Abstract
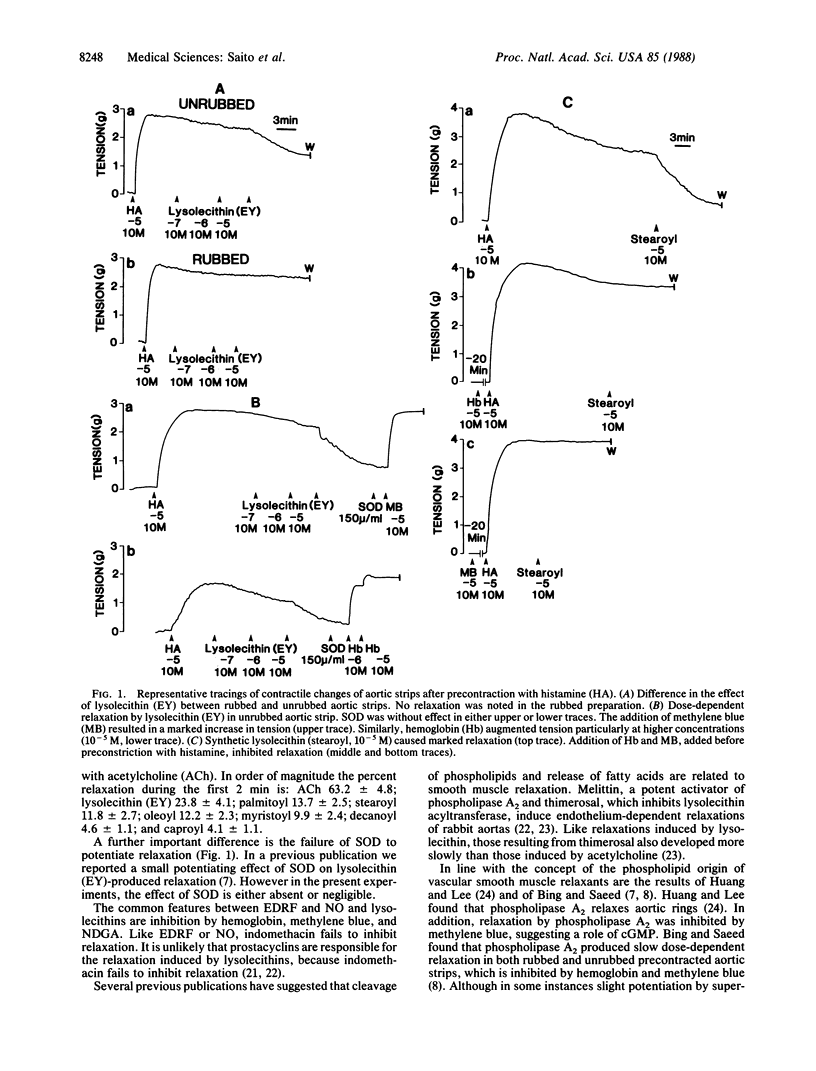
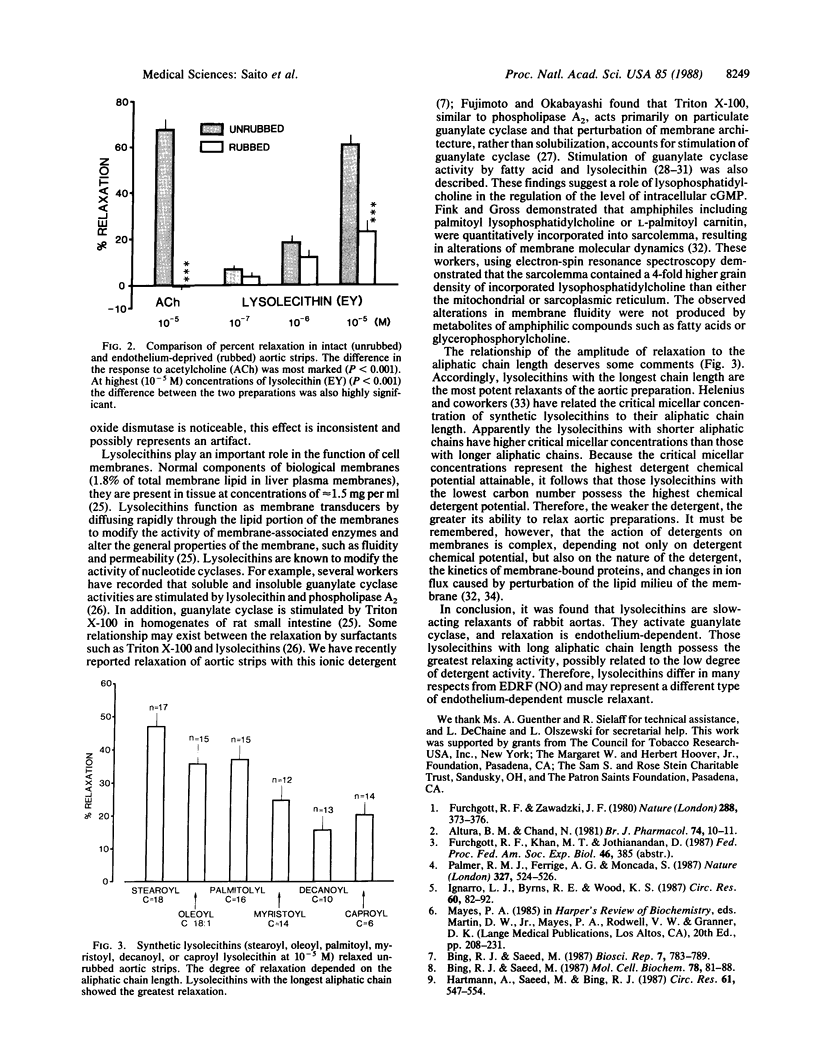
The effects of lysolecithin (lysophosphatidylcholine) derived from egg yolk as well as of synthetic lysolecithins with different aliphatic chain lengths on tension development of rabbit aortic strips were investigated. Lysolecithins caused slowly progressing, dose-dependent relaxation that was inhibited by hemoglobin, methylene blue, and nordihydroguiaretic acid. Indomethacin caused no inhibition of relaxation. The degree of relaxation was endothelium-dependent and appeared to be related to the activation of guanylate cyclase [GTP pyrophosphate-lyase (cyclizing), EC 4.6.1.2]. Superoxide dismutase failed to influence relaxation. Lysolecithins with the longest aliphatic chain were the most potent relaxants of aortic strips. The experiments suggest a role of lysolecithins through their weak detergent action on membrane dynamics of endothelial cells, resulting in the production of cyclic GMP and the relaxation of arterial smooth muscle. Lysolecithins differ in several respects from endothelium-derived relaxing factor. Endothelium-derived relaxing factor is an unstable humoral substance released from endothelium and is identical to nitric oxide, itself a labile substance causing vascular relaxation and cyclic GMP accumulation. Lysolecithins may represent a different type of endothelium-dependent muscle relaxant.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altura B. M., Chand N. Bradykinin-induced relaxation of renal and pulmonary arteries is dependent upon intact endothelial cells. Br J Pharmacol. 1981 Sep;74(1):10–11. doi: 10.1111/j.1476-5381.1981.tb09948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing R. J., Saeed M. A relaxing factor released by phospholipase A2 in the absence of endothelium. Mol Cell Biochem. 1987 Nov;78(1):81–88. doi: 10.1007/BF00224427. [DOI] [PubMed] [Google Scholar]
- Bing R. J., Saeed M. The role of lysolecithin in the relaxation of vascular smooth muscle. Biosci Rep. 1987 Oct;7(10):783–789. doi: 10.1007/BF01116751. [DOI] [PubMed] [Google Scholar]
- Fink K. L., Gross R. W. Modulation of canine myocardial sarcolemmal membrane fluidity by amphiphilic compounds. Circ Res. 1984 Nov;55(5):585–594. doi: 10.1161/01.res.55.5.585. [DOI] [PubMed] [Google Scholar]
- Fujimoto M., Okabayashi T. Proposed mechanisms of stimulation and inhibition of guanylate cyclase with reference to the actions of chlorpromazine, phosphoipases and Triton X-100. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1332–1336. doi: 10.1016/0006-291x(75)90173-4. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Cherry P. D., Zawadzki J. V., Jothianandan D. Endothelial cells as mediators of vasodilation of arteries. J Cardiovasc Pharmacol. 1984;6 (Suppl 2):S336–S343. doi: 10.1097/00005344-198406002-00008. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Burgwitz K., Frölich J. C. Effects of nonsteroidal phospholipase inhibitors and glucocorticoids on endothelium-dependent relaxations of rabbit aorta induced by different agents. J Cardiovasc Pharmacol. 1987 Sep;10(3):356–364. doi: 10.1097/00005344-198709000-00016. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Goppelt-Strübe M., Frölich J. C., Busse R. Inhibitors of acyl-coenzyme A:lysolecithin acyltransferase activate the production of endothelium-derived vascular relaxing factor. J Pharmacol Exp Ther. 1986 Jul;238(1):352–359. [PubMed] [Google Scholar]
- Förstermann U., Neufang B. Endothelium-dependent vasodilation by melittin: are lipoxygenase products involved? Am J Physiol. 1985 Jul;249(1 Pt 2):H14–H19. doi: 10.1152/ajpheart.1985.249.1.H14. [DOI] [PubMed] [Google Scholar]
- Glass D. B., Frey W., 2nd, Carr D. W., Goldberg N. D. Stimulation of human platelet guanylate cyclase by fatty acids. J Biol Chem. 1977 Feb 25;252(4):1279–1285. [PubMed] [Google Scholar]
- Gordon L. M., Sauerheber R. D., Esgate J. A., Dipple I., Marchmont R. J., Houslay M. D. The increase in bilayer fluidity of rat liver plasma membranes achieved by the local anesthetic benzyl alcohol affects the activity of intrinsic membrane enzymes. J Biol Chem. 1980 May 25;255(10):4519–4527. [PubMed] [Google Scholar]
- Gruetter C. A., Barry B. K., McNamara D. B., Gruetter D. Y., Kadowitz P. J., Ignarro L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cyclic Nucleotide Res. 1979;5(3):211–224. [PubMed] [Google Scholar]
- Gruetter C. A., Gruetter D. Y., Lyon J. E., Kadowitz P. J., Ignarro L. J. Relationship between cyclic guanosine 3':5'-monophosphate formation and relaxation of coronary arterial smooth muscle by glyceryl trinitrate, nitroprusside, nitrite and nitric oxide: effects of methylene blue and methemoglobin. J Pharmacol Exp Ther. 1981 Oct;219(1):181–186. [PubMed] [Google Scholar]
- Hartmann A., Saeed M., Bing R. J. Release of endothelium-derived relaxing factor from freshly harvested porcine endothelial cells. Circ Res. 1987 Oct;61(4):548–554. doi: 10.1161/01.res.61.4.548. [DOI] [PubMed] [Google Scholar]
- Helenius A., McCaslin D. R., Fries E., Tanford C. Properties of detergents. Methods Enzymol. 1979;56:734–749. doi: 10.1016/0076-6879(79)56066-2. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Asano T. Stimulation of human platelet guanylate cyclase by unsaturated fatty acid peroxides. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3657–3661. doi: 10.1073/pnas.74.9.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann S. Endothelium-induced relaxation by acetylcholine associated with larger rises in cyclic GMP in coronary arterial strips. J Cyclic Nucleotide Res. 1982;8(6):409–419. [PubMed] [Google Scholar]
- Huang H. C., Lee C. Y. Relaxant effect of phospholipase A2 from Vipera russelli snake venom on rat aorta. Eur J Pharmacol. 1985 Nov 26;118(1-2):139–146. doi: 10.1016/0014-2999(85)90672-7. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Buga G. M., Wood K. S. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987 Dec;61(6):866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Wood K. S. Endothelium-dependent modulation of cGMP levels and intrinsic smooth muscle tone in isolated bovine intrapulmonary artery and vein. Circ Res. 1987 Jan;60(1):82–92. doi: 10.1161/01.res.60.1.82. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Kadowitz P. J. The pharmacological and physiological role of cyclic GMP in vascular smooth muscle relaxation. Annu Rev Pharmacol Toxicol. 1985;25:171–191. doi: 10.1146/annurev.pa.25.040185.001131. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Waldman S. A., Ginsburg R., Molina C. R., Murad F. Effects of glyceryl trinitrate on endothelium-dependent and -independent relaxation and cyclic GMP levels in rat aorta and human coronary artery. J Cardiovasc Pharmacol. 1987 Jul;10(1):82–89. doi: 10.1097/00005344-198707000-00012. [DOI] [PubMed] [Google Scholar]
- Shier W. T., Baldwin J. H., Nilsen-Hamilton M., Hamilton R. T., Thanassi N. M. Regulation of guanylate and adenylate cyclase activities by lysolecithin. Proc Natl Acad Sci U S A. 1976 May;73(5):1586–1590. doi: 10.1073/pnas.73.5.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer H. A., Peach M. J. Calcium- and endothelial-mediated vascular smooth muscle relaxation in rabbit aorta. Hypertension. 1982 May-Jun;4(3 Pt 2):19–25. [PubMed] [Google Scholar]
- Wallach D., Pastan I. Stimulation of guanylate cyclase of fibroblasts by free fatty acids. J Biol Chem. 1976 Sep 25;251(18):5802–5809. [PubMed] [Google Scholar]
- Zwiller J., Ciesielski-Treska J., Mandel P. Effect of lysolecithin of guanylate and adenylate cyclase activities in neuroblastoma cells in culture. FEBS Lett. 1976 Oct 15;69(1):286–290. doi: 10.1016/0014-5793(76)80705-3. [DOI] [PubMed] [Google Scholar]