Abstract
Low to moderate doses of alcohol consumption induce heightened aggressive behavior in some, but not all individuals. Individual vulnerability for this nonadaptive behavior may be determined by an interaction of genetic and environmental factors to the sensitivity of alcohol’s effects on brain and behavior. We used a previously established protocol for alcohol oral self-administration and characterized alcohol-heightened aggressive (AHA) mice as compared to alcohol-non-heightened (ANA) counterparts. A week later, we quantified mRNA steady state levels of several candidate genes in the serotonin (5-HT) system in different brain areas. We report a regionally selective and significant reduction of all 5-HT receptor subtype transcripts, except for 5HT3, in the prefrontal cortex of AHA mice. Comparable gene expression profile was previously observed in aggressive mice induced by social isolation or by an anabolic androgenic steroid. Additional change in the 5-HT1B receptor transcripts was seen in the amygdala and hypothalamus of AHA mice. In both these areas, 5-HT1B mRNA was elevated when compared to ANA mice. In the hypothalamus, AHA mice showed also increased transcripts for 5-HT2A receptor. In the midbrain, 5-HT synthetic enzyme, 5-HT transporter, and 5-HT receptors mRNA levels were similar between groups. Our results emphasize a role for postsynaptic over presynaptic 5-HT receptors in individuals who showed escalated aggression after the consumption of a moderate dose of alcohol. This gene expression profile of 5-HT neurotransmission components in the brain of mice may suggest a vulnerability trait for alcohol-heightened aggression.
Keywords: aggression, violence, serotonin, 5-HT1B receptor, amygdala, hypothalamus, midbrain, real-time PCR, gene expression, transcription
Introduction
Following the consumption of alcohol, a small but significant subgroup of human and non-human primates, rats and mice will engage in heightened aggressive behavior and violent behavior (Fulwiler et al. 2005; Higley et al. 1996; Miczek et al. 1998a; Miczek et al. 2004b; Virkkunen 1996). Particularly within individuals with traits for impulsive and antisocial personality, as in type 2 alcoholics, the effects of alcohol in promoting violent behavior are prominent (Fulwiler et al. 2005; Pihl & LeMarquand 1998). The so-called serotonin (5-HT) deficiency hypothesis linking low levels of 5-HT metabolites to an increased propensity for violent behavior (Brown et al. 1982; Higley et al. 1996; Linnoila et al. 1983; Pihl & LeMarquand 1998), has been challenged on the basis of diverse roles of 5-HT receptors, transporters and enzymes in aggressive behavior (de Boer & Koolhaas 2005; Miczek et al. 2002; Nelson & Chiavegatto 2001).
In rodents, pharmacological manipulations targeting 5-HT receptors and transporter sites have been useful tools for modulating alcohol-heightened as well as other types of escalated aggressive behavior. Agonists at 5-HT1A and 5-HT1B receptors reduce both species-typical as well as escalated levels of aggression induced by moderate doses of alcohol (Centenaro et al. 2008; Chiavegatto et al. 2001; de Boer & Koolhaas 2005; Faccidomo et al. 2008; Fish et al. 1999; Miczek et al 1998b;), with 5-HT1B agonists showing higher behavioral selectivity for reducing aggressive relative to non-aggressive behaviors (Fish et al. 1999; Olivier & van Oorschot 2005). Anti-aggressive effects have also been reported for agonists at the 5-HT2 family of G-protein coupled receptors (de Almeida & Lucion 1994; Olivier et al. 1995), as well as for antagonists of ionotropic 5-HT3 receptors (McKenzie-Quirk et al. 2005), a putative site of action for alcohol (Lovinger & White 1991). Reduction of synaptic 5-HT reuptake by inhibition of 5-HT transporter sites also exerts anti-aggressive effects in clinical and preclinical settings (Ferris et al. 1997; New et al. 2004; Pinna et al. 2003; Reist et al. 2003). In alcohol-drinking mice, chronic treatment with citalopram, a 5-HT selective reuptake inhibitor, selectively reduced alcohol-heightened aggression to species-typical levels (Caldwell & Miczek 2008).
Whether or not the anti-aggressive effects of 5-HT manipulations are associated with increased or decreased 5-HT transmission as a common neurobiological mechanism remains to be resolved. Furthermore, different outcomes have been reported after the local infusion of 5-HT 1A or 1B agonists, depending on the specific population of 5-HT receptors (pre-or post-synaptic) targeted in one particular brain region (Bannai et al 2007; Faccidomo et al 2008). These data emphasize the increased complexity regarding the role of 5-HT in different brain areas through different 5-HT receptor subtypes in aggressive behavior.
One critical issue concerning alcohol-heightened aggression across different species is the fact that not all individuals will show escalated levels of aggressive behavior after consumption of alcohol (Miczek et al. 1992, 1998a, 2004a for a review). In mice and rats, approximately one third of the individuals may be characterized as alcohol-heightened aggressors (“AHA”). Individual vulnerability for alcohol-heightened aggression is likely determined by an interaction of genetic and environmental factors which determine sensitivity to alcohol’s effects on brain and behavior. Alterations in the expression of candidate genes in the 5-HT system may contribute for a behavioral phenotype of increased propensity to engage in high levels of aggressive behavior, as for example in socially isolated mice (Bibancos et al. 2007). Bibancos and colleagues (2007) reported a major suppression of post-synaptic transcripts for several 5-HT receptors in the prefrontal cortex (PFC), as well as few alterations in gene expression in the midbrain, hypothalamus and hippocampus of aggressive, isolated mice. Additionally, high doses of the anabolic-androgenic steroid (AAS) nandrolone in male mice induce behavioral disinhibition associated to reduced 5-HT receptor mRNA levels in the amygdala and PFC (Ambar & Chiavegatto, 2009).
In the present study, we systematically compared mice that engaged in alcohol-heightened aggression (AHA) vs. those which did not (“ANA”, alcohol non-heightened aggressors), in regard to the gene expression of several components of the 5-HT transmission, specifically 5-HT1 and 5-HT2 families of receptors, 5-HT3, 5-HT6, 5-HT7 receptors in different brain regions, and 5-HT transporter and the 5-HT synthetic enzyme (tryptophan hydroxylase 2) in the midbrain area. Now, we report a regionally selective and significant reduction of all 5-HT receptor subtypes transcripts, except for 5HT3, in the PFC of individuals who showed escalated aggression after the consumption of a moderate dose of alcohol.
Methods
Subjects
Six-week old CFW albino male mice (“residents”) were pair-housed with a female from the same strain (Charles River Breeding Laboratories, Wilmington, MA) for at least three weeks in 28×17×14 cm clear polycarbonate cages covered with a stainless steel wire lid. The floor of the cage was covered with wood shavings. Purina rodent chow was freely available through the cage lid, and water was available in a restricted manner (4 hours of access per day). The subjects were allowed to acclimate to the laboratory environment for at least one week prior to any manipulation.
Additional CFW male mice were used as stimulus animals (“intruders”) and were group-housed (n=6-8/cage) in large 44×24×14 cm clear polycarbonate cages lined with corn cob bedding. They had free access to food and water throughout the experiment. All mice were housed in a room with controlled temperature (21°C±1°C) and humidity (30-40%), on an inverse 12 hour light/dark photocycle (lights off at 06:30 h). All procedures followed the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and were approved by the Institutional Animal Care and Use Committee (IACUC) of Tufts University, USA.
Alcohol self-administration: acquisition and maintenance
Mice were restricted to 4h/day access to water, which began immediately following the daily experimental session (behavioral procedures occurred between 07:00 and 10:00 h). Under these conditions, mice gained weight at a slower rate than unrestricted animals. Prior to drinking sessions, the female and pups were removed from the home cage and were given access to water in a separate holding cage. As described and illustrated earlier (Miczek & de Almeida, 2001), an aluminum panel (16.5 × 3.8 × 15.9 cm) designed to record nose-poke responding and present liquid reinforcement was inserted into the home cage and affixed to the side walls with two thumb screws. Each panel contained 2 response units (3 × 2 × 2 cm) equipped with nose-poke photocell sensors and fluid delivery cups inside. The side of the active and inactive response units was counterbalanced across mice. Cue lights were positioned 5 cm above each of the response openings, with a house light in the center (devices from Med Associates, St. Albans, VT). The fluid delivery cup was connected to a syringe pump (Razel Scientific, St. Albans, VT), and all input and output devices were interfaced via Med Associates cards to a personal computer (running MED-PC for Windows, version 4.1; Med Associates, St. Albans, VT). Each nose poke in the active response unit resulted in an auditory click from a relay, and both the house light and the cue light above the active hole shut off during fluid delivery. Nose poke responses during the time-out period (1.26 sec during fluid delivery) and those in the inactive response unit were recorded, but were without consequence.
The procedure allowed for the voluntary consumption of precise, body weight-adjusted doses of alcohol or water in a regular manner. Every 5th response in the active response unit was reinforced by the delivery of 50 μl (in 1.26 sec) of 6% alcohol or water, i.e. a fixed ratio (FR) 5 schedule of reinforcement. In a procedure modified from previous studies (Miczek et al. 1998b; Miczek & de Almeida 2001), animals were trained to drink either water or alcohol. The currently implemented method established self-administration of water or alcohol with no previous sucrose/alcohol fading phase. In the first two days of acquisition, mice received 30 min self-administration sessions with water as the reinforcing fluid according to the FR5 schedule. From day 3 onwards, sessions were limited in time so that the accumulated fluid volume amounted to a 1.0 g/kg alcohol dose (approximately 8-11 reinforcements for mice weighing 25-31 g). Dose-effect studies have identified the 1.0 g/kg alcohol dose as the optimal dose for engendering heightened aggressive behavior in this strain of mice (Miczek et al. 1998b). Water or 6% alcohol were available on alternating days, such that mice would have two alcohol self-administration sessions/week (first alcohol session was on day 3). Within 10 days of training (5 days/week), the self-administration session would be completed on average in 2 min (range of 1-3.5 min), for both water and alcohol.
Resident-intruder confrontations
Approximately 3 weeks after being pair-housed with a female, each resident was examined for the display of aggressive behavior against a group-housed male intruder. The female and pups were removed, and an intruder was placed into the resident’s home cage (Miczek & O’Donnell 1978). Each encounter lasted 5 min after the first attack bite or was terminated after 5 min in the absence of attacks. Mice achieved stable rates of aggressive behavior against the same intruder in 4-6 aggressive confrontations (i.e. attack bites in three consecutive confrontations did not vary more than 15% from the individual’s average), with each confrontation scheduled in 24-48h intervals (Winslow & Miczek 1983). Residents were then repeatedly tested against the same intruder 15 min after the self-administration of water or 1.0 g/kg alcohol. Aggressive confrontations were carried out every 48-72h, alternating after water or alcohol consumption, for a total of 3 encounters in each condition (total of 6 tests). Confrontations were videotaped using a low-lux video camera (Panasonic BL-200) connected to a standard Panasonic VHS video cassette recorder and monitor. These encounters were subsequently analyzed by a reliable and blinded observer using a custom-designed keyboard connected to a computer running The Observer software (Noldus, The Observer v.5.0, Netherlands). The frequencies and durations of salient aggressive (attack bites, sideways threat, tail rattles, pursuit) and non-aggressive (grooming, rearing, walking, social contact) behaviors were quantified, based on the operational definitions by Grant & Mackintosh (1963) and Miczek & O’Donnell (1978). Inter- and intra-observer reliability for measuring each aggressive and non-aggressive act and posture ranged between r =0.86-0.96.
Characterization of Alcohol-Heightened Aggression
As previously described (Miczek et al. 1998b), the frequency of attacks displayed in the resident-intruder encounters after the self-administration of alcohol or water were compared. A statistical outlier criterion was used to identify those mice that were significantly more aggressive after consuming alcohol (AHA) from those that were not (ANA). If the number of attack bites during the alcohol tests exceeded the individuals’ average during water tests by 2 SD, then that mouse was considered an AHA. Mice that did not increase aggression above this criterion were considered ANA.
Brain samples
One week after the last behavioral test, AHA and ANA mice were decapitated in alternate order from 14:00 to 17:00 h, and the following brain areas were immediately dissected on ice with the help of magnifying lens, by a trained researcher (Ambar & Chiavegatto 2009; Bibancos et al. 2007): PFC (sectioned just anterior to the corpus callosum), striatum, hippocampus, hypothalamus, amygdala and midbrain (central portion around aqueduct, removing dorsally the colliculi and ventrally the peduncles and substantia nigra). Tissue samples were quickly submerged in a RNA stabilization solution (RNAlater®, Ambion, Austin, TX), maintained at room temperature for some hours and later stored at -80°C until use.
RNA isolation and cDNA synthesis
Brain samples were removed from the RNAlater®, immersed in TRIzol reagent (Invitrogen, Carlsbad, CA) and homogenized (Polytron PT10/35-Brinkmann, Westbury, NY) for 30 sec at maximum speed. Total RNA was isolated according to the manufacturer’s procedure and quantified in a spectrophotometer (NanoDrop® ND-1000, Wilmington, DE). The RNA purity was assessed by the OD ratio (260/280 nm) and its integrity was verified on ethidium bromide stained 1% agarose gel by rRNA 28S/18S comparison (Eagle Eye, Stratagene, La Jolla, CA). Only samples that met the criteria of quality (OD ratio and 28S/18S intensity > 1.8) were included in the experiments. Total RNA was treated with DNase I (Invitrogen, Brazil - 1U/μg of RNA, at 37°C for 20 min) to avoid DNA contamination of the samples. DNase I-treated total RNA (2μg) from both experimental groups were simultaneously reverse transcribed using oligo(dT) primer and SuperScript™ III reverse transcriptase (Invitrogen, Brazil) in a final volume of 20 μl.
Primers design
Specific primers for the serotonin receptors 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2C, 5-HT3A, 5-HT6 and 5-HT7 genes, the serotonin transporter 5-HTT (Slc6a4), tryptophan hydroxylase-2 (Tph2) and the control genes cyclophilin A (peptidylprolyl isomerase A: Ppia), hypoxanthine guanine phosphoribosyl transferase 1 (Hprt1), glyceraldehyde-3-phosphate dehydrogenase (Gapdh) and beta-actin (Actb) were designed using the Primer3 software (http://frodo.wi.mit.edu/primer3/) following previously reported criteria (Bibancos et al. 2007). Most forward and reverse primers were planned in different exons (except for the Htr2c, and the intronless genes: Htr1a and Htr1b). Primer specificity was confirmed by a Basic Local Alignment Search Tool (http://www.ncbi.nlm.nih.gov/BLAST) and primer pair characteristics as melting temperature and secondary structures analyzed by NetPrimer algorithms (PREMIER Biosoft International, Palo Alto, CA). Primers were synthesized by Invitrogen (Brazil) or IDT Technologies (Coralville, IA, USA) and tested in PCR reactions followed by electrophoresis on a 2% ethidium bromide-stained agarose gel. Only primers with no extra amplification products were used (Table 1).
Table 1.
Forward and reverse primer sequences for 5-HT-related and control transcripts
| Accession (NCBI) |
Gene | Foward / reverse primers (5′ → 3′) | Product size (bp) |
|---|---|---|---|
| NM_008308 | Htr1a | GGATGTTTTCCTGTCCTGGT / CACAAGGCCTTTCCAGAACT | 121 |
|
| |||
| NM_010482 | Htr1b | TCACATGGCCATTTTTGACT / CAGTTTGTGGAACGCTTGTT | 112 |
|
| |||
| NM_172812 | Htr2a | AGAACCCCATTCACCATAGC / ATCCTGTAGCCCGAAGACTG | 119 |
|
| |||
| NM_008312 | Htr2c | GATTGGACTGAGGGACGAAA / ATGAAGAATGCCACGAAGGA | 102 |
|
| |||
| NM_013561 | Htr3a | CTTCCCCTTTGATGTGCAG / CCACTCGCCCTGATTTATG | 139 |
|
| |||
| NM_021358 | Htr6 | CATAGCTCAGGCCGTATGTG / CGCATGAAGAGGGGATAGA | 111 |
|
| |||
| NM_008315 | Htr7 | TTCTGCAACGTCTTCATCG / ATTCTGCCTCACGGGGTA | 126 |
|
| |||
| NM_010484 | Slc6a4 | CAACTCCGGCTTTTCCAATA / ATTTCCGTTGGTGTTTCAGG | 176 |
| NM_173391 | Tph2 | TGCAAGCAAGAGGGTCAACT /CATGCTTCAATTCTCCGATG | 70 |
| NM_008907 | Ppia | AATGCTGGACCAAACACAAA / CCTTCTTTCACCTTCCCAAA | 101 |
|
| |||
| NM_013556 | Hprt1 | TGTTGTTGGATATGCCCTTG / GCGCTCATCTTAGGCTTTGT | 106 |
|
| |||
| NM_008084 | Gapdh | AGGAGCGAGACCCCACTAAC / GTGGTTCACACCCATCACAA | 179 |
|
| |||
| NM_007393 | Actb | GTGGGAATGGGTCAGAAGG / GGTCATCTTTTCACGGTTGG | 228 |
Real-time quantitative PCR
The quantitative analysis of mRNA transcripts was carried out in a Rotor Gene 3000 thermocycler (Corbett Life Science, Concord, Australia) using the SYBR green I methodology. The optimal concentration of cDNA and primers, as well as the maximum efficiency of amplification were obtained through 5-point twofold dilution curve analysis using RG-3000 software (Corbett Life Science, Concord, Australia) for each gene and for different brain regions. Therefore, each PCR reaction contained 12.5 ng of reverse-transcribed RNA, 200 nM of each specific primer, SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) and RNase-free water to a 20 μL final volume. cDNA samples from both experimental groups were amplified in the same run (using 0.1 ml plastic tubes) in triplicate for each gene. Samples without cDNA template (NTC) and no reverse-transcribed (no-RT) were included as negative controls in all runs. Cycling parameters were: 10 min at 95°C, followed by 40 cycles at 95°C for 15 sec and 60°C for 60 sec, with fluorescence acquiring at the end of each step. Melting curves were generated after each run to further confirm a single gene-specific peak and absence of primer-dimer formation by heating the samples stepwise from 55°C to 95°C, while continuously monitoring the fluorescence. Real time data were analyzed using RG-3000 software (Corbett Life Science, Concord, Australia) and an Excel worksheet. The relative amount of transcripts in different brain areas for both AHA and ANA groups was calculated (Vandesompele et al. 2002). Briefly, the arithmetic mean of each transcript’s replicated cycling threshold (Ct) value was transformed to a quantity relative to the sample with the highest level, taking into account the amplification efficiency of each transcript. GeNorm tool (http://medgen.ugent.be/~jvdesomp/genorm/) was used to identify the most stable and the optimal number of reference transcripts required for reliable normalization of our data in each brain region. This step is particularly important when studying a small difference of transcript amount as likely to occur in brain gene expression. Therefore, raw quantities were subsequently normalized by dividing by the geometric mean of the stable transcripts selected among Ppia, Hprt1, Gapdh and Actb.
Statistical analysis
After the behavioral characterization of mice as AHA or ANA, the two groups were compared with 2-way ANOVAs (with group and fluid - water vs. alcohol - as factors) for each aggressive and non-aggressive behavior, as well as the total sum of non-aggressive behaviors. Post-hoc comparisons were carried out for significant factors and interactions, using Holm-Sidak tests for multiple comparisons. Two-tailed Student’s t test was used to compare the normalized amount of each transcript between AHA and ANA groups. Differences were considered statistically significant if P<0.05. All data are expressed as means ± SEM.
Results
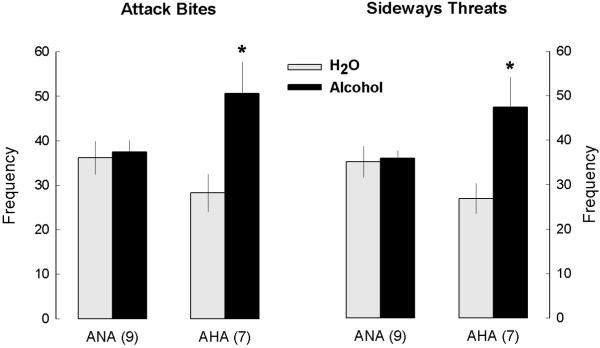
After detailed behavioral analyses of the aggressive encounters, 7 mice were characterized as AHA, and 9 as ANA after the consumption of 1.0 g/kg of alcohol. In total, 20 mice were tested for alcohol-heightened aggression, and 35% of the sample was characterized as AHA, a proportion typically found in CFW outbred male mice. Four out of the 20 mice were not further used for brain samples: two of them were never reliably aggressive; and two of them stopped fighting their intruders and for that reason would require additional time and additional confrontations to finish the characterization. We only include animals that were exposed to similar number of aggressive confrontations, so that additional experience in fighting would not be a variable in our study. Two-way ANOVAs confirmed significant interactions for attack bite (F1,28=5.40, p<0.05) and sideways threat frequencies (F1,28=6.05, p<0.05). After self-administration of 1.0 g/kg alcohol, AHA mice presented significantly higher frequencies of attack bites and threats when compared to ANA mice (p<0.05), as well as when compared to their own baseline values (after drinking water, p<0.01), as shown in Fig. 1. After drinking water, AHA and ANA mice showed similar levels of aggressive behaviors (Table 2A). There were no significant differences between AHA and ANA in non-aggressive behaviors (walking, rearing, grooming or social contact; Table 2B).
Figure 1.
Frequency of attack bites and sideways threats (means ± SEM) in mice characterized as “alcohol-heightened aggressors” (AHA) or “alcohol non-heightened aggressors” (ANA) over the course of six aggressive encounters after the self-administration of water (gray bars) or 1.0 g/kg alcohol (black bars). * increased levels of aggressive behavior in between-group and within-group comparisons for each separate behavior (p<0.05)
Table 2A. Aggressive and non-aggressive behaviors displayed by AHA and ANA mice during confrotations with an intruder after drinking water or 1.0 g/kg alcohol.
| Frequency of aggressive behaviors | ||||
|---|---|---|---|---|
| ANA mice (n=9) | AHA mice (n=7) | |||
| H2O | 1.0 g/kg alcohol | H2O | 1.0 g/kg alcohol | |
| BITES | 36.2 ± 3.8 | 37.5 ± 2.7 | 28.3 ± 4.3 | * 50.6 ± 7.2 |
| THREATS | 35.1 ± 3.6 | 35.9 ± 1.8 | 26.9 ± 3.4 | * 47.4 ± 6.9 |
| TAIL RATTLE | 20.9 ± 2.8 | 19.9 ± 1.5 | 17.2 ± 1.9 | 23.0 ± 2.0 |
| PURSUIT | 3.0 ± 1.1 | 3.5 ± 0.9 | 2.0 ± 0.7 | 3.6 ± 1.4 |
p<0.05 relative to ANA mice drinking alcohol, and relative to AHA mice tested after consuming water
Table 2B. Aggressive and non-aggressive behaviors displayed by AHA and ANA mice during confrotations with an intruder after drinking water or 1.0 g/kg alcohol.
| Duration of non-aggressive behaviors (s) | ||||
|---|---|---|---|---|
| ANA mice (n=9) | AHA mice (n=7) | |||
| H2O | 1.0 g/kg alcohol | H2O | 1.0 g/kg alcohol | |
| WALK | 70.8 ± 3.6 | 67.2 ± 2.9 | 71.2 ± 4.9 | 79.2 ± 6.5 |
| REAR | 46.3 ± 7.5 | 36.5 ± 4.3 | 51.1 ± 8.9 | 35.5 ± 6.3 |
| GROOM | 19.7 ± 4.1 | 29.1 ± 3.5 | 18.6 ± 4.1 | 23.8 ± 4.6 |
| CONTACT | 20.0 ± 5.4 | 16.7 ± 6.2 | 20.0 ± 4.7 | 9.7 ± 3.6 |
| TOTAL | 156.8 ± 9.7 | 149.6 ± 8.2 | 158.0 ± 11.7 | 148.1 ± 9.6 |
5-HT receptor mRNA levels in the PFC of AHA mice
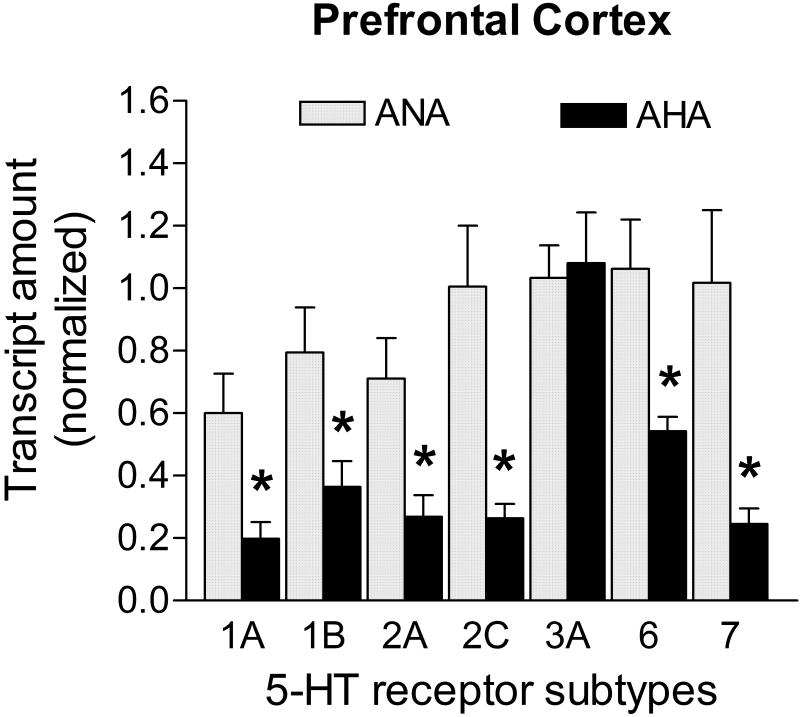
Transcript levels of 5-HT receptor subtypes 1A, 1B, 2A, 2C, 6 and 7 were statistically reduced in the PFC of AHA mice when compared to ANA mice (Fig. 2). The decrease in mRNA levels was -67.0% for 5-HT1A (p<0.05), -54.1% for 5-HT1B (p<0.05); -62.2% for 5-HT2A (p<0.05); -73.7% for 5-HT2C (p<0.05); -48.9% for 5-HT6 (p<0.05), and -75.8% for 5-HT7 (p<0.05). The remaining 5-HT receptor transcript quantified in this brain area, 5-HT3A, did not vary across groups (p>0.05; Fig. 2). In the PFC, mRNA levels of Ppia, Hprt1, Gapdh and Actb candidate control genes were not statistically different between groups (Ppia: 0.392 ± 0.099 vs. 0.606 ± 0.169; Hprt1: 0.395 ± 0.139 vs. 0.406 ± 0.136; Gapdh: 0.374 ± 0.134 vs. 0.339 ± 0.144; Actb: 0.381 ± 0.143 vs. 0.168 ± 0.062, for ANA n=8 vs. AHA n=5, respectively; p>0.05). Hprt1 and Gapdh showed the most stable levels in the prefrontal cortex (M=0.53, average pairwise variation according geNorm applet) and were thus used for the normalization of transcripts encoding 5-HT receptors.
Figure 2. mRNA levels of 5-HT receptors in the PFC of male mice displaying escalated aggression induced by low doses of alcohol.
Levels of transcripts for 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2C, 5-HT6 and 5-HT7 receptors were significantly reduced (decreases ranged from -48.9 to -75.8%) in AHA group (n=5) when compared to controls (n=8). Transcripts in the PFC were normalized against the geometric mean of Hprt1 and Gapdh mRNA levels. Data are expressed as mean and SEM, *p<0.05.
5-HT receptor mRNA levels in the striatum of AHA mice
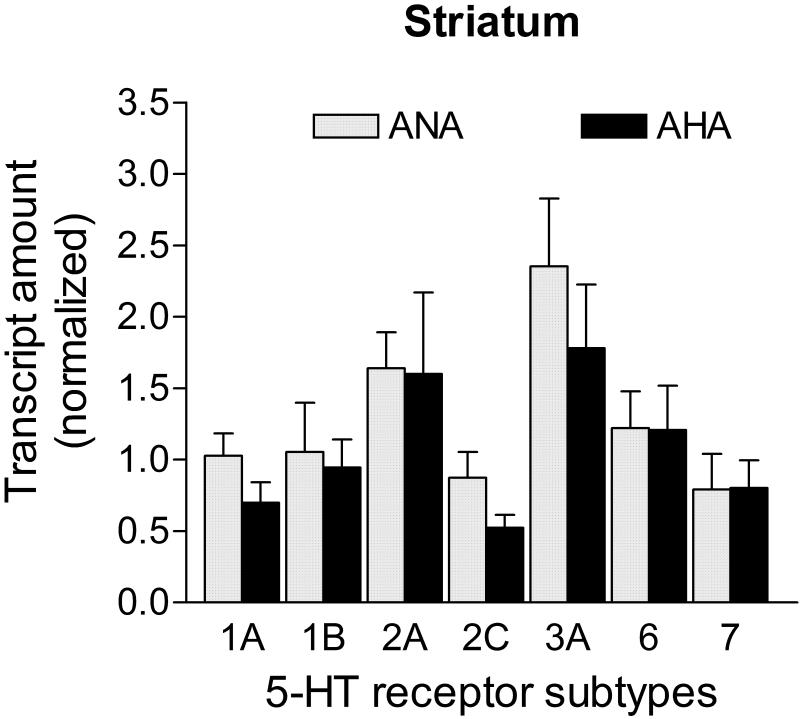
All 5-HT receptor subtype mRNA transcripts had similar levels between ANA and AHA mice in the striatum (p>0.05; Fig. 3). Transcripts for the candidate control genes did not show statistical differences across groups in the striatum (Ppia: 0.333 ± 0.122 vs. 0.268 ± 0.107; Hprt1: 0.341 ± 0.104 vs. 0.168 ± 0.076; Gapdh: 0.364 ± 0.100 vs. 0.227 ± 0.103; Actb: 0.430 ± 0.120 vs. 0.315 ± 0.156, for ANA n=9 vs. AHA n=5, respectively; p>0.05). The Hprt1 and Gapdh mRNAs were used to normalize 5-HT receptor transcripts in this brain area due to their stability (M= 0.38, geNorm applet).
Figure 3. mRNA levels of 5-HT receptors in the striatum of male mice displaying escalated aggression induced by low doses of alcohol.
Level of transcripts for all 5-HT receptors were similar between AHA (n=5) and ANA (n=9) mice (p>0.05). Transcripts in the striatum were normalized against the geometric mean of Hprt1 and Gapdh mRNA levels. Data are expressed as mean and SEM.
5-HT receptor mRNA levels in the hippocampus of AHA mice
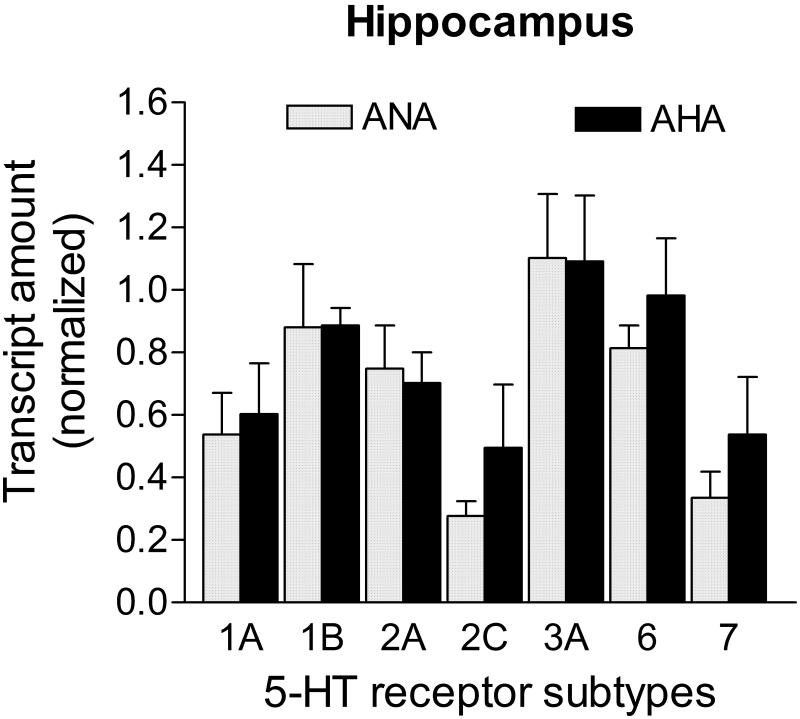
All 5-HT receptor subtype mRNA transcripts had similar levels between ANA and AHA mice in the hippocampus (p>0.05; Fig. 4). Transcripts for the candidate control genes did not show statistical differences between groups (Ppia: 0.328 ± 0.100 vs. 0.568 ± 0.160; Hprt1: 0.314 ± 0.108 vs. 0.462 ± 0.168; Gapdh: 0.268 ± 0.091 vs. 0.342 ± 0.141; Actb: 0.282 ± 0.123 vs. 0.230 ± 0.107, for ANA n=7 vs. AHA n=6, respectively; p>0.05). Most stable reference genes in the hippocampus were Ppia and Hprt1 (M=0.40, geNorm applet), and were both used to normalize 5-HT receptor mRNA levels in this brain area.
Figure 4. mRNA levels of 5-HT receptors in the hippocampus of male mice displaying escalated aggression induced by low doses of alcohol.
Level of transcripts for all 5-HT receptors were similar between AHA (n=6) and ANA (n=7) mice (p>0.05). Transcripts in the hippocampus were normalized against the geometric mean of Ppia and Hprt1 mRNA levels. Data are expressed as mean and SEM.
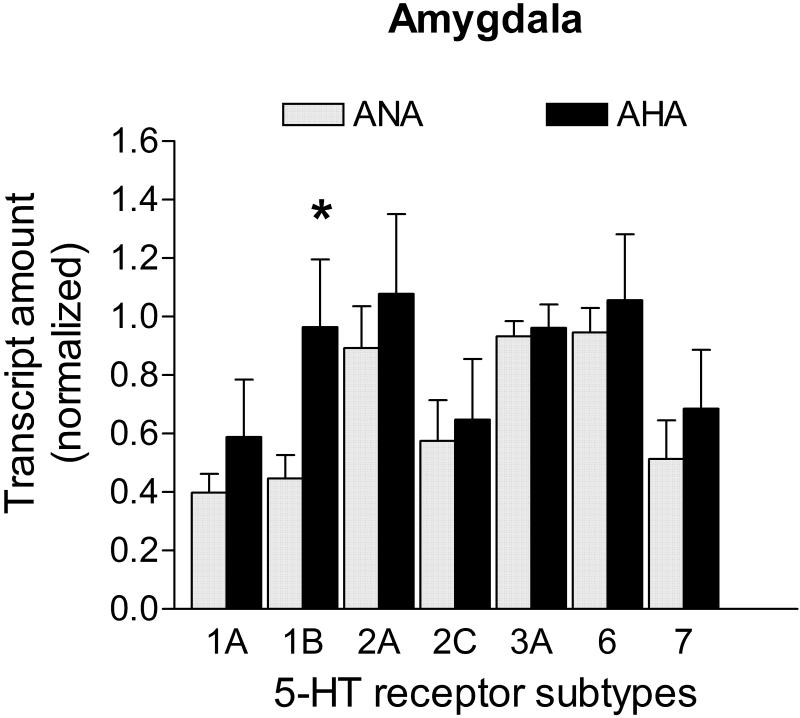
5-HT receptor mRNA levels in the amygdala of AHA mice
5-HT1B was the only 5-HT receptor transcript whose level was statistically different in the amygdala of AHA mice relative to the ANA group; it showed an increase of +115.8% (p<0.05; Fig. 5). Transcripts for the candidate control genes were not statistically different across groups (Ppia: 0.689 ± 0.079 vs. 0.596 ± 0.097; Hprt1: 0.528 ± 0.085 vs. 0.565 ± 0.130; Gapdh: 0.621 ± 0.079 vs. 0.558 ± 0.125; Actb: 0.576 ± 0.079 vs. 0.530 ± 0.121, for ANA n=7 vs. AHA n=6, respectively; p>0.05). In the amygdala, the geometric mean of all four genes was used to normalize the 5-HT receptor transcripts (M= 0.55).
Figure 5. mRNA levels of 5-HT receptors in the amygdala of male mice displaying escalated aggression induced by low doses of alcohol.
The 5-HT1B receptor transcript was the only altered transcript (+115.8%) in the amygdala of AHA mice (n=7) when compared to controls (n=6). Transcripts in the amygdala were normalized against the geometric mean of Ppia, Hprt1, Gapdh and Actb mRNA levels. Data are expressed as mean and SEM, * p<0.05.
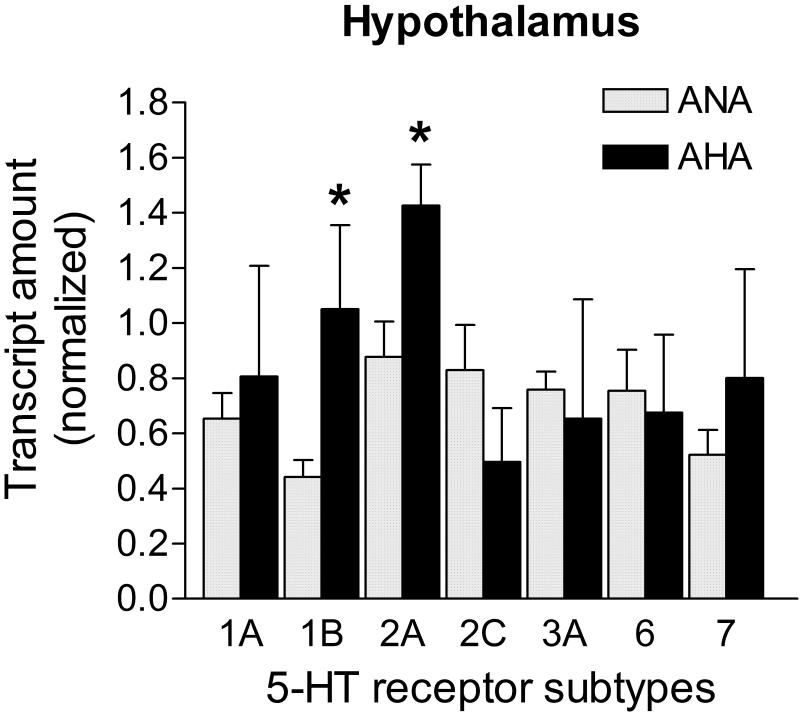
5-HT receptor mRNA levels in the hypothalamus of AHA mice
The level of 5-HT1B receptor transcripts was increased +137.6%, while the 5-HT2A receptor mRNA was elevated +62.5% in the hypothalamus of AHA mice when compared to ANA mice (p<0.05 for each; Fig. 6). The additional 5-HT receptor transcript levels investigated were similar across groups (p>0.05; Fig. 6). Transcripts for the candidate control genes did not show statistical differences between groups in the hypothalamus (Ppia: 0.700 ± 0.057 vs. 0.542 ± 0.078; Hprt1: 0.459 ± 0.104 vs. 0.204 ± 0.122; Gapdh: 0.521 ± 0.127 vs. 0.217 ± 0.097; Actb: 0.365 ± 0.098 vs. 0.152 ± 0.105; respectively for ANA n=8 vs. AHA n=5; p>0.05). Ppia transcripts showed less variability intra-group and were used to normalize 5-HT receptor mRNA levels in this brain area.
Figure 6. mRNA levels of 5-HT receptors in the hypothalamus of male mice displaying escalated aggression induced by low doses of alcohol.
The 5-HT1B receptor transcript level was significantly elevated (+137.6%), as well as the 5-HT2 receptor transcript (+62.5%) in the hypothalamus of AHA mice (n=5) when compared to the control group (n=8). The mRNA levels of the other receptors were similar between the groups (p>0.05). Transcripts in the hypothalamus were normalized against Ppia mRNA levels. Data are expressed as mean and SEM, * p<0.05.
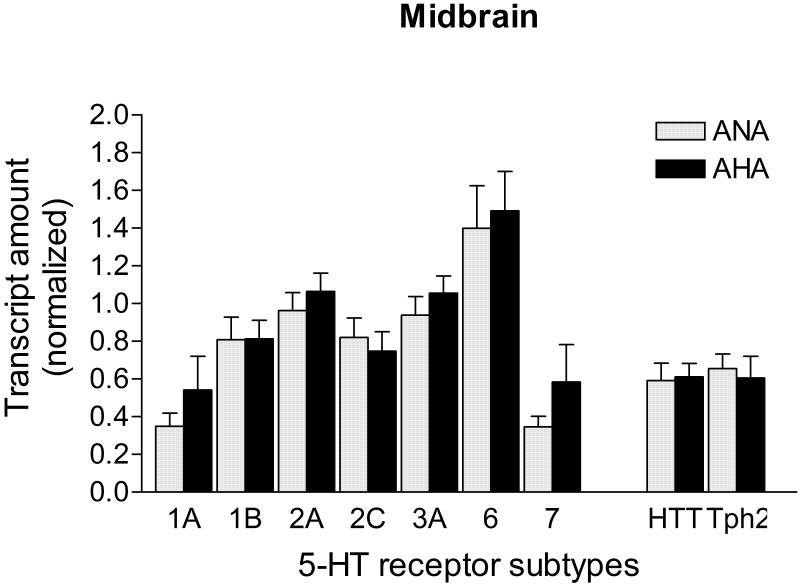
5-HT- related transcripts levels in the midbrain of AHA mice
The level of mRNA for all 5-HT receptors tested, as well as for the 5-HTT and Tph2 genes, did not vary across groups in the midbrain area (p>0.05; Fig. 7). Transcript levels of all 4 candidate control genes were similar between ANA and AHA mice in the midbrain (Ppia: 0.472 ± 0.080 vs. 0.421 ± 0.048; Hprt1: 0.388 ± 0.094 vs. 0.446 ± 0.099; Gapdh: 0.385 ± 0.097 vs. 0.442 ± 0.054; Actb: 0.296 ± 0.088 vs. 0.428 ± 0.124, ANA n=9 vs. AHA n=7, respectively; p>0.05). Hprt1 and Gapdh transcripts performed as the most stable for this structure (M=0.32) and were used to normalize the transcript amount of 5-HT receptors, Tph2 and 5-HTT.
Figure 7. mRNA levels of 5-HT receptors, 5-HTT and Tph2 in the midbrain of male mice displaying escalated aggression induced by low doses of alcohol.
Level of transcripts for all 5-HT receptor subtypes, the 5-HT transporter (HTT), and the 5-HT synthetic enzyme (Tph2) were similar between AHA (n=7) and ANA (n=9) mice (p>0.05). Transcripts in the midbrain were normalized against the geometric mean of Hprt1 and Gapdh mRNA levels. Data are expressed as mean and SEM.
Discussion
The present results confirm the PFC as a region of significance where 5HT-related transcripts are expressed less in those individuals in which alcohol escalates their aggressive behavior beyond the species-typical level. This anatomically selective pattern of mRNA expression changes may reflect the consequence of drinking and increased fighting or, alternatively, it may indicate a vulnerability trait of those individuals with a high propensity for escalated aggressive behavior after consuming a low to moderate dose of alcohol. Whether or not the currently measured mRNA for 5-HT receptor subtypes are cause or effect will require more detailed analysis of the emergence of alcohol-heightened aggression. We hypothesize that regulation of 5-HT receptors is impulse-dependent, and salient inputs trigger phasic elements in serotonergic activity.
We investigated several brain areas related to aggressive and emotional behaviors enriched in 5-HT receptors that could be implicated in alcohol-enhanced aggressive behavior. The mRNA transcripts harvested from each dissected brain region most likely originated from neural cell bodies expressing the corresponding gene (Ambar & Chiavegatto 2009; Bibancos et al. 2007). Because 5-HT neuronal cell bodies are found in raphè nuclei in the midbrain, 5-HT receptors mRNA obtained from PFC, striatum, hippocampus, amygdala, and hypothalamus consist mainly of postsynaptic 5-HT receptors from non-5-HT neural cells. Conversely, mRNA transcripts encoding 5-HT receptors, 5-HT transporter or 5-HT synthetic enzyme derived from the midbrain area are expected to be mainly derived from 5-HT neurons, but additional non-5-HT cell bodies from local circuits may be included (schematic drawing in Ambar & Chiavegatto 2009).
Serotonin itself has been implicated in the brain mechanisms underlying inhibitory response control (Soubrié 1986), and inadequate or excessive stimulation of 5-HT receptors in the PFC can functionally interfere with proper control of impulsive responding (de Almeida et al. 2006; Robbins 2005). Accordingly, impulsive aggressive personality disordered patients exhibit blunted PFC metabolism in response to a serotonergic challenge (New et al. 2002).
In the PFC, the characterized AHA mice display robust decreases ranging from -49% to -76% in all 5-HT receptor transcripts, with the exception of 5-HT3A, in comparison to ANA mice. A strong correlation between aggressive behavior and an overall reduction of 5-HT receptor mRNA levels in the PFC has already been demonstrated in a social isolation model in male inbred mice (Bibancos et al. 2007). In this model, the impaired PFC development in response to limited social and sensory stimulation during the early isolation period was reflected in the reduced amount of 5-HT receptor transcripts, an important example of genetic and environmental interaction. In the present study, it is possible that previous unidentified genetic alterations found in some individuals of the outbred stock of mice may account for this difference in expression that is correlated with the AHA phenotype. Additionally, increased aggressive behavior in male mice induced by prolonged administration of high doses of the AAS nandrolone is also correlated with reduced transcripts for 5-HT1A, 5-HT1B, 5-HT2A and 5-HT7 receptors in the PFC. Whether or not these are direct or indirect effects of the AAS upon 5-HT receptors modulation, the gene expression profile of 5-HT receptors in the PFC of these mice is also very similar to the one found in subjects vulnerable to alcohol-heightened aggression. Taken together, these data suggest that aggressive mice induced by either environmental or pharmacological manipulation display comparable reduction in several 5-HT receptor transcripts in the PFC.
Interestingly, all the reduced 5-HT receptors mRNA in the PFC code for proteins belonging to the G-protein type of receptors, which are not direct targets for acute effects of alcohol in neural cells. Conversely, AHA and ANA mice did not differ in the amount of 5-HT3A receptor mRNA, which encoded channel is a known site for allosteric modulation by alcohol (Dopico & Lovinger 2009), and a target for pharmacological modulation of alcohol-heightened aggression (McKenzie-Quirk et al. 2005). These data suggest that the observed changes in 5-HT receptor transcripts are unrelated to direct alcohol effects and may be part of the individual differences selected by our protocol. However, a recent study showed that the medial PFC of chronic alcohol-drinking mice, regardless of being AHA or ANA, does not respond neurochemically to a serotonergic pharmacological challenge as expected in naïve mice (Faccidomo et al. 2008). Therefore, this blunted response suggests a direct or indirect effect of alcohol upon medial PFC 5-HT neurotransmission unrelated to mechanisms involved in individual differences in alcohol-heightened aggression. In the present study, 5-HT transcripts in AHA mice were derived from total PFC, and are expected to encode mostly for postsynaptic 5-HT receptors in neural cells of different neurochemical phenotypes and locations, where these receptors are expressed and exert their physiological functions. Consequently, the significant reduction in PFC 5-HT receptor mRNA in AHA mice, if also verified by reduced 5-HT receptor proteins, might impact different neurotransmitter systems in distinct brain areas.
Amygdala hyperexcitability is suggested to participate in the neural basis of fear and anxiety disorders, impulse control, and aggressive behaviors (reviewed in Keele 2005; Davis et al. 2009). In agreement, Haller et al. (2006) found strong activation of the central amygdala in mice selected for high levels of aggressive behavior. In the amygdala of AHA mice, mRNA encoding 5-HT1B receptor was significantly elevated, being the only 5-HT receptor transcript changed in this brain area. Also, aggressively behaving dogs display higher 5-HT1B-positive neurons in the basolateral nuclear complex (BNC) of amygdala (Jacobs et al. 2007), which is correlated with higher number of neurons and increased volume of this area when compared with normally behaving dogs (Jacobs et al. 2006). Because we did not evaluate amygdala volume area or number of neurons in AHA and ANA mice, we cannot exclude that a similar increase may account for elevated 5-HT1B receptor mRNA. However, this appears not to be the case in the present study since other 5-HT receptor subtypes quantified, as well as all control transcripts, were similarly expressed between groups. In the BNC, the GABAergic inhibitory interneurons are important targets for 5-HT innervations, which have very dense local axonal arborizations that could amplify the effects of 5-HT in this brain area (Muller et al. 2007). If the 5-HT1B heteroreceptors encoded by the mRNA isolated from the total amygdala of AHA mice are found in these GABAergic interneurons, their activation would decrease the firing of these cells, due to their inhibitory nature. Consequently, we could speculate that the net stimulatory effect of elevated 5-HT1B receptors in the amygdala of AHA mice would potentially explain increased amygdala excitability and elevated aggressive behavior. This hypothesis deserves further investigation.
Serotonergic fibers and terminals are present throughout the hypothalamus (Steinbusch & Nieuwenhuys 1981), and 5-HT is implicated in neuroendocrine and autonomic mechanisms via different receptors (Jørgensen 2007). The increased steady state levels of 5-HT1B and 5-HT2A receptor mRNA in this area in AHA mice are difficult to correlate with their elevated aggression. The hypothalamus of social isolated aggressive male mice showed opposite findings (Bibancos et al. 2007), whereas the aggressive mice induced by AAS administration displayed reduced hypothalamic 5-HT1B mRNA and similar levels of 5-HT2A transcripts compared to controls (Ambar & Chiavegatto 2009). Therefore, it is possible that AHA mice have additional phenotypes in the hormonal or physiological domains, which we have not assessed, that might be in line with these gene expression alterations.
Hippocampus and striatum exhibited similar 5-HT receptor mRNA profiles in AHA and ANA mice; consequently these areas do not seem to participate in the vulnerability of enhanced aggression induced by alcohol regarding the 5-HT heteroreceptor transcription. At the midbrain level, all 5-HT-related transcripts were also quantitatively similar in AHA mice when compared to ANA mice. Because the 5-HT synthetic enzyme, the 5-HT transporter and the 5-HT1A and 5-HT1B autoreceptors function as a feedback mechanism to control the activity of 5-HT neurons, the genetic integrity of 5-HT machinery in their cell bodies is not associated with the AHA profile.
The present and previous studies (Miczek et al 1992, 1993; Van Erp & Miczek 1997) suggest that the aggression heightening effects of alcohol are independent of the baseline level of aggression. Our protocol of alcohol self-administration, coupled to mRNA quantification one week after the last aggressive confrontation, was effective to determine a gene expression profile that is associated with, and perhaps necessary for, the potentiating effects of alcohol on aggression, which exceeds the species-typical level of aggressive behavior. However, this experimental design cannot ascertain whether these alterations in gene expression were induced by alcohol transiently or permanently in selected predisposed animals, or if they represent a molecular signature of the vulnerability of alcohol-heightened aggression. In the latter case, the question that arises is what variables may contribute to these individual differences.
Acknowledgements
We are grateful to Dr. Jose Eduardo Krieger for laboratory facilities. This study was supported by grants from the National Institute of Health (AA013983, KAM), and the São Paulo State Foundation for Research Support (FAPESP: 06/06904-5). GA was a recipient of CAPES fellowship (Brazil) and SC is a research scholar of CNPq-Brazil.
References
- Ambar G, Chiavegatto S. Anabolic-androgenic steroid treatment induces behavioral disinhibition and downregulation of serotonin receptor messenger RNA in the prefrontal cortex and amygdala of male mice. Genes Brain Behav. 2009;8:161–173. doi: 10.1111/j.1601-183X.2008.00458.x. [DOI] [PubMed] [Google Scholar]
- Bannai M, Fish E, Faccidomo S, Miczek K. Anti-aggressive effects of agonists at 5-HT1B receptors in the dorsal raphe nucleus of mice. Psychopharmacology (Berl) 2007;193:295–304. doi: 10.1007/s00213-007-0780-5. [DOI] [PubMed] [Google Scholar]
- Bibancos T, Jardim D, Aneas I, Chiavegatto S. Social isolation and expression of serotonergic neurotransmission-related genes in several brain areas of male mice. Genes Brain Behav. 2007;6:529–539. doi: 10.1111/j.1601-183X.2006.00280.x. [DOI] [PubMed] [Google Scholar]
- Brown G, Goodwin F, Bunney WJ. Human aggression and suicide: their relationship to neuropsychiatric diagnoses and serotonin metabolism. Adv Biochem Psychopharmacol. 1982;34:287–307. [PubMed] [Google Scholar]
- Caldwell E, Miczek K. Long-term citalopram maintenance in mice: selective reduction of alcohol-heightened aggression. Psychopharmacology (Berl) 2008;196:407–416. doi: 10.1007/s00213-007-0972-z. [DOI] [PubMed] [Google Scholar]
- Centenaro L, Vieira K, Zimmermann N, Miczek K, Lucion A, de Almeida R. Social instigation and aggressive behavior in mice: role of 5-HT1A and 5-HT1B receptors in the prefrontal cortex. Psychopharmacology (Berl) 2008;201:237–248. doi: 10.1007/s00213-008-1269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavegatto S, Dawson V, Mamounas L, Koliatsos V, Dawson T, Nelson R. Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proc Natl Acad Sci U S A. 2001;98:1277–1281. doi: 10.1073/pnas.031487198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs. anxiety. Neuropsychopharm Rev. 2009 doi: 10.1038/npp.2009.109. doi:10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida R, Lucion A. Effects of intracerebroventricular administration of 5-HT receptor agonists on the maternal aggression of rats. Eur J Pharmacol. 1994;264:445–448. doi: 10.1016/0014-2999(94)00548-6. [DOI] [PubMed] [Google Scholar]
- De Almeida R, Rosa M, Santos D, Saft D, Benini Q, Miczek K. 5-HT(1B) receptors, ventral orbitofrontal cortex, and aggressive behavior in mice. Psychopharmacology (Berl) 2006;185:441–450. doi: 10.1007/s00213-006-0333-3. [DOI] [PubMed] [Google Scholar]
- de Boer S, Koolhaas J. 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol. 2005;526:125–139. doi: 10.1016/j.ejphar.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Dopico A, Lovinger D. Acute alcohol action and desensitization of ligand-gated ion channels. Pharmacol Rev. 2009;61:98–114. doi: 10.1124/pr.108.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccidomo S, Bannai M, Miczek K. Escalated aggression after alcohol drinking in male mice: dorsal raphé and prefrontal cortex serotonin and 5-HT(1B) receptors. Neuropsychopharmacology. 2008;33:2888–2899. doi: 10.1038/npp.2008.7. [DOI] [PubMed] [Google Scholar]
- Ferris C, Melloni RJ, Koppel G, Perry K, Fuller R, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish E, Faccidomo S, Miczek K. Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT(1B) receptor agonist CP-94,253. Psychopharmacology (Berl) 1999;146:391–399. doi: 10.1007/pl00005484. [DOI] [PubMed] [Google Scholar]
- Fulwiler C, Eckstine J, Kalsy S. Impulsive-aggressive traits, serotonin function, and alcohol-enhanced aggression. J Clin Pharmacol. 2005;45:94–100. doi: 10.1177/0091270004270457. [DOI] [PubMed] [Google Scholar]
- Grant E, Mackintosh J. A comparison of the social postures of some common laboratory rodents. Behaviour. 1963;21:246–295. [Google Scholar]
- Haller J, Tóth M, Halasz J, De Boer S. Patterns of violent aggression-induced brain c-fos expression in male mice selected for aggressiveness. Physiol Behav. 2006;88:173–182. doi: 10.1016/j.physbeh.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Higley J, Mehlman P, Poland R, Taub D, Vickers J, Suomi S, Linnoila M. CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biol Psychiatry. 1996;40:1067–1082. doi: 10.1016/S0006-3223(95)00675-3. [DOI] [PubMed] [Google Scholar]
- Jacobs C, Van Den Broeck W, Simoens P. Increased volume and neuronal number of the basolateral nuclear group of the amygdaloid body in aggressive dogs. Behav Brain Res. 2006;170:119–125. doi: 10.1016/j.bbr.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Jacobs C, Van Den Broeck W, Simoens P. Neurons expressing serotonin-1B receptor in the basolateral nuclear group of the amygdala in normally behaving and aggressive dogs. Brain Res. 2007;1136:102–109. doi: 10.1016/j.brainres.2006.11.096. [DOI] [PubMed] [Google Scholar]
- Jørgensen H. Studies on the neuroendocrine role of serotonin. Dan Med Bull. 2007;54:266–288. [PubMed] [Google Scholar]
- Keele N. The role of serotonin in impulsive and aggressive behaviors associated with epilepsy-like neuronal hyperexcitability in the amygdala. Epilepsy Behav. 2005;7:325–335. doi: 10.1016/j.yebeh.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin F. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci. 1983;33:2609–2614. doi: 10.1016/0024-3205(83)90344-2. [DOI] [PubMed] [Google Scholar]
- Lovinger D, White G. Ethanol potentiation of 5-hydroxytryptamine3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol Pharmacol. 1991;40:263–270. [PubMed] [Google Scholar]
- McKenzie-Quirk S, Girasa K, Allan A, Miczek K. 5-HT(3) receptors, alcohol and aggressive behavior in mice. Behav Pharmacol. 2005;16:163–169. doi: 10.1097/00008877-200505000-00005. [DOI] [PubMed] [Google Scholar]
- Miczek K, Barros H, Sakoda L, Weerts E. Alcohol and heightened aggression in individual mice. Alcohol Clin Exp Res. 1998a;22:1698–1705. [PubMed] [Google Scholar]
- Miczek K, de Almeida R. Oral drug self-administration in the home cage of mice: alcohol-heightened aggression and inhibition by the 5-HT1B agonist anpirtoline. Psychopharmacology (Berl) 2001;157:421–429. doi: 10.1007/s002130100831. [DOI] [PubMed] [Google Scholar]
- Miczek K, Faccidomo S, De Almeida R, Bannai M, Fish E, Debold J. Escalated aggressive behavior: new pharmacotherapeutic approaches and opportunities. Ann N Y Acad Sci. 2004a;1036:336–355. doi: 10.1196/annals.1330.021. [DOI] [PubMed] [Google Scholar]
- Miczek K, Fish E, De Almeida R, Faccidomo S, Debold J. Role of alcohol consumption in escalation to violence. Ann N Y Acad Sci. 2004b;1036:278–289. doi: 10.1196/annals.1330.018. [DOI] [PubMed] [Google Scholar]
- Miczek K, Fish E, De Bold J, De Almeida R. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology (Berl) 2002;163:434–458. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- Miczek K, Hussain S, Faccidomo S. Alcohol-heightened aggression in mice: attenuation by 5-HT1A receptor agonists. Psychopharmacology (Berl) 1998b;139:160–168. doi: 10.1007/s002130050701. [DOI] [PubMed] [Google Scholar]
- Miczek K, O’Donnell J. Intruder-evoked aggression in isolated and nonisolated mice: effects of psychomotor stimulants and L-dopa. Psychopharmacology (Berl) 1978;57:47–55. doi: 10.1007/BF00426957. [DOI] [PubMed] [Google Scholar]
- Miczek K, Weerts E, DeBold J. Alcohol, benzodiazepine-GABAA receptor complex and aggression: ethological analysis of individual differences in rodents and primates. J Stud Alcohol Suppl. 1993;11:170–179. doi: 10.15288/jsas.1993.s11.170. [DOI] [PubMed] [Google Scholar]
- Miczek K, Weerts E, Tornatzky W, DeBold J, Vatne T. Alcohol and “bursts” of aggressive behavior: ethological analysis of individual differences in rats. Psychopharmacology (Berl) 1992;107:551–563. doi: 10.1007/BF02245270. [DOI] [PubMed] [Google Scholar]
- Muller J, Mascagni F, McDonald A. Serotonin-immunoreactive axon terminals innervate pyramidal cells and interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;505:314–335. doi: 10.1002/cne.21486. [DOI] [PubMed] [Google Scholar]
- Nelson R, Chiavegatto S. Molecular basis of aggression. Trends Neurosci. 2001;24:713–719. doi: 10.1016/s0166-2236(00)01996-2. [DOI] [PubMed] [Google Scholar]
- New A, Buchsbaum M, Hazlett E, Goodman M, Koenigsberg H, Lo J, Iskander L, Newmark R, Brand J, O’Flynn K, Siever L. Fluoxetine increases relative metabolic rate in prefrontal cortex in impulsive aggression. Psychopharmacology (Berl) 2004;176:451–458. doi: 10.1007/s00213-004-1913-8. [DOI] [PubMed] [Google Scholar]
- New A, Hazlett E, Buchsbaum M, Goodman M, Reynolds D, Mitropoulou V, Sprung L, Shaw RJ, Koenigsberg H, Platholi J, Silverman J, Siever L. Blunted prefrontal cortical 18fluorodeoxyglucose positron emission tomography response to meta-chlorophenylpiperazine in impulsive aggression. Arch Gen Psychiatry. 2002;59:621–629. doi: 10.1001/archpsyc.59.7.621. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, van Oorschot R, Hen R. Serotonin receptors and animal models of aggressive behavior. Pharmacopsychiatry. 1995;28(Suppl 2):80–90. doi: 10.1055/s-2007-979624. [DOI] [PubMed] [Google Scholar]
- Olivier B, van Oorschot R. 5-HT1B receptors and aggression: a review. Eur J Pharmacol. 2005;526:207–217. doi: 10.1016/j.ejphar.2005.09.066. [DOI] [PubMed] [Google Scholar]
- Pihl R, LeMarquand D. Serotonin and aggression and the alcohol-aggression relationship. Alcohol Alcohol. 1998;33:55–65. doi: 10.1093/oxfordjournals.alcalc.a008348. [DOI] [PubMed] [Google Scholar]
- Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci U S A. 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reist C, Nakamura K, Sagart E, Sokolski K, Fujimoto K. Impulsive aggressive behavior: open-label treatment with citalopram. J Clin Psychiatry. 2003;64:81–85. [PubMed] [Google Scholar]
- Robbins T. Chemistry of the mind: neurochemical modulation of prefrontal cortical function. J Comp Neurol. 2005;493:140–146. doi: 10.1002/cne.20717. [DOI] [PubMed] [Google Scholar]
- Soubrié P. Serotonergic neurons and behavior. J Pharmacol. 1986;17:107–112. [PubMed] [Google Scholar]
- Steinbusch H, Nieuwenhuys R. Localization of serotonin-like immunoreactivity in the central nervous system and pituitary of the rat, with special references to the innervation of the hypothalamus. Adv Exp Med Biol. 1981;133:7–35. doi: 10.1007/978-1-4684-3860-4_1. [DOI] [PubMed] [Google Scholar]
- van Erp A, Miczek K. Increased aggression after ethanol self-administration in male resident rats. Psychopharmacology (Berl) 1997;131:287–295. doi: 10.1007/s002130050295. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virkkunen M, Eggert M, Rawlings R, Linnoila M. A prospective follow-up study of alcoholic violent offenders and fire setters. Arch Gen Psychiatry. 1996;53:523–529. doi: 10.1001/archpsyc.1996.01830060067009. [DOI] [PubMed] [Google Scholar]
- Winslow J, Miczek K. Habituation of aggression in mice: pharmacological evidence of catecholaminergic and serotonergic mediation. Psychopharmacology (Berl) 1983;81:286–291. doi: 10.1007/BF00427564. [DOI] [PubMed] [Google Scholar]