Summary
Background
Some encephalitides or seizure disorders once thought idiopathic now seem to be immune mediated. We aimed to describe the clinical features of one such disorder and to identify the autoantigen involved.
Methods
15 patients who were suspected to have paraneoplastic or immune-mediated limbic encephalitis were clinically assessed. Confocal microscopy, immunoprecipitation, and mass spectrometry were used to characterise the autoantigen. An assay of HEK293 cells transfected with rodent GABAB1 or GABAB2 receptor subunits was used as a serological test. 91 patients with encephalitis suspected to be paraneoplastic or immune mediated and 13 individuals with syndromes associated with antibodies to glutamic acid decarboxylase 65 were used as controls.
Findings
All patients presented with early or prominent seizures; other symptoms, MRI, and electroencephalography findings were consistent with predominant limbic dysfunction. All patients had antibodies (mainly IgG1) against a neuronal cell-surface antigen; in three patients antibodies were detected only in CSF. Immunoprecipitation and mass spectrometry showed that the antibodies recognise the B1 subunit of the GABAB receptor, an inhibitory receptor that has been associated with seizures and memory dysfunction when disrupted. Confocal microscopy showed colocalisation of the antibody with GABAB receptors. Seven of 15 patients had tumours, five of which were small-cell lung cancer, and seven patients had non-neuronal autoantibodies. Although nine of ten patients who received immunotherapy and cancer treatment (when a tumour was found) showed neurological improvement, none of the four patients who were not similarly treated improved (p=0.005). Low levels of GABAB1 receptor antibodies were identified in two of 104 controls (p<0.0001).
Interpretation
GABAB receptor autoimmune encephalitis is a potentially treatable disorder characterised by seizures and, in some patients, associated with small-cell lung cancer and with other autoantibodies.
Funding
National Institutes of Health.
Introduction
Synaptic plasticity is an essential property of neurons that is involved in memory, learning, and cognition. Plasticity depends on the interactions of ion channels and synaptic receptors, including excitatory glutamate NMDA receptors and AMPA receptors, and inhibitory GABAB receptors.1,2 In animal models, pharmacological or genetic disruption of these receptors result in seizures and changes in memory, learning, and behaviour.3–6 Immune responses against these receptors would therefore be expected to result in similar symptoms. Indeed, two disorders, one associated with antibodies to extracellular epitopes of the NR1 subunit of NMDA receptors7 and the other associated with antibodies to GluR1/2 subunits of AMPA receptors,8 have recently been identified. These disorders result in encephalitis with prominent psychiatric, behavioural, and memory problems, often accompanied by seizures. The antibodies implicated in these two autoimmune disorders cause a decrease in the amounts of the target receptor in cultured neurons, suggesting the antibodies are pathogenic. Patients with these syndromes often respond to treatment, and in some patients the immune response occurs as a paraneoplastic event. These findings, as well as the prevalence of some of these disorders (eg, anti-NMDA receptor encephalitis7,9,10), have raised the possibility that other syndromes in which memory and behaviour are impaired and seizures are common could also be immune mediated. In some of these syndromes an immune-mediated pathogenesis is suggested by the clinical response to immunotherapy, the CSF and MRI findings suggesting limbic encephalitis, and the detection of antibodies to unknown neuronal cell-surface antigens. We aimed to identify the autoantigen involved in a new disorder that has most of these suggestive features.
Methods
Study population
Between January, 2006, and June, 2009, we studied 410 patients with encephalitis suspected to be paraneoplastic or immune mediated. These patients were seen by the authors or by clinicians at other institutions and the patients' sera and CSF were sent for analysis of novel autoantibodies to the Center for Paraneoplastic Disorders at the University of Pennsylvania (PA, USA). We identified autoantibodies in the serum or CSF of 357 patients, including 275 patients with antibodies to NMDA receptors (including 75 patients previously reported7), 27 with antibodies to voltage-gated potassium channels, 19 with antibodies to glutamic acid decarboxylase 65 (GAD65), 15 with antibodies to AMPA receptors (including ten patients previously reported8), 11 with anti-Ma2 antibodies, eight with anti-HuD antibodies, and two with anti-CRMP5 antibodies (patients each had only one of these antibodies). Of the remaining 53 patients, 15 had serum or CSF antibodies with reactivity against neuronal cell-surface antigens predominantly in the neuropil of sectioned rat brain. Because of the serum and CSF findings and the response to immunotherapy and cancer treatment of the first of these patients to be clinically and immunologically studied (the index patient), we focused on these 15 patients. Clinical information about the patients was obtained by the investigators or provided by referring physicians. Patients were said to have neurologically improved if they were able to function independently or with little assistance when they returned home. Control samples were CSF or serum from 104 patients, including 91 randomly selected by use of an online random integer generator from the 410 individuals with encephalitis and 13 who had syndromes associated with GAD65 antibodies and who were not included in the group of 410 patients. These 13 patients were seen either by the study investigators or their serum, CSF, and clinical information were sent from other institutions to the primary investigator (JD) for study of disorders of unknown cause.
Studies were approved by the University of Pennsylvania Institutional Review Board, and written informed consent was obtained from all patients or their representatives.
Procedures
To establish whether serum or CSF contained antibodies to neural tissue, sagittal sections were taken from the brains of adult female Wistar rats; brains had been immersed in 4% paraformaldehyde at 4°C for 2 h, cryoprotected with 40% sucrose for 24 h, and snap frozen in chilled isopentane. Paraffin-embedded tumour tissue from patients was deparaffinised and the antigens retrieved.11 7 μm thick frozen (or 4 μm paraffin) tissue sections were incubated with 0.3% hydrogen peroxide for 20 min, with 10% goat serum in PBS for 1 h, and with patients' or control individuals' serum (1:250) or CSF (1:10) or a guineapig polyclonal antibody against an intracellular epitope of the GABAB1 receptor (1:200; AB2256, Millipore, Billerica, MA, USA) at 4°C overnight. After using the appropriate secondary antibodies (all 1:2000, diluted in PBS with 5% goat serum), labelling was developed with the avidin–biotin–peroxidase method. Results were photographed under a fluorescence microscope using Zeiss Axiovision software (Zeiss, Thornwood, NY, USA).
Immunohistochemistry with human tissue (small-cell lung cancer) was done by use of IgG purified from patients' or control individuals' sera and labelled with biotin.12 No secondary antibody was needed, thus avoiding background labelling caused by other human IgG in the tissue.
To identify the antigen and its localisation on cells in vitro, rat hippocampal neuronal cultures were prepared as reported previously.13 Live neurons grown on coverslips were incubated for 1 h at 37°C with patient or control serum (final dilution 1:200) or CSF (1:10). After removing the media and washing with PBS, neurons were fixed with 4% paraformaldehyde and were made permeable with 0.1% Triton X-100 (Sigma-Aldrich, St Louis, MO, USA). Neurons were single or double immunolabelled with a guineapig polyclonal GABAB1 receptor antibody (1:200), followed by the corresponding Alexa Fluor secondary antibodies (1:2000; Molecular Probes, Invitrogen, Eugene, OR, USA). Results were photographed as detailed above.
Rat hippocampal neurons were grown in 100 mm wells (106 neurons per well) and incubated at 37°C with filtered serum (1:500) for 1 h. Neurons were then washed with PBS, lysed with buffer (sodium chloride 150 mM, EDTA [edetic acid] 1 mM, tris(hydroxymethyl) aminomethane [Tris]-hydrochloric acid 100 mM, deoxycholate acid 0.5%, 1% Triton X-100, pH 7.5) containing protease inhibitors (P8340; Sigma-Aldrich), and centrifuged at 16.1×103 gravities for 20 min at 4°C. The supernatant was retained and incubated with protein A/G agarose beads (20423; Pierce, Rockford, IL, USA) overnight at 4°C, centrifuged, and the pellet containing the beads with patients' antibodies bound to the target cell-surface antigen was washed with PBS, aliquoted, and kept at −80°C. A 25 μL aliquot of this pellet was resuspended in Laemmli buffer, boiled for 10 min, separated in 4–15% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), and the proteins visualised with EZBlue gel staining (G1041; Sigma-Aldrich). Protein bands from the gels were cut and sent for mass spectrometry to the Proteomics Core Facility of the Genomics Institute at the Abramson Cancer Center (University of Pennsylvania, PA, USA). Protein bands were trypsin digested and analysed with a nanoLC/nanospray/LTQ mass spectrometer (Thermo Electron Corporation, San Jose, CA, USA) as reported previously.14 Briefly, a 3 μL trypsin-digested sample was injected with autosampler (Eksigent, Dublin, CA, USA). The digested samples were separated on a 10 cm C18 column, using nanoLC (Eksigent) with a 200 μL/min flow rate, and a 45 min gradient. Online nanospray was used to spray the separated peptides into a linear trap quadrupole, and raw data were obtained with Xcalibur software (Thermo Scientific, Waltham, MA, USA). The raw data files were searched against the National Center for Biotechnology Information and Swiss-Prot (Swiss Institute of Bioinformatics, Basel, Switzerland) databases with Mascot (Matrix Science, Boston, MA, USA). The cutoff score for definite protein identification was 70 or more.
After characterisation of the antigen, frozen samples of the pellets were separated in SDS-PAGE, transferred to nitrocellulose (162–0115; Bio-Rad, Hercules, CA, USA), and blotted with the polyclonal antibodies against GABAB1 (1:2000) or GABAB2 (1:1000) receptor subunits. The reactivity was developed by use of biotinylated anti-guineapig IgG made in goat (1:2000; Vector Laboratories, Burlingame, CA, USA) and the avidin–biotin–peroxidase diaminobenzidine method.
To determine the sensitivity and specificity of patients' antibodies for the GABAB receptor, we used a semi-quantitative confocal microscopy analysis similar to that used for other synaptic receptors.7,8 Live rat hippocampal neurons cultured for 14–21 days in vitro were incubated with patients' CSF (1:30 dilution in Neurobasal B27 medium; GIBCO, Invitrogen, Carlsbad, CA, USA) for 24 h, washed in PBS, fixed in paraformaldehyde (4% paraformaldehyde, 4% sucrose in PBS) for 5 min, made permeable with 0.25% Triton X-100 for 10 min, and blocked with 5% normal goat serum for 1 h. Neurons were incubated with a guineapig polyclonal antibody against an intracellular epitope of the GABAB receptor (1:1000; Invitrogen) and a mouse monoclonal antibody against the presynaptic marker Bassoon (1:200; Stressgen, Victoria, BC, Canada), washed, and incubated with the appropriate fluorescent-conjugated secondary antibodies (1:1000, Molecular Probes).
A laser-scanning confocal microscope (Leica TCS SP2; Leica, Deerfield, IL, USA) was used to obtain images. For each image, laser light levels and detector gain and offset were adjusted so that no pixel values were saturated. Images were automatically segmented with an iterative thresholding approach that finds maxima of fluorescence intensity,15 and areas of interest containing dendrites were selected, and the number of individual clusters along dendrites was quantified by use of ImageJ interactive software (Research Services Branch, National Institute of Mental Health, Bethesda, MD, USA) as described previously.7,8 The colocalisation of clusters labelled with patients' antibodies, commercial GABAB antibodies, and Bassoon was quantified using a software macro (written by EH) in ImageJ.
Owing to the reactivity of patients' antibodies with rat tissue and hippocampal neuronal cultures, and the homology between human and rat GABAB receptor sequences (the B1 receptor subunit has 91.3% cDNA sequence identity and 98.6% amino acid sequence identity in the two species),16 HEK293 cells were transfected with plasmids containing rodent GABAB1 or GABAB2 or plasmids without an insert (control), by use of a method previously reported.7 In other experiments, cells were transfected with GABAB1 and GABAB2 in equimolar ratios. Cells were then grown for 24 h before assessment. Transfected cells were fixed in 4% paraformaldehyde, made permeable with 0.1% Triton X-100, and then incubated overnight at 4°C with patients' serum (1:200) or CSF (100%) and the guineapig polyclonal GABAB1 receptor antibody (1:20 000) or a polyclonal GABAB2 receptor antibody (1:10 000, generated by SJM), washed in PBS, and incubated with the appropriate Alexa Fluor secondary antibodies (1:2000). Results were photographed as before.
Antibody titres were obtained by use of HEK293 cells expressing GABAB1/B2 incubated with serial dilutions of serum and CSF, starting at 1:1 dilution. Patients' antibody IgG subtypes in serum or CSF were identified by use of the HEK293 transfected cells and secondary anti-human antibodies specific for IgG1, IgG2, IgG3, or IgG4 (all 1:50; Sigma-Aldrich) as reported.17
Statistical analysis
The association between GABAB receptor antibodies and other autoantibodies (GAD65, N-type voltage-gated calcium channel, thyroid peroxidase, thyroglobulin, or SOX1) and that between neurological improvement and cancer treatment or immunotherapy were analysed with Fisher's two-sided exact test. The colocalisation of patients' antibodies with the polyclonal GABAB receptor antibodies or antibodies to the synaptic marker Bassoon was analysed with the Student's t test.
Role of the funding source
The study sponsor had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
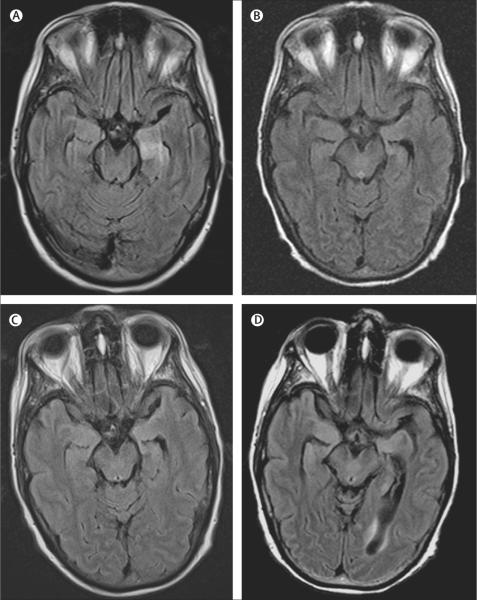
In May, 2008, a 60-year-old woman with a long history of smoking was admitted to hospital with confusion, memory problems, and new-onset generalised tonic-clonic and partial complex seizures refractory to treatment (index patient; patient 1). At examination, she was confused about the time and where she was and had poor concentration and short-term memory (table 1). Although she had saccadic pursuits with lateral gaze, no cranial nerve abnormalities were noted. Strength, sensation, reflexes, and coordination were normal. MRI of the brain showed increased fluid-attenuated inversion recovery (FLAIR) signal in the medial temporal lobe of both hemispheres, compatible with limbic encephalitis (table 2, figure 1A). Diffuse slowing and bilateral periodic lateralised epileptiform discharges were seen on encephalography (EEG). In the CSF there were nine white blood cells per μL, total protein concentration was 350 mg/L, and glucose concentration was 3.94 mmol/L; there were no oligoclonal bands and cytological findings were normal. PCR for herpes simplex virus, West Nile virus, and St Louis encephalitis were negative. The patient had hyponatraemia (119 mEq/L) caused by syndrome of inappropriate antidiuretic hormone secretion. Combined CT and fluorodeoxyglucose-PET showed mediastinal lymphadenopathy, which was proven by biopsy to be small-cell lung cancer. The patient was treated with antiepileptic drugs (levetiracetam, valproic acid, and phenytoin) and immunotherapy (intravenous immunoglobulins and corticosteroids), immediately followed by chemotherapy with cisplatin and etoposide. The patient's short-term memory and cognition improved, and seizures resolved. After chemotherapy the patient had standard prophylactic whole-brain radiation therapy. Brain MRI 1 month after symptom presentation showed improvement of the abnormal FLAIR signal (figure 1B); MRI at 3 months and 9 months were unchanged except for progressive general atrophy, probably secondary to radiation (figure 1C, D). 1 year after symptom presentation, the patient had only mild deficits in memory and cognition and lived independently.
Table 1.
Demographic features and symptoms
| Sex | Age (years) | Tumour by imaging or pathology | Presenting symptoms | Other clinical and immunological features | |
|---|---|---|---|---|---|
| Patient | |||||
| 1 | Female | 60 | SCLC | Subacute onset of complex partial seizures, confusion, memory impairment | SIADH |
| 2 | Male | 66 | SCLC | Subacute onset of seizures, confusion, memory deficit, behavioural problems | N-type VGCC antibodies |
| 3 | Female | 53 | SCLC | Rapidly progressive memory deficits, abnormal sleeping habits, followed by frequent seizures (focal, secondarily generalised), confusion, decline in mental status leading to coma | Pruritic rash with initial weakness |
| 4 | Male | 75 | Mediastinal adenopathy | Subacute onset of seizures, confusion, memory deficit, psychosis, encephalitis; died soon after presentation, before definitive diagnosis or treatment | Poor respiratory status, refused intubation |
| 5 | Male | 68 | Neuroendocrine tumour of the lung | Subacute onset of seizures, status epilepticus, confusion, memory deficit | .. |
| 6 | Female | 43 | CT and FDG/PET negative | Subacute onset of secondarily generalised tonic-clonic seizures, confusion, bizarre behaviours, delusions, paranoia, memory impairment | N-type VGCC antibodies |
| 7 | Male | 69 | CT and FDG/PET negative | Subacute onset of seizures, status epilepticus, severe encephalopathy, severe memory deficit, confusion | History of bipolar disorder |
| 8 | Female | 24 | CT and FDG/PET negative | Subacute onset of seizures, status epilepticus, confusion, memory deficit, fever; required intubation and ventilation owing to poor level of consciousness and airway protection | N-type VGCC antibodies |
| 9 | Male | 63 | CT and FDG/PET negative | Subacute onset of seizures, confusion, memory deficit, paranoia, psychosis, gustatory hallucinations | TPO and GAD65 antibodies; hypothyroidism and type 2 diabetes mellitus |
| 10 | Female | 45 | Benign ovarian mass | Subacute onset of complex partial and generalised seizures, confusion, short-term memory deficits | TPO and thyroglobulin antibodies in serum (not in CSF); no endocrinopathy |
| 11 | Female | 62 | CT chest, abdomen, pelvis negative | Subacute onset of generalised seizures, confusion, memory deficit, decreased level of consciousness, fluent aphasia, abnormal orolingual movements | .. |
| 12 | Male | 29 | CT and FDG/PET negative | Subacute onset of temporal lobe and generalised tonic-clonic seizures, confusion, memory deficits; no cognitive deterioration | Childhood seizures |
| 13 | Female | 30 | CT and FDG/PET negative | 3-month history of severe memory deficit, confusion, followed by seizures (generalised, subclinical) | GAD65 antibodies without endocrinopathy |
| 14 | Male | 69 | SCLC | Subacute onset of generalised tonic-clonic seizures, worsened short-term memory deficit, confusion | Mild short-term memory deficit from past history of subarachnoid haemorrhage |
| 15 | Male | 70 | SCLC | Subacute onset of seizures (partial motor and generalised); severe short-term memory loss, confusion, confabulation, visual hallucinations, disorientation, agitation | GAD65, TPO, and SOX1 antibodies; no endocrinopathy |
| Control | |||||
| 1 | Female | 63 | CT and FDG/PET negative | 1 year progression of cerebellar ataxia; normal mental status, no seizures, no muscle spasms or stiffness | GAD65 antibodies, adult-onset insulindependent diabetes mellitus |
| 2 | Female | 61 | CT and FDG/PET negative | 6 week history of gait disturbance, lower extremity myoclonus and stiffness; dysphagia, dysarthria, nystagmus, left gaze palsy. No seizures or cognitive symptoms | GAD65, TPO, and thyroglobulin antibodies (mild thyroid dysfunction) |
SCLC=small-cell lung cancer. SIADH=syndrome of inappropriate antidiuretic hormone. VGCC=voltage-gated calcium channel. FDG=fluorodeoxyglucose. TPO=thyroid peroxidase. GAD65=glutamic acid decarboxylase 65. SOX1=sex determining region Y-box 1.
Table 2.
Diagnostic tests, treatment, and outcome
| MRI | CSF | Serum antibody titres* | CSF antibody titres* | Chronological list of treatments | Outcome (duration of follow-up) | |
|---|---|---|---|---|---|---|
| Patient | ||||||
| 1 | FLAIR/T2 increased signal in medial temporal lobes | 9 WBC per μL; protein 350 mg/L; no OCBs | 640 | 160 | IVIg, corticosteroids, chemotherapy | Substantial improvement; mild residual short-term memory deficit; lives independently; seizure free (12 months) |
| 2 | Normal | Normal | 1280 | .. | Corticosteroids, IVIg, chemotherapy | Substantial improvement; died of metastatic disease (15 months) |
| 3 | FLAIR/T2 increased signal in medial temporal lobes | .. | 160 | .. | Tumour removal (lobectomy), IVIg | Partial improvement after tumour removal and IVIg (4 months); lost to follow-up |
| 4 | Normal | .. | 2560 | 640 | None | Died soon after presentation of rapidly progressive respiratory failure |
| 5 | FLAIR/T2 increased signal in medial temporal lobes | .. | 1280 | .. | Supportive | Died 6 months after symptom presentation; GABAB antibodies detected after patient's death in archived serum |
| 6 | FLAIR/T2 increased signal in small area of corpus callosum | 95 WBC per μL; protein 1040 mg/L; increased IgG index | .. | 640 | Corticosteroids, mycophenylate mofetil | Substantial improvement; lives independently; seizure free (9 months) |
| 7 | FLAIR/T2 increased signal in left medial temporal lobe | .. | .. | 640 | Corticosteroids, plasma exchange | Initial substantial response to corticosteroids; relapsed 1 month later; died after 5 months in ICU with refractory seizures, status epilepticus, and systemic complications; GABAB antibodies detected after patient's death in archived serum |
| 8 | FLAIR/T2 increased signal in medial temporal lobes | 19 WBC per μL; protein 460 mg/L | 5120 | 2560 | Corticosteroids, plasma exchange | Substantial improvement; mild residual short-term memory deficit; seizure free (3 months) |
| 9 | FLAIR/T2 increased signal in medial temporal lobes | 75 WBC per μL; protein 260 mg/L; OCBs present | Negative | 4 | Corticosteroids | Full recovery (41 months) |
| 10 | FLAIR/T2 increased signal in medial temporal lobes | 81 WBC per μL; protein 300 mg/L | 10240 | .. | Corticosteroids | Substantial improvement. Residual short-term memory deficit. Lives independently. Seizure free (72 months) |
| 11 | Normal | 20 WBC per μL; protein 220 mg/L | 40 | 40 | Corticosteroids | Full recovery (6 months) |
| 12 | FLAIR/T2 increased signal in left medial temporal lobe and insula | 950 WBC per μL; OCBs present | Negative | 10 | Symptomatic | Temporal lobe biopsy 20 months after symptom presentation showing reactive astrocytosis, without inflammation; no follow-up available after biopsy |
| 13 | FLAIR/T2 increased signal in medial temporal lobes | 4 WBC per μL; protein 1090 mg/L; 6 OCBs | Negative | 4 | Corticosteroids | Full recovery, except for infrequent brief episodes of visual hallucinations (10 months) |
| 14 | FLAIR/T2 increased signal in left medial temporal lobe | Traumatic; negative cytology | .. | 80 | Chemotherapy | Residual short-term memory deficit; seizures controlled; died of sepsis (3 months) |
| 15 | Normal | 0 WBC per μL; protein 950 mg/L | .. | 640 | IVIg, corticosteroids, chemotherapy | Seizures responded to antiepileptics; memory deficit persisted; died of cancer-related treatment (2 months) |
| Control | ||||||
| 1 | Normal | 3 WBC per μL; protein 780 mg/L; 1 OCB | Negative | 2 | IVIg | No seizures or cognitive deficits; limited response of cerebellar ataxia to IVIg (12 months) |
| 2 | Normal | 2 WBC per μL; protein 520 mg/L; OCBs present | Negative | 2 | IVIg, corticosteroids | No seizures or cognitive deficits; full recovery after steroids and IVIg (12 months) |
Titres defined as the reciprocal of the maximal dilution that gave positive immunostaining. FLAIR=fluid-attenuated inversion recovery. WBC=white blood cells (normal <4 per μL). OCB=oligoclonal band. IVIg=intravenous immunoglobulin. ICU=intensive care unit.
Figure 1. MRI of a patient with GABAB receptor antibodies and limbic encephalitis.
Axial fluid-attenuated inversion recovery (FLAIR) MRI from patient 1 at presentation (A) showed increased signal in the medial temporal lobes, which was more pronounced on the left. Repeat study at 1 month (B) showed improvement of the FLAIR signal that remained stable at 3 months and 9 months (C, D), with development of mild generalised atrophy (the patient received standard whole-brain radiation therapy as prophylaxis for small-cell lung cancer metastases).
Sera and CSF from the index patient and the 14 other patients (patients 1–15) showed a pattern of reactivity with the neuropil of rat brain (figure 2) that was different from that reported with antibodies against NR1 subunits of the NMDA receptor, GluR1/2 subunits of the AMPA receptor, or voltage-gated potassium channels.7,8,18 When non-fixed and non-permeabilised cultures of rat hippocampal neurons were incubated with patients' serum or CSF, intense reactivity with the cell surface was seen (figure 3A). Similar studies with serum or CSF from control individuals showed no reactivity with rat brain tissue (figure 2E) or cultures of neurons (figure 3B).
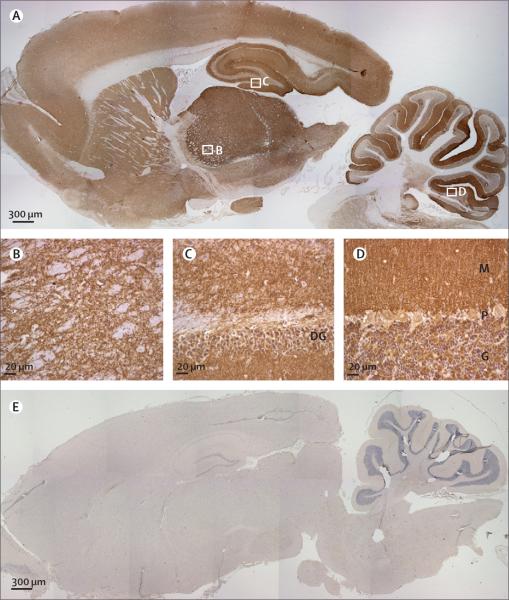
Figure 2. Immunolabelling of rat brain with patients' antibodies.
Sagittal section of rat brain immunolabelled with CSF of a patient with limbic encephalitis (A) and a control individual (E). Note the extensive staining in A of the neuropil of thalamus (B), hippocampus (C), cerebellum (D), and cerebral cortex. DG=dentate gyrus. M=molecular layer. P=Purkinje cell layer. G=granular cell layer. Avidin–biotin–peroxidase method; sections counterstained with haematoxylin.
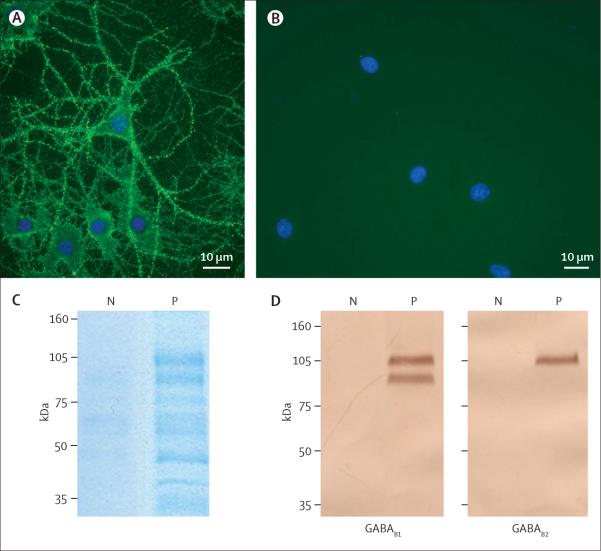
Figure 3. Culture of rat hippocampal neurons incubated (live, non-permeabilised) with the CSF of a patient with limbic encephalitis and a control individual.
Note the intense punctate reactivity of patient's antibodies with cell surface antigens (A) and the absence of reactivity in the control (B); nuclei of neurons stained with 4',6-diamidino-2-phenylindole (DAPI). The surface antigens were precipitated using the antibodies within the patient's serum, and then electrophoretically separated and visualised with EZBlue (C). Patient's antibodies (P) precipitated two main protein bands at about 105 kDa and 90 kDa; these bands are not seen in the precipitate using serum from a control individual (N). Sequencing of the 105 kDa band by use of mass spectrometry showed it contained the B1 and B2 subunits of the GABAB receptor (webappendix). The 90 kDa and other smaller bands were proteolytic fragments and patient's IgG products. Subsequent transfer of the gel to nitrocellulose and immunoblotting with antibodies specific for each of the GABAB (D) subunits confirmed that patient's antibodies precipitated the B1 and B2 subunits (105 kDa) and that the 90 kDa band was a proteolytic fragment of B1.
The GABAB receptor was identified as the target antigen by immunoprecipitation of the antigen with patients' serum samples and peptide sequence recognition (GABAB1 and GABAB2) by mass spectrometry (webappendix). Immunoprecipitates were obtained using the serum from four patients. Electrophoresis of the immunoprecipitates showed similar protein bands at about 90 kDa and 105 kDa (Figure 3C). The indicated protein bands contained sequences derived from GABAB1 and GABAB2 (protein scores for B1: 160, 225, 342, and 178; protein scores for B2: 1094, 1784, 1946, and 2653).
The results were confirmed by immunoblotting the immunoprecipitates with antibodies specific for GABAB1 and GABAB2. Immunoblot analysis confirmed that the band at about 105 kDa was recognised by anti-GABAB1 and anti-GABAB2 antibodies, and the band at about 90 kDa was recognised by anti-GABAB1 antibodies (figure 3D).
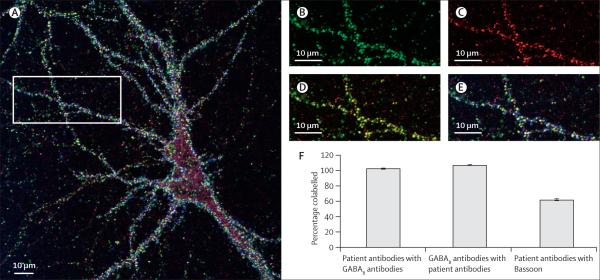
Colocalisation of patients' antibodies with the GABAB receptor and the synaptic and extrasynaptic location of the target receptors were noted on confocal microscopy. The colocalisation of patients' antibody clusters with GABAB receptor clusters (figure 4) was quantified for the dendrites of 23 neurons on four separate coverslips. 103% (SE 0.8%) of the clusters labelled with antibodies from patients colocalised with clusters labelled by the guineapig polyclonal GABAB receptor antibody, and 107% (SE 0.7%) of guineapig antibody-labelled clusters colocalised with those labelled by patients' antibodies (numbers slightly higher than 100% occur because of overlapping of a few clusters labelled by patient antibodies with two guineapig antibody-labelled clusters and vice versa). These results suggest that all patients' antineuronal cell-surface antibodies target the GABAB receptors and that almost all neuronal GABAB receptors are labelled by patients' antibodies. 62% (SE 1.3%) of GABAB receptor clusters labelled by patients' antibodies were also labelled by Bassoon, significantly fewer than those also labelled by guineapig GABAB receptor antibodies (Student's t test, p<0.0001), suggesting that patient antibodies bind both synaptic and extrasynaptic GABAB receptors.
Figure 4. Confocal image of a cultured triple labelled embryonic rat hippocampal neuron.
Patient's antibodies are in green, a guineapig polyclonal antibody against an intracellular epitope of the GABAB1 receptor is in red, and an antibody to the presynaptic marker Bassoon is in blue (A). Area of dendrite from the same neuron showing patient's antibody staining (B), guineapig polyclonal GABAB1 receptor antibody staining (C), both patient and guineapig antibody staining (D), and triple staining (E). The colocalisation of labelling of the dendrites of 23 neurons was quantified (F). This suggests that patients' antibodies bind both synaptic and extrasynaptic GABAB receptors.
The location of the epitope in GABAB1 was identified with HEK293 cells transfected with GABAB1, GABAB2, or both GABAB receptor subunits. All 15 patients had serum or CSF antibodies that reacted with GABAB1 (figure 5), and one had additional reactivity with the GABAB2 subunit (data not shown). Similar studies with the 104 control individuals showed that two patients, both with syndromes attributed to GAD65 autoimmunity, had GABAB1 receptor antibodies at low titres (CSF 1:2, serum negative), which did not bind at detectable levels to sections of rat brain (Fisher's exact test, p<0.0001, data not shown).
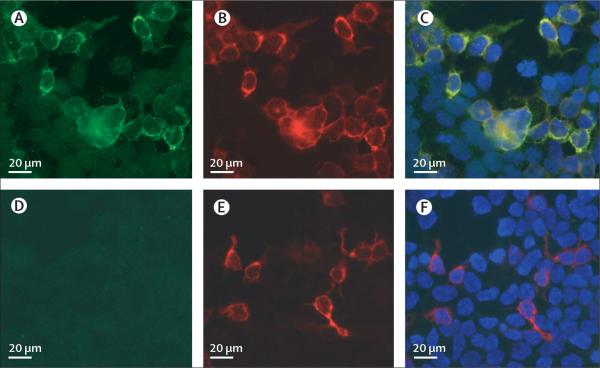
Figure 5. Detection of antibodies to the GABAB1 subunit using a HEK293 cell-based assay.
HEK293 cells transfected with the GABAB1 receptor subunit show reactivity with CSF from a patient with limbic encephalitis (A) and a polyclonal antibody against the B1 subunit of the GABAB receptor (B); both reactivities are merged in C. Similarly transfected cells do not react with CSF from a control individual (D) but do show reactivity with a polyclonal antibody against the B1 subunit of the GABAB receptor (E); reactivities merged in F. Immunofluorescent method.
Samples from the six patients for whom sufficient serum or CSF was available were analysed for antibody IgG subtypes. All six patients had IgG1 GABAB1 antibodies, two had additional IgG3, and one had IgG2 antibodies.
Table 1 shows demographic features and symptoms of the 15 patients and the two control individuals who had antibodies to GAD65. Among the 15 patients, median age was 62 years (range 24–75); eight were men. All patients had seizures, confusion, and memory deficits. In 13 patients the seizures were the presenting symptom; in two (patients 3 and 13) memory deficit and confusion were the presenting symptoms. After further clinical assessment most seizures appeared to have a temporal-lobe onset with secondary generalisation, and three patients had status epilepticus.
Ten patients had unilateral or bilateral increases in medial temporal lobe FLAIR/T2 signal consistent with limbic encephalitis, and one had a small area of increased FLAIR signal in the corpus callosum (table 2). Four patients had normal brain MRI.
CSF was abnormal in nine of ten patients for whom data were available. The most common CSF abnormality was lymphocytic pleocytosis in eight patients. EEG results were available from 12 patients: nine had temporal-lobe seizures, epileptiform discharges, or temporal-lobe slowing; two had generalised slowing; and one had no abnormalities. Several types of seizures were noted on EEG, including complex partial seizures (often of temporal-lobe onset), status epilepticus, and subclinical seizures.
The two control individuals with low titre GABAB1 antibodies developed different syndromes in association with high titre GAD65 antibodies in serum and CSF. Neither of these two patients developed seizures or limbic dysfunction (table 1). One had progressive cerebellar ataxia, and the other had gait instability, muscle stiffness, rigidity, myoclonus, and dysarthria, categorised as progressive encephalomyelitis with rigidity and myoclonus.
In addition to GABAB antibodies, seven of 15 patients had antibodies to one or more of the following: GAD65 (3 patients), thyroid peroxidase (3 patients), N-type voltage-gated calcium channels (3 patients), and SOX1 (1 patient). Only one of the three patients with GAD65 antibodies had endocrinopathy, and one of the three patients with voltage-gated calcium channel antibodies had small-cell lung cancer (table 1). The patient with SOX1 antibodies had small-cell lung cancer.
Seven patients had tumours (table 1), detected at the time of neurological symptom presentation. Of these patients, five had small-cell lung cancer, one had a lung tumour of neuroendocrine origin, and one had mediastinal adenopathy. No other systemic tumours were identified. Because most lung tumours were diagnosed by use of needle biopsy, no tissue was available for analysis of GABAB receptor expression. However, three of four small-cell lung cancers from control individuals without antibodies or encephalitis (archived tissue from the Division of Anatomic Pathology, University of Pennsylvania) showed reactivity with a guineapig polyclonal antibody to GABAB1 receptor and patients' biotinylated IgG, suggesting that these receptors are expressed by small-cell lung cancer (webappendix).
Five of the patients were young (median age 30 years, range 24–45), were non-smokers, and had negative cancer screening including CT/fluorodeoxyglucose-PET, and two of these patients had long-term follow-up (41 and 72 months), making the presence of cancer unlikely.
Nine of 15 patients had a neurological response to immunotherapy (six) or treatment of the tumour as well as immunotherapy (three). The median follow-up of these nine patients was 10 months (range 3–72 months). One patient (patient 2) later died of tumour progression (15 months) and one (patient 3) was lost to follow-up at 4 months. Six patients did not have sustained neurological improvement: three patients (patients 4, 14, and 15) died from tumour or chemotherapy-related complications soon after presentation of the disorder, two were diagnosed with GABAB receptor antibodies after death (patients 5 and 7), and one was lost to follow-up (patient 12). Of the latter three, only patient 7 was thought to have an autoimmune disorder, and therefore this patient received corticosteroids and plasma exchange; the other two patients did not receive immunotherapy. Overall, after excluding one non-assessable patient (patient 12) nine of the ten patients who received immunotherapy and cancer treatment (when a tumour was found) showed neurological improvement, while none of the four patients (patients 4, 5, 14, and 15) who did not receive immunotherapy or whose tumour treatment was not completed showed improvement (Fisher's exact test p=0.005).
Discussion
15 patients had autoimmune encephalitis associated with antibodies to extracellular epitopes of the GABAB receptor and nine responded to treatment. On the basis of clinical, MRI, and EEG findings, the brain regions most affected are the hippocampi and temporal lobes. Thus, it is not surprising that the resulting syndrome is similar to other types of limbic encephalitis (eg, encephalitis associated with antibodies against AMPA receptors or voltage-gated potassium channels), although some clinical and immunological features might suggest GABAB receptor autoimmunity. We have reported development of seizures in all patients, the association with lung cancer in seven patients (five pathologically confirmed as small-cell lung cancer), and the presence of autoantibodies of unclear relation to this type of limbic encephalitis in seven patients. Disruption of GABAB receptors by patients' antibodies is a possible explanation for the symptoms because pharmacological19–21 and genetic3,4 changes to these receptors in rodents result in phenotypes similar to limbic encephalitis, including prominent seizures, memory deficits, increased anxiety, and mood dysregulation.22 Moreover, in human beings, some GABAB receptor polymorphisms are associated with temporal-lobe epilepsy.23
GABAB receptors are G-protein-coupled receptors composed of two subunits, GABAB1 and GABAB2.19,24 GABAB receptors mediate presynaptic inhibition by at least two mechanisms: the activation of G-protein-coupled-inward rectifying potassium channels and the inhibition of calcium channels.25 These receptors also attenuate presynaptic firing frequencies.26 Postsynaptic GABAB receptors mediate inhibition by similar mechanisms27 and by inducing a slow inhibitory postsynaptic potential.28 GABAB receptors limit the duration of network high-activity states, preventing excessive neuronal synchronisation, and allowing new stimuli to break synchronous activity.29,30 GABAB receptors are widely distributed in the brain and spinal cord, but the highest levels of GABAB receptors are found in the hippocampus, thalamus, and cerebellum.31 In the current study, the corresponding areas of rat brain were more intensely immunolabelled by patients' antibodies. The main antigen recognised by the patients' antibodies, the GABAB1 subunit, is necessary for GABA binding and receptor function, whereas the GABAB2 subunit is required for localisation of the receptor to appropriate areas of the cell membrane and G-protein coupling.32,33
By use of a HEK293 cell-based assay we showed that the sera or CSF of all 15 patients had antibodies that reacted with GABAB1, with additional reactivity to GABAB2 in one patient. These findings suggest that HEK293 cells expressing GABAB1/B2 or GABAB1 could be used as a diagnostic test.
A third of patients with encephalitis and GABAB receptor antibodies had pathologically confirmed small-cell lung cancer (age range 53–70 years, all smokers). The involvement of this type of tumour in paraneoplastic disorders and its ability to express synaptic proteins, including GABAB receptors, suggests that it might trigger the immune response against these receptors. In a subgroup of patients with limbic encephalitis and small-cell lung cancer previously thought to be without antibodies or attributed to antibodies against intracellular antigens, GABAB receptor autoimmunity is probably involved,34 particularly in patients who improved after treatment of the tumour or immunotherapy.35,36 Moreover, GABAB receptor autoimmune encephalitis also seems to develop without cancer association. In this respect, GABAB receptor autoimmune encephalitis is similar to other synaptic autoimmunities of the CNS (those involving antibodies to NMDA receptors or AMPA receptors)8–10 or peripheral nervous system (those involving antibodies to acetylcholine receptors or P/Q-type voltage-gated calcium channels) that can develop with or without cancer.37 As occurs in some of these disorders,8 almost half of the patients with GABAB receptor autoimmune encephalitis (including five without tumours) had additional autoantibodies (to TPO, GAD65, SOX1, or N-type voltage-gated calcium channels), suggesting autoimmunity. The overlap with antibodies to GAD65 (an intracellular antigen) suggests that some patients with limbic encephalitis attributed to GAD65 autoimmunity might have GABAB receptor antibodies as a more likely cause of the symptoms.38,39 As more relevant cell-surface or synaptic autoantigens are identified, subsets of disorders with unclear definitions, such as steroid-responsive encephalitis or Hashimoto's encephalitis without thyroid peroxidase antibodies in the CSF, will probably be reclassified.
The small number of patients with GABAB receptor antibodies and the retrospective identification of patients prevented us from assessing the contribution of cancer treatment, immunotherapy, or both, to neurological improvement. Moreover, we were unable to correlate antibody titres with clinical outcome because we did not have serial serum or CSF samples. As this disorder becomes more widely recognised, additional symptoms are likely to be identified. On the basis of the distribution of GABAB receptors in the brain, one would expect that some patients might develop encephalitis or seizure disorders with less focal limbic dysfunction. This could be tested using HEK293 cells that express GABAB1/B2 or GABAB1, as described in this paper. By the time antibodies are detected the serum titres can be very low, and we suggest examining both serum and CSF. Identification of these antibodies should prompt the search for a small-cell lung cancer. Recognition of this disorder is important because it is potentially responsive to immunotherapy and treatment of the tumour. The binding of patients' antibodies to the GABAB receptor in live rat neurons, and the similarity of the syndrome to experimental phenotypes in which this receptor does not function properly, suggest the antibodies are pathogenic. Although GABAB1 receptor antibodies are mainly IgG1 and thus able to activate complement, the role of complement-mediated cytotoxicity is questionable in this potentially reversible disorder in which neurons are the main targets. Future studies should focus on the disease mechanism and effects of the antibodies.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health grants: R21 MH057683 (RB-G); NS046478, NS048045, NS051195, NS056359, and P01NS054900 (SJM); and RO1CA089054 and RO1CA107192 (JD).
Footnotes
Conflicts of interest RC has received honoraria from Boehringer-Ingelheim and Orion Pharma for projects unrelated to the current study. SJM has received reimbursement for travel and accommodation expenses as well as funding support from Pfizer. JD has received royalties from a patent related to Ma2 autoantibody test and has filed patent applications for NMDA and GABAB receptor autoantibody tests. JD has received funding from Euroimmun for projects unrelated to the current study. All other authors have no conflicts of interest.
References
- 1.Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–62. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 2.Rao VR, Finkbeiner S. NMDA and AMPA receptors: old channels, new tricks. Trends Neurosci. 2007;30:284–91. doi: 10.1016/j.tins.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Schuler V, Luscher C, Blanchet C, et al. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)) Neuron. 2001;31:47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- 4.Prosser HM, Gill CH, Hirst WD, et al. Epileptogenesis and enhanced prepulse inhibition in GABA(B1)-deficient mice. Mol Cell Neurosci. 2001;17:1059–70. doi: 10.1006/mcne.2001.0995. [DOI] [PubMed] [Google Scholar]
- 5.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–26. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–43. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 7.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–98. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65:424–34. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Florance NR, Davis RL, Lam C, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66:11–18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gable MS, Gavali S, Radner A, et al. Anti-NMDA receptor encephalitis: report of ten cases and comparison with viral encephalitis. Eur J Clin Microbiol Infect Dis. 2009;28:1421–29. doi: 10.1007/s10096-009-0799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cattoretti G, Pileri S, Parravicini C, et al. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol. 1993;171:83–98. doi: 10.1002/path.1711710205. [DOI] [PubMed] [Google Scholar]
- 12.Furneaux HM, Rosenblum MK, Dalmau J, et al. Selective expression of Purkinje-cell antigens in tumor tissue from patients with paraneoplastic cerebellar degeneration. N Engl J Med. 1990;322:1844–51. doi: 10.1056/NEJM199006283222604. [DOI] [PubMed] [Google Scholar]
- 13.Buchhalter JR, Dichter MA. Electrophysiological comparison of pyramidal and stellate nonpyramidal neurons in dissociated cell culture of rat hippocampus. Brain Res Bull. 1991;26:333–38. doi: 10.1016/0361-9230(91)90003-3. [DOI] [PubMed] [Google Scholar]
- 14.Strader MB, Tabb DL, Hervey WJ, Pan C, Hurst GB. Efficient and specific trypsin digestion of microgram to nanogram quantities of proteins in organic-aqueous solvent systems. Anal Chem. 2006;78:125–34. doi: 10.1021/ac051348l. [DOI] [PubMed] [Google Scholar]
- 15.Bergsman JB, Krueger SR, Fitzsimonds RM. Automated criteria-based selection and analysis of fluorescent synaptic puncta. J Neurosci Methods. 2006;152:32–39. doi: 10.1016/j.jneumeth.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Makoff A. Molecular cloning of human GABABR1 and its tissue distribution. Brain Res Mol Brain Res. 1999;64:137–40. doi: 10.1016/s0169-328x(98)00316-7. [DOI] [PubMed] [Google Scholar]
- 17.Tuzun E, Zhou L, Baehring JM, Bannykh S, Rosenfeld MR, Dalmau J. Evidence for antibody-mediated pathogenesis in anti-NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol. 2009;118:737–43. doi: 10.1007/s00401-009-0582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–77. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enna SJ, Bowery NG. GABA(B) receptor alterations as indicators of physiological and pharmacological function. Biochem Pharmacol. 2004;68:1541–48. doi: 10.1016/j.bcp.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 20.McNamara RK, Skelton RW. Baclofen, a selective GABAB receptor agonist, dose-dependently impairs spatial learning in rats. Pharmacol Biochem Behav. 1996;53:303–08. doi: 10.1016/0091-3057(95)02025-x. [DOI] [PubMed] [Google Scholar]
- 21.Arolfo MP, Zanudio MA, Ramirez OA. Baclofen infused in rat hippocampal formation impairs spatial learning. Hippocampus. 1998;8:109–13. doi: 10.1002/(SICI)1098-1063(1998)8:2<109::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 22.Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology. 2004;29:1050–62. doi: 10.1038/sj.npp.1300413. [DOI] [PubMed] [Google Scholar]
- 23.Gambardella A, Manna I, Labate A, et al. GABA(B) receptor 1 polymorphism (G1465A) is associated with temporal lobe epilepsy. Neurology. 2003;60:560–63. doi: 10.1212/01.wnl.0000046520.79877.d8. [DOI] [PubMed] [Google Scholar]
- 24.Emson PC. GABA(B) receptors: structure and function. Prog Brain Res. 2007;160:43–57. doi: 10.1016/S0079-6123(06)60004-6. [DOI] [PubMed] [Google Scholar]
- 25.Ladera C, del Carmen GM, Jose CM, et al. Pre-synaptic GABA receptors inhibit glutamate release through GIRK channels in rat cerebral cortex. J Neurochem. 2008;107:1506–17. doi: 10.1111/j.1471-4159.2008.05712.x. [DOI] [PubMed] [Google Scholar]
- 26.Kaneda K, Tachibana Y, Imanishi M, et al. Down-regulation of metabotropic glutamate receptor 1alpha in globus pallidus and substantia nigra of parkinsonian monkeys. Eur J Neurosci. 2005;22:3241–54. doi: 10.1111/j.1460-9568.2005.04488.x. [DOI] [PubMed] [Google Scholar]
- 27.Nicoll RA. My close encounter with GABA(B) receptors. Biochem Pharmacol. 2004;68:1667–74. doi: 10.1016/j.bcp.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 28.Kaneda K, Kita H. Synaptically released GABA activates both pre- and postsynaptic GABA(B) receptors in the rat globus pallidus. J Neurophysiol. 2005;94:1104–14. doi: 10.1152/jn.00255.2005. [DOI] [PubMed] [Google Scholar]
- 29.Mann EO, Kohl MM, Paulsen O. Distinct roles of GABA(A) and GABA(B) receptors in balancing and terminating persistent cortical activity. J Neurosci. 2009;29:7513–18. doi: 10.1523/JNEUROSCI.6162-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown JT, Davies CH, Randall AD. Synaptic activation of GABA(B) receptors regulates neuronal network activity and entrainment. Eur J Neurosci. 2007;25:2982–90. doi: 10.1111/j.1460-9568.2007.05544.x. [DOI] [PubMed] [Google Scholar]
- 31.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–67. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- 32.Couve A, Calver AR, Fairfax B, Moss SJ, Pangalos MN. Unravelling the unusual signalling properties of the GABA(B) receptor. Biochem Pharmacol. 2004;68:1527–1536. doi: 10.1016/j.bcp.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 33.Gassmann M, Shaban H, Vigot R, et al. Redistribution of GABAB(1) protein and atypical GABAB responses in GABAB(2)-deficient mice. J Neurosci. 2004;24:6086–97. doi: 10.1523/JNEUROSCI.5635-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alamowitch S, Graus F, Uchuya M, Rene R, Bescansa E, Delattre JY. Limbic encephalitis and small cell lung cancer. Clinical and immunological features. Brain. 1997;120:923–28. doi: 10.1093/brain/120.6.923. [DOI] [PubMed] [Google Scholar]
- 35.Fadul CE, Stommel EW, Dragnev KH, Eskey CJ, Dalmau J. Focal paraneoplastic limbic encephalitis presenting as orgasmic epilepsy. J Neurooncol. 2005;72:195–98. doi: 10.1007/s11060-004-2242-9. [DOI] [PubMed] [Google Scholar]
- 36.Mut M, Schiff D, Dalmau J. Paraneoplastic recurrent multifocal encephalitis presenting with epilepsia partialis continua. J Neurooncol. 2005;72:63–66. doi: 10.1007/s11060-004-2276-z. [DOI] [PubMed] [Google Scholar]
- 37.Wirtz PW, Bradshaw J, Wintzen AR, Verschuuren JJ. Associated autoimmune diseases in patients with the Lambert-Eaton myasthenic syndrome and their families. J Neurol. 2004;251:1255–59. doi: 10.1007/s00415-004-0528-7. [DOI] [PubMed] [Google Scholar]
- 38.Mata S, Muscas GC, Naldi I, et al. Non-paraneoplastic limbic encephalitis associated with anti-glutamic acid decarboxylase antibodies. J Neuroimmunol. 2008;199:155–59. doi: 10.1016/j.jneuroim.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Marchiori GC, Vaglia A, Vianello M, Bardin PG, Giometto B. Encephalitis associated with glutamic acid decarboxylase autoantibodies. Neurology. 2001;56:814. doi: 10.1212/wnl.56.6.814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.