Abstract
Background: Few studies have examined the associations of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) with biomarkers of chronic disease risk in populations with high intakes.
Objective: We examined the associations of red blood cell (RBC) EPA and DHA, as percentages of total fatty acids, with biomarkers of chronic disease risk across a wide range of EPA and DHA intakes.
Design: In a cross-sectional study of 357 Yup'ik Eskimos, generalized additive models were used to plot covariate-adjusted associations of EPA and DHA with chronic disease biomarkers. Linear regression models were used to test for the statistical significance of these associations.
Results: Means (5th–95th percentiles) for RBC EPA and DHA were 2.8% (0.5–5.9%) and 6.8% (3.3–9.0%), respectively. Associations of EPA and DHA were inverse and linear for triglycerides (β ± SE = −0.10 ± 0.01 and −0.05 ± 0.01, respectively) and positive and linear for HDL cholesterol (β ± SE = 2.0 ± 0.5 and 0.9 ± 0.6, respectively) and apolipoprotein A-I (β ± SE = 2.6 ± 0.8 and 1.7 ± 0.8, respectively). Positive linear associations of DHA with LDL and total cholesterol (β ± SE = 7.5 ± 1.4 and 6.80 ± 1.57, respectively) were observed; for EPA, these associations were nonlinear and restricted to concentrations ≈<5% of total fatty acids. Associations of EPA and DHA with C-reactive protein were inverse and nonlinear: for EPA, the association appeared stronger at concentrations ≈>3% of total fatty acids; for DHA, it was observed only at concentrations ≈>7% of total fatty acids.
Conclusion: Increasing EPA and DHA intakes to amounts well above those consumed by the general US population may have strong beneficial effects on chronic disease risk.
INTRODUCTION
The long chain omega-3 (n−3) polyunsaturated fatty acids (PUFAs) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), derived in the human diet primarily from fish and marine mammals, are associated with a reduced risk of cardiovascular disease (CVD) (1) and possibly cancer (2) and type 2 diabetes (3). Multiple interrelated mechanisms could explain the effects of EPA and DHA on disease risk, including 1) a shift from the arachidonic acid–derived eicosanoid pathway to the less inflammatory EPA-derived pathway (4), 2) transcriptional regulation of cytokine production (4), and 3) direct effects on the expression of several genes pertinent to the immune response, inflammation, lipid metabolism, and energy utilization (5, 6). As a consequence of these effects, diets rich in EPA and DHA are associated with several blood biomarkers of chronic disease risk, such as decreases in triglyceride concentrations (7–15), the number of small dense LDL particles (16–18), and proinflammatory cytokine and C-reactive protein (CRP) concentrations (4) and increases in HDL cholesterol (8–14) and antiinflammatory cytokine (4) concentrations.
The associations of EPA and DHA intakes with biomarkers of chronic disease risk have been studied primarily in populations with a relatively low and narrow range of EPA and DHA intakes. Only 3 published observational studies were based on populations with chronic, high intakes of EPA and DHA (12, 13, 19); however, none reached intakes as high as those of Yup'ik Eskimos and none examined whether associations remain constant or are nonlinear across a broad range of intakes. Because of their traditional diet, which is based largely on fish and other marine foods (20), Yup'ik Eskimos have a mean intake of EPA and DHA that is >20 times the current mean intake of the general US population (3.7 compared with 0.14 g/d in men and 2.4 compared with 0.09 g/d in women) (21). Studies of Yup'ik Eskimos offer a unique opportunity to examine how a broad range of EPA and DHA intakes (22) affect chronic disease biomarkers.
In this manuscript, we examined the associations of EPA and DHA with biomarkers of chronic disease risk in a population-based sample of Yup'ik Eskimos. We specifically test whether associations are linear across the wide range of EPA and DHA intakes or whether associations are stronger or weaker at high than at low intakes. These results can influence guidelines regarding the benefits or risks of increasing intakes of EPA and DHA to amounts consumed by Yup'ik Eskimos.
SUBJECTS AND METHODS
Participant recruitment and procedures
Data are from the Center for Alaska Native Health Research (CANHR) study, a cross-sectional community-based participatory research study of obesity and chronic disease risk factors in Yup'ik Eskimos. Study protocols were approved by the University of Alaska Institutional Review Board, the National and Area Indian Health Service Institutional Review Boards, and the Yukon Kuskokwim Human Studies Committee.
Participant recruitment and procedures are described in detail elsewhere (23, 24). In brief, between 2003 and 2006, a total of 1003 participants aged ≥14 y and residing in 10 communities in southwest Alaska were enrolled in the study. Extensive sociodemographic, medical, and other health-related data were collected through in-person interviews and self-administered questionnaires. Diet was assessed by using a 24-h dietary recall and a 3-d food record. Communities in the Yukon Kuskokwim River Delta prohibit consumption and possession of alcoholic beverages, which was therefore not assessed. Height, weight, body composition (through bioelectrical impedance), and blood pressure were obtained by trained clinical staff. Physical activity was measured by using pedometers. Blood samples collected in the field were separated into serum, plasma, or packed red blood cells (RBCs); frozen; stored locally at −20 °C for up to 6 d; and transferred to a central location and stored at −80 °C. RBC PUFAs are stable for ≥4 wk at −20 °C (25, 26).
Study sample
Blood samples used in this study were from a subset of 497 CANHR participants selected from 7 of the 10 participating communities. From 4 communities, we selected a random sample of 84 participants balanced across age strata (14–19, 20–49, and >50 y). The remaining 3 communities had <84 participants; thus, all participants were included in these analyses. We excluded 92 participants aged ≤18 y, 28 with CRP concentrations > 1 mg/dL (indicative of acute inflammation), and 20 with missing data on biochemical measurements, which left 357 for the analyses presented here.
Biochemical measurements
Triglyceride, total cholesterol, HDL-cholesterol, LDL-cholesterol, and apolipoprotein A-I (apo A-I) concentrations were measured with the Poly-Chem System Chemistry Analyzer (Polymedco Inc, Cortlandt Manor, NY). Leptin, adiponectin, and insulin were assayed by using human-specific radioimmunoassay kits (Linco Research Inc, St Charles, MO). Fasting blood glucose was measured with a Cholestech LDX analyzer (Hayward, CA). We estimated insulin resistance using the homeostasis model assessment of insulin resistance (HOMA-IR) index: [(fasting insulin (μU/mL) × fasting glucose (mg/dL)]/405 (27). CRP was measured with an Immulite Analyzer and high-sensitivity CRP reagents (Diagnostic Products Corporation, Los Angeles, CA). Insulin-like growth factor I (IGF-I), insulin-like growth factor binding protein-3 (IGFBP-3), interleukin-6 (IL-6), and soluble tumor necrosis factor receptor type 2 (sTNFR2) were assayed by using enzyme-linked immunosorbent assay kits (Biosource, Carlsbad, CA and Diagnostic Systems Laboratories, Inc, Webster, TX).
RBC fatty acid measurements
RBC fatty acids were analyzed as previously described (28). Briefly, fatty acids were extracted from washed RBCs with 2-propanol and chloroform according to Rose and Oklander (29). Fatty acids were converted to fatty acid methyl esters (FAMEs) by direct transesterification by using the method of LePage and Roy (30). FAMEs were recovered in hexane, dried under nitrogen (40 °C), and redissolved in hexane for gas chromatography analysis. The FAMEs of individual fatty acids were separated on a gas chromatograph [model 5890B; Hewlett-Packard (HP); Agilent, Santa Clara, CA] equipped with a flame ionization detector, automatic sampler (HP 7673), electronic pressure programming (HP), and Chemstation software (HP). Quantitative precision and identification were evaluated by using model mixtures of known FAMEs and an established control pool. The interassay CV was 2.7% for EPA and 2.0% for DHA. The RBC EPA and DHA composition is reported as a percentage of weight of total RBC fatty acids.
Statistical analyses
We examined the associations of RBC EPA and DHA with the following measures: systolic blood pressure (SBP), diastolic blood pressure (DBP), triglycerides, total cholesterol, LDL cholesterol, HDL cholesterol, apo A-I, glucose, insulin, HOMA-IR, IGF-I, IGFBP-3, CRP, IL-6, sTNFR2, leptin, and adiponectin. Triglycerides, HOMA-IR, CRP, IL-6, sTNFR2, and leptin were log transformed for analysis, and the results were back-transformed for ease of interpretation. Outliers for apo A-I (n = 2), glucose (n = 1), insulin (n = 1), adiponectin (n = 1), and sTNFR2 (n = 3) were excluded because they were >4 SD above the mean and were judged to be physiologically unreasonable. For IL-6 (n = 90), values below the limit of detection (LOD) of 0.02 pg/mL were replaced with the LOD divided by the square root of 2 (31).
Nonparametric generalized additive models (GAMs) with smoothing spline functions (32) were used to graphically display the associations of EPA and DHA with all measures. GAMs enable the fitted associations to take their natural shapes by relaxing assumptions about the form of the functional associations. Linear regression was used to model these associations and provide a statistical test of the linear and quadratic associations between RBC EPA and DHA and disease risk biomarkers. We compared the linear regression models with the GAMs and found that they well-characterize the data. All models were adjusted for age (continuous), sex, current smoking (yes or no) and body mass index (BMI; continuous). Control for dietary macronutrient intake and physical activity (pedometer counts per hour) did not affect the results; therefore, these variables were not included in the models presented. To test whether associations differed by sex and age, we used the likelihood ratio test to compare regression models with and without the interactions of RBC EPA and DHA with age (continuous) and sex by using a Bonferroni-adjusted α of P < 0.001 as the criterion of statistical significance. Results from regression models with significant interactions are given with and without interaction terms. GAMs corresponding to these models are shown as separate lines plotted by sex or, for age, plotted at ages 30 and 60 y.
All findings are reported for EPA and DHA separately for 2 reasons. First, RBC EPA increases linearly with intake, whereas RBC DHA plateaus at ≈9% of total membrane fatty acids (28), which suggests that RBC DHA does not reflect variability at high intakes (33–35). Second, there is evidence from previous studies that the effects for EPA and DHA on chronic disease biomarkers differ (17, 36–40). Statistical analyses were performed by using Stata/SE 10.0 (StataCorp LP, College Station, TX) and the package mgcv (32) in the R statistical software environment (http://www.R-project.org; 41).
RESULTS
The demographic and health-related characteristics of the study participants, stratified by sex, are shown in Table 1. Overall, the median age was 45 y; 59% were women and 70% were overweight or obese. RBC EPA and DHA (% of total fatty acids) ranged from 0.2% to 10% and from 2% to 10%, respectively, with means (5th–95th percentile) of 2.8% (0.5–5.9%) and 6.8% (3.3–9.0%). Compared with men, women were significantly more likely to be obese and nonsmokers. Both RBC EPA and DHA were significantly higher in women than in men.
TABLE 1.
Demographic and health-related characteristics of study participants by sex (n = 357)1
| Men | Women | Age-adjusted difference2 | P3 | |
| No. of participants | 147 | 210 | ||
| Age (y) | 45.4 ± 15.84 | 44.4 ± 15.34 | ||
| 18–29 y (%) | 19.7 | 19.5 | ||
| 30–54 y (%) | 49.0 | 52.9 | ||
| ≥55 y (%) | 31.3 | 27.6 | ||
| BMI (kg/m2) | 26.7 ± 4.3 | 30.0 ± 6.9 | .3 ± 0.6 | <0.0001 |
| <25 kg/m2 (%) | 37.4 | 24.3 | ||
| 25–29 kg/m2 (%) | 40.2 | 30.5 | ||
| 30–34 kg/m2 (%) | 17.0 | 23.3 | ||
| ≥35 kg/m2 (%) | 5.4 | 21.9 | ||
| Body fat (%) | 23.1 ± 6.7 | 37.1 ± 8.2 | 14.1 ± 0.8 | <0.0001 |
| Current smokers (%) | 36.7 | 20.0 | ||
| RBC EPA (% of total fatty acids) | 2.6 ± 1.9 | 3.0 ± 1.6 | 0.5 ± 0.1 | <0.01 |
| <1.0% of total fatty acids (%) | 24.5 | 14.8 | ||
| 1.0–2.9% of total fatty acids (%) | 42.2 | 39.5 | ||
| 3.0–4.9% of total fatty acids (%) | 19.7 | 30.9 | ||
| ≥5.0% of total fatty acids (%) | 13.6 | 14.8 | ||
| RBC DHA (% of total fatty acids) | 6.3 ± 1.8 | 7.2 ± 1.6 | 1.0 ± 0.1 | <0.0001 |
| <5.0% of total fatty acids (%) | 25.2 | 12.8 | ||
| 5.0–6.9% of total fatty acids (%) | 32.0 | 18.6 | ||
| 7.0–8.9% of total fatty acids (%) | 39.4 | 62.4 | ||
| ≥9.0% of total fatty acids (%) | 3.4 | 6.2 |
RBC, red blood cell; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
All values are means ± SEs.
Derived by using 2-sided, 2-sample t tests for age-adjusted difference between men and women.
Mean ± SD (all such values in this column).
The mean values of chronic disease biomarkers, stratified by sex, are shown in Table 2. Compared with men, women had significantly lower SBP and LDL cholesterol and higher HDL cholesterol, apo A-I, insulin, HOMA-IR index, and IGFBP-3. Leptin was 2.4-fold higher in women than in men. Other biomarker concentrations did not differ by sex, in contrast with differences found for CRP and adiponectin in other populations (42–44). Mean DBP and triglyceride, total cholesterol, glucose, IGF-I, CRP, IL-6, sTNFR2, and adiponectin concentrations did not differ by sex.
TABLE 2.
Means and distributions of chronic disease risk biomarkers by sex (n = 353–357)1
| Men | Women | Age-adjusted difference2 | P3 | |
| No. of participants | 147 | 210 | ||
| SBP (mm Hg) | 126.6 ± 13.94 | 122.4 ± 17.74 | −3.7 ± 0.05 | <0.05 |
| DBP (mm Hg) | 74.2 ± 8.5 | 72.3 ± 11.2 | −1.8 ± 1.1 | 0.10 |
| Triglycerides (mg/dL) | 86.2 (79.8, 93.2)5 | 79.8 (75.3, 84.6) | −6.3 (−13.5, 1.7) | 0.12 |
| Total cholesterol (mg/dL) | 224.2 ± 46.1 | 220.8 ± 45.1 | −2.2 ± 4.5 | 0.62 |
| LDL cholesterol (mg/dL) | 146.9 ± 39.4 | 138.2 ± 39.1 | −7.8 ± 4.0 | <0.05 |
| HDL cholesterol (mg/dL) | 57.6 ± 16.4 | 64.9 ± 17.4 | 7.7 ± 1.8 | <0.0001 |
| apo A-I (mg/dL) | 166.6 ± 24.5 | 177.7 ± 21.4 | 11.6 ± 2.3 | <0.0001 |
| Glucose (mg/dL) | 95.8 ± 10.5 | 94.8 ± 12.2 | −0.7 ± 1.1 | 0.57 |
| Insulin (μU/mL) | 13.9 ± 6.3 | 16.6 ± 7.9 | 2.7 ± 0.8 | <0.01 |
| HOMA-IR | 3.0 (2.8, 3.2) | 3.5 (3.3, 3.8) | 0.5 (0.2, 0.9) | <0.01 |
| IGF-I (ng/mL) | 255.0 ± 102.7 | 270.3 ± 103.2 | 11.7 ± 9.2 | 0.21 |
| IGFBP-3 (ng/mL) | 4101 ± 1035 | 4600 ± 940 | 478.4 ± 100.0 | <0.0001 |
| CRP (mg/dL) | 0.1 (0.08, 0.12) | 0.1 (0.08, 0.11) | 0.002 (−0.02, 0.03) | 0.90 |
| IL-6 (pg/mL)6 | 0.08 (0.06, 0.10) | 0.09 ( 0.07, 0.10) | 0.01 (−0.01, 0.04) | 0.39 |
| sTNFR2 (pg/mL) | 1999 (1817, 2199) | 2016 (1853, 2194) | 6.9 (−232.7, 278.9) | 0.96 |
| Leptin (ng/mL) | 3.3 (2.9, 3.8) | 14.8 (13.5, 16.2) | 14.6 (12.5, 17.0) | <0.0001 |
| Adiponectin (μg/mL) | 8.5 ± 4.2 | 8.7 ± 4.4 | 0.3 ± 0.5 | 0.48 |
Sample size varies slightly because of outliers and missing data. SBP, systolic blood pressure; DBP, diastolic blood pressure; apo A-I, apolipoprotein A-I; HOMA-IR, homeostasis model assessment of insulin resistance; IGF-I, insulin-like growth factor I; IGFBP-3, insulin-like growth factor binding protein-3; CRP, C-reactive protein; IL-6, interleukin-6; sTNFR2, soluble tumor necrosis factor receptor type 2.
Values are mean (±SE) age-adjusted differences or age-adjusted differences (95% CIs) for log-transformed variables.
Derived by using 2-sided, 2-sample t tests for age-adjusted differences between men and women.
Mean ± SD (all such values in this column).
Geometric mean for log-transformed variables; 95% CI in parentheses (all such values).
Values (n = 90) below the limit of detection (LOD) were replaced with the LOD divided by the square root of 2.
Associations of RBC EPA and DHA with biomarkers of chronic disease risk
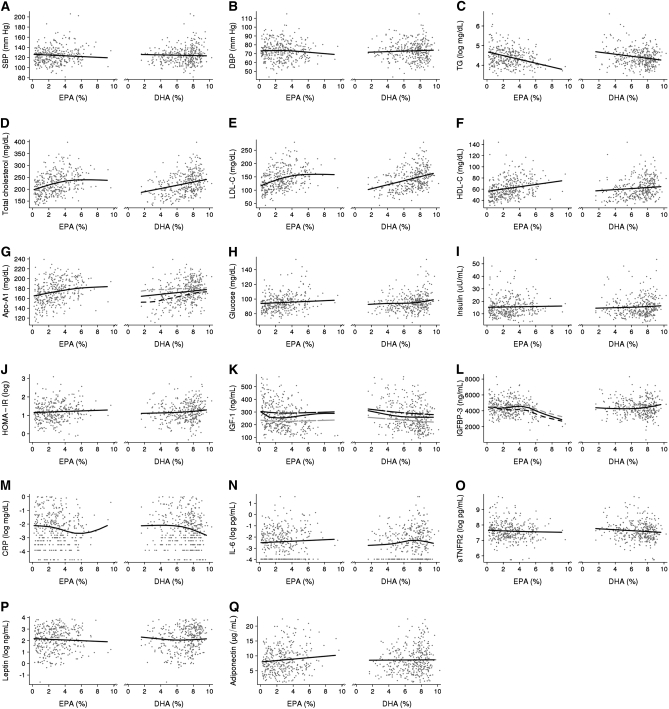
Scatter plots of RBC EPA and DHA versus disease biomarkers with fitted smooth curves estimated by using covariate-adjusted GAMs are shown in Figure 1. The results of simplified regression models corresponding to these figures are given in Table 3. There were no significant associations of RBC EPA or DHA with SBP, DBP, glucose, insulin, HOMA-IR, IL-6, sTNFR2, or adiponectin. The significant associations with serum lipids, IGF-I, IGFBP-3, CRP, and leptin are described below.
FIGURE 1.
A–Q: Scatter plots of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), as percentages of total fatty acids in red blood cells, compared with biomarkers of chronic disease risk with smooth curves (solid black line) estimated by using generalized additive models adjusted for age (continuous), sex, smoking (yes or no), and BMI (continuous). Generalized additive models with significant interactions of EPA and DHA with age (continuous; panel K) and sex (panels G and L) are shown as separate lines: for age, plotted at ages 30 (black long-dashed line) and 60 (gray long-dashed line) y; and for sex, plotted for men (black short-dashed line) and women (black short-dashed line). Sample size (n = 353–357) varies slightly because of outliers and missing data. SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; LDL-C, LDL cholesterol; HDL-C, HDL cholesterol; Apo-A1, apolipoprotein A-I; HOMA-IR, homeostasis model assessment of insulin resistance; IGF-1, insulin-like growth factor I; IGFBP-3, insulin-like growth factor binding protein-3; CRP, C-reactive protein; IL-6, interleukin-6; sTNFR2, soluble tumor necrosis factor receptor type 2.
TABLE 3.
Regression coefficients (±SE) for associations of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), as a percentage of total fatty acids in red blood cells, with biomarkers of chronic disease risk (n = 353–357)1
| EPA (20:5n−3) |
DHA (22:6n−3) |
|||||||
| Linear |
Quadratic |
Linear |
Quadratic |
|||||
| β ± SE | P | β ± SE | P | β ± SE | P | β ± SE | P | |
| SBP (mm Hg) | −0.7 ± 0.5 | 0.18 | −0.3 ± 0.6 | 0.55 | ||||
| DBP (mm Hg) | −0.3 ± 0.3 | 0.34 | 0.3 ± 0.3 | 0.42 | ||||
| Triglycerides (mg/dL)2 | −0.10 ± 0.01 | <0.0001 | −0.05 ± 0.01 | <0.001 | ||||
| Total cholesterol (mg/dL) | 17.0 ± 4.14 | <0.0001 | −1.5 ± 0.5 | <0.01 | 6.8 ± 1.6 | <0.0001 | ||
| LDL cholesterol (mg/dL) | 17.1 ± 3.6 | <0.0001 | −1.5 ± 0.5 | <0.01 | 7.5 ± 1.4 | <0.0001 | ||
| HDL cholesterol (mg/dL) | 2.0 ± 0.5 | <0.0001 | 0.9 ± 0.6 | 0.10 | ||||
| apo A-I (mg/dL) | 2.6 ± 0.8 | <0.001 | 1.7 ± 0.8 | <0.05 | ||||
| Glucose (mg/dL) | 0.5 ± 0.4 | 0.21 | 0.6 ± 0.4 | 0.13 | ||||
| Insulin (μU/mL) | 0.1 ± 0.2 | 0.65 | 0.2 ± 0.2 | 0.32 | ||||
| HOMA-IR2 | 0.01 ± 0.02 | 0.37 | 0.02 ± 0.02 | 0.16 | ||||
| IGF-I (ng/mL)3 | −15.4 ± 7.1 | <0.05 | 2.3 ± 0.9 | <0.05 | −5.3 ± 2.7 | <0.05 | ||
| IGFBP-3 (ng/mL) | 191.6 ± 93.2 | <0.05 | −36.2 ± 12.1 | <0.01 | −353.9 ± 177.3 | <0.05 | 33.7 ± 14.5 | <0.05 |
| CRP (mg/dL)2 | −0.09 ± 0.04 | <0.05 | 0.4 ± 0.2 | 0.08 | −0.04 ± 0.02 | <0.05 | ||
| IL-6 (pg/mL)2,4 | 0.03 ± 0.05 | 0.50 | 0.06 ± 0.05 | 0.22 | ||||
| sTNFR2 (pg/mL)2 | −0.02 ± 0.02 | 0.45 | −0.03 ± 0.02 | 0.19 | ||||
| Leptin (ng/mL)2 | −0.03 ± 0.02 | 0.13 | −0.2 ± 0.1 | <0.05 | 0.02 ± 0.01 | <0.05 | ||
| Adiponectin (μg/mL) | 0.2 ± 0.1 | 0.11 | 0.02 ± 0.15 | 0.91 | ||||
Sample size varies slightly because of outliers and missing data. SBP, systolic blood pressure; DBP, diastolic blood pressure; apo A-I, apolipoprotein A-I; HOMA-IR, homeostasis model assessment of insulin resistance; IGF-I, insulin-like growth factor I; IGFBP-3, insulin-like growth factor binding protein-3; CRP, C-reactive protein; IL-6, interleukin-6; sTNFR2, soluble tumor necrosis factor receptor type 2. Linear regression models were used to test whether the coefficients for linear and/or squared red blood cell EPA and DHA concentrations were statistically significant. Models were adjusted for age (continuous), sex, smoking (yes or no), and BMI (continuous).
Log-transformed values were used for the regression analysis.
Regression model additionally adjusted for IGFBP-3.
Values (n = 90) below the limit of detection (LOD) were replaced with the LOD divided by the square root of 2.
Serum lipids
GAMs showed positive approximately linear associations of both EPA and DHA with HDL cholesterol (Figure 1F) and apo A-I (Figure 1G, solid black line) and inverse approximately linear associations with triglyceride (Figure 1C). In linear regression models, these associations were statistically significant with the exception of DHA with HDL cholesterol (P = 0.1). Associations of DHA with total cholesterol (Figure 1D) and LDL cholesterol (Figure 1E) were positive and approximately linear, whereas for EPA they were positive and nonlinear; total cholesterol and LDL cholesterol increased with increasing intakes of EPA until reaching ≈5% of total fatty acids, with no association at higher intakes. In regression models (Table 3), the linear terms for DHA and quadratic terms for EPA were statistically significant.
IGF-I and IGFBP-3
GAMs showed nonlinear associations of both EPA and DHA with IGF-I (also controlled for IGFBP3; Figure 1K, solid black line) and IGFBP-3 (Figure 1L, solid black line). For EPA, associations with IGF-I appeared to be inverse at concentrations <2% of total fatty acids and flat or slightly positive at concentrations >2%; for IGFBP-3, these associations were flat for concentrations <6% and strongly inverse at concentrations >6% of total fatty acids. For DHA, associations with IGF-I appeared to be inverse at concentrations <6% of total fatty acids and flat at concentrations >6%; for IGFBP-3, these associations were flat at concentrations <6% and positive at concentrations >6% of total fatty acids. In linear regression models (Table 3), all quadratic terms were statistically significant except for DHA with IGF-I (P = 0.057).
C-reactive protein
GAMs showed inverse nonlinear associations of both EPA and DHA with CRP (Figure 1M). For EPA, the association appeared to be stronger at concentrations >3% of total fatty acids, with a positive association at concentrations >7%, due probably to a few outliers. For DHA, the association appeared to be flat at concentrations <7% of total fatty acids and strongly negative above 7%. In linear regression models (Table 3), only the linear term was significant for EPA, whereas for DHA the quadratic term was significant.
Leptin
GAMs showed weak, inverse associations of both EPA and DHA with leptin (Figure 1P). For DHA, the inverse association was restricted to concentrations <6% of total fatty acids. In linear regression models (Table 3), the association for EPA was not statistically significant and the quadratic term for DHA was significant.
Interaction of RBC EPA and DHA with age and sex
The results of the regression models for the associations of EPA and DHA with biomarkers that differed by age or sex are shown in Table 4. The inverse associations of both EPA and DHA with IGF-I were attenuated with increasing age. The association of EPA with IGFBP-3 was significant in women only; whereas, that of DHA with apo A-I was significant in men only. Whereas these interactions were statistically significant based on the linear regression models, the GAMs plotted by age (30 y compared with 60 y) for IGF-I (Figure 1K) and by sex for IGFBP-3 (Figure 1L) and apo A-I (Figure 1G) showed only marginal difference by age or sex.
TABLE 4.
Age- and sex-specific differences in the associations of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), as a percentage of total fatty acids in red blood cells, with insulin-like growth factor I (IGF-I), insulin-like growth factor binding protein-3 (IGFBP-3), and apolipoprotein A-I (apo A-I) (n = 355–356)1
| EPA (20:5n−3) |
DHA (22:6n−3) |
|||||||||
| Linear |
Quadratic |
Linear |
Quadratic |
|||||||
| β ± SE | P | β ± SE | P | P for interaction2 | β ± SE | P | β ± SE | P | P for interaction2 | |
| IGF-I (ng/mL)3 | −73.5 ± 21.7 | <0.01 | 7.3 ± 3.7 | <0.05 | −27.4 ± 6.6 | <0.0001 | ||||
| Age interaction | 1.5 ± 0.4 | <0.0001 | −0.14 ± 0.06 | <0.05 | <0.0001 | 0.6 ± 0.1 | <0.0001 | <0.0001 | ||
| IGFBP-3 (ng/mL) | ||||||||||
| Men | −77.7 ± 120.9 | 0.52 | −12.2 ± 15.1 | 0.42 | ||||||
| Women | 421.7 ± 136.4 | <0.01 | −58.4 ± 19.4 | <0.01 | <0.001 | |||||
| apo A-I (mg/dL) | ||||||||||
| Men | 4.6 ± 1.0 | <0.0001 | ||||||||
| Women | −0.6 ± 0.9 | 0.53 | <0.0001 | |||||||
Sample size varies slightly because of outliers and missing data. Linear regression models were adjusted for age (continuous), sex, smoking (yes or no), and BMI (continuous).
On the basis of a likelihood ratio test comparing regression models with and without the interactions of red blood cell EPA and DHA with age (continuous) and sex by using a Bonferroni-adjusted α of P < 0.001 as the criterion of statistical significance.
Regression model additionally adjusted for IGFBP-3.
DISCUSSION
In this population-based sample of Yup'ik Eskimos, associations of EPA and DHA with triglyceride, HDL cholesterol, and apo A-I were linear across a broad range of RBC EPA and DHA. However, several associations were nonlinear, notably CRP, for which inverse associations with EPA and DHA were stronger at high RBC concentrations. In contrast, the positive associations of EPA with total and LDL cholesterol were only observed at low concentrations.
A direct comparison of our findings with the literature is difficult for several reasons. Only 3 studies were based on populations with a broad range of EPA and DHA intakes (12, 13, 19), and none examined whether associations were nonlinear. Supplementation studies have examined the effects of high EPA and DHA intakes; however, most were small, short-term, and used a single dose delivered as fish oil (EPA+DHA). Even given the differences between this and previous studies, we and most other studies found no association of EPA and DHA with adiponectin (45–48) or blood pressure (13, 16, 37, 49–52). In the discussion of other biomarkers below, we describe only studies most informative for interpreting our results.
The findings of high EPA and DHA intakes associated with lower triglyceride and higher HDL cholesterol, total, and LDL cholesterol were generally consistent with published observational studies (7–15). Most supplementation studies report that purified EPA and/or DHA, fish oil, and n−3-enriched foods also lower triglycerides (16–18, 37, 38, 40, 46, 47, 50, 51, 53–59) but do not affect (37, 45–47, 50–53, 60) or moderately increase HDL cholesterol (18, 38, 40, 54–56, 59, 61), LDL cholesterol (16–18, 54, 57, 61), and total cholesterol (18, 61). We found that the associations of EPA and DHA with triglyceride and HDL cholesterol and those of DHA with total and LDL cholesterol were linear across a broad range of RBC concentrations; the associations of EPA with total and LDL cholesterol were decidedly nonlinear and restricted to RBC EPA <5%.
Findings from the few studies that examined the associations of EPA and DHA with apo A-I are not consistent with our findings of positive, linear associations. The single observational study (15) found associations that were positive for EPA and inverse for DHA, and most supplementation studies found that EPA and DHA decreased apo A-I (38, 39, 62). The reasons for the discrepancy between our and previous findings are not clear. Fish intake in the observational study was low and the supplementation studies were small and of short duration. EPA and DHA are associated with increased HDL cholesterol in most observational studies (8–14); thus, it is reasonable to expect a positive association with apo A-I. Note that the small, short-duration supplementation studies found inverse associations with apo A-I (38, 39) may not reflect effects of chronic high exposures.
Our findings of no associations of EPA and DHA with glucose, insulin, or HOMA-IR are consistent with those of most observational studies (12, 13, 49). Studies that used low-to-moderate doses (<3 g/d) of EPA and DHA reported no effect on fasting blood glucose and insulin (47, 52–54, 58, 59); studies using higher doses reported modestly increased glucose in overweight diabetic patients (45, 50, 57) and overweight hyperlipidemic men (37).
This was the first study to examine the associations of EPA and DHA with IGF-I and IGFBP-3. For IGF-I, there were inverse associations with EPA and DHA only at low RBC concentrations. For IGFBP-3, the associations were inverse for EPA and positive for DHA only at high RBC concentrations. There is indirect support for our IGF-I finding from a study that reported that fish-oil supplementation decreased plasma growth hormone concentrations (63). Our findings on IGF-I and IGFBP-3 need to be corroborated.
In agreement with many (46, 47, 49, 60, 64–68), but not all, observational (7, 69, 70) and supplementation studies (71, 72), we found no associations of EPA and DHA with IL-6 and sTNFR2. Our findings of inverse associations of EPA and DHA with CRP are consistent with those of most observational studies (19, 49, 64–66, 73). Conversely, few observational (7, 69, 70) and most supplementation (47, 52, 54, 60, 67, 68, 74–76) studies have found no effect on CRP. The lack of association in low-dose supplementation studies (<1 g/d of EPA+DHA) (52, 54, 60, 74) could be explained by the nonlinear associations of EPA and DHA with CRP: for EPA, the inverse association was stronger at RBC concentrations >3% of total fatty acids and for DHA it was restricted to concentrations >7%. Three of the 5 studies that used larger doses, 2 in young, normal-weight, healthy persons (47, 75) and the other in older, overweight, diabetic persons (68), reported no effects; however, a decrease in CRP concentrations was reported in older, hypertriglyceridemic men (72) and in hemodialysis patients (77). It is possible that the short-term effects of EPA and DHA on CRP are limited to populations with risk factors for systemic inflammation; nevertheless, the inconsistency between larger observational and small, short-duration supplementation studies requires further investigation.
Age- and sex-specific differences in associations of EPA and DHA with IGF-I, IGFBP-3, and apo A-I must be interpreted as preliminary. Given the relatively small sample size and number of models fitted, there is an inherent risk of overfitting data. We suggest that these findings be replicated in larger samples and/or different populations. Therefore, inferences based on these models are weak, and their biological relevance should be interpreted with caution.
This study had several important strengths. Our study population was ideal for testing associations with biomarkers: Yup'ik Eskimos have chronic, high, and broad ranges of EPA and DHA intakes. This was the first study to include a test for nonlinearity of associations of EPA and DHA with biomarkers. This study also had several limitations. First, we measured EPA and DHA in RBC membranes. RBC EPA increases linearly with dietary intake, whereas RBC DHA composition levels off at ≈9% of total fatty acids (28) and, at that level, there are individuals with high to very high DHA intakes. Therefore, compared with RBC DHA, EPA is a better biomarker of very high intakes of n−3 PUFAs (33–35). This should be considered when interpreting associations at high EPA intakes compared with high DHA intakes. Second, Yup'ik Eskimos have unique dietary and health characteristics (78); thus, our findings cannot be extrapolated to other populations without further investigation. Third, given the observational nature of the study, we cannot determine causality. Finally, although we adjusted for several confounders, it is possible that unmeasured factors could have affected RBC fatty acids and disease biomarkers.
This study extends earlier findings on associations of EPA and DHA intakes with disease biomarkers by describing these associations at very high RBC EPA and DHA intakes. Associations at low EPA and DHA intakes were generally consistent with the literature. We also showed that for some biomarkers, such as blood lipids, associations were linear across a broad range of EPA and DHA intakes, whereas for many others, including CRP and total and LDL cholesterol, associations were nonlinear. Our findings suggest that the beneficial effects on disease biomarkers associated with increasing EPA and DHA intakes within the low amounts consumed by the general US population continue to accrue up to the very high amounts consumed by Yup'ik Eskimos. At these high intakes, the reduction in CRP may be more pronounced, whereas the increases in total and LDL cholesterol may be attenuated. The high consumption of EPA and DHA by Yup'ik Eskimos may partially explain the relatively low prevalence of CVD and diabetes in this population (78).
Acknowledgments
We gratefully acknowledge all study participants and their communities and the CANHR research team, which made this study and manuscript possible. We thank Peter Havel and Kimber Stanhope from the Department of Nutrition, University of California–Davis for the hormone and lipid analyses, Charles Stephensen from the USDA Human Nutrition Research Center for the CRP analyses, and Irena King and her staff at the Fred Hutchinson Cancer Research Center for the red blood cell fatty acid analyses. We also thank Mario Kratz for his thoughtful input.
The authors’ responsibilities were as follows—ZM: analyzed and interpreted the data and wrote the manuscript; ARK: interpreted the data and wrote the manuscript; RG: analyzed the data and helped write the manuscript; BL and AB: collected and compiled the nutritional data; and BB and GVM (Principal Investigators): contributed to the data interpretation All authors contributed to the final draft of the manuscript. None of the authors had any conflicts of interest.
REFERENCES
- 1.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002;106:2747–57 [DOI] [PubMed] [Google Scholar]
- 2.Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther 1999;83:217–44 [DOI] [PubMed] [Google Scholar]
- 3.Nettleton JA, Katz R. n−3 long-chain polyunsaturated fatty acids in type 2 diabetes: a review. J Am Diet Assoc 2005;105:428–40 [DOI] [PubMed] [Google Scholar]
- 4.Fritsche K.Fatty acids as modulators of the immune response. Annu Rev Nutr 2006;26:45–73 [DOI] [PubMed] [Google Scholar]
- 5.Bouwens M, van de Rest O, Dellschaft N, et al. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am J Clin Nutr 2009;90:415–24 [DOI] [PubMed] [Google Scholar]
- 6.Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu Rev Nutr 2005;25:317–40 [DOI] [PubMed] [Google Scholar]
- 7.Ferrucci L, Cherubini A, Bandinelli S, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab 2006;91:439–46 [DOI] [PubMed] [Google Scholar]
- 8.Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis 2008;197:821–8 [DOI] [PubMed] [Google Scholar]
- 9.He K, Liu K, Daviglus ML, et al. Intakes of long-chain n−3 polyunsaturated fatty acids and fish in relation to measurements of subclinical atherosclerosis. Am J Clin Nutr 2008;88:1111–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motoyama KR, Curb JD, Kadowaki T, et al. Association of serum n−6 and n−3 polyunsaturated fatty acids with lipids in 3 populations of middle-aged men. Am J Clin Nutr 2009;90:49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dewailly EE, Blanchet C, Gingras S, et al. Relations between n−3 fatty acid status and cardiovascular disease risk factors among Quebecers. Am J Clin Nutr 2001;74:603–11 [DOI] [PubMed] [Google Scholar]
- 12.Dewailly E, Blanchet C, Gingras S, Lemieux S, Holub BJ. Cardiovascular disease risk factors and n−3 fatty acid status in the adult population of James Bay Cree. Am J Clin Nutr 2002;76:85–92 [DOI] [PubMed] [Google Scholar]
- 13.Dewailly E, Blanchet C, Lemieux S, et al. n−3 Fatty acids and cardiovascular disease risk factors among the Inuit of Nunavik. Am J Clin Nutr 2001;74:464–73 [DOI] [PubMed] [Google Scholar]
- 14.Okuda N, Ueshima H, Okayama A, et al. Relation of long chain n−3 polyunsaturated fatty acid intake to serum high density lipoprotein cholesterol among Japanese men in Japan and Japanese–American men in Hawaii: the INTERLIPID Study. Atherosclerosis 2005;178:371–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonaa KH, Bjerve KS, Nordoy A. Habitual fish consumption, plasma phospholipid fatty acids, and serum lipids: the Tromso study. Am J Clin Nutr 1992;55:1126–34 [DOI] [PubMed] [Google Scholar]
- 16.Kelley DS, Siegel D, Vemuri M, Mackey BE. Docosahexaenoic acid supplementation improves fasting and postprandial lipid profiles in hypertriglyceridemic men. Am J Clin Nutr 2007;86:324–33 [DOI] [PubMed] [Google Scholar]
- 17.Leigh-Firbank EC, Minihane AM, Leake DS, et al. Eicosapentaenoic acid and docosahexaenoic acid from fish oils: differential associations with lipid responses. Br J Nutr 2002;87:435–45 [DOI] [PubMed] [Google Scholar]
- 18.Maki KC, Van Elswyk ME, McCarthy D, et al. Lipid responses to a dietary docosahexaenoic acid supplement in men and women with below average levels of high density lipoprotein cholesterol. J Am Coll Nutr 2005;24:189–99 [DOI] [PubMed] [Google Scholar]
- 19.Niu K, Hozawa A, Kuriyama S, et al. Dietary long-chain n−3 fatty acids of marine origin and serum C-reactive protein concentrations are associated in a population with a diet rich in marine products. Am J Clin Nutr 2006;84:223–9 [DOI] [PubMed] [Google Scholar]
- 20.Bersamin A, Zidenberg-Cherr S, Stern JS, Luick BR. Nutrient intakes are associated with adherence to a traditional diet among Yup'ik Eskimos living in remote Alaska Native communities: the CANHR Study. Int J Circumpolar Health 2007;66:62–70 [DOI] [PubMed] [Google Scholar]
- 21.Johnson JS, Nobmann ED, Asay E, Lanier AP. Dietary intake of Alaska native people in two regions and implications for health: the Alaska Native Dietary and Subsistence Food Assessment Project. Int J Circumpolar Health 2009;68:109–22 [DOI] [PubMed] [Google Scholar]
- 22.Bersamin A, Luick BR, King IB, Stern JS, Zidenberg-Cherr S. Westernizing diets influence fat intake, red blood cell fatty acid composition, and health in remote Alaskan Native Communities in the Center for Alaska Native Health Study. J Am Diet Assoc 2008;108:266–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyer BB, Mohatt GV, Lardon C, et al. Building a community-based participatory research center to investigate obesity and diabetes in Alaska Natives. Int J Circumpolar Health 2005;64:281–90 [DOI] [PubMed] [Google Scholar]
- 24.Mohatt GV, Plaetke R, Klejka J, et al. The Center for Alaska Native Health Research study: a community-based participatory research study of obesity and chronic disease-related protective and risk factors. Int J Circumpolar Health 2007;66:8–18 [DOI] [PubMed] [Google Scholar]
- 25.Magnusardottir AR, Skuladottir GV. Effects of storage time and added antioxidant on fatty acid composition of red blood cells at -20° C. Lipids 2006;41:401–4 [DOI] [PubMed] [Google Scholar]
- 26.Otto SJ, Foreman-van Drongelen MM, van Houwelingen AC, Hornstra G. Effects of storage on venous and capillary blood samples: the influence of deferoxamine and butylated hydroxytoluene on the fatty acid. alterations in red blood cell phospholipids. Clin Chem Lab Med 1997;35:907–14 [DOI] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9 [DOI] [PubMed] [Google Scholar]
- 28.O'Brien DM, Kristal AR, Jeannet MA, Wilkinson MJ, Bersamin A, Luick B. Red blood cell 15N: a novel biomarker of dietary eicosapentaenoic acid and docosahexaenoic acid intake. Am J Clin Nutr 2009;89:913–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rose HG, Oklander M. Improved procedure for the extraction of lipids from human erythrocytes. J Lipid Res 1965;6:428–31 [PubMed] [Google Scholar]
- 30.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 1986;27:114–20 [PubMed] [Google Scholar]
- 31.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 1990;5:46–51 [Google Scholar]
- 32.Wood SN. Generalized additive models: an introduction with R. Boca Raton, FL: Chapman & Hall/CRC, 2006 [Google Scholar]
- 33.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n−3 fatty acids in humans. Am J Clin Nutr 2006;83:1467S–76S [DOI] [PubMed] [Google Scholar]
- 34.Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem 2006;52:2265–72 [DOI] [PubMed] [Google Scholar]
- 35.Brown AJ, Pang E, Roberts DCK. Erythrocyte eicosapentaenoic acid versus docosahexaenoic acid as a marker for fish and fish oil consumption. Prostaglandins Leukot Essent Fatty Acids 1991;44:103–6 [DOI] [PubMed] [Google Scholar]
- 36.Mori TA, Woodman RJ. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care 2006;9:95–104 [DOI] [PubMed] [Google Scholar]
- 37.Mori TA, Burke V, Puddey IB, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr 2000;71:1085–94 [DOI] [PubMed] [Google Scholar]
- 38.Bonaa KH, Bjerve KS, Nordoy A. Docosahexaenoic and eicosapentaenoic acids in plasma phospholipids are divergently associated with high density lipoprotein in humans. Arterioscler Thromb Vasc Biol 1992;12:675–81 [DOI] [PubMed] [Google Scholar]
- 39.Childs MT, King IB, Knopp RH. Divergent lipoprotein responses to fish oils with various ratios of eicosapentaenoic acid and docosahexaenoic acid. Am J Clin Nutr 1990;52:632–9 [DOI] [PubMed] [Google Scholar]
- 40.Egert S, Kannenberg F, Somoza V, Erbersdobler HF, Wahrburg U. Dietary α-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J Nutr 2009;139:861–8 [DOI] [PubMed] [Google Scholar]
- 41.R Development Core Team R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2009 [Google Scholar]
- 42.Cartier A, Cote M, Lemieux I, et al. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am J Clin Nutr 2009;89:1307–14. [DOI] [PubMed] [Google Scholar]
- 43.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999;257:79–83 [DOI] [PubMed] [Google Scholar]
- 44.Isobe T, Saitoh S, Takagi S, et al. Influence of gender, age and renal function on plasma adiponectin level: the Tanno and Sobetsu study. Eur J Endocrinol 2005;153:91–8 [DOI] [PubMed] [Google Scholar]
- 45.Mostad IL, Bjerve KS, Bjorgaas MR, Lydersen S, Grill V. Effects of n−3 fatty acids in subjects with type 2 diabetes: reduction of insulin sensitivity and time-dependent alteration from carbohydrate to fat oxidation. Am J Clin Nutr 2006;84:540–50 [DOI] [PubMed] [Google Scholar]
- 46.Kabir M, Skurnik G, Naour N, et al. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr 2007;86:1670–9 [DOI] [PubMed] [Google Scholar]
- 47.Damsgaard CT, Frøkier H, Andersen AD, Lauritzen L. Fish oil in combination with high or low intakes of linoleic acid lowers plasma triacylglycerols but does not affect other cardiovascular risk markers in healthy men. J Nutr 2008;138:1061–6 [DOI] [PubMed] [Google Scholar]
- 48.Kratz M, Swarbrick MM, Callahan HS, Matthys CC, Havel PJ, Weigle DS. Effect of dietary n−3 polyunsaturated fatty acids on plasma total and high-molecular-weight adiponectin conentrations in overweight to moderately obese men and women. Am J Clin Nutr 2008;87:347–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klein-Platat C, Drai J, Oujaa M, Schlienger JL, Simon C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am J Clin Nutr 2005;82:1178–84 [DOI] [PubMed] [Google Scholar]
- 50.Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr 2002;76:1007–15 [DOI] [PubMed] [Google Scholar]
- 51.Blonk MC, Bilo HJ, Nauta JJ, Popp-Snijders C, Mulder C, Donker AJ. Dose-response effects of fish-oil supplementation in healthy volunteers. Am J Clin Nutr 1990;52:120–7 [DOI] [PubMed] [Google Scholar]
- 52.Murphy KJ, Meyer BJ, Mori TA, et al. Impact of foods enriched with n−3 long-chain polyunsaturated fatty acids on erythrocyte n−3 levels and cardiovascular risk factors. Br J Nutr 2007;97:749–57 [DOI] [PubMed] [Google Scholar]
- 53.Williams CM, Moore F, Morgan L, Wright J. Effects of n−3 fatty acids on postprandial triacylglycerol and hormone concentrations in normal subjects. Br J Nutr 1992;68:655–66 [DOI] [PubMed] [Google Scholar]
- 54.Garg ML, Blake RJ, Clayton E, et al. Consumption of an n−3 polyunsaturated fatty acid-enriched dip modulates plasma lipid profile in subjects with diabetes type II. Eur J Clin Nutr 2007;61:1312–7 [DOI] [PubMed] [Google Scholar]
- 55.Schwellenbach LJ, Olson KL, McConnell KJ, Stolcpart RS, Nash JD, Merenich JA. The triglyceride-lowering effects of a modest dose of docosahexaenoic acid alone versus in combination with low dose eicosapentaenoic acid in patients with coronary artery disease and elevated triglycerides. J Am Coll Nutr 2006;25:480–5 [DOI] [PubMed] [Google Scholar]
- 56.Visioli F, Rise P, Plasmati E, Pazzucconi F, Sirtori CR, Galli C. Very low intakes of n−3 fatty acids incorporated into bovine milk reduce plasma triacylglycerol and increase HDL-cholesterol concentrations in healthy subjects. Pharmacol Res 2000;41:571–6 [DOI] [PubMed] [Google Scholar]
- 57.Boberg M, Pollare T, Siegbahn A, Vessby B. Supplementation with n−3 fatty acids reduces triglycerides but increases PAI-1 in non-insulin-dependent diabetes mellitus. Eur J Clin Invest 1992;22:645–50 [DOI] [PubMed] [Google Scholar]
- 58.Luo J, Rizkalla SW, Vidal H, et al. Moderate intake of n−3 fatty acids for 2 months has no detrimental effect on glucose metabolism and could ameliorate the lipid profile in type 2 diabetic men. Diabetes Care 1998;21:717–24 [DOI] [PubMed] [Google Scholar]
- 59.Sirtori CR, Crepaldi G, Manzato E, et al. One-year treatment with ethyl esters of n−3 fatty acids in patients with hypertriglyceridemia and glucose intolerance-Reduced triglyceridemia, total cholesterol and increased HDL-C without glycemic alterations. Atherosclerosis 1998;137:419–27 [DOI] [PubMed] [Google Scholar]
- 60.Fujioka S, Hamazaki K, Itomura M, et al. The effects of eicosapentaenoic acid-fortified food on inflammatory markers in healthy subjects—a randomized, placebo-controlled, double-blind study. J Nutr Sci Vitaminol (Tokyo) 2006;52:261–5 [DOI] [PubMed] [Google Scholar]
- 61.Theobald HE, Chowienczyk PJ, Whittall R, Humphries SE, Sanders TA. LDL cholesterol-raising effect of low-dose docosahexaenoic acid in middle-aged men and women. Am J Clin Nutr 2004;79:558–63 [DOI] [PubMed] [Google Scholar]
- 62.de Roos B, Geelen A, Ross K, et al. Identification of potential serum biomarkers of inflammation and lipid modulation that are altered by fish oil supplementation in healthy volunteers. Proteomics 2008;8:1965–74 [DOI] [PubMed] [Google Scholar]
- 63.Bhathena SJ, Berlin E, Judd JT, et al. Effects of omega 3 fatty acids and vitamin E on hormones involved in carbohydrate and lipid metabolism in men. Am J Clin Nutr 1991;54:684–8 [DOI] [PubMed] [Google Scholar]
- 64.Fernandez-Real JM, Broch M, Vendrell J, Ricart W. Insulin resistance, inflammation, and serum fatty acid composition. Diabetes Care 2003;26:1362–8 [DOI] [PubMed] [Google Scholar]
- 65.Lopez-Garcia E, Schulze MB, Manson JAE, et al. Consumption of (n−3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr 2004;134:1806–11 [DOI] [PubMed] [Google Scholar]
- 66.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n−3 and n−6 fatty acids in relation to inflammatory markers among US men and women. Circulation 2003;108:155–60 [DOI] [PubMed] [Google Scholar]
- 67.Yusof HM, Miles EA, Calder P. Influence of very long-chain n−3 fatty acids on plasma markers of inflammation in middle-aged men. Prostaglandins Leukot Essent Fatty Acids 2008;78:219–28 [DOI] [PubMed] [Google Scholar]
- 68.Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic Biol Med 2003;35:772–81 [DOI] [PubMed] [Google Scholar]
- 69.Yoneyama S, Miura K, Sasaki S, et al. Dietary intake of fatty acids and serum C-reactive protein in Japanese. J Epidemiol 2007;17:86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zampelas A, Panagiotakos DB, Pitsavos C, et al. Fish consumption among healthy adults is associated with decreased levels of inflammatory markers related to cardiovascular disease: the ATTICA Study. J Am Coll Cardiol 2005;46:120–4 [DOI] [PubMed] [Google Scholar]
- 71.Ciubotaru I, Lee YS, Wander RC. Dietary fish oil decreases C-reactive protein, interleukin-6, and triacylglycerol to HDL-cholesterol ratio in postmenopausal women on HRT. J Nutr Biochem 2003;14:513–21 [DOI] [PubMed] [Google Scholar]
- 72.Kelley DS, Siegel D, Fedor DM, Adkins Y, Mackey BE. DHA supplementation decreases serum C-reactive protein and other markers of inflammation in hypertriglyceridemic men. J Nutr 2009;139:495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Farzaneh-Far R, Harris WS, Garg S, Na B, Whooley MA. Inverse association of erythrocyte n−3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: the Heart and Soul Study. Atherosclerosis 2009;205:538–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Himmelfarb J, Phinney S, Ikizler TA, Kane J, McMonagle E, Miller G. Gamma-tocopherol and docosahexaenoic acid decrease inflammation in dialysis patients. J Ren Nutr 2007;17:296–304 [DOI] [PubMed] [Google Scholar]
- 75.Madsen T, Christensen JH, Blom M, Schmidt EB. The effect of dietary n−3 fatty acids on serum concentrations of C-reactive protein: a dose–response study. Br J Nutr 2003;89:517–22 [DOI] [PubMed] [Google Scholar]
- 76.Geelen A, Brouwer IA, Schouten EG, Kluft C, Katan MB, Zock PL. Intake of n−3 fatty acids from fish does not lower serum concentrations of C-reactive protein in healthy subjects. Eur J Clin Nutr 2004;58:1440–2 [DOI] [PubMed] [Google Scholar]
- 77.Saifullah A, Watkins BA, Saha C, Li Y, Moe SM, Friedman AN. Oral fish oil supplementation raises blood omega-3 levels and lowers C-reactive protein in haemodialysis patients a pilot study. Nephrol Dial Transplant 2007;22:3561–7 [DOI] [PubMed] [Google Scholar]
- 78.Boyer BB, Mohatt GV, Plaetke R, et al. Metabolic syndrome in Yup'ik Eskimos: The Center for Alaska Native Health Research (CANHR) Study. Obesity (Silver Spring) 2007;15:2535–40 [DOI] [PubMed] [Google Scholar]