrolateral medullo-spinal neurones in the rat. Exp Brain Res. 1992;90(1):147–152. doi: 10.1007/BF00229266. [DOI] [PubMed] [Google Scholar]
- Lovick TA. Integrated activity of cardiovascular and pain regulatory systems: role in adaptive behavioural responses. Prog Neurobiol. 1993a;40(5):631–644. doi: 10.1016/0301-0082(93)90036-r. [DOI] [PubMed] [Google Scholar]
- Lovick TA. The periaqueductal gray-rostral medulla connection in the defence reaction: efferent pathways and descending control mechanisms. Behav Brain Res. 1993b;58(1–2):19–25. doi: 10.1016/0166-4328(93)90087-7. [DOI] [PubMed] [Google Scholar]
- Lu J, Jhou TC, Saper CB. Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci. 2006;26(1):193–202. doi: 10.1523/JNEUROSCI.2244-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QP, Zhou Y, Han JS. Noxious stimulation accelerated the expression of c-fos protooncogene in cholecystokininergic and dopaminergic neurons in the ventral tegmental area. Peptides. 1993;14(3):561–566. doi: 10.1016/0196-9781(93)90145-7. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350(3):412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Matthews RT, German DC. Electrophysiological evidence for excitation of rat ventral tegmental area dopamine neurons by morphine. Neuroscience. 1984;11(3):617–625. doi: 10.1016/0306-4522(84)90048-4. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Miller JD, Farber J, Gatz P, Roffwarg H, German DC. Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and walking in the rat. Brain Res. 1983;273(1):133–141. doi: 10.1016/0006-8993(83)91101-0. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Franklin KB. 6-Hydroxydopamine lesions of the ventral tegmentum abolish D-amphetamine and morphine analgesia in the formalin test but not in the tail flick test. Brain Res. 1990;519(1–2):144–149. doi: 10.1016/0006-8993(90)90072-j. [DOI] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152(4):1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HF, Zhang JX, Harper RM. Cardiovascular-related discharge of periaqueductal gray neurons during sleep-waking states. Brain Res. 1990;532(1–2):242–248. doi: 10.1016/0006-8993(90)91766-a. [DOI] [PubMed] [Google Scholar]
- Nugent FS, Kauer JA. LTP of GABAergic synapses in the ventral tegmental area and beyond. J Physiol. 2008;586(6):1487–1493. doi: 10.1113/jphysiol.2007.148098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtori S, Takahashi K, Chiba T, Takahashi Y, Yamagata M, Sameda H, Moriya H. Fos expression in the rat brain and spinal cord evoked by noxious stimulation to low back muscle and skin. Spine. 2000;25(19):2425–2430. doi: 10.1097/00007632-200010010-00002. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Franklin KB. The development of a conditioned place preference to morphine: effects of microinjections into various CNS sites. Behav Neurosci. 1997;111(6):1324–1334. doi: 10.1037//0735-7044.111.6.1324. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J Comp Neurol. 2005;483(2):217–235. doi: 10.1002/cne.20417. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Ultrastructural evidence that non-dopamine cells in the rat ventral tegmental area synapse locally onto dopamine and GABA neurons. Soc Neurosci. 2006 Abstr 722.12. [Google Scholar]
- Omelchenko N, Sesack SR. Central gray afferents synapse onto dopamine and GABA neurons in the rat ventral tegmental area. Soc Neurosci. 2007a doi: 10.1002/jnr.22265. Abstr:780.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience. 2007b;146(3):1259–1274. doi: 10.1016/j.neuroscience.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse. 2009 doi: 10.1002/syn.20668. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton P, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Peters A, Palay SL, Webster H. The Fine Structure of the Nervous System: Neurons and Their Supporting Cells. New York: Oxford; 1991. [Google Scholar]
- Phillipson OT. A Golgi study of the ventral tegmental area of Tsai and interfascicular nucleus in the rat. J Comp Neurol. 1979;187(1):99–115. doi: 10.1002/cne.901870107. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. Is the short-latency dopamine response too short to signal reward error? Trends Neurosci. 1999;22(4):146–151. doi: 10.1016/s0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- Reichling DB, Basbaum AI. Collateralization of periaqueductal gray neurons to forebrain or diencephalon and to the medullary nucleus raphe magnus in the rat. Neuroscience. 1991;42(1):183–200. doi: 10.1016/0306-4522(91)90158-k. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7:191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Schurr A, Rigor BM, Ho BT, Dafny N. Periaqueductal gray neurons response to microiontophoretically injected morphine in naive and morphine-dependent rats. Brain Res Bull. 1981;6(6):473–478. doi: 10.1016/s0361-9230(81)80021-4. [DOI] [PubMed] [Google Scholar]
- Schurr A, Rigor BM, Ho BT, Dafny N. Electrophysiological support in favor of multiple opiate receptors in the caudate and the central gray of the rat. Comp Biochem Physiol C. 1982;73(2):323–330. doi: 10.1016/0306-4492(82)90129-0. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Bressler CN, Lewis DA. Ultrastructural associations between dopamine terminals and local circuit neurons in the monkey prefrontal cortex: a study of calretinin-immunoreactive cells. Neurosci Lett. 1995;200:9–12. doi: 10.1016/0304-3940(95)12076-g. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Miner LAH, Omelchenko N. Pre-embedding immunoelectron microscopy: applications for studies of the nervous system. In: Zaborszky L, Wouterlood F, Lanciego J, editors. Neuroanatomical Tract-Tracing 3: Molecules, Neurons, Systems. NY: Springer, New York; 2006. pp. 6–71. [Google Scholar]
- Shabat-Simon M, Levy D, Amir A, Rehavi M, Zangen A. Dissociation between rewarding and psychomotor effects of opiates: differential roles for glutamate receptors within anterior and posterior portions of the ventral tegmental area. J Neurosci. 2008;28(34):8406–8416. doi: 10.1523/JNEUROSCI.1958-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Goldman-Rakic PS. Silver-enhanced diaminobenzidine-sulfide (SEDS): a technique for high-resolution immunoelectron microscopy demonstrated with monoamine immunoreactivity in monkey cerebral cortex and caudate. J Histochem Cytochem. 1993;41(9):1393–1404. doi: 10.1177/41.9.8354879. [DOI] [PubMed] [Google Scholar]
- Smith WJ, Stewart J, Pfaus JG. Tail pinch induces fos immunoreactivity within several regions of the male rat brain: effects of age. Physiol Behav. 1997;61(5):717–723. doi: 10.1016/s0031-9384(96)00524-0. [DOI] [PubMed] [Google Scholar]
- Snowball RK, Dampney RA, Lumb BM. Responses of neurones in the medullary raphe nuclei to inputs from visceral nociceptors and the ventrolateral periaqueductal grey in the rat. Exp Physiol. 1997;82(3):485–500. doi: 10.1113/expphysiol.1997.sp004041. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Tr Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Rule learning and reward contingency are associated with dissociable patterns of dopamine activation in the rat prefrontal cortex, nucleus accumbens, and dorsal striatum. J Neurosci. 2006;26(34):8810–8818. doi: 10.1523/JNEUROSCI.1656-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfels GF, Heym J, Strecker RE, Jacobs BL. Behavioral correlates of dopaminergic unit activity in freely moving cats. Brain Res. 1983;258:217–228. doi: 10.1016/0006-8993(83)91145-9. [DOI] [PubMed] [Google Scholar]
- Takada M, Campbell KJ, Moriizumi T, Hattori T. On the origin of the dopaminergic innervation of the paraventricular thalamic nucleus. Neurosci Lett. 1990;115(1):33–36. doi: 10.1016/0304-3940(90)90513-9. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Preussler DW. Dopamine-containing ventral tegmental area neurons in freely moving cats: activity during the sleep-waking cycle and effects of stress. Exp Neurol. 1984;83:367–377. doi: 10.1016/S0014-4886(84)90105-5. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Mucha RF, O’Shaughnessy M, Bucenieks P. Reinforcing effects of brain microinjections of morphine revealed by conditioned place preference. Brain Res. 1982;243(1):107–117. doi: 10.1016/0006-8993(82)91124-6. [DOI] [PubMed] [Google Scholar]
- van der Plas J, Maes FW, Bohus B. Electrophysiological analysis of midbrain periaqueductal gray influence on cardiovascular neurons in the ventrolateral medulla oblongata. Brain Res Bull. 1995;38(5):447–456. doi: 10.1016/0361-9230(95)02012-g. [DOI] [PubMed] [Google Scholar]
- Veznedaroglu E, Milner TA. Elimination of artifactual labeling of hippocampal mossy fibers seen following pre-embedding immunogold-silver technique by pretreatment with zinc chelator. Microsc Res Tech. 1992;23(1):100–101. doi: 10.1002/jemt.1070230110. [DOI] [PubMed] [Google Scholar]
- Vianna DM, Brandao ML. Anatomical connections of the periaqueductal gray: specific neural substrates for different kinds of fear. Braz J Med Biol Res. 2003;36(5):557–566. doi: 10.1590/s0100-879x2003000500002. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kodama T, Hikosaka K. Increase of extracellular dopamine in primate prefrontal cortex during a working memory task. J Neurophysiol. 1997;78(5):2795–2798. doi: 10.1152/jn.1997.78.5.2795. [DOI] [PubMed] [Google Scholar]
- Wise RA. Opiate reward: sites and substrates. Neurosci Biobehav Rev. 1989;13(2–3):129–133. doi: 10.1016/s0149-7634(89)80021-1. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. Blockade of ionotropic glutamatergic transmission in the ventral tegmental area reduces heroin reinforcement in rat. Psychopharmacology (Berl) 2002a;164(2):144–150. doi: 10.1007/s00213-002-1190-3. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. GABAergic mechanisms of opiate reinforcement. Alcohol Alcohol. 2002b;37(5):485–494. doi: 10.1093/alcalc/37.5.485. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25(1):106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Shirouzu M, Tanaka M, Semba K, Fibiger HC. Dopaminergic neurons in the nucleus raphe dorsalis innervate the prefrontal cortex in the rat: a combined retrograde tracing and immunohistochemical study using anti-dopamine serum. Brain Res. 1989;496(1–2):373–376. doi: 10.1016/0006-8993(89)91091-3. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Grosu S, Williams EA, Qin S, Berod A. Neurons of origin of the neurotensinergic plexus enmeshing the ventral tegmental area in rat: retrograde labeling and in situ hybridization combined. Neuroscience. 2001;104(3):841–851. doi: 10.1016/s0306-4522(01)00118-x. [DOI] [PubMed] [Google Scholar]
- Zeng SL, Li YQ, Rao ZR, Shi JW. Projections from serotonin- and substance P-like immunoreactive neurons in the midbrain periaqueductal gray onto the nucleus reticularis gigantocellularis pars alpha in the rat. Neurosci Lett. 1991;131(2):205–209. doi: 10.1016/0304-3940(91)90614-y. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang H, Jin GZ, Zhang K, Zhen X. Single dose of morphine produced a prolonged effect on dopamine neuron activities. Mol Pain. 2008;4:57. doi: 10.1186/1744-8069-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]