Abstract
Angiogenesis is required for progression and metastasis of melanoma. Analysis of angiogenic molecules in benign and malignant tissues may allow identification of markers useful for prediction of sensitivity to antiangiogenic agents. We hypothesized that differential expression of VEGF and its receptors VEGF-R1, - R2, and -R3 would be higher in melanomas than nevi and higher in advanced melanoma. Using automated quantitative analysis (AQUA), we quantified VEGF, -R1, -R2 and -R3 expression in melanoma tissue microarrays composed of 540 nevi and 468 melanoma specimens (198 primaries, 270 metastases). VEGF, -R1, -R2 and -R3 expression was significantly higher in melanomas than nevi by unpaired t-tests (p <0.0001). VEGF-R2 expression was higher in metastatic specimens (p <0.0001), but VEGF-R3 expression was higher in primaries (p < 0.0001). VEGF was coexpressed with all three receptors when assessed by Spearman's rank correlation. VEGF, -R1, -R2 and -R3 expression is higher in melanomas than nevi. Higher expression of VEGF-R2 was found in metastases versus primaries, supporting the idea that selection for an angiogenic phenotype in metastatic melanoma is conferred via upregulation of VEGF-R2. However, higher expression of VEGF-R3 was seen on primary lesions, potentially implicating this receptor in initiation of lymphatic tumor spread. Clinical trials using antiangiogenic agents in melanoma should include correlative assays of VEGF, -R1, -R2 and -R3 as biomarkers of response to therapy, preferably using quantitative methods such as AQUA. Such assessments could assist with evaluation of these molecules as therapeutic targets in melanoma, ultimately facilitating improved selection of patients for treatment.
Keywords: VEGF, VEGF-R1, VEGF-R2, VEGF-R3, melanoma
Introduction
Melanoma is a relatively chemotherapy resistant disease that is associated with an extremely poor prognosis once systemic metastases develop. An estimated 62,480 new melanomas will be diagnosed in the United States in 2008, and an estimated 8,420 people in the United States will die of the disease. For patients with metastases, the median survival is in the range of 9 to 12 months (1). No therapy has been proven to prolong survival in patients with melanoma in randomized clinical trials, underscoring the need for the development of more effective treatments.
Angiogenesis has been recognized as an important process for the growth and invasion of malignant tumors, including melanoma. The vascular endothelial growth factor (VEGF) pathway plays a critical role in angiogenesis. This pathway has been the focus of much attention with the development of bevacizumab (Avastin, Genentech, San Francisco, CA), an antibody targeted against VEGF that has been shown to prolong progression free survival in kidney and breast cancers and overall survival in lung and colon cancers (2-3). The VEGF family consists of five isoforms, VEGF-A (known as VEGF), VEGF-B, VEGF-C, VEGF-D, and placental growth factor. VEGF-A, also known as vascular permeability factor or VEGF, was described as a potent endothelial cell mitogen which stimulates the proliferation and migration of endothelial cells. VEGF signals primarily through receptor tyrosine kinases VEGF receptor 1 (VEGF-R1, flt-1) and 2 (VEGF-R2, flk2/kdr). While VEGF-R1 has a much greater affinity for VEGF, ligand mediated autophosphorylation is weaker than that initiated by ligand binding to VEGF-R2. It has been postulated that VEGF-R1 may play a decoy role in VEGF signaling by regulating VEGF through decreased binding to VEGF-R2, and that the majority of effects of VEGF in malignancy are mediated through VEGF-R2. VEGF-A and primarily VEGF -C and -D signal through VEGF-R3; this pathway is thought to be essential for the development of lymphatic vasculature and for normal and tumor lymphangiogenesis (4).
Several studies have examined the expression of members of the VEGF signaling pathway in melanoma. Secretion of VEGF occurs during progression of early cutaneous melanocytic lesions, with low VEGF expression in benign nevi increasing significantly in dysplastic nevi and more so in malignant melanoma (5). The transition of melanomas from the radial to the aggressive vertical growth phases is also marked by increased VEGF production (6). Several groups have reported results of VEGF expression assessed by immunohistochemical staining in cohorts of melanoma tumor tissue (7-12). While most of these studies showed increased VEGF expression in malignant versus benign tumors, with higher expression associated with more invasive phenotypes, prevalence of tumors expressing VEGF varied widely, and no clear relationship with prognosis has been verified. In these tissues VEGF has traditionally been thought to signal in a paracrine fashion, recruiting endothelial cells for the formation of neovasculature to nourish growing tumors. However, previous identification of VEGF receptors in melanoma cell lines suggests VEGF may also signal in melanoma through an autocrine loop (13,14). Small studies have assessed the presence of VEGF-R1 and R2 on human melanoma tissue (7,15), and their results confirm the presence of these receptors in human melanomas as well as a possible relationship of VEGF-R2 expression with thicker and more invasive tumors.
Recognized for its distinct role in lymphangiogenesis, VEGF-R3 expression in malignant melanocytes is a focus of rising interest. VEGF-R3 expression has been seen on both tumor cells as well as blood vessels and lymphatics (16), where in benign melanocytic lesions, it is confined to lymphatic vessels only (17), implicating a role in metastatic spread. Mouawad et al. recently reported high levels of VEGFR-3 in melanoma tumor tissue, accompanied by significantly higher pre-treatment serum levels of VEGFR-3 in melanoma patients. Interestingly, median serum VEGFR-3 levels were increased in patients with high tumour burden as well as in non-responders compared to responders, and low VEGFR-3 related positively to disease free survival, underscoring the potential prognostic implications for expression of this receptor (18).
Given the probable diverse roles of VEGF and its receptors in promoting melanoma growth and metastases, we hypothesized that in melanoma, VEGF signaling may contribute substantially to tumor growth and metastasis. We thus sought to characterize expression of VEGF and its receptors VEGF-R1, VEGF-R2, and VEGF-R3 on a large number of human melanoma specimens and benign nevi and to correlate expression with survival, disease stage, age, gender, and the presence of known histologic prognostic factors such as Clark's level, Breslow depth, and the presence of ulceration or tumor infiltrating lymphocytes. We expected that higher expression levels of these molecules would be seen in malignant melanomas compared with benign nevi and that differential expression would be seen in primary and metastatic subsets. We were further interested in examining potential coexpression among the VEGF ligand and its receptors within melanomas. To accomplish this, we employed tissue microarrays of melanoma specimens using a newly developed, automated method of analysis (AQUA for Automated, QUantitative Analysis). This method provides precise, reproducible measurement of antigen levels, free of the subjectivity associated with pathologist-based scoring employed in traditional immunohistochemistry studies (19). AQUA analysis provides continuous output scores, as opposed to arbitrary nominal scores obtained with pathologist-based “by-eye” scoring of 0, 1, 2, or 3 or “positive” and “negative.” This is particularly important when therapeutic decisions are made based on immunohistochemistry under nonstandardized conditions. This method has been validated (19), has been proven to be more accurate than pathologist-based scoring of DAB stain, and has been used in several prior melanoma studies as previously described (20). Recent clinical trial designs, including melanoma trials, have planned for the incorporation of AQUA analysis of tissues from cancer patients treated with targeted therapies, with the goal of identifying markers that might predict response to treatment (21). We sought to use AQUA to examine a large historical cohort of 468 melanomas and 540 nevi. No previous studies to our knowledge have examined the expression of VEGF and its three major receptors in cohorts of clinical specimens using an automated method of expression analysis. Our results indicate higher expression of VEGF and all three of the receptors in melanomas compared with nevi. The expression of VEGF-R2 was higher in metastatic than in primary melanomas; however, for VEGF-R3, higher expression was seen in primary lesions.
Materials and Methods
Cell Lines and Western Blots
YUSAC, YUSOC, YUMAC, YUFIC and YUROB are cell lines derived from tumors of patients treated at Yale University. The MEL501 cell line was obtained from Dr. Steven Rosenberg at the Surgery Branch, National Cancer Institute, MD. Proteins from lysates were obtained using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Western blotting was performed by standard methods utilizing antibodies to VEGF (sc-152, Santa Cruz Biotechnology, 1:500), VEGF-R1 (sc-316, Santa Cruz Biotechnology, 1:500) VEGF-R2 (sc-6251, Santa Cruz Biotechnology, 1:500), VEGF-R3 (sc-321, Santa Cruz Biotechnology, 1:500) and β-Actin (A2066 or A5441, SIGMA, 1:200 or 1:5000 respectively). Detection of proteins was done utilizing peroxidase-conjugated anti-mouse or anti-rabbit IgG secondary antibodies (715-035-151 and 711-035-152, Jackson ImmunoResearch Laboratories, 1:5000) and ECL (PerkinElmer).
Tissue Microarray (TMA) Construction
TMAs were constructed as described (20). Cores from 198 primary melanomas and 270 metastatic melanomas, each measuring 0.6 mm in diameter, were spaced 0.8 mm apart on slides. Specimens and clinical information were collected with approval of a Yale University Institutional Review Board. The cohort has been used in prior publications (20). The specimens were resected between 1959 and 2000, with a follow-up range between 2 months and 40 years, and a mean follow-up time of 6.7 years. Age at diagnosis ranged from 18 to 91 (mean age 52.4 years). The cohort included 55% males and 45% females and contained lesions from patients with melanomas of various stages (i.e. early stage primary, or non-metastatic, lesions and late stage metastatic lesions). Treatment history was not available. No patients were treated with VEGF- or VEGF-receptor targeted therapy. Similarly a tissue microarray was made containing cores from 540 benign nevi. The nevus array contained 31 metastatic specimens from patients that were also represented on the melanoma array. Both arrays contained identical cell lines, cored from pellets, as previously described (20). The overlapping metastatic specimens and cell lines were used for normalization of the scores obtained from the benign and malignant arrays.
Immunohistochemistry
Staining was performed for automated analysis, as previously described (20). Briefly, slides were deparaffinized in xylene, and transferred through two changes of 100% ethanol. For antigen retrieval, slides were boiled in a pressure cooker containing 6.5mM sodium citrate (pH 6.0). Endogenous peroxidase activity was blocked in a mixture of methanol and 2.5% hydrogen peroxide for thirty minutes at room temperature. To reduce non-specific background staining, slides were incubated at room temperature for 30 minutes in 0.3% bovine serum albumin/1× Tris-buffered saline. Slides were incubated at 4°C overnight in a humidity tray with the primary antibodies (rabbit polyclonal anti-VEGF IgG at 1:50, rabbit polyclonal anti-VEGF-R1 at 1:200, rabbit polyclonal anti-VEGF-R3 at 1:500 and mouse monoclonal anti-VEGF-R2 IgG at 1:200). To create a tumor mask, slides were simultaneously incubated overnight with a primary anti-S100 antibody (mouse anti-human S100 for VEGF, VEGF-R1 and VEGF-R3 at 1:100, and rabbit anti-human S100 for VEGF-R2 at 1:500). Slides were rinsed three times in 1× Tris-buffered saline/0.05% Tween-20. For VEGF, VEGF-R1 and VEGF-R3, slides were incubated for 1 hour at room temperature with goat anti-rabbit HRP (Envision; DAKO Corp., Carpinteria, CA) to identify the target, and goat anti-mouse IgG conjugated to Alexa 546 (Molecular Probes, Inc. Eugene, OR) at a dilution of 1:100 to identify the mask. The same technique was used to assess VEGF-R2 expression, except that goat anti-mouse HRP and goat anti-rabbit IgG conjugated to Alexa 546 were used. The slides were washed again as above and incubated for ten minutes with Cy5 directly conjugated to tyramide (Perkin Elmer, Boston, MA) at a dilution of 1:50 for primary antibody identification. The slides were rinsed again and coverslips were mounted with ProLong Gold antifade reagent, which contained 4,6-diamidine-2-phenylindole (DAPI) to identify the nuclei.
Automated Image Acquisition
Images were acquired using automated, quantitative analysis, as described previously (20). Briefly, areas of tumor were distinguished from stroma by creating a mask with the S100 signal tagged with Alexa 546. Coalescence of S100 at the cell surface was used to identify cytoplasmic compartment within the tumor mask, while 4,6-diamidino-2-phenylindole (DAPI) was used to identify the nuclear compartment within the tumor mask. The target markers, VEGF, VEGF-R1, VEGF-R2 and VEGF-R3, were visualized with Cy5 (red). Cy5 was used because its emission peak is outside the color spectrum of tissue autoflourescence. Multiple monochromatic, high resolution (1024 × 1024 pixel 0.5-μm) grayscale images were obtained for each histospot, using the 10× objective of an Olympus AX-51 epifluorescence microscope (Olympus, Melville, NY) with an automated microscope stage and digital image acquisition driven by custom program and macro-based interfaces with IPLabs software (Scanalytics Inc., Fairfax, VA).
Algorithmic Image Analysis
Images were analyzed using algorithms that have been previously extensively described (19). Two images (one in-focus and one out-of-focus) were taken of the compartment specific tags and the target marker. A percentage of the out-of-focus image was subtracted from the in-focus image for each pixel, representing the signal to noise ratio of the image. An algorithm described as RESA (Rapid Exponential Subtraction Algorithm) was used to subtract the out-of-focus information in a uniform fashion for the entire microarray. Subsequently, the PLACE algorithm (Pixel Locale Assignment for Compartmentalization of Expression) was used to assign each pixel in the image to a specific subcellular compartment and the signal in each location was calculated. Pixels that could not accurately be assigned to a compartment were discarded. The data were saved and subsequently expressed as the average signal intensity per unit of compartment area. For the nuclear and membrane/cytoplasmic compartments, the image was measured on a scale of 0-255, and expressed as target signal intensity relative to the compartment area.
Statistical Analysis
The Statview and JMP5 (SAS Institute Inc., Cary, NC) software packages were used for data analyses. The associations between continuous AQUA scores of target expression and tumor stage, Clark's level, Breslow depth, age, gender, and presence of ulceration or tumor infiltrating lymphocytes were assessed using unpaired t-tests. The prognostic significance of the parameters was assessed using the Cox proportional hazards model with overall survival as an end point. Comparisons of expression between malignant and benign specimens and between primary and metastatic specimens were performed with unpaired t-tests. Spearman's non-parametric rank correlation coefficient was used to assess associations between expression of VEGF and its receptors.
Results
Lysates from a panel of melanoma cell lines were probed for VEGF, VEGF-R1, VEGF-R2 and VEGF-R3, and dominant bands were seen at 21 KD, 180 KD, 200 KD and 150 KD, consistent with reports in the literature (22). The intensity of the bands was more variable for VEGF-R1 and -R3, as shown in Figure 1.
Figure 1.
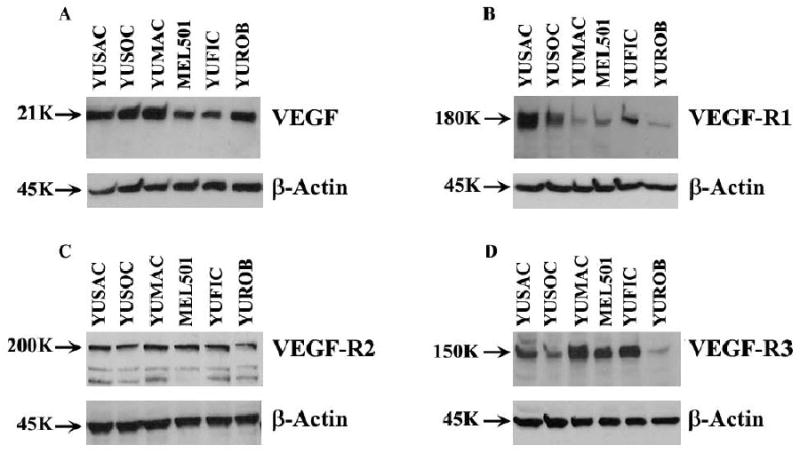
Expression of VEGF, VEGF-R1, VEGF-R2 and VEGF-R3 in melanoma cell lines. Protein was extracted from YUSAC, YUSOC, YUMAC, MEL501, YUFIC and YUROB melanoma cell lines and was subjected SDS-PAGE and Western blot analysis to detect VEGF (A), VEGF-R1 (B), VEGF-R2 (C) and VEGF-R3 (D).
To assess for intra-tumor heterogeneity, two separate arrays, each containing a core from a different area of the tumor for each patient, were used to evaluate the expression of each marker. None of the markers studied had significant amounts of nuclear staining, and thus only the membranous/cytoplasmic compartments were analyzed. Figure 2 shows regression plots for the scores of the two arrays for expression of: a)VEGF, b)VEGF-R1, c)VEGF-R2 and d) VEGF-R3 expression (R = 0.6, 0.5, 0.6 and 0.7, respectively).
Figure 2.
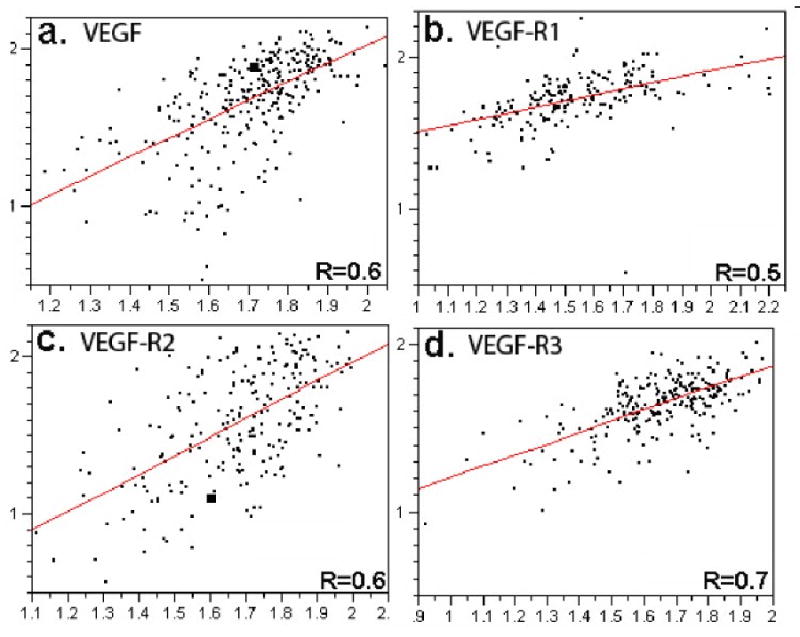
Regression plots for scores from the two sets of melanoma arrays for A) VEGF, B)VEGF-R1, C)VEGF-R2 and D)VEGF-R3. The y axis represents AQUA scores from one slide and the x axis from the second slide. Each array contains histospots from the same patients, taken from different areas of the tumor.
AQUA scores of melanomas ranged from 16.4 to 91.01 for VEGF with a median score of 52.4, from 12.23 to 130.95 for VEGF-R1 with a median score of 55.47, from 6.49 to 131.86 for VEGF-R2 with a median score of 46.2, and from 12.25 to 95.31 for VEGF-R3 with a median score of 47.47. Examples of strong staining for VEGF and its receptors are shown in Figure 3. As can be seen in this figure, staining was primarily cytoplasmic and membranous for VEGF and its receptors.
Figure 3.
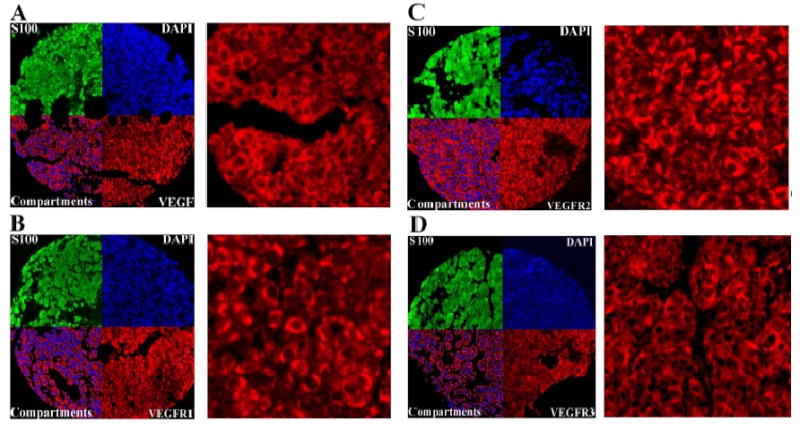
Cytoplasmic VEGF, VEGF-R1, VEGF-R2 and VEGF-R3 expression in melanoma histospots using S100 to define the tumor mask (green), 4,6-diamidine-2-phenylindole to define the nuclear compartment (blue), and Cy5 (red) for the target. Merged images displayed in the upper outer quadrants of panels A,B,C, and D show the amount of VEGF, VEGF-R1, VEGF-R2 and VEGF-R3, respectively, within the nuclear compartment and within the cytoplasmic compartment within the tumor mask at 10× magnification. Upper inner quadrants of panels A,B,C, and D show expression of VEGF, VEGF-R1, VEGF-R2 and VEGF-R3 at 60× magnification
For each of the four markers, the AQUA scores from both sets of slides were combined in the following manner: tumor spots were deemed uninterpretable if they had insufficient tumor cells, loss of tissue in the spot, or an abundance of necrotic tissue. For patients who had two interpretable histospots, a composite score was formed by taking the average of the two scores. For patients with only one interpretable core, the single score was used. The resulting combined data set included 380 patients for VEGF, 451 patients for VEGF-R1, 419 patients for VEGF-R2 and 339 patients for VEGF-R3. We assessed differences between benign and malignant tissues for VEGF and its receptors. Unpaired t tests showed that expression was significantly higher in malignant versus benign tissue cores for VEGF, VEGF-R1, VEGF-R2 and VEGF-R3 (p < 0.0001 for each), as shown in Figure 4. Expression of VEGF-R2 was significantly higher in metastatic compared to primary melanomas (p < 0.0001), while the inverse was seen for VEGF-R3 (p < 0.0001). Differential expression of VEGF and VEGF-R1 was not observed in primary versus metastatic specimens. By Cox univariate analysis of raw AQUA scores, we found no association between survival and expression of any of the four markers in either primary or metastatic specimens (p > 0.05 for all). Unpaired t-tests were used to assess the association between VEGF, VEGF-R1, VEGF-R2 and VEGF-R3 receptor expression and other commonly used clinical and pathologic variables. As stated above, expression of VEGF-R2 was higher in metastatic than primary melanomas, and vice versa for VEGF-R3. We found no association between expression of VEGF, VEGF-R1, VEGF-R2, or VEGF-R3 and age, Breslow depth, Clark's level, presence of ulceration or presence of tumor infiltrating lymphocytes. Using the Spearman Rank Correlation Test, we found strong associations between expression of all four markers, as shown in Table 1.
Figure 4.
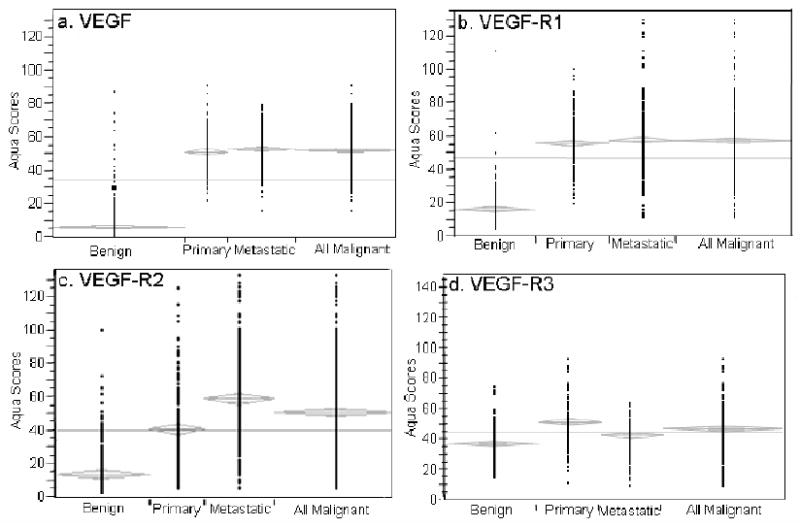
Expression of VEGF, VEGF-R1, VEGF-R2 and VEGF-R3 (P < 0.0001) was significantly higher in malignant than benign tissues. Expression of VEGF-R2 (P < 0.0001) was substantially higher in metastatic than primary specimens. Conversely, expression of VEGF-R3 (P < 0.0001) was more pronounced in primary versus metastatic spots.
Table 1.
Spearman Rank Correlations between variables.
| Spearman Rank Correlation for | Rho | P-Value |
|---|---|---|
| VEGF/VEGF-R1 | 0.588 | <.0001 |
| VEGF/VEGF-R2 | 0.487 | <.0001 |
| VEGF/VEGF-R3 | 0.381 | <.0001 |
| VEGF-R1/VEGF-R2 | 0.428 | <.0001 |
| VEGF-R1/VEGF-R3 | 0.493 | <.0001 |
| VEGF-R2/VEGF-R3 | 0.297 | <.0001 |
It is possible that sensitivity to agents targeted against VEGF or its receptors would be greater in tumors that over-express these targets. Therefore, we sought to classify tumors as high or low expressers, based on the range of expression in our large cohort of benign nevi, and the assumption that levels of expression in benign nevi are low from a biological perspective. We defined “low” or “normal” AQUA scores as those that fall at or below the 95th percentile score for nevi for each marker. This score was 20.03 for VEGF, 28.88 for VEGF-R1, 39.84 for VEGF-R2 and for 54.77 or VEGF-R3. Interestingly, VEGF and VEGF-R1 expression was high in 99.7 % and 96.4% of tumors using this definition. For VEGF-R2 approximately 59.9% of malignant tissues showed levels of expression above the 95th percentile for nevi, and 78% of metastatic spots were classified as high expressers compared to 45% of primary spots. For VEFG-R3, 30.5% of malignant tissues showed levels of expression above the 95th percentile for nevi.
Discussion
The purpose of this study was to quantitatively assess the expression of VEGF and its receptors using an objective, automated method and to characterize differences in expression between melanomas and nevi. Further, we evaluated associations with clinical and pathologic variables, and assessed co-expression among VEGF and its receptors. Expression of VEGF, VEGF-R1, VEGF-R2 and VEGF-R3 was significantly higher in malignant compared to benign specimens. When comparing unmatched (from different patients) primary and metastatic specimens, significant differences in expression persisted for VEGF-R2 and VEGF-R3. VEGF-R2 expression was higher in metastatic specimens; VEGF-R3 expression, however, was higher in primary specimens. Differences in VEGF and VEGF-R1 expression between primary and malignant specimens were much less pronounced. Although VEGF-R2 and VEGF-R3 expression was associated with disease stage, we found no correlation between the level of expression and survival within the primary or metastatic patient subsets. Our results were reproducible on a second array using different cores from the same patients.
These observations could reflect the roles of each of these molecules in melanoma tumorigenesis. The role of VEGF for the recruitment of endothelial cells to form neovasculature to nourish growing tumor is well established. In support of this, we found VEGF expression to be increased in malignant melanomas. Our discovery of high expression of VEGF-R1, R2 and R3 on malignant melanomas compared with benign nevi leads us to believe VEGF may be signaling through multiple receptors in the pathogenesis of melanoma. Further, strong associations between expression of all four markers were noted, as shown in Table 1, suggesting the activity of multiple receptors and possibly, the presence of autocrine signaling loops within the melanoma cells. Recent work by Sini et al (23) supports this conclusion, suggesting that combined inhibition of the three VEGFRs, compared with blocking antibodies against VEGF-R2 or VEGF-A alone, significantly reduced metastatic spread of melanoma cells and blocked function of peritumoral lymphatics. It has also been shown that VEGF acts as a potent tumor lymphangiogenesis factor and that tumor-derived VEGF promotes expansion of the lymphatic network within draining, sentinel lymph nodes, even before these tumors metastasized (24), indicating that lymph node lymphangiogenesis might contribute to further metastatic tumor spread. In the context of our findings, higher VEGF-R3 expression in melanocytes of early stage tumors could reflect a role for the melanocytes in lymphangiogenesis and subsequent metastasis in primary melanomas. Once the tumor becomes metastatic it is possible that VEGF-R3 plays a less central role in growth and therefore high levels of expression do not persist. It is plausible that at this point VEGF then signals through VEGF-R2 on melanoma metastases to continue growth through an autocrine mechanism and that survival of melanoma cells is dependent on this loop, as suggested in previous publications (25,26), at least partially supporting the tumor growth in conjunction with other pathways known to be active in melanoma. Our findings of higher VEGF-R2 on metastatic versus primary lesions would further support this hypothesis.
While expression of VEGF and VEGF receptors was associated with disease progression, no association with survival was seen. This is likely due to the fact that this autocrine loop is important for the metastatic process, but once the disease has metastasized, other mechanisms of proliferation and cell survival might be more important. In addition, environmental and stromal characteristics, such as immune cell activity, are likely to play a major role in tumor growth or suppression.
Several previous studies (5-12) have corroborated the presence of high expression of VEGF in melanoma tumor tissue. Straume et al showed that VEGF and its receptors are co-expressed on tumor cells (7), consistent with our results. Previous studies have not commented on higher expression of VEGF-R2 in metastatic lesions versus primaries, which we observed. Further, to the best of our knowledge, ours is the only large study to demonstrate higher expression of VEGF-R3 in primary as compared to metastatic melanomas.
Since ligand binding of VEGF to VEGF-R2 is thought to mediate the majority of VEGF effects in malignancy, finding higher expression in melanomas than in nevi is encouraging for the continued development of VEGF-R2 targeted agents in melanoma. Our data show that 59.9% of our melanomas have VEGF-R2 levels that exceed the 95th percentile of expression for nevi, suggesting that most melanomas have up-regulation of the VEGF-VEGF-R2 autocrine loop. 78% of metastatic spots were found to be high expressers compared to 45% of primary spots, indicating that up-regulation of these proteins is associated with tumor progression. Even larger differences between tumors and nevi were seen for VEGF and VEGF-R1.
Activity of agents targeted against the VEGF signaling pathway in melanoma has been implicated in several phase I/II trials. Much attention has been given to the Phase I/II trial of carboplatin, taxol and sorafenib (Nexavar, Bayer Pharmaceuticals Corporation, West Haven, CT and Onyx Pharmaceuticals, Emeryville, CA), a multi-tyrosine kinase inhibitor with activity against both the MAP kinase and VEGF-R2 signaling pathways. Preliminary results indicated partial responses in 11 of 32 evaluable patients, with 19 additional patients showing stabilization of disease as best response (27). Although sorafenib is a known B-raf inhibitor, no association was seen between B-raf mutation status and response. However, high VEGF-R2 expression of melanoma samples from treated patients was associated with response to this regimen (21). These results led to two multicenter, randomized phase III trials of carboplatin and taxol with or without sorafenib. In a trial of patients who had received prior chemotherapy, the addition of sorafenib, while resulting in a tolerable toxicity profile, did not result in improved response rates or progression free survival (28). The second trial, ECOG 2603, tested this same regimen of carboplatin, taxol +/- sorafenib in chemotherapy naïve patients, a distinct patient population. A planned interim analysis of the trial concluded that it would not meet the primary endpoint of improved overall survival in 800 patients with stage III or IV melanoma, resulting in a recommendation to terminate the trial1. Nonetheless, agents with antiangiogenic activity remain of interest in clinical trials for patients with metastatic melanoma. Most recently, Fruehauf reported the results of a single agent phase II trial with axitinib, an oral, potent and selective inhibitor of all three major VEGF receptors. Treatment was well tolerated, and an ORR of 15.6% (95% CI: 5.3, 312.8%) with response duration ranging from 2.3 to > 10.2 months was seen, with median PFS of 2.3 months (95% CI: 1.8, 5.7) and median OS of 6.8 months (95% CI: 5.2, 10.4; range 0.8-22.6 months). Axitinib therapy selectively decreased soluble VEGF-R2 and VEGF-R3 and increased VEGF in the blood, demonstrating activity against the targeted VEGF receptors (29). These results further underscore the probable role of VEGF and its several receptors in the pathogenesis of malignant melanoma. Additional ongoing trials of novel agents that target VEGF and its receptors include the study of IMC-1121B (Imclone, New York, NY) an antibody directed against VEGF-R2, with dacarbazine.
Our results may have important implications for the future design of clinical trials using agents targeted against the VEGF pathway. Differential expression between malignant and benign tissue of VEGF, VEGF-R1, VEGF-R2 and VEGF-R3 suggests these markers may play a role in disease progression and supports the current and future use of agents that target the VEGF pathway in melanoma. Currently, there are no proven biomarkers of efficacy of anti-VEGF therapy, although these biomarkers are needed to validate mechanistic hypotheses, to identify responsive patients and optimal doses, to predict efficacy of regimens that include anti-VEGF agents, and to detect and prevent tumor escape (2). Historically, assays for VEGF in tumor and serum of patients with melanoma as predictors of either survival or response to treatment have not produced consistent results in the literature in patients with melanoma (30-33). It is possible that serum VEGF levels are not indicative of VEGF activity within tumors. In addition, technical difficulties have been encountered with assays of serum levels of VEGF (34-35). Interestingly, recent data by Sabatino et al. (36) have reported the results of proteomic analysis of the serum of patients (predominantly with metastatic melanoma) who were treated with high-dose IL-2, using a customized, multiplex antibody targeted protein array platform to survey expression of soluble factors associated with tumor immunobiology. This analysis identified low serum VEGF as a significant predictor of response to IL-2 therapy, a finding which may potentially aid with the selection of patients for this treatment and thus deserves evaluation in larger prospective clinical trials. However, it remains unclear whether pre-treatment tumor or blood levels of VEGF and/or its receptors are routinely associated with response to therapy. If accurate measurements of protein expression could be obtained using advanced technologies, proven associations between pre-treatment expression and response would have invaluable implications for the improvement of patient selection, particularly therapies which directly target the VEGF pathway.
Assessment of the expression of VEGF and its receptors may be further useful to identify which targets are crucial in different stages of melanoma. For example, our results suggest that VEGF-R2 targeted agents may be useful in the metastatic setting, but VEGF-R3 may be the more opportune target in the adjuvant treatment of early stage disease; however, supportive correlative data from clinical trials of these agents in human beings are necessary for confirmation. While our findings of co-expression of VEGF and its receptors on melanoma tumor cells suggest the presence of an autocrine growth factor loop, VEGF is also secreted into the microenvironment, resulting in blood vessel formation. Further preclinical work is necessary to clarify whether VEGF and VEGF receptors function predominantly as initiators of growth and survival of the melanocytes or as pro-angiogenic molecules.
In summary, our studies show stronger expression of VEGF, VEGF-R1, VEGF-R2 and VEGF-R3 in melanomas than in benign nevi specimens, suggesting that these markers are associated with disease progression. VEGF-R3 expression was found to be higher in primary specimens, while VEGF-R2 expression was higher in metastatic specimens. Our data suggest that the VEGF signaling pathway certainly plays a role in the transformation from benign to malignant melanocytic tissues and as such members of this pathway remain important therapeutic targets. To our knowledge, our experiments comprise the largest studies of these molecules in cohorts of melanoma patients, and our method in particular has the advantage of objective, quantitative scoring of large numbers of tumor samples in a single assay. Based on our data, we propose that future trials of antiangiogenic agents in melanoma include correlative studies for the assessment of VEGF and/or its receptors in tumor samples to evaluate the predictive role of these molecules, with the ultimate goal of improved selection of patients for treatment.
Acknowledgments
Supported by NIH grant CA115756-01 (to H. Kluger).
Footnotes
“Nexxavar Fails Phase III Melanoma Trial,” http://www.pharmaprojects.com/news/3004_1605.htm, 4/30/2009.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3(1):24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Koralewski P, Pluzanska A, et al. A randomized, controlled, double-blind phase III study (AVOREN) of bevacizumab/interferon-a2a vs placebo/interferon- a2a as first-line therapy in metastatic renal cell carcinoma. J Clin Oncol. 2007;25:2s. Abstract. [Google Scholar]
- 4.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 5.Einspahr JG, Thomas TL, Saboda K, Nickolof BJ, Warneke J, Curiel-Lewandrowski C, Ranger-Moore J, Duckett L, Bangert J, Fruehauf JP, Alberts DS. Expression of vascular endothelial growth factor in early cutaneous melanocytic lesion progression. Cancer. 2007 Dec 1;110(11):2519–27. doi: 10.1002/cncr.23076. [DOI] [PubMed] [Google Scholar]
- 6.Erhard H, Rietveld FJ, van Altena MC, Brocker EB, Ruiter DJ, de Waal RM. Transition of horizontal to vertical growth phase melanoma is accompanied by induction of vascular endothelial growth factor expression and angiogenesis. Melanoma Res. 1997;7 2:S19–26. [PubMed] [Google Scholar]
- 7.Straume O, Akslen LA. Expression of vascular endothelial growth factor, its receptors (FLT-1, KDR) and TSP-1 related to microvessel density and patient outcome in vertical growth phase melanomas. Am J Pathol. 2001;159:223. doi: 10.1016/S0002-9440(10)61688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlaykova T, Laurila P, Muhonen T, et al. Prognostic value of tumour vascularity in metastatic melanoma and association of blood vessel density with vascular endothelial growth factor expression. Melanoma Res. 1999;9:59–68. doi: 10.1097/00008390-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Simonetti O, Lucarini G, Brancorsini D, et al. Immunohistochemical expression of vascular endothelial growth factor, matrix metalloproteinase 2, and matrix metalloproteinase 9 in cutaneous melanocytic lesions. Cancer. 2002;95:1963–70. doi: 10.1002/cncr.10888. [DOI] [PubMed] [Google Scholar]
- 10.Demirkesen C, Buyukpinarbasili N, Ramazanoglu R, Oguz O, Mandel NM, Kaner G. The correlation of angiogenesis with metastasis in primary cutaneous melanoma: a comparative analysis of microvessel density, expression of vascular endothelial growth factor and basic fibroblastic growth factor. Pathology. 2006;38:132–7. doi: 10.1080/00313020600557565. [DOI] [PubMed] [Google Scholar]
- 11.Bayer-Garner IB, Hough AJ, Jr, Smoller BR. Vascular endothelial growth factor expression in malignant melanoma: prognostic versus diagnostic usefulness. Mod Pathol. 1999;12:770–4. [PubMed] [Google Scholar]
- 12.Potti A, Moazzam N, Tendulkar K, Javed NA, Koch M, Kargas S. Immunohistochemical determination of vascular endothelial growth factor (VEGF) overexpression in malignant melanoma. Anticancer Res. 2003 Sep-Oct;23(5A):4023–6. [PubMed] [Google Scholar]
- 13.Gitay-Goren H, Halaban R, Neufeld G. Human melanoma cells but not normal melanocytes express vascular endothelial growth factor receptors. Biochem Biophys Res Commun. 1993;190:702–708. doi: 10.1006/bbrc.1993.1106. [DOI] [PubMed] [Google Scholar]
- 14.Graeven U, Fiedler W, Karpinski S, et al. Melanoma-associated expression of vascular endothelial growth factor and its receptors FLT-1 and KDR. J Cancer Res Clin Oncol. 1999;125:621–9. doi: 10.1007/s004320050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pisacane AM, Risio M. VEGF and VEGFR-2 immunohistochemistry in human melanocytic naevi and cutaneous melanomas. Melanoma Res. 2005 Feb;15(1):39–43. doi: 10.1097/00008390-200502000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Wobser M, Siedel C, Schrama D, Brocker EB, Becker JC, Vetter-Kauczok CS. Expression pattern of the lymphatic and vascular markers VEGFR-3 and CD31 does not predict regional lymph node metastasis in cutaneous melanoma. Arch Dermatol Res. 2006;297:352–7. doi: 10.1007/s00403-005-0633-1. [DOI] [PubMed] [Google Scholar]
- 17.Clarijs R, Schalkwijk L, Hofmann UB, Ruiter DJ, de Waal RM. Induction of vascular endothelial growth factor receptor-3 expression on tumor microvasculature as a new progression marker in human cutaneous melanoma. Cancer Res. 2002;62:7059–65. [PubMed] [Google Scholar]
- 18.Mouawad R, Spano JP, Comperat E, Capron F, Khayat D. Tumoural expression and circulating level of VEGFR-3 (Flt-4) in metastatic melanoma patients: Correlation with clinical parameters and outcome. Eur J Cancer. 2009 Jan 19; doi: 10.1016/j.ejca.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med. 2002;8:1323–7. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy MM, Divito KA, Sznol M, et al. Expression of tumor necrosis factor--related apoptosis-inducing ligand receptors 1 and 2 in melanoma. Clin Cancer Res. 2006;12:3856–63. doi: 10.1158/1078-0432.CCR-06-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jilaveanu L, Zito C, Lee SJ, Nathanson KL, Camp RL, Rimm DL, Flaherty KT, Kluger HM. Expression of sorafenib targets in melanoma patients treated with carboplatin, paclitaxel and sorafenib. Clin Cancer Res. 2009 Feb 1;15(3):1076–85. doi: 10.1158/1078-0432.CCR-08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C, Okruzhnov Y, Li H, Nicholas J. Human herpesvirus 8 (HHV-8)-encoded cytokines induce expression of and autocrine signaling by vascular endothelial growth factor (VEGF) in HHV-8-infected primary-effusion lymphoma cell lines and mediate VEGF-independent antiapoptotic effects. J Virol. 2001;75:10933–40. doi: 10.1128/JVI.75.22.10933-10940.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sini P, Samarzija I, Baffert F, Littlewood-Evans A, Schnell C, Theuer A, Christian S, Boos A, Hess-Stumpp H, Foekens JA, Setyono-Han B, Wood J, Hynes NE. Inhibition of multiple vascular endothelial growth factor receptors (VEGFR) blocks lymph node metastases but inhibition of VEGFR-2 is sufficient to sensitize tumor cells to platinum-based chemotherapeutics. Cancer Res. 2008 Mar 1;68(5):1581–92. doi: 10.1158/0008-5472.CAN-06-4685. [DOI] [PubMed] [Google Scholar]
- 24.Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–99. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graells J, Vinyals A, Figueras A, et al. Overproduction of VEGF concomitantly expressed with its receptors promotes growth and survival of melanoma cells through MAPK and PI3K signaling. J Invest Dermatol. 2004;123:1151–61. doi: 10.1111/j.0022-202X.2004.23460.x. [DOI] [PubMed] [Google Scholar]
- 26.Lacal PM, Ruffini F, Pagani E, D'Atri S. An autocrine loop directed by the vascular endothelial growth factor promotes invasiveness of human melanoma cells. Int J Oncol. 2005;27:1625–32. [PubMed] [Google Scholar]
- 27.Flaherty KT, Brose M, Schuchter L, et al. Phase I/II trial of BAY 43-9006, carboplatin (C) and paclitaxel (P) demonstrates preliminary antitumor activity in the expansion cohort of patients with metastatic melanoma (Abstract) J Clin Oncol. 2004;22:7507. [Google Scholar]
- 28.Hauschild A, Agarwala SS, Trefzer U, Hogg D, Robert C, Hersey P, Eggermont A, Grabbe S, Gonzalez R, Gille J, Peschel C, Schadendorf D, Garbe C, O'Day S, Daud A, White JM, Xia C, Patel K, Kirkwood JM, Keilholz U. Results of a Phase III, Randomized, Placebo-Controlled Study of Sorafenib in Combination With Carboplatin and Paclitaxel As Second-Line Treatment in Patients With Unresectable Stage III or Stage IV Melanoma. J Clin Oncol. 2009 Apr 6; doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 29.Fruehauf JP, Lutzky J, McDermott DF, Brown CK, Pithavala YK, Bycott PW, Shalinsky D, Liau KF, Niethammer A, Rixe O. Axitinib (AG-013736) in patients with metastatic melanoma: A phase II study. J Clin Oncol. 2008;26 May 20 suppl; abstr 9006. [Google Scholar]
- 30.Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19:577–83. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- 31.Tas F, Duranyildiz D, Oguz H, Camlica H, Yasasever V, Topuz E. Circulating serum levels of angiogenic factors and vascular endothelial growth factor receptors 1 and 2 in melanoma patients. Melanoma Res. 2006;16:405–11. doi: 10.1097/01.cmr.0000222598.27438.82. [DOI] [PubMed] [Google Scholar]
- 32.Brennecke S, Deichmann M, Naeher H, Kurzen H. Decline in angiogenic factors, such as interleukin-8, indicates response to chemotherapy of metastatic melanoma. Melanoma Res. 2005;15:515–22. doi: 10.1097/00008390-200512000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Osella-Abate S, Quaglino P, Savoia P, Leporati C, Comessatti A, Bernengo MG. VEGF-165 serum levels and tyrosinase expression in melanoma patients: correlation with the clinical course. Melanoma Res. 2002;12:325–34. doi: 10.1097/00008390-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Hormbrey E, Gillespie P, Turner K, et al. A critical review of vascular endothelial growth factor (VEGF) analysis in peripheral blood: is the current literature meaningful? Clin Exp Metastasis. 2002;19:651–63. doi: 10.1023/a:1021379811308. [DOI] [PubMed] [Google Scholar]
- 35.Webb NJ, Bottomley MJ, Watson CJ, Brenchley PE. Vascular endothelial growth factor (VEGF) is released from platelets during blood clotting: implications for measurement of circulating VEGF levels in clinical disease. Clin Sci (Lond) 1998;94:395–404. doi: 10.1042/cs0940395. [DOI] [PubMed] [Google Scholar]
- 36.Sabatino M, Kim-Schulze S, Panelli MC, Stroncek D, Wang E, Taback B, Kim DW, Deraffele G, Pos Z, Marincola FM, Kaufman HL. Serum vascular endothelial growthfactor and fibronectin predict clinical response to high-dose interleukin-2 therapy. J Clin Oncol. 2009 Jun 1;27(16):2645–52. doi: 10.1200/JCO.2008.19.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]


