Abstract
Neuroadaptations in the prefrontal cortex (PFC) are hypothesized to play an important role in the behavioral changes associated with repeated psychostimulant exposure, but there are few published studies that measure neuronal activity during the development and expression of sensitization. To address this, we recorded single neuron activity in the medial PFC (mPFC) of male rats that were exposed for five days to saline or amphetamine (AMPH; 1.0 mg/kg, i.p.) and then given saline or AMPH challenges following a three-day withdrawal. We found that rats exposed to AMPH developed locomotor sensitization to the drug that emerged on the fifth treatment session and became statistically significant at AMPH challenge. This was associated with no change in baseline (i.e., pre-injection) activity of mPFC neurons across the treatment or challenge sessions. Following the first AMPH injection, mPFC neurons responded primarily with reductions in firing, with the overall pattern and magnitude of responses remaining largely similar following repeated treatment. The exception was in the minority of cells that respond to AMPH with increases in firing rate. In this population, the magnitude of excitations peaked during the fifth AMPH exposure and was still relatively elevated at the AMPH challenge. Furthermore, these units increased firing during a saline challenge that was given to assess associative conditioning. These results suggest that AMPH-induced adaptations in mPFC function are not as apparent as AMPH-induced adaptations in behavior. When mPFC adaptations do occur, they appear limited to the population of neurons that increase their firing in response to AMPH.
Keywords: infralimbic, prelimbic, single-unit, electrophysiology, locomotor activity
When psychostimulant drugs are administered repeatedly, they have a significant potential to produce either a reduction (tolerance) or an increase (sensitization) in responsiveness after subsequent exposure to the same or lower doses. In laboratory animals, the most frequently studied effect with drugs such as cocaine and amphetamine (AMPH) is sensitization. In rats and mice, for example, repeated but intermittent exposure to these drugs results in augmentation of species-specific motor behaviors such as locomotion, head movement and sniffing, and stereotyped (i.e., repetitive) head, limb, and orofacial movements (Segal and Schuckit, 1983). The extent and duration of this sensitization is influenced by drug dose, treatment regimen, and environmental context (Robinson and Becker, 1986; Badiani and Robinson, 2004). Sensitization has also been described in humans, especially in relation to AMPH-induced psychosis (Robinson and Berridge, 1993) and AMPH-induced euphoria, drug “liking” and motor activation (Strakowski et al., 1996, 2001; Boileau et al., 2006).
It is hypothesized that these enduring behavioral changes are the result of drug-induced neuroadaptations, particularly within the brain's reward circuitry (Robinson and Kolb, 2004; Jones and Bonci, 2005; Kauer and Malenka, 2007). For example, increases in apical dendrite length, spine density, and the number of branched spines in the nucleus accumbens (NAc) and prefrontal cortex (PFC) have been demonstrated after both experimenter- (Robinson and Kolb, 1997, 1999; Singer et al., 2009) and self-administered (Robinson et al., 2001; Crombag et al., 2005) cocaine or AMPH. Repeated AMPH exposure has also been shown to increase the number of synapses onto spines in the infralimbic and prelimbic regions of the medial PFC (mPFC; Morshedi et al., 2009). These drug-induced changes have been shown to persist for long periods of time, as much as 3.5 years following AMPH administration in a study of the PFC in non-human primates (Selemon et al., 2007). Repeated exposure to cocaine or AMPH also has been shown to produce long-lasting changes in neuronal excitability and synaptic efficacy in the ventral tegmental area (VTA), NAc, and PFC (Onn and Grace, 2000; Thomas et al., 2001; Dong et al., 2005; Nogueira et al., 2006; Peterson et al., 2006; Kourrich et al., 2007; Chen et al., 2008; Ford et al., 2009).
The relationship between these drug-induced adaptations and the expression of behavioral sensitization is not clear, however, because the aforementioned studies utilized in vitro methods that required correlation of the observed anatomical or functional changes with the behavioral responses that animals expressed well before the neuroadaptations were assessed. For example, in the studies of neuronal function, one or several days elapsed between the last drug injection and the measurement of NAc or PFC activity. One method of more directly assessing this relationship is to perform in vivo electrophysiology recordings from behaving animals as they undergo repeated drug exposure and subsequent challenge injections. However, in the comparatively few studies where this was done in rats with a history of cocaine (Stalnaker et al., 2006; Sun and Rebec, 2006) or AMPH (Homayoun and Moghaddam, 2006) exposure, there was no description of neuronal responses as they related to drug-induced behavior and the expression of behavioral sensitization. One noteworthy exception to this is a recent report (Ball et al., 2006) of adaptations in the function of neurons in the dorsal striatum following repeated exposure to (±)3, 4-methylenedioxymethamphetamine (MDMA, or ecstasy). In this study, units recorded from rats that expressed locomotor sensitization to MDMA increased their firing rate to a greater extent following MDMA challenge compared to the first exposure to MDMA.
In the current study, we investigated the relationship between AMPH-induced behavioral sensitization and both baseline and AMPH-induced changes in mPFC activity using in vivo electrophysiological recording methods. We focused on the mPFC because a number of previous studies have highlighted its important role for not only the acute locomotor response to AMPH (Dunnett et al. 1984; Bast et al. 2002; Hall et al., 2009), but also the development and expression of locomotor sensitization following repeated AMPH exposure (Wolf et al., 1995; Cador et al., 1999; Bjijou et al. 2002). Recordings were obtained as rats behaved in an open-field arena before and after daily injections of saline or 1.0 mg/kg AMPH, with subsequent analyses performed on data from four recording sessions: treatment day 1, treatment day 5, and two separate challenge injection sessions. During these challenge sessions, which occurred on consecutive days and were preceded by three days during which rats remained in their home cage, rats were given either saline or AMPH to test for evidence of conditioning and locomotor sensitization, respectively.
Experimental Procedure
Subjects
Male Sprague-Dawley rats (n = 9), bred in our animal facility from stock rats obtained from Harlan (Indianapolis, IN), were housed individually starting at ∼2 months of age and were 3-5 months old (375-500g) at the beginning of experiments. They were maintained on a 12:12 h light:dark cycle (lights on at 0800) with food and water available ad libitum. Rats were handled five times for 15 min intervals prior to being used in experiments, which were conducted between 0900 and 1800 h. All experimental procedures were approved by the IACUC at the University of Illinois, Urbana-Champaign and were consistent with the Principles of Laboratory Animal Care (NIH Publication no. 85-23).
Electrode construction and surgical procedures
Electrode bundles were constructed of 16 or 32 formvar-coated nichrome microwires (25 μm diameter; A-M systems, Sequim, WA) that were cut and assembled into single cylindrical bundles. The bundles were placed in customized stainless steel tubing (22G; Small Parts, Miami Lakes, FL) before being attached with conductive epoxy to 10-pin connectors (Omnetics; Minneapolis, MN). Ground wires, which were constructed from Teflon-coated stainless steel wire (140 μm diameter; A-M systems) with insulation removed at the ends, were attached to a stainless steel machine screw (#0-80; Small Parts) and one channel of the connector. Impedances on recording electrodes were reduced to 0.3-1.0 MΩ by passing current through a saline solution (“saline bubbling”).
Electrode bundles were implanted unilaterally into the mPFC of rats that were anesthetized with ketamine and xylazine (100 and 10 mg/kg, i.m., respectively, followed by 25 mg/kg ketamine, i.p., boosters as needed). A moisturizing lubricant (Moisture Eyes PM; Bausch & Lomb, Rochester, NY) was applied to prevent corneal drying. Holes were drilled over the right mPFC (3.0 anterior-posterior and 1.3 medial-lateral to bregma; Paxinos and Watson, 1998) and at several other anterior and posterior skull positions for the insertion of stainless steel screws to anchor dental acrylic and the ground wires. In the hole overlying the mPFC, the dura was reflected with a 27G needle tip and electrode bundles were lowered 4.2 mm dorsal-ventral at a 9.5° angle towards the midline. When necessary, the craniotomy was sealed with silastic material (Kwik-Sil; WPI, Inc., Sarasota, FL) before the application of dental acrylic. An analgesic dose of carprofen (5.0 mg/kg, s.c.) was administered every 6-12 hours for the first day after surgery.
Electrophysiological techniques
Each microwire was connected to one channel of a 32-channel unity gain field effect transistor preamplifier. Signals from this headstage amplifier were transmitted via lightweight cabling through a multi-channel commutator (Plexon Inc.; Dallas, TX) that allowed the rat to move freely in the testing environment. For extracellular unit data, signals were amplified, bandpass filtered (250 Hz to 8 kHz) and digitized (40 kHz sampling rate). Signal acquisition and real-time spike sorting was accomplished via digital signal processors located within a Multichannel Acquisition Processor (MAP; Plexon, Inc.). For open-field chamber recordings, videos were captured and time-stamped (CinePlex; Plexon, Inc.) for later synchronization of behavior with neural activity (e.g., peri-event time histograms, or PETHs).
Spike activity was separated from background on each of the 16 or 32 channels using a combination of manual and semi-automated methods (Sort Client and Offline Sorter; Plexon, Inc.). Initially, an absolute refractory period of at least 1.1 ms and a waveform amplitude threshold of at least 2.5 standard deviations (SDs) higher than the noise amplitude were used to isolate single units from background; obvious noise artifacts were also removed from the data set. In addition to waveform template matching, clustering algorithms and principle component analysis (PCA) were used to optimize these discriminations. Spike activity was identified as originating from putative interneurons or pyramidal cells based on well-established electrophysiological criteria (Kim and Connors, 1993; Mainen and Sejnowski, 1996; Jung et al., 1998; Barthó et al., 2004) that included action potential waveform shape and spike duration, mean discharge rates, inter-spike interval histograms, and autocorrelograms. Specifically, single-unit activity was considered to be from putative interneurons if it had a high firing rate that followed a regular pattern, whereas pyramidal neurons were identified by burst firing patterns with peaks at 3-10 ms in their autocorrelograms (if the maximum peak is ≥ 50% of the maximum bin value of the first 50 ms) or regular-spiking with a slow-rising slope in their autocorrelogram (mode of interspike-interval histogram > 35 ms). Because well-separated putative interneurons made up a small sample of recorded cells (∼10 %), only data from putative pyramidal cells were used for further analyses.
Behavioral testing
Tests of locomotor activity were performed in an open-field arena, which consisted of a vinyl floor (41 cm × 41 cm) and 41 cm high Plexiglas walls. The arena was enclosed in a wooden black cubicle with a black curtain across the front. It was dimly illuminated with a Fiber-Lite illuminator (Dolan-Jenner, Lawrence, MA) that was mounted through holes in the ceiling of the surrounding cubicle. Also mounted to the cubicle ceiling was a small, high-resolution video camera. An audio speaker was located on the side of the cubicle near the top, and on the opposite side a small LED cue light was centered at the bottom of the arena.
Seven to ten days following surgery, rats were taken from the colony room to the testing room, the headstage was attached to the connectors implanted to their skull, and they were allowed to acclimate in a towel-lined holding pot. On this and subsequent test days, rats underwent two daily sessions. In a morning session, which was used to assess the stability of recorded units and also served to habituate animals to the recording procedures, rats rested in the holding pot and recordings lasted for ∼15 min. An afternoon session was used for testing and lasted for ∼1 hr 45 min. Following a ∼15 min period when units were discriminated and rats acclimated to the testing room, they were placed in the open-field arena for 30 min. They were then removed, injected (i.p.) with either 1.0 ml/kg saline or 1.0 mg/kg AMPH, and returned to the open-field for a 45 min post-injection recording period. At the conclusion of the session, rats were returned to the colony room.
On Day 1 (saline pre-test), all rats were injected with saline. On subsequent test days, five rats were given AMPH (AMPH group) and four rats were given saline (saline group) using a protocol we have used previously to demonstrate AMPH-induced locomotor sensitization and conditioned behavior (Hall et al., 2008). On days 2-6 (treatments 1-5), rats in the AMPH group were injected with 1.0 mg/kg AMPH and were presented with a compound stimulus (28 V, white LED and a 1 kHz, 55dB tone) that was presented in a 5 sec on/5 sec off pattern for the duration of the post-injection recording period. Rats in the saline group were injected with saline only (i.e., no compound stimulus was presented). Following a three-day withdrawal period during which they remained in the colony room, rats began the first of two daily challenge sessions. For the first challenge, all rats received saline but the AMPH group was presented with the compound stimulus that was previously associated with AMPH injections. For the second challenge session, rats in the saline group were given another saline injection whereas those in the AMPH group were given an AMPH challenge (1.0 mg/kg, i.p.). For both groups, the compound stimulus was presented for the duration of the post-injection period.
Histology
Following the last test session, rats were deeply anesthetized with chloral hydrate and marking lesions were produced by passing current (50 μA, cathodal; 5 s) through electrode channels where presumed mPFC neurons were recorded. Rats were then perfused transcardially with 0.9% saline and a solution of 5% potassium ferrocyanide in formalin, which reacted with metal deposits at the lesion site to produce a blue-green stain. Brains were removed and stored in formalin until they were frozen and sectioned (60 μm thickness) on a sliding microtome. A light microscope was used to analyze cresyl violet stained sections for electrode tracks and tip locations. Only data obtained from those electrodes determined to be in the vicinity of neurons in layer V of the prelimbic or infralimbic regions of the mPFC (as defined by Paxinos and Watson, 1998) were used for subsequent analysis. The anterior to posterior extent of these recording locations is shown schematically in Fig. 1.
Figure 1.
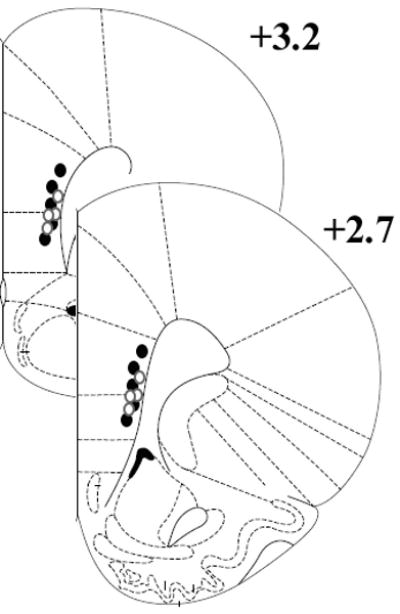
Schematic diagram of recording site locations in the ventral mPFC. Dots represent the approximate anterior to posterior extent of microwire electrode tip locations that were histologically verified to terminate in the prelimbic and infralimbic regions. The filled dots correspond to the AMPH-treated rats (n = 5), while the open dots correspond to the saline-treated rats (n = 4). These coronal section images were adapted from the stereotaxic atlas of Paxinos and Watson (1998).
Drugs
The d-AMPH sulfate used in this study was obtained from Sigma-Aldrich (St. Louis, MO). It was dissolved in sterile saline (0.9% NaCl), with the dose calculated based on the weight of the salt. All injections were given at a volume of 1 ml/kg.
Data analysis
Statistical analyses were conducted using SigmaStat 3.5 (Systat Software, Inc.; San Jose, CA). Locomotor activity before and after injections (saline or AMPH) was quantified by measurement of quadrant crossings in the open-field and summarized into mean values for the 45-min post-injection time period. These data were analyzed using two-way, mixed factor ANOVA (with session as the repeated factor) followed by Holm-Sidak post-hoc analysis.
Electrophysiological data were imported into NeuroExplorer (NEX Technologies, Littleton, MA) for summary and analysis using similar methods to those we (Gulley et al., 2002, 2004) and others (Homayoun and Moghaddam, 2006) have described previously. Units recorded on different treatment days were treated as independent units, although the anchoring of the electrode connectors leaves the possibility that the same or similar populations of cells were sampled across days. Analysis of baseline firing was done using the first and second 15-min time intervals of the 30-min pre-injection recording period. These were chosen because the first 15 min represents a period when rats were actively engaging in exploratory behavior. During the second 15 min, rats were relatively inactive, usually only exhibiting brief periods (< 5 s) of sniffing or head movements. Firing rate (spikes/s) was first analyzed using three-way ANOVA (group × session × baseline period) followed by two-way, mixed factor ANOVA (with baseline period as the repeated factor) for each of the treatment and challenge sessions. This was followed by Holm-Sidak post-hoc analysis where appropriate.
Changes in firing rate after saline or AMPH administration were analyzed by first determining if there was a consistent alteration in firing during the post-injection period compared to the baseline period preceding injection. Units were characterized as increased or decreased if the mean firing rate following injection was below or above, respectively, the 99% confidence interval of the baseline firing rate, (1) for at least four consecutive 5-min bins, or (2) for five of the nine 5-min bins that were recorded post-injection. Those units that did not meet this criterion were classified as not changed. Chi-square (χ2) tests were used to compare the distribution of these response types between treatment groups and within treatment groups across sessions. Bonferroni correction was used to determine the threshold for statistical significance as a control for Type 1 error introduced by the performance of multiple chi-square tests. Thus, the effective α level for these tests was p < 0.005. Mean firing rate for units in these categories was then analyzed using a three-way ANOVA (group × session × response type) followed by two-way ANOVA (group × response type) for each of the treatment and challenge sessions. Post-hoc comparisons were done with Holm-Sidak tests.
Burst firing was assessed using the interval specification algorithm in NeuroExplorer using the following parameters: maximum onset interspike (ISI) interval of 80 ms, minimum offset ISI interval of 160 ms, maximum between-burst interval of 6 msec, minimum burst duration of 10 msec, and a minimum of 3 spikes in a burst). Similar parameters have been used previously in an analysis of cocaine-induced changes in mPFC burst firing (Sun and Rebec, 2006). Analysis of burst rate (bursts/min) during the active and inactive baseline periods was done as described above for firing rate. For analysis of changes in burst activity following saline or AMPH injection, burst rate and % of spikes in bursts during the 5-min period just before injection was compared to that observed following saline or AMPH using a three-way ANOVA (group × session × time period). To address within- and between-group variability in burst activity during baseline and allow for a more direct comparison between groups and across recording sessions (Homayoun and Moghaddam, 2006), the post-injection burst activity for each unit was also normalized to its own activity prior to injection and expressed as a percentage of baseline. These data were analyzed with two-factor ANOVA (group × session) followed by Holm-Sidak post-hoc analysis.
In order to analyze responses of mPFC units to the light/tone cue presented following injections, PETHs with a bin size of 25 ms were constructed around 270 pairings with the light and tone cues. Evidence for robust changes related to these events was evaluated by calculating the firing rates for 150 ms after stimulus onset or offset and determining if these were at least 2 SDs above or below the mean firing rate during the previous 500 ms (Gulley et al., 2002).
Results
Locomotor behavior
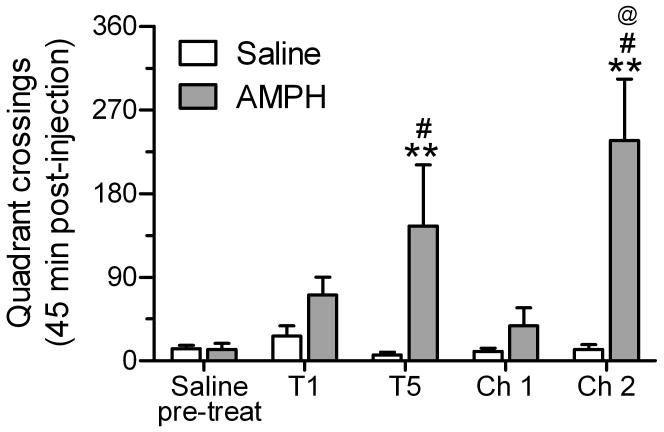
Analysis of quadrant crossing for rats in the saline and AMPH treatment groups revealed significant main effects of group (F1,40 = 11.8, p = 0.01) and session (F4,40 = 4.75, p < 0.01), along with a significant group × session interaction (F4,40 = 5.07, p < 0.01). As shown in Fig. 2, repeated treatment with saline did not change subsequent behavioral responses to saline on test days 1 and 5 or on either of the challenge sessions. AMPH (1.0 mg/kg, i.p.), however, produced an increase in locomotor activity during treatment 1, and subsequent repeated exposure induced sensitized locomotor behavior that began to appear on the fifth treatment day and became statistically significant on the AMPH challenge (i.e., challenge 2). To determine whether conditioning occurred in response to repeated AMPH exposure, a saline challenge was performed on the first day after withdrawal (i.e., challenge 1). The presence of conditioning was defined as an increase in locomotor activity on challenge 1 compared to the first injection with saline and to the activity in rats repeatedly exposed to saline. Although the number of quadrant crossings following saline challenge was greater (> 2-fold increase) than those observed after the first saline injection, this difference was not statistically significant (p > 0.67).
Figure 2.
Mean number of quadrant crossings in the open-field arena for the 45 min period after the first (T1) or fifth (T5) treatment and after challenge injections (Ch 1 and Ch 2) given following 3 days without treatment. Rats in the AMPH group (n = 5) received 1 mg/kg AMPH during tests 1 and 5 and during Ch 2; they received saline during Ch 1. Rats in the saline group (n = 4) received saline during all sessions. **p < 0.01, vs. saline group within-session; #p < 0.05, vs. AMPH group pre-test; @p < 0.05, vs. AMPH group during T1;
Basal mPFC activity
A total of 349 single units were recorded from the prelimbic and infralimbic regions of the mPFC during the first and fifth treatment days, as well as during challenge 1 and 2. During the 30 min they were in the open-field arena prior to injection, rats typically engaged in exploratory behaviors such as locomotion, rearing, and sniffing for the first 15 min (“active” period) and then rested with only occasional movement (e.g., sniffing, head movement, or brief body movements) for the last 15 min (“inactive” period). Table 1 shows the mean firing and burst rate for these periods of high and low spontaneous activity. Overall, there was little evidence of movement-related changes in activity the two treatment groups and there were no consistent changes in baseline activity across recording sessions. The one exception was the small, but significant increase in firing and burst rate that was observed in the inactive period on treatment 5 in rats from the saline-treated group. This statistically significant difference was revealed via significant main effects of session (firing rate: F3,696 = 5.88, p < 0.001; burst rate: F3,649 = 3.84, p < 0.05) and group (firing rate: F1,696 = 5.66, p < 0.05; burst rate: F3,696 = 5.06, p < 0.05) in the overall ANOVA and a significant group × baseline interaction (firing rate: F1,167 = 15.6, p < 0.001; burst rate: F1,161 = 8.68, p < 0.01) in follow-up analysis. Similarly, there was evidence of between-group differences in baseline mPFC activity on challenge 2 (main effect of group; firing rate: F1,160 = 4.07, p < 0.05; burst rate: F1,149 = 7.59, p < 0.01), with firing and burst rate reduced in AMPH-treated rats. This result was only significant when data were collapsed across the active and inactive baselines, however, as the group × baseline interactions on challenge 2 were not significant (p values > 0.59).
Table 1.
Mean (± SEM) firing and burst rates for mPFC neurons recorded 30 min prior to injection with saline or AMPH on treatment days 1 and 5 and challenge days 1 and 2. Data are presented for the first and second 15-min periods that rats where in the open-field arena, which encompassed periods of high (“active”) and low (“inactive”) spontaneous motor activity, respectively. The number of cells recorded during each of the sessions (n) is shown in the firing rate columns.
| Firing rate (spikes/s) | Burst rate (bursts/min) | |||
|---|---|---|---|---|
| Saline | AMPH | Saline | AMPH | |
| Treatment 1 | ||||
| (n) | (43) | (61) | ||
| active | 0.609 ± 0.136 | 0.605 ± 0.080 | 2.02 ±1.04 | 2.01 ± 0.44 |
| inactive | 0.650 ± 0.129 | 0.636 ± 0.067 | 2.01 ± 0.88 | 2.16 ± 0.31 |
| Treatment 5 | ||||
| (n) | (39) | (45) | ||
| active | 1.12 ± 0.060 | 0.907 ± 0.142 | 3.47 ± 1.52 | 3.65 ± 0.97 |
| inactive | 1.44 ± 0.060* | 0.769 ± 0.106 | 5.28 ± 1.80* | 2.96 ± 0.63 |
| Challenge 1 | ||||
| (n) | (34) | (46) | ||
| active | 0.659 ± 0.195 | 0.523 ± 0.079 | 2.61 ± 1.07 | 1.77 ± 0.43 |
| inactive | 0.644 ± 0.183 | 0.566 ± 0.062 | 2.47 ± 1.02 | 1.79 ± 0.36 |
| Challenge 2 | ||||
| (n) | (39) | (42) | ||
| active | 0.709 ± 0.155 | 0.448 ± 0.051 | 3.30 ± 0.96 | 1.15 ± 0.23 |
| inactive | 0.847 ± 0.163 | 0.498 ± 0.050 | 3.46 ± 0.86 | 1.32 ± 0.25 |
p < 0.05, compared to active baseline (Holm-Sidak post-hoc test)
mPFC activity after saline or AMPH treatment
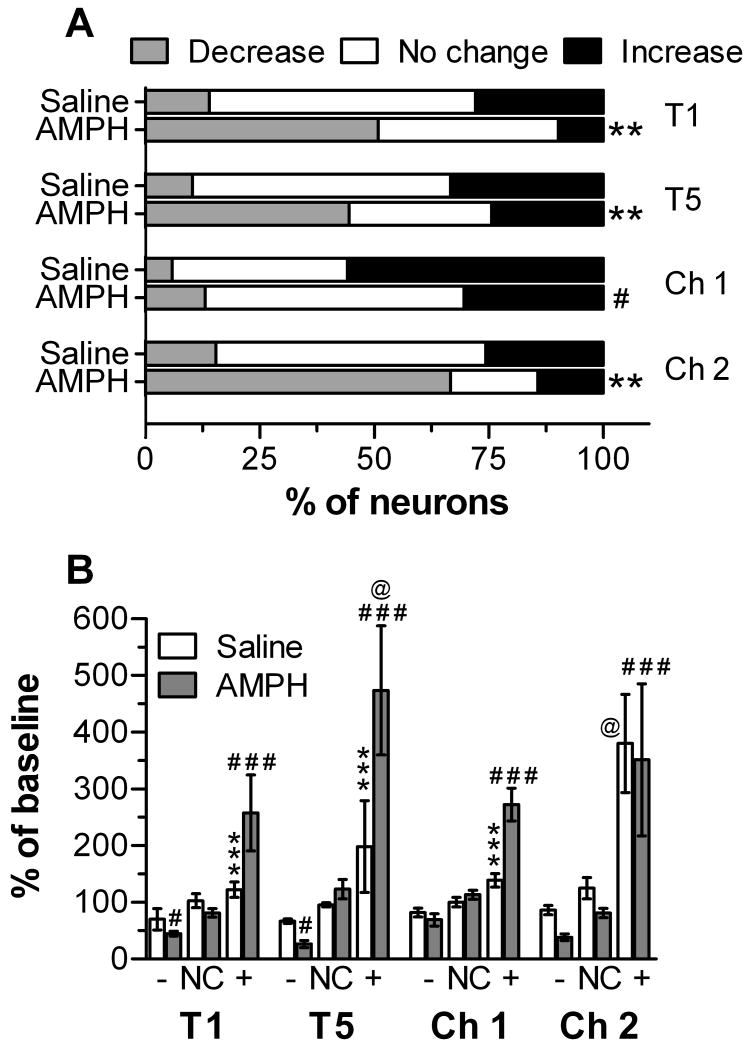
Following the first injection with 1 mg/kg AMPH (i.e., treatment 1), the majority of mPFC neurons significantly changed firing rate compared to their own baseline and to the firing of units recorded from rats given saline (Fig. 3A; χ2 = 16.3, p < 0.001). These changes were mostly reductions in firing, with smaller populations of cells either increased or not consistently changed. The responses in saline- compared to AMPH-treated rats were also significantly different following the fifth treatment (χ2 = 12.2, p = 0.002). During treatment 5 compared to treatment 1 in rats given AMPH, there tended to be fewer cells that did not consistently change and more cells that increased their firing post-injection (Fig. 3A). However, this change in the proportion of response types was not statistically significant. In response to the first challenge, where both groups received saline injections and the AMPH group was exposed to a light/tone cue previously associated with AMPH injection, there was not a significant difference in the proportion of responses between groups. There was, however, a significant change in the proportion of responses in the AMPH groups in comparison to those observed after the first treatment (χ2 = 18.4, p < 0.001). Specifically, following injection there were significantly more units that did not consistently change or increased their firing and significantly fewer cells that decreased their firing. During challenge 2, when both groups were presented the light/tone cue and both received the same injection type they had received on treatments 1 and 5, there was a significant group difference in the proportion of response types: 67% of the cells recorded from rats in the AMPH group exhibited decreases in firing following injection, whereas 59% of the cells recorded from rats in the saline group exhibited no consistent change (χ2 = 22.0, p < 0.001). This response pattern in the AMPH treatment group was not significantly different from that seen at treatment 1, however.
Figure 3.
Responses of mPFC neurons to saline and AMPH across treatment (T) and challenge (Ch) sessions. (A) Units were characterized as increased or decreased if mean firing rate was consistently above or below, respectively, the baseline firing rate during the 5-min period before injection (see Experimental Procedures for analysis details). **p < 0.001, χ2 statistic comparing proportion of responses in AMPH and saline groups; #p < 0.001, χ2 statistic comparing proportion of responses in AMPH group on Ch 1 and T1 (B) Firing rate, normalized to pre-injection baseline, during the 45-min period following saline or AMPH in units exhibiting a sustained decrease (-), increase (+), or no consistent change (NC). *p < 0.05 and ***p < 0.001, compared to units increasing firing rate following AMPH injection #p < 0.05 and ###p < 0.001, compared to units with no consistent change (NC) after AMPH injection; @p < 0.05, compared to firing rate during T1 for the same category of responses following the same injection type
As shown in Fig. 3B, there were significant group differences in the magnitude of firing rate changes following injections on the treatment and challenge days. A three-way ANOVA of these data revealed significant main effects of session (F3,339 = 2.68, p < 0.05) and response type (F2,339 = 66.4, p < 0.001), along with significant two-way interactions (group × session: F3,339 = 2.89, p < 0.05; group × response type: F2,339 = 9.25, p < 0.01; session × response type: F6,339 = 3.60, p < 0.01). Follow-up, two-way ANOVA analyses on data from individual sessions revealed that on treatments 1 and 5, the decreases or increases in baseline that were seen in units from AMPH-treated rats were significantly different from the post-injection firing observed in units classified as not changed (Fig. 3B). Furthermore, the magnitude of AMPH-induced excitation was greater on treatment 5 compared to treatment 1. For units classified as increased following AMPH injection, these normalized firing rates were also significantly different from those observed in saline-treated rats. When all rats were challenged with saline following the three-day withdrawal period (i.e., Ch 1), only the units recorded from rats in the AMPH treatment group and classified as increased following injection were significantly different from those classified as not changed. Furthermore, the magnitude of this increase was significantly higher in AMPH-treated compared to saline-treated rats. Following AMPH challenge (i.e., Ch 2), the magnitude of AMPH-induced decreases and increases in firing was similar to that seen on treatment 1, though only those classified as increased were significantly different from those classified as not changed. In the saline-group, the magnitude of the firing rate increase on challenge 2 was significantly greater than that observed during treatment 1. During this challenge session, these rats were given saline but they were also exposed to a light/tone cue for the first time.
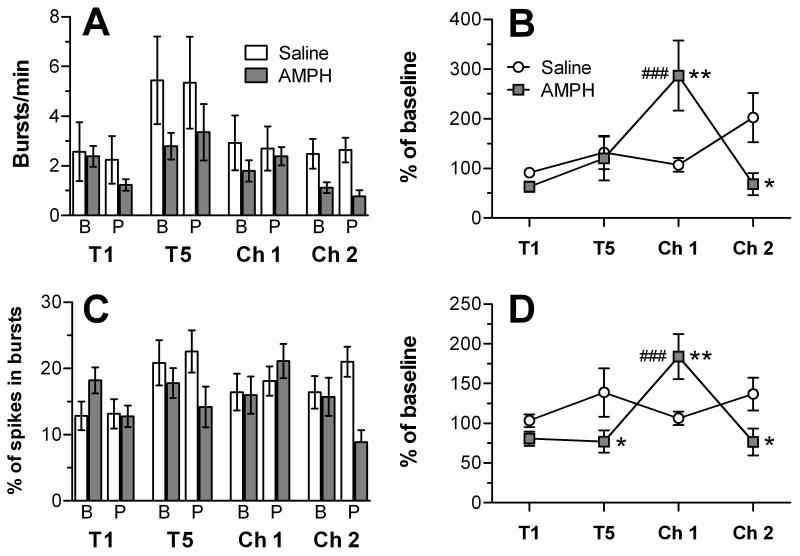
Analysis of burst firing in the mPFC neurons recorded here revealed that AMPH tended to decrease both burst firing rate (Fig. 4A) and the percentage of spikes in bursts (Fig. 4C) compared to baseline, but this effect was only evident on the first treatment day and the AMPH challenge session. An overall three-way ANOVA of these data revealed significant main effects of session for both measures (burst rate: F3,649 = 6.29, p < 0.001; percentage of spikes in bursts: F3,649 = 2.92, p < 0.05). In addition, there was a significant main effect of group for burst rate (F1,649 = 8.86, p < 0.01) and a significant session × group interaction for percentage of spikes in bursts (F3,649 = 3.37, p < 0.05). To account for both within- and between-group variability in bursting, data were normalized for each cell to its pre-injection baseline. A two-way ANOVA of the normalized burst rate revealed a significant main effect of session (F1,273 = 3.26, p < 0.05) and a significant group × session interaction (F3,273 = 5.16, p < 0.01). As shown in Fig. 4B, burst rate was significantly increased in the AMPH-treated group at challenge 1 when compared to the saline group and to the burst rate in cells recorded form the AMPH group at the first treatment. On challenge 2, the treatment groups were also significantly different from each other, with the saline group having a higher burst rate. As shown in Fig. 4D, the percentage of spikes in bursts was significantly reduced in the AMPH- compared to the saline-treated group during treatment 5 and challenge 2. At challenge 1, there was a significant increase in the percentage of spikes in bursts in units recorded from the AMPH-treated group compared to those treated with saline and to units recorded from the AMPH group after the first injection with AMPH.
Figure 4.
Burst rate and percent of spikes in bursts for mPFC neurons recorded before and after injection with saline or AMPH during treatment (T) and challenge (Ch) sessions. In (A) and (C), data are presented for the baseline (B) and post-injection (P) periods. In (B) and (D), burst rate and % of spikes in bursts, respectively, are normalized to the pre-injection baseline for each unit. ###p < 0.001, AMPH group compared to T1; *p < 0.05 and **p < 0.01, compared to saline group.
In order to assess the potential for modulations in mPFC firing rate that were specific to the cues presented to rats in the AMPH group following injections with AMPH on treatments 1 and 5 and challenge 2, or following saline on challenge 1, PETHs were constructed with cue onset or offset as the reference event. We found no evidence of statistically significant, cue-related modulations in firing during any of the treatment or challenge sessions (data not shown). When this same analysis was done for saline-treated rats on challenge 2, we also did not find any units that exhibited significant cue-related changes in firing (data not shown).
Discussion
The goal of this study was to investigate AMPH-induced adaptations in the function of mPFC neurons that, based on results from previous anatomical and in vitro electrophysiological studies, are expected to emerge along with the behavioral adaptations that typically result from repeated AMPH exposure (i.e., behavioral sensitization). This was accomplished by utilizing chronically implanted microwire electrodes to record the activity of mPFC neurons during a baseline period and after injections of either saline or AMPH in rats there were allowed to behave in an open-field arena. We found that during baseline recording (i.e., pre-injection), neurons in the mPFC generally did not modulate their spiking activity during periods of spontaneous movement relative to periods of inactivity. Furthermore, in rats given AMPH repeatedly, there was no consistent, statistically significant change in baseline firing or burst rate across treatments or AMPH challenges. Thus, repeated AMPH exposure did not produce a significant “hypofrontal” state as might be predicted from studies in humans with a long history of cocaine abuse (Goldstein and Volkow, 2002) and those in rodents showing that PFC-mediated glutamate release in the NAc is reduced in cocaine-sensitized rats (Pierce et al., 1996; Hotsenpiller et al., 2001; McFarland et al., 2003). Reduced baseline spiking activity in the mPFC has also been reported in rats with a relatively long history of cocaine self-administration experience (Sun and Rebec, 2006). Consistent with the present results, however, is the finding that repeated exposure to 0.5 or 2.0 mg/kg AMPH had no significant effect on baseline mPFC activity recorded from behaving rats (Homayoun and Moghaddam, 2006). Thus, the development of drug-induced hypofrontality in the mPFC may be influenced by the duration of drug exposure, whether the drug is self-administered or given non-contingently, and also by the type of drug.
Following its first injection, 1.0 mg/kg AMPH had primarily inhibitory effects on both firing rate and bursting in the mPFC. Mostly inhibitory responses have been described previously in anesthetized rats given a single injection of 1 or 2 mg/kg AMPH (Mora et al., 1976) and in brain slices where local application of AMPH leads to a depression of excitatory field potentials in layer V neurons that are evoked by layer II/III stimulation (Mair and Kauer, 2007). In recordings from awake, behaving rats, inhibitory responses also predominated following a single dose of 2 mg/kg AMPH, whereas excitatory response are more common following acute exposure to 0.5 mg/kg AMPH (Homayoun and Moghaddam, 2006). When taken with the latter study, the present results suggest the primary response in the mPFC to lower AMPH doses is opposite to that seen with higher doses. This may be relevant for the therapeutic effects of AMPH in disorders such as attention deficit hyperactivity (ADHD) where the effective dose range is typically 0.2-0.5 mg/kg (Solanto, 2000).
By the fifth injection of AMPH, when locomotor sensitization began to emerge, the pattern of mPFC unit responses to the drug was generally similar to that observed following the first injection – AMPH-induced inhibitions in firing rate predominated. However, the magnitude of excitatory responses, which represented 24% of the recorded neurons, was significantly increased from ∼ 267% of baseline to ∼474% of baseline on treatment 1 and 5, respectively. Following AMPH challenge, these drug-induced excitations were also of a greater magnitude (∼350% of baseline) compared to treatment 1. Importantly, this occurred when locomotor sensitization to AMPH was clearly evident. These changes in the magnitude of excitation were also associated with decreases in the rate of bursting during AMPH challenge and decreases in the number of spikes in bursts during treatment 5 and the AMPH challenge. It may be the case, therefore, that in behaving animals, the population of neurons excited by AMPH is more vulnerable to the plasticity induced by repeated AMPH exposure compared to the population inhibited by the drug. This hypothesis requires further study, particularly using techniques that allow for the simultaneous analysis of behavior and neuronal function.
Relative to units recorded from saline-treated rats, we observed increased burst activity associated with increases in locomotion following saline challenge in the AMPH-treated group. This may be related to an increased ability to process drug-paired information (Childress et al., 1999; Rebec and Sun, 2005; Sun and Rebec, 2006), as the PFC is well known to be involved in learning-related plasticity in rats (Mulder et al., 2003; Schoenbaum et al., 2003; Bouret and Sara, 2004). A specific role for associative conditioning in AMPH-induced adaptations in the mPFC was also highlighted in a recent study showing that drug-induced changes in dendritic morphology in the mPFC were only observed in those rats given systemic injections of AMPH and not those given local AMPH infusions into the ventral tegmental area (Singer et al., 2009). Both of these routes of AMPH exposure induce locomotor and neurochemical sensitization in the NAc, but only systemic exposure produces conditioned responding (Stewart and Vezina, 1991; Vezina, 1996). An alternative hypothesis is that the increased firing rate and burst activity we observed could have resulted in response to the novel situation presented by the first pairing of cues (tone and light) with saline rather then AMPH. While this explanation is plausible, it is noteworthy that saline-treated rats did not show a statistically significant increase in burst activity when they experienced this novel situation for the first time (i.e., challenge 2 where saline injection was paired to the tone and light cues). Thus, the changes in mPFC activity observed following saline challenge are more likely related to the conditioned behavior observed and may represent a change in responsiveness to the overall environmental context. The lack of temporal specificity we observed in our PETH analysis of cue-specific modulations in mPFC activity may also be a reflection of this more global response to the drug-associated context.
The PFC is part of a widely distributed neuronal network that has extensive reciprocal connections with both cortical and subcortical regions (Kolb, 1984). Thus, it is positioned to play a critical role in organizing behavior through functional regulation of numerous subcortical structures. The pyramidal cells in the mPFC, which are more than likely the source of the unit responses we recorded, are known to project densely to limbic structures such as the NAc, hippocampus, and amygdala (Sesack et al., 1989; Vertes, 2002; Heidbreder and Groenewegen, 2003). Moreover, the prelimbic and infralimbic areas receive the densest innervation of VTA dopamine efferents relative to dorsal regions of the mPFC (Lindvall et al., 1978). Thus, it was somewhat unexpected to find that neuroadaptations in the response to AMPH in the mPFC were not as apparent as those observed for AMPH-induced locomotor behavior. It is likely that AMPH-induced adaptations in the mPFC are dependent on the exposure dose, method of drug administration, and duration of withdrawal before challenge. Unfortunately, no other published studies have systematically described mPFC plasticity concurrently with AMPH-induced adaptations in the behavioral response. It has been reported, however, that higher doses of repeated AMPH or cocaine treatment, along with longer withdrawal time periods, altered the electrophysiological properties of mPFC neurons in vitro (Peterson et al, 2006), in anesthetized rats in vivo (Onn and Grace, 2000; Peterson et al., 2000; Trantham et al., 2002), and in awake rats (Homayoun and Moghaddam, 2006; Sun and Rebec, 2006). In the only other published report of mPFC recording in behaving rats, 2 mg/kg AMPH given once per day for 5 days was reported to shift mPFC responses to AMPH such that a greater number of AMPH-induced inhibitions were observed when a challenge injection of AMPH was given 10 days after the fifth drug treatment. A second AMPH challenge given 14 days after the first revealed that this response to AMPH persisted (Homayoun and Moghaddam, 2006). In this study, however, locomotor activity data were not presented, so it is unclear if the observed plasticity in the mPFC was associated with adaptations in the behavioral response to AMPH at both of the challenges. Because the recordings in this study were obtained while rats were in their home cages, it is possible that the inconsistencies with our results are related to the absence of AMPH-induced locomotor sensitization. The importance of environmental context for the development and expression of psychostimulant sensitization has been well documented (Badiani and Robinson, 2004), and single-unit recordings from the mPFC have highlighted its sensitivity to context-discrimination and sensorimotor information (Gemmell et al., 2002; Baeg et al., 2003; Euston and McNaughton, 2006; Cowen and McNaughton, 2007). Thus, it is conceivable that different neuroadaptations can be induced by repeated AMPH exposure and that they are influenced by the behavioral state of the animal.
In summary, we found that repeated exposure to AMPH, using a treatment schedule that resulted in locomotor sensitization to AMPH, leads to selective and relatively modest changes in mPFC function. In particular, AMPH-induced increases in activity were enhanced following five days of repeated treatment and persisted, but to a lesser extent, during an AMPH challenge given following a 3-day withdrawal period. AMPH-induced decreases in mPFC activity, which were the more frequently encountered type of response, were not significantly changed over the course of treatment or following challenge injections. When taken with other studies in behaving rats where repeated cocaine (Sun and Rebec, 2006) or AMPH (Homayoun and Moghaddam, 2006) were shown to produce more robust and longer lasting changes in baseline mPFC function and in the response of mPFC neurons to drug challenges, our findings suggest that AMPH-induced plasticity in the mPFC is highly dependent on treatment factors such as dose, duration of withdrawal, and the treatment environment. Furthermore, they highlight the importance of measuring the neuronal and behavioral consequences of repeated drug exposure concurrently. By doing so, it is possible to more directly correlate neural adaptations with the expression (or absence) of behavioral adaptations. This is particularly important in regards to the mPFC because it has been demonstrated that sensitizing regimens of AMPH treatment have long lasting effects on drug-induced locomotor behavior but do not significantly alter mPFC-sensitive behavioral tasks such as delay-discounting (Stanis et al., 2008), delayed non-matching to position (Featherstone et al., 2008), or delayed spatial alternation (Stefani and Moghaddam, 2002).
Acknowledgments
We thank Chris DeFalco, Colin Hu, Doug Schuweiler, and Martin D. White for their technical assistance. This work was supported in part by a grant from the NIH (DA 019876), an Arnold O. Beckman Award from the University of Illinois, Urbana-Champaign, and a predoctoral fellowship to JJS (T32 NIH/HD007333).
List of abbreviations
- ANOVA
analysis of variance
- AMPH
amphetamine
- NAc
nucleus accumbens
- PETH
peri-event time histogram
- MDMA
(±)3, 4-methylenedioxymethamphetamine
- mPFC
medial prefrontal cortex
- PFC
prefrontal cortex
- SD
standard deviation
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badiani A, Robinson TE. Drug-induced neurobehavioral plasticity: the role of environmental context. Behav Pharmacol. 2004;15:327–339. doi: 10.1097/00008877-200409000-00004. [DOI] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Huh K, Mook-Jung I, Kim HT, Jung MW. Dynamics of population code for working memory in the prefrontal cortex. Neuron. 2003;40:177–188. doi: 10.1016/s0896-6273(03)00597-x. [DOI] [PubMed] [Google Scholar]
- Ball KT, Budreau D, Rebec GV. Context-dependent behavioural and neuronal sensitization in striatum to MDMA (ecstasy) administration in rats. Eur J Neurosci. 2006;24:217–228. doi: 10.1111/j.1460-9568.2006.04885.x. [DOI] [PubMed] [Google Scholar]
- Bartho P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsaki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol. 2004;92:600–608. doi: 10.1152/jn.01170.2003. [DOI] [PubMed] [Google Scholar]
- Bast T, Pezze MA, Feldon J. Dopamine receptor blockade in the rat medial prefrontal cortex reduces spontaneous and amphetamine-induced activity and does not affect prepulse inhibition. Behav Pharmacol. 2002;13:669–673. doi: 10.1097/00008877-200212000-00010. [DOI] [PubMed] [Google Scholar]
- Bjijou Y, De Deurwaerdere P, Spampinato U, Stinus L, Cador M. D-amphetamine-induced behavioral sensitization: effect of lesioning dopaminergic terminals in the medial prefrontal cortex, the amygdala and the entorhinal cortex. Neuroscience. 2002;109:499–516. doi: 10.1016/s0306-4522(01)00508-5. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Cador M, Bjijou Y, Cailhol S, Stinus L. D-amphetamine-induced behavioral sensitization: implication of a glutamatergic medial prefrontal cortex-ventral tegmental area innervation. Neuroscience. 1999;94:705–721. doi: 10.1016/s0306-4522(99)00361-9. [DOI] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen SL, McNaughton BL. Selective delay activity in the medial prefrontal cortex of the rat: contribution of sensorimotor information and contingency. J Neurophysiol. 2007;98:303–316. doi: 10.1152/jn.00150.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Gorny G, Li Y, Kolb B, Robinson TE. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex. 2005;15:341–348. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- Dong Y, Nasif FJ, Tsui JJ, Ju WY, Cooper DC, Hu XT, Malenka RC, White FJ. Cocaine-induced plasticity of intrinsic membrane properties in prefrontal cortex pyramidal neurons: adaptations in potassium currents. J Neurosci. 2005;25:936–940. doi: 10.1523/JNEUROSCI.4715-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett SB, Bunch ST, Gage FH, Bjorklund A. Dopamine-rich transplants in rats with 6-OHDA lesions of the ventral tegmental area. I Effects on spontaneous and drug-induced locomotor activity. Behav Brain Res. 1984;13:71–82. doi: 10.1016/0166-4328(84)90031-7. [DOI] [PubMed] [Google Scholar]
- Euston DR, McNaughton BL. Apparent encoding of sequential context in rat medial prefrontal cortex is accounted for by behavioral variability. J Neurosci. 2006;26:13143–13155. doi: 10.1523/JNEUROSCI.3803-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone RE, Rizos Z, Kapur S, Fletcher PJ. A sensitizing regimen of amphetamine that disrupts attentional set-shifting does not disrupt working or long-term memory. Behav Brain Res. 2008;189:170–179. doi: 10.1016/j.bbr.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Ford KA, Wolf ME, Hu XT. Plasticity of L-type Ca2+ channels after cocaine withdrawal. Synapse. 2009;63:690–697. doi: 10.1002/syn.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmell C, Anderson M, O'Mara SM. Deep layer prefrontal cortex unit discharge in a cue-controlled open-field environment in the freely-moving rat. Behav Brain Res. 2002;133:1–10. doi: 10.1016/s0166-4328(01)00402-8. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley JM, Kosobud AE, Rebec GV. Behavior-related modulation of substantia nigra pars reticulata neurons in rats performing a conditioned reinforcement task. Neuroscience. 2002;111:337–349. doi: 10.1016/s0306-4522(02)00018-0. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Reed JL, Kuwajima M, Rebec GV. Amphetamine-induced behavioral activation is associated with variable changes in basal ganglia output neurons recorded from awake, behaving rats. Brain Res. 2004;1012:108–118. doi: 10.1016/j.brainres.2004.03.044. [DOI] [PubMed] [Google Scholar]
- Hall DA, Powers JP, Gulley JM. Blockade of D1 dopamine receptors in the medial prefrontal cortex attenuates amphetamine- and methamphetamine-induced locomotor activity in the rat. Brain Res. 2009;1300:51–57. doi: 10.1016/j.brainres.2009.08.084. [DOI] [PubMed] [Google Scholar]
- Hall DA, Stanis JJ, Marquez Avila H, Gulley JM. A comparison of amphetamine- and methamphetamine-induced locomotor activity in rats: evidence for qualitative differences in behavior. Psychopharmacology (Berl) 2008;195:469–478. doi: 10.1007/s00213-007-0923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. J Neurosci. 2006;26:8025–8039. doi: 10.1523/JNEUROSCI.0842-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr Opin Pharmacol. 2005;5:20–25. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Jung M, Qin Y, McNaughton B, Barnes C. Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cereb Cortex. 1998;8:437–450. doi: 10.1093/cercor/8.5.437. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kim HG, Connors BW. Apical dendrites of the neocortex: correlation between sodium- and calcium-dependent spiking and pyramidal cell morphology. J Neurosci. 1993;13:5301–5311. doi: 10.1523/JNEUROSCI.13-12-05301.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B. Functions of the frontal cortex of the rat: a comparative review. Brain Res. 1984;320:65–98. doi: 10.1016/0165-0173(84)90018-3. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A, Divac I. Organization of catecholamine neurons projecting to the frontal cortex in the rat. Brain Res. 1978;142:1–24. doi: 10.1016/0006-8993(78)90173-7. [DOI] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature. 1996;382:363–366. doi: 10.1038/382363a0. [DOI] [PubMed] [Google Scholar]
- Mair RD, Kauer JA. Amphetamine depresses excitatory synaptic transmission at prefrontal cortical layer V synapses. Neuropharmacology. 2007;52:193–199. doi: 10.1016/j.neuropharm.2006.07.004. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora F, Sweeney KF, Rolls ET, Sanguinetti AM. Spontaneous firing rate of neurones in the prefrontal cortex of the rat: evidence for a dopaminergic inhibition. Brain Res. 1976;116:516–522. doi: 10.1016/0006-8993(76)90500-x. [DOI] [PubMed] [Google Scholar]
- Morshedi MM, Rademacher DJ, Meredith GE. Increased synapses in the medial prefrontal cortex are associated with repeated amphetamine administration. Synapse. 2009;63:126–135. doi: 10.1002/syn.20591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder AB, Nordquist RE, Orgut O, Pennartz CM. Learning-related changes in response patterns of prefrontal neurons during instrumental conditioning. Behav Brain Res. 2003;146:77–88. doi: 10.1016/j.bbr.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Nogueira L, Kalivas PW, Lavin A. Long-term neuroadaptations produced by withdrawal from repeated cocaine treatment: role of dopaminergic receptors in modulating cortical excitability. J Neurosci. 2006;26:12308–12313. doi: 10.1523/JNEUROSCI.3206-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onn SP, Grace AA. Amphetamine Withdrawal Alters Bistable States and Cellular Coupling in Rat Prefrontal Cortex and Nucleus Accumbens Neurons Recorded In Vivo. J Neurosci. 2000;20:2332–2345. doi: 10.1523/JNEUROSCI.20-06-02332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th New York: Academic Press; 1998. [Google Scholar]
- Peterson JD, Wolf ME, White FJ. Repeated amphetamine administration decreases D1 dopamine receptor-mediated inhibition of voltage-gated sodium currents in the prefrontal cortex. J Neurosci. 2006;26:3164–3168. doi: 10.1523/JNEUROSCI.2375-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JD, Wolf ME, White FJ. Altered responsiveness of medial prefrontal cortex neurons to glutamate and dopamine after withdrawal from repeated amphetamine treatment. Synapse. 2000;36:342–344. doi: 10.1002/(SICI)1098-2396(20000615)36:4<342::AID-SYN11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebec GV, Sun W. Neuronal substrates of relapse to cocaine-seeking behavior: role of prefrontal cortex. J Exp Anal Behav. 2005;84:653–666. doi: 10.1901/jeab.2005.105-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47 1:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Segal DS, Schuckit MA. Animal models of stimulant-induced psychosis. In: Creese I, editor. Stimulants: Neurochemical, Behavioral, and Clinical Perspectives. New York: Raven Press; 1983. pp. 131–167. [Google Scholar]
- Selemon LD, Begovic A, Goldman-Rakic PS, Castner SA. Amphetamine sensitization alters dendritic morphology in prefrontal cortical pyramidal neurons in the non-human primate. Neuropsychopharmacology. 2007;32:919–931. doi: 10.1038/sj.npp.1301179. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Singer BF, Tanabe LM, Gorny G, Jake-Matthews C, Li Y, Kolb B, Vezina P. Amphetamine-induced changes in dendritic morphology in rat forebrain correspond to associative drug conditioning rather than nonassociative drug sensitization. Biol Psychiatry. 2009;65:835–840. doi: 10.1016/j.biopsych.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV. Clinical psychopharmacology of AD/HD: implications for animal models. Neurosci Biobehav Rev. 2000;24:27–30. doi: 10.1016/s0149-7634(99)00061-5. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Roesch MR, Franz TM, Burke KA, Schoenbaum G. Abnormal associative encoding in orbitofrontal neurons in cocaine-experienced rats during decision-making. Eur J Neurosci. 2006;24:2643–2653. doi: 10.1111/j.1460-9568.2006.05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanis JJ, Burns RM, Sherrill LK, Gulley JM. Disparate cocaine-induced locomotion as a predictor of choice behavior in rats trained in a delay-discounting task. Drug Alcohol Depend. 2008;98:54–62. doi: 10.1016/j.drugalcdep.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Effects of repeated treatment with amphetamine or phencyclidine on working memory in the rat. Behav Brain Res. 2002;134:267–274. doi: 10.1016/s0166-4328(02)00040-2. [DOI] [PubMed] [Google Scholar]
- Stewart J, Vezina P. Extinction procedures abolish conditioned stimulus control but spare sensitized responding to amphetamine. Behav Pharmacol. 1991;2:65–71. [PubMed] [Google Scholar]
- Strakowski SM, Sax KW, Rosenberg HL, DelBello MP, Adler CM. Human response to repeated low-dose d-amphetamine: evidence for behavioral enhancement and tolerance. Neuropsychopharmacology. 2001;25:548–554. doi: 10.1016/S0893-133X(01)00253-6. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Sax KW, Setters MJ, Keck PEJ. Enhanced response to repeated d-amphetamine challenge: evidence for behavioral sensitization in humans. Biol Psychiatry. 1996;40:872–880. doi: 10.1016/0006-3223(95)00497-1. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci. 2006;26:8004–8008. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Trantham H, Szumlinski KK, McFarland K, Kalivas PW, Lavin A. Repeated cocaine administration alters the electrophysiological properties of prefrontal cortical neurons. Neuroscience. 2002;113:749–753. doi: 10.1016/s0306-4522(02)00246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol. 2002;442:163–187. doi: 10.1002/cne.10083. [DOI] [PubMed] [Google Scholar]
- Vezina P. D1 dopamine receptor activation is necessary for the induction of sensitization by amphetamine in the ventral tegmental area. J Neurosci. 1996;16:2411–2420. doi: 10.1523/JNEUROSCI.16-07-02411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Dahlin SL, Hu XT, Xue CJ, White K. Effects of lesions of prefrontal cortex, amygdala, or fornix on behavioral sensitization to amphetamine: comparison with N-methyl-D-aspartate antagonists. Neuroscience. 1995;69:417–439. doi: 10.1016/0306-4522(95)00248-h. [DOI] [PubMed] [Google Scholar]