Abstract
Electrical impedance myography (EIM) is a new non-invasive technique for the evaluation of neuromuscular disease that relies upon the application and measurement of high-frequency, low-intensity electrical current. EIM assesses disease-induced changes to muscle’s normal composition and architecture, including myocyte atrophy and loss, edema, reinnervation, and the deposition of endomysial connective tissue and fat. With application of single-frequency electrical current, EIM can be used to help grade the severity of neuromuscular disease. Assessing electrical impedance across a spectrum of applied frequencies and with current flow at multiple orientations relative to the major muscle fiber direction can provide a more complete picture of muscle condition. EIM holds the promise of serving as an indicator of disease status, thus being useful in clinical trials work and in monitoring effectiveness of treatment in individual patients; ultimately, it may also find diagnostic application. Ongoing efforts have been focused on obtaining a deeper understanding of the basic mechanisms of impedance change, studying EIM in a variety of clinical contexts, and further refining the methods of EIM data acquisition and analysis.
Keywords: Electrical impedance, muscle, neuromuscular disease, technique, anisotropy
Electrical impedance myography (EIM) is a non-invasive, painless approach to muscle assessment based upon the application and measurement of high-frequency, low-intensity electrical current (Figure 1). In contrast to conventional needle electromyography (EMG) and most standard neurophysiological techniques, EIM does not focus on measuring the inherent electrical activity of the tissues. Rather, similar to diagnostic ultrasound, measurements are made over a small area of interest, with energy being applied to the body and the resultant surface patterns analyzed. Unlike ultrasound, however, in which energy is in the form of sound waves and the main interest is image reconstruction, in the case of EIM, electrical current is used and the output is a set of quantitative parameters describing the state of the muscle, with presently little emphasis on imaging (though this remains possible). Although still in a relatively early stage of development, EIM may have important clinical uses in the future, both as an indicator of neuromuscular disease status and as a diagnostic tool.
Figure 1.
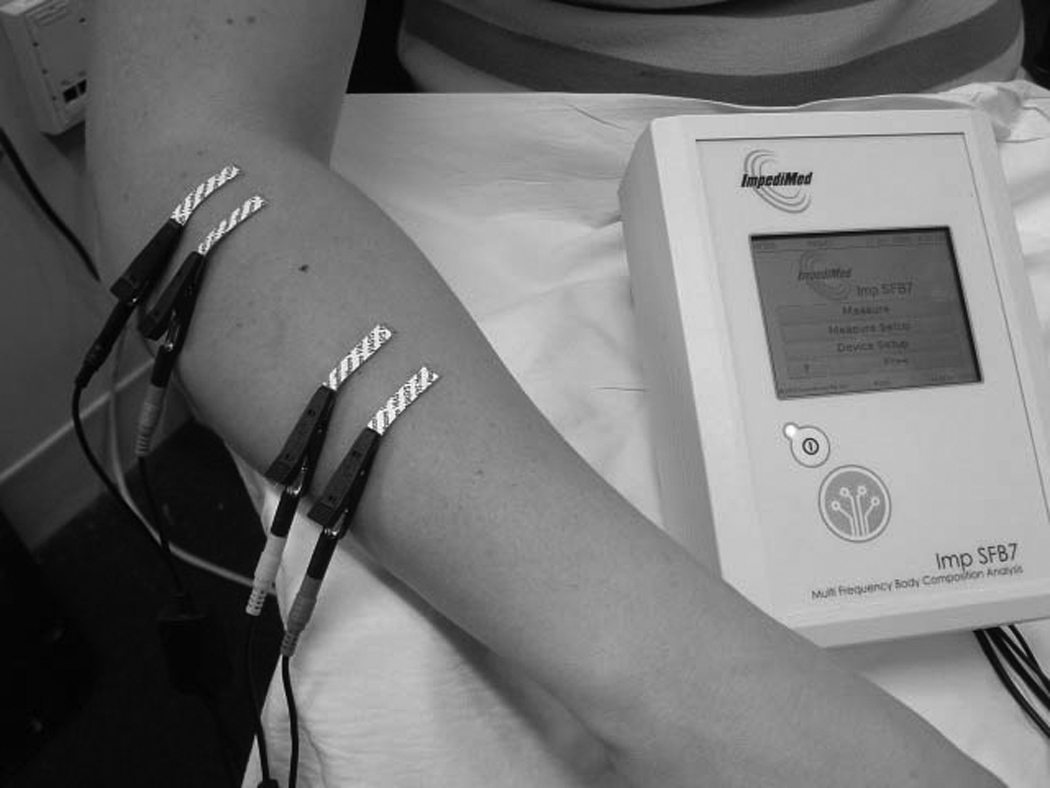
A basic approach to performing EIM measurements. In this figure, the two inner electrodes are for voltage measurement whereas the two outer ones inject the current. In other approaches, the current is injected further away, with electrodes placed on both hands and with the voltage electrodes in the same place. Each approach has certain advantages and disadvantages as discussed. The measurements here are being made with the ImpediMed Imp™ SFB7.
This review will begin with a brief history of bioelectricity in neuromuscular disease, exploring why electrical impedance measurements have been essentially overlooked as a potential means of muscle assessment. It will then detail the basic concepts underlying impedance measurements in general and the substantial challenges of applying them effectively to muscle. It will then review the data that we have collected over the past decade, the current state of the technology, the questions that remain as yet unanswered, and the future directions of research. Although EIM is still being refined, commercially available bioimpedance devices make it possible for any interested investigator to pursue clinical or basic research in this area.
A historical perspective on electricity in biomedical applications
By the late 19th and early 20th centuries, through the work of a number of investigators from Herman von Helmholtz to Edgar Adrian and Herbert Gasser, substantial progress had been made in understanding the basic character of neuronal and muscle depolarization.4 Ultimately, this culminated in the development of standard clinical neurophysiology, including nerve conduction studies and needle EMG by the mid 1940s, with additional contributions by many people, including Herbert Jasper, Fritz Bucthal, and James Golseth. As a result of these advances and eyeing a need to bring like-minded individuals together in helping establish its clinical value, James Golseth helped establish the American Association for Electromyography and Electrodiagnosis in 1951.
In parallel but in relative isolation to these developments, medical researchers in other areas of medicine were also interested in measuring electrical properties of tissues, but using electrical current to “probe” the tissue to reveal information about its integrity, density, and cellularity rather than recording its inherent electrical characteristics. For example, as early as 1901, King applied electrical current to patients with hypothyroidism, describing the disease as a low impedance state,22 and over the next two decades, electrical impedance measurements were performed on blood and other body fluids ex vivo to help characterize them in health and in disease. In 1926, it was demonstrated that electrical impedance could be used to detect a breast tumor15 and in the 1930s and 1940s impedance measurements were introduced for the study of blood flow, so called impedance plethysmography.26, 27 At about this time, impedance methods were also applied to nerve. Kenneth Cole and Howard Curtis in 1938 first described the change in conductivity in squid giant axon via impedance techniques,9 eventually leading to the Nobel-prize winning work of Hodgkin and Huxley the following decade. Kenneth Cole, working with his brother, Robert Cole, also helped researchers interpret impedance measurements in biological tissues by introducing simple circuit representations of the body and plots of reactance vs. resistance that continue to be utilized to this day (so-called Nyquist or “Cole-Cole plots”).8 By the 1950s and onward, the application of impedance methods in nutritional assessment, named simply “bioelectrical impedance analysis” (BIA), was established. And by the 1960s, researchers were measuring the impedance characteristics of various excised tissues.31 The past 3 decades have seen the establishment of BIA as a common method for nutritional assessment, including body fat analysis, as well as for lymphedema. A similar approach has also used in the grading of meat quality/fat content.23 More recently, other applications of bioimpedance have also been devised, including as a screening test for breast cancer (following up the work of the 1920s)46 and for the assessment of potentially malignant skin lesions.20 The technique of electrical impedance tomography, which is focused on image reconstruction, has also garnered considerable engineering interest over the past two decades and remains an intense area of research.5
Thus, during the past century these two forms of bioelectrical assessment, impedance analysis and standard clinical neurophysiology, have developed in relative isolation from one another, with the former being applied mostly to non-excitable tissues and the later to excitable ones. Surprisingly, outside the work of Curtis and Cole and a few others, there has been little effort to bridge this gap, perhaps in part because standard clinical neurophysiological testing works well and because it became clinically entrenched relatively early. Moreover, investigators and clinicians in neurology are naturally and reasonably drawn to the nervous system’s most unique feature: its ability to generate action potentials, something that impedance measurements for the most part ignore, being focused on tissue composition and architecture. One notable exception to this rule, however, is the pioneering work of David Holder and colleagues who have attempted to utilize electrical impedance methods to non-invasively identify action potentials in the brain.3
Electrical Impedance
All impedance methods rely upon the basic principle that if an alternating current is applied to a substance, energy will be dissipated as it travels through it, thus producing a measurable voltage. Moreover, the timing of the oscillations in the measured voltage will be out of phase with those of applied current. A variety of methods have been developed for the measurement of electrical impedance, such as the Wheatstone bridge, each with certain advantages and disadvantages depending on the frequency range of interest. Electrical impedance methods can be applied to virtually any substance or material, and thus have found widespread use in disciplines as disparate as medicine, metallurgy, geology, and forestry.
Figure 2A helps to illustrate how impedance measurements work. An alternating electrical current of known frequency, amplitude and timing (i.e., the waveform crosses the x-axis at a specific time), is applied to a substance. As the electrical current travels through the substance, it loses energy (due to the substance’s inherent resistance), thus reducing its amplitude. Moreover, the timing of the resulting voltage alternations is slightly delayed, with its no longer crossing the x-axis at the expected time, due to the substance’s inherent capacitive and inductive characteristics.
Figure 2.
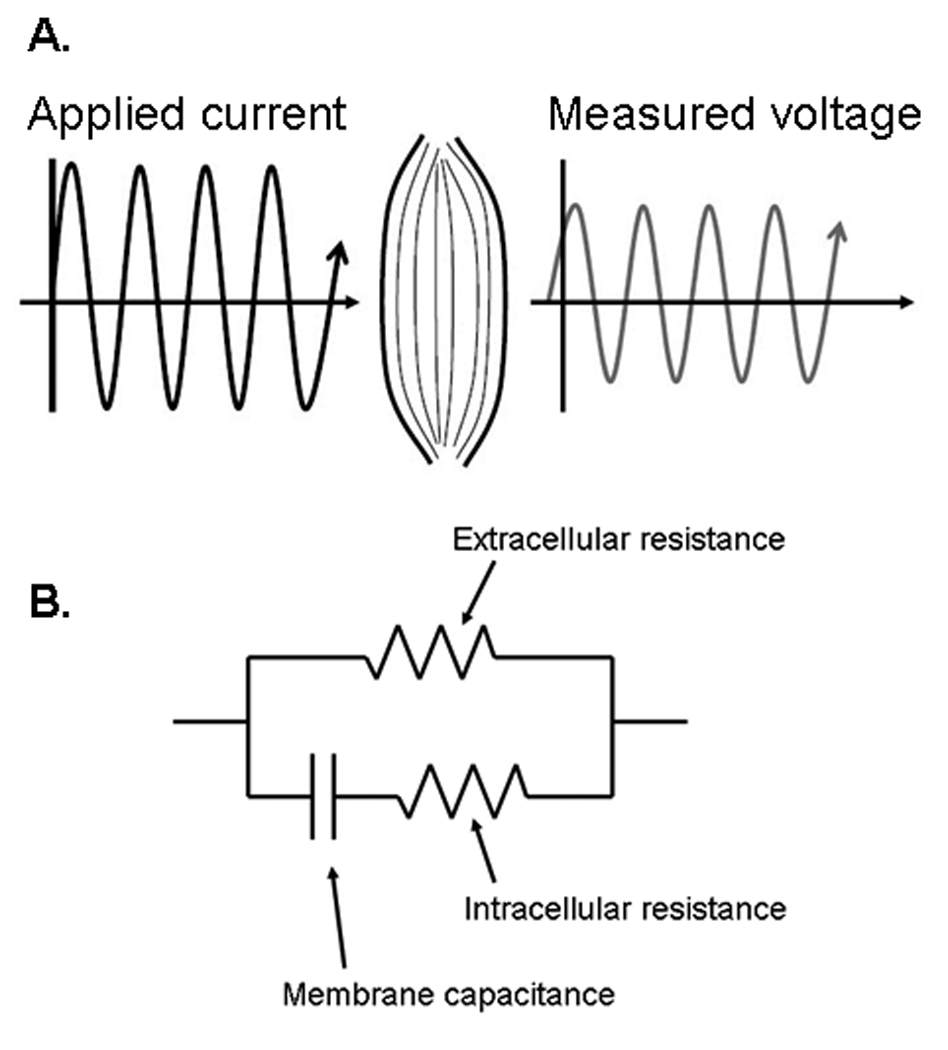
A. Basic concept of electrical impedance testing. Electrical current of a known frequency, amplitude is injected into a tissue. The tissue impacts the applied electrical current, reducing the measured voltage’s amplitude, secondary to the tissue’s resistance and slightly altering its timing, secondary to the tissue’s capacitance. The changes in electrical current flow reflect the properties of the tissue and thus can reveal information about tissue health or pathology. B. The “standard” basic equivalent circuit of bioelectrical impedance. The capacitor represents the cell membranes (in the case of muscle the sarcolemma) and the resistors the extra- and intra-cellular resistance. Although simplistic, this circuit diagram provides a basic handle with which to understand why changes in cellular structure may result in changes in the measured impedance.
Impedance can also be described mathematically. Ohm’s law states:
V, representing voltage; I, current flow, and R, the resistance; but this equation only describes a pure resistive circuit, without capacitors or inductors. For a more complex circuit (Figure 2) that includes such elements, the more equation becomes:
where Z represents the complex impedance of the circuit, a combination of its inherent resistance and its reactance (X), the latter due to the obstruction to current flow produced by the presence of capacitors and/or inductors in the circuit. There are thus two types of reactance: capacitive reactance (XC) and inductive reactance (XL). Together Z is a vector, expressed as a complex number (Z = R + jX) , composed of its resistance and its two forms of reactance in combination:
Fortunately, in medical applications this equation can be simplified by ignoring the XL term, since inductance is believed to play a minimal role in standard bioimpedance measurements, and the XC term can be simply written as X. Whereas the above equation can be applied to direct current circuits, it is most applicable to circuits utilizing alternating current, since in direct current circuits, current will simply stop flowing across the capacitor once it is fully charged; however, in alternating current circuits, current will flow indefinitely since it is constantly shifting directions. We can also calculate what is called the phase angle (θ) via standard trigonometric relationships by the formula θ = arctan (X/R). Thus, when discussing bioimpedance measurements, one of several values can be assessed: the raw resistance (R), reactance (X), both of which are measured in Ohms, or their combination, either as the impedance (Z) (also measured in Ohms) or as the phase angle (θ), measured in degrees. In practice, R is considerably larger than the X and the phase angle is relatively small (no more than about 15 degrees depending on the specific application). Moreover, the presence of a capacitor in the circuit confers an important frequency dependence that can also be described mathematically (for a full discussion of this topic see any of several basic physics textbooks or a multitude of web resources).
Basics of Bioimpedance
Essentially everything that lies under and between the electrodes contributes to the measured impedance of biological tissue. Saline alone is highly conductive. With the addition of extracellular matrices (e.g. proteoglycans, etc), the resistance and capacitance of the solution increase. When cells are added, their lipid bilayers serve as additional capacitors that will store and release charge with the reversing current flow. Intracellular components also contribute.
Figure 2B provides a simple circuit diagram that helps illustrate the basic concept of biologic impedance. It describes tissues as having two resistances, an intracellular and an extracellular, and a capacitor, consisting of the lipid bilayer making up the cell membranes. Such a circuit would be anticipated to behave differently depending upon the type and frequency of current injected. For example, with direct current application, current will initially flow through all 3 elements, until the capacitor is fully charged at which point the current will continue flow only through the extracellular resistor. With alternating current application at higher frequencies, current will flow increasingly through the intracellular resistive components that could not be accessed at lower frequencies, since the capacitor will become “invisible” to the current flow. This concept underlies a proposed 5-element circuit model, in which an additional resistor and capacitor are added in parallel, representing current flow through organelles within a cell.40
There are two basic approaches to measuring impedance: a 2-electrode approach and a 4-electrode approach. When using a 2-electrode approach, the voltage is measured across the entire circuit (Figure 3a). When using a 4-electrode approach, the voltage is measured across only a specific set of elements of the circuit of interest (Figure 3b). Most bioimpedance applications use this 4-electrode or tetrapolar approach, since, it can help isolate an area of interest and provide some protection against effects of electrode polarization.19 However, such measurements introduce other unknowns, including lack of knowledge of current paths prior to the current entering the region of interest; furthermore, they require a sensitive and accurate detector, since a preponderance of energy may be dissipated in regions outside the area of interest being evaluated by the voltage electrodes.19
Figure 3.
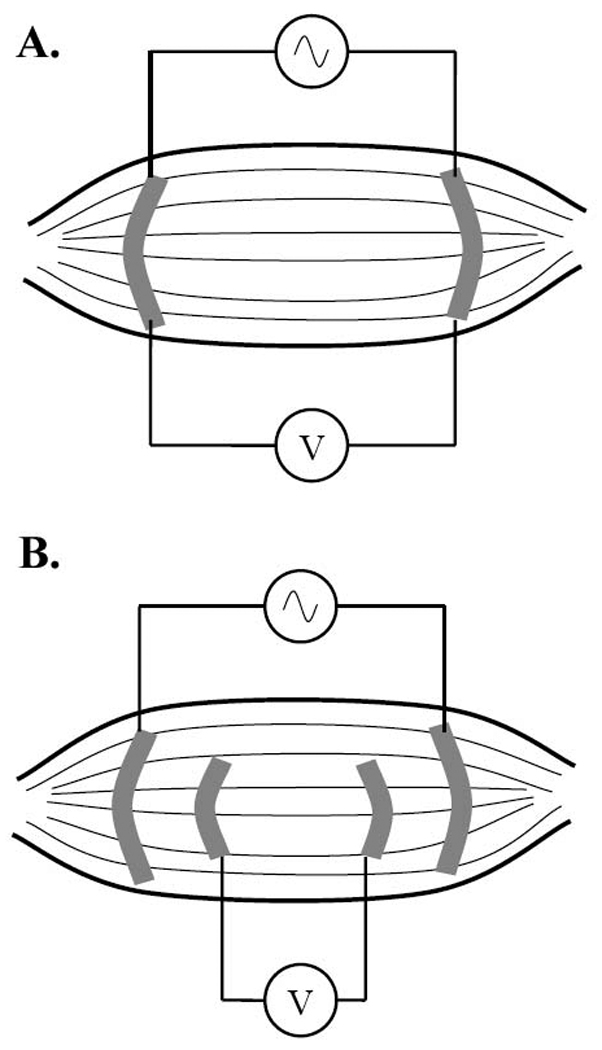
Two- versus four-electrode impedance methods. In the two-electrode approach (A.), current is injected at the same location where the intervening voltage is measured. In the four-electrode approach (B.), current is injected separately (see Figure 1).
Why should impedance measurements be useful in assessing tissue health? The simple answer is that changes in the composition and architecture of the tissue will affect the impedance values and their frequency dependences. For example, adding saline to a static tissue mass will lower the impedance of the system since there will be additional extracellular fluid through which the current can flow—such would be the case in an edematous state and explain the early findings of King in 190122 showing that the impedance of the body drops in hypothyroidism as edema develops. However, for a relatively constant amount of extracellular fluid, the amount of muscle mass as compared to fat begins to play a more important role, thus introducing the idea of using impedance methods for assessing body composition. Fat conducts electrical current poorly; lean muscle mass is far more effective. Thus, empirical relationships between height, weight, and impedance parameters can be established and have served as the basis of nutritional BIA.24 Similarly, for breast tumor detection, the dense cellular structure of the tumor has different impedance characteristics than that of the surrounding normal tissue.21 Clearly, other changes in the composition or structure of the tissue can impact the impedance signature.
Impedance measurements in muscle
The first reports of impedance being performed specifically on skeletal muscle date back to the late 1950s and early 1960s when Rush and others attempted to measure the impedance characteristics of a variety of body tissues.6, 14, 30, 31, 36 These measurements were typically performed directly on the excised tissue of freshly sacrificed animals, and the inherent electrical characteristics of the tissue, including its conductivity, were calculated. Epstein and Foster in 1983 provided additional measurements, including that of muscle permititivity, a measure of its capacitative characteristics .11
Skeletal muscle also possesses a relatively unique feature among tissues: its strong anisotropy, or directional dependence to current flow.30 A suspension of spherical cells—a truly isotropic medium--has no directional dependence, and applied current would be expected to flow similarly in any direction. However, because individual myocytes are cylindrical, current flows much more readily along than across them, giving different impedance values depending on the direction and measurement of the applied current. These directional dependences in current flow were identified by the early investigators, and separate conductivities for muscle were reported for measurements made longitudinally to (parallel) and transversely to (perpendicular) the major muscle fiber direction.16, 17, 29, 42
In the 1990s, two physicists at Northeastern University, Carl Shiffman and Ronald Aaron, continued this line of investigation by studying the characteristics of electrical current flow through muscles of healthy individuals using surface electrodes. They identified that electrical current flow through the thigh muscles could be effectively modeled,37 that anisotropy could be detected through the skin,2 and that electrode size and interelectrode distance substantially influenced impedance measurements.39 After coming upon their work in 1999, I suggested the idea of employing electrical impedance methods specifically for the non-invasive assessment of neuromuscular disease; we began to collaborate shortly thereafter.
General approaches to performing impedance measurement on muscle
Our initial method of muscle assessment and one that we continue to use in some clinical contexts is what we initially referred to as linear-EIM. In this technique, the current electrodes are placed on the hands or feet and a series of voltage electrodes (from 2 to 10 in number) is placed over a region of interest (what we refer to as our “far-current electrode” montage—see references 31 and 42 for examples). One major advantage of this approach is that by applying current as far as possible away from the electrode array, we can be relatively certain that the electrical current is flowing parallel to the long axis of the limb. The disadvantages of this approach are that it reduces muscle specificity, it is susceptible to variation in joint angle, is difficult to implement for distal muscles, and cannot be easily used if a joint replacement is present nearby, since it will markedly distort electrical current flow in the region of the muscle. Such an approach, however, has one other important advantage: it reduces substantially the contribution to measurements from the skin or subcutaneous fat,44 as current can be assumed to be flowing through the low-resistance muscles, with likely less than 1% of the electrical current traveling through the overlying skin-fat (the actual value is dependent on both the inherent resistivity of fat, which is about 10-fold greater than healthy muscle,13 and the relative volume of fat compared to muscle). If using more than 2 recording voltage electrodes, we measure the voltages across each pair and then calculate an average value for the entire muscle. In general, the electrical currents utilized for most of these EIM measurements are on the order of no more than a few milliamperes.
In contrast, we can also use a “near-current electrode” montage (Figure 1), in which the current electrodes are brought into close proximity with the muscle of interest. The anisotropy of the muscle can also be assessed by using this approach since the direction of applied current flow can be varied along with the voltage electrodes, by rotating the four-electrode set around a center point over the muscle of interest (Figure 4), and thus providing a detailed assessment of the impedance characteristics of the muscle in different directions.7 The one major disadvantage to this approach is the effect of the skin-fat layer the current must traverse. The skin and fat will cause the current to disperse to some extent before it reaches the muscle, making measurements of the muscle itself potentially more challenging, especially if there is excessive overlying tissue.
Figure 4.
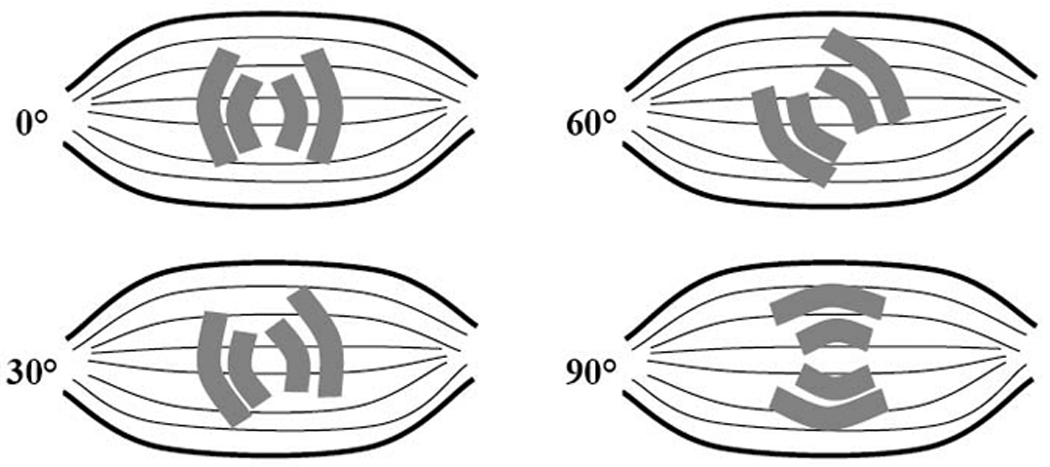
The basic concept of rotational EIM. The entire electrode array is rotated around a central point, allowing the anisotropy (directional dependence of current flow) of the muscle to be studied.
Single frequency EIM studies
Our initial focus was on using EIM to identify basic differences between impedance values in muscles of healthy individuals and those with generalized neuromuscular diseases, and our earliest system used single frequency current application at 50 kHz with an array of voltage electrodes over the muscle(s) of interest. 50 kHz is commonly used for most bioimpedance work since biological tissue tends to be most reactive at or near this frequency and most commercially available devices emit electrical current at only this one frequency. We were able to show major differences in the raw resistance and reactance values and the phase (averaged over the series of electrodes) in a group of neuromuscular disease subjects as compared to data from a limited group of normal subjects.32 The explanation for these differences is that the common pathological accompaniments of neuromuscular disease, such as muscle fiber atrophy, increased endomysial connective tissue, fat, and edema, all substantially alter the tissue’s impedance (this is discussed in detail below in the section entitled, “Mechanisms of impedance change in neuromuscular disease”). Building on this data, we also explored the potential for EIM as a measure of disease severity in myositis, showing that there was a good relationship between EIM measures and strength.43
That first study also included several patients with amyotrophic lateral sclerosis (ALS), one of whom we had the opportunity to study on repeated occasions. Interestingly, this individual demonstrated substantial declines in his measured phase from muscles that were not yet clinically affected, suggesting that the technique might be useful as a new outcome measure/biomarker of disease progression. Thus, we continued to collect EIM data on a group of 15 ALS patients longitudinally. Using this data, we performed a power analysis as if planning a drug trial in which 100 individuals received drug and 100 individuals received placebo, with study visits every 2 months for 1 year. Incorporating historical data from the “Celecoxib in ALS study”10 for standard measures such as quantitative muscle strength testing and ALS-functional rating scale (revised), EIM appeared potentially superior compared to these other measures, showing a 73% power to detect to 10% treatment effect, as compared to these other two measures which each had a 23% power to detect the same treatment effect of 10%.35
Although much of our focus has been on generalized disorders, single-frequency EIM may also be useful in evaluating more localized conditions. Utilizing EIM in a group of patients with unilateral radiculopathy, we demonstrated differences in the 50 kHz data between the two sides,33 with lower phase values on the affected side, usually specifically over the muscles demonstrating the most substantial clinical abnormalities. Other ongoing work also suggests that neurogenic change in muscles as small as abductor pollicis brevis may also be detectable, thus potentially reducing the need for needle electromyography in the diagnosis of carpal tunnel syndrome [unpublished observations]. However, localized conditions also present a clear challenge since electrical current flow cannot provide the muscle-specific accuracy possible with a needle. Deep muscles or muscles beneath thick subcutaneous fat (e.g., tibialis posterior or the glutei, respectively) may be especially difficult to evaluate wth EIM. While it remains theoretically possible to assess such regions, the technical challenges are substantial.
We have also evaluated 50 kHz EIM data across a large cohort of normal subjects and have identified substantial changes with advancing age1 and have recently shown that normal reference values can be easily generated using standard statistical approaches.34 Finally, we have had the opportunity to study 4 elderly subjects included in this cohort twice with several years between measurements and found that each experienced a substantial decline in phase during that period, paralleling the trend observed in the study population as a whole.1
Multifrequency measurements
EIM data can also be obtained using a range of applied current frequencies rather than a single one. Although most inexpensive commercial devices will only supply a single frequency of current at 50 kHz, at least one commercial multifrequency device is available (the ImpediMed Imp™ SFB7 from Impedimed, Inc), capable of applying current simultaneously at a multitude of frequencies from 2 kHz to 1MHz. Commercial impedance analyzers for non-medical applications can also be utilized for this purpose (an approach that we have also taken). It might be anticipated that probing muscle tissue with a spectrum of frequencies rather than just a single one would provide a richer assessment of the tissue. Indeed, we have found that the entire spectrum changes in diseased states.12 Figure 5 shows a comparison between the resistance, reactance, and phase vs. frequency in a healthy individual of 55 years and a similarly aged patient with inclusion body myositis (IBM). As can be seen, in the normal subject, the phase and reactance peaks near 50 kHz and gradually decreases at higher frequencies. In comparison, the patient with IBM shows a relatively low phase at 50 kHz and a prominent increase in phase at higher frequencies. As noted earlier, healthy tissue is most reactive at approximately 50 kHz. Changes in the cellular structure with disease, however, creates a shift in peak phase to much higher frequencies; however, measurement of electrical impedance at frequencies much above 1 MHz becomes increasingly challenging for technical reasons, and thus identifying a true peak is not a trivial matter. Of note, the absolute value of the 50 kHz phase is much lower in the diseased as compared to the healthy muscle, consistent with our earlier observations made with the application of a 50 kHz frequency alone. Also, the resistance is elevated; part of this change is purely geometrical—decreasing muscle volume means greater difficulty transmitting current through the tissue and hence a higher internal resistance to the current flow. However, part of this also likely reflects loss of intra- and extracellular water as the muscle fibers are replaced with adipose and connective tissue.
Figure 5.
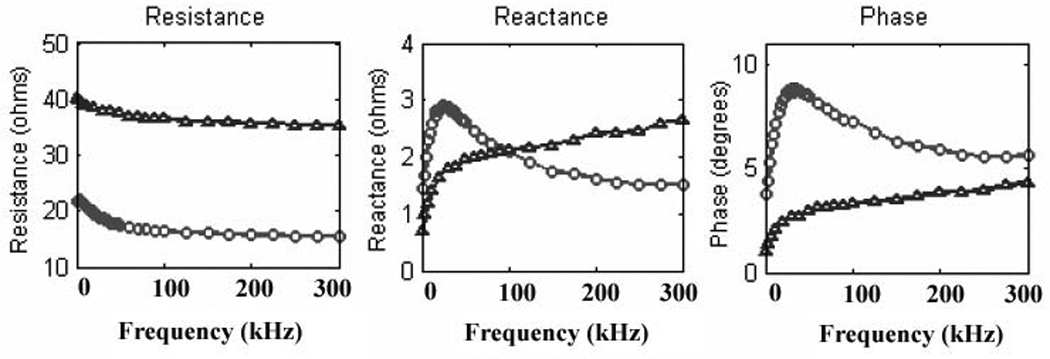
Archetypal changes in the multifrequency signature in normal muscle (circles) versus diseased, in this case inclusion body myositis (triangles). The normal low-frequency peak is lost, resistance increases and reactance decreases.
Although we can evaluate this multifrequency data by selecting and studying discrete frequencies within the spectrum, we are still seeking summary measures that effectively describe the entire frequency dependence of the tissue, such as the slope of phase vs. frequency.45 Approaches may also include log transformations of the resistance spectrum41 or possibly derivatives of the higher order polynomial phase and reactance curves. It may also be that the multifrequency spectrum will become even more important when taking into consideration measurement of the muscle anisotropy and changes during the contracted state.7
Rotational measurements
In rotational EIM we focus on assessing the muscle’s anisotropy and utilize the “near-current electrode montage,” placing the current electrodes in close proximity to the voltage electrodes. This allows us to effectively control the directional flow and measurement relative to the muscle fiber direction. By rotating the entire group of 4 electrodes simultaneously, we are able to interrogate the structure of the muscle at a variety of angles (Figure 4). At the present time, we are applying current at 5 angles, starting at 90° (perpendicular to the muscle fibers), 60°, 30°, 0° (i.e., parallel to the muscle fibers), −30°, and −60°.18 We also perform the same measurement at −90°, which is identical to the one obtained at 90° except that the electrodes are reversed. Again, we typically do this at multiple frequencies of current in order to obtain as complete an impedance picture of the muscle as possible.7
Once the rotation is completed, the raw impedance data (in the form of phase, resistance, and reactance) are plotted against the angle of applied current. Similar to our standard measurements using far-current electrodes, for any single angle of study (e.g., 0°, 30°, or 60°), disease can markedly impact the multifrequency data, with a reduced or absent peak in reactance at 50 kHz and elevations in values at higher frequencies. However, to simplify the study of anisotropy, we usually select just a single frequency, taking the individual angle data and normalizing it against the average angle data at that frequency and plotting it as the normalized anisotropy (Figure 6A). Through this means, we are able to demonstrate very consistent anisotropic patterns in healthy people at a given frequency, confirming the idea that reactance, and resistance to a lesser extent, appears to be highest when current flow is perpendicular to the muscle fibers (at 90° and −90°) and lowest when it is parallel (at 0°). Theoretical and empirical work, does demonstrate however, that the exact character of this relationship will be highly dependent on the relative size of these electrodes.7 Nonetheless, we have recently been able to show differences between normal muscle, denervated/reinnervated muscle, and myopathic muscle using this approach (Figure 6 B,C),18 and anticipate that with further refinement, such an approach may ultimately be practical for clinical neuromuscular disease assessment.
Figure 6.
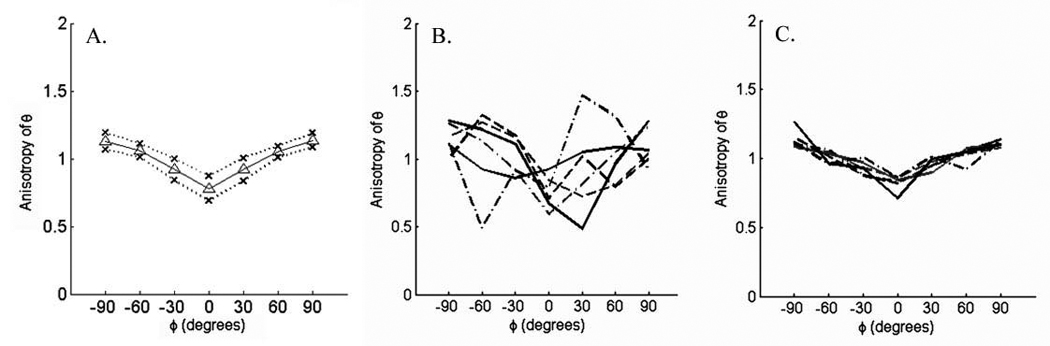
Anisotropy measurements on muscle using the approach outlined in Figure 4. A. Normalized data from a group of 15 normal subjects recording tibialis anterior; mean values +/− 1 standard deviation. B. Normalized data from a group of 7 patients with myopathy clinically affecting tibialis anterior; C. Normalized data from a group of 7 patients with ALS clinically affecting tibialis anterior.
Mechanisms of impedance change in neuromuscular disease
As suggested, several mechanisms are likely to account for changes in the impedance values in neuromuscular disease states. The simplest and most obvious explanation is that atrophy and loss of muscle fibers causes the basic “circuitry” of the muscle to change. With atrophy, resistance values will increase and reactance values will decrease. The elevation in resistance is partly due to the fact that the muscle itself is getting smaller, though intra- and extra-cellular fluid changes may also impact this. Changes in muscle composition also will play a role, with localized edema of muscle or increased fatty infiltration or connective tissue also impacting the measured electrical impedance. Why and how these changes should affect the multifrequency spectrum, however, remains incompletely elucidated. Thus, although simple atrophy likely plays an important role in the changes in signature in disease, it is likely only one piece of the puzzle; for example, disuse states show a very different picture than atrophy due to myopathy or neurogenic change, with relatively reduced resistance and a better preservation of the frequency relationships [unpublished results].
Another mechanistic effect worthy of further study is the change in anisotropy obtained in rotational EIM in different diseases. As noted above, we have recently observed that the anisotropic pattern in patients with neurogenic disease appear distinct from those with myopathic disease.18 Why this should be the case is uncertain, but with myopathy, inflammatory cells, fatty infiltration, and fibrosis all likely contribute to reductions in anisotropy (e.g., inflammatory cells are round and thus essentially isotropic); the muscle fibers themselves may become fragmented or entirely lost as well, contributing to this change. In comparison, in patients with neurogenic disease, we can observe increased normalized anisotropy, with minima at angles other than −90° (see Figure 6B) perhaps indicating distorted low impedance paths created by chronic reinnervation and type grouping. This work is still in its early stages, and considerable additional investigation will be needed to help fully understand these complex relationships.
Unanswered questions and future directions
A variety of questions about EIM remains unanswered and demand further study. These include: 1. the detailed mechanisms of how disease impacts the EIM signature and how it relates to standard EMG; 2. the effects of the skin-subcutaneous fat on the impedance measurements when “near-electrode” or rotational measurements are performed and how to account effectively for them; 3. the means of identifying individual muscles while using EIM; 4. the detailed effects of muscle excitation and contraction on the EIM signature. Although we have studied this last question to some extent,38 impedance changes with contraction are generally quite small (on the order of only a few per cent of the total impedance) and can vary considerably depending upon the exact location of the recording electrodes, making this work especially challenging. Still, the changes are readily detectable (Figure 7) and may produce fruitful future study.
Figure 7.
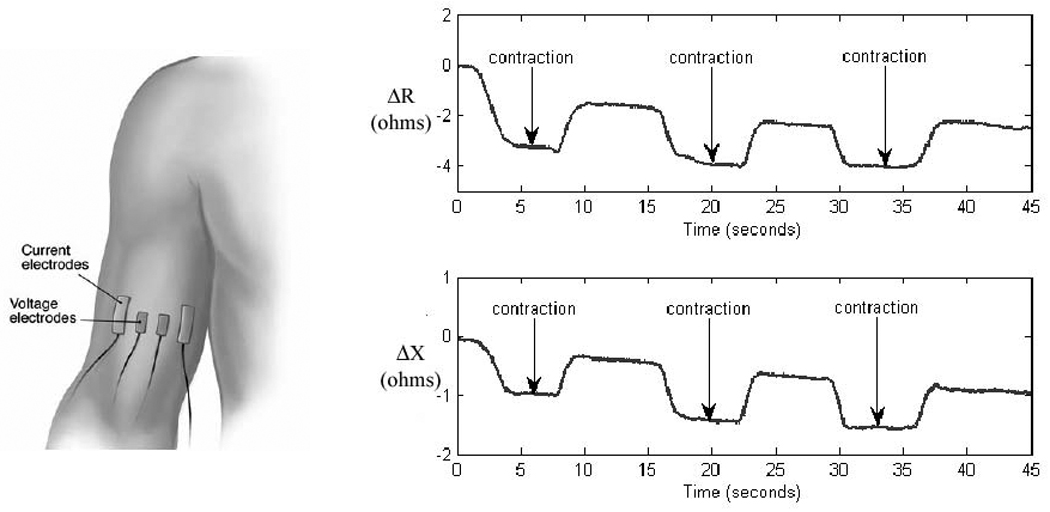
Example of dynamic-EIM being performed on biceps and the data obtained.. The time course in the changes observed appear to be due to changes in muscle fiber morphology with contraction and not clearly due to the firing of motor unit potentials per se.
Another point that deserves further investigation is the relationship between EIM and other methods of evaluating muscle beside EMG, such as ultrasound and magnetic resonance imaging (MRI). In certain respects, EIM has a closer relationship to both of these modalities than to EMG since, in all three techniques, energy is introduced into the body and its effects analyzed. Moreover, all 3 modalities are focused on muscle composition and structure rather than its electrical activity. MRI, ultrasound and EIM also share a sensitivity to increased intra- and extra-cellular water and fatty replacement of muscle. Unlike either MRI and ultrasound, EIM has the potential ability to focus more readily on muscle fiber organization and structure, via measurements of anisotropy. And like ultrasound (and EMG), EIM can be used flexibly at the bedside to study muscles as demanded by the examination and ongoing data analysis.
Our current efforts are moving in three separate directions. First, we recognize the critical importance of establishing a stronger theoretical and empirical understanding of the impedance data in normal and neuromuscular disease states. Accordingly, we have developed a full-fledged rodent research program,25 applying EIM to the rat gastrocnemius-soleus complex, with the intent of studying basic models of neuromuscular disease, including sciatic crush to assess neurogenic change, inflammatory myositis to assess the effects of primary muscle disease, and hind limb suspension to induce disuse, and their consequent effects on the observed impedance pattern. We are also specifically seeking impedance patterns that may help distinguish active from chronic neurogenic change. Additionally, we have also begun applying EIM to the mouse hind limb, which is considerably more challenging given its small size, but opens up the possibility of performing studies in a variety of transgenic and other disease models. As part of all this rodent work, we are using sophisticated methods to analyze current flow through muscle (via finite element analysis) and correlating our EIM data to quantified muscle pathology.
Second, we continue to employ EIM in the clinical setting, in a variety of disease states and are currently studying changes induced in hand muscles with localized nerve disease, changes with disuse after orthopedic injury, as well as ongoing studies in amyotrophic lateral sclerosis and spinal muscular atrophy. More recently, we have also begun to explore the use of quantifying dystonic muscle hypertrophy through the use of EIM. Of course, as the EIM techniques and their associated analytic approaches become further refined, it will be critical to compare EIM to other standard techniques for muscle assessment to determine its specificity and sensitivity to disease status.
Third, recognizing the limitations created by an absence of devices specifically geared toward muscle impedance measurements, we are working with a group of engineers to develop a robust, portable EIM system that can be applied readily at the bedside for rapid data acquisition.28 The expectation is that such a system will further improve the speed, repeatability and sensitivity of our measurements, while simultaneously allowing us to achieve rotational measurements with far greater angular resolution and precision than is currently possible. Our hope remains that that with further study and development, EIM will begin to serve a useful role in the diagnosis and subsequent management of patients with a variety of neuromuscular illnesses in the not too distant future.
Acknowledgments
The author would like to especially thank Carl Shiffman, Ronald Aaron, as well as Joel Dawson, Andrew Tarulli, Gregory Esper, Elizabeth Raynor, and Rachel Nardin for their help and encouragement with this work. This research was funded in part by grant R01 NS042037 from the National Institutes of Health.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- BIA
bioelectrical impedance analysis
- EIM
electrical impedance myography
- MRI
magnetic resonance imaging
References
- 1.Aaron R, Esper GJ, Shiffman CA, Bradonjic K, Lee KS, Rutkove SB. Effects of age on muscle as measured by electrical impedance myography. Physiol Meas. 2006;27:953–959. doi: 10.1088/0967-3334/27/10/002. [DOI] [PubMed] [Google Scholar]
- 2.Aaron R, Huang M, Shiffman CA. Anisotropy of human muscle via non-invasive impedance measurements. Phys Med Biol. 1997;42:1245–1262. doi: 10.1088/0031-9155/42/7/002. [DOI] [PubMed] [Google Scholar]
- 3.Ahadzi GM, Liston AD, Bayford RH, Holder DS. Neuromagnetic field strength outside the human head due to impedance changes from neuronal depolarization. Physiol Meas. 2004;25:365–378. doi: 10.1088/0967-3334/25/1/040. [DOI] [PubMed] [Google Scholar]
- 4.Bonner FJ, Jr, Devleschoward AB. AAEM minimonograph #45: the early development of electromyography. Muscle Nerve. 1995;18:825–833. doi: 10.1002/mus.880180805. [DOI] [PubMed] [Google Scholar]
- 5.Brown BH. Electrical impedance tomography (EIT): a review. J Med Eng Technol. 2003;27:97–108. doi: 10.1080/0309190021000059687. [DOI] [PubMed] [Google Scholar]
- 6.Burger HC, van Dongen R. Specific electrical resistance of body tissues. Phys Med Biol. 1961;5:431–447. doi: 10.1088/0031-9155/5/4/304. [DOI] [PubMed] [Google Scholar]
- 7.Chin AB, Garmirian LP, Nie R, Rutkove SB. Optimizing measurement of the electrical anisotropy of muscle. Muscle Nerve. 2008 doi: 10.1002/mus.20981. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole KS, Cole RH. Dispersion and absorption in dielectrics. I. Alternating current characteristics. J Chem Phys. 1941;9:341–351. [Google Scholar]
- 9.Cole KS, Curtis HJ. Electric imepdance of the squid giant axon during activity. Journal of General Physiology. 1939;22:649–670. doi: 10.1085/jgp.22.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cudkowicz ME, Shefner JM, Schoenfeld DA, Zhang H, Andreasson KI, Rothstein JD, Drachman DB. Trial of celecoxib in amyotrophic lateral sclerosis. Ann Neurol. 2006;60:22–31. doi: 10.1002/ana.20903. [DOI] [PubMed] [Google Scholar]
- 11.Epstein BR, Foster KR. Anisotropy in the dielectric properties of skeletal muscle. Med Biol Eng Comput. 1983;21:51–55. doi: 10.1007/BF02446406. [DOI] [PubMed] [Google Scholar]
- 12.Esper GJ, Shiffman CA, Aaron R, Lee KS, Rutkove SB. Assessing neuromuscular disease with multifrequency electrical impedance myography. Muscle Nerve. 2006;34:595–602. doi: 10.1002/mus.20626. [DOI] [PubMed] [Google Scholar]
- 13.Faes TJ, van der Meij HA, de Munck JC, Heethaar RM. The electric resistivity of human tissues (100 Hz-10 MHz): a meta-analysis of review studies. Physiol Meas. 1999;20:R1–R10. doi: 10.1088/0967-3334/20/4/201. [DOI] [PubMed] [Google Scholar]
- 14.Fatt P. An analysis of the transverse electrical impedance of striated muscle. Proc R Soc Lond B Biol Sci. 1964;159:606–651. doi: 10.1098/rspb.1964.0023. [DOI] [PubMed] [Google Scholar]
- 15.Fricke H, Morse S. The electric capacity of tumors of the breast. Cancer Research. 1926;16:340–376. [Google Scholar]
- 16.Gabriel C, Gabriel S, Corthout E. The dielectric properties of biological tissues: I. Literature survey. Phys Med Biol. 1996;41:2231–2249. doi: 10.1088/0031-9155/41/11/001. [DOI] [PubMed] [Google Scholar]
- 17.Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys Med Biol. 1996;41:2251–2269. doi: 10.1088/0031-9155/41/11/002. [DOI] [PubMed] [Google Scholar]
- 18.Garmirian LP, Chin AB, Rutkove SB. Discriminating Neurogenic from Myopathic Disease via Measurement of Muscle Anisotropy. Muscle Nerve. 2008 doi: 10.1002/mus.21115. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimnes S, Martinsen OG. Sources of error in tetrapolar impedance measurements on biomaterials and other ionic conductors. J Phys D: Appl Phys. 2007;40:9–14. [Google Scholar]
- 20.Har-Shai Y, Glickman YA, Siller G, McLeod R, Topaz M, Howe C, Ginzburg A, Zamir B, Filo O, Kenan G, Ullmann Y. Electrical impedance scanning for melanoma diagnosis: a validation study. Plast Reconstr Surg. 2005;116:782–790. doi: 10.1097/01.prs.0000176258.52201.22. [DOI] [PubMed] [Google Scholar]
- 21.Jossinet J. The impedivity of freshly excised human breast tissue. Physiol Meas. 1998;19:61–75. doi: 10.1088/0967-3334/19/1/006. [DOI] [PubMed] [Google Scholar]
- 22.King W. Electricity in Medicine and Surgery. New York: Boericke & Runyon; 1901. [Google Scholar]
- 23.Marchello MJ, Slanger WD. Use of bioelectrical impedance to predict leanness of Boston butts. J Anim Sci. 1992;70:3443–3450. doi: 10.2527/1992.70113443x. [DOI] [PubMed] [Google Scholar]
- 24.Mattsson S, Thomas BJ. Development of methods for body composition studies. Phys Med Biol. 2006;51:R203–R228. doi: 10.1088/0031-9155/51/13/R13. [DOI] [PubMed] [Google Scholar]
- 25.Nie R, Chin AB, Lee KS, Sunmonu NA, Rutkove SB. Electrical impedance myography: transitioning from human to animal studies. Clin Neurophys. 2006;117:1844–1849. doi: 10.1016/j.clinph.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 26.Nyboer J. Electrical impedance plethysmography; a physical and physiologic approach to peripheral vascular study. Circulation. 1950;2:811–821. doi: 10.1161/01.cir.2.6.811. [DOI] [PubMed] [Google Scholar]
- 27.Nyboer J. Plethysmography. In: Glasser O, editor. Impedance in Medical Physics. Chicago: Year Book Publishing; 1950. pp. 736–743. [Google Scholar]
- 28.Ogunnika OT, Scharfstein M, Cooper R, Ma H, Dawson JL, Rutkove SB. A Handheld Electrical Impedance Myography Probe for the Assessment of Neuromuscular Disease. In: Paolo Vicini JP, editor. 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; Vancouver, BC. 2008. Vol. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prokhorov E, Llamas F, Morales-Sanchez E, Gonzalez-Hernandez J, Prokhorov A. In vivo impedance measurements on nerves and surrounding skeletal muscles in rats and human body. Med Biol Eng Comput. 2002;40:323–326. doi: 10.1007/BF02344214. [DOI] [PubMed] [Google Scholar]
- 30.Rush S. Methods of measuring the resistivities of anisotropic conducting media J. Res. J Res NBS. 1962;66C:217–222. [Google Scholar]
- 31.Rush S, Abildskov J, McFee R. Resistivity of body tissues at low frequencies. Circ Res. 1963;12:40–50. doi: 10.1161/01.res.12.1.40. [DOI] [PubMed] [Google Scholar]
- 32.Rutkove SB, Aaron R, Shiffman CA. Localized bioimpedance analysis in the evaluation of neuromuscular disease. Muscle Nerve. 2002;25:390–397. doi: 10.1002/mus.10048. [DOI] [PubMed] [Google Scholar]
- 33.Rutkove SB, Esper GJ, Lee KS, Aaron R, Shiffman CA. Electrical impedance myography in the detection of radiculopathy. Muscle Nerve. 2005;32:335–341. doi: 10.1002/mus.20377. [DOI] [PubMed] [Google Scholar]
- 34.Rutkove SB, Fogerson PM, Garmirian LP, Tarulli AW. Reference values for 50-kHZ electrical impedance myography. Muscle Nerve. 2008 doi: 10.1002/mus.21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutkove SB, Zhang H, Schoenfeld DA, Raynor EM, Shefner JM, Cudkowicz ME, Chin AB, Aaron R, Shiffman CA. Electrical impedance myography to assess outcome in amyotrophic lateral sclerosis clinical trials. Clin Neurophysiol. 2007;118:2413–2418. doi: 10.1016/j.clinph.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwan HP. Electrical properties of tissue and cell suspensions. Adv Biol Med Phys. 1957;5:147–209. doi: 10.1016/b978-1-4832-3111-2.50008-0. [DOI] [PubMed] [Google Scholar]
- 37.Shiffman CA, Aaron R, Amoss V, Therrien J, Coomler K. Resistivity and phase in localized BIA. Phys Med Biol. 1999;44:2409–2429. doi: 10.1088/0031-9155/44/10/304. [DOI] [PubMed] [Google Scholar]
- 38.Shiffman CA, Aaron R, Rutkove SB. Electrical impedance of muscle during isometric contraction. Physiol Meas. 2003;24:213–234. doi: 10.1088/0967-3334/24/1/316. [DOI] [PubMed] [Google Scholar]
- 39.Shiffman CA, Aaron R. Angular dependence of resistance in non-invasive electrical measurements of human muscle: the tensor model. Phys Med Biol. 1998;43:1317–1323. doi: 10.1088/0031-9155/43/5/019. [DOI] [PubMed] [Google Scholar]
- 40.Shiffman CA, Kashuri H, Aaron R. Electrical impedance myography at frequencies up to 2 MHz. Physiol Meas. 2008;29:S345–S363. doi: 10.1088/0967-3334/29/6/S29. [DOI] [PubMed] [Google Scholar]
- 41.Stamoulis C, Betensky RA, Rutkove SB. Estimation of Disease Progression in Amyotrophic Lateral Sclerosis using Muscle Electrical Properties. Joint Statistical Meeting of the American Statistical Association; Denver, CO. 2008. [Google Scholar]
- 42.Stoy RD, Foster KR, Schwan HP. Dielectric properties of mammalian tissues from 0.1 to 100 MHz: a summary of recent data. Phys Med Biol. 1982;27:501–513. doi: 10.1088/0031-9155/27/4/002. [DOI] [PubMed] [Google Scholar]
- 43.Tarulli AW, Esper GJ, Lee KS, Aaron R, Shiffman CA, Rutkove SB. Electrical impedance myography in the bedside assessment of inflammatory myopathy. Neurology. 2005;65:451–452. doi: 10.1212/01.wnl.0000172338.95064.cb. [DOI] [PubMed] [Google Scholar]
- 44.Tarulli AW, Chin AB, Lee KS, Rutkove SB. Impact of skin-subcutaneous fat layer thickness on electrical impedance myography measurements: An initial assessment. Clin Neurophysiol. 2007;118:2393–2397. doi: 10.1016/j.clinph.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarulli AW, Shiffman CA, Aaron R, Chin AB, Rutkove SB. Multifrequency Electrical Impedance Myography In Amyotrophic Lateral Sclerosis. 13th International Conference on Electrical Bioimpedance and the 8th Conference on Electrical Impedance Tomography; Graz, Austria. Springer Berlin Heidelberg; 2007. [Google Scholar]
- 46.Trokhanova OV, Okhapkin MB, Korjenevsky AV. Dual-frequency electrical impedance mammography for the diagnosis of non-malignant breast disease. Physiol Meas. 2008;29:S331–S344. doi: 10.1088/0967-3334/29/6/S28. [DOI] [PubMed] [Google Scholar]


