Abstract
Background: Early nutrition may affect the risk of overweight in later life.
Objective: The objective was to explore the effect of the duration of breastfeeding (BF) and age at introduction of complementary feeding (CF) on body mass index (BMI) during childhood through adulthood.
Design: The study was based on a subsample of the Copenhagen Perinatal Cohort established in 1959–1961 (n = 5068). Information on BF and available information on CF (age of introduction of “spoon-feeding,” “vegetables,” “egg,” “meat,” and “firm food”) and several covariates were collected in infancy and linked with information on BMI from follow-up examinations in childhood and adulthood at age 42 y.
Results: The median (10th, 90th percentiles) durations of any BF and age at introduction of spoon-feeding were 2.50 (0.23, 6.50) and 3.50 (2.00, 6.00) mo, respectively. After 1 y of age and throughout childhood and adolescence, no association between BF and BMI was found in regression models also adjusted for age at introduction of spoon-feeding and covariates. The risk of overweight at age 42 y decreased or tended to decrease with increasing age (in mo) at introduction of spoon-feeding [odds ratio (OR): 0.94; 95% CI: 0.86, 1.02], vegetables (OR: 0.90; 95% CI: 0.81, 0.98), meat (OR: 0.93; 95% CI: 0.87, 1.00), and firm food (OR: 0.92; 95% CI: 0.86, 0.98) but not egg (OR: 0.98; 95% CI: 0.91, 1.05).
Conclusion: The findings of this study suggest that introduction of CF at a later age (within the range of 2 to 6 mo) is protective against overweight in adulthood but do not support a protective effect of a longer duration of BF.
See corresponding editorial on page 500
INTRODUCTION
The obesity epidemic is already evident in early childhood (1, 2) and is increasing at an accelerating rate (3), which highlights the need to identify early determinants. The idea that breastfeeding (BF) protects against obesity has therefore received considerable interest, and BF in the United States is included in recommendations to reduce the prevalence of childhood obesity (4). According to both a review (5) and an intervention study based on a cluster randomization to BF promotion (6), confounding and publication bias could, however, have led to the BF-obesity association. Furthermore, few studies have explored the persistency of the potential BF-obesity association into adulthood. On the basis of the Nurses’ Health Study, it was found that BF was associated with a reduced risk of obesity, as assessed by recall of ones own body shape, until 5 y of age, whereas no effect was seen at ages 10, 18, or 37–54 y (7).
A longer duration of BF is associated with a delayed introduction of complementary feeding (CF) (8–12), and an interaction between BF and CF has been reported such that early (<16 wk) introduction of CF was associated with an increased infancy weight gain only for those breastfed <20 wk (8). Despite this and the large number of studies addressing the issue of BF and obesity, only limited knowledge exists regarding the effect of age at introduction of CF on obesity. An analysis of data from 5 randomized trials of different dietary interventions, found infants introduced to CF at <12 wk of age to be heavier at 12 wk than those introduced to food later, but the difference did not persist until 18 mo of age (13). In agreement with this, an intervention study of early (3–4 mo) compared with later (6 mo) introduction to CF, including only formula-fed infants, also showed no differences in weight, length, or percentage body fat at 12 mo of age (14). Interestingly, a cohort study showed no effect of timing of CF on weight at 2 y of age (11), but, at age 7 y, early introduction of CF (<15 wk) was associated with an increased percentage body fat (15). In a study of US children, delayed introduction of CF was associated with a significant, but minor reduction in the risk of overweight (0.1% per month) at age 3–4 y (16). Other studies have not supported an association between timing of CF and body mass index (BMI; in kg/m2) (10, 17–19) or body fatness in childhood (9), but it is unknown whether an effect is seen after childhood.
In this study, we explored the effect of duration of BF and age at introduction of CF (indicated by age at introduction of “spoon-feeding,” “vegetables,“ “egg,” “meat,” and “firm food”) in relation to BMI from age 1 through 42 y.
SUBJECTS AND METHODS
The Copenhagen Perinatal Cohort
The Copenhagen Perinatal Cohort consists of 9125 individuals born at the Copenhagen University Hospital from 1959 to 1961 (20). The hospital preferentially admitted mothers for whom home delivery was “inadvisable” (primarily single mothers) and mothers with previous or expected pregnancy or delivery complications. Interviews on social, general medical, and obstetrical history were conducted before delivery when attending the hospital antenatal clinic and during the first few days after birth, when the infants were also examined. The mothers and their offspring were further invited to an examination at ages 1, 3, and 6 y at the hospital (20).
Follow-up examinations
Information on height and weight (7–14 y) was obtained from mandatory school health examinations for those attending school in the municipality of Copenhagen (1, 21) and for men from draft board examinations (mandatory except for men with documented disqualifying illness or disability—not including excess weight) (22). At the age of 20–34 y, a subsample participated in a research program on the effect of prenatal exposure to maternal prescribed medication (22). At the age of 41–43 y (referred to as 42 y), 98% of the study participants surviving infancy were still alive and traceable, and they were sent a questionnaire on lifestyle risk factors and health issues. This included weight, height, and waist circumference measured with an enclosed tape measure. The response rate was 54%. The follow-up examinations were approved by the local ethical committees, and participants gave written informed consent.
Information on BF and introduction of CF
Information on BF and CF was collected at the 1-y examination by a physician who interviewed the mother. The mothers were asked both about duration of exclusive BF and total duration of BF, but two-thirds of the mothers gave the same answer to these 2 questions. The findings are presented for duration of any BF, but the same pattern was seen when exclusive BF was used in the analysis instead (data not shown). Duration of BF was originally recorded on an 11-category scale, and the middle values were assigned for use in continuous analyses (1 wk, 3 wk, and 1.5, 2.5, 3.5, 4.5, 5.5, 6.5, 8.0, 10.5, and 13.0 mo). Twenty-five percent reported they had stopped BF in the first or second week of life (coded as 1 wk), which included those who did not start BF. It has, however, been reported that only 9% of the infants did not receive any breast milk at the age of 5 d and that the policy of the Maternity Departments at the hospital at that time was to encourage BF from a few hours after delivery, except for mothers with severe mental distress, tuberculosis, some forms of epilepsy, and mothers who had decided to adopt away their child (20). If adequate milk production was not established 3–4 d after birth, supplementary sugar water was given. Mothers of low-birth-weight or ill infants were encouraged to express milk for administration by tube or bottle. Infants who were not breastfed were given formula consisting of either a boiled 50% cow milk mixture diluted with barley water and added sugar or a commercial butter milk preparation (20).
Regarding CF, questions were asked about the age when the infants were introduced to 1) spoon-feeding, 2) vegetables, 3) egg, 4) meat, and 5) firm food, which was also described as “soaked bread and biscuits.” The age at which the infants started to eat with a spoon was used as the primary CF variable to focus on introduction of CF. Of those with information on all 5 questions (n = 4143), 79% were introduced to vegetables at the same age as they started spoon-feeding. Nineteen percent of the children started spoon-feeding before they were introduced to vegetables, egg, meat, or firm food, and they are therefore likely to have been introduced to gruel first.
Outcome variables
BMI from 1 to 42 y and overweight (BMI ≥ 25) at age 42 y were the outcome variables. The age intervals at the preschool follow-up examinations varied and only measurements performed within the age intervals ≥10 to <14 mo, ≥2.5 to <3.5 y, and ≥5.5 to <6.5 y were included and referred to as the 1-, 3-, and 6-y examination, respectively. From the school health records, BMI from age ≥6.5 to <7.5 y were included as a 7-y measurement and similarly for the 8–14-y measurements. All childhood BMI measurements were transformed to BMI z scores by using the British 1990 growth references (23) adjusted for sex and exact age. For adults (aged 20–34 and 42 y), no such reference is available; therefore, internal sex-specific BMI- z scores were created. Analyses including BMI at the draft board (range: 16–23 y) and the first adult follow-up examination (range: 20–34 y) were adjusted for current age. Adult waist circumference (42 y) was furthermore used as the outcome variable in both models with and without adjustment for current BMI.
Covariates
Potential confounders included in adjusted analyses were sex, maternal age (y), prepregnant BMI, gestational weight gain (kg, based on the following categories: <6, 7–8, 9–10, 11–12, 13–15, and ≥16 kg as originally recorded), birth weight (g), parental social class (1- to 8-point scale, 8 is the highest), breadwinner's education (1- to 4-point scale, 4 is the highest), single mother status (yes or no), preterm birth (yes or no, defined as a gestational age ≤37 wk due to the original categorization of the variable), and maternal smoking during pregnancy. These variables were all significantly associated with duration of BF, age at introduction of spoon-feeding (the available variable best reflecting introduction of CF) and/or offspring adult BMI (42 y) in bivariate analyses. Preliminary analyses suggested that parity was not a potential confounder, and it was therefore not included in the analyses.
Subjects available for analysis and sample attrition
The original cohort consisted of 9125 infants, of whom 996 were either stillborn, died during infancy, or were from twin or triple births and were excluded. These rates were high because of the hospitals’ admission criteria and high prevalence of preterm births that included infants born as early as 20 wk of gestation. Of the remaining 8129 individuals, this study included the 5068 individuals with information on BF, introduction of spoon-feeding, birth weight, and at least one BMI measurement from 1 y of age or older (273 subjects did not have such measurements). In a comparison of BMI z scores from 3 to 42 y between the 5068 included and the 3061 nonincluded individuals (including all available measurements at all ages), no significant differences were seen (data not shown). Birth weight and 1 y BMI z scores were, however, lower among those not included (mean ± SD): 3191 ± 593 compared with 3232 ± 588 g (P = 0.003) and 0.09 ± 1.12 compared with 0.26 ± 1.09 (P = 0.001), respectively.
Adult BMI (42 y) was considered the most important outcome of the study. Within the included subsample (n = 5068), those with information on adult BMI (42 y) had better social conditions in infancy and were breastfed for a longer time, whereas no difference was seen with respect to age at introduction of CF compared with those who did not participate in the last follow-up (Table 1). Birth weight and BMI through childhood did not differ, but at age 20–34 y the prevalence of obesity was lower among the men who participated in the last follow-up than in those who did not.
TABLE 1.
Baseline characteristics of the cohort (n = 5068) in relation to participation at the second adult follow-up examination (age 42 y)
| Women |
Men |
|||||||
| Participants |
Nonparticipants |
Participants |
Nonparticipants |
|||||
| n | Value | n | Value | n | Value | n | Value | |
| Maternal characteristics | ||||||||
| Age (y) | 1544 | 26.6 ± 6.71 | 890 | 26.0 ± 6.42 | 1331 | 26.3 ± 6.4 | 1276 | 26.2 ± 6.7 |
| Prepregnancy BMI (kg/m2) | 1433 | 21.7 ± 2.9 | 806 | 21.8 ± 3.0 | 1244 | 21.6 ± 2.8 | 1183 | 21.8 ± 3.1 |
| BMI ≥ 25 kg/m2 (%) | — | 11 | — | 12 | 10 | — | 11 | |
| BMI ≥ 30 kg/m2 (%) | — | 1 | — | 1 | 1 | — | 2 | |
| Smoking during pregnancy (%) | 1516 | 53 | 876 | 53 | 1318 | 48 | 1256 | 52 |
| Gestational weight gain (kg) | 961 | 11.3 ± 3.6 | 522 | 11.3 ± 3.5 | 839 | 11.7 ± 3.5 | 745 | 11.6 ± 3.6 |
| Social class (1- to 8-point scale) | 1482 | 4.14 ± 1.83 | 862 | 3.91 ± 1.753 | 1268 | 4.25 ± 1.83 | 1231 | 3.91 ± 1.794 |
| Breadwinner's education (1- to 4-point scale) | 1443 | 2.42 ± 0.69 | 832 | 2.38 ± 0.65 | 1252 | 2.48 ± 0.71 | 1276 | 2.38 ± 0.684 |
| Single mother status (%) | 1533 | 29 | 883 | 332 | 1327 | 30 | 1268 | 34 |
| Offspring characteristics | ||||||||
| Preterm (%)5 | 1260 | 17 | 730 | 17 | 1090 | 20 | 1014 | 19 |
| Breastfeeding <2 wk (%)6 | 1546 | 15 | 897 | 192 | 1338 | 17 | 1287 | 19 |
| Duration of any breastfeeding (mo)7 | 1309 | 3.63 ± 2.78 | 730 | 3.24 ± 2.543 | 1105 | 3.65 ± 2.77 | 1045 | 3.41 ± 2.68 |
| Timing of spoon-feeding (mo) | 1546 | 3.83 ± 1.47 | 897 | 3.81 ± 1.51 | 1338 | 3.75 ± 1.49 | 1287 | 3.80 ± 1.51 |
| Birth weight (g) | 1546 | 3171 ± 555 | 897 | 3158 ± 587 | 1338 | 3312 ± 583 | 1287 | 3273 ± 620 |
| 1-y BMI (kg/m2) | 1101 | 17.67 ± 1.50 | 691 | 17.61 ± 1.63 | 997 | 18.14 ± 1.51 | 900 | 18.15 ± 1.51 |
| 3-y BMI (kg/m2) | 758 | 15.86 ± 1.31 | 417 | 15.90 ± 1.40 | 653 | 16.03 ± 1.31 | 555 | 16.17 ± 1.44 |
| 6-y BMI (kg/m2) | 583 | 15.14 ± 1.40 | 312 | 15.27 ± 1.46 | 512 | 15.44 ± 1.26 | 429 | 15.47 ± 1.41 |
| 8-y BMI (kg/m2) | 402 | 15.61 ± 1.71 | 256 | 15.63 ± 1.72 | 330 | 15.66 ± 1.30 | 307 | 15.75 ± 1.50 |
| 11-y BMI (kg/m2) | 402 | 16.92 ± 2.47 | 250 | 16.77 ± 2.21 | 333 | 16.75 ± 1.90 | 299 | 16.91 ± 2.08 |
| 14-y BMI (kg/m2) | 368 | 18.96 ± 2.47 | 224 | 19.02 ± 2.62 | 298 | 18.51 ± 2.34 | 270 | 18.73 ± 2.54 |
| Draft (16–23 y) BMI (kg/m2) | — | — | — | — | 1115 | 21.80 ± 2.65 | 1010 | 21.69 ± 2.78 |
| Adult (20–34 y) BMI (kg/m2) | 289 | 22.54 ± 3.76 | 100 | 23.16 | 257 | 23.64 ± 2.81 | 150 | 23.78 ± 3.96 |
| BMI ≥ 25 kg/m2 (%) | — | 18 | — | 25 | 26 | — | 25 | |
| BMI ≥ 30 kg/m2 (%) | 5 | 7 | 2 | 62 | ||||
| Adult (42 y) BMI (kg/m2) | 1546 | 24.48 ± 4.88 | — | — | 1338 | 25.67 ± 3.69 | — | — |
| BMI ≥ 25 kg/m2 (%) | — | 34 | — | — | — | 51 | — | — |
| BMI ≥ 30 kg/m2 (%) | — | 12 | — | — | — | 11 | — | — |
| Adult waist circumference (cm) | 1518 | 85.9 ± 12.7 | — | — | 1318 | 95.2 ± 10.9 | — | — |
Mean ± SD (all such values).
Significantly different from participants (t test, Mann-Whitney U test, or chi-square test): 2P ≤ 0.05, 3P ≤ 0.01, 4P ≤ 0.001.
Born at gestational week ≤37 as explained in Subjects and Methods.
Presented separately because those not establishing breastfeeding were included in this group.
Does not include those who breastfed for <2 wk.
Statistical analyses
At all ages, all individuals with a BMI measurement were included because the subsample with information on BMI at all ages (n = 2) was too small for separate analysis. The estimates are therefore based on differing subsamples. The effects of duration of BF and age at introduction of CF on offspring BMI z scores were explored in linear regression models with and without adjustment for covariates. Preliminary analyses and visual inspection indicated that it was appropriate to include BF and CF as linear variables at all ages. Birth weight, maternal prepregnant BMI, and maternal age were also included as continuous variables, whereas the remaining covariates were included as categorical variables with dummy coding. Logistic regression was used to explore the effect of BF and CF on the risk of adult (42 y) overweight and obesity. The regression analyses were conducted by using SPSS (version 15.0; SPSS Inc, Chicago, IL).
RESULTS
The characteristics of the study participants and their mothers are shown in Table 1. The prevalence of overweight was low among the mothers (11%), but had increased to 34% and 51% in the adult (42 y) women and men, respectively. On the basis of the International Obesity Task Force's criteria for childhood overweight (24), the prevalence ranged from 3% to 5% in both girls and boys during the school years.
The median (10th, 90th percentiles) durations of any BF and age at introduction of spoon-feeding were 2.50 (0.23, 6.50) and 3.50 (2.00, 6.00) mo, respectively. The mean (±SD) age at introduction of CF for spoon-feeding, vegetables, egg, meat, and firm food were 3.80 ± 1.49, 4.05 ± 1.34, 6.08 ± 1.84, 6.87 ± 1.92, and 8.10 ± 2.08 mo, respectively. Duration of BF was positively correlated with introduction of all CF food items except firm food. Age at introduction of spoon-feeding, vegetables, egg, meat, and firm food were all positively correlated, but the correlations between spoon-feeding and vegetables (r = 0.80) and between egg and meat (r = 0.57) were especially high (Table 2). Duration of BF and age at introduction of spoon-feeding were not similarly related to covariates (data not shown). Mothers with high prepregnancy BMI breastfed for a shorter time and tended to start spoon-feeding at an earlier age. Infants from a high social class were breastfed longer, but were also introduced earlier to spoon-feeding. The same was seen for infants with the highest birth weight; they were breastfed for a longer period, but were also introduced earlier to spoon-feeding. Compared with normal-weight subjects, participants who were overweight or obese as adults (42 y) had a higher birth weight, had mothers who were younger, had mothers who were heavier before pregnancy, and had mothers from a lower social class (Table 3).
TABLE 2.
Spearman correlation coefficients between duration of any breastfeeding (in mo) and age (in mo) at the time of introduction of complementary feeding1
| Spoon-feeding | Vegetables | Egg | Meat | Firm food | |
| Breastfeeding | 0.17 (5068) | 0.20 (4898) | 0.06 (4392) | 0.07 (5444) | 0.02 (4339)2 |
| Spoon-feeding | 0.80 (4898) | 0.30 (4392) | 0.24 (4544) | 0.17 (4339) | |
| Vegetables | 0.34 (4291) | 0.28 (4455) | 0.17 (3967) | ||
| Egg | 0.57 (4216) | 0.28 (3967) | |||
| Meat | 0.37 (4130) |
n in parentheses. P < 0.001 for all, except where otherwise indicated.
P = 0.163.
TABLE 3.
Relation between covariates and offspring adult BMI category (age 42 y)
| Offspring adult BMI (kg/m2) |
||||||
| n | <18.5 | ≥18.5 to <25 | ≥25 to <30 | ≥30 | P1 | |
| Total sample (%) | 2884 | 2 | 56 | 31 | 11 | |
| Maternal characteristics | ||||||
| Age (y) | 2875 | 28.2 ± 7.92 | 26.7 ± 6.6 | 26.3 ± 6.5 | 25.4 ± 6.2 | 0.004 |
| Maternal BMI (kg/m2) | 2677 | 20.5 ± 2.0 | 21.4 ± 2.7 | 21.8 ± 2.8 | 23.1 ± 3.6 | <0.001 |
| Gestational weight gain (kg) | 1800 | 10.4 ± 3.9 | 11.4 ± 3.5 | 11.6 ± 3.6 | 11.9 ± 3.5 | 0.067 |
| Social status at birth (1- to 8-point scale) | 2750 | 4.53 ± 1.99 | 4.36 ± 1.88 | 4.09 ± 1.78 | 3.56 ± 1.56 | <0.001 |
| Breadwinner's education (1- to 4-point scale) | 2695 | 2.43 ± 0.65 | 2.51 ± 0.73 | 2.41 ± 0.68 | 2.25 ± 0.58 | <0.001 |
| Single mother (%) | 2860 | 17 | 28 | 31 | 34 | 0.041 |
| Smoking in pregnancy (%) | 2834 | 61 | 50 | 50 | 55 | 0.158 |
| Infant characteristics | ||||||
| Preterm (%)3 | 2350 | 19 | 17 | 20 | 19 | 0.363 |
| Birth weight (g) | 2842 | 2974 ± 537 | 3222 ± 563 | 3255 ± 582 | 3294 ± 586 | 0.002 |
| Duration of breastfeeding (mo) | 2884 | 2.32 ± 2.0 | 3.24 ± 2.90 | 2.97 ± 2.80 | 2.73 ± 2.58 | <0.001 |
| Timing of spoon-feeding (mo) | 2884 | 3.70 ± 1.26 | 3.85 ± 1.52 | 3.75 ± 1.42 | 3.64 ± 1.40 | 0.082 |
ANOVA, chi-square test, or Kruskal-Wallis test.
Mean ± SD (all such values).
Born at gestational week ≤37 as explained in Subjects and Methods.
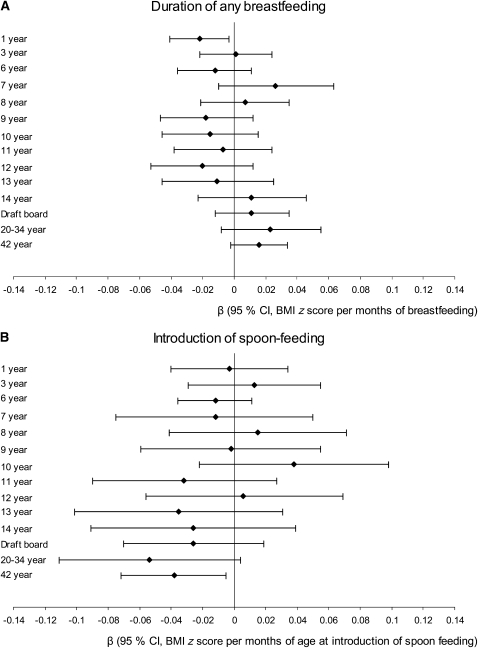
The longest duration of BF (P < 0.001) and latest introduction of spoon-feeding (P = 0.082) were seen among the normal-weight subjects (Table 3). When BMI z scores were regressed from 1 to 42 y on duration of BF and only adjusted for sex, a significant inverse association was seen at 1 y (β: −0.018; 95% CI: −0.032, −0.007) and at 42 y (β: −0.019; 95% CI: −0.031, −0.006) for BMI z scores per month of BF at 1 and 42 y, respectively. Restricting the same analyses to the sample with information on all covariates and age at introduction of spoon-feeding (but not adjusting for it), the associations persisted for BMI z scores at 1 y (β: −0.025; 95% CI: −0.043, −0.007), but not for those at 42 y (β: −0.005; 95% CI: −0.024, 0.013). When covariates were adjusted for the age at introduction of spoon-feeding, only the inverse association at 1 y remained significant (β: −0.022; 95% CI: −0.041, −0.003) (Figure 1).
FIGURE 1.
Results from regression analyses with BMI z scores from age 1 to 42 y as dependent variables and the duration of breastfeeding (A) and age at introduction of complementary feeding (B) as independent variables included in the same analysis. Each line represents the β coefficient with 95% CI from a single analysis. All models were further adjusted for sex, maternal age at birth, prepregnancy BMI, gestational weight gain, smoking during pregnancy, social class, breadwinner's education, single mother status, prematurity, and birth weight. The number of subjects varied from 367 (BMI at 7 y) to 1675 (BMI at 1 y).
When BMI z scores were regressed from 1 to 42 y on age at introduction of spoon-feeding and adjusted for only sex, significant or borderline significant inverse associations were seen at all ages (P < 0.100) at 1, 7, 8, 9, 11, 12, and 14 y; draft board; 20–34 y, and 42-y follow-up. Only the associations at age 14, 20–34, and 42 y persisted in unadjusted analyses of the reduced sample with information on all covariates (data not shown). When adjustment for covariates was carried out together with inclusion of BF, the inverse association at 20–34 y (P = 0.07) and 42 y persisted (P = 0.03) (Figure 1).
Although age at introduction of spoon-feeding did not seem to influence 1-y BMI z scores, it was explored whether the effect of spoon-feeding on adult BMI (42 y) was explained by an effect of infancy weight gain by including 1-y weight and exact age at the measurement in the model. With adult BMI z scores (42 y) as the dependent variable, the effect (β) of spoon-feeding was −0.048 (95% CI: −0.088, −0.007) and −0.046 (95% CI: −0.086, −0.006) when weight at 1 y was also included in the model (n = 992 in both models).
In the regression models shown in Figure 1, no significant interactions between duration of BF and age at introduction of spoon-feeding were seen (P > 0.05 at all ages) as explored by including the product of BF (mo) and spoon-feeding (mo) or the interaction term of BF <2 mo (46%) or ≥2 mo (54%) and spoon-feeding (mo). An analysis stratified on duration of BF further confirmed this finding: at 42 y, the association (β) of spoon-feeding (age in mo) with BMI (kg/m2) was −0.151 (95% CI: −0.369, 0.068) and −0.101 (95% CI: −0.304, 0.100) for individuals with BF <3 mo compared with ≥3 mo, respectively (P for interaction = 0.644)
The analyses with BMI z scores from 1 to 42 y as outcome were repeated with age at introduction of vegetables, egg, meat, and firm food as independent variables and adjusted for covariates and duration of BF. In these analyses, BF was again not associated with BMI after age 1 y (data not shown). Age at introduction of vegetables was inversely associated with BMI z scores at 20–34 y (β: −0.072; 95% CI: −0.133, −0.010) and 42 y (β: −0.064; 95% CI: −0.101, −0.027). Age at introduction of egg was not associated with BMI z scores at any age (data not shown). Age at introduction of meat was inversely associated with BMI z scores at age 42 y (β: −0.032; 95% CI: −0.059, −0.005). Age at introduction of firm food was inversely associated with BMI z scores at 1 y (β: −0.053; 95% CI: −0.082, −0.024), 10 y (β: −0.054; 95% CI: −0.096, −0.012), 11 y (β: −0.057; 95% CI: −0.101, −0.013), and borderline significant (P = 0.097) at 42 y (β: −0.022; 95% CI: −0.048, 0.004).
Waist circumference at age 42 y was also used as the outcome, with adjustment for all covariates and both with and without simultaneous adjustment for current BMI. Duration of BF was not associated with waist circumference in any of the models. Early introduction of CF (spoon-feeding and vegetables) was associated with larger waist circumference when not adjusted for BMI. After adjustment for BMI, the effect was reduced (data not shown) but remained significant for spoon-feeding (β: −0.25; 95% CI: −0.49, −0.01; waist circumference in cm per month of age at introduction of spoon-feeding).
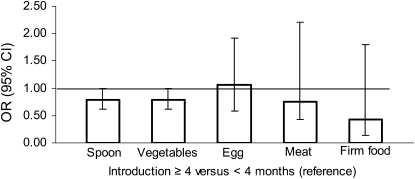
Logistic regression analyses with overweight and obesity at 42 y as outcome variables confirmed the findings from the linear regression analyses. In adjusted models, no effect was seen for duration of BF, whereas risk of overweight decreased with increasing age (mo) at introduction of vegetables (P = 0.014), meat (P = 0.044), and firm food (P = 0.012), but not spoon-feeding (P = 0.112) and egg (P = 0.527; Table 4). In a comparison of the effect of early (<4 mo) compared with late (≥4 mo) introduction of CF, a protective effect of late introduction was seen for vegetables (P = 0.046) and a similar trend was seen for spoon-feeding (P = 0.053; Figure 2).
TABLE 4.
Estimates from logistic regression analyses with adult (age 42 y) overweight and obesity as outcomes1
| OR (95% CI) |
|||
| n | Overweight | Obesity | |
| Age (mo) at introduction of spoon-feeding | 1305 | 0.94 (0.86, 1.02) | 0.94 (0.83, 1.08) |
| Duration of breastfeeding | 1.00 (0.96, 1.05) | 1.05 (0.98, 1.12) | |
| Age (mo) at introduction of vegetables | 1270 | 0.90 (0.81, 0.98) | 0.89 (0.76, 1.03) |
| Duration of breastfeeding | 1.01 (0.97, 1.06) | 1.06 (0.99, 1.14) | |
| Age (mo) at introduction of eggs | 1146 | 0.98 (0.91, 1.05) | 1.06 (0.96, 1.18) |
| Duration of breastfeeding | 0.99 (0.97, 1.06) | 1.02 (0.95, 1.10) | |
| Age (mo) at introduction of meat | 1188 | 0.93 (0.87, 1.00) | 0.97 (0.87, 1.08) |
| Duration of breastfeeding | 0.99 (0.95, 1.04) | 1.03 (0.96, 1.10) | |
| Age (mo) at introduction of firm food | 1132 | 0.92 (0.86, 0.98) | 1.00 (0.90, 1.11) |
| Duration of breastfeeding | 0.99 (0.94,1.03) | 1.03 (0.95, 1.11) | |
OR, odds ratio. All models were further adjusted for sex, maternal age at birth, prepregnancy BMI, gestational weight gain, smoking during pregnancy, social class, breadwinner's education, single mother status, prematurity, and birth weight. When the analyses were conducted with only the 1009 subjects for whom information on age at introduction of spoon-feeding, vegetables, eggs, meat, and firm food were available, the findings did not change.
FIGURE 2.
Results from logistic regression analyses with overweight [BMI (in kg/m2) ≥ 25] at age 42 y as the outcome variable and age [≥4 mo compared with <4 mo of age (reference)] at the time of introduction of complementary feeding (spoon, vegetables, eggs, meat, and firm food) as independent variables in separate analyses [odds ratios (ORs) and 95% CIs]. All models were further adjusted for sex, maternal age at birth, prepregnancy BMI, gestational weight gain, smoking during pregnancy, social class, breadwinner's education, single mother status, prematurity, birth weight, and duration of breastfeeding. The numbers of subjects in each analysis were as follows: 1305 for spoon-feeding, 1270 for vegetables, 1146 for egg, 1188 for meat, and 1132 for firm food.
Finally, the pattern of introduction of CF was described by the number of food items (spoon-feeding, vegetables, egg, meat, and firm food) introduced at 3, 4, 5, and 6 mo of age (Table 5). The OR for overweight increased with increasing number of food items introduced, but it was not significant.
TABLE 5.
Frequency distribution of the number of food items introduced at different ages and the association with risk of overweight at age 42 y1
| Number of food items | 3 mo (n = 1009) | 4 mo (n = 1009) | 5 mo (n = 1009) | 6 mo (n = 1009) |
| % | % | % | % | |
| 0 | 52 | 25 | 12 | 2 |
| 1 | 11 | 7 | 5 | 1 |
| 2 | 33 | 53 | 49 | 21 |
| 3 | 3 | 10 | 20 | 28 |
| 4 | 1 | 4 | 14 | 33 |
| 5 | 0 | 1 | 2 | 15 |
| OR (95% CI) for overweight (42 y)2 | 1.10 (0.97, 1.26) | 1.08 (0.95, 1.22) | 1.06 (0.94, 1.20) | 1.09 (0.97, 1.23) |
Values were calculated as the sum of spoon-feeding, vegetables, egg, meat, and firm food. OR, odds ratio.
All models were further adjusted for sex, maternal age at birth, prepregnancy BMI, gestational weight gain, smoking during pregnancy, social class, breadwinner's education, single mother status, prematurity, birth weight, and duration of breastfeeding.
DISCUSSION
Our study suggests that a longer duration of BF was associated with a lower BMI at 1 y of age, but no effect was seen later in childhood or in adulthood. A late introduction of CF was associated with a lower BMI at age 42 y, but, earlier in life, no effect of age at introduction of CF was seen.
Breastfeeding and obesity
Because most of the subjects in our cohort were breastfed for at least some days and because we do not have detailed information on who was never breastfed, our study explored the effect of duration of BF. A longer duration of BF was associated with a lower BMI at age 1y, which agrees with more recent studies (25). The associations we found between duration of BF and covariates were also as in more recent cohorts (26–28); a longer duration of BF was associated with better social conditions in infancy (higher social class, higher breadwinner's education, fewer single mothers), a lower prevalence of smoking during pregnancy, and a lower maternal prepregnancy BMI.
Our finding of no association between BF and obesity in adulthood is in line with other studies of varying size, design, and confounder control (7, 29–34), although one study suggested that BF was associated with an increased risk of obesity (35). Compared with many of these studies, an advantage of our study was the use of information on BF collected at the end of infancy compared with maternal recall later in childhood (31, 33, 34) or in adulthood (7, 35).
One important difference between studies with follow-up in childhood and adulthood relates to the increasing prevalence of obesity among children (2). In our study, the prevalence of overweight according to International Obesity Task Force criteria was 3–5% during the school years, which increased to 35% and 51% for women and men, respectively, at age 42 y. In our sample, overweight was therefore primarily developed in adulthood, and different mechanisms may exist for this compared with overweight developed during childhood in contemporary children. Some studies of contemporary children suggest that BF has its most protective effect in the upper end of the BMI distribution (36), with only a minor—if any—effect on the mean BMI (37, 38). Among children, the obesity epidemic is characterized by an increase in the upper end of the BMI distribution (39); consequently, we found that the possibility of a protective effect of BF in contemporary children should not be excluded. The composition of the current formula is closer to human milk than is the formula used in the 1960s; therefore, it does not seem likely that a change in formula composition explains the possible protective effect of BF in contemporary children.
Complementary feeding and obesity
At the 1-y examination, questions were asked about age at introduction of spoon-feeding, vegetables, egg, meat, and firm food. Except for egg, later introduction of CF was associated with a lower adult mean BMI (only borderline significant for meat) and a 6–10% lower risk of overweight per 1-mo delay (only borderline significant for spoon-feeding).
Food items introduced late in the weaning period (meat and firm food) were also associated with later obesity in separate analyses. This may have been due to the significant associations between the ages at the time of introduction of the different food items. Another interpretation is that early introduction of meat and firm food is a marker of a high intake of CF. The effect of early introduction of CF could then be more due to an adverse effect of eating a large quantity of CF at an early age than to an effect of age at which CF is introduced. The analyses exploring the effect of number of food items introduced at a specific age showed a pattern consistent with the latter hypothesis, but it was not significant. Our finding also suggests that early introduction of CF (only seen for the variable spoon-feeding) was associated with increased waist circumference, even after adjustment for BMI at age 42 y.
Other studies that explored a potential effect of age at introduction of CF on risk of obesity have included follow-up in childhood, the majority of which found no effects (9, 10, 17–19). Correspondingly, we found no consistent effect of age at introduction of CF on BMI in childhood. It may be that the effect of CF is at least partly expressed already during adolescence and that the effect did not become statistically significant because of a lower number of individuals than at the 42-y follow-up examination. Additional analyses of skewness to explore an effect on the distribution not reflected in the mean (based on the residuals from adjusted regression analyses; data not shown) of the BMI distributions at different ages showed no skewness during childhood but a marked skewness at the later follow-up examinations. There were, however, no difference in this skewness pattern between subjects introduced to CF at <4 mo and those introduced to CF at ≥4 mo.
Interestingly, and in support of a potential programming effect, a prospective Scottish study found that introduction of CF before 15 wk of age was associated with increased weight and percentage body fat at 7 y of age, whereas no effect on weight was seen at the age of 2 y (11). The analysis was adjusted for body weight at the age of introduction of CF, because weight at this time may be a potential confounder because heavier infants are introduced earlier to CF (11–13, 40) Similarly, it appeared in our cohort that an increase in infancy weight gain was not part of a mechanism explaining the association between CF and adult obesity.
It is possible that the early introduction of CF makes individuals more susceptible to an obesogenic environment, which would imply that the expression of the effect is dependent on environmental factors. The mechanism may involve permanent biological changes directly related to weight regulation, such as adipogenesis, appetite control, or effects on the central nervous system that are later associated with behavior leading to overweight and obesity. In a broader perspective, we believe that the observation fits well into other recent findings of obesity risk factors that act during fetal life and are not expressed until later in life. For example, in the same cohort, we reported a positive association between gestational weight gain and offspring BMI, which was not fully expressed until adulthood (41). It has also been reported that maternal smoking during pregnancy results in lower birth weight, but an increased risk of obesity later in childhood (42).
Regarding composition of CF and later obesity, interest has focused on the protein-adiposity hypothesis; ie, a high protein intake in early childhood increases the risk of adiposity in childhood (43). This hypothesis was supported by some studies that explored the role of protein intake at 12 mo of age (44, 45), but not by other studies of protein intake at 6 (44) or 9 (46) mo. In agreement with this finding, our data did not suggest a particular role of the food items egg and meat, which are more protein rich than the other food items. However, we did not have sufficient information on protein intake because the quantity of milk was not recorded.
Whereas residual confounding remains a major concern when addressing the effect of BF on obesity risk, it seems different with respect to CF, because later introduction of CF was associated with lower social class in infancy and not with maternal smoking during pregnancy. A high maternal prepregnancy BMI was associated with an earlier introduction of CF, but only weakly so. Therefore, it seems unlikely that residual confounding by social class, maternal smoking, or maternal BMI explained the observed association between introduction of CF and later obesity. A limitation of our data regarding adult BMI (42 y) was that weight and height were self-reported, and participation may be subject to some bias because of a lower participation rate among overweight subjects (Table 1). Selective underreporting by the heaviest subjects, however, is expected to lead to a weakening of the association. The potential bias with respect to participating in the follow-up will only affect the association if it is different among participants and nonparticipants, which we find unlikely.
According to current recommendations from the World Health Organization (WHO), infants should be exclusively breastfed for the first 6 mo of life, with introduction of CF and continued BF thereafter. It has been argued that the evidence for the benefits of this change in recommendation for infants in high-income countries compared with the previous WHO recommendation of exclusive BF for 4 to 6 mo is not strong, and that there is a need for more studies (47). One concern has been that exclusive BF may not provide sufficient energy at the age of 6 mo (48). Whether 6 mo is also the optimal time to introduce CF in formula-fed infants has received little attention (49). In our study, 17% of the participants were introduced to spoon-feeding before 3 mo, and 46% did not start until 4 mo of age or later. Our study therefore suggests that postponing the introduction of CF to the recommended 6 mo will be beneficial with respect to risk of later overweight. Too late an introduction of CF (>6 mo) may compromise the supply of total energy, protein, and some micronutrients (50, 51) and might also result in problems with acceptance of new tastes and textures (52).
Conclusions
In the Copenhagen Perinatal Cohort, born in 1959–1961 and followed into adulthood, longer duration of BF was associated with a lower BMI at the age of 1 y after several covariates and age at introduction of CF were taken into account; thereafter, however, the effect disappeared. The present study indicates that early introduction of CF may be associated with an increased BMI and risk of overweight in adulthood independent of BF and several covariates.
Acknowledgments
We are grateful for the collection of the data by the late Aage Willumsen and Bengt Zachau-Christiansen, and we thank June M Reinisch et al for contributing height and weight data on the subjects examined at draft and at age 20–34 y.
The authors’ responsibilities were as follows—LS-N, TIAS, and KFM: formulated the specific hypothesis of the study; LS-N: analyzed the data and drafted the manuscript; ELM: participated in data collection at the first adult follow-up examination, contributed knowledge about the cohort and data structure, and contributed to data interpretation and manuscript drafting; TIAS: initiated an obesity research program in this cohort, coordinated the last adult follow-up, and contributed to data interpretation and drafting of the manuscript; and KFM: contributed to data interpretation and manuscript drafting. All authors participated in preparing the final draft of the manuscript. No author had a financial or personal conflict of interest related to this research or its source of funding. TIAS has industrial collaborations (see http://www.ipm.regionh.dk/person/tias/Disclosures.html).
REFERENCES
- 1.Olsen LW, Baker JL, Holst C, Sørensen TIA. Birth cohort effect on the obesity epidemic in Denmark. Epidemiology 2006;17:292–5 [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes 2006;1:11–25 [DOI] [PubMed] [Google Scholar]
- 3.Jackson-Leach R, Lobstein T. Estimated burden of paediatric obesity and co-morbidities in Europe. Part 1. The increase in the prevalence of child obesity in Europe is itself increasing. Int J Pediatr Obes 2006;1:26–32 [DOI] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services, Centers for Disease Control and Prevention. Physical activity and good nutrition: essential elements to prevent chronic diseases and obesity. 2007. Available from: www.cdc.gov/nccdphp/publications/aag/pdf/dnpa.pdf (cited 6 December 2007) [PubMed]
- 5.Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Rockville, MD: Agency for Healthcare Research and Quality, 2007. (Evidence Report/Technology Assessment no. 153.) [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer MS, Matush L, Vanilovich I, et al. Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: evidence from a large randomized trial. Am J Clin Nutr 2007;86:1717–21 [DOI] [PubMed] [Google Scholar]
- 7.Michels KB, Willett WC, Graubard BI, et al. A longitudinal study of infant feeding and obesity throughout life course. Int J Obes (Lond) 2007;31:1078–85 [DOI] [PubMed] [Google Scholar]
- 8.Baker JL, Michaelsen KF, Rasmussen KM, Sørensen TIA. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am J Clin Nutr 2004;80:1579–88 [DOI] [PubMed] [Google Scholar]
- 9.Burdette HL, Whitaker RC, Hall WC, Daniels SR. Breastfeeding, introduction of complementary foods, and adiposity at 5 y of age. Am J Clin Nutr 2006;83:550–8 [DOI] [PubMed] [Google Scholar]
- 10.Kramer MS. Do breast-feeding and delayed introduction of solid foods protect against subsequent obesity? J Pediatr 1981;98:883–7 [DOI] [PubMed] [Google Scholar]
- 11.Forsyth JS, Ogston SA, Clark A, Florey CD, Howie PW. Relation between early introduction of solid food to infants and their weight and illnesses during the first two years of life. BMJ 1993;306:1572–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fewtrell MS, Lucas A, Morgan JB. Factors associated with weaning in full term and preterm infants. Arch Dis Child Fetal Neonatal Ed 2003;88:F296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan JB, Lucas A, Fewtrell MS. Does weaning influence growth and health up to 18 months? Arch Dis Child 2004;89:728–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta KC, Specker BL, Bartholmey S, Giddens J, Ho ML. Trial on timing of introduction to solids and food type on infant growth. Pediatrics 1998;102:569–73 [DOI] [PubMed] [Google Scholar]
- 15.Wilson AC, Forsyth JS, Greene SA, Irvine L, Hau C, Howie PW. Relation of infant diet to childhood health: seven year follow up of cohort of children in Dundee infant feeding study. BMJ 1998;316:21–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hediger ML, Overpeck MD, Kuczmarski RJ, Ruan WJ. Association between infant breastfeeding and overweight in young adults. JAMA 2001;285:2453–60 [DOI] [PubMed] [Google Scholar]
- 17.Zive MM, McKay H, Frank-Spohrer GC, Broyles SL, Nelson JA, Nader PR. Infant-feeding practices and adiposity in 4-y-old Anglo- and Mexican-Americans. Am J Clin Nutr 1992;55:1104–8 [DOI] [PubMed] [Google Scholar]
- 18.Maffeis C, Micciolo R, Must A, Zaffanello M, Pinelli L. Parental and perinatal factors associated with childhood obesity in north-east Italy. Int J Obes Relat Metab Disord 1994;18:301–5 [PubMed] [Google Scholar]
- 19.Reilly JJ, Armstrong J, Dorosty AR, et al. Early life risk factors for obesity in childhood: cohort study. BMJ 2005;330:1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zachau-Christiansen B, Ross EM. Babies: human development during the first year. Bristol, United Kingdom: JW Arrowsmith Ltd, 1975 [Google Scholar]
- 21.Rugholm S, Baker JL, Olsen LW, Schack-Nielsen L, Bua J, Sørensen TIA. Stability of the association between birth weight and childhood overweight during the development of the obesity epidemic. Obes Res 2005;13:2187–94 [DOI] [PubMed] [Google Scholar]
- 22.Reinisch JM, Mortensen EL, Sanders SA. The Prenatal Development Project. Acta Psychiatr Scand Suppl 1993;370:54–61 [DOI] [PubMed] [Google Scholar]
- 23.Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Arch Dis Child 1995;73:25–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dewey KG, Peerson JM, Brown KH, et al. Growth of breast-fed infants deviates from current reference data: a pooled analysis of US, Canadian, and European data sets. World Health Organization Working Group on Infant Growth. Pediatrics 1995;96:495–503 [PubMed] [Google Scholar]
- 26.Rasmussen KM. Association of maternal obesity before conception with poor lactation performance. Annu Rev Nutr 2007;27:103–21 [DOI] [PubMed] [Google Scholar]
- 27.Baker JL, Michaelsen KF, Sørensen TIA, Rasmussen KM. High prepregnant body mass index is associated with early termination of full and any breastfeeding in Danish women. Am J Clin Nutr 2007;86:404–11 [DOI] [PubMed] [Google Scholar]
- 28.Amir LH, Donath S. A systematic review of maternal obesity and breastfeeding intention, initiation and duration. BMC Pregnancy Childbirth 2007;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Victora CG, Barros F, Lima RC, Horta BL, Wells J. Anthropometry and body composition of 18 year old men according to duration of breast feeding: birth cohort study from Brazil. BMJ 2003;327:901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin RM, Holly JM, Middleton N, Davey SG, Gunnell D. Childhood diet and insulin-like growth factors in adulthood: 65-year follow-up of the Boyd Orr Cohort. Eur J Clin Nutr 2007;61:1281–92 [DOI] [PubMed] [Google Scholar]
- 31.Poulton R, Williams S. Breastfeeding and risk of overweight. JAMA 2001;286:1449–50 [PubMed] [Google Scholar]
- 32.Eriksson J, Forsen T, Osmond C, Barker D. Obesity from cradle to grave. Int J Obes Relat Metab Disord 2003;27:722–7 [DOI] [PubMed] [Google Scholar]
- 33.Parsons TJ, Power C, Manor O. Infant feeding and obesity through the lifecourse. Arch Dis Child 2003;88:793–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin RM, Smith GD, Mangtani P, Frankel S, Gunnell D. Association between breast feeding and growth: the Boyd-Orr cohort study. Arch Dis Child Fetal Neonatal Ed 2002;87:F193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin RM, Ben Shlomo Y, Gunnell D, Elwood P, Yarnell JW, Davey SG. Breast feeding and cardiovascular disease risk factors, incidence, and mortality: the Caerphilly study. J Epidemiol Community Health 2005;59:121–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toschke AM, von Kries R, Beyerlein A, Ruckinger S. Risk factors for childhood obesity: shift of the entire BMI distribution vs. shift of the upper tail only in a cross sectional study. BMC Public Health 2008;8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owen CG, Martin RM, Whincup PH, Smith G. D, Gillman MW, Cook DG. The effect of breastfeeding on mean body mass index throughout life: a quantitative review of published and unpublished observational evidence. Am J Clin Nutr 2005;82:1298–307 [DOI] [PubMed] [Google Scholar]
- 38.Grummer-Strawn LM, Mei Z. Does breastfeeding protect against pediatric overweight? Analysis of longitudinal data from the Centers for Disease Control and Prevention Pediatric Nutrition Surveillance System. Pediatrics 2004;113:e81–6 [DOI] [PubMed] [Google Scholar]
- 39.Bua J, Olsen LW, Sørensen TIA. Secular trends in childhood obesity in Denmark during 50 years in relation to economic growth. Obesity (Silver Spring) 2007;15:977–85 [DOI] [PubMed] [Google Scholar]
- 40.Ong KK, Emmett PM, Noble S, Ness A, Dunger DB. Dietary energy intake at the age of 4 months predicts postnatal weight gain and childhood body mass index. Pediatrics 2006;117:e503–8 [DOI] [PubMed] [Google Scholar]
- 41.Schack-Nielsen L, Michaelsen KF, Gamborg M, Mortensen EL, Sørensen TIA. Gestational weight gain in relation to offspring body mass index and obesity from infancy through adulthood. Int J Obes (Lond) (Epub ahead of print 17 November 2009). [DOI] [PubMed] [Google Scholar]
- 42.Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond) 2008;32:201–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rolland-Cachera MF, Deheeger M, Akrout M, Bellisle F. Influence of macronutrients on adiposity development: a follow up study of nutrition and growth from 10 months to 8 years of age. Int J Obes Relat Metab Disord 1995;19:573–8 [PubMed] [Google Scholar]
- 44.Gunther AL, Buyken AE, Kroke A. Protein intake during the period of complementary feeding and early childhood and the association with body mass index and percentage body fat at 7 y of age. Am J Clin Nutr 2007;85:1626–33 [DOI] [PubMed] [Google Scholar]
- 45.Scaglioni S, Agostoni C, Notaris RD, et al. Early macronutrient intake and overweight at five years of age. Int J Obes Relat Metab Disord 2000;24:777–81 [DOI] [PubMed] [Google Scholar]
- 46.Hoppe C, Molgaard C, Thomsen BL, Juul A, Michaelsen KF. Protein intake at 9 mo of age is associated with body size but not with body fat in 10-y-old Danish children. Am J Clin Nutr 2004;79:494–501 [DOI] [PubMed] [Google Scholar]
- 47.Fewtrell MS, Morgan JB, Duggan C, et al. Optimal duration of exclusive breastfeeding: what is the evidence to support current recommendations? Am J Clin Nutr 2007;85:635S–8S [DOI] [PubMed] [Google Scholar]
- 48.Reilly JJ, Wells JC. Duration of exclusive breast-feeding: introduction of complementary feeding may be necessary before 6 months of age. Br J Nutr 2005;94:869–72 [DOI] [PubMed] [Google Scholar]
- 49.Agostoni C, Decsi T, Fewtrell M, et al. Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 2008;46:99–110 [DOI] [PubMed] [Google Scholar]
- 50.Butte NF, Lopez-Alarcon MG, Garza C. Nutrient adequacy of exclusive breastfeeding for the term infant during the first six months of life. Geneva, Switzerland: World Health Organization, 2002 [Google Scholar]
- 51.Reilly JJ, Ashworth S, Wells JC. Metabolisable energy consumption in the exclusively breast-fed infant aged 3–6 months from the developed world: a systematic review. Br J Nutr 2005;94:56–63 [DOI] [PubMed] [Google Scholar]
- 52.Pridham KF. Feeding behavior of 6- to 12-month-old infants: assessment and sources of parental information. J Pediatr 1990;117:S174–80 [DOI] [PubMed] [Google Scholar]