Abstract
Purpose
The objective of this study was to examine the associations between paternal age and birth defects of unknown etiologies while carefully controlling for maternal age.
Methods
Using 1997–2004 data from the National Birth Defects Prevention Study, we fit logistic regression models with paternal and maternal age as continuous variables while adjusting for demographic and other factors.
Results
Elevated odds ratios for each year increase in paternal age were found for cleft palate (odds ratio (OR) = 1.02, 95% confidence interval (CI): 1.00, 1.04), diaphragmatic hernia (OR = 1.04, 95% CI: 1.02, 1.06), right ventricular outflow tract obstruction (OR = 1.03, 95% CI: 1.01, 1.04), and pulmonary valve stenosis (OR = 1.02, 95% CI: 1.01, 1.04). At younger paternal ages, each year increase in paternal age correlated with increased odds of having offspring with encephalocele, cataract, esophageal atresia, anomalous pulmonary venous return, and coarctation of the aorta, but these increased odds were not observed at older paternal ages. The effect of paternal age was modified by maternal age for gastroschisis, omphalocele, spina bifida, all orofacial clefts, and septal heart defects.
Conclusions
Our findings suggest that paternal age may be a risk factor for some multifactorial birth defects.
Keywords: congenital abnormalities, paternal age, maternal age, risk factors
Advanced paternal age is associated with increased DNA mutations and chromosomal aberrations in sperm (1, 2, 3, 4). The risk of miscarriage rises with paternal age (1, 5, 6, 7, 8), consistent with an increased frequency of genetic abnormalities in the embryo. Due to the increase in DNA mutations in sperm, older men are at higher risk of fathering children with certain autosomal dominant genetic disorders, such as achondroplasia, Apert syndrome, and Marfan syndrome (4, 9, 10, 11, 12). Genetic changes in sperm associated with advanced paternal age could lead to an increased risk for birth defects in offspring. Previous studies have found associations between advanced paternal age and several birth defects, including cleft lip (13, 14), cleft palate (13, 14, 15), all orofacial clefts (16, 17, 18, 19), hydrocephalus (17, 20), neural tube defects (21), hypospadias (22), craniosynostosis (22), congenital cataracts (21), limb reduction defects (21), malformations of the extremities (23), tracheo-esophageal fistula/esophageal atresia (24), pulmonic stenosis (17), atrial septal defects (ASDs) (25), ventricular septal defects (VSDs) (25), situs inversus (20), and congenital heart defects (overall) (14, 25). At the other end of the age spectrum, associations have been reported between young paternal age and neural tube defects (21, 24, 26), hypospadias (21), gastroschisis (24, 27, 28, 29, 30), ASDs (20, 25), VSDs (20, 25), and congenital heart defects (overall) (24, 31). However, these studies have not generally replicated each others’ findings. Furthermore, the definition of young or advanced paternal age varied between studies, as well as whether maternal and paternal ages were analyzed categorically or as continuous variables.
The associations between birth defects and paternal age need to be comprehensively evaluated, with careful attention paid to potential confounding by maternal age. Our study used data from the National Birth Defects Prevention Study (NBDPS), one of the largest series of cases with available paternal age (over 15,000 cases). We analyzed both paternal and maternal age as continuous variables and included paternal and maternal age interaction terms when needed.
METHODS
Design and study population
We analyzed data from the NBDPS, an ongoing, population-based, multicenter case-control study designed to investigate genetic and environmental risk factors for major birth defects. Case infants with specific major structural birth defects were identified through birth defects surveillance programs in Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, and Utah. Clinical geneticists reviewed case records, including hospital reports and medical records, to ensure the birth defect(s) met the NBDPS case definition. Cases included live born infants, stillborn infants, and terminations. Infants with recognized or strongly suspected single-gene disorders or chromosomal abnormalities were excluded. Control infants were live born infants with no major birth defects from the same geographic locations, identified through hospital or vital records. Mothers of case and control infants were interviewed by telephone, with response rates of 72% for case mothers and 69% for control mothers. The NBDPS was approved by the institutional review boards of participating study centers and the Centers for Disease Control and Prevention, and written informed consent was obtained from all mothers. Yoon et al. (32) describe the study methods in detail.
This analysis included infants born on or after October 1, 1997, with estimated delivery dates on or before December 31, 2004 and complete maternal interviews. Children whose mothers reported having type 1 or type 2 diabetes were excluded. Paternal and maternal ages at the estimated delivery date were calculated using information from maternal interviews. Analyses were performed for birth defect categories containing ≥100 case children (Table 1) and for aggregate defect categories, including heart defects (overall) and all orofacial clefts. In addition, we looked for associations with isolated defects (i.e., cases without multiple major defects) for all defect categories. We evaluated associations between paternal age and multiple major defects, comprising cases classified as multiple from all defect categories or complex from all defect categories except amnion rupture sequence and limb body wall complex. (“Complex” refers to groups of defects often found co-occurring but which are not associated with a known genetic mutation.) Cases categorized as complex were excluded from the analyses of diaphragmatic hernia, omphalocele, and spina bifida, since they consisted mainly of Pentalogy of Cantrell and OEIS (Omphalocele, Exstrophy, Imperforate anus, Spinal defects). Cases categorized as syndromic (due to a strong family history of congenital cataracts) were excluded from the analysis of cataracts, since some syndromic cases might be due to a single gene mutation and thus have a different etiology from other cataracts cases.
Table 1.
Number of Case Children in each Birth Defect Category Included in Current Study By Paternal Age, From the National Birth Defects Prevention Study 1997–2004.
| Birth defect category | Number of case children by paternal age (years) |
||||
|---|---|---|---|---|---|
| 24 and younger | 25–34 | 35 and older | Missing | Total | |
| Amniotic band syndrome, limb body wall complex | 67 (38%) | 82 (46%) | 19 (11%) | 9 (5%) | 177 |
| Anencephaly, craniorachischisis | 68 (23%) | 148 (50%) | 69 (23%) | 11 (4%) | 296 |
| Spina bifida | 133 (22%) | 297 (48%) | 157 (25%) | 31 (5%) | 618 |
| Encephalocele | 24 (21%) | 56 (49%) | 25 (22%) | 10 (9%) | 115 |
| Neural tube defects (overall) | 225 (22%) | 501 (49%) | 251 (24%) | 52 (5%) | 1029 |
| Hydrocephaly | 62 (24%) | 124 (48%) | 60 (23%) | 15 (6%) | 261 |
| Anophthalmos, microphthalmos | 25 (19%) | 64 (49%) | 36 (28%) | 5 (4%) | 130 |
| Cataracts | 32 (17%) | 103 (55%) | 50 (27%) | 2 (1%) | 187 |
| Anotia, microtia | 84 (24%) | 176 (50%) | 81 (23%) | 13 (4%) | 354 |
| Heart defects (overall) | 1,328 (20%) | 3,319 (50%) | 1,729 (26%) | 253 (4%) | 6,629 |
| Conotruncal heart defects | 245 (19%) | 652 (50%) | 362 (28%) | 41 (3%) | 1,300 |
| Transposition of the great arteries | 81 (20%) | 214 (53%) | 104 (26%) | 8 (2%) | 407 |
| Tetralogy of Fallot | 103 (18%) | 283 (49%) | 168 (29%) | 24 (4%) | 578 |
| Single ventricle/complex heart | 41 (23%) | 93 (51%) | 45 (25%) | 2 (1%) | 181 |
| Septal heart defects | 637 (22%) | 1,421 (48%) | 741 (25%) | 134 (5%) | 2,933 |
| Perimembranous VSD | 244 (21%) | 552 (47%) | 325 (28%) | 59 (5%) | 1,180 |
| Conoventricular VSD | 19 (19%) | 53 (52%) | 27 (26%) | 3 (3%) | 102 |
| Muscular VSD | 29 (16%) | 96 (52%) | 54 (30%) | 4 (2%) | 183 |
| ASD secundum | 284 (22%) | 631 (50%) | 288 (23%) | 61 (5%) | 1,264 |
| ASD NOS | 96 (23%) | 201 (48%) | 103 (25%) | 16 (4%) | 416 |
| Atrioventricular septal defects | 33 (21%) | 81 (52%) | 36 (23%) | 5 (3%) | 155 |
| Right ventricular outflow tract obstruction | 188 (19%) | 509 (50%) | 274 (27%) | 40 (4%) | 1,011 |
| Pulmonary atresia | 32 (25%) | 57 (44%) | 36 (28%) | 5 (4%) | 130 |
| Pulmonary valve stenosis | 127 (17%) | 388 (52%) | 197 (27%) | 28 (4%) | 740 |
| Left ventricular outflow tract obstruction | 171 (17%) | 542 (53%) | 274 (27%) | 28 (3%) | 1,015 |
| Aortic stenosis | 31 (14%) | 122 (57%) | 59 (27%) | 3 (1%) | 215 |
| Hypoplastic left heart syndrome | 48 (16%) | 165 (54%) | 79 (26%) | 12 (4%) | 304 |
| Coarctation of the aorta | 95 (18%) | 272 (51%) | 152 (29%) | 14 (3%) | 533 |
| Anomalous pulmonary venous return | 45 (25%) | 94 (52%) | 36 (20%) | 6 (3%) | 181 |
| Total anomalous pulmonary venous return | 41 (28%) | 72 (49%) | 30 (20%) | 5 (3%) | 148 |
| Heterotaxia with congenital heart defect | 37 (21%) | 88 (51%) | 34 (20%) | 14 (8%) | 173 |
| Cleft lip without cleft palate | 109 (20%) | 263 (49%) | 139 (26%) | 23 (4%) | 534 |
| Cleft lip and palate | 270 (27%) | 447 (45%) | 242 (24%) | 34 (3%) | 993 |
| Cleft palate | 138 (17%) | 419 (51%) | 226 (28%) | 31 (4%) | 814 |
| All orofacial clefts | 517 (22%) | 1,129 (48%) | 607 (26%) | 88 (4%) | 2,341 |
| Biliary atresia | 24 (25%) | 51 (53%) | 20 (21%) | 1 (1%) | 96 |
| Esophageal atresia | 63 (17%) | 173 (46%) | 122 (33%) | 17 (5%) | 375 |
| Jejunal/ileal atresia/stenosis | 55 (24%) | 110 (47%) | 61 (26%) | 7 (3%) | 233 |
| Duodenal atresia/stenosis | 26 (25%) | 51 (48%) | 27 (25%) | 2 (2%) | 106 |
| Anorectal atresia/stenosis | 130 (23%) | 250 (45%) | 151 (27%) | 23 (4%) | 554 |
| Hypospadias, 2nd/3rd degree | 176 (16%) | 549 (49%) | 364 (32%) | 40 (4%) | 1,129 |
| Bilateral renal agenesis/hypoplasia | 21 (22%) | 45 (48%) | 20 (21%) | 8 (9%) | 94 |
| Longitudinal limb deficiencies | 54 (23%) | 115 (50%) | 50 (22%) | 13 (6%) | 232 |
| Transverse limb deficiencies | 77 (21%) | 182 (50%) | 89 (24%) | 16 (4%) | 364 |
| Limb deficiencies (overall) | 138 (22%) | 311 (50%) | 145 (23%) | 31 (5%) | 625 |
| Craniosynostosis | 96 (14%) | 341 (51%) | 215 (32%) | 14 (2%) | 666 |
| Diaphragmatic hernia | 83 (19%) | 202 (47%) | 131 (30%) | 17 (4%) | 433 |
| Omphalocele | 42 (18%) | 128 (54%) | 62 (26%) | 5 (2%) | 237 |
| Gastroschisis | 354 (58%) | 202 (33%) | 32 (5%) | 24 (4%) | 612 |
| Multiple and complex defects* | 480 (23%) | 977 (46.7%) | 521 (24.9%) | 112 (5.4%) | 2,090 |
VSD, ventricular septal defect; ASD, atrial septal defect; NOS, not otherwise specified
Case children with multiple and complex defects are also included in individual defect categories. Complex defects refer to commonly co-occurring groups of defects (e.g., Pentalogy of Cantrell).
Statistical analyses and potential confounders
For each defect category, we first fit quadratic logistic regression models with defect status as the outcome and paternal age and maternal age as independent variables. Ages were modeled continuously with initial models including linear and quadratic main effects for maternal and paternal age as well as all two-way interactions between the age variables. To determine which birth defect categories required paternal and maternal age interaction terms, the −2 log likelihood ratio for models with and without the paternal and maternal age interaction terms were compared for each defect category. If the difference between the two −2 log likelihood ratios was equal to or less than the chi-square value (4 degrees of freedom, so chi-square = 9.48), then the interaction terms were dropped from the model for that defect category. If the difference was >9.48, then the interaction terms were individually removed from the model to determine which ones had to be kept in the model. Once final models for maternal and paternal age were derived, demographic and other variables were included in the models as main effect. Possible interaction terms between these factors and paternal age were evaluated, but none were required in any of the models.
Demographic and other covariates included in the models were paternal race and ethnicity (nonHispanic white, all other races and ethnicities), paternal education (0–12 years, >12 years), paternal birthplace (United States, outside United States), maternal alcohol use during pregnancy (yes, no), maternal smoking during pregnancy (yes, no), gravidity (primagravida, previous pregnancy), previous miscarriage or stillbirth (yes, no), pregnancy plurality (singleton, multiple), maternally reported paternal drug use (yes, no), periconceptional folic acid use (yes, no), use of assisted reproductive technology (yes, no), and maternal body mass index (BMI ≥ 30 kg/m2, BMI < 30 kg/m2). Demographic and other characteristics of cases and controls are described in Cogswell et al. (33).
Adjusted per year odds ratios (ORs) and 95% confidence intervals (CIs) were estimated for defect categories with final models having only a linear paternal age term and no interaction between paternal and maternal age. We calculated ORs and CIs at selected paternal age differences for the defect categories with final models that included quadratic paternal age effects. For defect categories with final models that included interactions between maternal and paternal age, we calculated ORs and CIs for selected combinations of these variables.
RESULTS
Using 1997–2004 NBDPS data, we investigated associations with paternal age for birth defect categories with ≥100 cases, with a control group of 5,839 nonmalformed live born infants (Table 1). The defect categories cleft palate, diaphragmatic hernia, right ventricular outflow tract obstruction (RVOTO), and pulmonary valve stenosis (PVS) showed significant linear associations with paternal age without requiring interaction terms or the quadratic term for paternal age in their models (Table 2). To examine defects which could have different phenotypes but a common etiology, we created a category including all cases with complex or multiple defects. A subset might have unrecognized single gene disorders associated with advanced paternal age, as seen for certain known single gene conditions. This category, multiple and complex defects (overall), showed significant interactions with paternal age without requiring either interaction terms or the quadratic term for paternal age in the model (Table 2). For each of these defect categories, we analyzed the cases with isolated defects, excluding case children with complex and multiple defects. Results from these analyses were similar to the results when all cases were included (Table 2). Analyses controlling only for maternal age also showed similar results (Supplementary Table 1).
Table 2.
Associations Between Paternal Age and Birth Defect Categories Without Paternal and Maternal Age Interaction Terms for All Defects in Category and for Isolated Defects Only, National Birth Defects Prevention Study 1997–2004.
| Defect category | All defects | Isolated defects only | ||||
|---|---|---|---|---|---|---|
| Adjusted odds ratio per year increase in paternal age* | 95% confidence interval | P | Adjusted odds ratio per year increase in paternal age* | 95% confidence interval | P | |
| Cleft palate | 1.02 | 1.00, 1.04 | .037 | 1.02 | 1.00, 1.04 | .039 |
| Diaphragmatic hernia† | 1.04 | 1.02, 1.06 | .001 | 1.04 | 1.01, 1.07 | .007 |
| PVS | 1.02 | 1.01, 1.04 | .011 | 1.03 | 1.01, 1.05 | .010 |
| RVOTO | 1.03 | 1.01, 1.04 | .002 | 1.03 | 1.01, 1.05 | .003 |
| Multiple and complex defects (overall) | 1.02 | 1.00, 1.03 | .007 | NA | NA | NA |
PVS, pulmonary valve stenosis; RVOTO, right ventricular outflow tract obstruction
Adjusted for maternal age, maternal age2, gravidity, periconceptional folic acid use, maternal body mass index, paternal birthplace, paternal education, paternal race and ethnicity, singleton/multiple birth, maternal smoking, maternal alcohol use, paternal drug use, use of assisted reproductive technology, and previous stillbirth or miscarriage
Cases categorized as complex (mostly Pentalogy of Cantrell) were excluded from analysis of diaphragmatic hernia
Models of the association of paternal age with encephalocele, cataracts, esophageal atresia, APVR, and coarctation of the aorta required the quadratic term for paternal age indicating that the per year change in the odds was not constant across paternal age. Adjusted ORs at paternal ages of 20, 30, and 40 years are shown in Table 3 for each of these defect categories. These ORs represent the change in odds between a single year difference in paternal age; for example, the odds for a father who is 20 years of age compared with one who is 19. At younger paternal ages (age 20 and, for some categories, age 30), the odds increased with each year increase in paternal age, whereas the odds did not increase with each year increase in paternal age at older paternal age (age 40) for all the defect categories in Table 3. However, although the per year increase in odds was lower for older paternal ages, the cumulative odds of having a child with encephalocele, cataracts, esophageal atresia, APVR, or coarctation of the aorta was estimated to be substantially greater among older as compared to younger fathers. Esophageal atresia is part of the VATER/VACTERL association (Vertebral, Anal, Cardiac, Tracheo-Esophageal, Renal, and Limb anomalies), which in some instances could be due to unrecognized single gene de novo mutations with an elevated advanced paternal age risk, and thus might be driving the association with paternal age. Therefore, we examined the relationship between paternal age and esophageal atresia excluding VATER/VACTERL cases and saw a slightly more significant association at younger paternal ages, suggesting that VATER/VACTERL cases are not the dominant factor in the association of paternal age with esophageal atresia. Results from analyses controlling only for maternal age and analyses of isolated defects only showed similar results (Supplementary Tables 2 and 3).
Table 3.
Associations Between Paternal Age and Birth Defect Categories With Quadratic Paternal Age Terms, National Birth Defects Prevention Study 1997–2004.
| Defect category | Paternal age | Adjusted odds ratio per year increase in paternal age* | 95% confidence interval | P |
|---|---|---|---|---|
| APVR | 20 | 1.11 | 1.01, 1.22 | 0.03 |
| 30 | 1.02 | 0.98, 1.07 | 0.27 | |
| 40 | 0.94 | 0.87, 1.02 | 0.15 | |
| Cataract† | 20 | 1.12 | 1.01, 1.23 | 0.03 |
| 30 | 1.04 | 1.00, 1.09 | 0.07 | |
| 40 | 0.97 | 0.91, 1.04 | 0.42 | |
| Coarctation of the aorta | 20 | 1.06 | 1.01, 1.12 | 0.03 |
| 30 | 1.03 | 1.00, 1.05 | 0.05 | |
| 40 | 0.99 | 0.95, 1.02 | 0.51 | |
| Encephalocele | 20 | 1.15 | 1.02, 1.29 | 0.03 |
| 30 | 1.03 | 0.97, 1.08 | 0.32 | |
| 40 | 0.92 | 0.83, 1.02 | 0.10 | |
| Esophageal atresia | 20 | 1.07 | 1.00, 1.14 | 0.04 |
| 30 | 1.03 | 1.00, 1.07 | 0.04 | |
| 40 | 1.00 | 0.96, 1.04 | 0.92 | |
| Esophageal atresia excluding | 20 | 1.09 | 1.01, 1.17 | 0.03 |
| VATER/VACTERL | 30 | 1.04 | 1.00, 1.08 | 0.03 |
| 40 | 1.00 | 0.95, 1.04 | 0.83 |
APVR, anomalous pulmonary venous return; VATER/VACTERL, Vertebral, Anal, Cardiac, Tracheo-Esophageal, Renal, and Limb anomalies
Adjusted for maternal age, maternal age2, gravidity, periconceptional folic acid use, maternal body mass index, paternal birthplace, paternal education, paternal race and ethnicity, singleton/multiple birth, maternal smoking, maternal alcohol use, paternal drug use, use of assisted reproductive technology, and previous stillbirth or miscarriage
Cases categorized as syndromic (due to a strong family history of congenital cataracts) were excluded from analysis of cataracts
For defect categories with paternal and maternal age interaction terms, we summarized the modeling results using graphs of the estimated ORs across a range of maternal and paternal ages (Figure 1). We calculated estimated ORs for these categories for specified differences in paternal age at selected values for maternal age (Table 4). Unlike most other defects studied, gastroschisis showed an association with young paternal age, but only when mothers were average (age 28) or above average age (age 35) (Figures 1A and Table 4). Furthermore, the combination of advanced paternal and maternal age appeared to have a protective effect for gastroschisis. In contrast, the combination of young maternal and paternal age was protective for omphalocele, spina bifida, all orofacial clefts, and septal heart defects. For omphalocele, increasing paternal age showed an association when maternal age was young, but correlated with decreasing odds when mothers were advanced age (Figures 1B and Table 4). Spina bifida was linked with increasing paternal age for younger but not older mothers (Figures 1C and Table 4). All orofacial clefts showed an association with increased paternal age when the mother was younger, but paternal age appeared to have more of a U-shaped effect when the mother was older (Figures 1D and Table 4). Septal heart defects were associated with increased paternal age when mothers were younger. Older mothers showed an increased odds of having a child with a septal heart defect, with a slight decrease with increasing paternal age (Figure 1E and Table 4). Results from analyses controlling only for maternal age and analyses of isolated defects only showed similar results (Supplementary Tables 4 and 5). The association with omphalocele appears slightly more significant when only isolated defects are included, indicating that cases with multiple defects are not the predominant factor in the association between paternal age and omphalocele.
Figure 1.
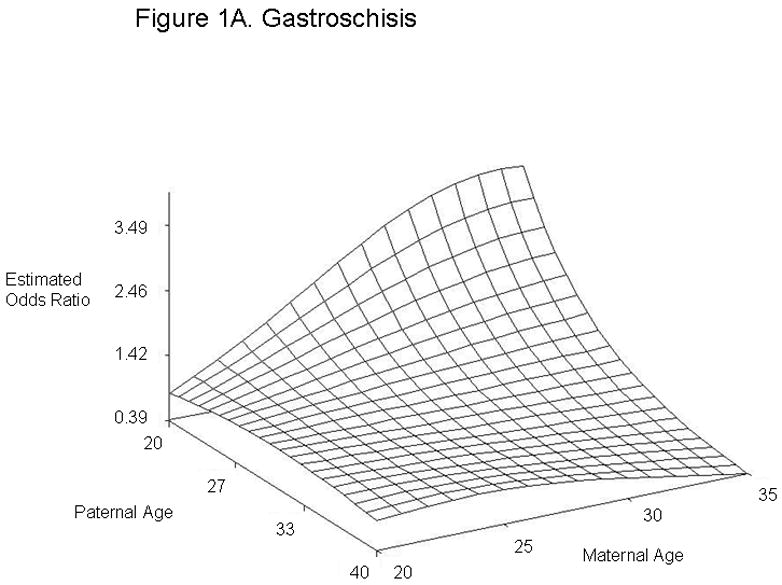
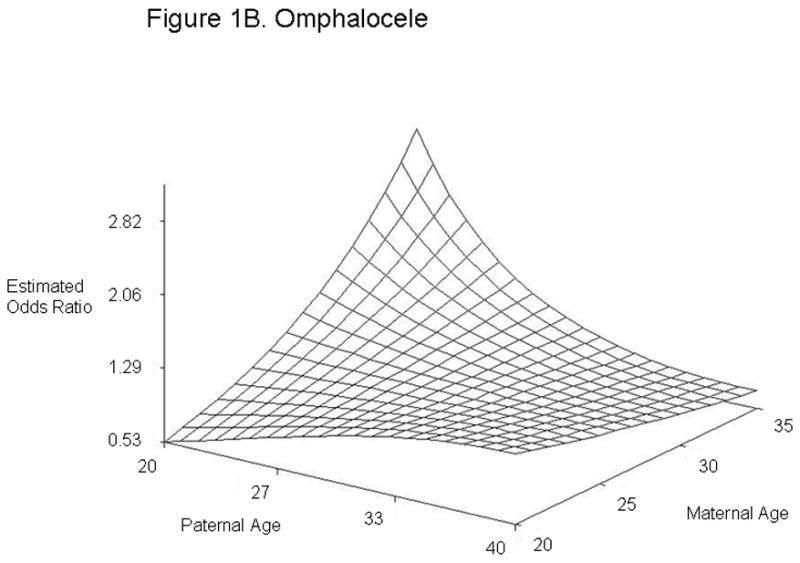
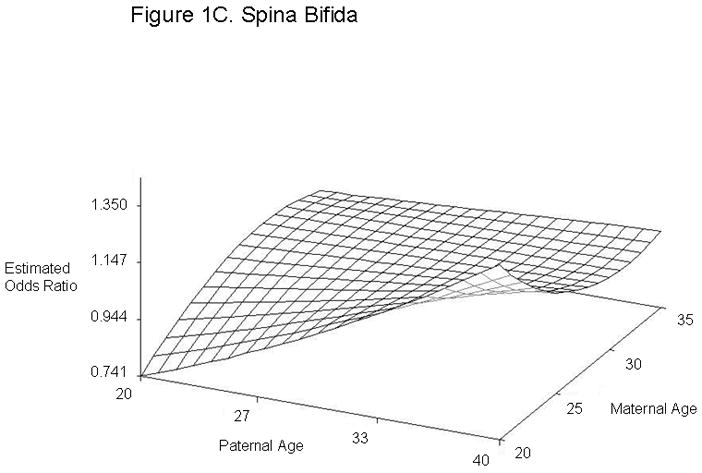
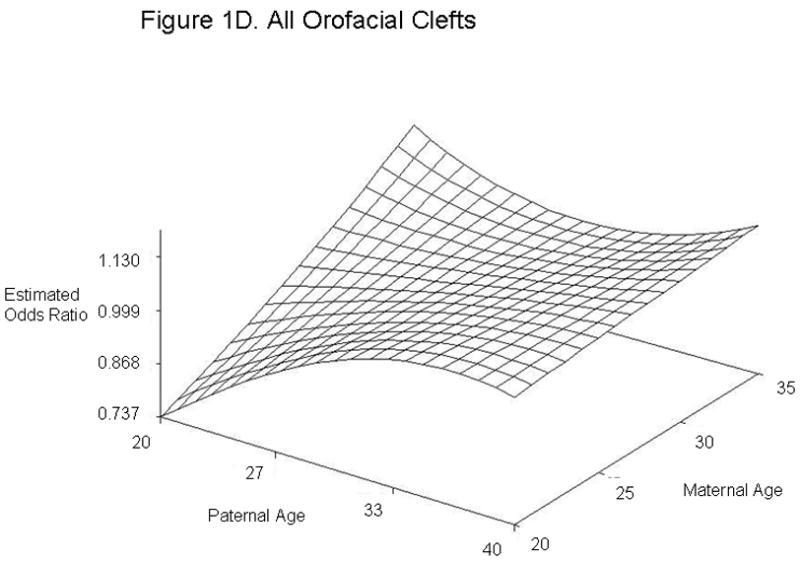
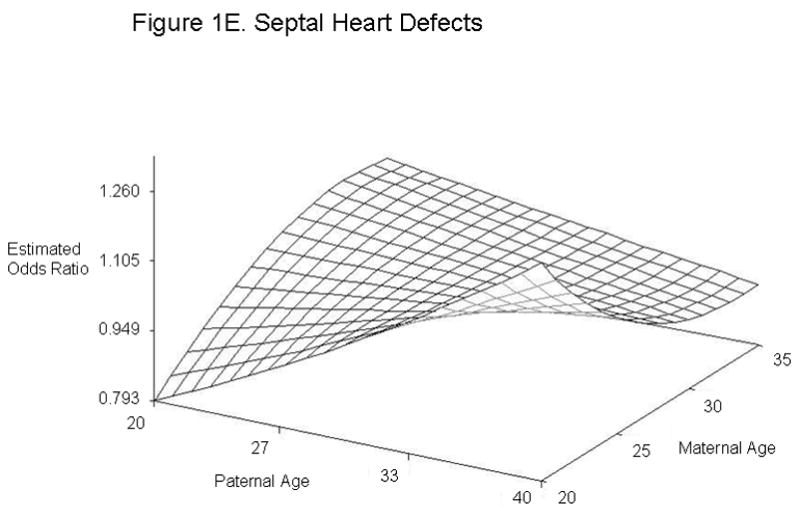
Estimated Odds Ratios of the Association Between Paternal Age (Referent = 30) and Gastroschisis (A), Omphalocele (B), Spina Bifida (C), All Orofacial Clefts (D), and Septal Heart Defects (E) From the National Birth Defects Prevention Study 1997–2004. Covariates included in the analyses were maternal age, maternal age2, gravidity, periconceptional folic acid use, maternal body mass index, paternal birthplace, paternal education, paternal race and ethnicity, singleton/multiple birth, maternal smoking, maternal alcohol use, paternal drug use, use of assisted reproductive technology, and previous stillbirth or miscarriage. The following maternal and paternal age interaction terms were included in the logistic regression models for each of the defects listed: maternal age x paternal age (gastroschisis, spina bifida, omphalocele, all orofacial clefts, and all septal defects); maternal age2 × paternal age (gastroschisis, spina bifida, and all septal defects); maternal age × paternal age2 (gastroschisis, omphalocele, and all orofacial clefts) and maternal age2 × paternal age2 (gastroschisis). Cases categorized as complex (mostly Pentalogy of Cantrell or OEIS (Omphalocele, Exstrophy, Imperforate anus, Spinal defects)) were excluded from analyses of omphalocele and spina bifida.
Table 4.
Estimated Odds Ratios and 95% Confidence Intervals at Different Paternal and Maternal Ages for Defect Categories With Paternal and Maternal Age Interaction Terms, National Birth Defects Prevention Study 1997–2004.
| Defect category | Paternal age (years)* | Maternal age (years) | Adjusted odds ratio† | 95% confidence interval |
|---|---|---|---|---|
| Gastroschisis‡ | 20 | 20 | 0.83 | 0.60, 1.15 |
| 40 | 20 | 0.83 | 0.47, 1.47 | |
| 20 | 28 | 2.67 | 1.61, 4.42 | |
| 40 | 28 | 0.80 | 0.54, 1.21 | |
| 20 | 35 | 3.47 | 1.02, 11.74 | |
| 40 | 35 | 0.39 | 0.18, 0.82 | |
| Omphaloceleठ| 20 | 20 | 0.53 | 0.28, 1.01 |
| 40 | 20 | 1.23 | 0.62, 2.42 | |
| 20 | 28 | 1.29 | 0.72, 2.30 | |
| 40 | 28 | 0.92 | 0.64, 1.33 | |
| 20 | 35 | 2.82 | 1.14, 6.94 | |
| 40 | 35 | 0.72 | 0.47, 1.08 | |
| Spina bifidaठ| 20 | 20 | 0.74 | 0.54, 1.02 |
| 40 | 20 | 1.35 | 0.98, 1.86 | |
| 20 | 28 | 0.97 | 0.78, 1.21 | |
| 40 | 28 | 1.03 | 0.82, 1.28 | |
| 20 | 35 | 0.97 | 0.71, 1.33 | |
| 40 | 35 | 1.03 | 0.75, 1.40 | |
| All orofacial clefts‡ | 20 | 20 | 0.74 | 0.58, 0.93 |
| 40 | 20 | 1.04 | 0.80, 1.35 | |
| 20 | 28 | 0.93 | 0.73, 1.17 | |
| 40 | 28 | 1.07 | 0.94, 1.23 | |
| 20 | 35 | 1.13 | 0.75, 1.69 | |
| 40 | 35 | 1.11 | 0.92, 1.34 | |
| Septal defect‡ | 20 | 20 | 0.79 | 0.66, 0.96 |
| 40 | 20 | 1.26 | 1.04, 1.53 | |
| 20 | 28 | 1.02 | 0.91, 1.16 | |
| 40 | 28 | 0.98 | 0.87, 1.10 | |
| 20 | 35 | 1.07 | 0.92, 1.24 | |
| 40 | 35 | 0.93 | 0.80, 1.08 |
Referent paternal age was 30 years
Adjusted for maternal age, maternal age2, gravidity, periconceptional folic acid use, maternal body mass index, paternal birthplace, paternal education, paternal race and ethnicity, singleton/multiple birth, maternal smoking, maternal alcohol use, paternal drug use, use of assisted reproductive technology, and previous stillbirth or miscarriage
The following maternal and paternal age interaction terms were included in the logistic regression models for each of the defects listed: maternal age × paternal age (gastroschisis, spina bifida, omphalocele, all orofacial clefts, and all septal defects); maternal age2 × paternal age (gastroschisis, spina bifida, and all septal defects); maternal age × paternal age2 (gastroschisis, omphalocele, and all orofacial clefts) and maternal age2 × paternal age2 (gastroschisis)
Cases categorized as complex (mostly Pentalogy of Cantrell or OEIS (Omphalocele, Exstrophy, Imperforate anus, Spinal defects)) were excluded from analyses of omphalocele and spina bifida
In their study, Archer et al. (27) detected a selection bias, whereby younger mothers were more likely to have missing paternal age information and thus be excluded from their study. The percentage of cases with missing paternal age information ranged from 11.2 % to 55.0 % for different defect categories in their study. The percentage of cases and controls with missing paternal age information, categorized by maternal age in our study, is shown in Table 5. For each maternal age group, approximately the same percentage of cases and controls were missing information: 1.3 % to 8.4 % of cases and 1.2 % to 8.8 % of controls. While the younger mothers in both the case and control groups had the highest percentage of missing paternal age information, the percentages were lower than in Archer et al. (27).
Table 5.
Number and Percentage of Cases and Controls Missing Paternal Age, Categorized by Maternal Age, National Birth Defects Prevention Study 1997–2004.
| Maternal age | Case | Control | ||
|---|---|---|---|---|
| N | % | N | % | |
| 21 and under | 264 | 8.4 | 100 | 8.8 |
| 22–26 | 133 | 3.5 | 60 | 4.2 |
| 27–30 | 72 | 2.2 | 16 | 1.2 |
| 31–33 | 32 | 1.3 | 15 | 1.6 |
| 34 and over | 85 | 2.8 | 17 | 1.6 |
| Total | 586 | 3.7 | 208 | 3.6 |
DISCUSSION
We detected increased odds of having offspring with cleft palate, diaphragmatic hernia, RVOTO, PVS, and multiple or complex defects (overall) with each year increase in paternal age. While the incremental increase per year in the ORs does not appear large, consistent with previous findings, the difference in odds faced by men several years apart in age could be substantial. For example, we found an OR of 1.04 (95% CI: 1.02, 1.06) per year increase in paternal age for diaphragmatic hernia. This means that a 40 year old father would have twice the odds of having a child with diaphragmatic hernia compared with a 20 year old. Our findings are consistent with previous studies which found associations between advanced paternal age and offspring with cleft palate (13, 17, 30).
We found an increased OR with each year increase in paternal age for APVR, cataracts, coarctation of the aorta, encephalocele, and esophageal atresia, but only when fathers were younger. At older paternal ages, the ORs did not change with increasing paternal age, but instead reached a plateau. Yang et al. (24) also observed a correlation between increasing paternal age and offspring with esophageal atresia.
Young maternal age has been established as a risk factor for gastroschisis (30, 34, 35). Young paternal age appears not to contribute further to this elevated risk, as evidenced by the non-significant OR observed when both parents were young. However, younger paternal age showed an association with gastroschisis when the mother was aged 28 (average maternal age in our study) and 35 (advanced maternal age). Thus, both young paternal and maternal age could be independent risk factors for gastroschisis, with the maternal factor predominating when both parents are young. Our findings are consistent with earlier studies which identified an association between young paternal age and gastroschisis (25, 29, 30). Previous studies hypothesized that the increased risk for younger mothers could be due to behavioral factors and exposures more common among this age group, such as illicit drug use, smoking, diet, and infections (36), as well as the combination of younger maternal age and lower BMI (37). Younger fathers might share some of these risk factors and could influence the behavior of mothers whose age would otherwise place them in a lower risk category. However, our study cannot differentiate whether the increased odds with younger paternal age is due to biological or lifestyle factors, especially since most of these paternal exposures were not ascertained.
Omphalocele, spina bifida, all orofacial clefts, and septal heart defects mainly showed associations with advanced paternal age when maternal age was young. Omphalocele, all orofacial clefts, and septal heart defects were associated with the combination of younger paternal age and advanced maternal age. These findings suggest that a difference in age between the mother and father could be associated with these defects. Different exposures more common at either end of the age spectrum could have similar effects, for example, illicit drugs and prescription medications. However, our study cannot distinguish whether the increased odds are due to environmental or biological factors. Interestingly, previous studies have reported spina bifida or neural tube defects in offspring to be associated with both younger paternal age (21, 24, 26, 30) and older paternal age (21). Offspring with orofacial clefts have been associated with advanced paternal age (19, 30), while offspring with omphalocele have been associated with younger paternal age (24). Although we observed associations with paternal age for the combined category of septal heart defects, we did not see significant associations for ASDs or VSDs individually. This could be due to inadequate sample size to detect an association in the individual categories or could indicate that our findings are spurious. However, in support of our results, previous studies have described associations between advanced paternal age and offspring with ASDs and VSDs (20, 25).
Multiple or complex defects could be due to unrecognized syndromes with monogenic etiologies. Thus, the association with advanced paternal age, which has already been linked to certain single gene conditions, would not be surprising. Consistent with this, Harville et al. (15) found that older parents were more likely than younger parents to have offspring with cleft palate accompanied by other defects. Zhu et al. (23) found an association between advanced paternal age and syndromes affecting multiple systems. The associations with advanced paternal age that we observed for the multiple and complex defects (overall) category, as well as for the individual defect categories, could reflect a subset of these defects with a monogenic etiology. NBDPS excludes birth defects with a known single gene etiology; however, defects could be due to novel, unrecognized, or currently unknown genetic anomalies.
Compared with previous work, our study had important strengths. Due to the relatively large sample size, we were able to model both paternal and maternal age as continuous variables, using linear and quadratic terms, and to include potential interactions between maternal and paternal age. In contrast, most previous studies only considered paternal and maternal age categorically and did not include interaction terms between maternal and paternal age. Others have shown that residual confounding could occur in studies on the effect of paternal age when maternal age was controlled for categorically (38, 39); this was not an issue with our analyses. The increased sample size in our study allowed evaluation of individual birth defects, rather than grouped outcomes, providing greater likelihood of observing risks specific to certain defects. We could control for several demographic, behavioral, and medical factors that might affect the outcomes, a unique feature of this study. All case records in the NBDPS are reviewed by a medical geneticist and specific diagnostic criteria must be met for inclusion. Thus, the defect categories in our study might represent more homogenous groups than those in other studies. The population-based selection of cases and controls might reduce selection bias, and response rates by maternal age were similar. Also, unlike many risk factors, paternal age reporting should not be affected by recall bias.
Our study had some limitations. The number of multiple comparisons evaluated in our modeling approach likely produced some spurious findings. Paternal age information was provided by the mother and could not be independently verified. A larger percentage of younger mothers had missing paternal age information, and some centers did not interview mothers who were under 18 years of age. While the percentage of missing paternal age overall was low, Archer et al. (27) found that bias could exist even when only a small percentage of paternal ages are missing. We did not have enough information to calculate the prevalence by maternal age for cases with and without paternal age information, as Archer et al. (27) did. If present, this selection bias could have decreased associations seen with younger paternal age and increased associations with advanced paternal age. With the exception of maternal age, all confounders were dichotomized, because further subdividing confounders into multiple categories would have reduced our sample size and power to detect associations. However, controlling for some confounders categorically might have been better in terms of biological plausibility. For example, we examined paternal race and ethnicity as two categories, nonHispanic whites and all other races and ethnicities. As shown in Supplementary Table 6, a larger percentage of nonHispanic Black, Hispanic, and Native American/Alaskan Native fathers were 24 and under and a lower percentage were 25–34 and 35 and over, compared with nonHispanic White and Asian/Pacific Islander fathers, which could result in population stratification for defect categories more prevalent in certain races and ethnicities. However, when we performed analyses using either the categories nonHispanic whites, nonHispanic blacks, and all other races and ethnicites or nonHispanic whites, Hispanics, and all other races and ethnicities, we did not see any significant differences compared with when race and ethnicity were dichotomized (data not shown).
Current guidelines on genetic risk assessment and counseling for advanced paternal age are general and provide no clear definition of what age constitutes advanced paternal age (40, 41). No screening or diagnostic test panels specifically target conditions associated with advanced paternal age. Our findings on gastroschisis, omphalocele, orofacial clefts (overall), spina bifida, and septal heart defects indicate that maternal and paternal age should be considered together in assessing risk.
In summary, our study indicates that paternal age is associated with certain birth defects, and this association could provide clues to the etiology of these conditions. Ultimately this might lead to consideration of paternal as well as maternal age in counseling couples about risk for affected offspring.
Supplementary Material
Acknowledgments
The authors acknowledge Dr. Matthew Strickland for assistance with statistical methods, Dr. Jennita Reefhuis and Dr. Suzanne Gilboa for advice on data analysis, Dr. Sheree Boulet for assistance with data replication, and other National Birth Defects Prevention Study investigators for their valuable contributions. This research was supported in part by a grant from the National Institute of Environmental Health Sciences (P30ES10126). Conflict of interest: none declared.
ABBREVIATIONS
- APVR
anomalous pulmonary venous return
- ASD
atrial septal defect
- BMI
body mass index
- CI
confidence interval
- NBDPS
National Birth Defects Prevention Study
- OEIS
Omphalocele, Exstrophy, Imperforate anus, Spinal defects
- OR
odds ratio
- PVS
pulmonary valve stenosis
- RVOTO
right ventricular outflow tract obstruction
- VATER/VACTERL
Vertebral, Anal, Cardiac, Tracheo-Esophageal, Renal, and Limb anomalies
- VSD
ventricular septal defect
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Puscheck EE, Jeyendran RS. The impact of male factor on recurrent pregnancy loss. Curr Opin Obstet Gynecol. 2007;19:222–228. doi: 10.1097/GCO.0b013e32813e3ff0. [DOI] [PubMed] [Google Scholar]
- 2.Sartorelli EM, Mazzucatto LF, de Pina-Neto JM. Effect of paternal age on human sperm chromosomes. Fertil Steril. 2001;76:1119–1123. doi: 10.1016/s0015-0282(01)02894-1. [DOI] [PubMed] [Google Scholar]
- 3.Schmid TE, Eskenazi B, Baumgartner A, et al. The effects of male age on sperm DNA damage in healthy non-smokers. Hum Reprod. 2007;22:180–187. doi: 10.1093/humrep/del338. [DOI] [PubMed] [Google Scholar]
- 4.Wyrobek AJ, Eskenazi B, Young S, et al. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci U S A. 2006;103:9601–9606. doi: 10.1073/pnas.0506468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de la Rochebrochard E, Thonneau P. Paternal age and maternal age are risk factors for miscarriage; results of a multicentre European study. Hum Reprod. 2002;17:1649–1656. doi: 10.1093/humrep/17.6.1649. [DOI] [PubMed] [Google Scholar]
- 6.Kleinhaus K, Perrin M, Friedlander Y, et al. Paternal age and spontaneous abortion. Obstet Gynecol. 2006;108:369–377. doi: 10.1097/01.AOG.0000224606.26514.3a. [DOI] [PubMed] [Google Scholar]
- 7.Maconochie N, Doyle P, Prior S, et al. Risk factors for first trimester miscarriage--results from a UK-population-based case-control study. BJOG. 2007;114:170–186. doi: 10.1111/j.1471-0528.2006.01193.x. [DOI] [PubMed] [Google Scholar]
- 8.Slama R, Bouyer J, Windham G, et al. Influence of paternal age on the risk of spontaneous abortion. Am J Epidemiol. 2005;161:816–823. doi: 10.1093/aje/kwi097. [DOI] [PubMed] [Google Scholar]
- 9.Tolarova MM, Harris JA, Ordway DE, et al. Birth prevalence, mutation rate, sex ratio, parents’ age, and ethnicity in Apert syndrome. Am J Med Genet. 1997;72:394–398. doi: 10.1002/(sici)1096-8628(19971112)72:4<394::aid-ajmg4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 10.Risch N, Reich EW, Wishnick MM, et al. Spontaneous mutation and parental age in humans. Am J Hum Genet. 1987;41:218–248. [PMC free article] [PubMed] [Google Scholar]
- 11.Crow JF. The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet. 2000;1:40–47. doi: 10.1038/35049558. [DOI] [PubMed] [Google Scholar]
- 12.Rolf C, Nieschlag E. Reproductive functions, fertility and genetic risks of ageing men. Exp Clin Endocrinol Diabetes. 2001;109:68–74. doi: 10.1055/s-2001-14825. [DOI] [PubMed] [Google Scholar]
- 13.Bille C, Skytthe A, Vach W, et al. Parent’s age and the risk of oral clefts. Epidemiology. 2005;16:311–316. doi: 10.1097/01.ede.0000158745.84019.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollier LM, Leveno KJ, Kelly MA, et al. Maternal age and malformations in singleton births. Obstet Gynecol. 2000;96:701–706. doi: 10.1016/s0029-7844(00)01019-x. [DOI] [PubMed] [Google Scholar]
- 15.Harville EW, Wilcox AJ, Lie RT, et al. Epidemiology of cleft palate alone and cleft palate with accompanying defects. Eur J Epidemiol. 2007;22:389–395. doi: 10.1007/s10654-007-9129-y. [DOI] [PubMed] [Google Scholar]
- 16.Polednak AP. Paternal age in relation to selected birth defects. Hum Biol. 1976;48:727–739. [PubMed] [Google Scholar]
- 17.Savitz DA, Schwingl PJ, Keels MA. Influence of paternal age, smoking, and alcohol consumption on congenital anomalies. Teratology. 1991;44:429–440. doi: 10.1002/tera.1420440409. [DOI] [PubMed] [Google Scholar]
- 18.Rittler M, Lopez-Camelo J, Castilla EE. Sex ratio and associated risk factors for 50 congenital anomaly types: clues for causal heterogeneity. Birth Defects Res A Clin Mol Teratol. 2004;70:13–19. doi: 10.1002/bdra.10131. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez BS, Lopez ML, Rico MA, et al. Oral clefts: a retrospective study of prevalence and predisposal factors in the State of Mexico. J Oral Sci. 2008;50:123–129. doi: 10.2334/josnusd.50.123. [DOI] [PubMed] [Google Scholar]
- 20.Lian ZH, Zack MM, Erickson JD. Paternal age and the occurrence of birth defects. Am J Hum Genet. 1986;39:648–660. [PMC free article] [PubMed] [Google Scholar]
- 21.McIntosh GC, Olshan AF, Baird PA. Paternal age and the risk of birth defects in offspring. Epidemiology. 1995;6:282–288. doi: 10.1097/00001648-199505000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Singer S, Bower C, Southall P, et al. Craniosynostosis in Western Australia, 1980–1994: a population-based study. Am J Med Genet. 1999;83:382–387. doi: 10.1002/(sici)1096-8628(19990423)83:5<382::aid-ajmg8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 23.Zhu JL, Madsen KM, Vestergaard M, et al. Paternal age and congenital malformations. Hum Reprod. 2005;20:3173–3177. doi: 10.1093/humrep/dei186. [DOI] [PubMed] [Google Scholar]
- 24.Yang Q, Wen SW, Leader A, et al. Paternal age and birth defects: how strong is the association? Hum Reprod. 2007;22:696–701. doi: 10.1093/humrep/del453. [DOI] [PubMed] [Google Scholar]
- 25.Olshan AF, Schnitzer PG, Baird PA. Paternal age and the risk of congenital heart defects. Teratology. 1994;50:80–84. doi: 10.1002/tera.1420500111. [DOI] [PubMed] [Google Scholar]
- 26.Kazaura M, Lie RT, Skjaerven R. Paternal age and the risk of birth defects in Norway. Ann Epidemiol. 2004;14:566–570. doi: 10.1016/j.annepidem.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Archer NP, Langlois PH, Suarez L, et al. Association of paternal age with prevalence of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2007;79:27–34. doi: 10.1002/bdra.20316. [DOI] [PubMed] [Google Scholar]
- 28.Kazaura MR, Lie RT, Irgens LM, et al. Increasing risk of gastroschisis in Norway: an age-period-cohort analysis. Am J Epidemiol. 2004;159:358–363. doi: 10.1093/aje/kwh051. [DOI] [PubMed] [Google Scholar]
- 29.Vu LT, Nobuhara KK, Laurent C, et al. Increasing prevalence of gastroschisis: population-based study in California. J Pediatr. 2008;152:807–811. doi: 10.1016/j.jpeds.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 30.Materna-Kiryluk A, Wisniewska K, Badura-Stronka M, et al. Parental age as a risk factor for isolated congenital malformations in a Polish population. Paediatr Perinat Epidemiol. 2009;23:29–40. doi: 10.1111/j.1365-3016.2008.00979.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhan SY, Lian ZH, Zheng DZ, et al. Effect of fathers’ age and birth order on occurrence of congenital heart disease. J Epidemiol Community Health. 1991;45:299–301. doi: 10.1136/jech.45.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon PW, Rasmussen SA, Lynberg MC, et al. The National Birth Defects Prevention Study. Public Health Rep. 2001;116 (Suppl 1):32–40. doi: 10.1093/phr/116.S1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cogswell ME, Bitsko RH, Anderka M, et al. Control Selection and Participation in an Ongoing, Population-based, Case-Control Study of Birth Defects: The National Birth Defects Prevention Study. Am J Epidemiol. 2009 doi: 10.1093/aje/kwp226. [DOI] [PubMed] [Google Scholar]
- 34.Reefhuis J, Honein MA. Maternal age and non-chromosomal birth defects, Atlanta--1968–2000: teenager or thirty-something, who is at risk? Birth Defects Res A Clin Mol Teratol. 2004;70:572–579. doi: 10.1002/bdra.20065. [DOI] [PubMed] [Google Scholar]
- 35.Werler MM, Mitchell AA, Shapiro S. Demographic, reproductive, medical, and environmental factors in relation to gastroschisis. Teratology. 1992;45:353–360. doi: 10.1002/tera.1420450406. [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen SA, Frias JL. Non-genetic risk factors for gastroschisis. Am J Med Genet C Semin Med Genet. 2008;148C:199–212. doi: 10.1002/ajmg.c.30175. [DOI] [PubMed] [Google Scholar]
- 37.Siega-Riz AM, Herring AH, Olshan AF, et al. The joint effects of maternal prepregnancy body mass index and age on the risk of gastroschisis. Paediatr Perinat Epidemiol. 2009;23:51–57. doi: 10.1111/j.1365-3016.2008.00990.x. [DOI] [PubMed] [Google Scholar]
- 38.Slama R, Werwatz A. Controlling for continuous confounding factors: non- and semiparametric approaches. Rev Epidemiol Sante Publique. 2005;53(Spec No 2):2S65–80. [PubMed] [Google Scholar]
- 39.Kazaura MR, Lie RT. Down’s syndrome and paternal age in Norway. Paediatr Perinat Epidemiol. 2002;16:314–319. doi: 10.1046/j.1365-3016.2002.00446.x. [DOI] [PubMed] [Google Scholar]
- 40.ACOG committee opinion. Advanced paternal age: risks to the fetus. Number 189, October 1997. Committee on Genetics. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 1997;59:271–272. [PubMed] [Google Scholar]
- 41.Toriello HV, Meck JM. Statement on guidance for genetic counseling in advanced paternal age. Genet Med. 2008;10:457–460. doi: 10.1097/GIM.0b013e318176fabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


