Abstract
Deep brain stimulation (DBS) is an established treatment for advanced Parkinson’s disease (PD). The procedure entails intracranial implantation of an electrode in a specific brain structure followed by chronic stimulation. Although the beneficial effects of DBS on motor symptoms in PD are well known, it is often accompanied by cognitive impairments the origin of which is not fully understood. To explore the possible contribution of the surgical procedure itself, we studied the effect of electrode implantation in the subthalamic nucleus (STN) on regional neuroinflammation and memory function in rats implanted bilaterally with stainless steel electrodes. Age-matched sham and intact rats were used as controls. Brains were removed one week or eight weeks post implantation and processed for in vitro autoradiography with [3H]PK11195, an established marker of microglial activation. Memory function was assessed by the novel object recognition test (ORT) before surgery and two and eight weeks after surgery. Electrode implantation produced region-dependent changes in ligand binding density in the implanted brains at one week as well as eight weeks post implantation. Cortical regions showed more intense and widespread neuroinflammation than striatal or thalamic structures. Furthermore, implanted animals showed deficits in ORT performance two and eight weeks post implantation. Thus, electrode implantation resulted in a widespread and persistent neuroinflammation and sustained memory impairment. These results suggest that the insertion and continued presence of electrodes in the brain, even without stimulation, may lead to inflammation-mediated cognitive deficits in susceptible individuals, as observed in patients treated with DBS.
Keywords: Deep brain stimulation, brain injury, Peripheral Benzodiazepine Receptor, translocator protein, Autoradiography, Novel Object Recognition
Introduction
Deep brain stimulation (DBS) is an increasingly popular therapeutic approach for diverse neurological disorders including Parkinson’s Disease (PD) (Benabid et al., 2005; Limousin and Martinez-Torres, 2008), depression (Marangell et al., 2007), obsessive compulsive disorder (Lipsman et al., 2007) and epilepsy (Vonck et al., 2003; Vonck et al., 2007). This approach involves chronic implantation of an electrode in a specific brain structure followed by chronic electrical stimulation.
While DBS relieves motor symptoms, there is a steady increase in reports of cognitive impairments associated with this procedure in PD (Daniele et al., 2003; Dujardin et al., 2001; Funkiewiez et al., 2004; Saint-Cyr et al., 2000; Trepanier et al., 2000; Witt et al., 2008; York et al., 2008) as well as Huntington disease (Fasano et al., 2008), essential tremor (Fields et al., 2003) and dystonia (Kiss et al., 2007). Previous studies explained the cognitive impairment accompanying DBS in PD patients as a result of the electrical stimulation of the subthalamic nucleus (Alegret et al., 2001; Ardouin et al., 1999; Dujardin et al., 2001; Saint-Cyr et al., 2000; Trepanier et al., 2000) which alters the basal ganglia - anterior cingulate cortex circuit activity (Cilia et al., 2007a; Kalbe et al., 2009; Schroeder et al., 2003). In contrast, there is also evidence of cognitive decline after DBS surgery both “on” and “off” stimulation, compared to pre-surgical performance, suggesting effects related to the surgical procedure rather than the stimulation. However, the relative contribution of the surgery would be hard to assess under these conditions due to possible lingering effects of the chronic stimulation.
Postmortem analyses of brain biopsies from patients treated with DBS demonstrate a local brain tissue reaction to the electrode characterized by the presence of activated astrocytes (Boockvar et al., 2000; Burbaud et al., 2002; Haberler et al., 2000; Henderson et al., 2001; Henderson et al., 2002; Jarraya et al., 2003; Nielsen et al., 2007; Pilitsis et al., 2008) and activated microglia (Chou et al., 2004; Haberler et al., 2000; Henderson et al., 2002; Jarraya et al., 2003; Nielsen et al., 2007; Pilitsis et al., 2008). These findings were very similar, regardless of the disease, the electrode location and the duration of the implantation. Very similar inflammatory responses were observed following implantation of cerebrospinal fluid shunt devices (Del Bigio, 1998) and recording electrodes used for localization of epileptogenic tissue (Stephan et al., 2001) in humans, which do not involve electrical stimulation. Animal studies with various intracranial implants similarly report activated astrocytes (Kim et al., 2004; Lenarz et al., 2007; Leung et al., 2008; McConnell et al., 2007; Mokry et al., 2000; Stice et al., 2007; Szarowski et al., 2003; Turner et al., 1999) and activated microglia (Biran et al., 2007; Biran et al., 2005; Griffith and Humphrey, 2006; Kim et al., 2004; Leung et al., 2008; McConnell et al., 2007; Mokry et al., 2000; Szarowski et al., 2003) in close proximity to the implant site.
Since neuroinflammation can cause cognitive impairment in humans (Hoogman et al., 2007; Pikis et al., 1996; Schmidt et al., 2006) and in animals exposed to Lipopolysaccharide (LPS) (Hauss-Wegrzyniak et al., 2000) or ischemia (Langdon et al., 2008; Liu et al., 2007), we set out to test the hypothesis that implantation-induced neuroinflammation is not limited to the implantation site and may spread through brain regions playing a critical role in cognitive functioning, thereby leading to memory deficits. To facilitate quantitative regional measurement of neuroinflammation, we chose to employ [3H]PK11195, an established marker of neuroinflammation which labels peripheral benzodiazepoine receptors (PBR) on astrocytes and microglia (James et al., 2006; Lang, 2002).
Materials and Methods
Electrodes
Three types of electrodes were used: 1. Bipolar twisted electrodes with an insulated strand diameter of 0.28mm (Plastics One, part no. MS303/1), referred to as “thick” electrodes in throughout the text. 2. Bipolar twisted electrode with an insulated strand diameter of 0.15mm (Plastics One, part no. MS303/3), referred to as “thin” electrodes throughout the text. 3. Bipolar concentric electrode with an inner electrode projection of 1mm, inner insulated electrode diameter of 0.15mm and outer electrode diameter of 0.4mm (Plastics One, part no. MS303/8). These are referred to as concentric electrodes throughout the text. All electrodes are constructed of stainless steel and coated with polyimide.
Animals
Four months old Sprague Dawley male rats were housed in the Sheba animal facility under controlled light/dark cycle with food and water available ad libitum. Animals were housed singly after surgery to prevent them from removing each others’ implants. Animals were maintained for one week or eight weeks post implantation surgery. Parallel groups of age matched non-surgical controls were maintained under the same conditions.
Experiments were conducted in accordance with international standards on animal welfare and were approved by the Sheba Medical Center and Bar Ilan University institutional animal care and use committees. Adequate measures were taken to minimize pain or discomfort
Thirty eight animals were used for the study and were divided into the following experimental groups:
Experiment 1
Four months old male Sprague Dawley rats were implanted bilaterally with “thick” (N=7) or “thin” (N=5) electrodes in the subthalamic nucleus (STN). The control group (N=7) consisted of intact (N=3) and sham-operated (N=4) age-matched animals. Implanted and control animals were maintained for one week.
Experiment 2
Four months old male Sprague Dawley rats (N=9) were implanted bilaterally with concentric electrodes in the subthalamic nucleus. The control group (N=10) consisted of sham treated (N=4) and intact (N=6) age matched animals. Implanted and control animals were maintained for eight weeks. These animals were tested in the object recognition test (ORT) pre- implantation (N=10) and two weeks (N=16) and eight weeks (N=17) post implantation.
Electrode implantation surgery
Rats were anesthetized with intraperitoneal (i.p.) Equithesin (120mg/Kg Chloral Hydrate, 25.2mg/Kg Nembutal) and placed in a stereotaxic apparatus (Kopf instruments, Tujunga, California). A midline longitudinal incision was performed, the skin was retracted and the skull exposed. Two holes for electrodes were drilled into the skull using a dental drill (Elektrotechnisches, Leutkirch, Germany). The center of the drilled holes was positioned at stereotaxic coordinates +3 mm forward of bregma, and ±2.4 mm lateral to bregma according to the rat brain atlas (Paxinos and Watson, 1998). The dura was exposed and stainless steel electrodes coated with polyimide (Plastics One, Roanoke, Virginia, USA) were inserted at an angle of 38°, passing through cortex, striatum and thalamus and terminating in the STN, mimicking the surgical path employed in patients with PD (Benabid et al., 2009). Three additional holes were drilled into the skull and were used for surgical bone screws (Small Parts Inc., Miramar, Florida, USA). Acrylic dental adhesive (Major Dental, Moncalieri, Italy) was applied as a slurry around the bone screws to cover the skull and used to firmly secure the electrodes. The skin was sutured and an antibiotic ointment was applied to the wound. Sham rats were anesthetized, a midline longitudinal incision was performed, the skin was sutured and an antibiotic ointment was applied to the wound.
Tissue processing
Rats were decapitated one week or eight weeks post implantation surgery and the brains were quickly removed, rinsed, separated along the midline and the two separated hemispheres frozen in powdered dry ice. Long-term storage of brains was at −80°c. The frozen brains were sectioned in a cryostat (Leica, Nussloch, Germany) in the sagittal plane. Sections (20μm) were produced at a cutting temperature of −15°c and 10 consecutive series collected at 200μm intervals by thaw mounting onto coated glass slides, starting from the temporal cortex towards the midline for each hemisphere.
Histological Staining
One series of brain sections was stained with Hematoxylin & Eosin. Electrode track verification was carried out using the stained sections and a rat brain atlas (Paxinos and Watson, 1998).
In vitro autoradiography
On the day of the assay, sections were removed from the −80°c freezer and allowed to reach room temperature. Peripheral-type benzodiazepine receptors were labeled with [3H]PK11195 (Perkin Elmer, Waltham, Massachusetts, USA, Specific activity 84.8Ci/mmol; 1mCi/mL) using a methodology adapted from the literature (Guilarte et al., 1995; Raghavendra Rao et al., 2000). Briefly, sections were first pre-incubated in PBS, pH 7.4 (Sigma-Aldrich, Rehovot, Israel) for 15min at room temperature, followed by 30min incubation at room temperature with the radioactive ligand. Total binding was determined with 1nM [3H]PK11195 whereas nonspecific binding was determined on consecutive slides in the presence of excess (20μM) unlabeled PK11195 (Sigma-Aldrich, Rehovot, Israel). Sections were then washed twice for 6min in ice-cold (4°c) PBS and dipped in ice cold (4°c) double distilled water prior to drying to remove buffer salts. The dried sections were exposed to Biomax MR films (Kodak, Rochester, NY, USA) for 21 days alongside calibrated tritium microscales (Amersham, Buckinghamshire, England).
Quantitative image analysis
The films were scanned and digitized using an HP scanner. The intensity and spread of the neuroinflammatory response were assessed through regional density measurements of anatomically defined structures. Mean grey level of each structure was measured using the ROI manager tool in ImageJ and was translated to radioactivity level through calibration standard curve using the tritium microscale measurements. Brain regions were identified in reference to a rat brain atlas (Paxinos and Watson, 1998). For analysis, the brains were divided into four consecutive anatomical levels from lateral to medial as follows: Level 1: temporal cortex. Level 2: entorhinal cortex, insular cortex, occipital cortex, parietal cortex, perirhinal cortex, striatum, subiculum and ventral hippocampus. Level 3: dorsolateral prefrontal cortex, frontal cortex, cornu ammoni 1 (CA1), cornu ammoni 3 (CA3) and dentate gyrus (DG) in the hippocampus, laterodorsal thalamic nucleus-ventrolateral part (LDVL), occipital cortex, parietal cortex, striatum, substantia nigra (SN), ventral pallidum/substantia innominata (VPSI) and ventral posteromedial thalamic nucleus (VPM). Level 4: frontal cortex and medial prefrontal cortex (mPFC). Overall, [3H]PK11195 binding was measured in 19 distinct anatomical regions which were previously found to be differentially sensitive to LPS injection and traumatic brain injury (Biegon et al., 2002; Grossman et al., 2003). The peri-lesional area was defined as an area around the center of the electrode’s track (0.5mm in each direction) and was divided into three sub regions: cortex, striatum and thalamus. Grey levels of sections that represent non specific binding were measured for all structures together at the same level. Non specific binding levels were subtracted from the total binding to obtain the specific binding in each brain structure. In order to illustrate the spread of neuroinflammation within the cortex and within the striatum of implanted animals, plot profiles of [3H]PK11195 density versus distance were obtained using the plot profile tool in ImageJ software. Lines were drawn on sagittal sections containing the electrode’s track. The line in the cortex was drawn from the parietal cortex (above the ventricle), through the frontal cortex and prefrontal cortex and terminated at the edge of the section. The line in the striatum was drawn from the ventricle through the striatum and terminated at the corpus callosum.
Object Recognition Test
The object recognition test was performed as originally described by Ennaceur and Delacour (Ennaceur and Delacour, 1988). Briefly, in the first day of the test animals were placed in the testing cage (a plastic box of 72 × 47 × 34cm) for one hour habituation. On the following day (pre- implantation, two weeks and eight weeks post implantation) rats were placed in the same cage with two identical objects. The cumulative time spent by the rat exploring the objects was recorded during a 5 min interval. Four hours later, the animals were reintroduced into the cage for a 5min test. One of the two identical objects was replaced by a novel object. To offset location bias, the novel object was placed at the location of the old object which the rat spent less time exploring (less than 45%) during the familiarization phase. The time (out of 5min total) spent exploring each of the objects was recorded. The outcome measure was percentage of time spent exploring the novel object during the testing phase, whereby normal healthy rodents will spend relatively more time exploring a novel object than a familiar, i.e “memorized” object. The proportion of time spent exploring the novel object during the testing phase was compared to the proportion of time spent exploring the object at the same location during the familiarization phase.
Statistical analysis
We used Q-Q plots to analyze the data distribution and it was found to be normal, justifying the use of analyses of variance. The effect of electrode implantation on regional density of PBR was examined using repeated measures ANOVA (repeated measure =region) including regions represented in all hemispheres (10 regions in experiment 1 and eighteen regions in experiment 2) and by two way ANOVA (treatment X region). Posthoc comparisons for all regions were performed using the Fisher PLSD. The effect of electrode implantation on PBR density in peri-lesional areas was examined only in the implanted animals by one way ANOVA (by region). Significance was preset at p < 0.05. The effect of electrode implantation on the performance of the object recognition test was examined using paired or unpaired T-test as appropriate.
Results
Effects of thick and thin electrode implantation on PBR density one week post implantation (Experiment 1)
There were no significant differences between hemispheres of sham animals compared with hemispheres of intact animals by repeated measures ANOVA, thus results from these hemispheres were pooled and considered as control hemispheres. Electrode implantations resulted in significant increases in the density of PBR indicative of neuroinflammation one week post implantation (significant region X treatment interaction by two way ANOVA, F=4.62 P<0.0001). Changes observed in hemispheres implanted with thick electrode compared to control hemispheres showed a region dependent pattern with the largest and most significant increase (177%) detected in the frontal cortex. Smaller but statistically significant increases were observed in the medial prefrontal cortex, parietal cortex and striatum (31%–61%). Non significant elevations in PBR density were seen in the dorsolateral prefrontal cortex, temporal cortex, insular cortex, ventral pallidum/substantia innominata, thalamic nuclei and substantia nigra. Significant decreases in PBR density were detected in the subiculum, entorhinal cortex and the perirhinal cortex (21%–50%). Ventral hippocampus, occipital cortex, dentate gyrus, CA1 and CA3 showed trends towards a decrease in density which were not statistically significant (Table 1, Figures 1 and 2).
Table 1.
Electrode implantation and PBR densities at one week after surgery
| Control | Thick electrode | Thick vs. Control | Thin electrode | Thin vs. Control | ||||
|---|---|---|---|---|---|---|---|---|
| Brain region | mean | ± SE | mean | ± SE | % change | mean | ± SE | % change |
| CA1 | 8.0 | ± 0.4 | 7.1 | ± 0.7 | −11 | 10.2 | ± 1.7c | 26 |
| CA3 | 6.4 | ± 0.4 | 5.8 | ± 0.6 | −10 | 8.4 | ± 1.2#a | 32 |
| DG | 10.9 | ± 0.5 | 9.5 | ± 0.8 | −13 | 13.3 | ± 2.0a | 22 |
| DLPFC | 9.5 | ± 1.0 | 11.4 | ± 0.5# | 21 | 13.1 | ± 1.0* | 39 |
| Ent Cx | 11.0 | ± 1.5 | 6.9 | ± 0.8* | −37 | 12.4 | ± 1.2b | 13 |
| Frontal Cx | 8.5 | ± 0.6 | 23.6 | ± 2.9** | 178 | 23.6 | ± 2.2** | 177 |
| Insular Cx | 9.6 | ± 0.9 | 10.9 | ± 1.5 | 14 | 13.7 | ± 1.4** | 44 |
| LDVL | 6.1 | ± 0.8 | 6.7 | ± 0.9 | 10 | 6.8 | ± 1.1 | 12 |
| mPFC | 9.3 | ± 1.5 | 15.0 | ± 1.6* | 61 | 11.3 | ± 0.5 | 22 |
| Occipital Cx | 7.6 | ± 0.5 | 6.7 | ± 0.5 | −12 | 10.6 | ± 0.8**b | 39 |
| Parietal Cx | 8.3 | ± 0.7 | 11.5 | ± 1.2* | 39 | 16.2 | ± 1.6**a | 96 |
| PRh Cx | 9.2 | ± 1.8 | 4.6 | ± 0.9* | −50 | 8.7 | ± 0.8b | −6 |
| Subiculum | 9.4 | ± 0.8 | 7.5 | ± 0.5* | −21 | 10.1 | ± 1.3a | 7 |
| SN | 8.3 | ± 0.8 | 8.9 | ± 0.8 | 7 | 11.4 | ± 2.5 | 38 |
| Striatum | 7.9 | ± 0.6 | 10.3 | ± 0.6** | 31 | 11.6 | ± 1.1** | 47 |
| Temporal Cx | 7.7 | ± 0.7 | 9.0 | ± 1.1 | 18 | 15.0 | ± 1.8**b | 96 |
| vHipp | 9.8 | ± 0.8 | 8.6 | ± 0.8 | −12 | 9.3 | ± 1.1 | −5 |
| VPM | 6.1 | ± 0.8 | 6.7 | ± 0.8 | 10 | 7.9 | ± 1.1 | 28 |
| VPSI | 6.6 | ± 1.5 | 7.5 | ± 0.8 | 14 | 10.4 | ± 2.3 | 59 |
Animals were killed one week after bilateral implantation of thick or thin twisted electrodes. Values represent specifically bound radioactivity in nCi/mg from 14 control hemispheres (N=6 intact and 8 sham operated hemispheres), 14 hemispheres implanted with thick electrode and 9 hemispheres implanted with thin electrode with 6–14 measurements/region. CA, cornu ammoni; Cx, cortex; DG, dentate gyrus; DLPFC, dorsolateral prefrontal cortex; Ent, Entorhinal; LDVL, laterodorsal thalamic nucleus-ventrolateral part; mPFC, medial prefrontal cortex; PRh, perirhinal; SN, substantia nigra; vHipp, ventral hippocampus; VPM, ventral postermedial thalamic nucleus; VPSI, ventral pallidum/substantia innominata.
p<0.05,
p<0.01,
p<0.1, electrode vs. control,
p< 0.05,
p<0.01
p<0.1 thick electrode vs. thin electrode by posthoc analysis following two way ANOVA (by region X treatment).
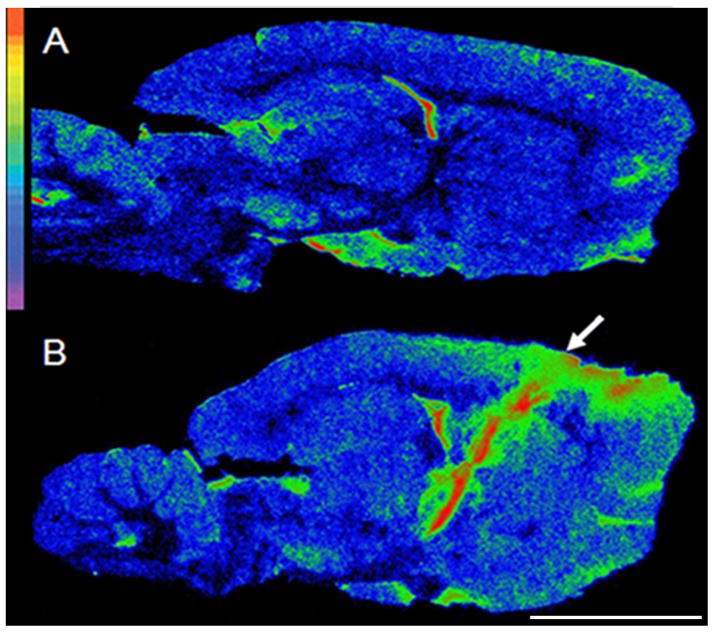
Figure 1.
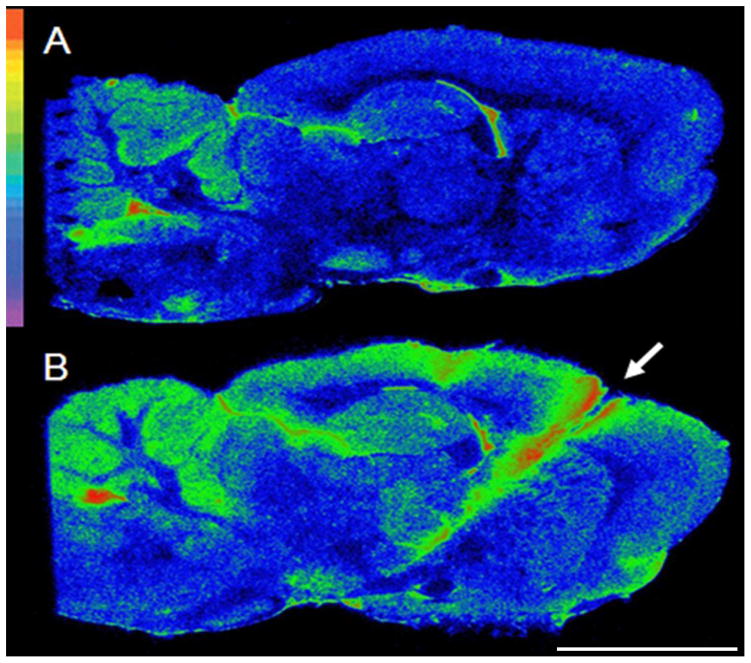
Regional neuroinflammation at the level of the STN one week following electrode implantation. Autoradiograms of sagittal brain sections from: A= an intact animal, B= an animal implanted with thick electrodes. Autoradiograms were pseudocolored using the rainbow spectrum (top left) with red representing the highest value. The electrode insertion point is marked by an arrow. Scale bar: 0.5cm.
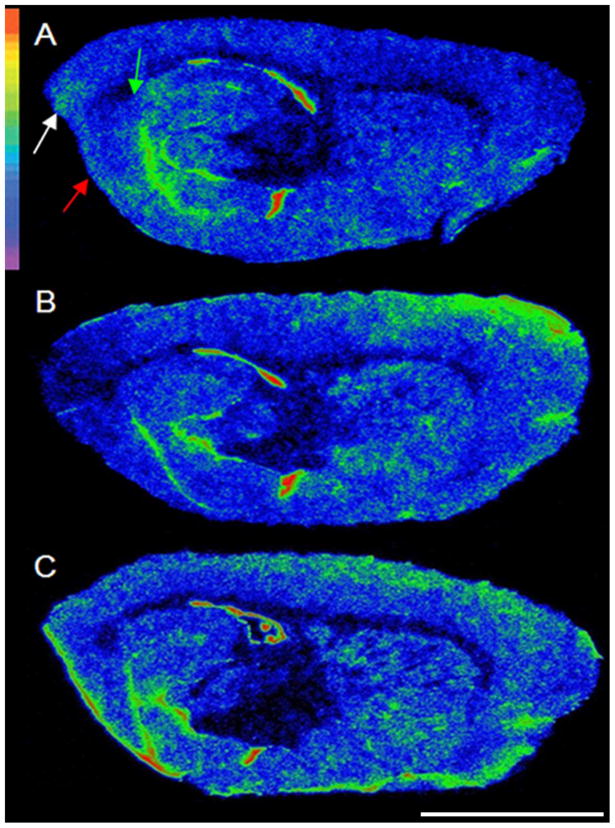
Figure 2.
Regional neuroinflammation lateral to the electrode track one week following electrode implantation. Autoradiograms of sagittal brain sections from A= a sham animal B= an animal implanted with thick electrodes C=an animal implanted with thin electrodes. Autoradiograms were pseudocolored using the rainbow spectrum (top left). Note the decrease in PBR binding in the entorhinal cortex (red arrow), perirhinal cortex (white arrow) and subiculum (green arrow) with the thick, but not the thin, electrode. Scale bar: 0.5cm.
A similar pattern of increases in PBR density was observed in hemispheres implanted with thin electrode (compared to control hemispheres) with the largest increases in the frontal cortex (177%). Smaller but significant increases were seen in parietal, temporal, insular, occipital and dorsolateral prefrontal cortex and in striatum (38%–96%). Large increases were also observed in the ventral pallidum/substantia innominata (58%) and substantia nigra (38%) but did not reach significance due to higher measurement variability in these small regions. Changes in the CA3, thalamic nuclei, CA1, dentate gyrus, medial prefrontal cortex, entorhinal cortex, subiculum, ventral hippocampus and perirhinal cortex were small and not statistically significant (Table 1, Figure 2).
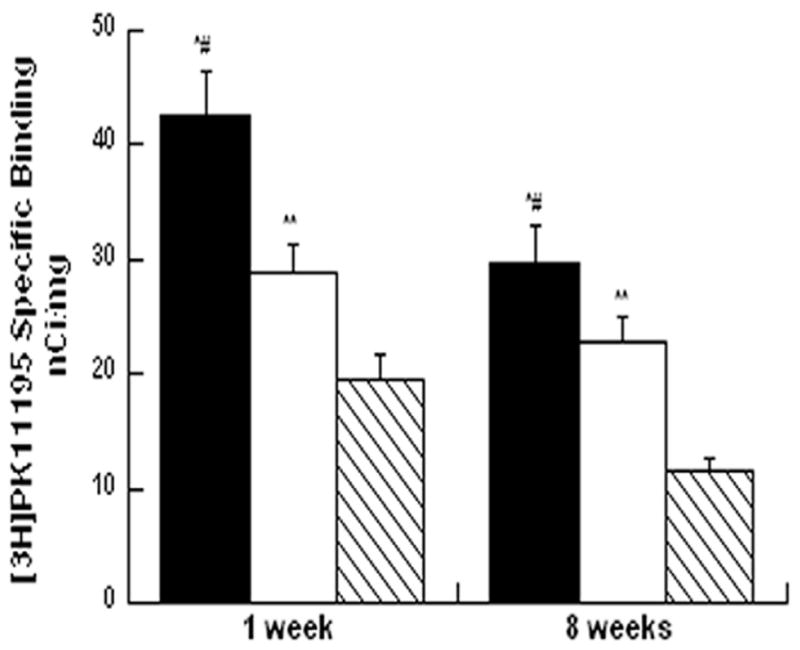
Further characterization of the regional profile of the neuroinflammation revealed that cortical increases in PBR density are larger and spread further from the site of implantation compared to the striatum when measured one week post implantation (Figure 3). PBR density in the peri-lesional cortex was higher than the peri-lesional striatum (30% difference, P< 0.0001) and the peri-lesional thalamus (50% difference, P< 0.0001). In addition, PBR density in the striatum was higher than the peri-lesional thalamus (30% difference, P=0.0002) (Figure 6).
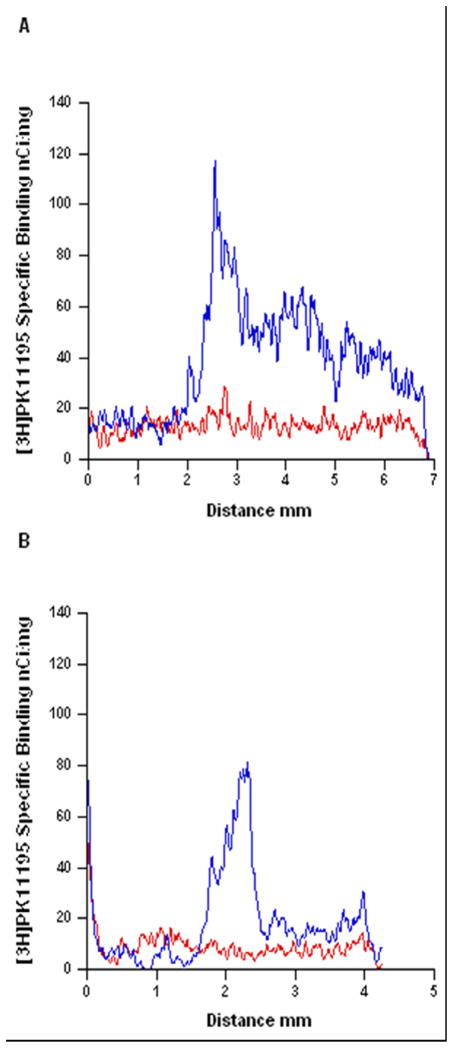
Figure 3.
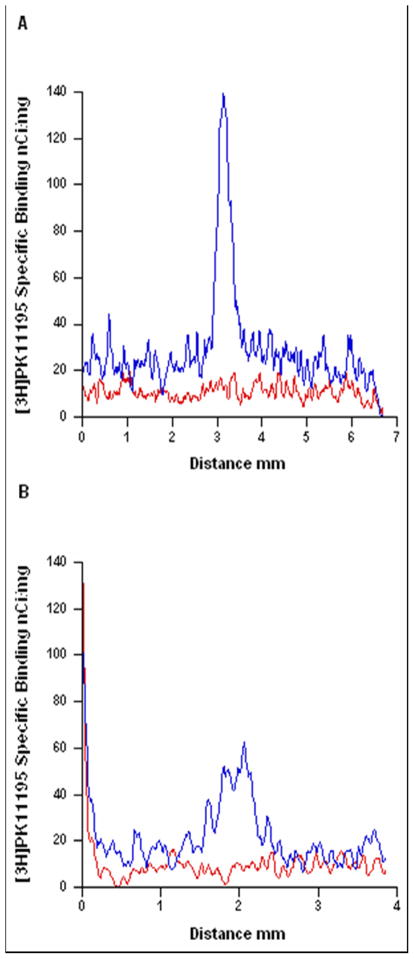
The spread of neuroinflammation within the cortex and striatum one week following electrode implantation. Line plot profile of [3H]PK11195 specific binding versus distance in the cortex (A) and striatum (B) of an intact animal (red line) and an implanted animal (blue line). The line in the cortex was drawn from the parietal cortex (above the ventricle), through the frontal cortex and prefrontal cortex and terminated at the edge of the section. The line in the striatum was drawn from the ventricle through the striatum and terminated at the corpus callosum.
Figure 6.
Neuroinflammation in the peri-lesional areas one week and eight weeks following electrode implantation. Bars depict means±SEM of peri-lesional PBR density in cortex (N=11–16, black bars), striatum (N=10–16, white bars) and thalamus (6–15, hatched bars). ap<0.0001, cortex compared to striatum bp<0.0001 cortex compared to thalamus cp< 0.0002 striatum compared to thalamus, by one way ANOVA (by region).
Effects of electrode implantation on PBR density eight weeks post implantation (Experiment 2)
Like in the short-term study above, there were no significant differences between hemispheres of sham animals compared with hemispheres of intact animals by repeated measures ANOVA, and results from these hemispheres were pooled and considered as control hemispheres. Increased density of PBR indicative of neuroinflammation was observed in hemispheres implanted with concentric electrodes compared with control (sham and intact) hemispheres eight weeks post implantation (significant region X treatment interaction by two way ANOVA F=5.56 P<0.0001). The largest significant increase was found in the frontal cortex (56%). Smaller but significant increases were seen in the striatum, medial prefrontal cortex, substantia nigra, ventral posteromedial thalamic nucleus, parietal and insular cortex (10%–29%). Non-significant changes were seen in the ventral pallidum/substantia innominata (17%) entorhinal cortex (11%) and perirhinal cortex (−11%) (Table 2, Figure 4).
Table 2.
Electrode implantation and PBR densities at eight weeks after surgery
| Control | Electrode | Electrode vs. Control | |||
|---|---|---|---|---|---|
| Brain region | mean | ± SE | mean | ±SE | % change |
| CA1 | 8.2 | ± 0.4 | 8.0 | ± 0.3 | −2 |
| CA3 | 8.2 | ± 0.4 | 8.2 | ± 0.3 | 0 |
| DG | 12.0 | ± 0.4 | 12.5 | ± 0.4 | 4 |
| DLPFC | 10.2 | ± 0.4 | 10.8 | ± 0.5 | 7 |
| Ent Cx | 14.9 | ± 0.7 | 16.5 | ± 0.6# | 11 |
| Frontal Cx | 10.3 | ± 0.4 | 16.1 | ± 1.2** | 59 |
| Insular Cx | 9.8 | ± 0.3 | 10.9 | ± 0.3* | 10 |
| LDVL | 6.7 | ± 0.4 | 7.1 | ± 0.4 | 6 |
| mPFC | 9.2 | ± 0.6 | 11.3 | ± 0.9* | 23 |
| Occipital Cx | 9.5 | ± 0.3 | 9.5 | ± 0.2 | 0 |
| Parietal Cx | 9.9 | ± 0.2 | 11.0 | ± 0.3** | 11 |
| PRh Cx | 12.7 | ± 0.8 | 11.3 | ± 0.6 | −11 |
| SN | 8.7 | ± 0.5 | 10.5 | ± 0.5** | 21 |
| Striatum | 7.9 | ± 0.2 | 10.2 | ± 0.3** | 29 |
| Subiculum | 10.5 | ± 0.4 | 10.2 | ± 0.3 | −3 |
| Temporal Cx | 9.3 | ± 0.3 | 9.2 | ± 0.5 | 0 |
| vHipp | 10.2 | ± 0.4 | 9.7 | ± 0.5 | −4 |
| VPM | 6.3 | ± 0.3 | 7.4 | ± 0.3* | 18 |
| VPSI | 5.4 | ± 0.3 | 6.3 | ± 0.4# | 17 |
Animals were killed eight weeks after bilateral implantation of concentric electrodes. Values represent specifically bound radioactivity in nCi/mg from 20 control hemispheres (N=11 intact and 9 sham operated hemispheres) and 18 implanted hemispheres with 8–20 measurements/region. CA, cornu ammoni; Cx, cortex; DG, dentate gyrus; DLPFC, dorsolateral prefrontal cortex; Ent, Entorhinal; LDVL, laterodorsal thalamic nucleus-ventrolateral part; mPFC, medial prefrontal cortex; PRh, perirhinal; SN, substantia nigra; vHipp, ventral hippocampus; VPM, ventral postermedial thalamic nucleus; VPSI, ventral pallidum/substantia innominata.
p< 0.05,
p<0.01,
p < 0.1electrode vs. control, posthoc analysis following two way ANOVA (by region X treatment).
Figure 4.
Regional neuroinflammation at the level of the STN eight weeks following electrode implantation. Autoradiograms of sagittal brain sections from: A= an intact animal, B= an implanted animal. Autoradiograms were pseudocolored using the rainbow spectrum (top left) with red representing the highest value. Electrode insertion point is marked by a white arrow. Scale bar: 0.5cm.
Density profile analysis eight weeks post implantation shows that the distribution of PBR within the cortex is more restricted relative to the distribution one week post implantation, although it is still higher than striatum (Figure 5).
Figure 5.
The spread of neuroinflammation within the cortex and the striatum eight weeks following electrode implantation. Line plot profile of [3H]PK11195 specific binding versus distance in the cortex (A) and striatum (B) of an intact animal (red line) and an implanted animal (blue line).
PBR density in the peri-lesional cortex was higher than the peri-lesional striatum (20% difference, P< 0.0001) and the peri-lesional thalamus (60% difference, P< 0.0001). PBR density in the peri-lesional striatum was significantly higher than in the peri-lesional thalamus (50% difference, P=0.0001) (Figure 6).
Effects of electrode implantation surgery on ORT performance
When tested before randomization to treatment, the animals spent 58.6% (±5.8) of total time exploring the novel object during the testing phase in comparison to 35.7% (±2.6) of total time spent exploring the old object in the same location during the familiarization phase. This difference was statistically significant (T=4.2, P<0.01) (Figure 7A).
Figure 7.
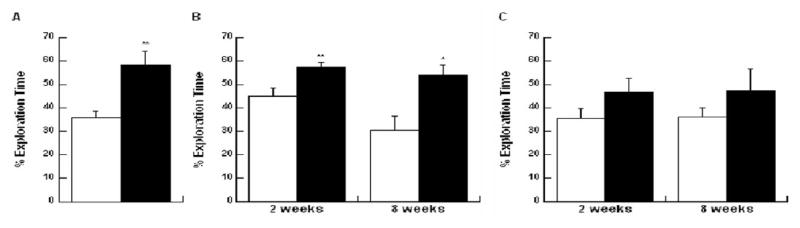
ORT performance two weeks and eight weeks following electrodes implantation. Bars depict means±SEM of percentage of time exploring the familiar (white bars) or novel (black bars) object. A. Performance in the ORT pre-implantation (N=10) B. Performance of control animals two and eight weeks post sham procedure (N=9 at two weeks, N=8 at eight weeks) C. Performance of implanted animals two and eight weeks post implantation (N=7 at two weeks, N=9 at eight weeks). *p<0.05, **p<0.005 by paired t-test.
Control (sham and intact) animals showed the expected increase in exploration of the novel object when tested again 2 weeks or 8 weeks later. Two weeks after the procedure, the control animals spent 57.2% (±2) of total time exploring the novel object during the testing phase in comparison to 44.8% (±3.5) of total time spent exploring the old object in the same location during the familiarization phase (T=3.9, P<0.005). Eight weeks post implantation the control rats spent 54.2% (±4.2) of total time exploring the novel object during the testing phase in comparison to 30.6% (±6.1) of total time spent exploring the old object in the same location during the familiarization phase (T=2.9, P=0.02) (Figure 7B).
In contrast, the implanted animals did not show a significant preference for the novel object at both time points; spending 46.6% (±5.9) with the novel object compared to 35.2% (±4.3) of total time exploring the old object when tested two weeks post implantation (T=1.4, P=0.2). Eight weeks post implantation implanted animals spent 47.3% (±9.3) of total time exploring the novel object compared to 36.1% (±4) of total time spent exploring the old object (T=1.3, P=0.2) (Figure 7C).
Total exploration time of implanted animals in the familiarization phase was not significantly different from control rats at two weeks (29.8±3.3 and 37.8±5.6 respectively, T=1.23, P=0.23) and eight weeks (21.9±2.4 and 25.5±9 respectively; T=0.38, P=0.71) post implantation; suggesting there was no significant motor deficit or general decrease in spontaneous exploration in the implanted animals.
Discussion
The current study shows for the first time that chronic implantation of electrodes in the STN produces a memory deficit as well as persistent and widespread neuroinflammation in rats, which extends beyond the electrode track in a region-selective manner. Widespread neuroinflammation appears to be a general feature of the chronic implantation procedure since it was found in rats implanted with three different types of electrodes varying in thickness and shape. Unlike previous studies which used immunohistochemistry and investigated only the local reaction to the implant (Biran et al., 2005; Griffith and Humphrey, 2006; Kim et al., 2004; Leung et al., 2008; McConnell et al., 2007; Mokry et al., 2000; Stice et al., 2007; Szarowski et al., 2003; Turner et al., 1999), we measured the intensity of neuroinflammation in multiple regions throughout the brain; using quantitative in vitro autoradiography with [3H]PK11195 for PBR labeling (Dubois et al., 1988; Myers et al., 1991a; Myers et al., 1991b; Pappata et al., 2000; Stephenson et al., 1995). Among the brain regions traversed by the electrode, the cortex showed the most intense and widespread increase in PBR (nearly two fold) one week after the surgery. This time point corresponds to the maximal microglial reaction and PBR density in other models of acute brain injury and neurotoxicity (Maeda et al., 2007; Miyazawa et al., 1995). Significant increases in PBR were found extending from the point of insertion in the frontal cortex anteriorly (to dorsolateral prefrontal and insular cortex), posteriorly (to parietal and occipital cortex), laterally (to temporal cortex) and medially (to medial prefrontal cortex). Smaller though significant increases were seen in the striatum (30%–50%) and the thalamus demonstrated even smaller increases (10%–28%) which were not statistically significant. This regional pattern of sensitivity to inflammatory insult is similar to the one we reported previously in acute models of pure global neuroinflammation (LPS injection in the cisterna magna, (Biegon et al., 2002) and closed head injury (Grossman et al., 2003)) which do not involve insertion of a foreign object into the brain; suggesting that the vulnerability of the cortex and relative resistance of the striatum and thalamus are the results of an inherent regional sensitivity to neuroinflammatory challenge. Consistently with earlier reports, we found large elevations in PBR density in close proximity to the electrode track one week post implantation (Biran et al., 2007; McConnell et al., 2007; Szarowski et al., 2003). Measurement of PBR density in peri-lesional areas also revealed an enhanced sensitivity of the cortex relative to the striatum and the thalamus.
A similar regional pattern of increases in PBR density was found in brain hemispheres examined eight weeks post implantation, though the effects were generally smaller than those observed one week post implantation. The frontal cortex again showed the largest response (>50% increase) compared to striatum (<30%), with small though significant increases in remote cortical areas including medial prefrontal cortex and insular cortex. Two regions which showed a trend towards an increase at this time point were the substantia nigra and substantia innominata/ventral pallidum. It is noteworthy that the substantia nigra and substantia innominata were among the regions most vulnerable to inflammation induced acutely by delivering LPS non invasively into the cisterna magna (Biegon et al 2002) as well as after intraparenchymal injection to the substantia nigra (Kim et al., 2000) and substantia innominata (Willard et al., 2000).
Our observations of persistent neuroinflammation near the electrode are in agreement with previous observations of sustained neuroinflammation adjacent to the implant site measured 4–12 weeks post implantation (Biran et al., 2007; Biran et al., 2005; Griffith and Humphrey, 2006; Kim et al., 2004; Lenarz et al., 2007; Leung et al., 2008; McConnell et al., 2007; Mokry et al., 2000; Stice et al., 2007; Szarowski et al., 2003; Turner et al., 1999). However our results show that even along the electrode track, there is regional sensitivity (higher sensitivity of the peri-lesional cortex relative to striatum and thalamus) which is sustained through the later time point as well.
Interestingly, the implantation of a thick electrode (but not a thin electrode) also resulted in reductions in PBR density compared to control hemispheres in regions distant from the implant site such as entorhinal cortex, perirhinal cortex and subiculum one week post implantation. A possible explanation for this finding could be the migration of microglia from remote regions towards the lesion site as shown by Carbonell et al. following focal brain injury (Carbonell et al., 2005), which could be more pronounced with a thicker electrode at short time intervals (Szarowski et al., 2003).
On the behavioral level, the implanted animals did not show any gross pathology when examined one week or more after surgery and their spontaneous locomotion was similar to controls. However, performance in the ORT was disrupted relative to control rats at two weeks as well as eight weeks post implantation. These findings are in line with a recent study showing that chronic unilateral microdialysis cannulation of the striatum led to widespread reduction of cortical glucose metabolism and memory deficits in the ORT at 3,7, 14 and 56 days after implantation while general locomotion was not affected at any of the time points (Frumberg et al., 2007). In addition, it was previously shown that neuroinflammation can lead to memory impairment (Hauss-Wegrzyniak et al., 2000; Langdon et al., 2008; Liu et al., 2007). Hence, we hypothesis that the presence of memory deficits and absence of motor deficits observed in the implanted animals are a consequence of the implantation-induced region-selective neuroinflammation. Among the regions that showed high neuroinflammation as a result of the electrode implantation there are several regions that mediate cognitive functions, e.g the insular cortex, which is involved in the object recognition test performance (Balderas et al., 2008; Bermudez-Rattoni et al., 2005). Importantly, the electrode trajectory in brains of PD patients traverses the prefrontal cortex (Benabid et al., 2009) which is responsible for many cognitive functions in humans (Funahashi, 2001; Godefroy, 2003; Smith and Jonides, 1999). Indeed, the most prevalent cognitive deficits observed in PD patients treated with DBS are related to prefrontal dysfunctions (Campbell et al., 2008; Cilia et al., 2007b; De Gaspari et al., 2006; Dujardin et al., 2001). In fact, a path through the prefrontal (“non-eloquent”) cortex is favored in most intracranial neurosurgical procedures. However, we do not have direct evidence of cortical neuroinflammation from biopsies obtained from patients treated with DBS, since published studies focused only on the area adjacent to the electrode’s contacts, which are usually located subcortically (Boockvar et al., 2000; Burbaud et al., 2002; Chou et al., 2004; Haberler et al., 2000; Henderson et al., 2001; Nielsen et al., 2007; Pilitsis et al., 2008).
Taken together, these observations suggest that the cognitive deficits observed in DBS patients may result at least in part from neuroinflammation caused by the surgical procedure, irrespective of the target region and its direct stimulation. If this is indeed the case, it may also explain the increased cognitive vulnerability of older patients to DBS (Saint-Cyr et al., 2000; Trepanier et al., 2000), since it was shown that a neuroinflammatory challenge in aged mice produces a disproportionately high induction of proinflammatory cytokines in microglia (Henry et al., 2009). In addition, it was shown by post mortem analysis that activated microglia are present in the cortex of PD brains (Imamura et al., 2003). Therefore, the disease itself may also contribute to the neuroinflammation seen in PD patients treated with DBS.
Perhaps more importantly, these observations support an investigation of the ability of inhibitors of microglial activation (e.g. minocycline, a derivative of the antibiotic tetracycline (Kim et al., 2009; Liu et al., 2007; Yrjanheikki et al., 1998) to prevent or ameliorate cognitive deficits associated with DBS (Cai et al., 2008; Liu et al., 2007). Further experiments in animal models and patients with DBS are needed to validate and translate these hypotheses.
Acknowledgments
We would like to thank Dr. Spiegelman from the department of neurosurgery in Sheba medical center for helpful discussions. Supported in part by NIH RO1 NS050285 to Anat Biegon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alegret M, Junque C, Valldeoriola F, Vendrell P, Pilleri M, Rumia J, Tolosa E. Effects of bilateral subthalamic stimulation on cognitive function in Parkinson disease. Arch Neurol. 2001;58:1223–1227. doi: 10.1001/archneur.58.8.1223. [DOI] [PubMed] [Google Scholar]
- Ardouin C, Pillon B, Peiffer E, Bejjani P, Limousin P, Damier P, Arnulf I, Benabid AL, Agid Y, Pollak P. Bilateral subthalamic or pallidal stimulation for Parkinson’s disease affects neither memory nor executive functions: a consecutive series of 62 patients. Ann Neurol. 1999;46:217–223. doi: 10.1002/1531-8249(199908)46:2<217::aid-ana11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Balderas I, Rodriguez-Ortiz CJ, Salgado-Tonda P, Chavez-Hurtado J, McGaugh JL, Bermudez-Rattoni F. The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learn Mem. 2008;15:618–624. doi: 10.1101/lm.1028008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 2009;8:67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Chabardes S, Seigneuret E. Deep-brain stimulation in Parkinson’s disease: long-term efficacy and safety - What happened this year? Curr Opin Neurol. 2005;18:623–630. doi: 10.1097/01.wco.0000186839.53807.93. [DOI] [PubMed] [Google Scholar]
- Bermudez-Rattoni F, Okuda S, Roozendaal B, McGaugh JL. Insular cortex is involved in consolidation of object recognition memory. Learn Mem. 2005;12:447–449. doi: 10.1101/lm.97605. [DOI] [PubMed] [Google Scholar]
- Biegon A, Alvarado M, Budinger TF, Grossman R, Hensley K, West MS, Kotake Y, Ono M, Floyd RA. Region-selective effects of neuroinflammation and antioxidant treatment on peripheral benzodiazepine receptors and NMDA receptors in the rat brain. J Neurochem. 2002;82:924–934. doi: 10.1046/j.1471-4159.2002.01050.x. [DOI] [PubMed] [Google Scholar]
- Biran R, Martin DC, Tresco PA. The brain tissue response to implanted silicon microelectrode arrays is increased when the device is tethered to the skull. J Biomed Mater Res A. 2007;82:169–178. doi: 10.1002/jbm.a.31138. [DOI] [PubMed] [Google Scholar]
- Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol. 2005;195:115–126. doi: 10.1016/j.expneurol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Boockvar JA, Telfeian A, Baltuch GH, Skolnick B, Simuni T, Stern M, Schmidt ML, Trojanowski JQ. Long-term deep brain stimulation in a patient with essential tremor: clinical response and postmortem correlation with stimulator termination sites in ventral thalamus. Case report J Neurosurg. 2000;93:140–144. doi: 10.3171/jns.2000.93.1.0140. [DOI] [PubMed] [Google Scholar]
- Burbaud P, Vital A, Rougier A, Bouillot S, Guehl D, Cuny E, Ferrer X, Lagueny A, Bioulac B. Minimal tissue damage after stimulation of the motor thalamus in a case of chorea-acanthocytosis. Neurology. 2002;59:1982–1984. doi: 10.1212/01.wnl.0000038389.30437.1e. [DOI] [PubMed] [Google Scholar]
- Cai ZY, Yan Y, Sun SQ, Zhang J, Huang LG, Yan N, Wu F, Li JY. Minocycline attenuates cognitive impairment and restrains oxidative stress in the hippocampus of rats with chronic cerebral hypoperfusion. Neurosci Bull. 2008;24:305–313. doi: 10.1007/s12264-008-0324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MC, Karimi M, Weaver PM, Wu J, Perantie DC, Golchin NA, Tabbal SD, Perlmutter JS, Hershey T. Neural correlates of STN DBS-induced cognitive variability in Parkinson disease. Neuropsychologia. 2008;46:3162–3169. doi: 10.1016/j.neuropsychologia.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell WS, Murase S, Horwitz AF, Mandell JW. Migration of perilesional microglia after focal brain injury and modulation by CC chemokine receptor 5: an in situ time-lapse confocal imaging study. J Neurosci. 2005;25:7040–7047. doi: 10.1523/JNEUROSCI.5171-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou KL, Forman MS, Trojanowski JQ, Hurtig HI, Baltuch GH. Subthalamic nucleus deep brain stimulation in a patient with levodopa-responsive multiple system atrophy. Case report J Neurosurg. 2004;100:553–556. doi: 10.3171/jns.2004.100.3.0553. [DOI] [PubMed] [Google Scholar]
- Cilia R, Siri C, Marotta G, De Gaspari D, Landi A, Mariani CB, Benti R, Isaias IU, Vergani F, Pezzoli G, Antonini A. Brain networks underlining verbal fluency decline during STN-DBS in Parkinson’s disease: an ECD-SPECT study. Parkinsonism Relat Disord. 2007a;13:290–294. doi: 10.1016/j.parkreldis.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Cilia R, Siri C, Marotta G, De Gaspari D, Landi A, Mariani CB, Benti R, Isaias IU, Vergani F, Pezzoli G, Antonini A. Brain networks underlining verbal fluency decline during STN-DBS in Parkinson’s disease: an ECD-SPECT study. Parkinsonism Relat Disord. 2007b;13:290–294. doi: 10.1016/j.parkreldis.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Daniele A, Albanese A, Contarino MF, Zinzi P, Barbier A, Gasparini F, Romito LM, Bentivoglio AR, Scerrati M. Cognitive and behavioural effects of chronic stimulation of the subthalamic nucleus in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2003;74:175–182. doi: 10.1136/jnnp.74.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gaspari D, Siri C, Di Gioia M, Antonini A, Isella V, Pizzolato A, Landi A, Vergani F, Gaini SM, Appollonio IM, Pezzoli G. Clinical correlates and cognitive underpinnings of verbal fluency impairment after chronic subthalamic stimulation in Parkinson’s disease. Parkinsonism Relat Disord. 2006;12:289–295. doi: 10.1016/j.parkreldis.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR. Biological reactions to cerebrospinal fluid shunt devices: a review of the cellular pathology. Neurosurgery. 1998;42:319–325. doi: 10.1097/00006123-199802000-00064. discussion 325–316. [DOI] [PubMed] [Google Scholar]
- Dubois A, Benavides J, Peny B, Duverger D, Fage D, Gotti B, MacKenzie ET, Scatton B. Imaging of primary and remote ischaemic and excitotoxic brain lesions. An autoradiographic study of peripheral type benzodiazepine binding sites in the rat and cat. Brain Res. 1988;445:77–90. doi: 10.1016/0006-8993(88)91076-1. [DOI] [PubMed] [Google Scholar]
- Dujardin K, Defebvre L, Krystkowiak P, Blond S, Destee A. Influence of chronic bilateral stimulation of the subthalamic nucleus on cognitive function in Parkinson’s disease. J Neurol. 2001;248:603–611. doi: 10.1007/s004150170139. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fasano A, Mazzone P, Piano C, Quaranta D, Soleti F, Bentivoglio AR. GPi-DBS in Huntington’s disease: Results on motor function and cognition in a 72-year-old case. Mov Disord. 2008 doi: 10.1002/mds.22116. [DOI] [PubMed] [Google Scholar]
- Fields JA, Troster AI, Woods SP, Higginson CI, Wilkinson SB, Lyons KE, Koller WC, Pahwa R. Neuropsychological and quality of life outcomes 12 months after unilateral thalamic stimulation for essential tremor. J Neurol Neurosurg Psychiatry. 2003;74:305–311. doi: 10.1136/jnnp.74.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frumberg DB, Fernando MS, Lee DE, Biegon A, Schiffer WK. Metabolic and behavioral deficits following a routine surgical procedure in rats. Brain Res. 2007;1144:209–218. doi: 10.1016/j.brainres.2007.01.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S. Neuronal mechanisms of executive control by the prefrontal cortex. Neurosci Res. 2001;39:147–165. doi: 10.1016/s0168-0102(00)00224-8. [DOI] [PubMed] [Google Scholar]
- Funkiewiez A, Ardouin C, Caputo E, Krack P, Fraix V, Klinger H, Chabardes S, Foote K, Benabid AL, Pollak P. Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:834–839. doi: 10.1136/jnnp.2002.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy O. Frontal syndrome and disorders of executive functions. J Neurol. 2003;250:1–6. doi: 10.1007/s00415-003-0918-2. [DOI] [PubMed] [Google Scholar]
- Griffith RW, Humphrey DR. Long-term gliosis around chronically implanted platinum electrodes in the Rhesus macaque motor cortex. Neurosci Lett. 2006;406:81–86. doi: 10.1016/j.neulet.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Grossman R, Shohami E, Alexandrovich A, Yatsiv I, Kloog Y, Biegon A. Increase in peripheral benzodiazepine receptors and loss of glutamate NMDA receptors in a mouse model of closed head injury: a quantitative autoradiographic study. Neuroimage. 2003;20:1971–1981. doi: 10.1016/j.neuroimage.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Kuhlmann AC, O’Callaghan JP, Miceli RC. Enhanced expression of peripheral benzodiazepine receptors in trimethyltin-exposed rat brain: a biomarker of neurotoxicity. Neurotoxicology. 1995;16:441–450. [PubMed] [Google Scholar]
- Haberler C, Alesch F, Mazal PR, Pilz P, Jellinger K, Pinter MM, Hainfellner JA, Budka H. No tissue damage by chronic deep brain stimulation in Parkinson’s disease. Ann Neurol. 2000;48:372–376. [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Vannucchi MG, Wenk GL. Behavioral and ultrastructural changes induced by chronic neuroinflammation in young rats. Brain Res. 2000;859:157–166. doi: 10.1016/s0006-8993(00)01999-5. [DOI] [PubMed] [Google Scholar]
- Henderson JM, O’Sullivan DJ, Pell M, Fung VS, Hely MA, Morris JG, Halliday GM. Lesion of thalamic centromedian--parafascicular complex after chronic deep brain stimulation. Neurology. 2001;56:1576–1579. doi: 10.1212/wnl.56.11.1576. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Pell M, O’Sullivan DJ, McCusker EA, Fung VS, Hedges P, Halliday GM. Postmortem analysis of bilateral subthalamic electrode implants in Parkinson’s disease. Mov Disord. 2002;17:133–137. doi: 10.1002/mds.1261. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman M, van de Beek D, Weisfelt M, de Gans J, Schmand B. Cognitive outcome in adults after bacterial meningitis. J Neurol Neurosurg Psychiatry. 2007;78:1092–1096. doi: 10.1136/jnnp.2006.110023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106:518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- James ML, Selleri S, Kassiou M. Development of ligands for the peripheral benzodiazepine receptor. Curr Med Chem. 2006;13:1991–2001. doi: 10.2174/092986706777584979. [DOI] [PubMed] [Google Scholar]
- Jarraya B, Bonnet AM, Duyckaerts C, Houeto JL, Cornu P, Hauw JJ, Agid Y. Parkinson’s disease, subthalamic stimulation, and selection of candidates: a pathological study. Mov Disord. 2003;18:1517–1520. doi: 10.1002/mds.10607. [DOI] [PubMed] [Google Scholar]
- Kalbe E, Voges J, Weber T, Haarer M, Baudrexel S, Klein JC, Kessler J, Sturm V, Heiss WD, Hilker R. Frontal FDG-PET activity correlates with cognitive outcome after STN-DBS in Parkinson disease. Neurology. 2009;72:42–49. doi: 10.1212/01.wnl.0000338536.31388.f0. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Kim MJ, Park JM, Lee SH, Kim YJ, Ryu S, Kim YH, Yoon BW. Reduced neurogenesis after suppressed inflammation by minocycline in transient cerebral ischemia in rat. J Neurol Sci. 2009;279:70–75. doi: 10.1016/j.jns.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YT, Hitchcock RW, Bridge MJ, Tresco PA. Chronic response of adult rat brain tissue to implants anchored to the skull. Biomaterials. 2004;25:2229–2237. doi: 10.1016/j.biomaterials.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Kiss ZH, Doig-Beyaert K, Eliasziw M, Tsui J, Haffenden A, Suchowersky O. The Canadian multicentre study of deep brain stimulation for cervical dystonia. Brain. 2007;130:2879–2886. doi: 10.1093/brain/awm229. [DOI] [PubMed] [Google Scholar]
- Lang S. The role of peripheral benzodiazepine receptors (PBRs) in CNS pathophysiology. Curr Med Chem. 2002;9:1411–1415. doi: 10.2174/0929867023369745. [DOI] [PubMed] [Google Scholar]
- Langdon KD, Granter-Button S, Corbett D. Persistent behavioral impairments and neuroinflammation following global ischemia in the rat. Eur J Neurosci. 2008;28:2310–2318. doi: 10.1111/j.1460-9568.2008.06513.x. [DOI] [PubMed] [Google Scholar]
- Lenarz M, Lim HH, Lenarz T, Reich U, Marquardt N, Klingberg MN, Paasche G, Reuter G, Stan AC. Auditory midbrain implant: histomorphologic effects of long-term implantation and electric stimulation of a new deep brain stimulation array. Otol Neurotol. 2007;28:1045–1052. doi: 10.1097/MAO.0b013e318159e74f. [DOI] [PubMed] [Google Scholar]
- Leung BK, Biran R, Underwood CJ, Tresco PA. Characterization of microglial attachment and cytokine release on biomaterials of differing surface chemistry. Biomaterials. 2008;29:3289–3297. doi: 10.1016/j.biomaterials.2008.03.045. [DOI] [PubMed] [Google Scholar]
- Limousin P, Martinez-Torres I. Deep brain stimulation for Parkinson’s disease. Neurotherapeutics. 2008;5:309–319. doi: 10.1016/j.nurt.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsman N, Neimat JS, Lozano AM. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: the search for a valid target. Neurosurgery. 2007;61:1–11. doi: 10.1227/01.neu.0000279719.75403.f7. discussion 11–13. [DOI] [PubMed] [Google Scholar]
- Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- Maeda J, Higuchi M, Inaji M, Ji B, Haneda E, Okauchi T, Zhang MR, Suzuki K, Suhara T. Phase-dependent roles of reactive microglia and astrocytes in nervous system injury as delineated by imaging of peripheral benzodiazepine receptor. Brain Res. 2007;1157:100–111. doi: 10.1016/j.brainres.2007.04.054. [DOI] [PubMed] [Google Scholar]
- Marangell LB, Martinez M, Jurdi RA, Zboyan H. Neurostimulation therapies in depression: a review of new modalities. Acta Psychiatr Scand. 2007;116:174–181. doi: 10.1111/j.1600-0447.2007.01033.x. [DOI] [PubMed] [Google Scholar]
- McConnell GC, Schneider TM, Owens DJ, Bellamkonda RV. Extraction force and cortical tissue reaction of silicon microelectrode arrays implanted in the rat brain. IEEE Trans Biomed Eng. 2007;54:1097–1107. doi: 10.1109/TBME.2007.895373. [DOI] [PubMed] [Google Scholar]
- Miyazawa N, Diksic M, Yamamoto Y. Chronological study of peripheral benzodiazepine binding sites in the rat brain stab wounds using [3H] PK-11195 as a marker for gliosis. Acta Neurochir (Wien) 1995;137:207–216. doi: 10.1007/BF02187195. [DOI] [PubMed] [Google Scholar]
- Mokry J, Karbanova J, Lukas J, Paleckova V, Dvorankova B. Biocompatibility of HEMA copolymers designed for treatment of CNS diseases with polymer-encapsulated cells. Biotechnol Prog. 2000;16:897–904. doi: 10.1021/bp000113m. [DOI] [PubMed] [Google Scholar]
- Moro E, Scerrati M, Romito LM, Roselli R, Tonali P, Albanese A. Chronic subthalamic nucleus stimulation reduces medication requirements in Parkinson’s disease. Neurology. 1999;53:85–90. doi: 10.1212/wnl.53.1.85. [DOI] [PubMed] [Google Scholar]
- Morrison CE, Borod JC, Perrine K, Beric A, Brin MF, Rezai A, Kelly P, Sterio D, Germano I, Weisz D, Olanow CW. Neuropsychological functioning following bilateral subthalamic nucleus stimulation in Parkinson’s disease. Arch Clin Neuropsychol. 2004;19:165–181. doi: 10.1016/S0887-6177(03)00004-0. [DOI] [PubMed] [Google Scholar]
- Myers R, Manjil LG, Cullen BM, Price GW, Frackowiak RS, Cremer JE. Macrophage and astrocyte populations in relation to [3H]PK 11195 binding in rat cerebral cortex following a local ischaemic lesion. J Cereb Blood Flow Metab. 1991a;11:314–322. doi: 10.1038/jcbfm.1991.64. [DOI] [PubMed] [Google Scholar]
- Myers R, Manjil LG, Frackowiak RS, Cremer JE. [3H]PK 11195 and the localisation of secondary thalamic lesions following focal ischaemia in rat motor cortex. Neurosci Lett. 1991b;133:20–24. doi: 10.1016/0304-3940(91)90047-w. [DOI] [PubMed] [Google Scholar]
- Nielsen MS, Bjarkam CR, Sorensen JC, Bojsen-Moller M, Sunde NA, Ostergaard K. Chronic subthalamic high-frequency deep brain stimulation in Parkinson’s disease--a histopathological study. Eur J Neurol. 2007;14:132–138. doi: 10.1111/j.1468-1331.2006.01569.x. [DOI] [PubMed] [Google Scholar]
- Pappata S, Levasseur M, Gunn RN, Myers R, Crouzel C, Syrota A, Jones T, Kreutzberg GW, Banati RB. Thalamic microglial activation in ischemic stroke detected in vivo by PET and [11C]PK1195. Neurology. 2000;55:1052–1054. doi: 10.1212/wnl.55.7.1052. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Pikis A, Kavaliotis J, Tsikoulas J, Andrianopoulos P, Venzon D, Manios S. Long-term sequelae of pneumococcal meningitis in children. Clin Pediatr (Phila) 1996;35:72–78. doi: 10.1177/000992289603500204. [DOI] [PubMed] [Google Scholar]
- Pilitsis JG, Chu Y, Kordower J, Bergen DC, Cochran EJ, Bakay RA. Postmortem study of deep brain stimulation of the anterior thalamus: case report. Neurosurgery. 2008;62:E530–532. doi: 10.1227/01.neu.0000316024.81786.78. discussion E532. [DOI] [PubMed] [Google Scholar]
- Pillon B, Ardouin C, Damier P, Krack P, Houeto JL, Klinger H, Bonnet AM, Pollak P, Benabid AL, Agid Y. Neuropsychological changes between “off” and “on” STN or GPi stimulation in Parkinson’s disease. Neurology. 2000;55:411–418. doi: 10.1212/wnl.55.3.411. [DOI] [PubMed] [Google Scholar]
- Raghavendra Rao VL, Dogan A, Bowen KK, Dempsey RJ. Traumatic brain injury leads to increased expression of peripheral-type benzodiazepine receptors, neuronal death, and activation of astrocytes and microglia in rat thalamus. Exp Neurol. 2000;161:102–114. doi: 10.1006/exnr.1999.7269. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Trepanier LL, Kumar R, Lozano AM, Lang AE. Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson’s disease. Brain. 2000;123 (Pt 10):2091–2108. doi: 10.1093/brain/123.10.2091. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Heimann B, Djukic M, Mazurek C, Fels C, Wallesch CW, Nau R. Neuropsychological sequelae of bacterial and viral meningitis. Brain. 2006;129:333–345. doi: 10.1093/brain/awh711. [DOI] [PubMed] [Google Scholar]
- Schroeder U, Kuehler A, Lange KW, Haslinger B, Tronnier VM, Krause M, Pfister R, Boecker H, Ceballos-Baumann AO. Subthalamic nucleus stimulation affects a frontotemporal network: a PET study. Ann Neurol. 2003;54:445–450. doi: 10.1002/ana.10683. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Stephan CL, Kepes JJ, SantaCruz K, Wilkinson SB, Fegley B, Osorio I. Spectrum of clinical and histopathologic responses to intracranial electrodes: from multifocal aseptic meningitis to multifocal hypersensitivity-type meningovasculitis. Epilepsia. 2001;42:895–901. doi: 10.1046/j.1528-1157.2001.042007895.x. [DOI] [PubMed] [Google Scholar]
- Stephenson DT, Schober DA, Smalstig EB, Mincy RE, Gehlert DR, Clemens JA. Peripheral benzodiazepine receptors are colocalized with activated microglia following transient global forebrain ischemia in the rat. J Neurosci. 1995;15:5263–5274. doi: 10.1523/JNEUROSCI.15-07-05263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice P, Gilletti A, Panitch A, Muthuswamy J. Thin microelectrodes reduce GFAP expression in the implant site in rodent somatosensory cortex. J Neural Eng. 2007;4:42–53. doi: 10.1088/1741-2560/4/2/005. [DOI] [PubMed] [Google Scholar]
- Szarowski DH, Andersen MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, Turner JN, Shain W. Brain responses to micro-machined silicon devices. Brain Res. 2003;983:23–35. doi: 10.1016/s0006-8993(03)03023-3. [DOI] [PubMed] [Google Scholar]
- Trepanier LL, Kumar R, Lozano AM, Lang AE, Saint-Cyr JA. Neuropsychological outcome of GPi pallidotomy and GPi or STN deep brain stimulation in Parkinson’s disease. Brain Cogn. 2000;42:324–347. doi: 10.1006/brcg.1999.1108. [DOI] [PubMed] [Google Scholar]
- Turner JN, Shain W, Szarowski DH, Andersen M, Martins S, Isaacson M, Craighead H. Cerebral astrocyte response to micromachined silicon implants. Exp Neurol. 1999;156:33–49. doi: 10.1006/exnr.1998.6983. [DOI] [PubMed] [Google Scholar]
- Vonck K, Boon P, Goossens L, Dedeurwaerdere S, Claeys P, Gossiaux F, Van Hese P, De Smedt T, Raedt R, Achten E, Deblaere K, Thieleman A, Vandemaele P, Thiery E, Vingerhoets G, Miatton M, Caemaert J, Van Roost D, Baert E, Michielsen G, Dewaele F, Van Laere K, Thadani V, Robertson D, Williamson P. Neurostimulation for refractory epilepsy. Acta Neurol Belg. 2003;103:213–217. [PubMed] [Google Scholar]
- Vonck K, Boon P, Van Roost D. Anatomical and physiological basis and mechanism of action of neurostimulation for epilepsy. Acta Neurochir Suppl. 2007;97:321–328. doi: 10.1007/978-3-211-33081-4_35. [DOI] [PubMed] [Google Scholar]
- Willard LB, Hauss-Wegrzyniak B, Danysz W, Wenk GL. The cytotoxicity of chronic neuroinflammation upon basal forebrain cholinergic neurons of rats can be attenuated by glutamatergic antagonism or cyclooxygenase-2 inhibition. Exp Brain Res. 2000;134:58–65. doi: 10.1007/s002210000446. [DOI] [PubMed] [Google Scholar]
- Witt K, Daniels C, Reiff J, Krack P, Volkmann J, Pinsker MO, Krause M, Tronnier V, Kloss M, Schnitzler A, Wojtecki L, Botzel K, Danek A, Hilker R, Sturm V, Kupsch A, Karner E, Deuschl G. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study. Lancet Neurol. 2008;7:605–614. doi: 10.1016/S1474-4422(08)70114-5. [DOI] [PubMed] [Google Scholar]
- York MK, Dulay M, Macias A, Levin HS, Grossman R, Simpson R, Jankovic J. Cognitive declines following bilateral subthalamic nucleus deep brain stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2008;79:789–795. doi: 10.1136/jnnp.2007.118786. [DOI] [PubMed] [Google Scholar]
- Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]