Abstract
Xenograft outcomes are dictated by xenoantigen expression, for example, Gal α 1, 3Gal (Gal), but might also depend on differing vascular responses. We investigated whether differential vascular gene expression in kidney and cardiac xenografts correlate with development of thrombotic microangiopathy (TM) and consumptive coagulation (CC). Immunosuppressed baboons underwent miniswine or hDAF pig kidney (n = 6) or heart (n = 7), or Gal-transferase gene-knockout (GalT-KO) (thymo)kidney transplantation (n = 14). Porcine cDNA miniarrays determined donor proinflammatory, apoptosis-related and vascular coagulant/fibrinolytic gene expression at defined time points; validated by mRNA, protein levels and immunopathology. hDAF-transgenic and GalT-KO xenografts, (particularly thymokidneys) exhibited prolonged survival. CC was seen with Gal-expressing porcine kidneys (3 of 6), only 1 of 7 baboons post-cardiac xenotransplantation and was infrequent following GalT-KO grafts (1 of 14). Protective-type genes (heme oxygenase-I, superoxide dismutases and CD39) together with von Willebrand factor and P-selectin were upregulated in all renal grafts. Transcriptional responses in Gal-expressing xenografts were comparable to those seen in the infrequent GalT-KO rejection.
In cardiac xenografts, fibrin deposition was associated with increased plasminogen activator inhibitor-1 expression establishing that gene expression profiles in renal and cardiac xenografts differ in a quantitative manner. These findings suggest that therapeutic targets may differ for renal and cardiac xenotransplants.
Keywords: Gene expression, heart, kidney, vasculature, xenotransplantation
Introduction
A central role for the endothelium has been proposed for the regulation of hemostasis, local immune and inflammatory reactions, and in graft rejection (1,2). Upon activation, vascular endothelial cells alter their quiescent anticoagulant surface phenotype to promote thrombosis and vascular occlusion (3). This transformation is associated with multiple inflammatory mediators and consequent signaling events (4,5). Organ-specific vascular reactions (6) might be relevant to the differential forms of graft injury seen in clinical and experimental transplantation (Tx) (7–9). For example, as with warm and cold ischemia followed by reperfusion (7,10,11), injury can be far more pronounced in lungs and hearts when compared to kidneys and the liver (12). Severe preservation injury is in turn associated with heightened rejection reactions (12–15).
The pig-to-primate xenotransplantation combination is termed discordant, and is associated with hyperacute rejection (5,16). This develops as a consequence of binding of natural xenoantibodies to Gala1,3Gal (Gal) with complement activation in wild-type xenografts transplanted into unmodified recipients (17–19). When this modality of rejection is appropriately managed by graft or recipient modification, further humoral xenograft rejection associated with elicited antibodies can be observed. This delayed xenograft rejection or acute humoral xenograft rejection (AHXR) is analogous, to some extent, to the vascular rejection process seen in allografts (5,14,20). In the xenograft, specific molecular incompatibilities in the initiation and regulation of coagulation and complement activation pathways by the graft endothelium (18) might exacerbate inflammatory and thrombotic processes (5). Xenograft vascular injury can therefore precipitate a situation characterized by progressive endothelial cell activation with thrombotic microangiopathy (TM) and result in consumptive coagulopathy (CC) (19,21).
How organ heterogeneity impacts upon xenograft outcomes has been largely unexplored. We have studied genes in the graft that might be differentially altered after Tx of porcine kidneys or hearts into baboons, in the variable setting of various xenograft rejection mechanisms, associated CC and TM. Our data strongly suggest that altered vascular responses may impact xenograft survival and the associated different incidences of systemic complications in kidneys or hearts.
Methods
Animals
Baboons (Papio anubis, n = 27) of known ABO blood group and of body weight 8–17 kg (Biological Resources, or Mannheimer Foundation) were used as recipients. Massachusetts General Hospital (MGH) MHC-inbred miniature swine, either unmodified (n = 6) or homozygous for α 1, 3-galactosyltransferase gene-knockout (GalT-KO) (n = 14) (22), of blood group O, 1.5–4 months old, 6.5–40 kg body weight (Charles River Laboratories, Wilmington, MA) or Large White/Landrace crossbreed pigs transgenic for human decay-accelerating factor (hDAF) (n = 7) (Novartis Pharma, Madison, WI) of blood group O, 1.5–3.5 months old, weighing 9–35 kg, served as controls and as donors of (thymo)kidneys and/or hearts.
All experiments were conducted according to the NIH Guidelines for Care and Use of Laboratory Animals and were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
Experimental groups
Group 1 (kidney xenografts; n = 6): Recipients consisted of baboons postunilateral native nephrectomy that received either MGH (n = 3) or hDAF kidney (n = 3) grafts from Gal-positive swine.
Group 2 (cardiac xenografts: n = 7): Recipients underwent heterotopic Tx (in the abdomen) of either MGH (n = 3) or hDAF (n = 4) Gal-positive hearts.
Group 3 (GalT-KO renal grafts with/without thymus; n = 14): Baboons had unilateral native nephrectomy with the contralateral ureter ligated and divided (organ-perfusion supporting grafts); 11 renal xenotransplants were performed in combination with vascularized thymus from GalT-KO donors, 5 as thymokidneys and 6 as vascularized thymic lobe grafts (23). The remaining three kidney transplants were performed without cotransplantation of vascularized thymus tissue. As additional controls, kidney and heart samples of age-, gender- and genotype matched, nontransplant tissues from naïve, untreated swine were studied.
Surgical procedures
Anesthesia and all applied surgical procedures have been described (24,25).
Extracorporeal immunoadsorption in baboons
Where required, anti-Gal antibody (Ab) was depleted from the baboon's circulation was performed, as described (26–28).
Conditioning regimen in baboons
The conditioning regimen in baboons was applied as reported (23,29,30). The regimen based on initial T-cell depletion and maintenance anti-CD154 monoclonal antibody and mycophenolate mofetil therapy. Animals undergoing the concomitant Tx of vascularized thymus tissue underwent thymectomy and splenectomy, prior to the xeno organ Tx procedure.
Monitoring and supportive therapy
Monitoring and supportive therapy was conducted as described (30).
Assays for detection of baboon anti-gal antibody and of antibody directed against porcine non-gal determinants
Details of these methods have been reported previously (26,27).
Differential gene expression analysis in porcine renal and cardiac xenografts by DNA arrays
We developed a DNA array with immobilized oligonucleotides that recognize the 3′ end of mRNAs of 68 sequences from selected proinflammatory, apoptosis-related genes and coagulant/fibrinolytic genes (Table 1). For each gene, 20 fmol of two different 60 bp oligonucleotides (MWG Biotech, Huntsville, AL) were spotted onto hybridization membranes (GenScreen plus, Bio-Rad Laboratories, Hercules, CA) with a 1536-pin replicator (V and P Scientific, San Diego, CA). The selected oligonucleotides satisfied the following criteria: −Tm within 85–95°C; absence of secondary structure; and absence of cross-hybridization verified by querying each oligonucleotide against the expressed sequence tag (EST) data base. Blank spots without oligonucleotides were included for evaluation of background caused by nonspecific interactions of individual probes with the membrane.
Table 1.
Listing of porcine genes investigated by cDNA microarray
| Gene | Accession |
|---|---|
| Alpha-B crystalline | Z98816 |
| Proapoptotic Bak | AJ001204 |
| Antiapoptotic Bcl-xL | AJ001203 |
| BiP | J03214 M21051 |
| Caspase-3 | AB029345 |
| CD39 | AJ133746 |
| CD40 | AF248545 |
| CD 40L | AF2639 |
| CD47/IAP | AF332698 |
| CD95, APO1 | AJ001202 |
| Cox-1 | AF207823 |
| Cox-2 | AF207824 |
| E-selectin | L39076 |
| Factor VIII | U49517 |
| Fas ligand | AF397407 |
| Heme oxygenase | X60677 S54783 |
| Heat shock protein 47 | AF178675 |
| Heat shock protein 70 | X68213 |
| Heat shock protein 72 | M29506 |
| Heat shock protein 90 | U94395 |
| ICAM-1 | AF156712 |
| Interleukin 1-alpha | X52731 |
| Interleukin-12 mRNA | U08317 |
| Interleukin-13 mRNA | AF385626 |
| Interleukin-15 mRNA | U58142 |
| Interleukin-18 mRNA | AF176949 |
| Interleukin-1 beta | M86725 |
| Interleukin-1 beta CE | AB027296 |
| Interleukin 2 | X58428 |
| Interleukin-4 | X68330 |
| Interleukin 5 | AJ133452 |
| Interleukin-7 | AB035380 |
| Interleukin-8 | X61151 |
| Integrin beta-1 | U91517 |
| Integrin beta-1 subunit | AF192528 |
| Integrin beta 3 | AB048866 |
| NADPH-cytochrome P-450 oxidoreductase | L33893 |
| Nitric oxide synthase (NOS) | U59924 |
| Platelet-derived growth factor receptor beta | AF347050 |
| P53 protein | AF124298 |
| Plasminogen activator inhibitor-1 | Y11347 |
| Plasminogen activator | X02724 |
| CD31 protein (PECAM-1) | X98505 |
| Putative inhibitor of apoptosis (PIAP) | U79142 |
| Protein C mRNA | AF191307 |
| Protein S (PROS) | L31379 |
| P-selectin | L39075 |
| Superoxide dismutase 1 (SOD1) Cu-Zn-superoxide dismutase | AF396674 AJ010339 |
| Superoxide dismutase 2 (SOD2) Mn-superoxide dismutase | AF396673 X64057 |
| Apoptosis inhibitor survivin mRNA | AF195781 |
| Transforming growth factor beta 2 | X70142 S48994 |
| TGF-beta type III receptor | L07595 |
| TGF-beta 3 | X14150 |
| TGF-beta 1 | X12373 |
| TNF-alpha mRNA for tumor necrosis factor alpha | X57321 |
| Type-1 cytochrome c | D73391 |
| Type-2 cytochrome c | D73392 |
| VCAM | L43124 |
| von Willebrand factor | S78431 |
| von Willebrand factor precursor(vWF) | AF052036 |
| GPIIIa mRNA | AF170527 |
| GPIIb | AF170526 |
| Aminopeptidase | A U66371 |
| Hog cholera virus polyprotein | AF001291 |
Total RNA was isolated from heart and kidney grafts functioning in baboons, and at later time-points, when undergoing rejection (Table 2). For the array analysis, total mRNA was used to produce labeled reagents with (33P) dCTP (Prime –It II) cDNA. Labeled cDNA was purified from free (33P) dCTP by a QiaQuick PCR column (Qiagen, Valencia, CA) and heat-denatured before hybridization to the membrane array. After washing and exposure, the spot reading on the membranes was performed with the phosphorImager. In a single experiment, two or three identical membranes were hybridized and analyzed. The cDNA from 6 experimental kidney transplants, 6 control porcine kidneys (MGH miniature or hDAF-transgenic), 7 experimental heart transplants and 6 controls porcine hearts (MGH miniature or hDAF-transgenic) were labeled and used separately for hybridization.
Table 2.
Relative gene expression levels in rejecting kidney and heart grafts (relative to control organs)
| Encoded protein1 | Kidney | Heart | Galt-KO kidney |
|---|---|---|---|
| CD31 (PECAM) | +4.1 (±0.8) | → | +3.9 (±0.5) |
| von Willebrand factor (vWf) | +3.5 (±0.4) | +3.4 (±0.8) | +3.7 (±0.6) |
| Superoxide dismutases | +3.6 (±0.8) | → | +3.3 (±0.5) |
| Tissue factor | +3.2 (±0.4) | → | +3.4 (±0.4) |
| Heme oxygenase-1 | +2.1 (±0.2) | → | +2.2 (±0.4) |
| Heat shock protein-72 | +2.1 (±0.4) | → | +2.1 (±0.3) |
| Nitric oxide synthase | +2.1 (±0.33) | → | +2.1 (±0.5) |
| CD39 | +2.0 (±0.2) | +4.1 (±0.5) | +2.1 (±0.2) |
| PAI-1 | → | +3.7 (±0.2) | → |
PAI-1 = plasminogen activator inhibitor-1.
PECAM = platelet endothelial cell adhesion molecules (CD31).
→ = no substantive change in the content of specific mRNA by miniarray.
Superoxide dismutases, heme oxygenase-1, CD39 and nitric oxide synthase are potential ‘protective factors’ that are upregulated in response to some stresses. CD39, which is expressed on endothelium, prevents platelet aggregation. CD31/PECAM, vWF, tissue factor and PAI-1 are factors that promote coagulation and thrombosis.
The data were imported into Microsoft Excel spreadsheets, and were normalized using a set of highly and steadily expressed genes. Probes with hybridization below background noise were excluded from the analysis. The readings of each oligonucleotide probe averaged among replicate arrays hybridized with the same cDNA were considered independent. The hypothesis that a gene was differentially expressed in the experimental histologically rejected (R) or non-rejected (NR) heart or kidney sample as compared with the relevant control (C) was tested by calculating the corresponding p-value using the native Excel t-test option.
Northern blotting
Total RNA (2 μg) was separated on a 0.7% agarose gel, denatured, and blotted onto a nylon membrane (GeneScreen Plus, NEN du Pont, Boston, MA). Hybridization with the radioactively labeled oligonucleotide probes was performed according to standard protocols in Quick Hybrid solution (Stratagene, La Jolla, CA).
Western blotting
Frozen left ventricular tissue was homogenized in RIPA buffer (150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM TRIS pH 8.0 with protease inhibitors). Homogenates were centrifuged, supernatant protein concentration was determined, and SDS-PAGE performed with 100 μg of protein. Following transfer to a PVDF membrane (Millipore, Billerica, MA) and blocking with 2% BSA at room temperature, the membrane was incubated overnight at 4°C with the following primary Abs, mouse anti-pig NTPDase-1/CD39 (in house reagent), rabbit anti-vWF (A0082, Dako, Denmark), rabbit anti-P-Selectin (553716, Pharmigen, CA), rabbit anti- plasminogen activator inhibitor (PAI)-1 (American Diagostica Inc., CT), rabbit anti- heme oxygenase (HO)-1(Stressgen Biotechnology Corp., San Diego, CA, SPE 895). Following incubation with appropriate HRP conjugates secondary antibody, proteins have been visualized by enhanced chemiluminesence (Pierce, Rockford, IL).
Histopathology of recovered kidney and heart tissue biopsies
Tissue samples of kidneys and ureter, or of atrial and ventricular tissues of the hearts, were processed and stained with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) for light microscopy as described previously (30).
Immunofluorescence
Processing and staining for immunofluorescence was performed as previously reported (30).
Electron microscopy
Assessment by electron microscopy was performed as reported previously (30).
Statistical analysis
Student's t-tests were used when two ordinal variables are used or analysis of variance when three or more ordinal variables were compared. Statistical significance determined by p < 0.05.
Immunohistochemistry
Heart and kidney samples were embedded in freezing medium, snap-frozen in isopentane in liquid nitrogen, and stored at −80°C until used. Sections (5 μm) were obtained, air-dried, fixed in acetone, and stained using the three-step method. Primary Abs included: – mouse monoclonal anti-pig NTPDase1/CD39 (4D-11); rabbit-anti-von Willebrand factor (vWF) (Dako, Glostrup, Denmark); rabbit anti-P-selectin (Pharmingen, Franklin Lakes, NJ); and rabbit antiplasminogen activator inhibitor-1 (PAI-1) (American Diagnostica). Secondary Abs included: – horse anti-mouse (BA-2001, Vector) and goat anti-rabbit (BA-1000, Vector, Burlingame, CA). Slides were incubated with avidin-biotin complex with HRP (Dako) and visualized with DAB (Vector). Sections were counterstained with hematoxylin solution (Gill's II, American Master Tech, Lodi, CA). Negative controls were stained with the non-specific isotype-matched primary Ab or preimmune sera.
Results
Graft survival
Pig orthotopic renal (group 1) and heterotopic (nonorgan-perfusion supporting) cardiac (group 2) xenografts survived for up to a maximal time of 29 and 28 days, respectively. Within these groups, rejection of hDAF kidneys was delayed longer than the wild-type MGH kidneys (median survival 29 vs. 7 days); failure of the MGH kidneys arose from several different causes, whereas failure of hDAF kidneys was uniformly from AHXR. There was little difference in survival of heart grafts from these two sources (median 27 vs. 24 days); AHXR was the prime reason for termination of the experiment in seven cases.
In six cases in the combined groups, the experiment was terminated following the death of the baboon from infection (n = 3) or other complications (28,30). CC developed in three baboon recipients of kidneys, proving fatal in two cases, with one baboon undergoing euthanasia.
The experimental results of group 3 have been reported previously (23). Four of 11 baboons bearing vascularized thymic grafts, either thymokidney or vascularized thymic lobe, maintained xenogeneic renal function longer than 50 days. Three of four long-term survivors maintained normal serum creatinine levels for 56, 69 and 83 days, respectively, before expiring from causes unrelated to rejection. The fourth baboon experienced an apparent rejection crisis between days 53 and 65, which reversed with anti-T-cell rejection therapy; subsequently the animal expired on day 81 from pneumonia. Five other baboons died from other causes before day 50, but with normal creatinine levels. Neither hyperacute nor irreversible rejection was observed in any animals bearing vascularized thymic grafts, except for two baboons whose immunosuppression was terminated at an early time-point due to severe diarrhea (B115) and a gastric ulcer (B129) that required the cessation of immunosuppression. In contrast, all baboon recipients of kidneys alone, treated similarly rejected their renal grafts within 34 days.
Anti-Gal and anti-nonGal antibody responses
Anti-Gal Ab remained at low levels in all baboons in groups 1 and 2 (not shown). There were no correlations between serum anti-Gal IgM or IgG levels and changes in gene expression profiles. Antibody to non-Gal pig determinants did not develop in any baboon in groups 1–3, until discontinuation of anti-CD154 mAb therapy (postgraftectomy) (30).
Hematological parameters
Changes in fibrinogen after xenografting with evidence for consumption (largely in group 1) are depicted for all baboons in Figure 1. Details on fibrinogen levels, fibrin split products, platelet counts and prothrombin time measurements after Tx has been reported for groups 1 and 2 previously (30).
Figure 1. Coagulation parameters.
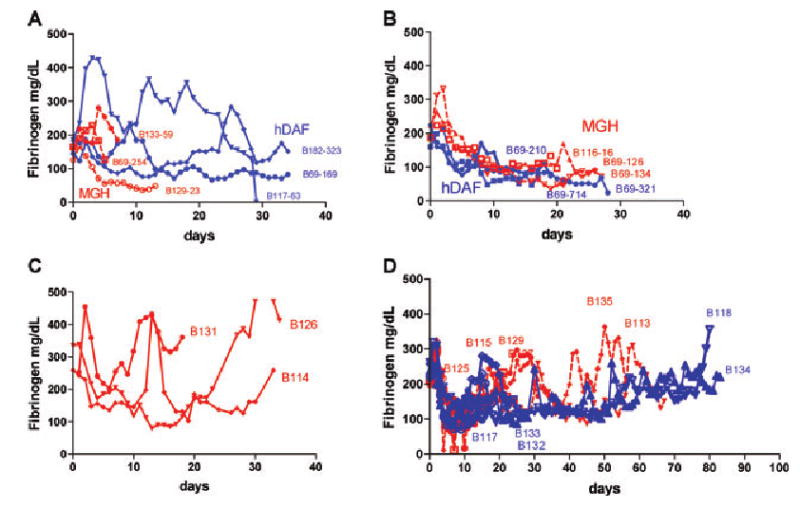
Changes in plasma fibrinogen (in mg/dL) in baboons; (A) Non GalT-KO pig kidney recipients (group 1) (_ _ MGH pig, — — hDAF pig), (B) Pig heart recipients (group 2) (_ _ MGH pig, — — hDAF pig), (C) GalT-KO kidney without thymic tissue transplantation, (D) GalT-KO kidney with vascularized thymus (— — thymokidney, _ _ vascularized thymic lobe).
In group 3, recipients of kidneys alone had fibrinogen levels that decreased slowly, with only 1 of 3 animals decreasing to a level <100 mg/dL (Figure 1C). The other animals maintained fibrinogen >100 mg/dL throughout, despite rejection via predominantly cellular mechanisms. Animals that received thymus plus kidney transplants (both vascularized thymic lobe [VTL] and thymokidney [TK] and TK) had a slow decline in average fibrinogen levels with the nadir at day 7 (103 mg/dL ± 26). Thereafter, fibrinogen levels increased to >150 mg/dL by day 15 and remained fairly stable thereafter (Figure 1D). Creatinine levels were stable in these animals during this period.
Differential gene expression in porcine renal and cardiac xenografts
We observed that there were 18 specific mRNAs were present at different levels in porcine kidney and heart xenografts, when compared to native, nongrafted kidneys and hearts from control pigs (Table 2 and Figure 2). We also detected genes where expression was considerably lower than in naïve controls, as well as other genes that were induced after Tx (not shown). We then analyzed mRNA expression in control (C), NR and R xenografts by northern blotting (Figure 3). These data largely validate the miniarray studies (see later).
Figure 2. Miniarrays and differential gene expression profiles.
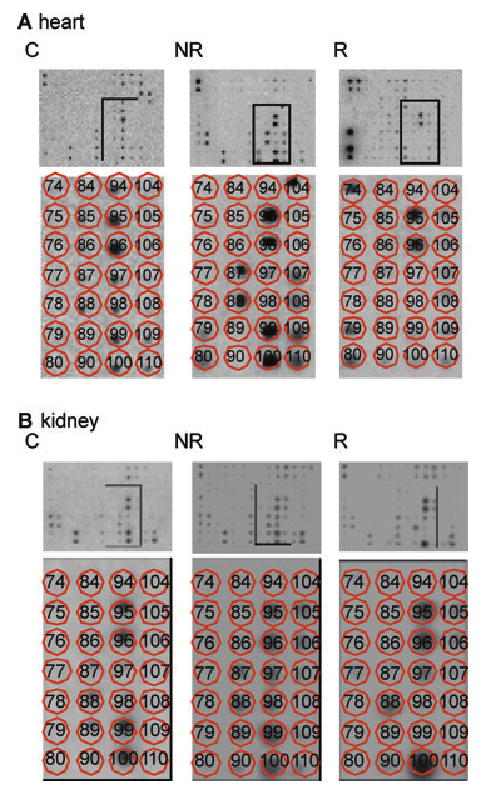
Identical miniarray membranes were probed with individual 33P-labeled cDNA prepared from (A) kidney xenografts and (B) heart xenografts. Arrays contained oligonucleotide probes that were specific for the differentially expressed clones. Probes were radioactively labeled, differentially expressed cDNA fragments for the respective genes. Bottom panels of (A) and (B) show an enlargement of the boxed portions in the top panels. Matrix overlay maps the individual oligonucleotides for each of the genes (C = controls; NR = nonrejected; R = rejected).
Figure 3. Representative northern blot analyses confirming changes in mRNA levels.
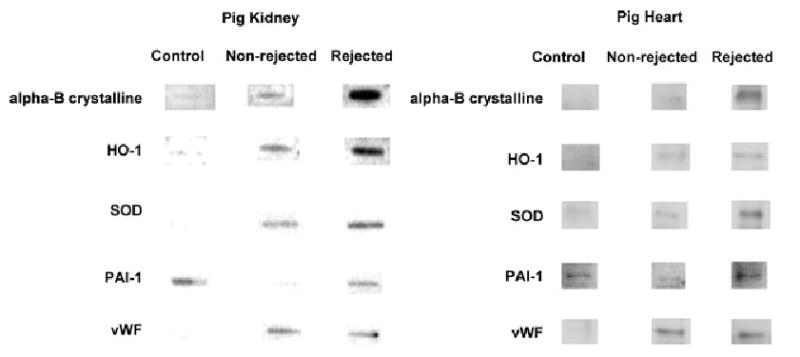
Total RNA samples (2 μg) from pig kidney and heart grafts and the respective nontransplanted control samples analyzed by the miniarrays, were also examined by northern blot analysis. Corresponding genes to the probes are identified to the left of the autoradiographs. See text for details (Abbreviations: Table 2).
In both MGH miniature porcine as well as in the hDAF-transgenic renal xenografts undergoing rejection, we noted factors that were substantively expressed in the setting of immune xenogeneic reactions. Amongst others, these included: –vWf, the two superoxide dismutases, CD39, Platelet endothelial cell adhesion molecule-1(PECAM-1)/CD31, heme oxygenase-I, heat shock protein-72 and nitric oxide synthases (Tables 2, 3 and Figure 3). The relatively higher levels of expression of vWF, PECAM-1/CD31 and P-selectin at the time of rejecting kidney grafts, were associated with the observed heightened platelet deposition and more prominent CC in these baboon recipients. Such immunopathological changes with platelet sequestration were not seen in heart xenografts.
Table 3.
Relative gene expression patterns in rejecting galt-ko kidney xenografts
| Encoded protein | Gene expression levels (relative to nonrejecting grafts) |
|---|---|
| Heat shock and stress-inducible proteins | |
| Heat shock protein 90 | +4.1 (±0.7) |
| Heat shock protein 70 | +3.8 (±1.1) |
| BiP | +3.6 (±0.5) |
| Homeostasis-related genes | |
| Integrin beta-1 | −3.1 (±0.4) |
| CD39 | +2.8 (±0.1) |
| P-selectin mRNA | +2.6 (±0.6) |
| CD31 protein (PECAM-1) | +2.6 (±0.3) |
| Miscellaneous | |
| IL-8 | −2.9 (±0.5) |
| IL-1α | +2.9 (±0.6) |
| IL-1β | +2.7 (±0.8) |
| TNF-α | +2.1 (±0.4) |
In cardiac xenografts, there were comparable alterations of expression in CD39 and vWF. We noted transient changes in plasminogen activator inhibitor-1 (PAI-1) in the transplanted cardiac tissues followed by later increases in this fibrinolytic inhibitor resulting in high levels of expression at rejection; in renal xenografts, PAI-1 expression became very low postimplantation, prior to rejection (Table 2 and Figure 3). Heightened levels of PAI-1 reexpression could result in preferential inhibition of fibrinolysis and was, in fact, associated with greater fibrin deposition in the cardiac grafts. Although these dramatic changes at the RNA level were not seen in the miniarray studies of the renal grafts, minor fluctuations in PAI-1 expression were detected on northern analyses of rejected xenografts (Figure 3).
Changes at the protein level for the selected, differentially expressed genes in the same groups were also evaluated. The above results were validated by western blotting (not shown).
These data suggest that coagulation and fibrinolysis appear differentially regulated in renal and cardiac xenograft-specific manners. This hypothesis was further supported by our data showing that gene expression in the GalT-KO kidneys was comparable to that seen in unmodified or hDAF-transgenic Gal epitope expressing kidneys.
To investigate the mechanism of xenograft rejection and loss of GalT-KO xenografts in more detail, we searched for changes in specific RNA levels in R versus the NR grafts in group 3. Such changes could provide an early indication of potential xenograft vascular injury, prior to the detection of immunopathological or functional changes.
Table 3 summarizes the observed changes in specific RNA content in the GalT-KO rejection group compared to the nonrejection group. As in Gal-positive kidneys, CD39, P-selectin and PECAM-1/CD31 were upregulated to high levels during the time of rejection in GalT-KO grafts. The mRNA level for select chaperone proteins, such as Hsp90, Hsp70 and Bip, were noted to increase in response to injury. IL-1 and TNF-α were also noted to be expressed at high levels. Integrin β-1 and IL-8 mRNA levels were decreased when compared to NR comparable organs.
Histopathology, immunohistopathology and ultrastructure of pig organ xenografts and native baboon tissue biopsies
Light microscopy of biopsies of NR grafts taken 14 days after organ Tx indicated minimal-to-moderate changes of AHXR in group 1 (kidneys) and no abnormalities in the cardiac biopsies of group 2 (Figure 4A, E). Minimal fibrin deposition was noted in renal grafts at this time (Figure 4C) with no abnormalities in heart grafts (Figure 4G). In contrast, biopsies of rejecting grafts indicated a range of histopathological features from moderate to severe AHXR (Figure 4B, F) with extensive fibrin deposition in both kidney and cardiac grafts (Figure 4D, H).
Figure 4. Histopathological examination and immunofluorescence of functioning and rejected renal and cardiac xenografts.
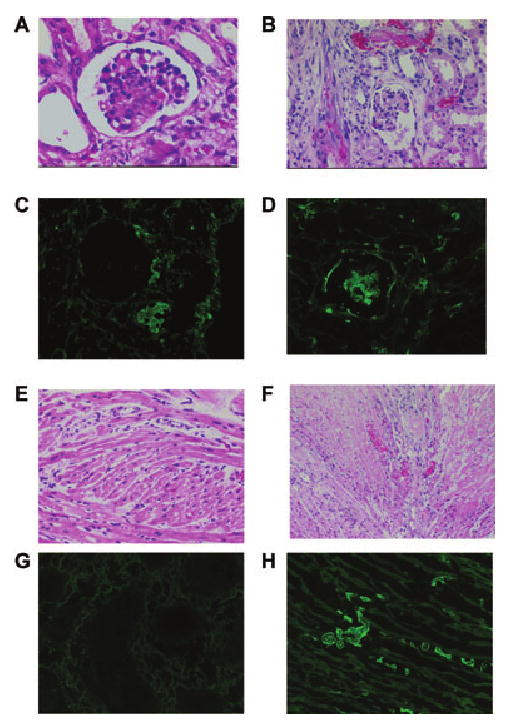
The functioning kidney xenograft (A) shows mild features of acute humoral xenograft rejection (AHXR) whereas the rejected kidney xenograft (B) has moderate features of AHXR (interstitial hemorrhage and glomerular thrombi). Immunofluorescence of a functioning kidney xenograft (C) shows minimal fibrin deposition while the rejected kidney tissues exhibit high amounts of fibrin deposition in the glomeruli (D). Histopathology of the functioning heart xenograft reveals no signs of AHXR (E), whereas the rejected heart xenograft (F) has signs of moderate AHXR (intracellular and interstitial edema and patchy necrosis). Immunofluorescence of a functioning heart xenograft (G) reveals no signs of fibrin deposition, whereas the rejected heart xenograft (H) shows extensive intravascular fibrin deposition.
In group 3, renal xenografts from animals receiving a regimen directed toward tolerance induction showed minimal evidence of rejection histologically and only mild, focal TM (23). Immunohistochemistry showed no evidence of IgG deposition. GalT-KO kidneys without combined thymic tissue Tx showed severe cellular and humoral rejection with bright staining for IgG in glomeruli and peritubular capillaries.
In contrast, in the excised kidney grafts of group 1, immunofluorescence showed IgM deposition in all cases with no or minimal IgG, fibrin (as above), or C3 and C4d deposition (not shown). There were no direct correlations between Ig deposition in the graft and biochemical and hematological changes observed in the baboons.
Although electron microscopic studies indicated endothelial cell activation within xenografts, there was an absence of features suggesting significant immune complex deposition in any of the native baboon organs (not shown). In group 2, immunofluorescence showed IgM deposition in 3 of 7 cases, IgG in 2 of 7, fibrin in one, no C3 deposition, but C4d deposition in cases of moderate-to-severe AHXR. As in group 1, electron microscopic studies indicated vascular injury and endothelial cell activation within the pig organs (not shown).
Immunohistological studies demonstrated upregulation of vWF in the porcine kidneys (Figure 5A) and hearts (Figure 5B) transplanted into baboons. Heightened P-selectin staining in porcine renal xenografts (Figure 5C), relative to the lower level of expression in cardiac xenografts (Figure 5D), was observed prior to rejection. These data indicated both upregulation of expression as well as accompanying, heightened platelet sequestration in kidneys. Renal xenograft staining for PAI-1 (Figure 5E) did not increase in functioning grafts (NR) and was comparable to basal expression in the native kidneys until fully established rejection was noted when fluctuations were noted. In contrast, more marked increases of PAI-1 staining were notable in functioning cardiac grafts (Figure 5F). Heme oxygenase-1 expression was increased in both renal (Figure 5G) and cardiac (Figure 5H) grafts. Heightened CD39 expression was pronounced in renal grafts (Figure 5I), where it was also associated with vWf expression with extensive platelet sequestration. Some increments of CD39 staining were also seen in cardiac xenografts (Figure 5J), but the expression of this ectoenzyme was lost when rejection was fully established in both kidneys and hearts with extensive tissue destruction (not shown).
Figure 5. Immunohistological expression of representative genes in transplanted porcine renal and cardiac xenografts.

Selected immunohistological confirmation of the tabulated gene microarray studies (Table 2) demonstrating upregulation of von Willebrand factor (vWF) in porcine kidneys (A) and hearts (B) transplanted into baboons. Heightened P-selectin staining was present in porcine renal xenografts (C) relative to cardiac xenografts (D) prior to rejection, indicating both upregulation of endothelial cell expression as well as heightened platelet sequestration in kidneys, relative to hearts. Renal graft staining for plasminogen activator inhibitor-1 (PAI-1) (E) did not increase in functioning grafts and was comparable to the basal expression in the native kidneys, until fully established rejection was noted, whereas increases of plasminogen activator inhibitor-1 staining were notable in functioning cardiac grafts (F). Heme oxygenase-1 (HO-1) expression was increased in both renal (G) and cardiac (H) xenografts. Heightened CD39 expression was pronounced in renal grafts (I), where it was associated with vWf expression with extensive platelet sequestration. Some increment of CD39 staining was also seen in the cardiac xenografts (J). Expression of this ectoenzyme was lost when rejection became fully established in both kidneys and hearts with extensive tissue destruction (not shown).
Discussion
We have studied differential vascular responses of kidney and heart grafts in defined xenotransplantation models. Our data suggest that the distinct differences in the incidence and pattern of thrombocytopenia and CC are associated with differential gene responses in the vasculature of different organs. This study also provides early evidence for comparable vascular responses in those GalT-KO and Gal-expressing renal xenografts that are subject to rejection, albeit with differing kinetics.
AHXR is currently the major immunological barrier to successful discordant xenotransplantation in the pig-to-nonhuman primate model. Anti-Gal and non-Gal epitope immunoglobulin deposition on the vascular endothelium and the associated thrombotic manifestations within the xenograft are of pathological importance (5,8,9,31–36). The immunological response to the xenograft vasculature has the capacity to modulate the activation of coagulation factors at this interface. This process has the potential to generate serious systemic hemostatic abnormalities, with localized xenograft vascular injury progressing to a form of CC (21,37). The immunosuppressive therapy and conditioning regimen used in the present studies do not induce coagulation disturbances in the absence of a xenograft (38).
Our data suggest that isolated kidney xenografts, when compared to hearts, are associated with the more frequent development of CC, noted previously during AHXR of renal xenografts (39,40), as also seen in humoral rejection of human allografts (41). In addition, ex vivo hemoperfusion of porcine kidneys by human volunteers has also been shown to result in substantive levels of thrombocytopenia with rapid onset of vascular injury (42,43); similar events have also been described with ex vivo porcine liver hemoperfusion (44). Other studies in nonhuman primates have described the development of vascular thrombosis leading to xenograft nonfunction (45,46). Coagulation disturbances associated with pulmonary xenograft dysfunction develop within hours of graft reperfusion (47). CC in pulmonary xenotransplantation may represent a unique and/or accelerated version of this process (48). Additionally, systemic hemorrhage is a complication of liver xenotransplantation, and occurs because of a decrease in the number and function of circulating platelets in the recipient (49). These and other data reinforce the hypothesis that xenograft vascular injury, in particular to kidneys and lungs, is associated with substantive thrombotic abnormalities and CC (50).
In kidney xenografts, the genes that were upregulated following Tx include: vWf, P-selectin, PECAM-1/CD31, superoxide dismutases, CD39, heme oxygenase-I, heat shock protein-72 and nitric oxide synthases. It is possible that heightened vWf and P-selectin expression in the setting of inflammation may have pathological significance for heightened platelet deposition within the injured renal xenograft. How PECAM-1 is upregulated in this setting will be the focus of future work.
In contrast, cardiac xenografts appear less likely to initiate systemic coagulation problems. In these grafts, we observed comparable types of alterations in CD39 and vWf expression in addition changes in PAI-1 expression. PAI-1 does seem to be initially down regulated with grafting and is reconstituted at both mRNA level (by gene chip and northern analysis in a far more marked extent in hearts relative to kidneys). The heightened overall expression of PAI-1, an inhibitor of plasminogen activators, would promote preferential fibrin deposition within the vasculature and possibly contribute to TA in the cardiac xenografts. In this context, higher levels of fibrin and yet markedly less platelet deposition were noted in the cardiac grafts when contrasted to the renal grafts.
We suggest further that the pig xenograft vasculature appears to be thrombophilic in baboons. Indeed, CC does not appear to develop to the same extent in comparable experiments in treated primates exposed to allografts (51) nor to thymokidneys from GalT-KO donors, in which the regimen directed toward tolerance induction appears to reduce the prevalence of CC in recipients. The presence and nature of a vascularized xenograft, immunoglobulin deposition and putative molecular barriers or incompatibilities between pig and primate, individually or together, are linked to development of CC (4,5,19). However, microarray studies on rejecting GalT-KO porcine kidneys parallel those of genetically unmodified or transgenic hDAF pigs. This supports the contention that coagulation changes may develop independently of the presence of anti-Gal Abs and indicates that comparable vascular transcriptional changes occur within Gal expressing and GalT-KO xenografts.
Although vascular endothelial cells share common features and functions in kidneys and hearts, it is clear that there is significant functional, structural and anatomic heterogeneity of these cells. The possibility we suggest above, that normally the coagulation system may be regulated in a tissue-specific manner, has been previously proposed following analysis of gene-targeting studies in animals (51). Mice have been bred with a thrombomodulin gene containing a point mutation that specifically deletes the anticoagulant activity of the protein (52). These mice exhibit 10 to 30 times as much fibrin in heart, lungs and kidneys when compared to naïve wild-type mice. In contrast, fibrin deposition is preferentially increased only in the heart and lungs of mice that lack tissue-type plasminogen activator and urokinase-type plasminogen activator (52).
In organs such as the heart and lungs, thrombomodulin and plasminogen activators play a synergistic role in tempering the procoagulant and antifibrinolytic forces. In other organs, such as the kidney, mainly anticoagulant mechanisms appear to counterbalance any thrombotic tendencies without the need for fibrinolytic factors (53). In addition to these natural anticoagulants, there are described organ-specific and species-specific endothelial cell antigens that could be targets of xenoreactive antibodies and also impact upon organ graft outcome (54,55).
Our findings could also be impacted by the critical interplay of genetic and environmental factors, for example, viral infections. Studies at our center using hearts from cytomegalovirus-negative pigs transplanted into baboons that received a CD40-CD154 costimulatory blockade regimen after depletion of baboon anti-Gal Abs, resulted in prolonged xenograft survival of up to 139 days (56). However, these grafts still exhibited platelet-rich fibrin thrombi with microvascular occlusion. Studies in our laboratory on grafts from GalT-KO, cytomegalovirus-negative pigs that did not develop hyperacute rejection or AHXR still showed features of ischemic microvascular occlusion (57).
In conclusion, pathways of vascular injury and thrombotic/fibrinolytic disturbances in xenotransplantation may be viewed as a consequence of rejection responses, as modified by organ-specific vascular endothelial characteristics. The proposal that disordered thromboregulation and abnormal fibrinolysis might occur in an organ-specific manner may be important for the selective targeting of nonobvious genes in the future generation of transgenic pigs in xenotransplantation research.
Abbreviations
- Ab
antibody
- AHXR
acute humoral xenograft rejection
- CC
consumptive coagulopathy
- Gal
Galα 1–3Gal
- GalT-KO
α 1,3-galactosyltransferase gene-knockout
- hDAF
human decay-accelerating factor
- PAI-1
plasminogen activator inhibitor-1
- TM
thrombotic microangiopathy
- Tx
transplantation
- vWF
von Willebrand factor
References
- 1.Cotran RS, Pober JS. Endothelial activation and inflammation. Prog Immunol. 1989;8:747. [Google Scholar]
- 2.Pober JS, Cotran RS. The role of endothelial cells in inflammation. Transplantation. 1990;50:537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Bevalicqua MPGM. Modulation of endothelial cell procoagulant and fibrinoltic activities by inflammatory mediators. In: US R, editor. Endothelial Cells. Boca Raton: CRC Press; 1988. pp. 107–118. [Google Scholar]
- 4.Bach FH, Robson SC, Ferran C, et al. Endothelial cell activation and thromboregulation during xenograft rejection. Immunol Rev. 1994;141:5–30. doi: 10.1111/j.1600-065x.1994.tb00870.x. [DOI] [PubMed] [Google Scholar]
- 5.Sachs DH, Sykes M, Robson SC, Cooper DK. Xenotransplantation. Adv Immunol. 2001;79:129–223. doi: 10.1016/s0065-2776(01)79004-9. [DOI] [PubMed] [Google Scholar]
- 6.Stevens T, Rosenberg R, Aird W, et al. NHLBI workshop report: Endothelial cell phenotypes in heart, lung, and blood diseases. Am J Physiol Cell Physiol. 2001;281:C1422–C1433. doi: 10.1152/ajpcell.2001.281.5.C1422. [DOI] [PubMed] [Google Scholar]
- 7.Robson SC, Candinas D, Hancock WW, Wrighton C, Winkler H, Bach FH. Role of endothelial cells in transplantation. Int Arch Allergy Immunol. 1995;106:305–322. doi: 10.1159/000236861. [DOI] [PubMed] [Google Scholar]
- 8.Platt JL, Lin SS, McGregor CGA. Acute vascular rejection. Xenotransplantation. 1998;5:169–175. doi: 10.1111/j.1399-3089.1998.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 9.Platt JL. New directions for organ transplantation. Nature. 1998;392(6679 Suppl):11–17. doi: 10.1038/32023. [DOI] [PubMed] [Google Scholar]
- 10.Bilzer M, Gerbes AL. Preservation injury of the liver: Mechanisms and novel therapeutic strategies. J Hepatol. 2000;32:508–515. doi: 10.1016/s0168-8278(00)80404-3. [DOI] [PubMed] [Google Scholar]
- 11.Game DS, W AN, Lechler RI. Rejection mechanisms in transplantation. Wiener Klinische Wochenschrift. 2001;113:832–838. [PubMed] [Google Scholar]
- 12.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160–166. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 13.Lu CY, Penfield JG, Kielar ML, Vazquez MA, Jeyarajah DR. Hypothesis: Is renal allograft rejection initiated by the response to injury sustained during the transplant process? Kidney Int. 1999;55:2157–2168. doi: 10.1046/j.1523-1755.1999.00491.x. [DOI] [PubMed] [Google Scholar]
- 14.Platt JL, Lin SS, McGregor CG. Acute vascular rejection. Xenotransplantation. 1998;5:169–175. doi: 10.1111/j.1399-3089.1998.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 15.Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169–173. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 16.Calne RY. Organ transplantation between widely disparate species. Transplant Proc. 1970;2:550–556. [PubMed] [Google Scholar]
- 17.Bach FH, Winkler H, Ferran C, Hancock WW, Robson SC. Delayed xenograft rejection. Immunol Today. 1996;17:379–384. doi: 10.1016/0167-5699(96)10024-4. [DOI] [PubMed] [Google Scholar]
- 18.Robson SC, Cooper DK, d'Apice AJ. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation. 2000;7:166–176. doi: 10.1034/j.1399-3089.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 19.Robson SC. Acute vascular rejection/delayed xenograft rejection and consumptive coagulopathy in xenotransplantation. Curr Opin Organ Transplant. 2003;8:76–82. [Google Scholar]
- 20.Platt JL, Lin SS. The future promises of xenotransplantation. Ann N Y Acad Sci. 1998;862:5–18. doi: 10.1111/j.1749-6632.1998.tb09112.x. [DOI] [PubMed] [Google Scholar]
- 21.Ierino FL, Kozlowski T, Siegel JB, et al. Disseminated intravascular coagulation in association with the delayed rejection of pig-to-baboon renal xenografts. Transplantation. 1998;66:1439–1450. doi: 10.1097/00007890-199812150-00006. [DOI] [PubMed] [Google Scholar]
- 22.Kolber-Simonds D, Lai L, Watt SR, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 24.Kozlowski T, Shimizu A, Lambrigts D, et al. Porcine kidney and heart transplantation in baboons undergoing a tolerance induction regimen and antibody adsorption. Transplantation. 1999;67:18–30. doi: 10.1097/00007890-199901150-00004. [DOI] [PubMed] [Google Scholar]
- 25.Cooper DKC, Ye Y, Niekrasz M. Heart transplantation in primates. In: Cramer DV, Podesta LG, Makowska L, editors. Handbook of Animal Models in Transplantation Research. Boca Raton: CRC Press; 1994. pp. 173–200. [Google Scholar]
- 26.Kozlowski T, Ierino FL, Lambrigts D, et al. Depletion of anti-Gal(alpha)1–3Gal antibody in baboons by specific alpha-Gal immunoaffinity columns. Xenotransplantation. 1998;5:122–131. doi: 10.1111/j.1399-3089.1998.tb00018.x. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Lorf T, Sablinski T, et al. Removal of anti-porcine natural antibodies from human and nonhuman primate plasma in vitro and in vivo by a Galalpha1–3Galbeta1–4betaGlc-X immunoaffinity column. Transplantation. 1998;65:172–179. doi: 10.1097/00007890-199801270-00005. [DOI] [PubMed] [Google Scholar]
- 28.Kozlowski T, Monroy R, Xu Y, et al. Anti-Gal(alpha)1–3Gal antibody response to porcine bone marrow in unmodified baboons and baboons conditioned for tolerance induction. Transplantation. 1998;66:176–182. doi: 10.1097/00007890-199807270-00006. [DOI] [PubMed] [Google Scholar]
- 29.Buhler L, Yamada K, Kitamura H, et al. Pig kidney transplantation in baboons: Anti-Gal(alpha)1–3Gal IgM alone is associated with acute humoral xenograft rejection and disseminated intravascular coagulation. Transplantation. 2001;72:1743–1752. doi: 10.1097/00007890-200112150-00007. [DOI] [PubMed] [Google Scholar]
- 30.Knosalla C, Gollackner B, Buhler L, et al. Correlation of biochemical and hematological changes with graft failure following pig heart and kidney transplantation in baboons. Am J Transplant. 2003;3:1510–1519. doi: 10.1046/j.1600-6135.2003.00258.x. [DOI] [PubMed] [Google Scholar]
- 31.Platt JL. Xenotransplanting hepatocytes: The triumph of a cup half full. Nat Med. 1997;3:26–27. doi: 10.1038/nm0197-26. [DOI] [PubMed] [Google Scholar]
- 32.Platt JL. Genetic engineering: The triumph or the bane of xenotransplantation? Transplantation. 1998;66:939–940. doi: 10.1097/00007890-199810150-00026. [DOI] [PubMed] [Google Scholar]
- 33.Platt JL. Xenotransplantation. New risks, new gains. Nature. 2000;407:27–30. doi: 10.1038/35024181. [DOI] [PubMed] [Google Scholar]
- 34.Platt JL. Knocking out xenograft rejection. Nat Biotechnol. 2002;20:231–232. doi: 10.1038/nbt0302-231. [DOI] [PubMed] [Google Scholar]
- 35.Bach FH, Robson SC, Winkler H, et al. Barriers to xenotransplantation. Nat Med. 1995;1:869–873. doi: 10.1038/nm0995-869. [DOI] [PubMed] [Google Scholar]
- 36.Bach FH, Fishman JA, Daniels N, et al. Uncertainty in xenotransplantation: Individual benefit versus collective risk. Nat Med. 1998;4:141–144. doi: 10.1038/nm0298-141. [DOI] [PubMed] [Google Scholar]
- 37.Kozlowski T, Fuchimoto Y, Monroy R, et al. Apheresis and column absorption for specific removal of Gal-alpha-1,3 Gal natural antibodies in a pig-to-baboon model. Transplant Proc. 1997;29:961. doi: 10.1016/s0041-1345(96)00299-0. [DOI] [PubMed] [Google Scholar]
- 38.Buhler L, Basker M, Alwayn IP, et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 2000;70:1323–1331. doi: 10.1097/00007890-200011150-00010. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg JC, Broersma RJ, Bullemer G, Mammen EF, Lenaghan R, Rosenberg BF. Relationship of platelets, blood coagulation, and fibrinolysis to hyperacute rejection of renal xenografts. Transplantation. 1969;8:152–161. doi: 10.1097/00007890-196908000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Broersma RJ, Bullemer GD, Rosenberg JC, Lenaghan R, Rosenberg BF, Mammen EF. Coagulation changes in hyperacute rejection of renal xenografts. Thromb Diath Haemorrh Suppl. 1969;36:333–340. [PubMed] [Google Scholar]
- 41.Dragun D, Muller DN, Brasen JH, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 42.Breimer ME, Bjorck S, Svalander CT, Bengtsson A, Rydberg L, Lienkarlsen K. Extracorporeal (ex vivo) connection of pig kideys to humans 1. Clinical data and studies of platelet destruction. Xenotransplanation. 1996;3:328–339. doi: 10.1111/j.1399-3089.1996.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 43.Bustos M, Saadi S, Platt JL. Platelet-mediated activation of endothelial cells: Implications for the pathogenesis of transplant rejection. Transplantation. 2001;72:509–515. doi: 10.1097/00007890-200108150-00025. [DOI] [PubMed] [Google Scholar]
- 44.Collins BH, Chari RS, Magee JC, et al. Immunopathology of porcine livers perfused with blood of humans with fulminant hepatic failure. Transplant Proc. 1995;27:280–281. [PubMed] [Google Scholar]
- 45.Zaidi A, Schmoeckel M, Bhatti F, et al. Life-supporting pig-to-primate renal xenotransplantation using genetically modified donors. Transplantation. 1998;65:1584–1590. doi: 10.1097/00007890-199806270-00008. [DOI] [PubMed] [Google Scholar]
- 46.Schmoeckel M, Bhatti FN, Zaidi A, et al. Orthotopic heart transplantation in a transgenic pig-to-primate model. Transplantation. 1998;65:1570–1577. doi: 10.1097/00007890-199806270-00006. [DOI] [PubMed] [Google Scholar]
- 47.Pfeiffer S, Zorn GL, 3rd, Blair KS, et al. Hyperacute lung rejection in the pig-to-human model 4: Evidence for complement and antibody independent mechanisms. Transplantation. 2005;79:662–671. doi: 10.1097/01.tp.0000148922.32358.bf. [DOI] [PubMed] [Google Scholar]
- 48.Pfeiffer S, Zorn GL, 3rd, Zhang JP, et al. Hyperacute lung rejection in the pig-to-human model. III. Platelet receptor inhibitors synergistically modulate complement activation and lung injury. Transplantation. 2003;75:953–959. doi: 10.1097/01.TP.0000058517.07194.90. [DOI] [PubMed] [Google Scholar]
- 49.Ramirez P, Chavez R, Majado M, et al. Transgenic pig-to-baboon liver xenotransplantation: Clinical, biochemical, and immunologic pattern of delayed acute vascular rejection. Transplant Proc. 2002;34:319–320. doi: 10.1016/s0041-1345(01)02834-2. [DOI] [PubMed] [Google Scholar]
- 50.Cowan PJ, Aminian A, Barlow H, et al. Renal xenografts from triple-transgenic pigs are not hyperacutely rejected but cause coagulopathy in non-immunosuppressed baboons. Transplantation. 2000;69:2504–2515. doi: 10.1097/00007890-200006270-00008. [DOI] [PubMed] [Google Scholar]
- 51.Kawai T, Sogawa H, Boskovic S, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4:1391–1398. doi: 10.1111/j.1600-6143.2004.00523.x. [DOI] [PubMed] [Google Scholar]
- 52.Weiler-Guettler H, Christie PD, Beeler DL, et al. A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J Clin Invest. 1998;101:1983–1991. doi: 10.1172/JCI2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenberg RD, Aird WC. Vascular-bed–specific hemostasis and hypercoagulable states. N Engl J Med. 1999;340:1555–1564. doi: 10.1056/NEJM199905203402007. [DOI] [PubMed] [Google Scholar]
- 54.Murray AG, Khodadoust MM, Pober JS, Bothwell AL. Porcine aortic endothelial cells activate human T cells: Direct presentation of MHC antigens and costimulation by ligands for human CD2 and CD28. Immunity. 1994;1:57–63. doi: 10.1016/1074-7613(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 55.Savage CO, Hughes CC, McIntyre BW, Picard JK, Pober JS. Human CD4+ T cells proliferate to HLA-DR +allogeneic vascular endothelium. Identification of accessory interactions. Transplantation. 1993;56:128–134. doi: 10.1097/00007890-199307000-00024. [DOI] [PubMed] [Google Scholar]
- 56.Kuwaki K, Knosalla C, Dor FJ, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-human CD154 mAb-based regimen. Am J Transplant. 2004;4:363–372. doi: 10.1111/j.1600-6143.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 57.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: Initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]


