Abstract
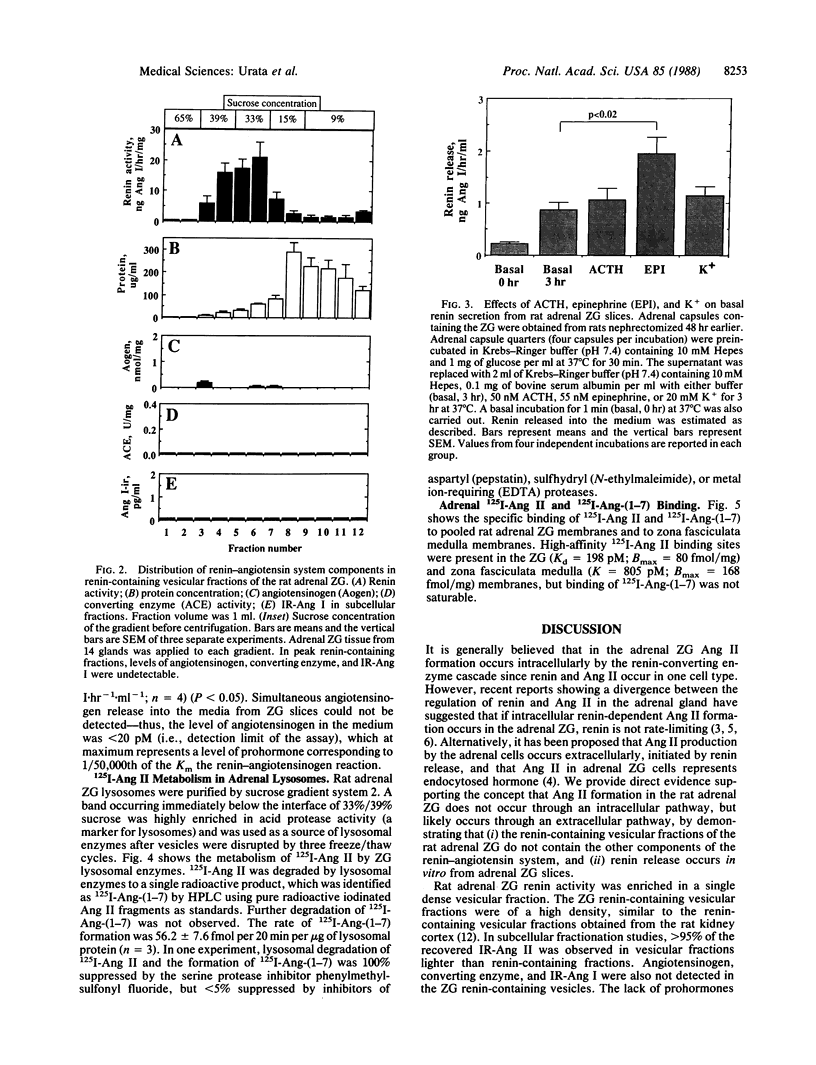
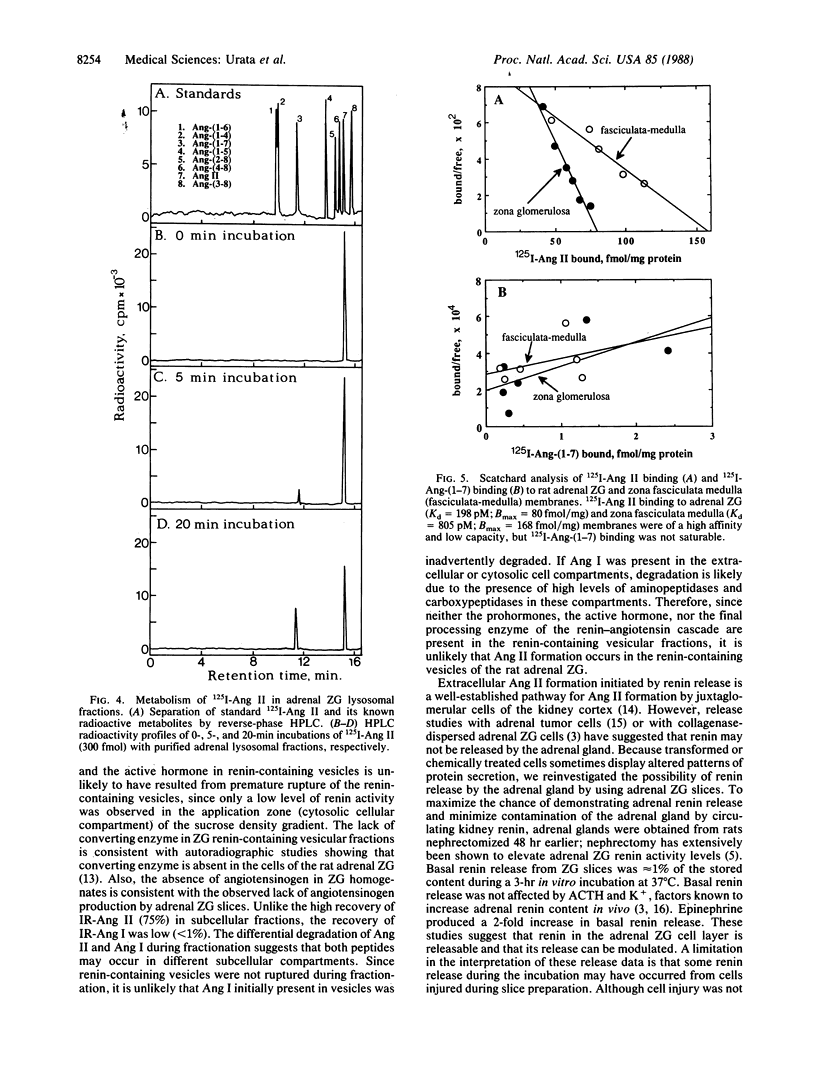
Based on the observation that high levels of renin and angiotensin II (Ang II) are found in the adrenal zona glomerulosa (ZG), it has been postulated that Ang II is formed intracellularly by the renin-converting enzyme cascade in this tissue. To test this hypothesis, we examined renin-angiotensin system components in subcellular fractions of the rat adrenal ZG. Renin activity and immunoreactive-Ang II (IR-Ang II) were observed in vesicular fractions but were not colocalized. In addition, angiotensinogen, angiotensin I, and converting enzyme were not observed in the renin or IR-Ang II-containing vesicular fractions. These data do not support the hypothesis that Ang II is formed intracellularly within the renin-containing vesicles of the ZG. Rather, since modulatable renin release from adrenal ZG slices was observed and renin activity was found in dense vesicular fractions (33-39% sucrose), it is likely that Ang II formation in the ZG is extracellular and initiated by the release of vesicular renin. Receptor-mediated endocytosis and subsequent degradation of Ang II in ZG lysosomes have been shown by others. The presence of IR-Ang II in light vesicular fractions (15% sucrose) and the finding of a high correlation between ZG IR-Ang II and Ang II receptor levels suggest that the primary occurrence of this peptide in the ZG is by receptor-mediated endocytosis. In ZG lysosomal fractions 125I-labeled Ang II was degraded to 125I-labeled des-[Phe8]Ang II. Since Ang II antibodies do not recognize des-[Phe8]Ang II, these findings explain why IR-Ang II in the ZG is due predominantly to Ang II and not to its C-terminal immunoreactive fragments.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilera G., Schirar A., Baukal A., Catt K. J. Circulating angiotensin II and adrenal receptors after nephrectomy. Nature. 1981 Feb 5;289(5797):507–509. doi: 10.1038/289507a0. [DOI] [PubMed] [Google Scholar]
- Baba K., Doi Y., Franco-Saenz R., Mulrow P. J. Mechanisms by which nephrectomy stimulates adrenal renin. Hypertension. 1986 Nov;8(11):997–1002. doi: 10.1161/01.hyp.8.11.997. [DOI] [PubMed] [Google Scholar]
- Bianchi C., Gutkowska J., De Léan A., Ballak M., Anand-Srivastava M. B., Genest J., Cantin M. Fate of [125I]angiotensin II in adrenal zona glomerulosa cells. Endocrinology. 1986 Jun;118(6):2605–2607. doi: 10.1210/endo-118-6-2605. [DOI] [PubMed] [Google Scholar]
- Doi Y., Atarashi K., Franco-Saenz R., Mulrow P. Adrenal renin: a possible regulator of aldosterone production. Clin Exp Hypertens A. 1983;5(7-8):1119–1126. doi: 10.3109/10641968309048845. [DOI] [PubMed] [Google Scholar]
- Husain A., Bumpus F. M., De Silva P., Speth R. C. Localization of angiotensin II receptors in ovarian follicles and the identification of angiotensin II in rat ovaries. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2489–2493. doi: 10.1073/pnas.84.8.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain A., DeSilva P., Speth R. C., Bumpus F. M. Regulation of angiotensin II in rat adrenal gland. Circ Res. 1987 May;60(5):640–648. doi: 10.1161/01.res.60.5.640. [DOI] [PubMed] [Google Scholar]
- Husain A., Pajka S. F., Taylor S. M., Speth R. C. Monoiodinated angiotensin II is a potent, full agonist analog of angiotensin II. J Pharmacol Exp Ther. 1986 Oct;239(1):71–77. [PubMed] [Google Scholar]
- Khosla M. C., Nishimura H., Hasegawa Y., Bumpus F. M. Identification and synthesis of [1-asparagine, 5-valine, 9-glycine] angiotensin I produced from plasma of American eel Anguilla rostrata. Gen Comp Endocrinol. 1985 Feb;57(2):223–233. doi: 10.1016/0016-6480(85)90267-9. [DOI] [PubMed] [Google Scholar]
- Kono T., Taniguchi A., Imura H., Oseko F., Khosla M. C. Biological activities of angiotensin II-(1-6)-hexapeptide and angiotensin II-(1-7)-heptapeptide in man. Life Sci. 1986 Apr 21;38(16):1515–1519. doi: 10.1016/0024-3205(86)90565-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nakamaru M., Misono K. S., Naruse M., Workman R. J., Inagami T. A role for the adrenal renin-angiotensin system in the regulation of potassium-stimulated aldosterone production. Endocrinology. 1985 Nov;117(5):1772–1778. doi: 10.1210/endo-117-5-1772. [DOI] [PubMed] [Google Scholar]
- Naruse M., Shizume K., Inagami T. Renin and angiotensins in cultured mouse adrenocortical tumour cells. Acta Endocrinol (Copenh) 1985 Apr;108(4):545–549. doi: 10.1530/acta.0.1080545. [DOI] [PubMed] [Google Scholar]
- Peach M. J. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1977 Apr;57(2):313–370. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- Pucell A. G., Bumpus F. M., Husain A. Rat ovarian angiotensin II receptors. Characterization and coupling to estrogen secretion. J Biol Chem. 1987 May 25;262(15):7076–7080. [PubMed] [Google Scholar]
- Ryan J. W. Renin-like enzyme in the adrenal gland. Science. 1967 Dec 22;158(3808):1589–1590. doi: 10.1126/science.158.3808.1589. [DOI] [PubMed] [Google Scholar]
- Sagnella G., Price R., Peart W. Subcellular distribution and storage form of rat renal renin. Hypertension. 1980 Sep-Oct;2(5):595–603. doi: 10.1161/01.hyp.2.5.595. [DOI] [PubMed] [Google Scholar]
- Strittmatter S. M., De Souza E. B., Lynch D. R., Snyder S. H. Angiotensin-converting enzyme localized in the rat pituitary and adrenal glands by [3H]captopril autoradiography. Endocrinology. 1986 Apr;118(4):1690–1699. doi: 10.1210/endo-118-4-1690. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., Erdös E. G., Chiang T. S. New enzymatic route for the inactivation of angiotensin. Nature. 1968 Jun 29;218(5148):1224–1226. doi: 10.1038/2181224a0. [DOI] [PubMed] [Google Scholar]