Abstract
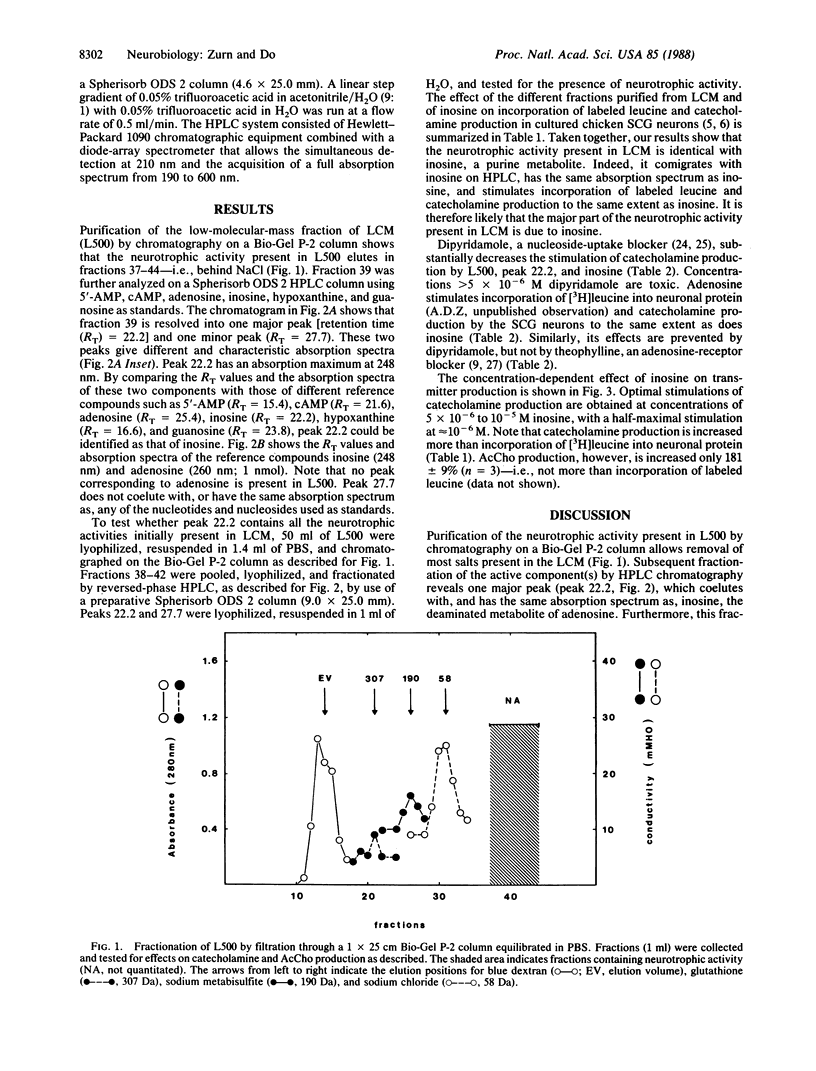
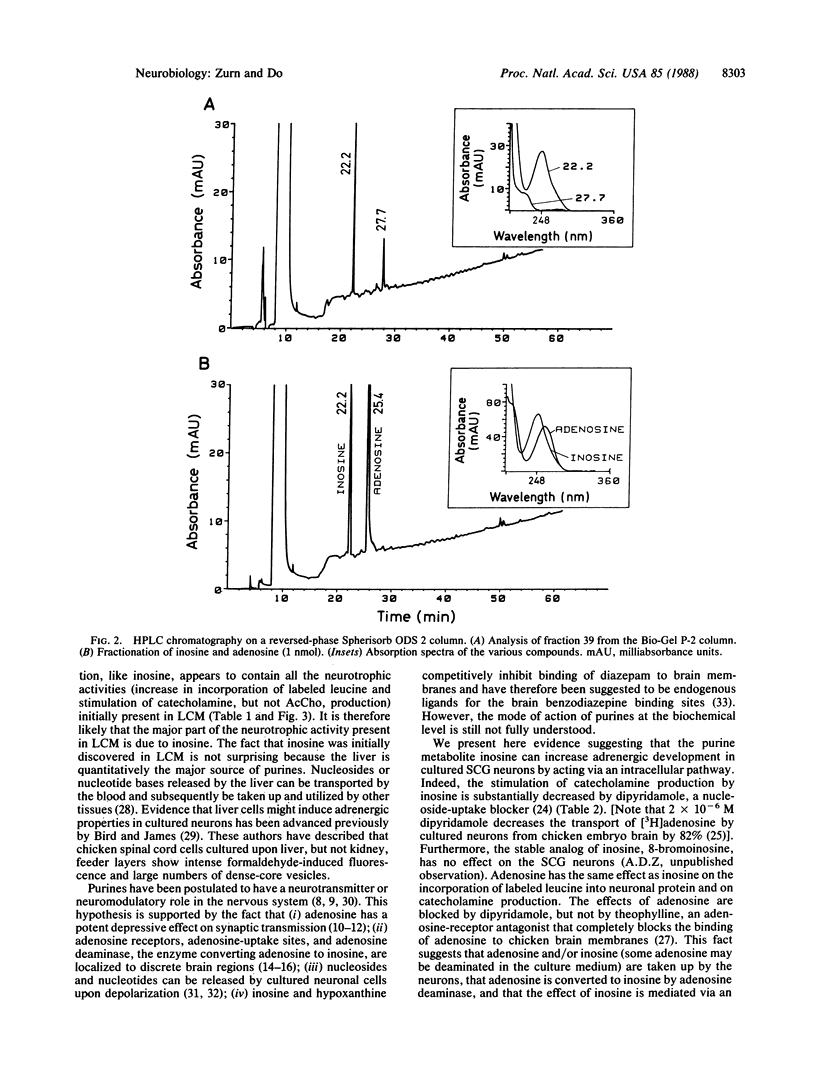
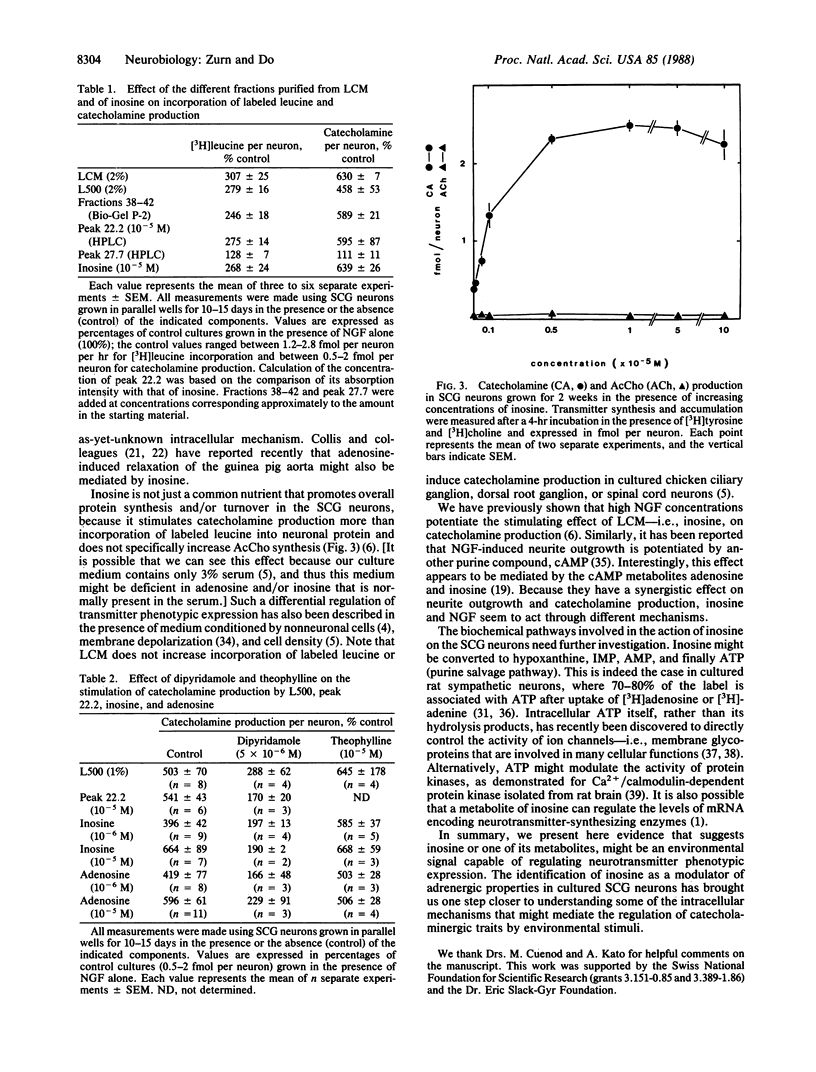
Purines are ubiquitous endogenous cellular metabolites that have been postulated as neurotransmitters or neuromodulators in the nervous system. Recently, we showed that a low-molecular-mass component present in liver-conditioned medium selectively enhances the adrenergic properties of dissociated chicken sympathetic neurons in culture. We report here that this substance is inosine, a purine metabolite. Indeed, analysis of the low-molecular-mass fraction of liver-conditioned medium by HPLC shows that the neurotrophic activity coelutes with and has the same absorption spectrum as inosine. Inosine increases incorporation of [3H]leucine into neuronal protein and stimulates catecholamine, but not acetylcholine, production by the sympathetic neurons in a dose-dependent fashion (half-maximal stimulation at 10(-6) M). This effect can be blocked by 5 x 10(-6) M dipyridamole, an inhibitor of nucleoside transport. Inosine therefore appears to be capable of modulating adrenergic phenotypic expression in cultured sympathetic neurons by acting via an as-yet-unknown intracellular pathway.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano T., Spector S. Identification of inosine and hypoxanthine as endogenous ligands for the brain benzodiazepine-binding sites. Proc Natl Acad Sci U S A. 1979 Feb;76(2):977–981. doi: 10.1073/pnas.76.2.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Barnes E. M., Jr, Thampy K. G. Subclasses of adenosine receptors in brain membranes from adult tissue and from primary cultures of chick embryo. J Neurochem. 1982 Sep;39(3):647–652. doi: 10.1111/j.1471-4159.1982.tb07941.x. [DOI] [PubMed] [Google Scholar]
- Bird M. M., James D. W. The culture of previously dissociated embryonic chick spinal cord cells on feeder layers of liver and kidney, and the development of paraformaldehyde induced fluorescence upon the former. J Neurocytol. 1975 Dec;4(6):633–646. doi: 10.1007/BF01181626. [DOI] [PubMed] [Google Scholar]
- Bisserbe J. C., Patel J., Marangos P. J. Autoradiographic localization of adenosine uptake sites in rat brain using [3H]nitrobenzylthioinosine. J Neurosci. 1985 Feb;5(2):544–550. doi: 10.1523/JNEUROSCI.05-02-00544.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black I. B., Adler J. E., Dreyfus C. F., Friedman W. F., LaGamma E. F., Roach A. H. Biochemistry of information storage in the nervous system. Science. 1987 Jun 5;236(4806):1263–1268. doi: 10.1126/science.2884727. [DOI] [PubMed] [Google Scholar]
- Braas K. M., Newby A. C., Wilson V. S., Snyder S. H. Adenosine-containing neurons in the brain localized by immunocytochemistry. J Neurosci. 1986 Jul;6(7):1952–1961. doi: 10.1523/JNEUROSCI.06-07-01952.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braumann T., Jastorff B., Richter-Landsberg C. Fate of cyclic nucleotides in PC12 cell cultures: uptake, metabolism, and effects of metabolites on nerve growth factor-induced neurite outgrowth. J Neurochem. 1986 Sep;47(3):912–919. doi: 10.1111/j.1471-4159.1986.tb00697.x. [DOI] [PubMed] [Google Scholar]
- Collis M. G., Brown C. M. Adenosine relaxes the aorta by interacting with an A2 receptor and an intracellular site. Eur J Pharmacol. 1983 Dec 9;96(1-2):61–69. doi: 10.1016/0014-2999(83)90529-0. [DOI] [PubMed] [Google Scholar]
- Collis M. G., Palmer D. B., Baxter G. S. Evidence that the intracellular effects of adenosine in the guinea-pig aorta are mediated by inosine. Eur J Pharmacol. 1986 Feb 11;121(1):141–145. doi: 10.1016/0014-2999(86)90404-8. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T. V. The physiological role of adenosine in the central nervous system. Int Rev Neurobiol. 1985;27:63–139. doi: 10.1016/s0074-7742(08)60556-5. [DOI] [PubMed] [Google Scholar]
- Erny R. E., Berezo M. W., Perlman R. L. Activation of tyrosine 3-monooxygenase in pheochromocytoma cells by adenosine. J Biol Chem. 1981 Feb 10;256(3):1335–1339. [PubMed] [Google Scholar]
- Fukada K. Purification and partial characterization of a cholinergic neuronal differentiation factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8795–8799. doi: 10.1073/pnas.82.24.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furshpan E. J., Potter D. D., Matsumoto S. G. Synaptic functions in rat sympathetic neurons in microcultures. III. A Purinergic effect on cardiac myocytes. J Neurosci. 1986 Apr;6(4):1099–1107. doi: 10.1523/JNEUROSCI.06-04-01099.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger J. D., Nagy J. I. Distribution of adenosine deaminase activity in rat brain and spinal cord. J Neurosci. 1986 Sep;6(9):2707–2714. doi: 10.1523/JNEUROSCI.06-09-02707.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. R., Synder S. H. Autoradiographic localization of adenosine receptors in rat brain using [3H]cyclohexyladenosine. J Neurosci. 1982 Sep;2(9):1230–1241. doi: 10.1523/JNEUROSCI.02-09-01230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann S. R., Joshi H. C., Schechter A., Fletcher J. R., Bothwell M. Synergistic effects of cyclic AMP and nerve growth factor on neurite outgrowth and microtubule stability of PC12 cells. J Cell Biol. 1985 Mar;100(3):916–927. doi: 10.1083/jcb.100.3.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M. J., Bronner-Fraser M. Neural tube-derived factors influence differentiation of neural crest cells in vitro: effects on activity of neurotransmitter biosynthetic enzymes. Dev Biol. 1986 Sep;117(1):45–54. doi: 10.1016/0012-1606(86)90346-5. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Adler J. E., Bohn M. C., Black I. B. Substance P in principal sympathetic neurons: regulation by impulse activity. Science. 1981 Oct 16;214(4518):335–336. doi: 10.1126/science.6169153. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Maurer K., Gensheimer H. P., Schwabe U. Dual actions of adenosine on rat peritoneal mast cells. Naunyn Schmiedebergs Arch Pharmacol. 1987 May;335(5):555–560. doi: 10.1007/BF00169124. [DOI] [PubMed] [Google Scholar]
- Lou L. L., Lloyd S. J., Schulman H. Activation of the multifunctional Ca2+/calmodulin-dependent protein kinase by autophosphorylation: ATP modulates production of an autonomous enzyme. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9497–9501. doi: 10.1073/pnas.83.24.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti P. J., Hof P. R., Martin J. L. Adenosine stimulates glycogenolysis in mouse cerebral cortex: a possible coupling mechanism between neuronal activity and energy metabolism. J Neurosci. 1986 Sep;6(9):2558–2562. doi: 10.1523/JNEUROSCI.06-09-02558.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains R. E., Patterson P. H. Primary cultures of dissociated sympathetic neurons. I. Establishment of long-term growth in culture and studies of differentiated properties. J Cell Biol. 1973 Nov;59(2 Pt 1):329–345. doi: 10.1083/jcb.59.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H., Chun L. L. The induction of acetylcholine synthesis in primary cultures of dissociated rat sympathetic neurons. I. Effects of conditioned medium. Dev Biol. 1977 Apr;56(2):263–280. doi: 10.1016/0012-1606(77)90269-x. [DOI] [PubMed] [Google Scholar]
- Prestwich S. A., Forda S. R., Dolphin A. C. Adenosine antagonists increase spontaneous and evoked transmitter release from neuronal cells in culture. Brain Res. 1987 Mar 3;405(1):130–139. doi: 10.1016/0006-8993(87)90997-8. [DOI] [PubMed] [Google Scholar]
- Pritchard J. B., O'Connor N., Oliver J. M., Berlin R. D. Uptake and supply of purine compounds by the liver. Am J Physiol. 1975 Oct;229(4):967–972. doi: 10.1152/ajplegacy.1975.229.4.967. [DOI] [PubMed] [Google Scholar]
- Roos H., Pfleger K. Kinetics of adenosine uptake by erythrocytes, and the influence of dipyridamole. Mol Pharmacol. 1972 Jul;8(4):417–425. [PubMed] [Google Scholar]
- Silinsky E. M. On the mechanism by which adenosine receptor activation inhibits the release of acetylcholine from motor nerve endings. J Physiol. 1984 Jan;346:243–256. doi: 10.1113/jphysiol.1984.sp015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. H. Adenosine as a neuromodulator. Annu Rev Neurosci. 1985;8:103–124. doi: 10.1146/annurev.ne.08.030185.000535. [DOI] [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature. 1985 Aug 22;316(6030):736–738. doi: 10.1038/316736a0. [DOI] [PubMed] [Google Scholar]
- Su C. Purinergic neurotransmission and neuromodulation. Annu Rev Pharmacol Toxicol. 1983;23:397–411. doi: 10.1146/annurev.pa.23.040183.002145. [DOI] [PubMed] [Google Scholar]
- Thampy K. G., Barnes E. M., Jr Adenosine transport by primary cultures of neurons from chick embryo brain. J Neurochem. 1983 Mar;40(3):874–879. doi: 10.1111/j.1471-4159.1983.tb08061.x. [DOI] [PubMed] [Google Scholar]
- Tolkovsky A. M., Suidan H. S. Adenosine 5'-triphosphate synthesis and metabolism localized in neurites of cultured sympathetic neurons. Neuroscience. 1987 Dec;23(3):1133–1142. doi: 10.1016/0306-4522(87)90187-4. [DOI] [PubMed] [Google Scholar]
- Wolinsky E. J., Patterson P. H. Potassium-stimulated purine release by cultured sympathetic neurons. J Neurosci. 1985 Jul;5(7):1680–1687. doi: 10.1523/JNEUROSCI.05-07-01680.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Z. G., Smith J., Le Douarin N. M. Differentiation of catecholaminergic cells in cultures of embryonic avian sensory ganglia. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8800–8804. doi: 10.1073/pnas.82.24.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurn A. D. A new low-molecular-weight component promoting adrenergic development in cultured chick sympathetic neurons. J Neurosci. 1987 Nov;7(11):3566–3573. doi: 10.1523/JNEUROSCI.07-11-03566.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurn A. D., Mudry F. Conditions increasing the adrenergic properties of dissociated chick superior cervical ganglion neurons grown in long-term culture. Dev Biol. 1986 Oct;117(2):365–379. doi: 10.1016/0012-1606(86)90306-4. [DOI] [PubMed] [Google Scholar]


