Summary
Background
The hypothalamic-pituitary adrenal (HPA) axis is critical for biobehavioral adaptation to challenge and appears dysregulated in a range of psychiatric disorders. Its precise role in psychopathology remains unclear and discrepant and difficult to explain findings abound in the clinical literature. Basic research suggests this system is sensitive to psychosocial cues, but psychosocial milieu factors are rarely controlled or examined in psychiatric studies using biological probes of the HPA axis. To test the hypothesis that psychological factors might complicate HPA study results even in direct, pharmacological challenge paradigms, endocrine responses to corticotropin-releasing hormone (CRH) were examined under two different cognitive preparation conditions.
Methods
Healthy subjects (n=32) received standard instructions or a cognitive intervention (CI) prior to injection with CRH and placebo, given on separate days in random order. The CI combined access to control over drug exposure with novelty reduction and coping enhancement. Blood samples were obtained via intravenous catheter before and after CRH.
Results
Cognitive intervention reduced corticotropin (ACTH) levels, but only when CRH was given first (intervention by order interaction). It did not reduce cortisol response. The CI and visit (1st or 2nd) both impacted cortisol levels on placebo day.
Conclusions
Modifiable psychological factors may amplify or inhibit HPA axis activity in pharmacological activation paradigms, including CRH stimulation tests. The factors manipulated by the CI (novelty/familiarity, control and coping) may have particular salience to the HPA axis. Differential sensitivity to such factors could impact results in studies applying biological HPA probes to psychiatric populations.
Keywords: stress; cortisol; ACTH, corticotropin-releasing hormone; control; coping
Introduction
The hypothalamic-pituitary adrenal (HPA) stress axis has been studied for decades, as a central mediator of an organism’s capacity to adapt to environmental challenge (McEwen et al., 1997) and a candidate pathophysiological factor in the development or expression of stress-related psychiatric disorders (Yehuda, 2002; Young et al., 1994). Our understanding of stress neurobiology has evolved tremendously, from Selye’s description of a “general adaptation syndrome” (Selye, 1936) to our current understanding of a highly flexible and adaptive system that is critical to survival in the face of overwhelming stress, but also critical to fine tuning of adaptive responses to nuanced changes in environmental demands (McEwen, 2002). The HPA axis is shaped by complex interactions between genetic and environmental/developmental factors (Heim et al., 2008) and it in turns shapes an organism’s behavioral adaptive capacities throughout adult life. HPA abnormalities have been demonstrated in patients with depression, anxiety disorders, psychosis, and personality disorders (Abelson and Curtis, 1996; Carrasco et al., 2007; Heuser et al., 1994; Lammers et al., 1995), and alterations within this system are implicated in the etiology of depression and PTSD (Yehuda, 2002; Young et al., 1994). Understanding of the HPA axis is clearly important in efforts to understand both psychopathology and adaptive functioning.
Basic research has demonstrated that HPA axis activation in the face of overwhelming challenge, direct physical threat, or novel threat exposures occurs via direct connections from peripheral sensory inputs through thalamus to hypothalamus (Herman et al., 2003). Activation is also modulated by complex processing of indirect inputs through limbic-associated channels, which allow activation by psychological ‘anticipatory’ signals that predict exposure to threat (Herman et al., 2003). Learning shapes system responsivity through these indirect inputs, providing pathways for modulation by prior experience, access to safety or coping responses, or social support (Herman et al., 2003; Levine, 2000). Human research has shown that direct homeostatic threat, such as that associated with surgery, is a highly potent HPA activator (Gholami et al., 2004). In the psychosocial realm, lack of control over potential challenges and social evaluative threat appear to be particularly salient to HPA axis activation, whereas level of subjective distress itself is not (Dickerson and Kemeny, 2004).
The psychiatric HPA literature has evolved somewhat independently of this basic work, growing out of the original demonstration of cortisol escape from dexamethasone suppression in patients with endogenous depression (Carroll et al., 1981). This generated hope for a biological test for depression as a disease state and led to on ongoing search for specific, brain-based dysregulations that might contribute to HPA dysfunction and play pathophysiological roles in psychiatric disorders. Increasingly sophisticated biological probes, including corticotropin releasing hormone (CRH) stimulation tests, combined CRH/dexamethasone tests, ACTH stimulation, and metyrapone have been used to more precisely dissect the nature of HPA axis dysregulation in psychiatric disorders and identify abnormalities that might contribute to disease etiology or course (Heuser et al., 1994; Holsboer, 2001). This literature has generally assumed that the biological probes utilized tap into fundamental dysregulations within the HPA axis itself (Heim et al., 2008).
HPA axis activity in healthy humans, however, is sensitive to psychosocial cues, which raises the possibility that unrecognized but salient environmental aspects of experimental paradigms could influence responses to biological probes in studies of psychiatric patients. If so, then reputed HPA axis abnormalities could actually reflect differential sensitivity to contextual cues rather than to the specific probes utilized. It is possible that laboratory measured responses even to direct probes like a CRH stimulation test, could be influenced by individual differences in reactivity to the environment or psychosocial context even in the absence of CRH receptor sensitivity differences. Abnormalities detected in psychiatric disorders could then be due to disorder-related dysregulation in extra-hypothalamic modulatory circuits rather than to abnormalities within primary structures of the HPA axis. We have consistently demonstrated HPA axis abnormalities in patients with panic disorder, for example (Abelson and Curtis, 1996; Abelson et al., 1994; Abelson et al., 1996; Curtis et al., 1997), but our data suggest to us that these abnormalities may be due to reactivity to the testing paradigm rather than to altered basal activity within the HPA axis or altered sensitivity to specific biological probes.
We have recently replicated our finding that manipulation of cognitive expectancies – shifting psychological experience of study context – can alter HPA axis reactivity to pharmacological probes (Abelson et al., 2008). Utilizing the same cognitive manipulation in both panic patients and in healthy controls, we have been able to alter cortisol responses to two different pharmacological challenges – one that stimulates respiration but does not directly impact HPA axis activity (doxapram, (Abelson et al., 1996)) and one that directly stimulates HPA activity through pituitary receptors (pentagastrin (Abelson and Liberzon, 1999)). We therefore wondered whether HPA reactivity was similarly subject to cognitive modulation in models more commonly used to identify HPA dysregulation in psychiatric patients. If so, then disorder-based differences in the cognitive expectancies brought into the experimental context could be the source of apparent HPA axis abnormalities. If this is indeed the case, then the search for neurobiological mediators of these abnormalities might have to be considerably broadened beyond the classical HPA axis neurocircuitry, to include upstream cortical structures. Here we report the impact of a cognitive intervention on ACTH and cortisol responses to CRH – an extensively used HPA probe that, when given at low doses, produces cortisol responses comparable to those produced by pentagastrin, through a similar mechanism (direct stimulation at the pituitary).
Methods and Materials
Subjects
Thirty-three healthy, normal weight (+30%/−10% of ideal body weight), 18 to 45 year old subjects were recruited by advertisement. They were non-smokers without history of substance dependence or recent (6 months) abuse, not pregnant or lactating, with negative urine drug screens, no exposure (past month) to psychoactive medication, limited alcohol use (<8 drinks/week, mean = 1.5), no clinically significant abnormalities on screening laboratory tests, and no history of psychiatric disorder (per SCID-IV). Females were pre-menopausal, not using birth control pills, and studied within 10 days of menstruation onset. The study was IRB approved and subjects provided written, informed consent.
Design and Procedures
Subjects were admitted twice to a General Clinical Research Center (GCRC), receiving intravenous injection of placebo on one visit and ovine corticotropin releasing hormone (CRH) on the other (1 to 10 days apart). Subjects were told they might receive either substance on both visits. They were randomly assigned to instruction conditions (Cognitive Intervention or Standard Instructions) and order of drug administration, with constraint to insure equal sex distribution across groups. Subjects and nurses were blind to instruction group and drug.
Instructions were administered identically on both days at 1300h. An intravenous catheter was inserted into an antecubital vein at ~1330h. Subjects rested for 1.5h, reading or doing homework, to accommodate to the setting. Baseline blood samples were obtained at 1500h and 1520h. At 1520h, the investigator entered the room briefly, turning on a bedside infusion pump and, for subjects in the Cognitive Intervention (CI) group, a light on top (see below). He returned at 1530h to inject (out of the subject’s awareness, over 10 seconds) the placebo (0.9% saline) or CRH (0.3 mcg/kg; Ferring, Parsippany, NJ). CRH was prepared 1h before injection and refrigerated until used. Blood was drawn into iced vacuum tubes 5, 10, 15, 30, 60, and 120min after drug administration, spun in a refrigerated centrifuge within 5m, separated and frozen (−70°C). Physical and emotional symptoms were recorded using an Acute Panic Inventory and visual analog scales.
Instructions
The investigator administered instructions twice (on both placebo and CRH days) via a 5min audio tape for standard instructions or a 9min tape and 3min discussion for the CI. The standard instructions (SI) described apparatus and procedures, listed side effects of CRH, and disclosed risks (Abelson et al., 2005). Subjects were told drug would be delivered while the bedside infusion pump was on (between 1520h and 1540h). An indicator light attached to the pump informed subjects whether they had permission to utilize pump controls to slow down or stop the infusion. They were asked not to do so unless really necessary, but assured that if the light was lit, this decision was in their hands. If the light was off, they were told they did not have this option. For SI subjects the indicator light remained off; for CI subjects it was turned on. CI subjects also received (a) more detailed description of expectable responses to all aspects of the research experience (e.g., sensations associated with IV insertion or intravenous fluid flow) and to the CRH (to reduce novelty) and (b) coaching to attribute responses (physical and emotional) to normal reactions to CRH and study conditions rather than anything dangerous, unexpected or unusual (to facilitate cognitive coping).
Measures and assays
Baseline measures included: Beck Depression Inventory (BDI), Anxiety Sensitivity Index (ASI), an ego resilience scale, and Spielberger State/Trait Anxiety Inventory (STAI). Symptoms, emotions and cognitions were recorded with each blood sample using an acute panic inventory (API) and visual analog scales (VAS). The API measured DSM-IV symptoms of panic on a 4-point scale, primarily capturing physical side effects (e.g., flushing, sweating, shortness of breath, racing heart). The VAS measured emotions or cognitions on 100-mm visual analog lines (“not at all” to “most ever”). Primary dependent variables were symptom intensity (sum of API symptom ratings) and subjective anxious distress (sum of VAS ratings of “anxious,” “nervous,” and “fearful” minus a rating of “calm”). Separate VAS items measured perceived dangerousness of CRH, novelty of the experience, supportiveness of staff, and sense of control (Abelson et al., 2005). Cortisol was assayed using the Coat-A-Count assay and ACTH using an Immulite Chemoluminescent system from Diagnostic Products Corporation (Los Angeles). Sensitivities were 1 µg/dL for cortisol and 10 pg/ml for ACTH. Coefficients of variation were 5 to 9 %. Equal numbers of subjects from each group were included in all assay runs.
Analyses
Symptom intensity and anxious distress levels were identical across groups at baseline and essentially zero. To determine whether CRH produced side effects that could differentiate it from placebo, we calculated a maximum symptom response to each injection (maximum post-injection value minus mean of two pre-injection measures). We analyzed these in repeated measures analyses of variance (ANOVAs) with Drug (CRH vs. Placebo) as a within subject measure and Instructions (CI vs. SI) and Order (Placebo 1st versus CRH 1st) as between subject measures.
We already knew that ACTH and cortisol would decline over placebo day and rise dramatically following CRH, so hormone analyses focused on the impact of Instructions and Order of drug administration within each experimental day. We ran separate repeated measure ANOVAs for placebo and CRH days, with Time (hormone levels from 30min before to 2h after injection) as a within subjects variable, and Instructions (CI vs. SI) and Order (Placebo 1st vs. CRH 1st) as between subject variables. Analyses of raw data are presented; results were essentially identical with log transformation.
For additional analyses we calculated cortisol and ACTH area under the curve (AUC), using trapezoidal approximation, examining both total secretion (AUC from first to last sample) and AUC response (AUC of post-injection levels corrected for mean baseline).
Results
Preliminary analyses
One female subject was dropped from the SI/CRH first group (incomplete delivery of CRH due to IV problem). Groups did not differ in gender, age, weight, CRH dose, or psychometrically measured depression, anxiety, anxiety sensitivity or recent subjective stress (Table 1). They also did not differ in weekly exercise, 6 month anxiety rating, or ego resilience (p>.65, data not shown). Males and females did not differ on any hormonal measures on either placebo or CRH infusion days (repeated measures ANOVAs, p values for sex main effects were .15 to .72, p values for sex-by-time interactions were .16 to .92). There were no sex differences in anxious distress or symptom responses to CRH or placebo (p values from .17 to .94). No subject with control chose to slow or stop the infusion.
Table 1.
Demographic and baseline data for the four experimental groups.
| Placebo First | CRH First | ||||
|---|---|---|---|---|---|
| Standard Instruction | Cognitive Intervention | Standard Instruction | Cognitive Intervention | p | |
| n | 9 | 8 | 7 | 8 | |
| Sex (F/M) | 5/4 | 3/5 | 2/5 | 4/4 | .70 |
| Age (years) | 22.9 (4.0) | 23.5 (4.0) | 23.0 (4.6) | 22.3 (7.0) | .97 |
| Weight (kg) | 69.6 (15.1) | 70.8 (12.2) | 73.9 (4.5) | 71.5 (11.6) | .91 |
| CRH Dose (mcg) | 20.9 (4.5) | 21.2 (3.7) | 22.1 (1.4) | 21.4 (3.5) | .91 |
| Beck Depression | 1.0 (1.2) | 1.9 (1.8) | 0.30 (0.76) | 1.3 (1.6) | .23 |
| Trait Anxiety | 26.7 (5.0) | 27.6 (5.3) | 23.6 (2.1) | 28.4 (5.9) | .27 |
| ASI | 12.1 (5.8) | 13.3 (9.7) | 10.1 (5.1) | 16.3 (8.5) | .47 |
| Stress | 3.7 (1.4) | 5.3 (1.5) | 4.9 (1.3) | 4.3 (1.8) | .19 |
Numbers represent group means (±SD), except for “n”, and “sex”, where number of subjects is presented. F = females, M = males; CRH = corticotropin releasing hormone; ASI = Anxiety Sensitivity Index. Trait Anxiety was measured using the Spielberger State-Trait Anxiety Scale. Stress was measured using a 10 point Likert scale of subjectively experienced stress in the prior week.
Symptoms and Anxiety
CRH produced significantly greater side effects (API symptom intensity) than did placebo (1.3 ± 1.4 and 0.4 ± 1.0, respectively, t=3.70, p=.0008, df=31), though only two subjects spontaneously reported awareness that they had received drug. The 1.3 point increase over baseline reflects an average appearance of one CRH-related symptom (feeling chilled was most common), rated as mild. ANOVA examining Instruction and Order effects confirmed the CRH-placebo difference (F(1,28)=12.5, p<.002), with greater symptoms to CRH than placebo seen in all conditions. There was also an Instruction-by-Order interaction (F(1,28)=6.1, p=.02), because under Standard Instructions symptom responses (to placebo and CRH) were greater when placebo was given first, whereas with Cognitive Intervention symptom responses were greater when CRH was given first. CRH did not produce significantly more anxiety than placebo (mean increase of 16.8 ± 25.5 vs. 11.4 ± 22.8, respectively, t = 1.07, p = .30, df = 31). A parallel ANOVA on anxious distress response showed no significant effects. (See Supplemental Figures 1 & 2).
Responses to CRH
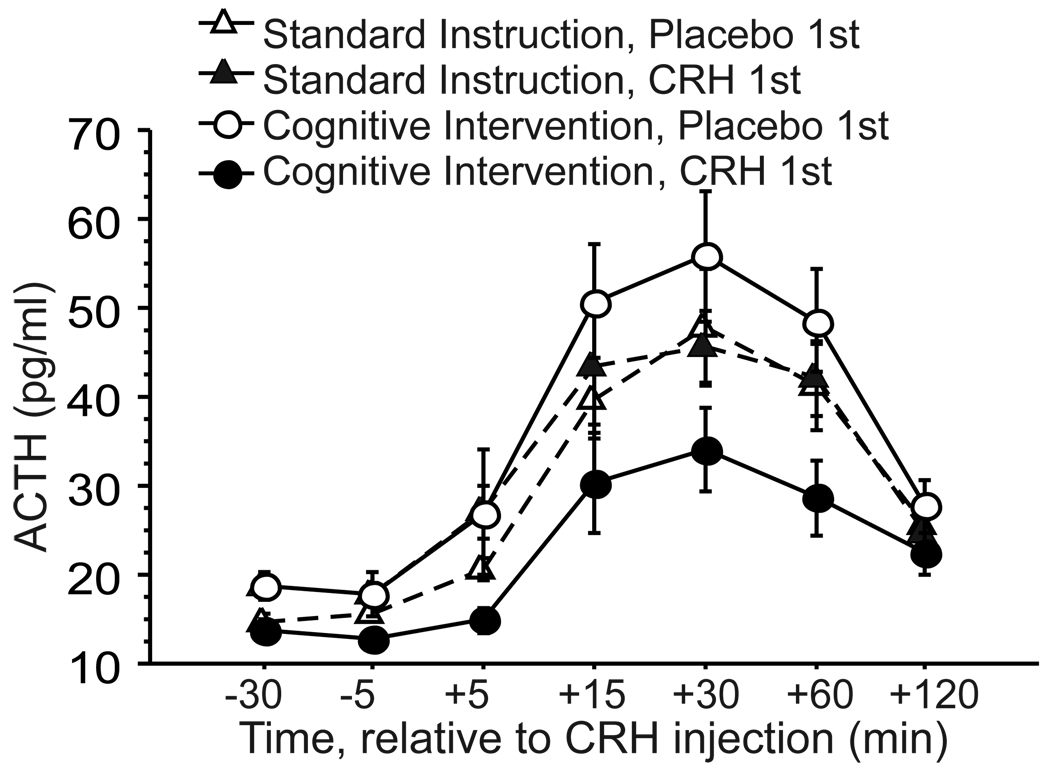
ACTH secretion was significantly altered by the cognitive intervention, though this effect was dependent upon order of drug administration. As expected, ACTH rose in all groups following CRH (main effect of Time (F(6,168)=60.48, p<.0001). The Instruction-by-Order interaction was also significant (F(1,28)=7.04, p=.01); the main effect of Order (F(1,28)=3.76, p=.06) and the Order-by-Time interaction (F(6,168)=1.97, p=.07) approached significance. The data (Figure 1) indicate that these results are due to a striking difference between the two CI groups that depended upon drug order. The CI reduced ACTH levels and response to CRH in those who received CRH first, but clearly did not do so in those who received placebo first. Order of drug administration had no impact under Standard Instructions. Follow up ANOVA comparing all four groups in total ACTH secretion (AUC), showed a significant difference between groups on this measure (F(3,28)=3.66, p=.02), due to significantly lower total ACTH secretion in the CI/CRH 1st group relative to the other groups (t(14)=3.03, p=.009 vs. CI/placebo 1st; t(22)=2.62, p=.02 vs. combined SI groups). The two SI groups did not differ from each other (p=.85) and the CRH/placebo 1st group did not differ from the combined SI groups (p=.15). These analyses suggest that the primary finding is that the CI significantly reduced ACTH during CRH stimulation when CRH was given on first visit, but had no effect when CRH was given after a placebo visit, though it was administered identically on both visits.
Figure 1.
Corticotropin (ACTH) responses to CRH injection in healthy subjects randomly assigned to standard instructions or cognitive intervention (mean ± SE). Half of each instruction group received CRH on first visit (CRH 1st) and half received it on second visit (Placebo 1st).
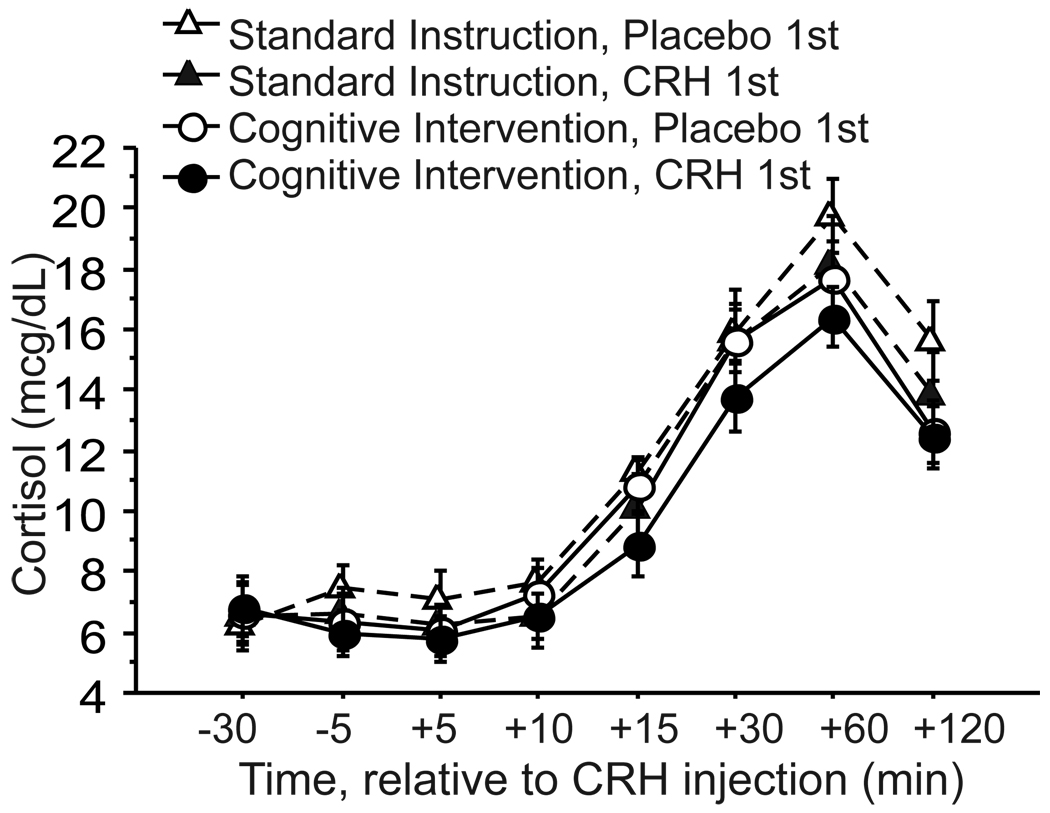
Cortisol responses to CRH were not affected by the CI or by the Order of drug administration (Figure 2). The CI group that received CRH on first visit had lower cortisol levels following CRH than the other three groups, but the only significant effect in the overall RM-ANOVA was a main effect of Time (F(7,196)=167.0, p<.0001). Effect of Instructions and Order were not significant (F(1,28)=1.51, p=.23; F(1,28)=1.36, p=.25, respectively); and there were no significant interaction effects (Instruction-by-Order p=.92, Instruction-by-Time p=.23, Order-by-Time p=.72, and Instruction-by-Order-by-Time p=.87).
Figure 2.
Cortisol responses to CRH in healthy subjects randomly assigned to standard instructions or cognitive intervention (mean ± SE). Half of each instruction group received CRH on first visit (CRH 1st) and half received it on second visit (Placebo 1st).
Responses to Placebo
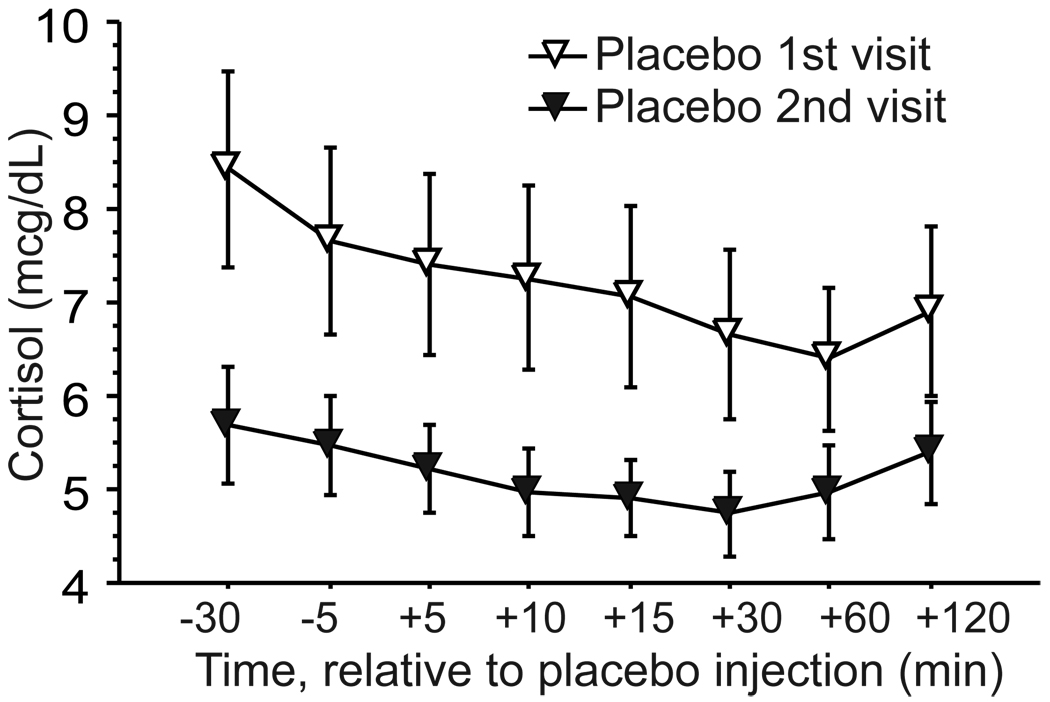
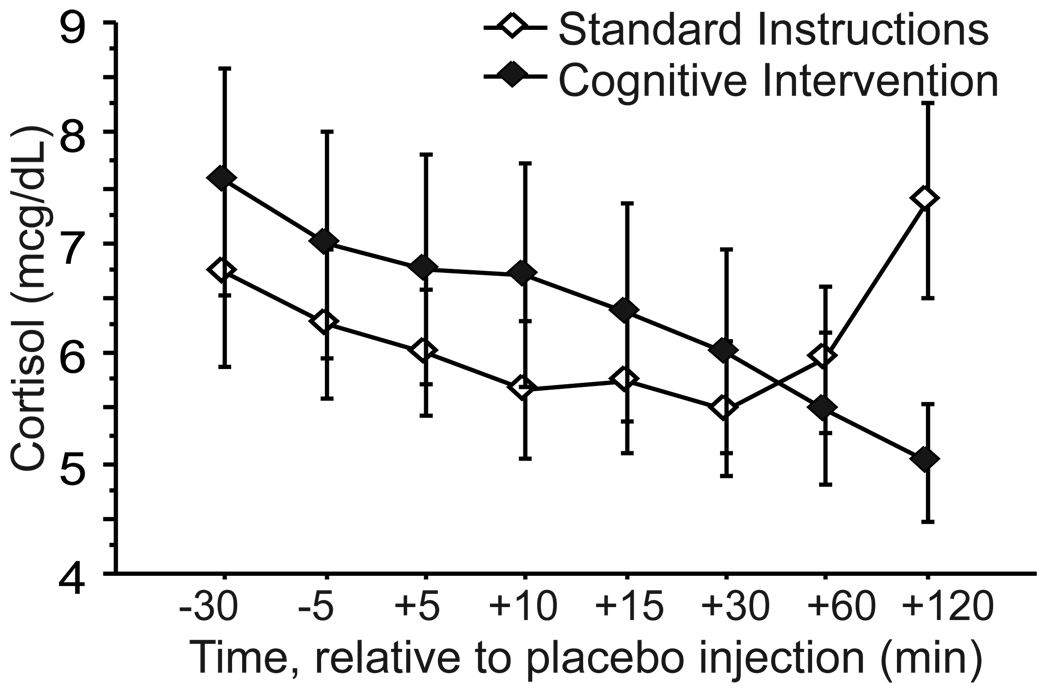
Cortisol levels declined over time on placebo day, as expected, with some increase at the end of the sampling period in some groups. The change over time was slight, but consistent, and the main effect of Time was significant in the RM-ANOVA (F(7,196)=2.22, p=.03). There was also a significant main effect of Order (F(1,28)=4.57, p=.04), due to higher placebo-day cortisol levels when placebo was administered on first visit than when given on second visit (Figure 3). The Time-by-Instruction interaction was also significant (F(7,196)=3.34, p=.002), due to late elevations in cortisol that disrupted its normal diurnal decline in the two SI groups, which were absent in both CI groups (see Figure 4). (See Supplemental Figure 3 for 4 group breakdown).
Figure 3.
Cortisol responses (mean ± SE) to placebo injection in healthy subjects who received placebo on first or second visit to the research unit. Instruction groups are combined in this figure (see Supplemental Figure 3 for graph with instruction groups separated).
Figure 4.
Cortisol responses (mean ± SE) to placebo injection in healthy subjects receiving standard instructions or cognitive intervention. Placebo first and CRH first groups are combined in this figure (see Supplemental Figure 3 for graph with these groups separated).
ACTH patterns on placebo day paralleled those of cortisol, with lower levels on second visit than first visit, and slight rises at the end of the day in SI but not CI groups, but there were no significant effects in the RM-ANOVA (Time F(6,168)=1.41, p=.21; Instruction F(1,28)=0.20, p=.66; Order F(1,28)=1.01, p=.32).
Manipulation Checks
The CI targeted familiarity/novelty and sense of control. Contrary to expectations, CI subjects rated (VAS) experiences in the research unit as more novel/unusual than did SI subjects (F(1,27)=4.69, p=.04). Regardless of instructions, subjective novelty was higher at the beginning of first visit than second (t(31)=3.13, p=.004).
Consistent with expectations, CI subjects tended to report a greater sense of control than SI subjects (F(1,28)=3.41, p=.07). This trend was due to low perceived control in the SI group receiving first visit placebo. The CI groups reported identical subjective control at all time points, regardless of drug Order, and these groups combined experienced significantly more control than the SI-placebo first group at all time points except baseline on placebo day (p’s from .004 to .05). Sense of control increased over placebo day in the CI groups, but decreased in the SI groups (Instruction-by-Time interaction F(2,56)=5.39, p=.007). By end of placebo day, the CI subjects had significantly greater perceived control than SI subjects (F(1,28)=7.24, p=.01), regardless of drug Order. (See Supplemental Figure 4).
There were no significant effects of Instructions or drug Order on perceived dangerousness of CRH or on sense of social support provided by staff or the Investigator.
Correlations
Regressions revealed no significant relationships between HPA axis measures (CRH-day baseline ACTH and cortisol, AUC total, AUC response) and Depression score, Trait Anxiety, Anxiety Sensitivity, Subjective Anxious Distress or Symptom Intensity responses to CRH, perceived novelty, social support, or sense of control. Perceived dangerousness of CRH did predict CRH-day HPA axis activity (r=.50, p=.004; r=.69, p<.0001 for ACTH and cortisol AUC responses, respectively), even though subjects rated dangerousness in a narrow range at the low end of the scale (mean=7.6±7.3 from 0–100 potential range). Because the CI impacted both sense of control and cortisol on placebo day, we also examined the relationship between these variables. Magnitude of decline in sense of control during placebo day significantly predicted final cortisol level on that day (r=.51, p=.004).
Discussion
A brief cognitive intervention, designed to reduce novelty and enhance sense of control and coping, significantly modulated ACTH response to low dose CRH infusion in healthy subjects when CRH was given during first visit to the experimental context. This suggests that apparent HPA sensitivity even to a direct pituitary activator like CRH can potentially be modulated by verbal instructions in single visit experiments. The data also show that cortisol levels over the course of an experiment in which a drug injection might occur (placebo day) can be affected by prior experience in the study setting and are influenced by subjects’ perceived sense of control, which can be manipulated through preparation instructions. These findings indicate that psychological manipulation of expectancies related to novelty, control, and coping can influence HPA axis measures in laboratory studies, even when primary biological activators like CRH are used. This has implications for understanding the psychobiology of stress neurocircuitry in general and for interpretation of HPA dysregulation detected in psychiatric populations using biological probes.
Before addressing implications, it is important to acknowledge a need for replication. The observation of a cognitive intervention effect on ACTH only in subjects receiving CRH on first visit to the laboratory, but not those receiving it on second visit, left us with a small cell size for the key finding. Given potential skepticism that a brief, verbal discussion can significantly alter ACTH responses to a pituitary stimulant as direct and potent as CRH, replication with a larger sample is necessary. However, this effect was robust, despite the small sample, and quite consistent within the first visit CRH group (there were no outliers and variance is fairly small). It is also consistent with the clear and replicated effect of a similar intervention on the HPA response to pentagastrin, which also stimulates ACTH release through direct effect on the pituitary (Abelson et al., 2008). The finding is also consistent with a growing literature documenting the impact of psychological modulation on HPA axis activity across species (Ellenbogen et al., 2006; Gaab et al., 2005; Herman et al., 2003). Existing literature also supports the particular salience of novelty, control and coping to this system (Abelson et al., 2008; Dickerson and Kemeny, 2004; Herman et al., 2003), further supporting the possibility that an intervention focused on these factors might be able to modulate HPA responses even to direct, pharmacological activators. Replication efforts should address additional weaknesses. They should equalize the time spent with subjects, since in the current design the cognitive intervention took slightly more time to administer than the standard instructions. They should also include direct measurement of cortisol binding globulin (CBG), as there is growing recognition that CBG levels vary between sexes and across the menstrual cycle, and can influence results in HPA axis studies (Kumsta et al., 2007). We conducted additional analyses on these data to insure that group differences in CBG levels could not explain our results (Abelson, 2009) (see supplementary materials on-line), but future studies should include direct measurement and analytic control of this variable.
If replicable, this finding has important implications for HPA axis studies of psychiatric disorders. If manipulations of cognitive set can impact results in a CRH stimulation test, then pre-existing individual or group differences in cognitive set might also influence such results. If so, then abnormal responses to CRH in psychiatric patients could be due to disorder-related differences in cognitive set/expectancies rather than to differential sensitivity of CRH receptors. Such effects would likely be mediated via indirect input to the hypothalamus from limbic and prefrontal cortical areas (Jankord and Herman, 2008). Disorder-related dysregulation in such extra-hypothalamic areas might contribute, for example, to attentional or memory biases (Bradley et al., 1995; McNally, 1998) that influence how the experimental context or instructions are experienced or perceived. These in turn may impact modulatory input to the HPA axis and contribute to the detection of HPA axis abnormalities in psychiatric patients by altering reactivity to CRH even without changes in CRH or glucocorticoid receptor sensitivity. In a recent review of HPA data in Panic Disorder (Abelson et al., 2007), we concluded that apparent HPA abnormalities in panic patients, and the considerable inconsistencies in this literature, could well be due to a hypersensitivity to novelty cues in the panic patients, interacting with study variations in the extent of novelty exposure and the kind of preparation provided to subjects. The current data support this possibility by showing that sensitivity of the HPA axis to contextual factors is a normal phenomenon and that altering familiarity with context or preparedness to control/cope can impact HPA reactivity data even in healthy subjects and even when using a very direct probe like CRH.
The HPA axis has been studied more extensively in PTSD and depression than in panic, and evidence supports a more central role for HPA axis dysregulation in those disorders (Holsboer, 2001; Yehuda, 2002). However, even for PTSD and MDD, the notion that a simple defect within the HPA axis might provide a primary, etiologic pathophysiological factor is being questioned. Growing evidence suggests that hypercortisolemia and resistance to feedback inhibition in depression may not be directly linked to the disorder itself, but may at least partially be a consequence of early life trauma (Heim et al., 2008). Inconsistent HPA findings in PTSD may not be surprising if this system is highly context sensitive, since PTSD patients may have a specific deficit in context processing (Liberzon and Sripada, 2008). A recent, non-replication of low basal cortisol and feedback hyper-suppression findings in PTSD led to the conclusion that HPA axis abnormalities reported in PTSD likely reflect other risk factors rather than the presence of PTSD itself (Metzger et al., 2008). HPA axis dysregulation could be a consequence of interacting genetic and developmental factors that create deficits in adaptive capacities, reflected in poor coping in the face of environmental challenge, and detected in laboratory studies that expose subjects to novel and sometimes challenging experiences. Such biobehavioral adaptive deficits could create a general vulnerability to a multitude of psychiatric disorders, contributing to the pervasiveness of HPA abnormalities across disorders. Given this system’s role in shaping responses to environmental challenge, the wide range of environments that psychiatric patients have experienced over their developmental years, and the widely varying environments to which we expose them in experiments, it does not seem surprising that individual differences are seen in HPA studies and that the psychiatric HPA literature is full of contradictions and inconsistencies. The relevance of these results to psychiatric disorders must be tempered by awareness that we studied healthy subjects. Direct examination of HPA context sensitivity across clinical populations – comparing patients to controls in differing laboratory contexts and to themselves in novel versus familiar contexts such as their homes – is needed to better inform interpretation of HPA data from laboratory studies of patients. Heightened attention to relatively ignored aspects of experimental designs – such as prior experience in laboratory settings and the precise nature of instructions/preparation provided – might clarify some existing inconsistencies in the literature. Some documented abnormalities in some patient groups might “disappear” if they are studied in contexts that are highly familiar to them.
Our data cannot identify the psychological mechanisms through which cognitive set might alter responses to CRH. The impact of a verbal manipulation on ACTH response to first visit CRH suggests, however, that there are “top-down” pathways through which higher order cognitive/emotional inputs can modulate pituitary response to direct stimulation. The fact that this effect was only evident on first visit suggests a novelty-related, psychological amplification effect that augments response on first visit, with cognitive preparation able to override or block this amplification. Novelty alone cannot fully explain our results, however, since we did not see a smaller response to CRH when it was given on second visit under standard instructions. Also, the CI did not reduce perceived novelty and perceived novelty did not predict any HPA measure. However, novelty is a known activator of the HPA axis, perceived novelty was higher on first visit than second, and the CI specifically tried to reduce novelty by thoroughly preparing subjects for their laboratory experiences. Alternatively, the CI effect might be mediated by enhancement of sense of control, which may be less salient to subjects when they have had prior experience with the study procedures. The CI did in fact enhance sense of control, though this variable also failed to predict ACTH responses. The best subjective correlate of HPA axis response was perceived dangerousness of CRH, but this measure was not impacted by the CI. Measurement error may have contributed to the failure to find more significant subjective correlates. Our measure of perceived novelty has not been previously used and may not have reliably captured the relevant experience. Future work might include better established measures of novel experience. We suspect that as yet unidentified interactions between preparation, expectancies, uncertainty, and first visit novelty – perhaps involving unmeasured aspects of automatic processing (Ellenbogen et al., 2006) or anticipatory cognitive appraisal (Gaab et al., 2005) – are able to psychologically modulate HPA axis sensitivity when CRH is given on first exposure to the research setting. If replicable, further work will be needed to dissect the intervention components (novelty reduction, control, coping) and identify the relevant factors.
Placebo-day data support the relevance of laboratory novelty to HPA axis activation by showing higher cortisol levels on first compared to second visit. Cortisol levels declined over time on placebo day, regardless of visit, as would be expected on the basis of normal diurnal decline and accommodation to setting novelty. Intriguingly, this decline was disrupted by a late cortisol rise under standard instructions, not seen with cognitive intervention. This rise corresponded in time to a transition to less frequent sampling, which meant that the nurse no longer remained at the bedside between sample collections. This may be an example of the type of “context” change that is usually ignored but may be salient. Though speculative, we wonder whether this context shift was experienced differently by the two groups, enhancing uncertainty and perceived lack of control under SI, but having less effect on the more thoroughly prepared CI subjects. Sense of control in fact declined over time in SI groups on placebo day, but rose in CI groups. Sense of control is known to modulate cortisol release (Dickerson and Kemeny, 2004) and isolated manipulation of control directly modulates cortisol in our pentagastrin model (Abelson et al., 2008). Declining sense of control from baseline to last sampling point on placebo day directly predicted cortisol level at that last sampling point. These findings support the importance of sense of control in shaping HPA axis activity, corroborating that the HPA axis is sensitive to subtle but perhaps fairly specific psychological phenomena in healthy humans and supporting the hypothesis that unattended shifts in experimental contexts might influence HPA data in human laboratory studies.
Failure to see modulation of the cortisol response to CRH in the first visit group was surprising, given significant modulation of ACTH and robust cognitive intervention effects on cortisol in the pentagastrin model (Abelson et al., 2008). Dose and potency issues may be salient. CRH in this study produced a somewhat larger cortisol response (14 to 20 mcg/dL) than was seen in our recent pentagastrin replication study (8 to 14 mcg/dL). Even at the low dose utilized here, CRH may produce a maximal cortisol response that is less amenable to psychological modulation. Replication using a smaller dose is needed. In contrast to cortisol, the ACTH response to this dose of CRH is still quite far from its ceiling (Keller-Wood et al., 1983). We may thus be within a still dynamic segment of the ACTH range with this level of CRH stimulation, but having produced a robust and prolonged ACTH surge using oCRH, we may have pushed cortisol beyond its dynamic range. On placebo day, when cortisol release is not driven by exogenous CRH, we can see effects of novelty and cognitive manipulation. On placebo day, ACTH levels were also in a much lower range, close to the assay detection limit, where variability undermines the robustness of statistical analyses.
Our results are consistent with basic science literature that recognizes the importance of both rapid ‘reactive’ pathways to HPA axis activation, and higher level ‘anticipatory’ pathways of modulation that involve prefrontal-limbic circuits that can both amplify and inhibit hypothalamic output (Herman et al., 2003; Jankord and Herman, 2008). They are also consistent with growing evidence from both animal (Amat et al., 2006; Diorio et al., 1993; Herman et al., 2003; Quirk and Gehlert, 2003; Quirk et al., 2003) and human studies (Bishop, 2007; Liberzon et al., 2007; Lieberman et al., 2007; Ochsner et al., 2002; Taylor et al., 2003) that cortical activity can modulate (or dampen) limbic and hypothalamic activity. We hypothesize that cognitive intervention engages prefrontal circuits to inhibit hypothalamic output, and that top-down modulation of hypothalamic output is “in play” even when probing the system with a direct pituitary activator. Future studies will directly explore the circuitry involved, and will explore our ability to specifically and efficiently activate top-down inhibitory systems – with the goal of developing training techniques that may be able to buffer the detrimental health effects of stress that are mediated by HPA axis activation. Such techniques may also have relevance for treating psychiatric disorders, where inefficiencies in ‘top-down’ inhibitory circuits may contribute to psychiatric symptoms and/or treatment-resistance (Abelson et al., 2007; Monk et al., 2008; Rauch et al., 2006).
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abelson JL. Cognitive modulation of endocrine responses to CRH stimulation, revisited – An exercise in understanding potential sex and CBG effects. Poster presented at the 40th Annual ISPNE Conference; July 23, 2009; San Francisco, CA. 2009. [Google Scholar]
- Abelson JL, Curtis GC. Hypothalamic-pituitary-adrenal axis activity in panic disorder: 24-hour secretion of corticotropin and cortisol. Arch Gen Psychiatry. 1996;53:323–331. doi: 10.1001/archpsyc.1996.01830040059010. [DOI] [PubMed] [Google Scholar]
- Abelson JL, Khan S, Liberzon I, Erickson TM, Young EA. Effects of perceived control and cognitive coping on endocrine stress responses to pharmacological activation. Biol Psychiatry. 2008;64:701–707. doi: 10.1016/j.biopsych.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abelson JL, Khan S, Liberzon I, Young EA. HPA axis activity in patients with panic disorder: review and synthesis of four studies. Depress Anxiety. 2007;24:66–76. doi: 10.1002/da.20220. [DOI] [PubMed] [Google Scholar]
- Abelson JL, Liberzon I. Dose response of adrenocorticotropin and cortisol to the CCK-B agonist pentagastrin. Neuropsychopharmacology. 1999;21:485–494. doi: 10.1016/S0893-133X(98)00124-9. [DOI] [PubMed] [Google Scholar]
- Abelson JL, Liberzon I, Young EA, Khan S. Cognitive modulation of the endocrine stress response to a pharmacological challenge in normal and panic disorder subjects. Arch Gen Psychiatry. 2005;62:668–675. doi: 10.1001/archpsyc.62.6.668. [DOI] [PubMed] [Google Scholar]
- Abelson JL, Nesse RM, Vinik AI. Pentagastrin infusions in patients with panic disorder II. Neuroendocrinology. Biol Psychiatry. 1994;36:84–96. doi: 10.1016/0006-3223(94)91188-6. [DOI] [PubMed] [Google Scholar]
- Abelson JL, Weg JG, Nesse RM, Curtis GC. Neuroendocrine responses to laboratory panic: Cognitive intervention in the doxapram model. Psychoneuroendocrinology. 1996;21:375–390. doi: 10.1016/0306-4530(96)00005-4. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Bradley BP, K M, Williams R. Implicit and explicit memory for emotion-congruent information in clinical depression and anxiety. Behaviour Research and Therapy. 1995;33:755–770. doi: 10.1016/0005-7967(95)00029-w. [DOI] [PubMed] [Google Scholar]
- Carrasco JL, Diaz-Marsa M, Pastrana JI, Molina R, Brotons L, Lopez-Ibor MI, Lopez-Ibor JJ. Hypothalamic-pituitary-adrenal axis response in borderline personality disorder without post-traumatic features. British Journal of Psychiatry. 2007;190:357–358. doi: 10.1192/bjp.bp.106.022590. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Feinberg M, Greden JF, Tarika J, Albala AA, Haskett RF, James NM, Kronfol Z, Lohr N, Steiner M, de Vigne JP, Young E. A specific laboratory test for the diagnosis of melancholia: Standardization, validation, and clinical utility. Arch Gen Psychiatry. 1981;38:15–22. doi: 10.1001/archpsyc.1981.01780260017001. [DOI] [PubMed] [Google Scholar]
- Curtis GC, Abelson JL, Gold PW. ACTH and cortisol responses to CRH: Changes in panic disorder and effects of alprazolam treatment. Biol Psychiatry. 1997;41:76–85. doi: 10.1016/s0006-3223(95)00578-1. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen MA, Schwartzman AE, Stewart J, Walker CD. Automatic and effortful emotional information processing regulates different aspects of the stress response. Psychoneuroendocrinology. 2006;31:373–387. doi: 10.1016/j.psyneuen.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Nater UM, Ehlert U. Psychological determinants of the cortisol stress response: the role of anticipatory cognitive appraisal. Psychoneuroendocrinology. 2005;30:599–610. doi: 10.1016/j.psyneuen.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Gholami B, King AP, Harden YM, Khan SA, Henke PK, Upchurch GR, Graham L, Liberzon I. Acute HPA axis responses to major abdominal surgery and post-operative psychiatric and medical outcomes. Biol Psychiatry. 2004;55:122S–123S. [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: A refined laboratory test for psychiatric disorders. J Affective Dis. 1994;62:77–91. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. Journal of Affective Disorders. 2001;62:77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Annals NY Acad Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Wood ME, Shinsakov J, Dallman MF. Integral as well as proportional adrenal responses to ACTH. Am J Physiol. 1983;245:R53–R59. doi: 10.1152/ajpregu.1983.245.1.R53. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Entringer S, Hellhammer DH, Wust S. Cortisol and ACTH responses to psychosocial stress are modulated by corticosteroid binding globulin levels. Psychoneuroendocrinology. 2007;32:1153–1157. doi: 10.1016/j.psyneuen.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Lammers CH, Garcia-Borreguero D, Schmider J, Gotthardt U, Dettling M, Holsboer F, Heuser IJE. Combined dexamethasone/corticotropin-releasing hormone test in patients with schizophrenia and in normal control: II. Biol Psychiatry. 1995;38:803–807. doi: 10.1016/0006-3223(95)00065-8. [DOI] [PubMed] [Google Scholar]
- Levine S. Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. European Journal of Pharmacology. 2000;405:149–160. doi: 10.1016/s0014-2999(00)00548-3. [DOI] [PubMed] [Google Scholar]
- Liberzon I, King AP, Britton JC, Phan L, Abelson JL, Taylor SF. Paralimbic and medial prefrontal cortical involvement in neuroendocrine responses to traumatic stimuli. Am J Psychiatry. 2007;164:1250–1258. doi: 10.1176/appi.ajp.2007.06081367. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Sripada C. The functional neuroanatomy of PTSD: A critical review. Progress in Brain Research. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: the good and bad sides of the response to stress. Metabolism. 2002;51:2–4. doi: 10.1053/meta.2002.33183. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, Weiss JM. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Research - Brain Research Reviews. 1997;23:79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Information-processing abnormalities in anxiety disorders: Implications for cognitive neuroscience. Cognition and Emotion. 1998;12:479–495. [Google Scholar]
- Metzger LJ, Carson MA, Lasko NB, Paulus LA, Orr SP, Pitman RK, Yehuda R. Basal and suppressed salivary cortisol in female Vietnam nurse veterans with and without PTSD. Psychiatry Research. 2008;161:330–335. doi: 10.1016/j.psychres.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Chen G, McClure-Tone EB, Ernst M, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Gehlert DR. Inhibition of the amygdala: key to pathological states? Ann N Y Acad Sci. 2003;985:263–272. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Selye H. A syndrome produced by diverse noxious agents. Nature. 1936;138:32–33. [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18:650–659. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Current status of cortisol findings in post-traumatic stress disorder. Psychiatr Clin North Am. 2002;25:341–368. doi: 10.1016/s0193-953x(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Young EA, Haskett RF, Grunhaus L, Pande A, Murphy-Weinberg V, Watson SJ, Akil H. Increased evening activation of the hypothalamic-pituitary-adrenal axis in depressed patients. Arch Gen Psychiatry. 1994;51:701–707. doi: 10.1001/archpsyc.1994.03950090033005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.