Abstract
Background
In rodents, cocaine self-administration under a fixed-ratio schedule and with timeout intervals limited to the duration of the infusions is characterized by an initial burst of drug intake (loading) followed by more stable infusion rates (maintenance). We sought to examine whether similar phases might characterize self-regulated cocaine use in humans.
Methods
31 Non-treatment seeking, cocaine dependent subjects participated in three (8, 16, and 32 mg/70 kg/infusion), self-regulated, two-hour cocaine self-administration sessions under a fixed-ratio 1, 5-min timeout schedule. Data were assessed for visual (e.g., by graphs of cumulative numbers of infusions) and statistical evidence of change in phase (by step-function analyses of individual infusion rates).
Results
Graphs of cumulative infusions over time suggested a single, linear rate of self-administration over two hours at each cocaine dose. Statistical analyses of infusion data by generalized estimating equation (GEE) models also failed to support a loading/maintenance pattern (suggesting, if anything, the possibility of increasing infusion rates over time).
Conclusions
Our findings fail to support the existence of distinct loading and maintenance phases of self-regulated cocaine administration in humans at behaviorally relevant doses. Several factors may account for these observations including differences between humans and rodents in self-regulated drug intake.
Keywords: Cocaine Self-Administration, Loading, Maintenance, Self-Regulation
1. Introduction
The self-regulation of drug administration, and potential underlying mechanisms, has been studied extensively in non-humans (de la Garza et al., 1981; Gerber and Wise, 1989; Lynch and Carroll, 2001; Lynch et al., 1998; Pickens and Thompson, 1968; Tornatzky and Miczek, 2000). To date, however, only limited effort has been devoted to the study of self-regulated drug intake in humans (Donny et al., 2003; Fischman, 1989; Fischman and Foltin, 1992; Fischman and Schuster, 1982; Kalayasiri et al., 2007b; Lynch et al., 2006; Paly et al., 1982; Sughondhabirom et al., 2005). Despite a relative paucity of data, human laboratory studies have nonetheless suggested that self-administration behavior in experienced drug users employing behaviorally relevant cocaine doses closely resembles that observed in non-human laboratory animals in several respects. For example, self-administration occurs with regularity (Tsibulsky and Norman, 1999), is relatively stable for a given individual, and can be highly variable across individuals (Flory and Woods, 2003; Foltin and Fischman, 1997). Another similarity is that self-administration behavior in rats, monkeys, and humans is dose-dependent, with rates of responding varying according to an inverted U-shaped function (comprised of ascending and descending limbs) (Flory and Woods, 2003; Johanson, 1982; Pickens and Thompson, 1968; Sughondhabirom et al., 2005). Thus, despite the relative scarcity of data, clear similarities appear to exist across species.
However, the extent to which the other aspects of self-administration behavior are shared between humans and non-humans is currently unclear. For example, when rodents are given access to psychomotor stimulants, an initial rapid burst of self-administration (“loading” phase) is observed, followed by a period of slower and more stably spaced drug intake (“maintenance” phase). The transition between loading and maintenance phases is apparent in the sharp and readily observed change in slope of plots depicting cumulative drug infusions over time. This biphasic pattern has been consistently observed with cocaine (Ettenberg et al., 1982; Pickens and Thompson, 1968; Wilson et al., 1971; Wise et al., 1995) and has been replicated using cocaine analogs such as WIN 35,428 (β-CFT; (−)-3β-(4-fluorophenyl)tropane-2β-carboxylic acid methyl ester) (Norman et al., 2004). Studies is non-human primates, however, have been both less systematic and perhaps as a result, less consistent. For example, some studies have revealed patterns of responding consistent with loading / maintenance (Wilson et al., 1971), while others have been less clear (Johanson, 1982), conspicuous for the apparent absence of such a pattern (Goldberg et al., 1971; Goldberg and Kelleher, 1976; also L. Howell, personal communication).
The mechanistic basis of this loading and maintenance pattern has yet to be established, but researchers have hypothesized that it reflects efforts to achieve a relatively constant level of drug intake over a specified time period (e.g., per hour and per day) (Pickens and Thompson, 1968; Wilson et al., 1971) or “to maintain cocaine levels above a minimum trigger point” Tsibulsky and Norman, 1999).
To examine the self-regulation of drug administration in humans, we developed and validated a paradigm of ad libitum cocaine self-administration in which experienced users are allowed control over the frequency of drug intake (i.e., as is possible in preclinical models of operant drug self-administration). Our prior work has shown these methods to be procedurally feasible, medically safe, pharmacologically valid, and test-retest reliable (Kalayasiri et al., 2007b; Lynch et al., 2006; Sughondhabirom et al., 2005). We have previously applied these methods to the study of cocaine-induced subjective effects (Lynch et al., 2008; Lynch et al., 2006), their modulation by genetic and pharmacologic factors (Kalayasiri et al., 2007b), the impact of pharmacologic interventions, such as disulfiram (Kalayasiri et al. 2007), and the role of cocaine self-administration and abstinence on sleep and cognition (Morgan and Malison, 2008; Morgan et al., 2006; Pace-Schott et al., 2008). In the current study, we specifically sought to evaluate whether self-regulated cocaine administration in humans at previously validated and behaviorally relevant doses is also characterized by distinct loading and maintenance phases of drug intake. To our knowledge, this is the first study that has explored this question in humans.
2. Materials and Methods
Subjects
We performed a secondary analysis of data obtained from 31 participants in our prior cocaine self-administration studies (Kalayasiri et al., 2007a; Kalayasiri et al., 2007b; Lynch et al., 2006; Sughondhabirom et al., 2005). Subjects (10 female, 21 male; 12 European American, 19 African American; ages 18-45 yr, mean ± SD age = 38 ± 6 yrs) were assessed for eligibility by unstructured psychiatric interview, physical and neurological examinations, ECG, and routine laboratory testing (i.e., blood chemistries, hematology, and urinalysis). Inclusion criteria included a history of cocaine abuse or dependence (DSM IV) of at least 2-year duration, cocaine use via a high potency, rapid-onset route (i.e., smoked or intravenous), a history of regular, recent use of cocaine in quantities exceeding those available during the study, as well as objective evidence of recent use by urine toxicology testing (i.e., benzoylecgonine positivity). Candidates with significant psychiatric, medical, or neurological illness, either by history or clinical examination (e.g., nonsubstance-related Axis I disorders, cardiac conditions, seizures, etc.), those seeking drug treatment, those dependent on other substances (except for nicotine), and, for females, those with a positive serum β-HCG (i.e., pregnancy) test were excluded. Subjects were experienced (age of first use 20 ± 4 yrs), frequent (6 ± 1 days / week), and heavy ($60 ± 56 spent per day) users of cocaine. Subjects were studied as inpatients and were paid for their participation. Studies/methods were reviewed and approved by the Yale Human Investigations Committee.
Self-Administration Methods
All participants were studied using the same human laboratory paradigm of self-regulated cocaine administration, as previously described (Kalayasiri et al., 2007a; Kalayasiri et al., 2007b; Lynch et al., 2006; Sughondhabirom et al., 2005). Specifically, subjects were allowed to self-select the timing of three cocaine doses (8, 16, and 32 mg per 70 kg body weight per infusion, hereafter referred to as 8, 16, and 32 mg) by pressing a button on a corded infusion pump (Abbott Pain Manager II, Abbott Laboratories) under a fixed-ratio (FR) 1, 5-minute timeout (TO) schedule (i.e., each button press or “response” yielded a cocaine “infusion,” except during the 5 minutes immediately following an infusion). Subjects could press the button during the TO, but infusions were not delivered. They had access to a single cocaine dose per laboratory session, with sessions conducted most often on consecutive weekdays. Thus, self-administration (response and infusion) data were available for all three cocaine doses in all individuals. Subjects were blind to dose (randomized) and self-administration (i.e., FR1, 5min TO) schedules, but were aware of session duration (two hours) and time (per a wall clock in the room). As a safety feature of the paradigm, subjects had access to the pump withheld for predetermined elevations in cardiac vital signs (HR > 75% age-corrected maximum, SBP>170 mmHg, DBP>100 mmHg). These and other aspects regarding the safety, validity, and test-retest reliability of the methods have been previously reviewed in detail (Kalayasiri et al., 2007b; Lynch et al., 2006; Sughondhabirom et al., 2005). Self-administration sessions were conducted on the Yale Center for Clinical Investigation's Hospital Research Unit.
Data Analysis
By virtue of the FR1, 5min TO schedule, self-administration data consisted of two outcomes: responses (button presses) and infusions (presses associated with a drug infusion), the latter being the primary outcome measure of interest with respect to drug loading and maintenance (Tsibulsky and Norman, 1999). Infusion data were evaluated by both graphical depictions and statistical analyses. Graphical depictions, by convention (Norman and Tsibulsky, 2006), employed plots of cumulative infusion data over time (0-120 min; with first infusions anchored at time 0 min). For formal statistical analyses, however, infusion data were coded as a binary outcome (0 = infusion absent vs. 1 = present) for each of 24 five-minute ‘bins’ (i.e., bin 1=0-5 min, bin 2=5-10 min, ..., bin 24=116-120 min). Given that essentially all subjects (30-31 out of 31) pressed for cocaine immediately upon receipt of the pump button (and therefore received an initial infusion during bin 1), statistical analyses / model fittings were conducted using bins 2-24 (i.e., so as to address the artificially elevated incidence of infusions resulting from the synchronized “starting line” effect in bin 1). To account for within-subject correlations and for missing data (i.e., bins during which the infusion pump was withheld due to vital sign elevations), generalized estimating equation (GEE) methods, with the logit link function, were used for all modeling (SAS PROC GENMOD). Based on predictions of two stable (i.e., linear) self-administration phases of differing rate (i.e., greater for loading than maintenance), we conducted a “step-point analysis,” in which binned infusion data were fit to a step function (i.e., with infusion rates significantly higher for all bins before as compared to all bins after the “step”). The best-fitting “step point” for each dose over the two-hour session was determined by deviance statistics. In contrast to cumulative infusion data, where two linear and positively sloping components are present, step function modeling of binned infusion data assumes two linear phases of zero slope and differing y-axis intercepts (loading > maintenance). Thus, a significant descending “step” (i.e., decrease in infusion frequency) for each cocaine dose was hypothesized.
3. Results
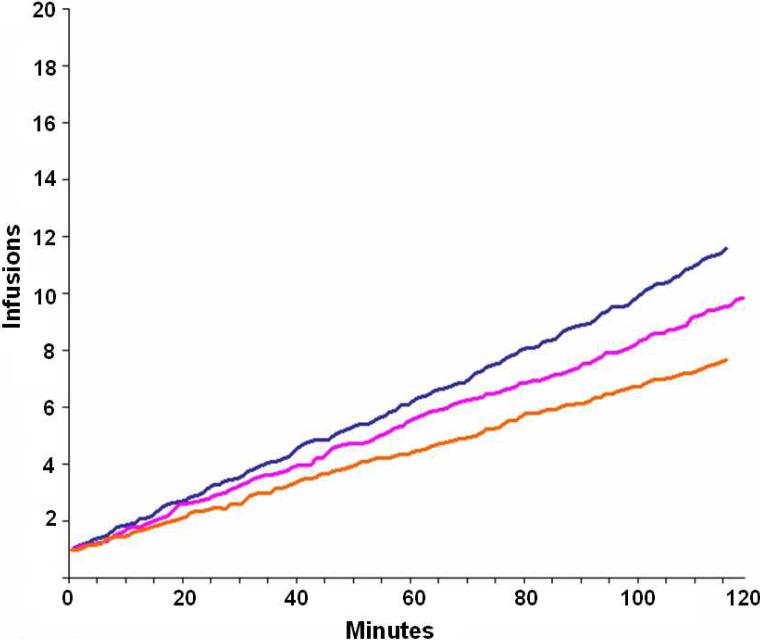
Consistent with our previous observation that cocaine intake appeared to be regulated, the present analysis revealed that subjects obtained more infusions at lower as compared to higher doses (i.e., 359 infusions for 8 mg, 292 for 16 mg, and 237 for 32 mg). Thus, 888 infusions were available for graphical and statistical analyses in our 31 subjects over a total of 2232 bins (31 subjects × 3 doses × 24 five-minute bins/dose). Consistent with preclinical paradigms in rodents, self-administration behavior in our human subjects showed a non-linear relationship to unit cocaine dose, as evidenced by mean inter-infusion intervals of 13, 16, and 19 minutes for 8, 16, and 32 mg/70 kg doses, respectively (e.g., see Figure 2, Tsibulsky and Norman, 1999). Vital sign elevations produced 105 bins of missing data (4.7% of total; 12 for 8 mg, 19 for 16 mg, and 74 for 32 mg). Group plots of cumulative infusion data for each cocaine dose (8, 16, and 32 mg) are presented in Figure 1a. As seen in the figure, distinct loading / maintenance phases are not apparent for any of the three cocaine doses.
Fig. 1a.
Average number of cumulative cocaine infusions for 8 mg (blue line), 16 mg (pink line) and 32 mg (orange line) depicted over time (i.e., 120 min self-administration period). Cumulative infusion curves appear linear and without evidence of discrete loading/maintenance phases.
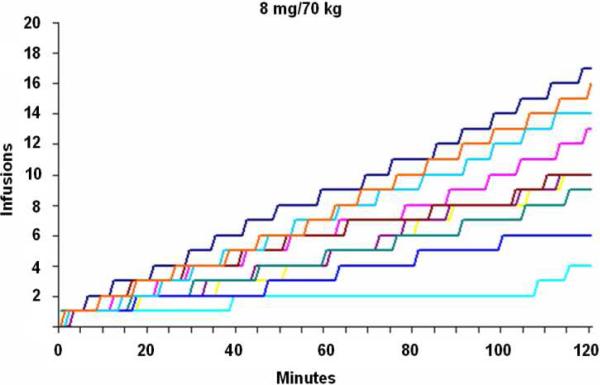
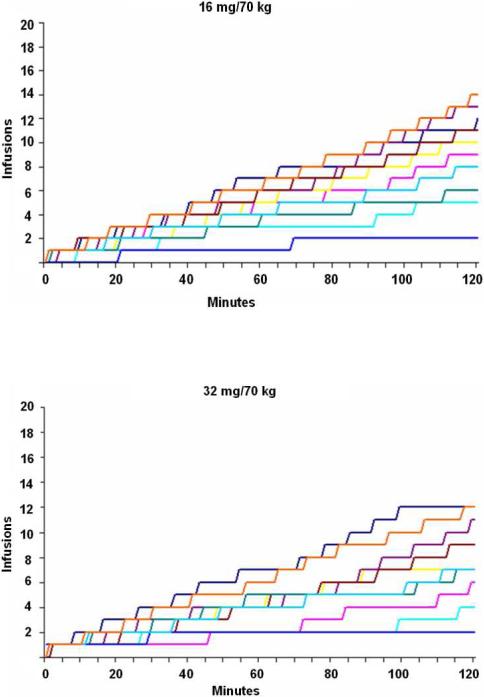
To exclude the possibility that differences in the timing of transitions from loading to maintenance across subjects might have obscured the detection of otherwise obvious discrete phases, cumulative infusion data were inspected for all subjects individually, as well (Figure 1b depicts representative data in 10 subjects for 8, 16, and 32 mg). As for group-wise depictions, however, no change in self-administration rate over the two-hour period was apparent.
Fig. 1b.
Cumulative cocaine infusions for each of 10 representative subjects depicted over time (i.e., 120 min self-administration period) for 8 mg (top), 16 mg (middle), and 32 mg (bottom) doses. Individual cumulative infusion curves are without evidence of distinct loading/maintenance phases.
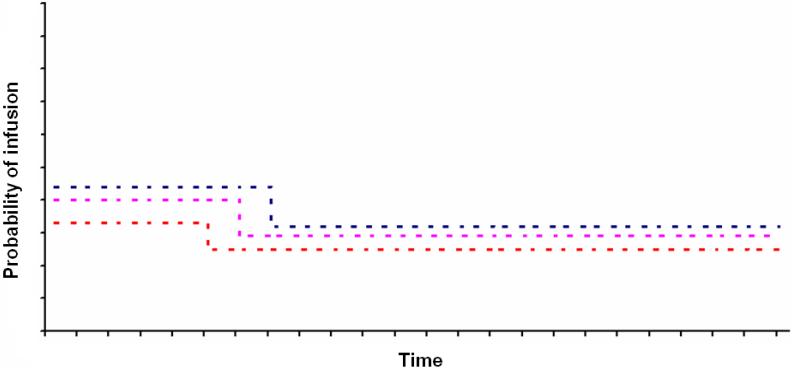
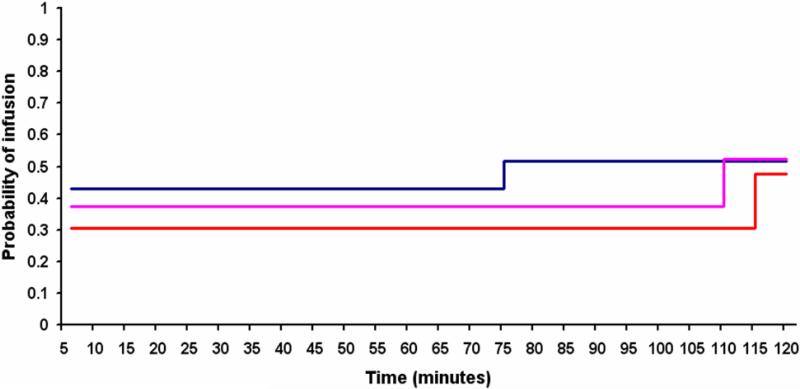
Though visually indiscernible, statistical analyses did reveal significant (or nearly significant), best-fitting “step points” for each cocaine doses (at 71-75 min for 8 mg, p < 0.0001; at 106-110 min for 16 mg, p< 0.001; and at 111- 115 min for 32 mg, p<0.054). Quite unexpectedly, however, and contrary to hypothesized directions of change, results indicated statistically higher infusion frequencies following as compared to preceding significant steps (i.e., opposite of that depicted in Figure 2a). As such, model fitting approaches also failed to find evidence in support of distinct loading and maintenance phases. To rule-out the possibility that the 5-minute timeout might have imposed a ‘ceiling’ on infusion rates (i.e., which could conceivably have masked subjects’ efforts to load during earlier self-administration bins), we also modeled the probability of responses (i.e., button presses) exceeding infusions by identical step-point analyses (predicting that thwarted ‘loading’ efforts would be associated with higher response rates during earlier as compared to later sessions bins). However; results revealed the same pattern for responses as for infusions, with best-fitting “step ups” for each dose (106-110 min for 8 mg, p = 0.0007; 101-105 min for 16 mg, p < 0.0001; and 106-110 min for 32 mg, p < 0.0001). Thus, graphical and statistical analyses were consistent in their failure to find evidence of distinct loading and maintenance phases.
Fig. 2a.
Graph depicting hypothesized probabilities of cocaine infusions over time for 8 mg (blue dashed line), 16 mg (pink dashed line), and 32 mg (orange dashed line) doses as fit by a step-function model. Specifically, loading to maintenance phases are predicted to be associated with descending “steps” for each cocaine dose (i.e., with the probability of infusions being higher for lower as compared to higher doses, with the probability of infusions being higher for loading as compared to maintenance periods, and with the transition being later for lower as compared to higher doses).
4. Discussion
To our knowledge, this is the first study to assess whether self-regulated cocaine administration in humans is associated with distinct phases of drug “loading” and “maintenance”. Our evaluation of data in 31 experienced users who self-selected the timing of three different cocaine doses over two-hour sessions did not reveal such a pattern. Graphs of cumulative infusions, which readily reveal such phases in non-humans, showed no evidence of respective loading/maintenance periods. Moreover, statistical analyses argue persuasively against sequential periods of rapid followed by slower drug intake. In fact, unexpectedly, our model fitting results point to an opposite pattern of statistically higher (albeit visually unimpressive) probabilities of infusions over time (i.e., with best-fitting transition times ranging from 71-115 min for 8-32 mg doses, respectively). As such, under the human laboratory conditions employed, we do not find distinct loading and maintenance phases in experienced cocaine users.
Although reasons for these negative results are unclear, several factors could account for our findings. Perhaps principal among such possibilities are differences is self-administration methods across species. Certainly, response rates in both humans and non-humans can be exquisitely sensitive to self-administration schedules and drug doses. Simultaneously, interspecies differences in physiology, pharmacokinetics, and even research ethics can create challenges in establishing precisely equivalent conditions under which self-administration behavior might be fairly and comparably evaluated with certainty. In this regard, we consider several such factors that might help to explain these apparent differences.
First, and in contrast to preclinical paradigms where cocaine is most often available to non-humans under a simple FR1 schedule (i.e., every response is associated with an infusion), issues of subject safety dictated the inclusion of a 5-minute timeout in our human paradigm (i.e., so as to allow sufficient time for subjects to experience and investigators to monitor the effects of an initial infusion before access to another was possible). Since drug regulation is best revealed in situations in which access is unrestricted (Carroll and Bickel, 1998), the presence of a timeout could conceivably have limited subjects’ infusions at earlier session times. However, our analyses of response data were fundamentally in agreement with those for infusions, arguing against the possibility that the timeout played a significant role.
A second potential factor is the duration and the number of our self-administration sessions. Although two hours is a sufficient period of time for the detection of loading/maintenance phases in rodents (e.g., where cocaine half-life is roughly 8-10 min), cocaine's slower pharmacokinetics in humans (e.g., 45-60 min half-life) might be associated with longer loading intervals and delayed transitions to maintenance. Certainly, cocaine binges in humans are not limited to two hours’ periods of time, often lasting more hours or even days longer (Ward et al., 1997). Thus, we cannot rule out the possibility that longer drug self-administration periods might have revealed two phases. In addition, although our cocaine subjects were highly experienced users of the drug, the human laboratory methods and hospital environment were also highly novel. Thus, it is also possible that efforts to orient/train/habituate subjects (i.e., a single safety/eligibility session) and/or expose individuals to the specific cocaine amounts (i.e., a single two-hour session per dose) were insufficient.
In addition to the former, a third factor that must also be considered is the possibility that chronic drug use and/or the nature of human addiction leads to an alteration in self-administration behavior in which phasic consumption is absent. Preclinical studies are most often conducted in drug-naïve non-humans, and though measures are often obtained after periods of stable self-administration, it is not clear whether such states are comparable to those in cocaine-abusing and/or dependent humans.
A fourth and potentially confounding influence could also be the increased infusion rates observed during the last minutes of the self-administration paradigm. We speculate that subjects’ awareness of session duration and time resulted in increased response rates at session end (i.e., a sort of “last call” effect). Alternatively, were self-administration rates in humans influenced by efforts to attain desirable (i.e., positive) subjective drug effects (e.g., euphoria or ‘high’), and a progressive tolerance to the latter, then secondary increases in self-administration rates might be observed over time as subjects attempt to sustain such states. Though intriguing to speculate about the potential mechanisms underlying these statistically discernable effects, we hesitate to expound given their modest, visually unimpressive, and perhaps clinically questionable magnitude (Figure 1a). Given the latter, however, we believe it is unlikely that such effects obscured the detection of loading / maintenance phases.
Finally, and perhaps most importantly, we wonder about the equivalence and/or comparability of the unit cocaine doses used in human vs. non-human paradigms. Preclinical studies in non-humans can show variable evidence of loading and maintenance depending upon the specific doses employed. In rodents, for example, the unit dose ranges most commonly studied (e.g., 0.2-1.0 mg/kg IV) are associated with distinct loading/maintenance phases (Peoples et al., 2004b), while larger and less commonly studied doses (e.g., 2.0 mg/kg IV or higher) are not, apparently producing drug-loading with a single dose (Andrew Norman, personal communication). A similar dose-dependency has also been suggested in at least one study of non-human primates (Wilson et al., 1971), with doses of 0.1-0.4 mg/kg/injection appearing to be associated with discrete phases, while other below (0.025-0.050 mg/kg/injection) or above (0.8 – 2.0 mg/kg/injection) not. While the doses employed in our human paradigm match the former well from a dose/weight perspective (i.e., 8-32 mg/70 kg or roughly 0.1-0.5 mg/kg IV in our study), others have suggested that the higher rodent doses might be behaviorally more relevant from the standpoint of self-administration rate (Mantsch et al., 2004). For example, doses of 0.5 and 0.7 mg/kg in rats are associated with inter-infusion intervals of only 2-5 minutes (Mantsch et al., 2004) and 6-8.5 minutes (Peoples et al., 2004a), respectively. In contrast, comparable (or even somewhat lower) mg/kg cocaine doses in our human subjects were associated with mean inter-infusion intervals of 13, 16, and 19 minutes (0.1, 0.2, and 0.5 mg/kg, respectively), intervals closer to those (10-15 min) produced by higher (e.g., 2.0 mg/kg) rodent doses. Thus, neither can we exclude the possibility that smaller unit cocaine doses (e.g., 1-4 mg/70 kg) might have yielded different results in our paradigm / population.
Importantly, we do not believe that limitations of sample size and/or self-administration outcomes (i.e., numbers of infusions) were a factor in our inability to identify loading/maintenance effects. Quite to the contrary, our dataset provided sufficient statistical power to unambiguously detect increases in infusion (and response) rates over time – a pattern opposite that hypothesized (and one confirmed by other model fitting approaches as well; data not shown; available upon request).
In conclusion, under the human laboratory conditions employed, the current study did not find evidence of loading and maintenance phases of drug intake in humans, a pattern that has been previously well demonstrated in non-humans. Future studies may benefit from more liberal self-administration schedules, longer session durations, blinding with respect to session durations/times, and/or the use of lower unit doses of cocaine. Such modifications may ultimately enable more definitive replication, or refutation, of our negative results.
Fig. 2b.
Graph depicting actual probabilities of cocaine infusions over time (minutes) for 8 mg (blue line), 16 mg (pink line), and 32 mg (orange line) doses as best-fit by “step-point” analyses. In contrast to predictions, step-function modeling revealed statistically significant ascending “steps” for each cocaine dose.
Acknowledgements
We gratefully acknowledge the excellent technical assistance of Jessica Bells. We are grateful for the support of the National Institute on Drug Abuse (DA018978, WJL; DA00397, DA15857, DA12283, RTM), the Integrated Mentored Patient-Oriented Research Training program in Psychiatry (IMPORT; R25 MH071584), the Yale Center for Clinical Investigation, and the Department of Mental Health and Addiction Services (DMHAS) of the State of Connecticut. This publication was also made possible by CTSA Grant Number UL1RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Carroll ME, Bickel WK. Behavioral-Environmental Determinants of the reinforcing functions of cocaine. In: Higgins S, Katz JL, editors. Cocaine abuse: basic determinants, cllinical applications, and policy New York Plenum. 1998. pp. 81–105. [Google Scholar]
- de la Garza R, Bergman J, Hartel CR. Food deprivation and cocaine self-administration. Pharmacology, biochemistry, and behavior. 1981;15:141–144. doi: 10.1016/0091-3057(81)90353-1. [DOI] [PubMed] [Google Scholar]
- Donny EC, Bigelow GE, Walsh SL. Choosing to take cocaine in the human laboratory: effects of cocaine dose, inter-choice interval, and magnitude of alternative reinforcement. Drug and alcohol dependence. 2003;69:289–301. doi: 10.1016/s0376-8716(02)00327-7. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology. 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Fischman MW. Relationship between self-reported drug effects and their reinforcing effects: studies with stimulant drugs. NIDA research monograph. 1989;92:211–230. [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Self-administration of cocaine by humans: a laboratory perspective. Ciba Foundation symposium. 1992;166:165–173. doi: 10.1002/9780470514245.ch10. discussion 173-180. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Schuster CR. Cocaine self-administration in humans. Federation proceedings. 1982;41:241–246. [PubMed] [Google Scholar]
- Flory GS, Woods JH. The ascending limb of the cocaine dose-response curve for reinforcing effect in rhesus monkeys. Psychopharmacology. 2003;166:91–94. doi: 10.1007/s00213-002-1336-3. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. A laboratory model of cocaine withdrawal in humans: intravenous cocaine. Experimental and clinical psychopharmacology. 1997;5:404–411. doi: 10.1037//1064-1297.5.4.404. [DOI] [PubMed] [Google Scholar]
- Gerber GJ, Wise RA. Pharmacological regulation of intravenous cocaine and heroin self-administration in rats: a variable dose paradigm. Pharmacology, biochemistry, and behavior. 1989;32:527–531. doi: 10.1016/0091-3057(89)90192-5. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Hoffmeister F, Schlichting UU, Wuttke W. A comparison of pentobarbital and cocaine self-administration in rhesus monkeys: effects of dose and fixed-ratio parameter. The Journal of pharmacology and experimental therapeutics. 1971;179:277–283. [PubMed] [Google Scholar]
- Goldberg SR, Kelleher RT. Characteristics of behavior controlled by cocaine injections. Psychopharmacology bulletin. 1976;12:43–44. [PubMed] [Google Scholar]
- Johanson CE. Behavior maintained under fixed-interval and second-order schedules of cocaine or pentobarbital in rhesus monkeys. The Journal of pharmacology and experimental therapeutics. 1982;221:384–393. [PubMed] [Google Scholar]
- Kalayasiri R, Morgan P, Pittman B, Gueorguieva R, Coric V, Bhagwagar Z, Cubells JF, Malison RT. 69 th College on Problems of Drug Dependence. Quebec, Canada: 2007a. Disulfiram enhances paranoia during “binge” cocaine self-administration. [Google Scholar]
- Kalayasiri R, Sughondhabirom A, Gueorguieva R, Coric V, Lynch WJ, Lappalainen J, Gelernter J, Cubells JF, Malison RT. Dopamine beta-hydroxylase gene (DbetaH) -1021C-->T influences self-reported paranoia during cocaine self-administration. Biological psychiatry. 2007b;61:1310–1313. doi: 10.1016/j.biopsych.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Experimental and clinical psychopharmacology. 2001;9:131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Kalayasiri R, Sughondhabirom A, Pittman B, Coric V, Morgan PT, Malison RT. Subjective responses and cardiovascular effects of self-administered cocaine in cocaine-abusing men and women. Addiction biology. 2008;13:403–410. doi: 10.1111/j.1369-1600.2008.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, LaBounty LP, Carroll ME. A novel paradigm to investigate regulation of drug intake in rats self-administering cocaine or heroin intravenously. Experimental and clinical psychopharmacology. 1998;6:22–31. doi: 10.1037//1064-1297.6.1.22. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Sughondhabirom A, Pittman B, Gueorguieva R, Kalayasiri R, Joshua D, Morgan P, Coric V, Malison RT. A paradigm to investigate the regulation of cocaine self-administration in human cocaine users: a randomized trial. Psychopharmacology. 2006;185:306–314. doi: 10.1007/s00213-006-0323-5. [DOI] [PubMed] [Google Scholar]
- Mantsch J, Yuferov V, Mathieu-Kia A, Ho A, Kreek M. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Malison RT. Pilot study of lorazepam and tiagabine effects on sleep, motor learning, and impulsivity in cocaine abstinence. The American journal of drug and alcohol abuse. 2008;34:692–702. doi: 10.1080/00952990802308221. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: Evidence for occult insomnia. Drug and alcohol dependence. 2006;82:238–249. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Norman AB, Buesing WR, Norman MK, Tabet MR, Tsibulsky VL. The self-administration of WIN 35,428 and cocaine: comparisons of satiety threshold and elimination half-life in rats. European journal of pharmacology. 2004;483:281–287. doi: 10.1016/j.ejphar.2003.10.040. [DOI] [PubMed] [Google Scholar]
- Norman AB, Tsibulsky VL. The compulsion zone: a pharmacological theory of acquired cocaine self-administration. Brain Res. 2006;1116:143–152. doi: 10.1016/j.brainres.2006.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Morgan PT, Malison RT, Hart CL, Edgar C, Walker M, Stickgold R. Cocaine users differ from normals on cognitive tasks which show poorer performance during drug abstinence. The American journal of drug and alcohol abuse. 2008;34:109–121. doi: 10.1080/00952990701764821. [DOI] [PubMed] [Google Scholar]
- Paly D, Jatlow P, Van Dyke C, Jeri FR, Byck R. Plasma cocaine concentrations during cocaine paste smoking. Life sciences. 1982;30:731–738. doi: 10.1016/0024-3205(82)90606-3. [DOI] [PubMed] [Google Scholar]
- Peoples L, Lynch K, Lesnock J, Gangadhar N. Accumbal neural responses during the initiation and maintenance of intravenous cocaine self-administration. J Neurophysiol. 2004a;91:314–323. doi: 10.1152/jn.00638.2003. [DOI] [PubMed] [Google Scholar]
- Peoples LL, Lynch KG, Lesnock J, Gangadhar N. Accumbal neural responses during the initiation and maintenance of intravenous cocaine self-administration. Journal of neurophysiology. 2004b;91:314–323. doi: 10.1152/jn.00638.2003. [DOI] [PubMed] [Google Scholar]
- Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. The Journal of pharmacology and experimental therapeutics. 1968;161:122–129. [PubMed] [Google Scholar]
- Sughondhabirom A, Jain D, Gueorguieva R, Coric V, Berman R, Lynch WJ, Self D, Jatlow P, Malison RT. A paradigm to investigate the self-regulation of cocaine administration in humans. Psychopharmacology. 2005;180:436–446. doi: 10.1007/s00213-005-2192-8. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Cocaine self-administration “binges”: transition from behavioral and autonomic regulation toward homeostatic dysregulation in rats. Psychopharmacology. 2000;148:289–298. doi: 10.1007/s002130050053. [DOI] [PubMed] [Google Scholar]
- Tsibulsky VL, Norman AB. Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Res. 1999;839:85–93. doi: 10.1016/s0006-8993(99)01717-5. [DOI] [PubMed] [Google Scholar]
- Ward AS, Haney M, Fischman MW, Foltin RW. Binge cocaine self-administration in humans: intravenous cocaine. Psychopharmacology. 1997;132:375–381. doi: 10.1007/s002130050358. [DOI] [PubMed] [Google Scholar]
- Wilson MC, Hitomi M, Schuster CR. Psychomotor stimulant self administration as a function of dosage per injection in the rhesus monkey. Psychopharmacologia. 1971;22:271–281. doi: 10.1007/BF00401789. [DOI] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology. 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]