Abstract
The cellular mechanisms underlying typical absence seizures, which characterize various idiopathic generalized epilepsies, are not fully understood, but impaired GABAergic inhibition remains an attractive hypothesis. In contrast, we show here that extrasynaptic GABAA receptor–dependent ‘tonic’ inhibition is increased in thalamocortical neurons from diverse genetic and pharmacological models of absence seizures. Increased tonic inhibition is due to compromised GABA uptake by the GABA transporter GAT–1 in the genetic models tested, and GAT–1 is critical in governing seizure genesis. Extrasynaptic GABAA receptors are a requirement for seizures in two of the best characterized models of absence epilepsy, and the selective activation of thalamic extrasynaptic GABAA receptors is sufficient to elicit both electrographic and behavioural correlates of seizures in normal animals. These results identify an apparently common cellular pathology in typical absence seizures that may have epileptogenic significance, and highlight novel therapeutic targets for the treatment of absence epilepsy.
Keywords: extrasynaptic, tonic current, GAT–1, thalamus, spike–and–wave discharge, GAERS, stargazer, lethargic, GHB, THIP
Typical absence seizures characterize numerous idiopathic generalized epilepsies and appear in the EEG as bilaterally synchronous spike–and–wave discharges (SWDs) accompanied by behavioural arrest1,2. Whilst absence seizures are known to arise in thalamo–cortical networks2-4, the underlying cellular mechanisms are not fully understood. Impaired GABAergic inhibition remains an attractive hypothesis5,6, and GABAA receptor (GABAAR) subunit mutations have been identified in human cohorts with typical absence seizures, albeit as part of a complex phenotype7-9. However, although some of these mutations compromise GABAAR function in heterologous expression systems10, only modest changes in GABAAR inhibition have so far been identified in the thalamo–cortical network of animals with spontaneous SWDs11-13. Furthermore, systemic or intra–thalamic administration of agents that promote GABAergic inhibition, including the anti–epileptic drugs vigabatrin and tiagabine, initiate or exacerbate seizures in patients and animals14-18. Thus, augmented rather than impaired GABAAR inhibition may be a feature of absence seizures.
Activation of GABAARs generates two types of inhibition: the transient activation of synaptic GABAARs (sGABAARs) elicits inhibitory post–synaptic currents (IPSCs), or ‘phasic’ inhibition; and the activation of peri– or extrasynaptic GABAARs (eGABAARs) by ambient GABA causes a persistently active or ‘tonic’ current19,20. Since in thalamocortical (TC) neurons, major players in thalamo–cortical networks during SWDs, >90% of GABAAR inhibition is tonic21-24, we have examined the prospect of aberrant tonic inhibition in experimental absence seizures. Our data indicate that enhanced tonic GABAA inhibition is a common feature of diverse genetic and pharmacological models of typical absence epilepsy, and may be a requirement for the appearance of absence seizures.
RESULTS
Enhanced tonic GABAA current in genetic models of absence
Genetic absence epilepsy rats from Strasbourg (GAERS) are a well established polygenic model of absence epilepsy, that exhibit bilateral spontaneous SWDs and accompanying behavioural arrest from approximately postnatal day (P)30 (ref. 16). In TC neurons, tonic GABAA currents are generated by extrasynaptic receptors containing the δ subunit21-23, and in rats significant levels of δ subunit are apparent only from ~P12 (ref. 25). Therefore, we measured tonic GABAA current amplitude from TC neurons in slices of the somatosensory ventrobasal (VB) thalamus of GAERS from P14 onward, and compared it to non–epileptic control (NEC) animals of the same age. No significant difference in tonic current amplitude was observed at P14–16 (P > 0.05 for each day) (Fig. 1a,b). At P17, however, there was an approximate two–fold increase in tonic current amplitude in GAERS compared to NEC (P < 0.05), that was sustained in subsequent days (Fig. 1a,b), and was independent of whole–cell capacitance (Supplementary Results and Supplementary Fig. 1a). Comparison of spontaneous IPSC (sIPSC) parameters in GAERS and NEC at the same ages revealed no consistent differences (Supplementary Table 1), in agreement with previous data obtained from younger GAERS12. Interestingly, there was a significantly smaller sIPSC peak amplitude, frequency, charge transfer and total current in GAERS at P18, but these changes were not maintained at later ages (Supplementary Table 1). These results show that TC neurons of GAERS exhibit a selective enhancement of eGABAAR function prior to seizure onset.
Figure 1.
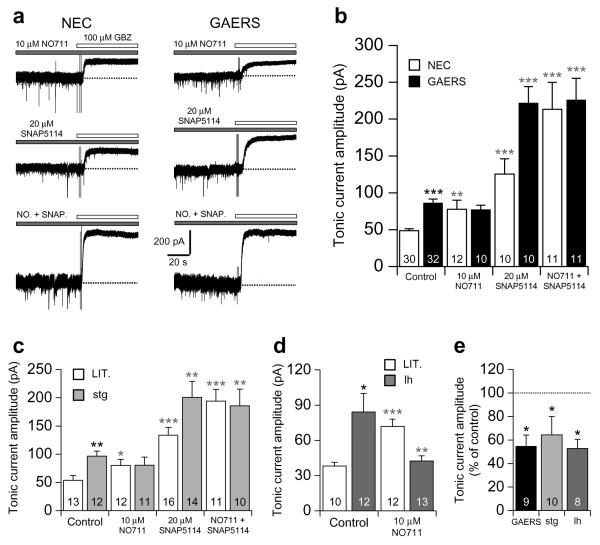
Increased tonic GABAA inhibition in genetic and pharmacological models of absence seizures. (a) Representative current traces from TC neurons of P14 (upper panels) and P17 (lower panels) NEC and GAERS, indicating the presence of tonic GABAA currents following the focal application of 100 μM gabazine (GBZ, white bars). Dotted lines indicate the continuation of the initial baseline current for each neuron. (b) Comparison of the tonic current amplitude in NEC (white columns) and GAERS (black columns) at the ages indicated (P14 to P29/30). (c) Comparison of tonic current amplitude in post–seizure stargazer (stg, P19–21, light grey column), lethargic (lh, P27–30, grey column) and tottering (tg, P26–28, dark grey column) mice to respective control littermates of the same age (LIT., white columns). (d) Representative current trace from a normal Wistar rat TC neuron showing the effect of 3 μM THIP on baseline current in the presence of 0.5 μM TTX. The dotted line indicates the initial baseline current. (e) Comparison of tonic current amplitude in varying concentrations of THIP. (f) Representative current traces, in the presence of 0.5 μM TTX, from two different Wistar TC neurons showing the effect of 3 mM GHB (right) on tonic current amplitude compared to control (left). (g) Comparison of the effects of varying concentrations of GHB on tonic current amplitude, and block of GHB–induced increases by the GABABR antagonist CGP55845 (10 μM) and the putative GHB receptor antagonist NCS–382 (1 mM). Values were normalised to the average tonic current amplitude under control conditions. Experiments in (d–g) were performed on P21–26 Wistar rats. * P < 0.05, ** P < 0.01, *** P < 0.001. For (b), (c), (e) and (g), the number of recorded neurons is as indicated.
We then tested whether increased tonic GABAA current also occurs in the mutant mouse strains stargazer, lethargic and tottering, monogenic models of absence epilepsy that exhibit seizures as part of a complex phenotype26. In pre–seizure mutant mice, tonic current amplitude measured from TC neurons in slices of VB thalamus was not significantly different compared to control littermates (P > 0.05) (Supplementary Fig. 1b), whereas it was significantly larger in post–seizure stargazer and lethargic mice (P < 0.01 and P < 0.05, respectively) (Fig. 1c). However, in post–seizure tottering mice, although tonic current amplitude was not significantly different to control littermates, it was of similar magnitude to post–seizure stargazer and lethargic mice (Fig. 1c). Enhanced tonic current amplitude was still apparent in stargazer and lethargic mice when normalized to whole–cell capacitance (Supplementary Results and Supplementary Fig. 1d). Comparison of sIPSC properties revealed no difference between mutant and control littermates either pre– or post–seizure (Supplementary Table 2). Thus, as in GAERS, eGABAAR function is selectively enhanced in stargazer and lethargic, but not tottering, mice.
SWD–inducing agents enhance the tonic GABAA current
The systemic or intra–thalamic administration of γ–hydroxybutyric acid (GHB), a weak GABABR agonist, is an established model of typical absence seizures27,28, and the systemic administration of the δ subunit–selective agonist THIP (Gaboxadol) has been shown to induce SWDs29. Therefore, we tested if these agents enhance the tonic GABAA current in TC neurons. In the presence of TTX (0.5 μM), application of 0.1–10 μM THIP increased tonic current amplitude in TC neurons of the VB from normal P21–26 Wistar rats (all P < 0.001) (Fig. 1d,e) as described previously for mice21,23. Similarly, in the presence of TTX, application of 0.3–3 mM GHB, concentrations that induce SWDs in vivo27, significantly increased tonic current amplitude in vitro (P < 0.05–0.001) (Fig. 1f,g), despite a reduction in IPSC frequency (data not shown)30. The action of 3 mM GHB was completely blocked by the GABABR antagonist CGP55845 (10 μM), and the putative GHB antagonist NCS–382 (1 mM) (Fig. 1g). Indeed, application of CGP55845 alone significantly reduced tonic current amplitude to 74.0 ± 10.8% of control (P < 0.05) (Fig. 1g), indicating that facilitation of eGABAARs by GABABRs contributes approximately one quarter of the tonic GABAA current in normal Wistars under control conditions.
These data, together with those from GAERS, stargazer and lethargic, show that enhanced eGABAAR activity in TC neurons characterizes diverse genetic and pharmacological models of typical absence seizures.
GAT–1 malfunction underlies aberrant tonic GABAA inhibition
We next examined the mechanism(s) giving rise to enhanced tonic GABAA current in GAERS. Elevated GABA levels have been observed in the ventral thalamus of adult GAERS compared to NEC31, and may arise due to reduced GABA uptake32. Therefore, we tested the contribution of aberrant GABA uptake to enhanced tonic current in P18–21 GAERS compared to age–matched NEC using concentrations of GABA transporter blockers selective for GAT–1 and GAT–3 (ref. 33). In NEC, application of the GAT–1 blocker NO711 (10 μM) significantly increased tonic current compared to control conditions (P < 0.01) (Fig. 2a,b), and application of the GAT–3 blocker SNAP5114 (20 μM) increased tonic current (P < 0.001) to a greater extent than NO711, in agreement with the greater abundance of GAT–3 in the thalamus34,35. Co-application of NO711 and SNAP5114 in NEC increased tonic current (P < 0.001) to a greater extent than would be expected by simply summing the effects of NO711 and SNAP5114 alone, suggesting that block of GAT–1 causes a compensatory increase in uptake by GAT–3, and vice versa. In GAERS, application of NO711 had no effect on tonic current (P > 0.05) (Fig. 2a,b), but application of SNAP5114 caused a large increase (P < 0.001). Co–application of NO711 and SNAP5114 in GAERS significantly increased tonic current (P < 0.001), but the increase was similar to that observed following application of SNAP5114 alone, and following co–application of NO711 and SNAP5114 in NEC. Compromised GABA uptake by GAT–1 is therefore responsible for enhanced tonic current since (i) block of GAT–1 in GAERS has no effect on tonic current amplitude, (ii) block of GAT–1 in NEC increases tonic current amplitude to similar values to that seen in GAERS under control conditions, and (iii) the compensatory increase in uptake by GAT–1 following block of GAT–3 is lost in GAERS.
Figure 2.
Aberrant GABA uptake by GAT–1 underlies enhanced tonic inhibition in GAERS, stargazer and lethargic. (a) Representative current traces in P18–21 NEC and GAERS showing the effects of block of GAT–1 alone (following bath application of 10 μM NO711, upper traces), GAT–3 alone (20 μM SNAP5114, middle traces), and GAT–1 and GAT–3 together (NO. + SNAP., lower traces), on tonic current amplitude, revealed by focal application of 100 μM GBZ (white bars). (b) Comparison of the effects of application of NO711 and SNAP5114 alone, and their co–application, on tonic current amplitude in NEC (white columns) and GAERS (black columns). (c) Comparison of the effect of NO711 and SNAP5114 alone, and their co–application, on tonic current amplitude in P19–21 stargazer (stg) mice (light grey columns) and control littermates (LIT., white columns). (d) Comparison of the effect of NO711 on tonic current amplitude in P27–30 lethargic (lh) mice (grey columns) and control littermates (LIT., white columns). (e) Comparison of the effect of bath application of 10 μM CGP55845 on tonic current amplitude in GAERS, stargazer and lethargic. Values were normalised to the average tonic current amplitude in the absence of CGP55845. (b), (c) and (d) * P < 0.05, ** P < 0.01 and *** P < 0.001, mutant vs. non–mutant animals under control conditions; * P < 0.05, ** P < 0.01 and *** P < 0.001, drug vs. non–drug for each strain. (e) * P < 0.05, control vs. CGP55845. For (b–e), the number of recorded neurons is as indicated.
We also tested whether increased vesicular GABA release, overexpression of eGABAARs, mis–expression of sGABAARs, or aberrant taurine transport contribute to enhanced tonic current in GAERS, but none of these cellular mechanisms were implicated (Supplementary Results and Supplementary Fig. 2). However, since our data in normal Wistar rats show that activation of GABABRs contributes to tonic GABAA current (Fig. 1g), we tested whether modulation of eGABAARs by GABABRs occurs in GAERS. In P18–21 GAERS, CGP55845 (10 μM) significantly reduced tonic current amplitude to 54.7 ± 9.5% of control (P < 0.05) (Fig 2e), indicating that facilitation of eGABAAR function by GABABR activation contributes almost half of the tonic current in GAERS.
To determine if compromised GAT–1 activity is restricted to neurons that participate in seizure genesis, we tested the effects of NO711 on tonic GABAA current in dentate gyrus granule cells (DGGCs) of GAERS, since the hippocampal formation is not involved in the generation or maintenance of SWDs16. Under control conditions, no tonic current was observed in DGGCs of either P18–21 GAERS or NEC (data not shown) and sIPSCs were similar in the two strains (Supplementary Table 3). Application of 10 μM NO711, however, induced a tonic current of similar amplitude in both GAERS and NEC (Supplementary Fig. 3), indicating that GAT–1 activity is normal in the dentate gyrus of GAERS.
We also tested whether compromised GAT–1 activity underlies enhanced eGABAAR function in stargazer and lethargic mice. In stargazer, similar to GAERS, there was no effect of GAT–1 block by NO711 on tonic current amplitude, and block of GAT–3 alone by SNAP5114 caused a similar increase to co–application of NO711 and SNAP5114 together (P < 0.01 and 0.001, respectively) (Fig. 2c). In stargazer littermates, NO711 increased tonic current (P < 0.05) to the same level as in stargazer under control conditions. In contrast, in lethargic mice, NO711 significantly decreased tonic current amplitude compared to control conditions (P < 0.01), and in lethargic littermates NO711 increased tonic current (P < 0.001) to similar values to those seen in lethargic under control conditions (Fig. 2d). Thus, in stargazer mice, as in GAERS, increased eGABAAR activity is caused by a failure of GABA uptake by GAT–1. However, in lethargic mice, GAT–1 appears to be a source of ambient GABA apparently indicating that GABA transport by GAT–1 is reversed36. To test if GABABR activation contributes to enhanced tonic inhibition in stargazer and lethargic, we measured tonic current amplitude in the presence of CGP55845 (10 μM). Similar to GAERS, application of CGP55845 reduced tonic current amplitude to 64.4 ± 15.7% in stargazer and 52.7 ± 7.9% in lethargic (both P < 0.05) (Fig. 2e).
In summary, these data show that increased tonic GABAA current in TC neurons of GAERS, stargazer and lethargic is caused by loss of GABA uptake by GAT–1. However, GAT–1 function in DGGCs is unaltered, and GAT–1 expression levels are normal in thalamus and cortex of GAERS (Supplementary Results and Supplementary Fig. 4). We suggest that the resultant increase in ambient GABA in the thalamus leads to enhanced tonic current through direct activation of eGABAARs, and a GABABR–dependent facilitation of eGABAAR function.
Thalamic GAT–1 controls absence seizures
In light of the fact that compromised GAT–1 activity causes enhanced tonic inhibition in TC neurons of GAERS, stargazer and lethargic, we would predict that GAT–1 knockout (GAT–1 KO) mice should exhibit both enhanced tonic inhibition and spontaneous SWDs. Tonic current is indeed increased in cerebellar and cortical neurons of GAT–1 KO mice37,38, but TC neurons have not been tested. Similarly, GAT–1 KO mice have increased susceptibility to pentylenetetrazole–induced seizures37, but the presence of spontaneous SWDs has not been determined. In TC neurons of P68–74 GAT–1 KO mice, tonic current amplitude was significantly larger compared to age–matched wildtype (WT) littermates (P < 0.01) (Fig. 3a,b), and normalized tonic current amplitude was also larger (P < 0.01) (data not shown). Furthermore, sIPSC properties were also significantly different (P < 0.05) (Supplementary Table 3). Freely moving GAT–1 KO mice exhibited spontaneous absence seizures, with SWDs having a mean frequency of 5.2 ± 0.1 Hz (range 4.7–5.7 Hz, n = 10 SWDs in each of eight animals) (Fig. 3c), that were blocked by systemic administration of the anti–absence drug ethosuximide (ETX, 200 mg kg–1 i.p., P < 0.001) (Fig. 3d). Interestingly, ETX (750 μM) and another anti–absence drug sodium valproate (500 μM) had no effect on tonic current amplitude (Supplementary Fig. 5). Thus, as predicted, GAT–1 KO mice exhibit both enhanced tonic inhibition in TC neurons and spontaneous SWDs.
Figure 3.
Role of thalamic GAT–1 in the generation of SWDs. (a) Representative current traces from P68–74 wildtype (WT, left) and GAT–1 knockout (GAT–1 KO, right) mice indicating the presence of tonic currents following the focal application of 100 μM GBZ (white bars). (b) Comparison of tonic current amplitude in WT (white column) and GAT–1 KO (black column) mice. Number of recorded neurons are as indicated. (c) Simultaneous, bilateral (L = left, R = right hemispheres) EEG traces from a GAT–1 KO mouse showing spontaneous SWDs under control conditions. Below is a spectrogram corresponding to the R trace. (d) Comparison of the effect of ETX (200 mg kg−1 i.p.) on the total time (over 1 hr) spent in seizure. Number of recorded animals is as indicated. (e) Simultaneous, bilateral EEG traces from a normal Wistar rat following intra–thalamic administration of aCSF (top traces) and then 200 μM NO711 (bottom traces). Below is a spectrogram for the lowest trace L. At the bottom is an enlargement of the single SWD indicated (●). Calibration bars for the enlarged SWD; vertical 200 μV, horizontal 1 s. (f) Graph showing the effects of intra–thalamic administration of NO711 on the time (20 min periods) spent in seizure, compared to aCSF administration. (g) and (h) Comparison of the effects of systemic ETX (100 mg kg−1 i.p.) administration on total time (over 2 hrs) spent in seizure (g), and total number of SWDs (h), during intra–thalamic NO711 administration. * P < 0.05, ** P < 0.01 and *** P < 0.001.
We further tested the role of thalamic GAT–1 in the generation of SWDs using intra–thalamic reverse microdialysis of the selective GAT–1 inhibitor NO711 (200 μM, effective concentration ≤20 μM, see Methods for details) in normal Wistar rats. Application of NO711 induced both behavioural and electrographic correlates of seizures in all rats tested (Fig. 3e,f and Supplementary Movie 1), and SWDs had a mean frequency of 8.7 ± 1.3 Hz (range 5.0–15.3 Hz, n = 10 SWDs from each of five animals). Systemic application of ETX (100 mg kg–1 i.p.) significantly reduced NO711–induced seizures in all animals tested (Fig. 3g,h) (P < 0.05). Thus, intra–thalamic NO711 administration is sufficient for the appearance of absence seizures, and these two sets of experiments show that thalamic GAT–1 is critical in controlling the generation of SWDs.
eGABAARs are a requirement for seizure generation
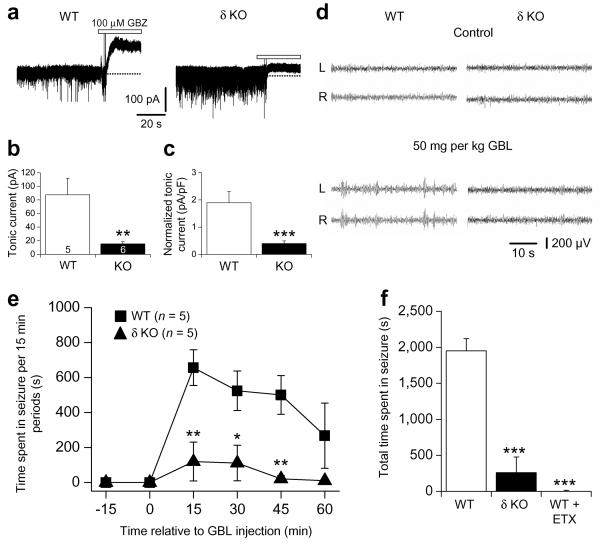
To assess the importance of enhanced eGABAAR function to seizure generation in two well established models of absence epilepsy, GHB and GAERS, we performed two sets of experiments. Firstly, we determined whether animals without thalamic eGABAARs were resistant to the pharmacological induction of absence seizures. GABAAR δ subunit knockout (δ KO) mice have reduced tonic inhibition in TC neurons (P < 0.01–0.001) (Fig. 4a–c)39, whereas sIPSCs are largely unaffected (Supplementary Table 3), and preliminary findings indicate these mice are resistant to the induction of SWDs by GHB and low concentrations of pentylenetetrazole40. In WT littermates, systemic administration of the GHB pro–drug γ–butyrolactone (GBL, 50 mg kg–1 i.p.)41 readily induced absence seizures (Fig. 4d,e) that were largely abolished following administration of ETX (200 mg kg–1 i.p., P < 0.001) (Fig. 4f). However, GBL administration only rarely induced SWDs in δ KO mice (Fig. 4d-f).
Figure 4.
δ subunit knockout mice exhibit reduced tonic inhibition and reduced sensitivity to GBL–induced SWDs. (a) Representative current traces from P23–30 wildtype (WT, left) and δ subunit knockout (δ KO, right) mice revealing tonic currents following the focal application of 100 μM GBZ (white bars). (b) Comparison of tonic current amplitude in WT (white column) and δ KO (black column) mice. Number of recorded neurons are as indicated. (c) Comparison of normalised tonic current amplitude for the same neurons as in (b). (d) Simultaneous, bilateral EEG traces from WT (left) and δ KO (right) mice under control conditions (top) and following injection of GBL (50 mg kg−1 i.p., bottom). (e) Graph showing the effects of GBL on the time (15 min periods) spent in seizure for WT compared to δ KO mice. (f) Comparison of the total time spent in GBL–induced seizure (over 1 hr) between WT and δ KO mice, and the effect of ETX (200 mg kg−1 i.p.) on seizures in WT mice. Number of recorded animals in (f) is the same as in (e). * P < 0.05, ** P < 0.01 and *** P < 0.001.
In the second set of experiments, we intra–thalamically injected δ subunit–specific antisense oligodeoxynucleotides (ODNs) (Fig. 5d) in GAERS in order to knock–down δ subunit expression and therefore decrease the number of eGABAARs42. In animals treated with antisense ODNs (1 or 2 nmol site−1), spontaneous seizures were significantly reduced (P < 0.05–0.01) 1–2 days after injection (Fig. 5a,b) compared to control. By comparison, injection of a missense ODN (1–2 nmol site−1) had no effect on spontaneous seizures (Fig. 5a,b). Importantly, injection of the missense ODN (2 nmol site−1) also had no effect on tonic inhibition in P28–32 GAERS, compared to age–matched, untreated animals, whereas the antisense ODN (2 nmol site−1) significantly reduced tonic current amplitude (P < 0.05) (Fig. 5c). Also, neither missense or antisense ODNs had any effect on sIPSCs (Supplementary Table 3). The results from these two sets of experiments demonstrate that enhanced eGABAAR function in the thalamus is important for the appearance of absence seizures in two of the best characterized models of absence epilepsy.
Figure 5.
Spontaneous absence seizures in GAERS are reduced by intra–thalamic injection of δ subunit–specific antisense ODNs. (a) Graph showing the effect of intra–thalamic injection in GAERS of 1 and 2 nmol site−1 δ subunit–specific antisense ODNs, and 1–2 nmol site−1 non–specific missense ODN, on the time spent in seizure. Values were normalised to the time spent in seizure prior to ODN injection. (b) Comparison of the total number of SWDs following antisense (1 nmol site−1, light grey column; 2 nmol site−1, grey column) and missense (white column) ODN administration. Values were normalised to the number of seizures prior to ODN injection. (c) Effect of 2 nmol site−1 missense (white column) and 2 nmol site−1 antisense (grey column) administration on tonic current amplitude. Values were normalised to the average tonic current amplitude in age–matched, untreated GAERS. (d) Brain section showing that the spread of 2 nmol biotinylated antisense ODN is restricted to the VB thalamus 24 hrs after unilateral injection into the right hemisphere. Arrows indicate the termination of the cannulae in both hemispheres. (b) ‡ and *, P < 0.05 1 and 2 nmol antisense ODN, respectively; ** P < 0.01. (b) and (c) * P < 0.05 and ** P < 0.01. Number of animals in (b) as in (a). Number of recorded neurons in (c) is as indicated.
Enhanced eGABAAR function is sufficient for absence seizures
To directly test if enhanced eGABAAR function in TC neurons is sufficient for absence seizures, we used intra–thalamic administration of the eGABAAR agonist THIP (30–100 μM, effective concentration ≤3–10 μM, see Methods for details) by reverse microdialysis in normal Wistar rats to determine whether selectively increasing tonic inhibition initiates absence seizures in animals that do not normally exhibit them. THIP induced SWDs in only one out of six animals at low concentrations (30 μM, data not shown), but robustly induced the electrographic and behavioural correlates of absence seizures at concentrations of 70 and 100 μM (Fig. 6a,b and Supplementary Movie 2). THIP–induced SWDs had a frequency of 6.1 ± 0.9 Hz (range 5.2–7.5 Hz, n = 10 SWDs in each of four animals), increased in duration during the recording period (Fig. 6a), and were blocked by ETX administration (100 mg kg−1 i.p., P < 0.05–0.01) (Fig. 6c,d). Since THIP at the concentrations used is selective for δ subunit–containing eGABAARs43, our data show that enhanced eGABAAR function in TC neurons is sufficient for the appearance of absence seizures.
Figure 6.

Selective activation of thalamic eGABAARs initiates absence seizures. (a) Simultaneous, bilateral EEG traces showing representative examples of SWDs in the first (upper traces) and second (lower traces) hour following administration of 100 μM THIP in the VB. Below is a spectrogram corresponding to the lower R trace. (b) Graph showing the effects of intra–thalamic administration of 70 and 100 μM THIP on the time (20 min periods) spent in seizure, compared to aCSF injection. (c) and (d) Comparison of the effects of systemic ETX (100 mg kg−1 i.p.) administration on total time (over 2 hrs) spent in seizure (c) and total number of SWDs (d) during intra–thalamic THIP administration. (b) ** and ‡‡ P < 0.01, 100 and 70 μM THIP vs. aCSF, respectively. (c) and (d) * P < 0.05 and ** P < 0.01. Number of animals in (c) and (d) as indicated in (b).
DISCUSSION
Here, we show that enhanced eGABAAR function is a common pathophysiological mechanism in all the diverse genetic and pharmacological models of absence epilepsy tested, except tottering mice (Supplementary Discussion). However, in agreement with previous studies, we rarely observed changes in phasic inhibition (Supplementary Discussion). Our data also indicate that enhanced tonic inhibition is sufficient for seizure genesis, and a requirement for the appearance of seizures in some models. Previous studies have implicated eGABAARs and GAT–1 in the generation of convulsive seizures (Supplementary Discussion), but our data directly demonstrate a role for both in absence seizures. Moreover, GAT–1 KO mice and intra–thalamic NO711 may represent new models of absence epilepsy. Compromised GAT–1 activity underlies increased tonic inhibition in GAERS, stargazer and lethargic, but how the genetic variants in these models lead to GAT–1 malfunction is unknown. GAT–1 is a target for intracellular modulation, at least in neurons44-47, but in the thalamus GABA uptake is governed exclusively by astrocytes34. Thus, thalamic cellular pathology in absence seizures may be astrocyte–specific.
The potential contribution of eGABAARs to absence seizure generation is considered in the Supplementary Discussion. Surprisingly, in GAERS, stargazer, lethargic and GHB models, a novel GABABR–dependent facilitation of eGABAAR function appears to contribute to tonic GABAA inhibition. Although the mechanism(s) underlying this facilitation are unknown, our findings help to explain the sensitivity of seizures to GABABR modulation, a defining criterion for models of absence epilepsy, but do not detract from classical pre– and post–synaptic GABABR contributions to seizure genesis (Supplementary Discussion).
In conclusion, our data show that enhanced eGABAAR function occurs in diverse models of absence seizures and appears to be important in seizure genesis. Furthermore, thalamic GAT–1 critically controls the appearance of absence seizures. We therefore suggest that thalamic eGABAARs and GABA transporters may be potential targets for the generation of novel anti–absence therapies.
METHODS
Electrophysiological recordings
We prepared horizontal slices containing the VB and dentate gyrus in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986 and associated procedures, as described previously22. Whole–cell patch clamp recordings were made from visualised VB TC neurons and DGGCs held at 33 ± 1 °C, and pipettes were attached to the headstage of either a Multiclamp 700B preamplifier, controlled by Multiclamp Commander software, or an Axopatch 200A preamplifier (Molecular Devices). Whole–cell capacitance was measured in response to small (5 mV) voltage pulses. Experimental data were digitized at 20 kHz (Digidata 1322, Molecular Devices), acquired using pClamp 9.0 software (Molecular Devices) and stored on a personal computer. Experiments were performed on only a single neuron within a slice, after which the slice was discarded. Drugs, except focal applications of GBZ, were bath applied in the recording medium.
We analysed data using LabView based software (National Instruments). Tonic currents were observed as an outward shift in baseline current following application of 100 μM GBZ, and tonic and phasic currents measured as described22. Comparison of tonic current amplitude and IPSC properties between mutant and control animals, and between neurons recorded under control conditions and in the presence of drugs, was made using Student’s unpaired t–test. Significance was set at P < 0.05. Data are presented as mean ± s.e.m.
EEG recordings in behaving animals
All surgical procedures were performed in accordance with the UK Animals (Scientific Procedures) Act 1986 and associated procedures. We anaesthetised male and female rats and mice of the relevant strains with a mixture of isoflurane and N2O, and implanted rats with six screw electrodes placed bilaterally over the frontal cortex, parietal cortex and cerebellum, and mice with four screws bilaterally over the parietal cortex and cerebellum. For injection of anti– and missense ODNs and reverse microdialysis experiments, two guide cannulae were implanted over the VB thalamus (AP −3.1, L 3.0, V 4.0; ref. 48) and permanently fixed to the skull with methylacrylic cement. The position of cannulae was checked post hoc (e.g. Fig. 5d) and data from animals with incorrectly positioned cannulae were not included for further analysis. Anti– and missense ODNs were injected one week after recovery from implantation, and the spread of injected ODNs determined using a unilaterally injected biotinylated antisense ODN visualised by the avidin–biotin–horseradish peroxidase complex procedure. Labelling in the injected hemisphere occured not only in the VB but also in the nucleus reticularis thalami, caudate putamen, central amygdala and some regions of neocortex. However, binding in the caudate putamen, amygdala and neocortex was mirrored in the non-injected hemisphere and is therefore non–specific (Fig. 5d). For tonic current measurements after ODN injection, injected animals were sacrificed 22–26 hrs after injection and slices prepared as above. Reverse microdialysis experiments were performed following the bilateral insertion of microdialysis probes (CMA/12, 2 mm length and 500 μm outer diameter; Carnegie Medicin), connected to a two–channel liquid swivel (Carnegie Medicin), to a depth 2 mm below the end of the cannulae.
We made EEG recordings using a Neurolog (Digitimer Ltd) or Plexon (model REC/64) amplifier, and analysed data using pClamp 9.0 (Molecular Devices) or Plexon software, respectively. Spontaneous or GBL–induced seizures in mice were recorded for a period of 1 hr. Control recordings were made prior to the injection of ODNs, and experiments started 1 d after injection. For reverse microdialysis experiments, EEG recordings were made first without probes (30 mins), second with probes infusing aCSF (20 mins), and third with probes infusing the relevant drug dissolved in aCSF (120 mins). Although high concentrations of each drug were used, reverse microdialysis reduces the effective concentration of administered drug to ≤10% (ref. 49), therefore the final concentrations were selective for their desired targets. During the recording session, we video monitored animals to record the behavioural components of absence seizures. Data were quantified as the time spent in seizure during 15 min periods for mice, and 20 min periods for rats, and the total number of SWDs was also calculated in some instances. The effect of ETX on spontaneous and drug–induced seizures was tested by i.p. injection at doses of 100–200 mg kg−1 in a volume of 1 ml kg−1. Drug effects were assessed by repeated measures ANOVA with post-hoc Tukey HSD when significant differences were found (P < 0.05). The effects of ETX on seizures were compared using Student's paired t–test (P < 0.05). Data are presented as mean ± s.e.m.
For further details see Supplementary Methods.
Supplementary Material
ACKNOWLEDGMENTS
We thank P. Blanning for his help in genotyping mice, D. Belelli who kindly provided the genotyping protocol for the δ subunit KO mice, and K. Thomas for initial discussions on the antisense ODN experiments. H. Parri, S. Hughes and N. Leresche commented on a previous version of the manuscript.
D.W.C. is a research Fellow of Epilepsy Research U.K. (grant P0802), and G.O. was supported by a Fellowship of the Italian Ministry for University and Scientific Research. This work was also supported by the Wellcome Trust (grant 71436) and the European Union (grant HEALTH F2–2007–202167).
REFERENCES
- 1.Commission on Classification and Terminology of the International League Against Epilepsy Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 2.Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat. Rev. Neurosci. 2002;3:371–382. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- 3.McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu. Rev. Physiol. 2001;63:815–846. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- 4.Blumenfeld H. Cellular and network mechanisms of spike-wave seizures. Epilepsia. 2005;46(Suppl. 9):21–33. doi: 10.1111/j.1528-1167.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 5.von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261:361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- 6.Huntsman MM, Porcello DM, Homanics GE, DeLorey TM, Huguenard JR. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science. 1999;283:541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- 7.Wallace RH, et al. Mutant GABAA receptor γ2-subunit in childhood absence epilepsy and febrile seizures. Nat. Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- 8.Kananura C, et al. A splice-site mutation in GABRG2 associated with childhood absence epilepsy and febrile convulsions. Arch. Neurol. 2002;59:1137–1141. doi: 10.1001/archneur.59.7.1137. [DOI] [PubMed] [Google Scholar]
- 9.Maljevic S, et al. A mutation in the GABAA receptor α1-subunit is associated with absence epilepsy. Ann. Neurol. 2006;59:983–987. doi: 10.1002/ana.20874. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald RL, Gallagher MJ, Feng H-J, Kang J. GABAA receptor epilepsy mutations. Biochem. Pharmacol. 2004;68:1497–1506. doi: 10.1016/j.bcp.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 11.Caddick SJ, et al. Excitatory but not inhibitory synaptic transmission is reduced in lethargic (Cacnb4lh) and tottering (Cacna1atg) mouse thalami. J. Neurophysiol. 1999;81:2066–2074. doi: 10.1152/jn.1999.81.5.2066. [DOI] [PubMed] [Google Scholar]
- 12.Bessaïh T, et al. Nucleus-specific abnormalities of GABAergic synaptic transmission in a genetic model of absence seizures. J. Neurophysiol. 2006;96:3074–3081. doi: 10.1152/jn.00682.2006. [DOI] [PubMed] [Google Scholar]
- 13.Tan HO, et al. Reduced cortical inhibition in a mouse model of familial childhood absence epilepsy. Proc. Natl. Acad. Sci. USA. 2007;104:17536–17541. doi: 10.1073/pnas.0708440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosford DA, Wang Y, Cao Z. Differential effects mediated by GABAA receptors in thalamic nuclei in lh/lh model of absence seizures. Epilepsy Res. 1997;27:55–65. doi: 10.1016/s0920-1211(97)01023-1. [DOI] [PubMed] [Google Scholar]
- 15.Hosford DA, Wang Y. Utility of the lethargic (lh/lh) mouse model of absence seizures in predicting the effects of lamotrigine, vigabatrin, tiagabine, gabapentin, and topiramate against human absence seizures. Epilepsia. 1997;38:408–414. doi: 10.1111/j.1528-1157.1997.tb01729.x. [DOI] [PubMed] [Google Scholar]
- 16.Danober L, Deransart C, Depaulis A, Vergnes M, Marescaux C. Pathophysiological mechanisms of genetic absence epilepsy in the rat. Prog. Neurobiol. 1998;55:27–57. doi: 10.1016/s0301-0082(97)00091-9. [DOI] [PubMed] [Google Scholar]
- 17.Perucca E, Gram L, Avanzini G, Dulac O. Antiepileptic drugs as a cause of worsening seizures. Epilepsia. 1998;39:5–17. doi: 10.1111/j.1528-1157.1998.tb01268.x. [DOI] [PubMed] [Google Scholar]
- 18.Ettinger AB, et al. Two cases of nonconvulsive status epilepticus in association with tiagabine therapy. Epilepsia. 1999;40:1159–1162. doi: 10.1111/j.1528-1157.1999.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 19.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat. Rev. Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 20.Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56:763–770. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics. J. Neurosci. 2005;25:11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J. Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia F, et al. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J. Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- 24.Bright DP, Aller MI, Brickley SG. Synaptic release generates a tonic GABAA receptor-mediated conductance that modulates burst precision in thalamic relay neurons. J. Neurosci. 2007;27:2560–2569. doi: 10.1523/JNEUROSCI.5100-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J. Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fletcher CF, Frankel WN. Ataxic mouse mutants and molecular mechanisms of absence epilepsy. Hum. Mol. Genet. 1999;8:1907–1912. doi: 10.1093/hmg/8.10.1907. [DOI] [PubMed] [Google Scholar]
- 27.Snead OC., III The γ-hydroxybutyrate model of absence seizures: correlation of regional brain levels of γ-hydroxybutyric acid and γ-butyrolactone with spike wave discharges. Neuropharmacology. 1991;30:161–167. doi: 10.1016/0028-3908(91)90199-l. [DOI] [PubMed] [Google Scholar]
- 28.Banerjee PK, Hirsch E, Snead OC., III γ-hydroxybutyric acid induced spike and wave discharges in rats: relation to high-affinity [3H]γ-hydroxybutyric acid binding sites in the thalamus and cortex. Neuroscience. 1993;56:11–21. doi: 10.1016/0306-4522(93)90557-v. [DOI] [PubMed] [Google Scholar]
- 29.Fariello RG, Golden GT. The THIP-induced model of bilateral synchronous spike and wave in rodents. Neuropharmacology. 1987;26:161–165. doi: 10.1016/0028-3908(87)90204-8. [DOI] [PubMed] [Google Scholar]
- 30.Le Feuvre Y, Fricker D, Leresche N. GABAA receptor-mediated IPSCs in rat thalamic sensory nuclei : patterns of discharge and tonic modulation by GABAB autoreceptors. J. Physiol. 1997;502:91–104. doi: 10.1111/j.1469-7793.1997.091bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richards DA, Lemos T, Whitton PS, Bowery NG. Extracellular GABA in the ventrolateral thalamus of rats exhibiting spontaneous absence epilepsy: a microdialysis study. J. Neurochem. 1995;65:1674–1680. doi: 10.1046/j.1471-4159.1995.65041674.x. [DOI] [PubMed] [Google Scholar]
- 32.Sutch RJ, Davies CC, Bowery NG. GABA release and uptake measured in crude synaptosomes from Genetic Absence Epilepsy Rats from Strasbourg (GAERS) Neurochem. Int. 1999;34:415–425. doi: 10.1016/s0197-0186(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 33.Borden LA. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem. Int. 1996;29:335–356. doi: 10.1016/0197-0186(95)00158-1. [DOI] [PubMed] [Google Scholar]
- 34.De Biasi S, Vitellaro-Zuccarello L, Brecha NC. Immunoreactivity for the GABA transporter-1 and GABA transporter-3 is restricted to astrocytes in the rat thalamus. A light and electron-microscopic immunolocalization. Neuroscience. 1998;83:815–828. doi: 10.1016/s0306-4522(97)00414-4. [DOI] [PubMed] [Google Scholar]
- 35.Pow DV, et al. Differential expression of the GABA transporters GAT-1 and GAT-3 in brains of rats, cats, monkeys and humans. Cell Tissue Res. 2005;320:379–392. doi: 10.1007/s00441-004-0928-0. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, Wang W, Díez-Sampdero A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron. 2007;56:851–865. doi: 10.1016/j.neuron.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiu C-S, et al. GABA transporter deficiency causes tremor, ataxia, nervousness, and increased GABA-induced tonic conductance in cerebellum. J. Neurosci. 2005;25:3234–3245. doi: 10.1523/JNEUROSCI.3364-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bragina L, et al. GAT-1 regulates both tonic and phasic GABAA receptor-mediated inhibition in the cerebral cortex. J. Neurochem. 2008;105:1781–1793. doi: 10.1111/j.1471-4159.2008.05273.x. [DOI] [PubMed] [Google Scholar]
- 39.Herd MB, et al. Inhibition of thalamic excitability by 4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridine-3-ol : a selective role for δ-GABAA receptors. Eur. J. Neurosci. 2009;29:1177–1187. doi: 10.1111/j.1460-9568.2009.06680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banerjee PK, Mihalek RM, Homanics GE, Olsen RW. Mice lacking the γ-aminobutyric acid type A receptor δ-subunit show altered susceptibility to epileptic seizures. Soc. Neurosci. Abs. 2000;661.13 [Google Scholar]
- 41.Aizawa M, Ito Y, Fukuda H. Pharmacological profiles of generalized absence seizures in lethargic, stargazer and γ-hydroxybutyrate-treated model mice. Neurosci. Res. 1997;29:17–25. doi: 10.1016/s0168-0102(97)00066-7. [DOI] [PubMed] [Google Scholar]
- 42.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizures susceptibility and anxiety. Nat. Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 43.Stórustovu S, Ebert B. Pharmacological characterization of agonists at δ-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J. Pharm. Exp. Ther. 2006;316:1351–1359. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- 44.Quick MW, Corey JL, Davidson N, Lester HA. Second messengers, trafficking-related proteins, and amino acid residues that contribute to the functional regulation of the rat brain GABA transporter GAT1. J. Neurosci. 1997;17:2967–2979. doi: 10.1523/JNEUROSCI.17-09-02967.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beckman ML, Bernstein EM, Quick MW. Multiple G protein-coupled receptors initiate protein kinase C redistribution of GABA transporters in hippocampal neurons. J. Neurosci. 1999;19:RC9 1–6. doi: 10.1523/JNEUROSCI.19-11-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang D, Deken SL, Whitworth TL, Quick MW. Syntaxin 1A inhibits GABA flux, efflux, and exchange mediated by the rat brain GABA transporter GAT1. Mol. Pharmacol. 2003;64:905–913. doi: 10.1124/mol.64.4.905. [DOI] [PubMed] [Google Scholar]
- 47.Hu J, Quick MW. Substrate-mediated regulation of γ-aminobutyric acid transporter 1 in rat brain. Neuropharmacology. 2008;54:309–318. doi: 10.1016/j.neuropharm.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. second edition Academic Press Inc; San Diego CA: 1986. [Google Scholar]
- 49.Juhász G, Kékesi K, Emri Zs., Soltesz I, Crunelli V. Sleep-promoting action of excitatory amino acid antagonists: a different role for thalamic NMDA and non-NMDA receptors. Neurosci. Letts. 1990;114:333–338. doi: 10.1016/0304-3940(90)90586-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.