Abstract
Background: Estimates of obesity-associated deaths in the United States for 1991 were published by Allison et al (JAMA 1999;282:1530–8) and subsequently for 2000 by Mokdad et al (JAMA 2004;291:1238–45). Flegal et al (JAMA 2005;293:1861–7) then published lower estimates of obesity-associated deaths for 2000. All 3 studies incorporated data from the first National Health and Nutrition Examination Survey (NHANES I).
Objective: The objective was to clarify the effects of methodologic differences between the 3 studies in estimates of obesity-associated deaths in the US population by using NHANES I hazard ratios.
Design: The earlier reports used imputed smoking data for much of the NHANES I sample rather than the available reported data and applied a method of calculating attributable fractions that did not adjust for the effects of age, sex, and smoking on mortality in the target US population and did not account for effect modification by age. The effects of these and other methodologic factors were examined.
Results: The NHANES I hazard ratios in the earlier reports were too low, probably because of the imputed smoking data. The low hazard ratios obscured the magnitude and direction of the bias arising from the incompletely adjusted attributable fraction method. When corrected hazard ratios were used, the incompletely adjusted attributable fraction method overestimated obesity-associated mortality in the target population by >100,000 deaths.
Conclusion: Methodologic sources of bias in the reports by Allison et al and Mokdad et al include the assessment of smoking status in NHANES I and the method of calculating attributable fractions.
INTRODUCTION
Several different estimates of obesity-associated deaths in the United States have been published (1–3). Allison et al (1) estimated deaths associated with overweight and obesity for the year 1991; Mokdad et al (2) updated the estimates to the year 2000. Flegal et al (3) also estimated obesity-associated deaths for the year 2000 with a different method of estimation and for the most part different data sources. All 3 studies incorporated the first National Health and Nutrition Examination Survey (NHANES I) as one of their data sources. After the article by Flegal et al (3) was published, the Centers for Disease Control and Prevention (CDC) changed its estimate of obesity-associated deaths, writing that “CDC will state, ‘The latest study based on a nationally representative sample of U.S. adults estimates that about 112,000 deaths are associated with obesity each year in the United States.’.” (4).
Estimates in the 2 earlier reports (1, 2) were considerably higher than those by Flegal et al (3). To clarify the sources of these differences, we first briefly reviewed the methods and results of the aforementioned publications (1–3). We then compared estimates of obesity-associated deaths for the US population in 1991 and 2000 using hazard ratios derived from the NHANES I data with various approaches to explain the differences and to show the effects of several sources of bias.
METHODS
Methods and results reported by the 3 studies
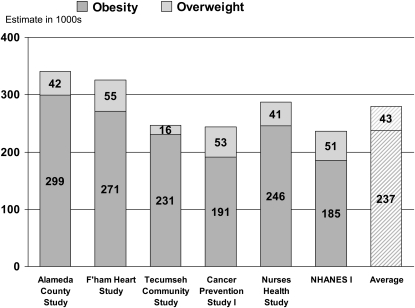
Comparative information about the 3 studies is summarized in Table 1. The report by Allison et al (1) represented an important advance in methodology, combining hazard ratios from epidemiologic cohorts with national obesity prevalence estimates and vital statistics data to make estimates for the US population. Allison et al (1) derived hazard ratios from 6 different epidemiologic cohorts, including NHANES I. For each derivation cohort, hazard ratios, adjusted for age, sex, and smoking, were calculated for 9 levels of body mass index (BMI; in kg/m2), with a BMI of 23 to <25 as the reference category. Overweight was defined as a BMI of 25 to <30 and obesity as a BMI ≥ 30. The hazard ratios from each cohort were combined with BMI prevalence estimates from NHANES III (1988–1994) and the total number of deaths in the United States in 1991 to estimate overweight and obesity-attributable deaths for the target population (the US population) in 1991. The 6 estimates were averaged to arrive at a final number (Figure 1). The average value for obesity and overweight-associated deaths combined was 280,184. For obesity-associated deaths alone, the average was 237,199.
TABLE 1.
Selected characteristics of the 3 studies1
| Allison et al (1) | Mokdad et al (2) | Flegal et al (3) | |
| Data sets used as derivation cohorts to estimate hazard ratios | |||
| Alameda County study, 1965–1975 | ✓ | ✓ | |
| Framingham Heart Study, 1948–1980 | ✓ | ✓ | |
| Tecumseh Community Study, 1959–1985 | ✓ | ✓ | |
| Cancer Prevention Study I, 1960–1972 | ✓ | ✓ | |
| Nurses’ Health Study, 1976–1992 | ✓ | ✓ | |
| NHANES I, 1971–1992 | ✓ | ✓ | ✓ |
| NHANES II, 1976–1992 | ✓ | ||
| NHANES III, 1988–2000 | ✓ | ||
| Target year for which deaths are estimated | |||
| 1991 | ✓ | ||
| 2000 | ✓ | ✓ | |
| Data sets used to estimate prevalence of BMI in target year for target population | |||
| NHANES III (1988–1994) | ✓ | ||
| NHANES 1999–2000 | ✓ | ||
| NHANES 1999–2002 | ✓ | ||
| Reference BMI category | |||
| 23 to <25 kg/m2 | ✓ | ✓ | |
| 18.5 to <25 kg/m2 | ✓ | ||
| Hazard ratios in derivation cohorts adjusted for | |||
| Age, sex, smoking | ✓ | ✓ | |
| Age, sex, smoking, alcohol, race-ethnicity group | ✓ | ||
| Attributable fraction in target population adjusted for | |||
| No adjustment | ✓ | ✓ | |
| Age, sex, smoking, alcohol, race-ethnicity group | ✓ | ||
| Effect modification by age | |||
| Not allowed for | ✓ | ✓ | |
| Allowed for | ✓ | ||
| NHANES I smoking data | |||
| Missing smoking data statistically imputed | ✓ | ✓ | |
| No imputed data | ✓ |
NHANES, National Health and Nutrition Examination Survey.
FIGURE 1.
Published estimates of overweight- and obesity-associated deaths in 1991 taken from Allison et al (1). The figure shows estimates based on hazard ratios from the Alameda County Study, the Framingham (F'ham) Heart Study, the Tecumseh Community Study, the American Cancer Society Cancer Prevention Study 1, the Nurses’ Health Study, the first National Health and Nutrition Examination Survey (NHANES I) Epidemiologic Follow-up Study, and the average over all 6 cohorts.
To update these results to the year 2000, Mokdad et al (2) used the same derivation cohorts, hazard ratios, and attributable fraction method used by Allison et al (1) but used the prevalence of BMI levels from NHANES 1999–2000 and the number of deaths in the United States in 2000. After correcting some calculation errors (5), Mokdad et al estimated 414,123 deaths in 2000 associated with overweight and obesity combined—an increase of 47% compared with the estimate of Allison et al (1) for 1991. This increase was partly due to the 12% increase in the absolute number of deaths between 1991 and 2000 (6) and partly due to the 33% increase in the prevalence of obesity (7). The age-adjusted death rate in the United States fell from 925.5/100,000 in 1991 to 872.0/100,000 in 2000, but the total number of US deaths among adults increased due to the increased size of the population (6). Changes in other risk factors such as smoking do not enter into the calculations.
Mokdad et al (2) then averaged their initial estimate for the year 2000 with the Allison et al (1) estimate for 1991. This reduced their estimate of overweight and obesity-associated deaths from 414,123 to 347,154, which then was rounded upward to 350,000. Mokdad et al (2) next added 15,000 more deaths to account for additional deaths due to poor diet and physical inactivity that were independent of overweight and obesity, to arrive at a final value of 365,000 for diet- and inactivity-associated deaths, of which 350,000 were overweight- and obesity-associated deaths.
Flegal et al (3) used a different reference category of BMI 18.5 to <25 and a different method of estimating attributable fractions (described in more detail below). They estimated hazard ratios adjusted for age, sex, smoking, race, and alcohol consumption based on the combined NHANES I, II, and III mortality follow-up data and applied hazard ratios to data for the US population in 2000. Flegal et al (3) estimated that 112,000 obesity-associated deaths occurred in the US target population in 2000.
NHANES I smoking data
In NHANES I, there were 2 initial questions on cigarette smoking. By design, ≈50% of the NHANES I sample (the “detailed sample”) received a more detailed examination that included the smoking questions, whereas the other subsample (the “nutrition sample”) was not asked smoking questions at baseline and thus had missing information about smoking at baseline (8, 9).
The responses to the baseline smoking questions in the 2 subsamples are shown in Table 2. If the answer to the first question (“Have you smoked at least 100 cigarettes in your entire life?”) was negative, further smoking-behavior questions were not asked, so that never-smokers in the detailed sample did not answer the second question (“Do you smoke cigarettes now?”). More than 50% of the survey participants (7494 of 14,407) were in the nutrition sample that was not asked the smoking questions. At the first follow-up in 1982–1984, retrospective smoking information was collected, mostly from participants themselves (10). This retrospective information has been validated and used in numerous analyses to fill in most of the missing smoking data and provide a more complete smoking data set without statistical imputation (10–15). Flegal et al (3) used the complete reported smoking data, including both the baseline and retrospective data.
TABLE 2.
Original baseline smoking data from the first National Health and Nutrition Examination Survey (NHANES I)
| Subsample | Have you smoked ≥100 cigarettes in your entire life? | Do you smoke cigarettes now? | n | Baseline smoking classification |
| Detailed sample | Yes | Yes | 2587 | Current smokers |
| Yes | No | 1496 | Former smokers | |
| No | Not asked | 2822 | Never-smokers | |
| Nutrition sample | Blank (did not answer) | Not asked | 8 | Missing smoking data |
| Not asked | Not asked | 7494 | Missing smoking data |
Allison et al (1) statistically imputed the smoking status for the large number of NHANES I participants who had missing smoking data. The smoking data reported at baseline included 4318 “nonsmokers” (2822 never-smokers and 1496 former smokers), but the report by Allison et al (1) included only 1496 nonsmokers, so it is not clear whether Allison et al (1) perhaps also imputed smoking data for the 2822 never-smokers and for the 7502 participants who did not have smoking data reported at baseline.
Attributable fraction calculations
Partially adjusted method
The 2 earlier reports (1, 2) used a computing formula to estimate the attributable fraction (AF) of deaths associated with obesity:
where P(Ei) is the prevalence of BMI exposure level i, and Ri is the unadjusted hazard ratio (relative risk) of mortality associated with exposure level i. The formula used by Allison et al (1) is algebraically identical to this formula.
Allison et al (1) used hazard ratios (relative risks) that were adjusted for age, sex, and smoking but used the attributable fraction formula given above, which is only appropriate for unadjusted relative risks. The use of adjusted, rather than unadjusted, relative risks in this formula is known to lead to bias in the attributable fraction estimates (16, 17). We have referred to this approach as the “partially adjusted” method (18), because it only partially adjusts for confounding factors (eg, age, sex, and smoking). Although the hazard ratios in the derivation cohorts are adjusted for these confounding factors, the attributable fraction estimates are not adjusted for the effect of those same factors on mortality in the target population. In addition, this approach of Allison et al (1) and Mokdad et al (2) does not allow for effect modification by age (18, 19). Both earlier reports (1, 2) used the partially adjusted method.
Fully adjusted method
A complete adjustment for confounding factors takes into account the effect of the confounding factors themselves on mortality in the target population as well as on relative risks for BMI in the derivation cohort. Flegal et al (3) used a method to estimate attributable fractions that adjusts for confounding in the target population and allows for effect modification by age (3, 20, 21). For convenience, we will refer to this method here as the “fully adjusted” method. In this approach, the association of BMI with mortality is modeled by using Cox proportional hazard models within separate age groups to adjust for age and allows for the possibility of effect modification by age. The hazard ratios from the Cox model are then applied to the distribution of the model covariates in the general (target) population. The hazard ratios are used to calculate an estimated risk rc for each individual with a given set of covariates c in the target population based on the values of all the covariates for that individual, not just BMI.
A counterfactual risk rc* was also calculated for each individual from the same coefficients, setting BMI equal to the reference category and keeping all other covariates the same for that individual. This is a hypothetical estimate of an individual's mortality risk if the individual's BMI had been moved to the reference BMI category, but all of the individual's other covariates had remained the same. All individuals with identical covariates c will have identical values of rc and rc*; the prevalence of that combination of covariates is pc.
The mortality rate for a given age group is I ∑ rc pc, where I is the mortality rate for individuals who are at the reference levels of BMI and all other covariates, and the sum is over all risk factor combinations. The hypothetical counterfactual mortality rate from moving all participants to the reference weight category is I ∑rc*pc. Because the factor I cancels out, the attributable fraction depends only on the hazard ratios and prevalences of the covariate categories and can be calculated as follows:
where R =∑ rc pc and R* = ∑ rc*pc. This approach accounts for confounding of the BMI-mortality hazard ratios in the derivation cohort by all covariates in the model and for the independent effect of the covariates on mortality in the target population (21). Contrary to what has been stated elsewhere (22, p 46), the article by Flegal et al (3) did not use the weighted sum method.
Statistical analyses
Analyses were conducted with PC-SAS (version 9.1; SAS Institute, Cary, NC) and SUDAAN (version 9.03. Research Triangle Institute, Research Triangle Park, NC). All analyses used sample weights. We calculated hazard ratios (relative risks) for BMI categories from the NHANES I data using Cox proportional hazards models with adjustment for age, sex, and smoking status. We used the data set without exclusions, because, as we showed elsewhere (20), the attributable fractions for BMI categories did not show large or systematic changes after simultaneous exclusion of ever smokers, participants with a history of cancer or cardiovascular disease, and persons who died early in the follow-up period or were measured at older ages. We first calculated excess deaths from these hazard ratios by using the partially adjusted method used in the earlier reports (1, 2). We then calculated excess deaths using the approach described by Flegal et al (3), first with the same confounding factors and reference category as used by Allison et al (1) and then with additional confounders and a different reference category.
RESULTS
We calculated hazard ratios from NHANES I for the same BMI categories as used by Allison et al (1), adjusted for age, sex, and smoking, first with only the original baseline reported smoking data (Table 3) and then with the complete reported smoking data, including both baseline and retrospective information. The published hazard ratios from Allison et al (1) for NHANES I are also shown in Table 3. At higher BMI levels, the published hazard ratios (1) for the full sample appear too low and do not agree with the hazard ratios calculated with the reported smoking data. The differences between the hazard ratios indicate that the NHANES I data set used by Allison et al (1) is not the same as the NHANES I data set used by Flegal et al (3). The data set used by Allison et al (1) included both reported and imputed smoking data, and the main difference is probably due to some problem in the statistical imputation of smoking data for much of the sample by Allison et al (1). The report by Mokdad et al (2) used the hazard ratio estimates published by Allison et al (1) and thus was also affected by these differences. We also included in Table 3 the correct hazard ratios for participants who reported at baseline that they had never smoked as well as the published hazard ratios from Allison et al (1) for “nonsmokers.” These 2 sets of hazard ratios are similar to each other and both agree well with the correct hazard ratios for the full sample, which further indicates that the problems seem to lie with the published hazard ratios for the full sample from Allison et al (1).
TABLE 3.
Hazard ratios for BMI categories from the first National Health and Nutrition Examination Survey (NHANES I), adjusted for age, sex, and smoking status
| Calculated with reported smoking data |
Published by Allison et al (1) |
||||
| BMI category (in kg/m2) | With baseline smoking data | With baseline and retrospective smoking data | Never-smokers according to baseline smoking data | Entire sample with use of reported and imputed smoking data | Nonsmokers only |
| <23 | 1.19 | 1.22 | 1.22 | 1.04 | 1.24 |
| 23 to <25 | 1 | 1 | 1 | 1 | 1 |
| 25 to <26 | 1.02 | 1.04 | 0.78 | 0.96 | 0.88 |
| 26 to <27 | 1.06 | 1.11 | 1.27 | 1.11 | 0.91 |
| 27 to <28 | 0.89 | 0.84 | 0.75 | 0.96 | 0.95 |
| 28 to <29 | 1.28 | 1.35 | 1.15 | 1.4 | 1.16 |
| 29 to <30 | 1.27 | 1.09 | 1.16 | 1.06 | 1.26 |
| 30–35 | 1.72 | 1.52 | 1.68 | 1.33 | 1.61 |
| >35 | 2.23 | 2.38 | 2.21 | 1.68 | 2.24 |
Using the partially adjusted method, we calculated obesity-associated deaths for the target population aged ≥25 y in 1991 with the NHANES I hazard ratios calculated with the complete smoking data. The resulting estimate of 314,000 obesity-associated deaths was almost 70% higher than the published estimate by Allison et al (1) of 185,000 obesity-associated deaths for the same target population based on NHANES I hazard ratios. Thus, when the partially adjusted method was used, the low NHANES I hazard ratios used in the earlier reports (1, 2) resulted in considerably lower estimates of obesity-associated deaths in the target population than if the correct hazard ratios had been used.
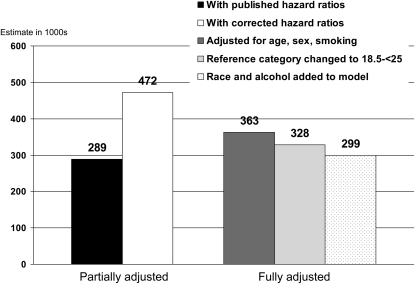
Comparisons of estimates of obesity-associated deaths in 2000 are shown in Figure 2. 2 estimates of obesity-associated deaths for 2000 from NHANES I hazard ratios from the partially adjusted method, using the low hazard ratios and then the corrected hazard ratios calculated with the complete smoking data, are shown. Using the low hazard ratios produced an estimate of 289,000. When the corrected hazard ratios with both baseline and retrospective smoking data were used, however, the partially adjusted method led to an estimate of 472,000, >60% or 183,000 deaths higher.
FIGURE 2.
Effects of methodologic differences on estimates of obesity [BMI (in kg/m2) ≥30]-associated deaths in 2000 based on first National Health and Nutrition Examination Survey (NHANES I) hazard ratios. The fully adjusted estimates use the complete reported smoking data.
We next compared the estimates from the partially adjusted method with estimates from the fully adjusted method when the identical data set was used as the derivation cohort for both estimates. This allows for a direct comparison between the 2 methods with other sources of differences held constant. Applying the fully adjusted method used by Flegal et al (3) with the same adjustment factors and the same reference category as used in the earlier reports (1, 2) resulted in an estimate of 363,000 deaths. The partially adjusted estimate for the target population in 2000 of 472,000 was >100,000 deaths higher than the fully adjusted estimate of 363,000. Thus, the partially adjusted method led to a large overestimate of obesity-associated deaths in the target population relative to the fully adjusted method. The incorrect hazard ratios obscured the magnitude, and even the direction, of the bias due to the use of the partially adjusted method. The bias arises both from incomplete adjustment for confounding factors and from failure to allow for effect modification by age.
The effects of other less influential methodologic differences between the Flegal et al report (3) and the 2 previous reports (1, 2) are also shown in Figure 2. Changing the reference category to BMI 18.5 to <25 decreased the estimate for 2000 by 36,000 deaths to 328,000. Further adjustment for race and alcohol consumption decreased the estimate for obesity-associated deaths to 299,000. This is the previously published estimate based on NHANES I shown in the report by Flegal et al (3).
Competing biases of over- and underestimation
The estimate of 289,000 obesity-associated deaths in 2000 based on NHANES I as the derivation cohort obtained by the methods of Allison et al (1) resembled the estimate by Flegal et al (3) of 299,000 deaths, also based on NHANES I as the derivation cohort. This similarity arises primarily because of competing biases: overestimation from partial adjustment of the attributable fraction, offset by underestimation due to the smaller hazard ratios calculated from data with imputed smoking status. These errors nearly cancelled each other out in this analysis of NHANES I data, as shown in Table 4.
TABLE 4.
Comparisons of estimates of obesity-associated deaths in 2000 based on the first National Health and Nutrition Examination Survey (NHANES I) hazard ratios, with adjustment for age, sex, and smoking and a BMI reference category (in kg/m2) of 23 to <25
| NHANES I hazard ratios | Attributable fraction method | Estimate | Bias relative to fully adjusted method with the correct hazard ratios |
| Calculated by using complete smoking data | Fully adjusted | 363,000 | — |
| Calculated by using complete smoking data | Partially adjusted | 472,000 | 109,000 |
| Published by Allison et al (1) | Partially adjusted | 289,000 | −74,000 |
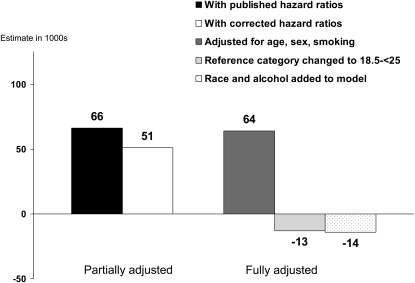
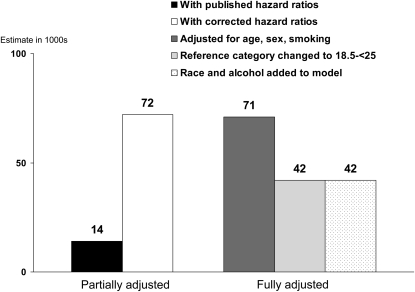
Similar estimates for overweight (BMI 25 to <30) and for a low BMI (<23 for the first 3 estimates shown and <18.5 for the fourth and fifth estimates) are shown in Figures 3 and 4. All estimates of attributable deaths for both overweight and low BMI were small. For overweight, the differences between the published and the recalculated hazard ratio estimates were slight, and the results of the partially adjusted method showed only slight differences depending on which hazard ratios were used. There was little difference between the partially adjusted and the fully adjusted estimates. Changing the reference category from BMI 23 to <25 to BMI 18.5 to <25 decreased the estimated excess deaths for overweight from a small positive number to a small negative number. For low weight, use of the corrected NHANES I hazard ratios increased the estimate from the partially adjusted method, but there was little effect of using the fully adjusted method, of changing the reference category, or of including additional covariates in the model.
FIGURE 3.
Effects of methodologic differences on estimates of overweight [BMI (in kg/m2) ≥30]-associated deaths in 2000 based on first National Health and Nutrition Examination Survey (NHANES I) hazard ratios. The fully adjusted estimates use the complete reported smoking data.
FIGURE 4.
Effects of methodologic differences on estimates of low BMI–associated deaths in 2000 based on the first National Health and Nutrition Examination Survey (NHANES I) hazard ratios. The fully adjusted estimates use the complete reported smoking data.
Effects of changing reference categories
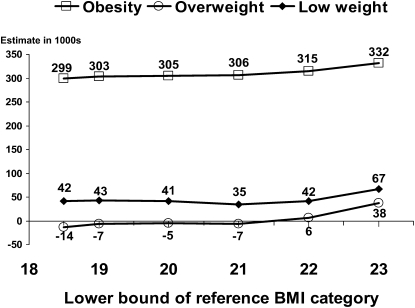
The study by Flegal et al (3) used a reference category of BMI 18.5 to <25; the 2 earlier studies used a reference category of BMI 23 to <25 (1, 2). The effects of varying the lower bound of the reference category on estimates of obesity-associated, overweight-associated, and low weight–associated deaths, with the upper bound held constant at a BMI of 25, are shown in Figure 5. Although differences in estimates were not large in any case, the major change was seen between a reference category of BMI 22 to <25 and a reference category of BMI 23 to <25. Changing the lower bound from a BMI of 22 to BMI levels <22 had little effect on the relative risks for the overweight and obese categories, particularly at older ages in which most deaths occur, and thus had little effect on estimates of excess deaths in those categories. Changing the lower bound from a BMI of 22 to BMI levels <22 led to increased relative risks but a decreased prevalence for the low-weight category and had little effect on the estimate of excess deaths at low BMI because these effects approximately counterbalanced each other.
FIGURE 5.
Effects of varying the lower bound of the reference BMI category on estimates of obesity-associated deaths in 2000 calculated by using the methods of Flegal et al (3) and the corrected first National Health and Nutrition Examination Survey (NHANES I) hazard ratios. The upper bound of the reference category is fixed at a BMI (in kg/m2) of 25.
DISCUSSION
The reports by Allison et al (1), Mokdad et al (2), and Flegal et al (3) all used hazard ratios from NHANES I (the derivation cohort) to estimate obesity-associated deaths in the US population (the target population). Flegal et al (3) used a statistical method to calculate obesity-associated deaths that was different from the partially adjusted method used in the other 2 articles. Several articles (23–25) have compared the NHANES I–based estimates from the several studies and have concluded from the similarity between those estimates that the differences in the statistical methods between the reports had little effect on the estimates. We showed why this conclusion is incorrect.
The NHANES I hazard ratios used in the earlier reports (1, 2) were too low, probably because of the use of incorrect smoking data. Once the hazard ratios are corrected, the statistical methods can be compared directly, and it becomes apparent that the partially adjusted method used in the earlier reports (1, 2) leads to considerably higher estimates for the target population than does the fully adjusted method used by Flegal et al (3). For obesity-associated deaths in 2000 based on NHANES I hazard ratios, the partially adjusted method led to overestimates of ≈30% (>100,000 deaths) relative to the fully adjusted method with the same data, holding all other factors constant. Thus, when the identical data are used, the difference in the statistical methods has a large effect on the estimates.
The bias in the partially adjusted method arises from the failure to account for the confounding effects of age, sex, and smoking on mortality in the US population. In addition, the earlier reports (1, 2) did not account for effect modification by age (18, 19). The bias caused by partial adjustment of attributable fractions has been repeatedly noted (16, 17, 26, 27). Besides NHANES I, the earlier reports (1, 2) also used the partially adjusted method of calculating attributable fractions with hazard ratios, adjusted for age, sex, and smoking, from 5 other derivation cohorts to estimate obesity-associated deaths for the same target population. Even if the adjusted hazard ratios for the derivation cohorts are correct, the attributable fraction method causes bias in the estimates for the target population, because of the characteristics of the target population itself. The bias results from failure to adjust for confounding by age, sex, and smoking in the target population, not in the derivation cohorts. In the US population, age and sex are associated both with BMI and with mortality (6, 7, 18), but the partially adjusted method fails to take this into account. Because all the estimates are for the same target population with the same confounding characteristics, all the estimates would be expected to show bias due to use of the partially adjusted method, relative to use of the fully adjusted method for the same derivation cohort. In addition, however, when there is effect modification by age, differences between the derivation cohort and the target population can increase the bias from partial adjustment (18, 19). Thus, the use of partial adjustment with hazard ratios from the other cohorts, which are less representative of the target population than NHANES is, may have led to even more pronounced bias than illustrated above in our analysis of estimates based on NHANES I hazard ratios.
The NHANES I example illustrates additional factors that affect the estimates of obesity-associated deaths. When relative risks are relatively small (generally <2), modest differences may have a large effect on estimates of obesity-associated deaths (3). Estimates are also affected by the absolute numbers of deaths in different years in the target population and by changes in the prevalence of obesity. The choice of confounding variables to include in the models also can affect the estimates. Flegal et al (3) reported that, for their full sample, estimates of obesity-associated deaths ranged from 138,000 for a simple model to 79,000 excess deaths for a more complex model. Although we currently only addressed bias due to the statistical method used to derive estimates, additional bias may have arisen from the use of hazard ratios that are not representative of the target population.
It has been suggested that it might be inappropriate to include the lower half (BMI < 21) of the normal-weight category (BMI 18.5 to <25) in the reference category (28). Use of the more restrictive reference category of BMI 23 to <25 will increase the number of attributable deaths. As we showed here, the effect was not, as might be surmised, due to the exclusion of BMI < 21 from the reference category, but rather primarily simply due to the exclusion of BMI 22 to <23 from the reference category. Thus, in this instance, there appears to be little reason for concern about including the lower BMI values. Flegal et al (3) also reported only a small difference in estimates between a reference category of BMI 21 to <25 and a reference category of 18.5 to <25.
The earlier reports (1, 2) used somewhat earlier cohorts that are less representative of the US target population, did not properly adjust attributable fractions for confounding, and did not allow for effect modification by age. These factors may be among the largest contributors to the differences. More minor differences result from differences in the reference BMI category and from increases in the absolute numbers of deaths in the United States over time. In summary, the differences in estimates between the 3 reports can be explained in terms of the timing and sources of the survey data and many methodologic differences, including especially the method of adjustment of attributable fractions for confounding and effect modification.
Although the quantitative estimates differed between the 3 studies (1–3), all agreed in finding that obesity was associated with excess mortality in the US population. These estimates of obesity-associated deaths depend on estimates of relative risks, which are measures of association that do not necessarily indicate causal relations. In addition, these estimates use the assumption that relative risks from past cohorts are applicable to the present day. These estimates, like other attributable fraction estimates, are based on the predicted numbers of deaths if a factor had not been present. Thus, they estimate the health burden associated with the presence of an exposure but not necessarily the effects of possible interventions to prevent or reduce exposure (29, 30). These estimates should be interpreted cautiously.
The problem of bias introduced by misapplication of attributable fraction methods has the potential to affect results of analyses that are used to make policy and should be addressed. Errors in the calculation and interpretation of attributable fractions are common, and the direction and magnitude of the effects are not completely predictable (27). Further difficulties arise in attempting to use attributable fraction methods to compare or rank the burden of different risk factors (31). Two workshops have been held to address issues related to the calculation and interpretation of attributable health burden estimates (32, 33). The development and evaluation of methods to estimate the effect on mortality of interventions on lifestyle-related factors remains an important challenge.
Acknowledgments
We thank Karen Steinberg, Centers for Disease Control and Prevention, and Judith Stern, University of California at Davis, for their comments on the manuscript.
The authors’ responsibilities were as follows—KMF: designed the study and wrote the first draft of the manuscript; KMF and BIG: conducted the statistical analysis; and KMF, BIG, DFW, and MHG: analyzed and interpreted the data and critically revised the manuscript for important intellectual content. None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1.Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA 1999;282:1530–8 [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA 2004;291:1238–45 [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA 2005;293:1861–7 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Frequently asked questions about calculating obesity-related risk. Available from: http://www.cdc.gov/PDF/Frequently_Asked_Questions_About_Calculating_Obesity-Related_Risk.pdf (cited 20 January 2009)
- 5.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Correction: actual causes of death in the United States, 2000. JAMA 2005;293:293–4 [DOI] [PubMed] [Google Scholar]
- 6.Minino AM, Arias E, Kochanek KD, Murphy SL, Smith BL. Deaths: final data for 2000. Natl Vital Stat Rep 2002;50:1–119 [PubMed] [Google Scholar]
- 7.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA 2002;288:1723–7 [DOI] [PubMed] [Google Scholar]
- 8.Engel A, Murphy RS, Maurer K, Collins E. Plan and operation of the HANES I augmentation survey of adults 25-74 years United States, 1974-1975. Vital Health Stat 1 1978;Jun:1–110 [PubMed] [Google Scholar]
- 9.Miller HW. Plan and operation of the health and nutrition examination survey. United states—1971-1973. Vital Health Stat 1 1973;Feb:1–46 [PubMed] [Google Scholar]
- 10.Machlin SR, Kleinman JC, Madans JH. Validity of mortality analysis based on retrospective smoking information. Stat Med 1989;8:997–1009 [DOI] [PubMed] [Google Scholar]
- 11.Ballard-Barbash R, Schatzkin A, Taylor PR, Kahle LL. Association of change in body mass with breast cancer. Cancer Res 1990;50:2152–5 [PubMed] [Google Scholar]
- 12.Harris DM, Russell LB. Hospitalizations attributable to arthritis, smoking, and hypertension: a comparison based on NHEFS and NHANES III. Arthritis Rheum 2005;53:543–8 [DOI] [PubMed] [Google Scholar]
- 13.Liszka HA, Mainous AG, III, King DE, Everett CJ, Egan BM. Prehypertension and cardiovascular morbidity. Ann Fam Med 2005;3:294–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore AA, Giuli L, Gould R, et al. Alcohol use, comorbidity, and mortality. J Am Geriatr Soc 2006;54:757–62 [DOI] [PubMed] [Google Scholar]
- 15.Reichman ME, Hayes RB, Ziegler RG, et al. Serum vitamin A and subsequent development of prostate cancer in the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Cancer Res 1990;50:2311–5 [PubMed] [Google Scholar]
- 16.Benichou J. A review of adjusted estimators of attributable risk. Stat Methods Med Res 2001;10:195–216 [DOI] [PubMed] [Google Scholar]
- 17.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health 1998;88:15–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flegal KM, Graubard BI, Williamson DF. Methods of calculating deaths attributable to obesity. Am J Epidemiol 2004;160:331–8 [DOI] [PubMed] [Google Scholar]
- 19.Flegal KM, Williamson DF, Pamuk ER, Rosenberg HM. Estimating deaths attributable to obesity in the United States. Am J Public Health 2004;94:1486–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flegal KM, Graubard BI, Williamson DF, Gail MH. Impact of smoking and preexisting illness on estimates of the fractions of deaths associated with underweight, overweight, and obesity in the US population. Am J Epidemiol 2007;166:975–82 [DOI] [PubMed] [Google Scholar]
- 21.Graubard BI, Flegal KM, Williamson DF, Gail MH. Estimation of attributable number of deaths and standard errors from simple and complex sampled cohorts. Stat Med 2007;26:2639–49 [DOI] [PubMed] [Google Scholar]
- 22.Hu FB. Obesity epidemiology. New York, NY: Oxford University Press, 2007 [Google Scholar]
- 23.Hoerger TJ. Controversies in obesity mortality: a tale of two studies. Health Promotion Economics Issue Brief 2006;1:1 [Google Scholar]
- 24.Manson JE, Bassuk SS, Hu FB, Stampfer MJ, Colditz GA, Willett WC. Estimating the number of deaths due to obesity: can the divergent findings be reconciled? J Womens Health (Larchmt) 2007;16:168–76 [DOI] [PubMed] [Google Scholar]
- 25.McHugh MD. Fit or fat? A review of the debate on deaths attributable to obesity. Public Health Nurs 2006;23:264–70 [DOI] [PubMed] [Google Scholar]
- 26.Greenland S. Bias in methods for deriving standardized morbidity ratio and attributable fraction estimates. Stat Med 1984;3:131–41 [DOI] [PubMed] [Google Scholar]
- 27.Steenland K, Armstrong B. An overview of methods for calculating the burden of disease due to specific risk factors. Epidemiology 2006;17:512–9 [DOI] [PubMed] [Google Scholar]
- 28.Lewis CE, McTigue KM, Burke LE, et al. Mortality, health outcomes, and body mass index in the overweight range. A Science Advisory From the American Heart Association Circulation2009;119:3263–71 [DOI] [PubMed] [Google Scholar]
- 29.Hernán MA, Taubman SL. Does obesity shorten life? The importance of well-defined interventions to answer causal questions. Int J Obes 2008;32(suppl 3):S8–14 [DOI] [PubMed] [Google Scholar]
- 30.Levine B. What does the population attributable fraction mean? Prev Chronic Dis 2007;4:A14. [PMC free article] [PubMed] [Google Scholar]
- 31.Land M, Vogel C, Gefeller O. Partitioning methods for multifactorial risk attribution. Stat Methods Med Res 2001;10:217–30 [DOI] [PubMed] [Google Scholar]
- 32.Institute of Medicine Workshop on estimating the contribution of lifestyle-related factors to preventable death. Washington, DC: National Academies Press, 2005 [Google Scholar]
- 33.Steinberg KK, Dietz WH. Workshop on estimating the health burden of overweight and obesity. Int J Obes (Lond) 2008;32(suppl 3):S1–3 [DOI] [PubMed] [Google Scholar]