Abstract
Although there is evidence that caveolae and cholesterol play an important role in myocyte signalling processes, details of the mechanisms involved remain sparse. In this paper we have studied for the first time the clinically relevant intact coronary artery and measured in situ Ca2+ signals in individual myocytes using confocal microscopy. We have examined the effect of the cholesterol-depleting agents, methyl-cyclodextrin (MCD) and cholesterol oxidase, on high K+, caffeine and agonist-induced Ca2+ signals. We find that cholesterol depletion produces a stimulus-specific alteration in Ca2+ responses; with 5-HT (10 μM) and endothelin-1 (10 nM) responses being selectively decreased, the phenylephrine response (100 μM) increased and the responses to high K+ (60 mM) and caffeine (10 mM) unaffected. Agonist-induced Ca2+ signals were restored when cholesterol was replenished using cholesterol-saturated MCD. In additional experiments, enzymatically isolated myocytes were patch clamped. We found that cholesterol depletion caused a selective modification of ion channel function, with whole cell inward Ca2+ current being unaltered, whereas outward K+ current was increased, due to BKCa channel activation. There was also a significant decrease in cell capacitance. These data are discussed in terms of the involvement of caveolae in receptor localisation, Ca2+ entry pathways and SR Ca2+ release, and the role of these in agonist signalling.
Keywords: Vascular smooth muscle, Coronary artery, Cholesterol, Lipid rafts
1. Introduction
Lipid rafts are highly dynamic, cholesterol and sphingolipid-enriched domains that compartmentalize cellular processes [1,2]. The high concentration of cholesterol results in a region more ‘ordered’ and less fluid than the surrounding membrane. While there may be speculation about the exact physical nature of rafts [3], it is accepted that they have associated with them (or excluded from them) a range of signalling components, including ion channels, receptors and enzymes, and hence the interest in them as modulators of contractility [2,4]. Caveolae, 50–100 nM flask-like invaginations of the surface membrane, may be regarded as a type of raft where the structure is stabilized by proteins from the caveolin and cavin families [5–7] and are abundant in vascular smooth muscle. Depletion of cell membrane cholesterol disrupts caveolae and may lead to alterations in cell signalling.
Given the importance of the coronary artery to health and the association of hypercholesterolemia with atherosclerosis, endothelial dysfunction and impaired vascular smooth muscle function [8–10], it is of interest and significance to determine the effects of cholesterol manipulation on this vessel. Additionally, while coronary vessels may share many properties with others in the vascular system, they exhibit specific adaptations to their specialized location; for example, the orientation of smooth muscle cells within the coronary artery differs from most other vessels in that there are irregularly orientated oblique bundles, believed to convey increased resistance against the stretch which occurs with each heart beat [11]. It has also been reported that coronary endothelial cells possess distinct properties compared with other vascular beds [12]. Therefore clearly in order to better understand the contractility and regulation of coronary smooth muscle, there is a need to study the coronary vasculature itself, rather than making assumptions based on comparisons with smooth muscle from other vascular beds.
In addition, much previous work investigating lipid rafts and vascular Ca2+ signalling has been carried out on isolated myocytes, which may not respond to agonists as they would in intact tissue, or used global measurements of Ca2+, thus masking any differences in responses between myocytes. We have therefore used confocal microscopy to determine the effects of cholesterol manipulation in single myocytes but from intact coronary artery preparations.
In this study we have examined the effects of methyl-cyclodextrin (MCD) and cholesterol oxidase, cholesterol-depleting agents, on agonist-induced and caffeine and high K+ stimulated in situ [Ca2+]i signalling in Fluo-4 loaded rat coronary artery myocytes from an intact preparation, using confocal microscopy. In additional experiments, enzymatically isolated myocytes were patch clamped and the effects of MCD and Ca2+-activated K+ channel blockers on whole cell outward K+ and inward Ca2+ current were examined. Modulation of cell membrane cholesterol altered coronary artery myocyte Ca2+ signalling in a stimulus-specific manner and differentially regulated ion channel function, indicating the importance of cholesterol and caveolae in the maintenance of myocyte signalling processes.
2. Materials and methods
2.1. Confocal microscopy
Wistar rats (150 g) were anaesthetized (CO2) and humanely killed by cervical dislocation in accordance with Schedule 1 of the UK Animals (Scientific Procedures) Act of 1986. The heart was placed into a modified Krebs solution of composition (mM): NaCl 130, KCl 5.8, MgCl2 1.2, HEPES 10, glucose 8, and CaCl2 2.5 (adjusted to pH 7.4). The septal coronary artery (4–6 mm) was dissected, cleared of adhering tissue and incubated with 20 μM Fluo-4 AM for 3 h at room temperature in the presence of 0.25% of the non-ionic detergent Pluronic F-127. The tissue was then placed in Krebs solution to allow de-esterification of the dye. Vessels were mounted under a small amount of isometric tension between two fixed aluminium foil clips at the bottom of the chamber, on the stage of an Olympus inverted microscope and maintained at room temperature. Experiments were performed using an Ultraview LCI spinning (Nipkow) disc, widefield confocal microscope (PerkinElmer, Cambridge, UK), equipped with an Orca ER cooled CCD camera (Hamamatsu Photonics, UK) and a 60× water immersion objective (N.A. 1.20). Mean fluorescence intensity was measured on-line from regions of interest drawn over individual cells using UltraView software. Movement artefacts were rarely a problem when measuring from individual cells in a vessel under isometric tension. Many of the contractile stimuli applied to the tissues produced non-synchronous Ca2+ waves in only a subset of the cells under observation and overall showed minimal movement. If substantial movement occurred, measurements were not made. The numerical data obtained were saved to an ASCII file for further analysis using Origin 7.0 software. The peak amplitude of the [Ca2+]i signal was expressed as normalised pseudo ratio of Fluo-4 fluorescence (F/F0).
2.2. Electrophysiology
For cell isolation, the heart was placed into ice cold Hanks solution of the following composition (mM): NaCl 137, KCl 5.6, Na2HPO4 0.42, NaH2PO4 0.44, MgCl2 1, HEPES 10, NaHCO3 4.2, glucose 8, and CaCl2 2 (adjusted to pH 7.4 with NaOH) and the septal coronary artery dissected as before. Smooth muscle cells were isolated as follows: arteries were placed in low Ca2+ (100 μM) Hanks solution containing 1.2 mg/ml papain (pre-activated with 1 mg/ml DTT for 5 min at 35 °C) and incubated at 35 °C, for 15 min. Arteries were transferred to low Ca2+ Hanks solution containing 1.2 mg/ml collagenase and 1 mg/ml hyaluronidase and incubated for 10 min (35 °C). The vessel was dispersed with gentle trituration using a fire-polished micropipette in Ca2+-free Hanks solution, containing 1 mg/ml BSA. Cells were maintained in Ca2+-free solution at 4 °C until required (0–5 h). Only spindle-shaped cells were used in experiments.
Transmembrane ionic currents were studied using the conventional patch clamp technique. Patch pipettes were pulled from thick walled borosilicate glass capillaries (Harvard Apparatus, UK) using a PP830 puller (Narishige, Japan) and the tips were heat-polished using a MF830 micro-forge (Narishige, Japan) to achieve 3–6 MΩ resistance. Patch pipettes were filled with a solution containing (mM) KCl 130, NaCl 15, MgCl2 0.3, HEPES 10, ATP 7, EGTA 0.005 (pH adjusted to 7.2 with KOH) for measurement of outward current. For inward Ca2+ current measurements, Cs+ replaced K+ in the pipette. Whole cell currents were recorded using the EPC-9 patch clamp amplifier controlled by the Pulse software (HEKA Elektronik, Germany). During the experiment, cells were superfused with physiological saline solution at room temperature and held at −70 mV holding potential. Current–voltage curves were constructed from the peak outward currents recorded in response to voltage pulses ranging from −60 to +70 mV in 10 mV increments. Cell capacitance and access conductance were measured and corrected automatically before each sweep. Whole cell currents are expressed as current density (peak current/cell capacitance, pA/pF). Additionally, the effect of selective antagonists was assessed using a protocol where cells were repeatedly depolarised to +50 mV for 750 ms, from a holding potential of −70 mV, at 20 s intervals, allowing outward current and cell capacitance to be monitored continuously with time.
2.3. Cholesterol manipulation
Membrane cholesterol was extracted using methyl-cyclodextrin (MCD), a cyclic oligosaccharide which sequesters cholesterol as previously described [13–15] or using cholesterol oxidase. In intact tissues, 15 mM MCD dissolved in physiological solution (2% MCD) was applied to the tissue for 10 min at room temperature, followed by a 20 min washout period, which has been shown to lower cholesterol by approximately 30% [15]. Agonist responses were compared before and after MCD application. The effect of cholesterol oxidase (2 U/ml), also dissolved in physiological solution, was examined in the same way. Control experiments were carried out to ensure the repeatability of the agonist responses. Membrane cholesterol was replenished by applying cholesterol-saturated MCD (0.5%) to the tissue for 10 min at room temperature.
On isolated cells, 2% MCD solution was applied to the cell under study for a period of 10 min at room temperature. In one protocol, a control I–V curve was obtained, followed by MCD exposure, a 5 min washout period and a second I–V curve was recorded. Alternatively, the protocol of repeated depolarisations to +50 mV, at 20 s intervals was used, allowing outward current and cell capacitance to be monitored continuously with time. Control recordings were taken for 2 min, followed by continued recordings during a 10 min MCD exposure.
The effectiveness of MCD was compared at both room temperature and at 30 °C in the isolated smooth muscle cell preparations. Under both conditions, identical results were obtained (data not shown). Therefore, room temperature was chosen for the subsequent experiments, since the integrity of the isolated cells was preserved for longer periods than at the higher temperature, allowing the more lengthy voltage clamp protocols to be completed.
2.4. Drugs and solutions
Unless otherwise specified, chemicals were obtained from Sigma (UK). Pluronic F-127 and Fluo-4 AM were obtained from Invitrogen. A stock solution of TRAM-34 (10 mM) was prepared in DMSO and apamin (1 mM) was prepared in 0.05 M acetic acid. Both were diluted in physiological salt solution as required. All other compounds were made in aqueous solution.
2.5. Statistics
Data are presented as mean ± SEM, where n = number of cells tested from a minimum of three coronary arteries. The Student t-test was used for statistical comparisons. A value of P < 0.05 was considered significant.
3. Results
3.1. Effect of agonist stimulation
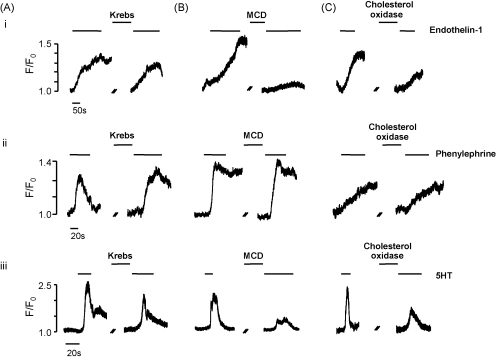
Isolated intact segments of coronary artery were stimulated with 5-HT (10 μM), endothelin-1 (ET-1, 10 nM) and phenylephrine (PE, 100 μM) and the resulting Ca2+ transients were measured in individual smooth muscle cells within the intact vessel wall. In a series of control experiments we validated the use of sequential agonist applications. We found that the Ca2+ signals generated by ET-1 and PE were reversible and repeatable (Fig. 1Ai and ii), with no significant differences between applications. The response to 5-HT showed some desensitisation; the peak response to 10 μM was reduced by 35.7 ± 7% (n = 21) in the second administration (Fig. 1Aiii). Therefore in the following experiments the effect of cholesterol extraction on 5-HT was evaluated in paired experiments by comparing the resulting change in Ca2+ signal with the reduction in signal produced by the second control application of 5-HT.
Fig. 1.
Experimental traces showing (A) sequential application of agonist (i) 10 nM ET-1, (ii) 100 μM PE and (iii) 10 μM 5-HT. (B) The effect of cholesterol depletion using MCD and (C) the effect of cholesterol depletion with cholesterol oxidase, on the response of coronary artery myocytes to (i) 10 nM ET-1, (ii) 100 μM PE and (iii) 10 μM 5-HT.
3.2. Effect of cholesterol extraction
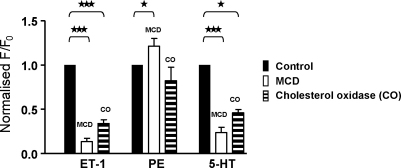
Endothelin-1 produced a relatively slowly developing rise in Ca2+ which reached a plateau during a 2–3 min application. Incubation with MCD greatly reduced this response, as shown in Fig. 1Bi (typical of 26 other cells) and produced a mean inhibition of 86.6 ± 4%. Phenylephrine (100 μM) produced a more rapid rise in Ca2+, attaining a plateau within 30 s to 1 min. MCD-induced cholesterol extraction led to a small but significant increase in the PE response, as shown in Fig. 1Bii (21.3 ± 8% increase, n = 17, P = 0.044). Application of 5-HT also produced a rapid rise in Ca2+ that reached a peak within 20 s. Incubation with MCD greatly reduced the 5-HT response, producing a significant inhibition of 76.3 ± 5% (n = 20, Fig. 1Biii). These data show that incubation with MCD leads to an agonist-specific alteration in myocyte Ca2+ signalling. The mean data for all three agonists are represented in Fig. 2.
Fig. 2.
Mean data showing the effect of cholesterol depletion (using MCD and cholesterol oxidase) on the response of coronary artery myocytes to 10 nM ET-1, 100 μM PE and 10 μM 5-HT. ***p < 0.0001 and *p < 0.05.
Cholesterol oxidase (2 U/ml) was used as an alternative method of reducing cholesterol content, and representative traces are shown in Fig. 1C. Mirroring the effect of MCD, this treatment also caused a significant inhibition of the 5-HT (53.9 ± 4%, n = 22) and ET-1 (66.0 ± 4%, n = 14) responses and little change with PE (n = 22). The degree of inhibition was less striking than with MCD, presumably because cholesterol oxidase was unable to achieve the same degree of caveolar disruption within the time frame of the experiment. The mean data are shown in Fig. 2. Subsequent experiments were performed using 5-HT.
3.3. Cholesterol replenishment
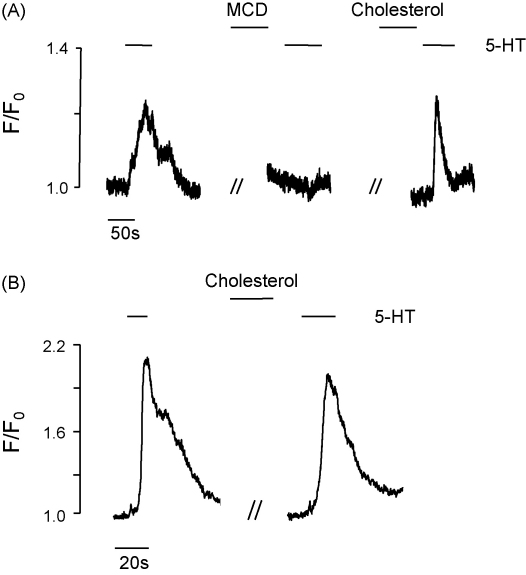
In order to test the specificity and reversibility of cholesterol extraction with MCD, cholesterol was replenished using cholesterol-saturated MCD (0.5%). As shown in Fig. 3A, MCD significantly decreased the Ca2+ response to 5-HT as expected, but subsequent responsiveness to 5-HT could be restored after cholesterol was added back (in 4 of 16 cells, full restoration was achieved; mean restoration of 50.0 ± 9%, n = 16). Application of cholesterol-saturated MCD alone had no effect on the 5-HT response (Fig. 3B, 33.4 ± 8% inhibition, n = 27, not significantly different when compared to the second application of 5-HT in the control experiment, 35.7 ± 7%). These data suggest that the inhibition seen with MCD is due to cholesterol extraction and lipid raft disruption, rather than a non-specific effect of MCD.
Fig. 3.
Experimental trace showing (A) the effect of cholesterol depletion (using MCD) and subsequent cholesterol replenishment (using cholesterol-saturated MCD) on the response of coronary artery myocytes to 10 μM 5-HT and (B) effect of cholesterol-saturated MCD alone on the response of coronary artery myocytes to 10 μM 5-HT.
3.4. Removal of extracellular calcium
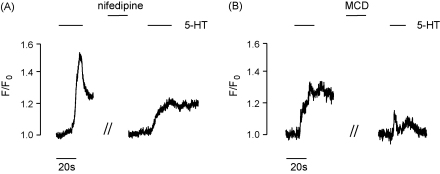
Agonists can affect both Ca2+ release from the sarcoplasmic reticulum (SR) and extracellular Ca2+ entry. The 5-HT response was therefore also examined in Ca2+-free extracellular solution (2 mM EGTA) or after treatment with nifedipine (10 μM), in order to eliminate Ca2+ entry through L-type Ca2+ channels. The effects of cholesterol depletion on SR Ca2+ release and Ca2+ channel entry processes were examined. Nifedipine inhibited the 5-HT maximal response by 58.5 ± 5% (n = 17, Fig. 4A). This concentration of nifedipine was sufficient to inhibit the response to high K+ (60 mM) by 80% (data not shown). The nifedipine-resistant portion of the 5-HT response was inhibited a further 63.5 ± 6% (n = 15) in the presence of MCD (Fig. 4B). Similarly, a 72.3 ± 4% (n = 32) inhibition of the maximal response to 5-HT was observed under Ca2+-free conditions. The remaining 5-HT response, that demonstrated resistance to the lack of extracellular Ca2+, was also inhibited upon treatment with MCD (78.2 ± 6% inhibition, n = 15).
Fig. 4.
Experimental trace showing (A) the effect of 10 μM nifedipine on the response to 10 μM 5-HT and (B) the susceptibility of the nifedipine-resistant portion of the response to treatment with MCD.
3.5. Caffeine and high K+
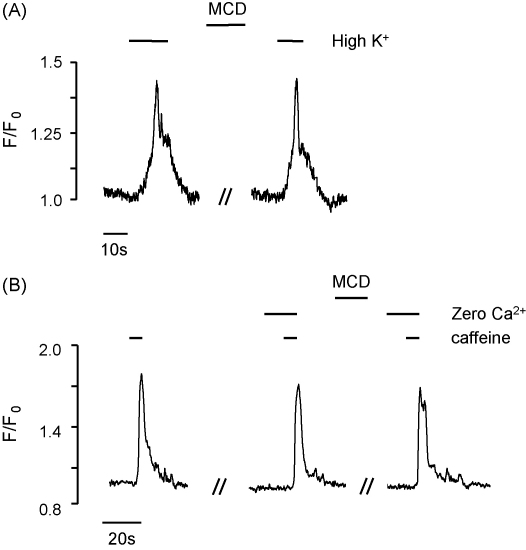
Intact segments of coronary artery were stimulated with 60 mM high K+ or 10 mM caffeine using the protocol described above for agonists, and the resulting Ca2+ transients measured in individual smooth muscle cells within the vessel wall. The response to high K+ was reversible and repeatable, with no significant difference between the first and second application (n = 21, data not shown). Incubation with MCD did not alter the Ca2+ response to high K+ stimulation (Fig. 5A, 7.4 ± 13% increase, n = 16). Caffeine produced a brief and rapid rise in Ca2+ as shown in Fig. 5B. This response was also both reversible and repeatable with no significant difference between the first and second application (8.3 ± 5% inhibition, n = 15, data not shown). Application of MCD did not significantly alter the response to caffeine (1.8 ± 4% inhibition, n = 26, data not shown). The effect of caffeine was also evaluated in Ca2+-free extracellular solution (2 mM EGTA) in order to better examine the effects of cholesterol depletion on Ca2+ release from the intracellular stores. Under these conditions, MCD still had no effect on the caffeine response (8.5 ± 7.4%, n = 28, Fig. 5B), suggesting little effect of MCD on SR Ca2+ release.
Fig. 5.
Experimental trace showing (A) the effect of MCD on the response of coronary artery myocytes to 60 mM high K+ solution and (B) the effect of 10 mM caffeine on [Ca2+]i in the presence and absence of extracellular Ca2+. The Ca2+-free solution was perfused for 5 min prior to addition of caffeine.
3.6. Electrophysiology
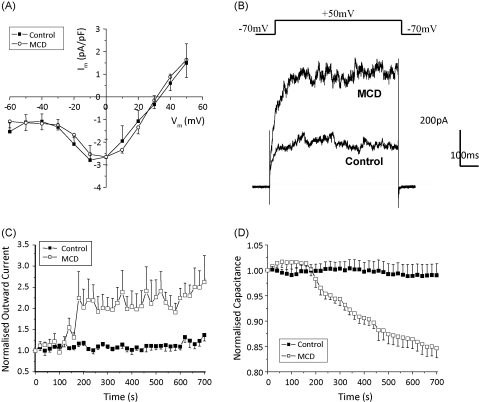
The effect of MCD was also evaluated in electrophysiological studies on freshly isolated coronary artery myocytes. When Cs+ was used in the pipette solution (instead of K+), no outward current was apparent. A small inward current (2.1 ± 0.1 pA/pF) was observed, peaking at 0 mV and sensitive to inhibition with nifedipine and potentiation with Bay K8644 (n = 4, data not shown). Experiments evaluating the effect of cholesterol extraction were carried out in the presence of 1 μM Bay K8644. Treatment with MCD for 10 min did not significantly alter the inward current over the entire voltage range tested, as shown in Fig. 6A (peak current at 0 mV: control 2.66 ± 0.8 pA/pF versus 2.65 ± 0.4 pA/pF, n = 3).
Fig. 6.
(A) Mean inward current–voltage relationship in the absence and presence of MCD. (B) Representative traces, recorded from the same cell, showing the outward current generated by a +50 mV depolarisation in the absence and presence of MCD. (C) Effect of MCD on outward K+ current and (D) Effect of MCD on cell capacitance.
In voltage clamp experiments where cells were dialysed with K+-containing pipette solution, a large, sustained whole cell outward current was elicited from cells exposed to pulses ranging from −20 mV to +70 mV. Extraction of membrane cholesterol with MCD led to a significant increase in the whole cell outward current and a significant decrease in cell capacitance. Fig. 6B shows selected traces recorded from the same cell in response to +50 mV voltage clamp pulses under control conditions and after cholesterol extraction. Fig. 6C and D shows the mean effect of MCD on cell capacitance and outward current, respectively. Cell capacitance was measured before each voltage sweep, allowing it to be monitored throughout the time course of the experiment. We used paired t-tests to compare the capacitance of every cell before and after depletion of cholesterol. Size variations between cells meant there was cell-to-cell variation in capacitance. However, paired comparisons revealed a statistically significant decrease in cell capacitance after cholesterol depletion (Fig. 6D, n = 18, P < 0.0001). Control superfusion of cells with physiological saline solution instead of MCD for 10 min produced no change in cell capacitance (Fig. 6D). Similarly, a paired t-test was used to compare the outward current generated by each cell before and after 10 min treatment with MCD and demonstrated a significant increase of 2.5 ± 0.4-fold (Fig. 6C, n = 6, P = 0.012). Again, control superfusion of cells with physiological saline solution instead of MCD for 10 min produced no change in outward current.
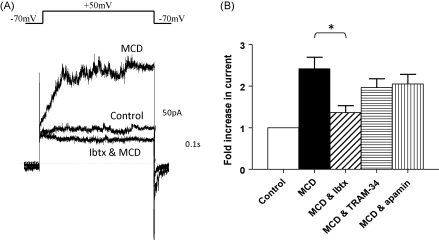
MCD has been reported to elevate basal intracellular Ca2+ [16]. Therefore, in order to determine whether this elevation of outward current in the presence of MCD is due to activation of Ca2+-dependent K+ currents, we examined the effects of 3 selective channel blockers: iberiotoxin (100 nM) to block large conductance (BKCa) channels, TRAM-34 (100 nM) to block intermediate conductance (IKCa) channels and apamin (100 nM) to block small conductance (SKCa) channels. The main protocol used to assess the inhibitory nature of these antagonists involved pre-treatment with the blocker and then a challenge with MCD. We also confirmed the result by using MCD first to increase outward current and then applying the blocker on top. Iberiotoxin pre-treatment significantly inhibited the increase in outward current observed with MCD (2.42 ± 0.28-fold, n = 11 versus 1.37 ± 0.16-fold, n = 5, P = 0.029). When cholesterol was depleted with MCD first, iberiotoxin entirely ablated the MCD-induced increase in outward current (Fig. 7A, n = 3). TRAM-34 failed to alter the increase in K+ current observed with MCD using either protocol (Fig. 7B, 2.42 ± 0.28-fold versus 1.97 ± 0.21-fold, n = 5, P = 0.32). Similarly, apamin failed to alter the increase in K+ current observed with MCD (Fig. 7B, 2.42 ± 0.28-fold versus 2.1 ± 0.23-fold, n = 5, P = 0.43). It was confirmed that the DMSO and apamin vehicle controls had no effect on outward current.
Fig. 7.
(A) Representative traces, recorded from the same cell, showing that the increase in outward K+ current induced by MCD is blocked by 100 nM iberiotoxin and (B) mean data showing the effects of 100 nM iberiotoxin, 100 nM TRAM-34 and 100 nM apamin on MCD-induced increases in outward K+ current.
4. Discussion
In this study we manipulated the cell membrane cholesterol content of in situ coronary artery myocytes, using MCD or cholesterol oxidase, and observed stimulus-specific alteration in the [Ca2+]i responses. The Ca2+ rises in responses to 5-HT and ET-1 were significantly reduced when cholesterol levels were lowered, whereas the responses to PE, caffeine and high K+ were not. Cholesterol extraction also caused a selective modification of ion channel function, with L-type Ca2+ channel activity unchanged, but K+ channel function significantly increased due to BKCa channel activity. We found no evidence for loading of the SR or change in ryanodine receptor-mediated (RyR) Ca2+ release processes. A large variety of signalling proteins relevant to the contractile process are associated with caveolae; such as, GPCRs [16–18] and ion channels [19–22], and as such, are susceptible to the modulation of cholesterol. We suggest that the differences in agonist responses are a consequence of the different signalling cascades as well as caveolar and non-caveolar localisation of receptor subtypes.
Treatment with MCD selectively extracts cholesterol from the plasma membrane [13–14] and leads to the disruption of caveolae, which importantly may be reversed by the replenishment of cholesterol [15,23,24]. As a cholesterol-extracting agent, MCD has been widely used to evaluate the role of lipid rafts/caveolae in various biological systems and more recently, its effects on smooth muscle contraction have been demonstrated [15,16,23,25–28]. As we show here for coronary artery and in agreement with the previous studies noted above, the effects of MCD are both selective and reversible. They are also similar, we show, to those of the more slowly acting cholesterol oxidase. The decrease we found in membrane capacitance with MCD is consistent with removal of caveolae from the myocyte membrane; previously demonstrated at the electron micrographic level [16,23,29] and by internalisation of dextran-conjugated fluorescent indicators [27]. Thus, although cholesterol depletion may have other effects, we conclude that the appropriateness of MCD for investigating the effects of cholesterol manipulation and caveolae disruption has been demonstrated. Although previous studies have examined the effects of MCD in a number of blood vessels, this is the first study on coronary arteries. In addition there is only one other paper [16] that has investigated changes in Ca2+ signals in single myocytes in situ. Given the importance of the coronary circulation, the changes that can occur with isolation of single cells and the difficulties of interpreting global (photometric) Ca2+ signals, our data add significant insight to previous studies.
Before considering the data obtained with the three different agonists, we will discuss the mechanistic findings from measuring membrane currents and manipulating Ca2+ entry. The response of these coronary artery myocytes to high K+ depolarisation was impervious to MCD treatment, suggesting that L-type Ca2+ channel function was unaltered. Measurements of inward current also showed no effect of MCD treatment even when augmented with Bay K8644, and over the entire voltage range studied. Thus direct effects on L-type Ca2+ channels do not appear to underlie the effects of MCD, although indirect effects subsequent to increased outward current appear likely as discussed next. The whole cell outward K+ current was strongly potentiated by MCD at voltages positive to −30 mV, an effect we found to be mediated by BKCa channels. These data are consistent with those produced in arterial smooth muscle cells from caveolin-1 knockout (Cav-1−/−) mice, where outward current was enhanced in the knockout compared to control [30]. There are reports of caveolar localisation for BKCa channels, although the effects appear to be tissue-specific [27,31]. That outward current increases when cholesterol is depleted and caveolae/lipid rafts are disrupted, suggests that the localisation of K+ channel subunits to these membrane domains may be a mechanism by which K+ channel function is limited and regulated. A similar regulatory mechanism has been proposed for eNOS [32,33]; although it is notable that in this example cholesterol extraction does not produce the same effects as Cav-1 gene disruption [34,35].
Given the comparative sizes of the whole cell outward and inward currents measured in these coronary artery myocytes (10–15-fold larger outward current at 0 mV), it is clear that there is a strong hyperpolarising drive and suppression of excitability in these cells which will be increased with cholesterol depletion and/or caveolar disruption. This hyperpolarisation of the smooth muscle cells will decrease the opening of the voltage-dependent Ca2+ channels and would be expected therefore to reduce the Ca2+ signal produced in coronary arterial myocytes by agonists working through this pathway, i.e. stimulating Ca2+ entry. Support for such a mechanism comes from work on cerebral artery from Cav-1 deficient mice; diminished membrane depolarisation and Ca2+ influx in response to vascular pressure increases and attenuated vasoconstriction were reported [36]. This indicates a physiological role for caveolae in mediating the myogenic response, and for their disruption to decrease Ca2+ entry in response to certain stimuli. The mechanism is however unlikely to explain the decreased response to ET-1, as this is not considered to be mediated by L-type Ca2+ entry [37–39] and as discussed below effects on other entry mechanisms are likely to underlie ET-1 Ca2+ responses.
Cholesterol extraction did not affect the release of Ca2+ from RyR on the SR, as demonstrated by the unchanged caffeine responses. Although the SR membrane is low in cholesterol and MCD is not readily able to cross the plasma membrane, loading of the SR with Ca2+ due to an elevation of basal Ca2+ and an increased response to caffeine (and cirazoline) post-MCD, was reported in tail arteries [16]. We found no sustained rise in basal Ca2+ or increased caffeine response subsequent to MCD. The reason for the sustained elevation of basal Ca2+ in tail artery is unclear but it may be due to the much longer exposure (1 h) to MCD that was used by Dreja et al. Thus the reduced Ca2+ signals in response to 5-HT and ET-1 that we find are unlikely to be accounted for by an alteration in SR luminal Ca2+ content or RyR-mediated SR release. This therefore leads to the suggestion that it is Ca2+ entry mechanisms (but not via direct effects on L-type Ca2+ channels) and/or IP3-mediated SR Ca2+ release mechanisms that are impaired. Cav-1−/− mice, which lacked caveolae and exhibited impaired Ca2+ signalling in vascular smooth muscle [40], also had no difference in L-type Ca2+ channel function or SR load between knockout and control animals [30]; findings which are consistent with our data and conclusion.
Via activation of phospholipase C, 5-HT, ET-1 and PE all cause a rise in IP3 and release of Ca2+ from IP3-sensitive intracellular stores [39,41,42]. Subsequent depletion of SR Ca2+ may trigger store-operated Ca2+ entry in order to replenish the store (see Ref. [43] for review). Our experiments carried out with MCD in the presence of nifedipine demonstrated that the remaining 30–40% of the Ca2+ entry response to 5-HT was also reduced by cholesterol extraction. These data suggest that, in this tissue, store-operated channels (SOC; such as TRPC1) and/or receptor-operated channels (ROC; such as NSCC1 & 2 or TRPC3 & 6) are activated by 5-HT and are susceptible to the effects of cholesterol depletion.
The above discussion points to distinct mechanisms within the myocytes that may be affected by caveolar disruption with MCD treatment. Although the effects of cholesterol extraction need not all be via caveolae disruption, we have focussed on this to simplify the discussion. We found that extraction of membrane cholesterol reduced the Ca2+ signals produced by 5-HT and ET-1, but not those of PE. The contractile responses to 5-HT and ET-1 are mediated by the 5-HT2A and ETA receptors, respectively, both of which have been reported to localise to caveolae (5-HT2A: [16,17,44] and ETA: [16,18,45]), and thus effects of caveolar disruption on these receptor signalling pathways would be expected to underlie the effects of MCD. For 5-HT we showed that around 60% of the rise in [Ca2+]i it produces is due to L-type Ca2+ entry, as it was inhibited by nifedipine. This component of the Ca2+ signal will therefore be sensitive to MCD and cholesterol oxidase as outward current is increased. The remaining, i.e. non-L-type, Ca2+ entry is due to receptor-operated and store-operated channels. As discussed above our data show that these entry mechanisms are also sensitive to MCD. The response to ET-1 is dependent on extracellular Ca2+, but not sensitive to blockade of L-type Ca2+ channels. Instead, the response is mediated by a mixture of SOC and ROC channels [37–39,45–47]. In rat tail artery, the cholesterol depletion-mediated reduction in ET-1 response was due to the disruption of the caveolar localisation of the TRPC1 channel and the subsequent inhibition of store-operated Ca2+ entry [45]. Thus, the inhibition of the ET-1 response by cholesterol depletion in rat coronary artery is likely due to inhibition of ROC and/or SOC pathways.
The response to phenylephrine was not decreased and in fact was slightly increased after exposure to MCD treatment in rat coronary arterial myocytes, with robust Ca2+ signals being maintained after cholesterol depletion. The simplest explanation of our PE data would be that its receptors, α1-adrenceptors, are not present in caveolae and hence their disruption does not change their efficacy. There is some evidence to support this, i.e. this receptor does not localise to caveolae and that the PE response is insensitive to cholesterol depletion; [16,48,49] but other studies have observed a reduced response to PE after exposure to MCD [28,50,51]. The differences may reflect tissue and species differences. Our data are consistent with, but do not prove, the suggestion that in rat coronary artery the α1-adrenoceptor predominantly has a non-caveolar localisation and its signalling is not greatly affected by membrane potential and Ca2+ entry, and hence is little affected by cholesterol manipulation. It may be that in rat coronary artery myocytes the SOC and/or NSCC currents activated by PE differ from those activated by ET-1 and 5-HT and in addition to α1-adrenoceptors not being localised to caveolae, this provides a clear difference between the signalling pathways of 5-HT and ET-1 (sensitive to cholesterol depletion) and PE (resistant to the effects of cholesterol depletion), which otherwise activate many of the same signalling pathways. The small increase in Ca2+ response found may be due to redistribution of a small caveolar α1-adrenoceptor pool to this non-caveolar domain, or other factors favouring coupling between the receptor and Ca2+ signalling.
This study has shown that modification of cell membrane cholesterol levels in rat coronary artery smooth muscle cells in situ causes agonist and stimulus-specific alteration of [Ca2+]i signalling and differentially regulates ion channel function. These data increase the evidence that cholesterol plays an important role in the maintenance of myocyte signalling processes. Increasingly caveolae are being implicated in the genesis of a variety of disease states, ranging from cardiovascular and neurological diseases to cancer and immune system failures (see Ref. [52] for review). Clearly further work is required to fully understand the role of cholesterol/caveolae in modulating cell signalling in smooth muscle and other cell types and may in future provide the basis for new therapeutic interventions in these emerging fields.
Acknowledgement
This work was supported by the British Heart Foundation (PG/04/044/16954).
References
- 1.Brown D.A., London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 2.Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 3.Laude A.J., Prior I.A. Plasma membrane microdomains: organization, function and trafficking. Mol. Membr. Biol. 2004;21:193–205. doi: 10.1080/09687680410001700517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connell K.M., Martens J.R., Tamkun M.M. Localization of ion channels to lipid Raft domains within the cardiovascular system. Trends Cardiovasc. Med. 2004;14:37–42. doi: 10.1016/j.tcm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Quest A.F., Leyton L., Parraga M. Caveolins, caveolae, and lipid rafts in cellular transport, signalling and disease. Biochem. Cell Biol. 2004;82:129–144. doi: 10.1139/o03-071. [DOI] [PubMed] [Google Scholar]
- 6.Hill M.M., Bastiani M., Luetterforst R. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113–124. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastiani M., Liu L., Hill M.M. MURC/cavin-4 and cavin family members form tissue-specific caveolar complexes. J. Cell Biol. 2009;185:1259–1273. doi: 10.1083/jcb.200903053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox D.A., Cohen M.L. Selective enhancement of 5-hydroxytryptamine-induced contraction of porcine coronary artery by oxidized low-density lipoprotein. J. Pharmacol. Exp. Ther. 1996;276:1095–1103. [PubMed] [Google Scholar]
- 9.Bowles D.K., Heaps C.L., Turk J.R., Maddali K.K., Price E.M. Hypercholesterolemia inhibits L-type calcium current in coronary macro-, not microcirculation. J. Appl. Physiol. 2004;96:2240–2248. doi: 10.1152/japplphysiol.01229.2003. [DOI] [PubMed] [Google Scholar]
- 10.Van A.T., Fransen P., Guns P.J., Herman A.G., Bult H. Altered Ca2+ handling of smooth muscle cells in aorta of apolipoprotein E-deficient mice before development of atherosclerotic lesions. Cell Calcium. 2007;41:295–302. doi: 10.1016/j.ceca.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Higuchi K., Hashizume H., Aizawa Y., Ushiki T. Scanning electron microscopic studies of the vascular smooth muscle cells and pericytes in the rat heart. Arch. Histol. Cytol. 2000;63:115–126. doi: 10.1679/aohc.63.115. [DOI] [PubMed] [Google Scholar]
- 12.Aird W.C. Phenotypic heterogeneity of the endothelium. II. Representative vascular beds. Circ. Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 13.Kilsdonk E.P.C., Yancey P.G., Stoudt G.W. Cellular cholesterol efflux mediated by cyclodextrins. J. Biol. Chem. 1995;270:17250–17256. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- 14.Yancey P.G., Rodrigueza W.V., Kilsdonk E.P. Cellular cholesterol efflux mediated by cyclodextrins. Demonstration of kinetic pools and mechanism of efflux. J. Biol. Chem. 1996;271:6026–6034. doi: 10.1074/jbc.271.27.16026. [DOI] [PubMed] [Google Scholar]
- 15.Smith R.D., Babiychuk E.B., Noble K., Draeger A., Wray S. Increased cholesterol decreases uterine activity: functional effects of cholesterol in pregnant rat myometrium. Am. J. Physiol. 2005;288:C982–C988. doi: 10.1152/ajpcell.00120.2004. [DOI] [PubMed] [Google Scholar]
- 16.Dreja K., Voldstedlund M., Vinten J., Tranum-Jensen J., Hellstrand P., Sward K. Cholesterol depletion disrupts caveolae and differentially impairs agonist-induced arterial contraction. Arterioscler. Thromb. Vasc. Biol. 2002;22:1267–1272. doi: 10.1161/01.atv.0000023438.32585.a1. [DOI] [PubMed] [Google Scholar]
- 17.Cogolludo A., Moreno L., Lodi F. Serotonin inhibits voltage-gated K+ currents in pulmonary artery smooth muscle cells: role of 5-HT2A receptors, caveolin-1, and KV1.5 channel internalization. Circ. Res. 2006;98:931–938. doi: 10.1161/01.RES.0000216858.04599.e1. [DOI] [PubMed] [Google Scholar]
- 18.Chun M., Liyanage U.K., Lisanti M.P., Lodish H.F. Signal transduction of a G protein-coupled receptor in caveolae: colocalization of endothelin and its receptor with caveolin. Proc. Natl. Acad. Sci. U.S.A. 1994;91:11728–11732. doi: 10.1073/pnas.91.24.11728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohn M., Furstenau M., Sagach V. Ignition of calcium sparks in arterial and cardiac muscle through caveolae. Circ. Res. 2000;87:1034–1039. doi: 10.1161/01.res.87.11.1034. [DOI] [PubMed] [Google Scholar]
- 20.Martens J.R., Sakamoto N., Sullivan S.A., Grobaski T.D., Tamkun M.M. Isoform-specific localization of voltage-gated K+ channels to distinct lipid raft populations. Targeting of Kv1.5 to Caveolae. J. Biol. Chem. 2001;276:8409–8414. doi: 10.1074/jbc.M009948200. [DOI] [PubMed] [Google Scholar]
- 21.Sampson L.J., Hayabuchi Y., Standen N.B., Dart C. Caveolae localize protein kinase A signaling to arterial ATP-sensitive potassium channels. Circ. Res. 2004;95:1012–1018. doi: 10.1161/01.RES.0000148634.47095.ab. [DOI] [PubMed] [Google Scholar]
- 22.Wang X.L., Ye D., Peterson T.E. Caveolae targeting and regulation of large conductance Ca(2+)-activated K+ channels in vascular endothelial cells. J. Biol. Chem. 2005;280:11656–11664. doi: 10.1074/jbc.M410987200. [DOI] [PubMed] [Google Scholar]
- 23.Babiychuk E.B., Smith R.D., Burdyga T.V., Babiychuk V.S., Wray S., Draeger A. Membrane cholesterol selectively regulates smooth muscle phasic contraction. J. Membr. Biol. 2004;198:95–101. doi: 10.1007/s00232-004-0663-1. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J., Kendrick A., Quenby S., Wray S. Contractility and calcium signalling of human myometrium are profoundly affected by cholesterol manipulation: implications for labour? Reprod. Sci. 2007;14:456–466. doi: 10.1177/1933719107306229. [DOI] [PubMed] [Google Scholar]
- 25.Cristofaro V., Peters C.A., Yalla S.V., Sullivan M.P. Smooth muscle caveolae differentially regulate specific agonist induced bladder contractions. Neurourol. Urodyn. 2007;26:71–80. doi: 10.1002/nau.20361. [DOI] [PubMed] [Google Scholar]
- 26.Gosens R., Stelmack G.L., Dueck G. Caveolae facilitate muscarinic receptor-mediated intracellular Ca2+ mobilization and contraction in airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L1406–L1418. doi: 10.1152/ajplung.00312.2007. [DOI] [PubMed] [Google Scholar]
- 27.Shmygol A., Noble K., Wray S. Depletion of membrane cholesterol eliminates the Ca2+-activated component of outward potassium current and decreases membrane capacitance in rat uterine myocytes. J. Physiol. 2007:445–456. doi: 10.1113/jphysiol.2007.129452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schach C., Firth A.L., Xu M. Regulation of pulmonary vasoconstriction by agonists and caveolae. Exp. Lung Res. 2008;34:195–208. doi: 10.1080/01902140801925471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potocnik S.J., Jenkins N., Murphy T.V., Hill M.A. Membrane cholesterol depletion with beta-cyclodextrin impairs pressure-induced contraction and calcium signalling in isolated skeletal muscle arterioles. J. Vasc. Res. 2007;44:292–302. doi: 10.1159/000101451. [DOI] [PubMed] [Google Scholar]
- 30.Cheng X., Jaggar J.H. Genetic ablation of caveolin-1 modifies Ca2+ spark coupling in murine arterial smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2309–H2319. doi: 10.1152/ajpheart.01226.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lam R.S., Shaw A.R., Duszyk M. Membrane cholesterol content modulates activation of BK channels in colonic epithelia. Biochim. Biophys. Acta. 2004;1667:241–248. doi: 10.1016/j.bbamem.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Cardena G., Martasek P., Masters B.S. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J. Biol. Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 33.Michel J.B., Feron O., Sacks D., Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J. Biol. Chem. 1997;272:15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- 34.Razani B., Engelman J.A., Wang X.B. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J. Biol. Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 35.Blair A., Shaul P.W., Yuhanna I.S., Conrad P.A., Smart E.J. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J. Biol. Chem. 1999;274:32512–32519. doi: 10.1074/jbc.274.45.32512. [DOI] [PubMed] [Google Scholar]
- 36.Adebiyi A., Zhao G., Cheranov S.Y., Ahmed A., Jaggar J.H. Caveolin-1 abolishment attenuates the myogenic response in murine cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1584–H1592. doi: 10.1152/ajpheart.00584.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chabrier P.E., Auguet M., Roubert P. Vascular mechanism of action of endothelin-1: effect of Ca2+ antagonists. J. Cardiovasc. Pharmacol. 1989;13(Suppl. 5):S32–S35. doi: 10.1097/00005344-198900135-00009. [DOI] [PubMed] [Google Scholar]
- 38.Chen C., Wagoner P.K. Endothelin induces a nonselective cation current in vascular smooth muscle cells. Circ. Res. 1991;69:447–454. doi: 10.1161/01.res.69.2.447. [DOI] [PubMed] [Google Scholar]
- 39.Furutani H., Zhang X.F., Iwamuro Y. Ca2+ entry channels involved in contractions of rat aorta induced by endothelin-1, noradrenaline, and vasopressin. J. Cardiovasc. Pharmacol. 2002;40:265–276. doi: 10.1097/00005344-200208000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Drab M., Verkade P., Elger M. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 41.Wilson S.M., Mason H.S., Ng L.C. Role of basal extracellular Ca2+ entry during 5-HT-induced vasoconstriction of canine pulmonary arteries. Br. J. Pharmacol. 2005;144:252–264. doi: 10.1038/sj.bjp.0706077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villalba N., Stankevicius E., Garcia-Sacristan A., Simonsen U., Prieto D. Contribution of both Ca2+ entry and Ca2+ sensitization to the alpha1-adrenergic vasoconstriction of rat penile small arteries. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1157–H1169. doi: 10.1152/ajpheart.01034.2006. [DOI] [PubMed] [Google Scholar]
- 43.Leung F.P., Yung L.M., Yao X., Laher I., Huang Y. Store-operated calcium entry in vascular smooth muscle. Br. J. Pharmacol. 2008;153:846–857. doi: 10.1038/sj.bjp.0707455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhatnagar A., Sheffler D.J., Kroeze W.K., Compton-Toth B., Roth B.L. Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Galphaq-coupled protein receptors. J. Biol. Chem. 2004;279:34614–34623. doi: 10.1074/jbc.M404673200. [DOI] [PubMed] [Google Scholar]
- 45.Bergdahl A., Gomez M.F., Dreja K. Cholesterol depletion impairs vascular reactivity to endothelin-1 reducing store-operated Ca2+ entry dependent on TRPC-1. Circ. Res. 2003;93:839–847. doi: 10.1161/01.RES.0000100367.45446.A3. [DOI] [PubMed] [Google Scholar]
- 46.Enoki T., Miwa S., Sakamoto A. Long-lasting activation of cation current by low concentration of endothelin-1 in mouse fibroblasts and smooth muscle cells of rabbit aorta. Br. J. Pharmacol. 1995;115:479–485. doi: 10.1111/j.1476-5381.1995.tb16358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minowa T., Miwa S., Kobayashi S. Inhibitory effect of nitrovasodilators and cyclic GMP on ET-1-activated Ca(2+)-permeable nonselective cation channel in rat aortic smooth muscle cells. Br. J. Pharmacol. 1997;120:1536–1544. doi: 10.1038/sj.bjp.0701059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darblade B., Caillaud D., Poirot M. Alteration of plasmalemmal caveolae mimics endothelial dysfunction observed in atheromatous rabbit aorta. Cardiovasc. Res. 2001;50:566–576. doi: 10.1016/s0008-6363(01)00251-6. [DOI] [PubMed] [Google Scholar]
- 49.Bailey S.R., Mitra S., Flavahan S., Bergdall V.K., Flavahan N.A. In vivo endothelial denudation disrupts smooth muscle caveolae and differentially impairs agonist-induced constriction in small arteries. J. Cardiovasc. Pharmacol. 2007;49:183–190. doi: 10.1097/FJC.0b013e318031d5dd. [DOI] [PubMed] [Google Scholar]
- 50.Je H.D., Gallant C., Leavis P.C., Morgan K.G. Caveolin-1 regulates contractility in differentiated vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H91–H98. doi: 10.1152/ajpheart.00472.2003. [DOI] [PubMed] [Google Scholar]
- 51.Shaw L., Sweeney M.A., O’Neill S.C., Jones C.J., Austin C., Taggart M.J. Caveolae and sarcoplasmic reticular coupling in smooth muscle cells of pressurised arteries: the relevance for Ca2+ oscillations and tone. Cardiovasc. Res. 2006;69:825–835. doi: 10.1016/j.cardiores.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 52.Michel V., Bakovic M. Lipid rafts in health and disease. Biol. Cell. 2007;99:129–140. doi: 10.1042/BC20060051. [DOI] [PubMed] [Google Scholar]