Abstract
Sirtuin1 (SIRT1) deacetylase and poly(ADP-ribose)-polymerase-1 (PARP-1) respond to environmental cues, and both require NAD+ cofactor for their enzymatic activities. However, the functional link between environmental/oxidative stress-mediated activation of PARP-1 and SIRT1 through NAD+ cofactor availability is not known. We investigated whether NAD+ depletion by PARP-1 activation plays a role in environmental stimuli/oxidant-induced reduction in SIRT1 activity. Both H2O2 and cigarette smoke (CS) decreased intracellular NAD+ levels in vitro in lung epithelial cells and in vivo in lungs of mice exposed to CS. Pharmacological PARP-1 inhibition prevented oxidant-induced NAD+ loss and attenuated loss of SIRT1 activity. Oxidants decreased SIRT1 activity in lung epithelial cells; however increasing cellular NAD+ cofactor levels by PARP-1 inhibition or NAD+ precursors was unable to restore SIRT1 activity. SIRT1 was found to be carbonylated by CS, which was not reversed by PARP-1 inhibition or selective SIRT1 activator. Overall, these data suggest that environmental/oxidant stress-induced SIRT1 down-regulation and PARP-1 activation are independent events despite both enzymes sharing the same cofactor.
Keywords: SIRT1, oxidants, NAD+, PARP-1, cigarette smoke
INTRODUCTION
SIRT1 is a class III histone deacetylase important in inflammation, aging, senescence, and apoptosis. The role of SIRT1 in diseases where oxidative stress plays an important role, including cancer, type II diabetes, cardiovascular diseases, and chronic obstructive pulmonary disease (COPD), is currently being investigated [1]. SIRT1 transfers the acetyl group of substrate proteins to NAD+, generating two metabolites; 2′-O-acetyl-ADP-ribose and nicotinamide. Nicotinamide is an inhibitor of SIRT1 and precursor for NAD+ synthesis through the NAD+ salvage pathway. Activation of SIRT1 by its cofactor has been studied in caloric restriction and upregulation of the NAD+ salvage pathway [2, 3]. We and others have shown that SIRT1 activity is decreased in lungs of smokers and patients with COPD via oxidative/carbonyl stress-dependent mechanism [4–6]. However, it is not known whether the reduced activity of SIRT1 is due to decreased NAD+ level caused by oxidative stress and cigarette smoke (CS).
Oxidative/environmental stresses, such as cigarette smoke (CS), activates the NAD+-dependent enzyme poly(ADP-ribose)-polymerase-1 (PARP-1). PARP-1 is a nuclear protein involved in DNA repair, chromatin remodeling, and has co-repressor/co-activator activities. When activated, PARP-1 synthesizes poly(ADPribose) chains from NAD+, which non-covalently modifies proteins. Activation of PARP-1 can be cytoprotective or lead to necrosis by depleting NAD+ [9]. SIRT1 may be regulated by PARP-1 activation and subsequent NAD+ depletion in response to environmental/oxidative stresses, which may have subsequent effects on inflammatory mediators release, stress responses, and senescence via RelA/p65 and p53 regulation.
In this study, we hypothesized that increasing intracellular NAD+ levels through inhibition of PARP-1 would restore activity to SIRT1 in response to oxidative stress and CS exposure in lung epithelial cells. We tested this hypothesis by increasing cellular NAD+ levels through PARP-1 inhibitor and NAD+ precursors, and examined the interplay of PARP-1 and SIRT1 activity in response to oxidative stress.
MATERIALS AND METHODS
Reagents
Biochemical reagents used in this study were purchased from Sigma Chemicals Co., St. Louis, MO. Antibodies used: human SIRT1 (Cell Signaling Technology, Danvers, MA), PARP-1 (Cell Signaling Technology), pADPr (Santa Cruz Biotechnology, Santa Cruz, CA) for immunoblotting and human SIRT1 (Abcam, Cambridge, MA) for immunoprecipitation. Sirtinol and 3-aminobenzamide were purchased from EMD Chemicals, Merck KGaA (Darmstadt, Germany). SRT1720 was synthesized by Life Chemicals (Ottawa, Canada) with >95% purity measured by GC-mass spectrometry, as previously described [8]. FK866 was purchased from Cayman Chemical (Ann Arbor, MI).
Mouse cigarette smoke exposure
Eight- to ten-week-old male C57BL6/J mice (6–8 per group) mice (Jackson Laboratory, Bar Harbor, ME) were used in the study. All animal procedures were approved by the Committee on Animal Research of the University of Rochester. In brief, mice were exposed to CS using research-grade cigarettes (2R4F, University of Kentucky, Lexington, KY) at a concentration of 300 mg/m3 TPM for two 1 h CS exposures 1 h apart for 3 consecutive days or daily for 8 weeks [9]. Mice sacrificed by exsanguination 24 h after the last exposure. The lungs were removed en bloc and frozen for immunoblot analysis.
Protein extraction from lung tissue
One hundred milligrams of lung tissue was homogenized in 0.5 ml of ice-cold RIPA buffer as described previously [4,5,9].
Cell culture and treatments
Human bronchial epithelial cell line BEAS-2B were grown in DMEM-F12 (Mediatech, Manassas, VA). The cells (1% FBS) were treated with CSE (0.1% - 1.5%) or H2O2 (150 μM) for 6 or 24 h in the presence or absence of PARP-1 inhibitor 3-aminobenzamide (3-ABA, 1 mM) pre-treatment for 1 h at 37°C. Cells were also pre-treated for 1 h with SIRT1 inhibitor sirtinol (10 μM) or a specific and selective SIRT1 activator SRT1720 (5 μM) with 3-ABA (1 mM) prior to CSE or H2O2 treatment. Cells were also pre-treated for 1 h with NAD+ (1 mM), tryptophan (5 mM), nicotinamide (NAM; 1 mM), inhibitor of NAMPT, FK866 (10 nM) and nicotine (1 μM) followed by treatment with CSE (1%) for 6 and 24 h.
Cigarette smoke extract (CSE) preparation
Quantification of NAD+
NAD+ levels were measured using a commercial NAD+/NADH quantification kit according to the manufacturer’s instructions (BioVision, Mountain View, CA).
Immunoblot analysis
Protein levels were measured using the bicinchoninic acid kit (Pierce, Rockford, IL), and immunoblotting was performed as previously described [4,5].
SIRT1 Activity Assay
SIRT1 was immunoprecipitated from whole cell extracts and SIRT1 activity was assayed using a deacetylase colorimetric activity assay kit (Biomol International, Plymouth Meeting, PA).
Enzyme-linked immunosorbant assay (ELISA) for IL-8
The levels of IL-8 in the culture medium were determined by ELISA using the duo antibody kit from R&D Systems Inc. (Minneapolis, MN).
SIRT1 carbonylation assay
SIRT1 was immunoprecipitated and electrophoresed as described above. Membranes were first probed with anti-SIRT1 antibody and then derivitized with 2,4-dinitrophenylhydrazine, as described [10]. The membranes were incubated overnight with anti-dinitrophenyl antibody to determine SIRT1 carbonylation.
Statistical Analysis
The results are shown as the mean ± SEM. Statistical analysis of significance was calculated using one-way Analysis of Variance (ANOVA) by STATVIEW; p>0.05 considered non-significant.
RESULTS
Oxidative stress and cigarette smoke decrease cellular NAD+ levels through activation of PARP-1
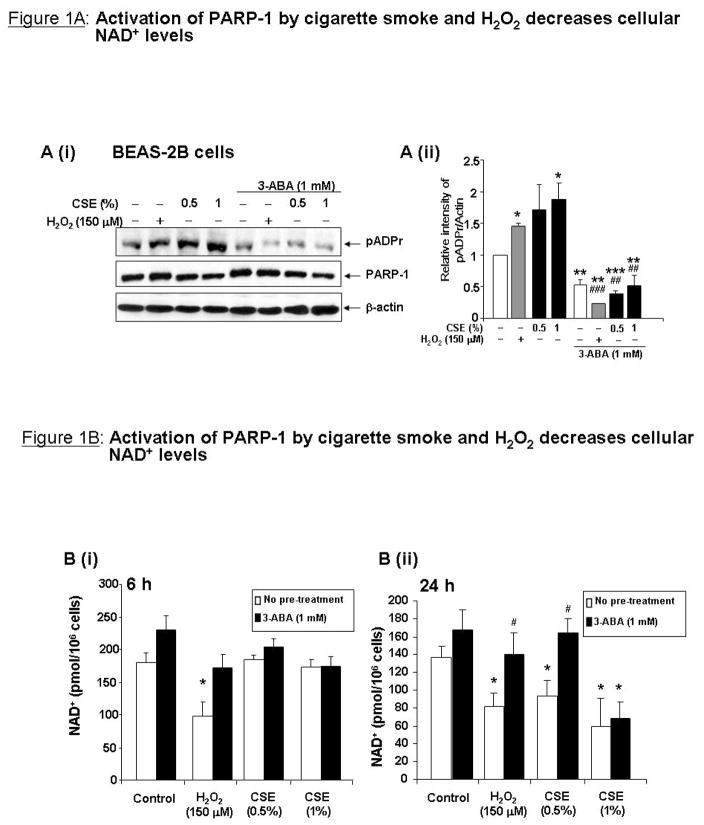
Human bronchial epithelial BEAS-2B cells were pre-treated with PARP-1 inhibitor 3-aminobenzamide (3-ABA; 1 mM) followed by H2O2 (150 μM) or CSE (0.5% and 1%) for 24 h. PARP-1 activation was assessed by immunoblotting poly(ADP-ribose) (pADPr) from whole cell extracts. H2O2 and CSE significantly increased pADPr formation, which was attenuated to basal levels by 3-ABA, indicating that PARP-1 was activated by these stimuli (Figure 1A). Next it was determined whether PARP-1 activation would lead to NAD+ depletion. NAD+ was extracted from BEAS-2B cells pre-treated with the 3-ABA (1 mM) for 1 h before CSE (0.5 and 1%) or H2O2 (150 μM) treatments for 1, 3, 6 or 24 h. H2O2 significantly decreased NAD+ levels at 1 and 3 h (data not shown), and at 6 and 24h, which were restored by 3-ABA (Figure 1B). Treatment with CSE (0.5 or 1%) significantly decreased cellular NAD+ levels only at 24 h, which was attenuated by 3-ABA for CSE (0.5%) treatment (Figure 1B). Total NAD nucleotides were decreased in response to H2O2 and CSE at 24 h, while NADH levels were unchanged (data not shown), suggesting that NAD+ was consumed rather than converted to NADH.
Figure 1. Activation of PARP-1 by cigarette smoke and H2O2 decreases cellular NAD+ levels.
A (i) Bronchial epithelial cells (BEAS-2B) were treated with H2O2 (150 μM) or CSE (0.5 and 1%) for 24 h with or without a 1 h pre-treatment with PARP-1 inhibitor 3-ABA (1 mM). Whole cell extracts were used for immunoblot analysis for PARP-1 and pADPr. Densitometry is shown in (ii). (B) NAD+ levels from BEAS-2B cell treated with CSE or H2O2 for 6 h (i) or 24 h (ii) were calculated using a NAD+/NADH quantification kit. Data are expressed in pmol of NAD+/106 cells. (C) Whole lung homogenates from C57BL6/J mice exposed to CS for 3 days (i) or 8 weeks (ii) were used for immunoblot analysis for PARP-1 and pADPr. (D) NAD+ levels from mouse lung homogenates were measured as in (B). Data are expressed in pmol/mg protein. All data are representative of 3 separate experiments. *p<0.05, **p<0.01 relative to control and #p<0.05 relative to corresponding treatment of H2O2 or CSE.
PARP-1 activation and NAD+ depletion by CS was also observed in vivo in mouse lung. C57BL6/J mice exposed to CS for 3 days or 8 weeks showed increased levels of pADPr as compared to air-exposed mice in whole lung homogenates (Figure 1C). CS exposure for 8 weeks, but not for 3 days, significantly decreased cellular NAD+ levels as compared to air exposed mice (Figure 1D).
Inhibition of PARP-1 attenuates loss of SIRT1 activity in response to H2O2, but not CSE
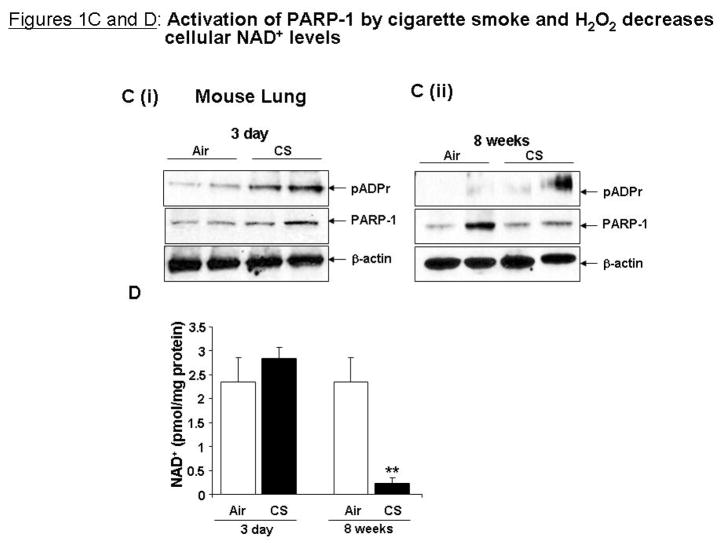
As NAD+ levels were restored through inhibition of PARP-1, it was next investigated whether SIRT1 activity could also be restored in response to oxidative/CS stress by PARP-1 inhibition. BEAS-2B cells were pre-treated for 1 h with 3-ABA (1 mM) before treatment with H2O2 (150 μM) or CSE (0.5 and 1%) for 24 h, treatment conditions where 3-ABA could rescue NAD+ levels. SIRT1 protein levels were significantly decreased in response to CSE in BEAS-2B cells; however H2O2 did not decrease SIRT1 protein levels in BEAS-2B cells (Figure 2A). 3-ABA did not affect SIRT1 protein levels in response to H2O2 or CSE, implying that NAD+ levels do not affect SIRT1 protein levels. SIRT1 activity was assessed by measuring levels of acetylation of p53 on the K382 residue. CSE and H2O2 significantly increased acetylation on p53 on K382, which was not changed by 3-ABA (Figure 2A). H2O2 and CSE significantly decreased intrinsic SIRT1 deacetylase activity at 24 h (Figure 2B). 3-ABA prevented loss of SIRT1 activity in response to H2O2, but not in response to CSE. These data suggest that PARP-1 inhibition does not affect SIRT1 intrinsic activity in response to CS, and that other mechanisms may be involved in CS-induced decrease in SIRT1 activity. CSE also activated PARP-1 and reduced SIRT1 activity in human umbilical vein endothelial cells (HUVEC) and human monocyte/macrophage cell line (MonoMac6) (data not shown), showing that these phenomena are not cell-specific.
Figure 2. PARP-1 inhibition increases SIRT1 activity in response to H2O2 but not in response to CSE.
(A) BEAS-2B cells were pre-treatment with 3-ABA (1 mM) for 1 h then treated with H2O2 (150 μM) or CSE (0.5 and 1%) for 24 h (n=3). (B) SIRT1 deacetylase activity was determined. (C) Cell culture media was used for IL-8 assay by ELISA. Cells were pre-treated with the combination of 3-ABA (1 mM) and sirtinol (10 μM) for 1 h. All data are representative of 3 separate experiments. *p<0.05, **p<0.01 and ***p<0.001 relative to control and ##p<0.05 and ###p<0.001 relative to corresponding treatment of CSE or H2O2.
Since SIRT1 deacetylates the RelA/p65 subunit of NF-κB and regulates pro-inflammatory cytokine release in response to CSE [4] and PARP-1 is a co-activator for NF-κB-dependent gene transcription in response to inflammatory stimuli [11], the effect of PARP-1 inhibition on CSE-dependent pro-inflammatory release was investigated. Interleukin-8 (IL-8) was measured in cell culture media from cells treated with 3-ABA (1 mM) for 1 h and CSE (0.5 and 1%) or H2O2 (150 μM) for 24 h. TNF-α (10 ng/ml) treatment for 24 h was used as a positive control for IL-8 release. CSE dose-dependently increased IL-8 release, which was blocked by 3-ABA (Figure 2C). To determine whether the effect of 3-ABA on CS-induced cytokine release was due to inhibition of PARP-1 activity or increased SIRT1 activity, IL-8 release was measured from cells pre-treated for 1 h with 3-ABA (1 mM) and SIRT1 inhibitor sirtinol (10 μM) and treated with H2O2, TNF-α, or CSE for 24 h. 3-ABA and sirtinol significantly decreased cytokine release in response to CSE (0.5 and 1%), however there was no difference in IL-8 release from cells pre-treated with 3-ABA or sirtinol + 3-ABA (Figure 2C). These data suggest that the decreased cytokine release resulting from 3-ABA pre-treatment was due to inhibition of PARP-1, not increased SIRT1 activity.
Altering cellular NAD+ levels does not alleviate decreased SIRT1 activity in response to CSE
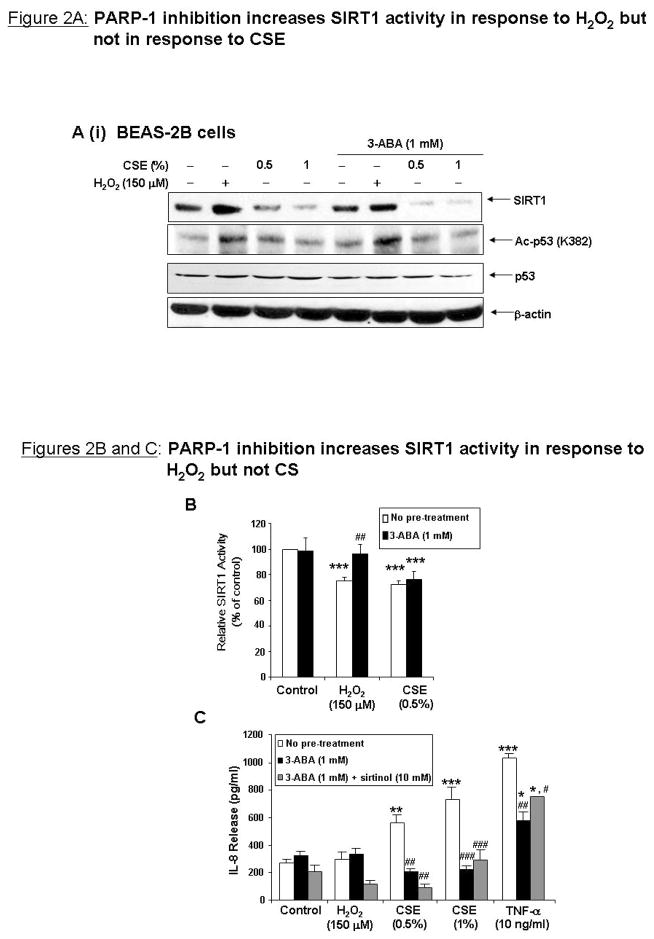
Since CS decreased cellular NAD+ levels, it was next investigated whether increasing the cellular NAD+ content could prevent oxidant-induced loss of SIRT1 activity. BEAS-2B cells were treated for 6 or 24 h with NAD+ (1 mM), L-tryptophan (Trp, 5 mM), precursor of de novo NAD+ biosynthesis and nicotinamide (NAM; 1 mM), an endogenous inhibitor of SIRT1 and precursor for the NAD+ salvage pathway. Cells were treated with an inhibitor of the NAD+ salvage pathway, FK866 (10 nM), as a negative control. NAD+ and Trp increased cellular NAD+ levels after 6 h of treatment, however by 24 h, NAD+ levels were similar to control treated cells (Figure 3A), suggesting that the earlier time-point was most effective. BEAS-2B cells were pre-treated for 1 h with NAD+, NAM, Trp, or FK866 and treated with CSE (1%) for 6 h, or with nicotine (1 μM) for 6 h. SIRT1 levels were decreased by CSE, and were not restored by raising cellular NAD+ levels (Figure 3B). Nicotine, which is structurally similar to NAD+ and present in CS, did not decrease SIRT1 levels, suggesting that the complex mixture of reactive aldehydes and oxidants present or derived from CS are responsible for SIRT1 down-regulation. NAD+, Trp, and NAM all increased basal levels of pADPr, possibly by providing increased substrate for PARP-1 (Figure 3B). The level of pADPr was modestly increased by CSE; however NAD+, Trp, or NAM did not decrease pADPr levels. PARP-1 levels did not change with either of the treatments in response to CSE. Overall, these data suggest PARP-1 is activated by CSE even when intracellular NAD+ levels are elevated by exogenous NAD+ or NAD+ precursors.
Figure 3. Increasing cellular NAD+ levels does not restore SIRT1 activity in response to CS.
BEAS-2B cells were pre-treated for 1 h with NAD+ (1 mM), tryptophan (5 mM), nicotinamide (NAM; 1 mM), inhibitor of nicotinamide phosphoribosyltransferase FK866 (10 nM) and nicotine (1 μM) before treatment with CSE (1%) for 6 and 24 h (n=3). (A) NAD+ levels were measured. Data is presented as fold increase NAD+ as compared to control. (B) Immunoblot analysis for pADPr, PARP-1, and SIRT1. (C) SIRT1 deacetylase activity was determined. *p<0.05, **p<0.01 and ***p<0.001 relative to control and ##p<0.05 and ###p<0.001 relative to corresponding treatment of CSE.
CSE significantly decreased SIRT1 activity; however there was no significant difference between cells treated with CSE with or without NAD+ or Trp pre-treatment (Figure 3C). Treatment of NAM or FK866 decreased SIRT1 activity as NAM is an endogenous inhibitor of SIRT1 and FK866 depletes NAD+ levels; although SIRT1 activity was not further decreased by these agents in combination with CSE. Altogether these data suggest that loss of SIRT1 activity by CSE is not entirely due to NAD+ depletion, but by mechanisms independent of the cells’ metabolic activity.
SIRT1 activators or PARP-1 inhibitors do not restore SIRT1 activity in response to CSE as SIRT1 is covalently modified via carbonylation
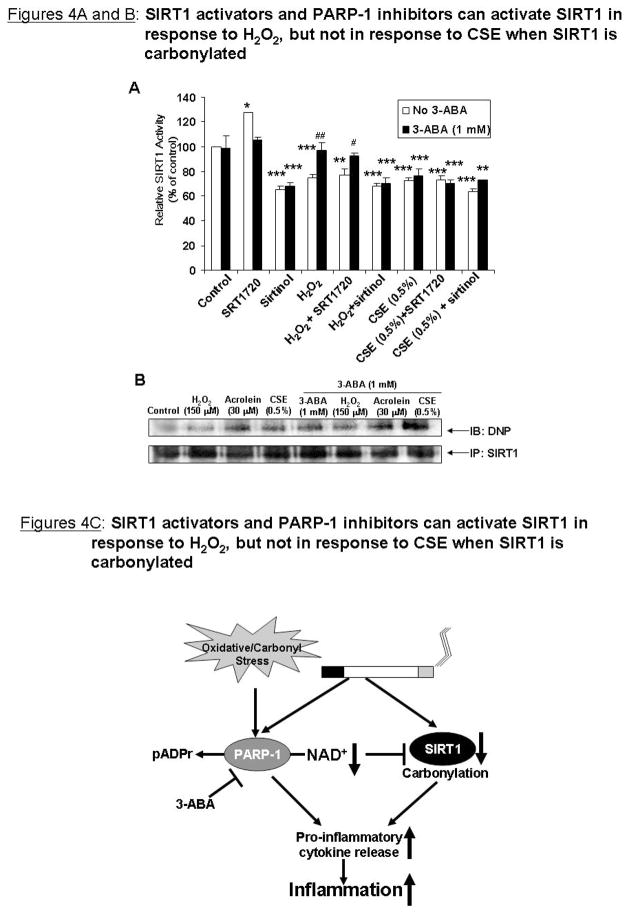
Bronchial epithelial cells were pre-treated for 1 h with 3-ABA (1 mM) in combination with SRT1720 (5 μM), a specific and potent SIRT1 activator [8], and then treated with H2O2 (150 μM) or CSE (0.5 %) for 24 h. Cells pre-treated with sirtinol (10 μM) were used as a negative control. SRT1720 increased SIRT1 activity whereas sirtinol decreased SIRT1 activity (Figure 4A). H2O2 decreased SIRT1 activity, which was restored by 3-ABA. SRT1720 only restored SIRT1 activity in response to H2O2 in combination with 3-ABA, but not alone. CSE decreased SIRT1 activity, but was not restored by SRT1720, 3-ABA, or SRT1720 + 3-ABA, implying that even with a specific activator, increasing cofactor levels can not restore SIRT1 activity in response to CSE.
Figure 4. SIRT1 activators and PARP-1 inhibitors can not activate SIRT1 in response to CSE when SIRT1 is carbonylated.
(A) BEAS-2B cells were pre-treated for 1 h with 3-ABA (1mM) with or without SIRT1 activator SRT1720 (5 μM) or sirtinol (10 μM) before treatment with CSE (0.5%) and H2O2 (150 μM). SIRT1 deacetylase activity was measured. (B) SIRT1 was immunoprecipitated from cells pre-treated with 3-ABA (1 mM) for 1 h before acrolein (30 μM), H2O2 (150 μM), and CSE (0.5%) treatment for 24 h. 100 μg of immunoprecipitated SIRT1 protein was used for immunoblotting (IB). Carbonylation was detected by derivitizing the samples with 2,4-dinitrophenylhydrazine and immunoblotting with an anti-dinitrophenyl (DNP) antibody. All data are representative of 3 separate experiments. *p<0.05, **p<0.01, ***p<0.001 relative to control, and #p<0.05, ##<0.01 relative to H2O2 treatment. (C) Schematic for oxidant-induced activation of PARP-1 and inhibition of SIRT1.
It has been previously shown that oxidative stress increases covalent carbonyl modifications on SIRT1 [4] and that carbonylation can inhibit protein functions [12]. SIRT1 was immunoprecipitated from BEAS-2B cells treated with 3-ABA (1 mM) and H2O2 (150 μM), CSE (0.5%), or acrolein (30 μM) for 24 h. Total carbonylation on SIRT1 was measured by derivitizing immunoprecipitated SIRT1 with 2,4-dinitrophenylhydrazine. Acrolein and CSE increased carbonylation on SIRT1 (Figure 4B). Pre-treatment with 3-ABA had no effect on carbonylation of SIRT1 in response to acrolein or CSE, suggesting that the presence of these covalent modifications occur despite cofactor availability. H2O2 slightly increased carbonylation on SIRT1, which may explain why SRT1720 and 3-ABA could restore SIRT1 activity.
DISCUSSION
As both SIRT1 and PARP-1 are nuclear enzymes that use the same nuclear NAD+ cofactor pool, and both respond to similar stimuli in cellular functions [13], the purpose of this study was to determine whether oxidants/CS activation of PARP-1, depletes NAD+ and inhibits SIRT1, and whether inhibiting PARP-1 would restore SIRT1 activity in response to oxidative/CS stress in lung epithelial cells as these cells are first target of environmental inhaled agents.
SIRT1 is emerging as a major therapeutic target, therefore understanding its modulation by environmental/oxidative stress is crucial. CS is a complex mixture of different chemical compounds and oxidants/free radicals, and has been shown to activate PARP-1 and down-regulate SIRT1 protein level and activity [4, 5, 14, 15]. Our data show that NAD+ levels were time-dependently decreased by CSE in epithelial cells in vitro. NAD+ depletion by PARP-1 activation can lead to senescence or cell death, which can also be induced by oxidants and CS [16,17]. NAD+ levels were not restored by PARP-1 inhibition in response to CSE, suggesting that CSE may inhibit NAD+ biosynthesis. Alternatively, CS inactivates AMP-activated protein kinase (AMPK), a metabolic sensor that increases NAD+ levels [18, 19]. Inhibition of AMPK by CS would explain why 3-ABA did not restore NAD+ levels in response to high concentration of CSE. PARP-1 activation was also observed in vivo in lungs of mice exposed to CS for 3 days and 8 weeks. CS exposed mouse lung also showed time-dependent decrease in NAD+ levels, with significant loss of NAD+ at 8 weeks. Whether NAD+ levels remain depleted in vivo in long-term exposures remains to be determined.
In this study SIRT1 activity was assessed by two measures: acetylation of p53 and intrinsic deacetylase activity, both of these showed no increase of SIRT1 activity in response to CSE and PARP-1 inhibition. However, PARP-1 inhibition was able to attenuate H2O2-induced reduction in SIRT1 intrinsic activity. Our findings are supported by a recent study where a similar observation was shown in transformed U937 cells treated with high H2O2 concentration and 3-ABA [6]. It is known that caloric restriction increases SIRT1 activity by up-regulating SIRT1 levels and skewing the NAD+/NADH ratio to higher NAD+ levels and decreases NAM [3]. However, elevation of cellular NAD+ levels by exogenous NAD+ or NAD+ precursors had no effect on CSE-induced reduction in SIRT1 activity. It has also been shown that enhancing the NAD+ salvage pathway to activate SIRT1 enhances replicative lifespan in vascular smooth muscle cells [20]. To activate the NAD+ salvage pathway, epithelial cells were pre-treated with NAM before CS treatment. NAM significantly raised cellular NAD+ levels; however, SIRT1 activity was decreased as it is a well known inhibitor of SIRT1 [21]. Furthermore, pre-treatment with the NAMPT inhibitor FK866 decreased SIRT1 activity. Nicotine, a component of CS which is structurally similar to NAD+ had no effect on SIRT1 activity. All of these effects on SIRT1 activity were observed while PARP-1 was active as levels of pADPr were seen to increase with CSE treatment and no NAD+ depletion was observed at early time points. Hence these data implicates that CSE decreases SIRT1 activity even during conditions of excess NAD+ cofactor.
Increasing evidence has shown PARP-1 to be a modulator of inflammation. PARP-1 knockout mice show protection from inflammatory disease models including LPS-induced septic shock and myocardial infarction [22]. In our study, PARP-1 inhibition was shown to decrease pro-inflammatory cytokine release in response to CSE and TNF-α in epithelial cells, implicating PARP-1 as a key regulator of CSE-mediated NF-κB activation. Acetylation of PARP-1 enhances its co-activation ability and that SIRT1 deacetylates PARP-1, decreasing its activity [23]. It is possible that CS down-regulates SIRT1, increasing acetylation of PARP-1, leading to increased co-activation of NF-κB and enhanced inflammation. Use of a specific SIRT1 activator, SRT1720, could not prevent loss of SIRT1 activity in response to H2O2 or CSE alone, but in combination with 3-ABA, SRT1720 prevented loss of SIRT1 activity in response to H2O2. One possible explanation was that CSE had post-translationally modified SIRT1 such that SRT1720 and 3-ABA had no effect on it. Carbonylation is an irreversible covalent modification on proteins which can affect protein function and stability [12]. CSE and acrolein, an alkylating agent found in CS, both increased carbonylation on SIRT1. SIRT1 was also carbonylated in cells pre-treated with 3-ABA and treated with CSE or acrolein. As H2O2 showed less carbonylation on SIRT1 than acrolein or CSE, these data suggest that when SIRT1 is carbonylated it is not responsive to the increased NAD+ from PARP-1 inhibition.
Altogether our data shows that oxidative stress and CS simultaneously activates PARP-1 and decreases SIRT1 activity, leading to increased cytokine release (Figure 4C). Modulation of SIRT1 activity has many clinical applications including COPD, type II diabetes, cardiovascular diseases and cancer where oxidative stress occurs [1], therefore further characterization of oxidants/aldehyde/CS-induced down-regulation of SIRT1 is critical for development of new therapeutics. Our data suggests that PARP-1 inhibitor and SIRT1 activator may not be effective therapeutics in conditions where oxidative/carbonyl stress occurs unless oxidative post-translational modifications are reversed or prevented.
Acknowledgments
This work was supported by NIH R01-HL085613, 1R01HL097751-01, NIEHS ES01247 and T32-ES07026.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zeng L, Chen R, Liang F, Tsuchiya H, Murai H, Nakahashi T, Iwai K, Takahashi T, Kanda T, Morimoto S. Silent information regulator, sirtuin 1, and age-related diseases. Geriatr Gerontol Int. 2009;9:7–15. doi: 10.1111/j.1447-0594.2008.00504.x. [DOI] [PubMed] [Google Scholar]
- 2.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 3.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: Understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 4.Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:861–870. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: Implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol. 2007;292:L567–76. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- 6.Nakamaru Y, Vuppusetty C, Wada H, Milne JC, Ito M, Rossios C, Elliot M, Hogg J, Kharitonov S, Goto H, Bemis JE, Elliott P, Barnes PJ, Ito K. A protein deacetylase SIRT1 is a negative regulator of metalloproteinase-9. FASEB J. 2009;23:2810–2819. doi: 10.1096/fj.08-125468. [DOI] [PubMed] [Google Scholar]
- 7.Huang Q, Shen HM. To die or to live: The dual role of poly(ADP-ribose) polymerase-1 in autophagy and necrosis under oxidative stress and DNA damage. Autophagy. 2009;5:273–276. doi: 10.4161/auto.5.2.7640. [DOI] [PubMed] [Google Scholar]
- 8.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao H, Edirisinghe I, Rajendrasozhan S, Yang SR, Caito S, Adenuga D, Rahman I. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1174–86. doi: 10.1152/ajplung.00439.2007. [DOI] [PubMed] [Google Scholar]
- 10.Murtaza I, Wang HX, Feng X, Alenina N, Bader M, Prabhakar BS, Li PF. Down-regulation of catalase and oxidative modification of protein kinase CK2 lead to the failure of apoptosis repressor with caspase recruitment domain to inhibit cardiomyocyte hypertrophy. J Biol Chem. 2008;283:5996–6004. doi: 10.1074/jbc.M706466200. [DOI] [PubMed] [Google Scholar]
- 11.Hassa PO, Buerki C, Lombardi C, Imhof R, Hottiger MO. Transcriptional coactivation of nuclear factor-kappaB-dependent gene expression by p300 is regulated by poly(ADP)-ribose polymerase-1. J Biol Chem. 2003;278:45145–45153. doi: 10.1074/jbc.M307957200. [DOI] [PubMed] [Google Scholar]
- 12.Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA. Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem. 2008;283:21837–21841. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolthur-Seetharam U, Dantzer F, McBurney MW, de Murcia G, Sassone-Corsi P. Control of AIF-mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell Cycle. 2006;5:873–877. doi: 10.4161/cc.5.8.2690. [DOI] [PubMed] [Google Scholar]
- 14.Kamp DW, Srinivasan M, Weitzman SA. Cigarette smoke and asbestos activate poly-ADP-ribose polymerase in alveolar epithelial cells. J Investig Med. 2001;49:68–76. doi: 10.2310/6650.2001.34092. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Conner H, Kobayashi T, Kim H, Wen F, Abe S, Fang Q, Wang X, Hashimoto M, Bitterman P, Rennard SI. Cigarette smoke extract induces DNA damage but not apoptosis in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2005;33:121–129. doi: 10.1165/rcmb.2003-0341OC. [DOI] [PubMed] [Google Scholar]
- 16.Hageman GJ, Larik I, Pennings HJ, Haenen GR, Wouters EF, Bast A. Systemic poly(ADP-ribose) polymerase-1 activation, chronic inflammation, and oxidative stress in COPD patients. Free Radic Biol Med. 2003;35:140–148. doi: 10.1016/s0891-5849(03)00237-5. [DOI] [PubMed] [Google Scholar]
- 17.Aoshiba K, Tamaoki J, Nagai A. Acute cigarette smoke exposure induces apoptosis of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1392–401. doi: 10.1152/ajplung.2001.281.6.L1392. [DOI] [PubMed] [Google Scholar]
- 18.Yuan H, Shyy JY, Martins-Green M. Second-hand smoke stimulates lipid accumulation in the liver by modulating AMPK and SREBP-1. J Hepatol. 2009;51:535–547. doi: 10.1016/j.jhep.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho C, van der Veer E, Akawi O, Pickering JG. SIRT1 markedly extends replicative lifespan if the NAD+ salvage pathway is enhanced. FEBS Lett. 2009;583:3081–3085. doi: 10.1016/j.febslet.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 21.Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson MP. Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromolecular Med. 2009;11:28–42. doi: 10.1007/s12017-009-8058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassa PO, Hottiger MO. The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front Biosci. 2008;13:3046–3082. doi: 10.2741/2909. [DOI] [PubMed] [Google Scholar]
- 23.Rajamohan SB, Pillai VB, Gupta M, Sundaresan NR, Konstatin B, Samant S, Hottiger MO, Gupta MP. SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly (ADP-ribose) polymerase 1. Mol Cell Biol. 2009;29:4116–4129. doi: 10.1128/MCB.00121-09. [DOI] [PMC free article] [PubMed] [Google Scholar]