Abstract
Prostate gland development is a complex process that involves coordination of multiple signaling pathways including endocrine, paracrine, autocrine, juxtacrine and transcription factors. To put this into proper context, the present manuscript will begin with a brief overview of the stages of prostate development and a summary of androgenic signaling in the developing prostate, which is essential for prostate formation. This will be followed by a detailed description of other transcription factors and secreted morphogens directly involved in prostate formation and branching morphogenesis. Except where otherwise indicated, results from rodent models will be presented since studies that examine molecular signaling in the developing human prostate gland are sparse at the present time.
Keywords: prostate development, androgen receptor, Hox genes, Nkx3.1, FoxA1, Notch, Fgf10, Shh, Bmp4, Bmp7, TgfB1, Wnt genes
Stages of prostatic development
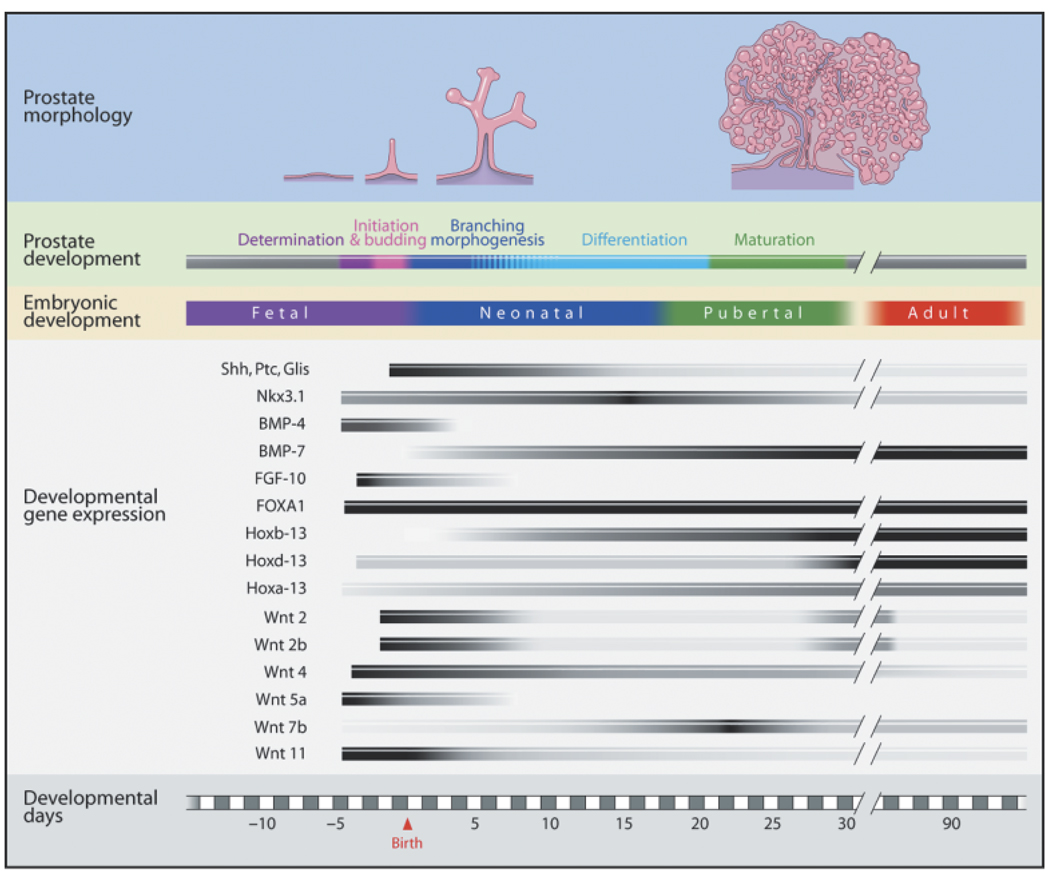
In contrast to most male accessory sex glands, which develop embryologically from the Wolffian ducts (mesodermal), the prostate gland originates from the urogenital sinus (UGS) and is an endodermal structure. Although the developmental process is continuous, it can be categorized in five distinct stages involving determination, initiation or budding, branching morphogenesis, differentiation, and pubertal maturation (Fig. 1). Determination of the prostate occurs before clear morphological evidence of a developing structure and involves expression of molecular signals that commit a specific field of UGS cells to a prostatic cell fate. Phenotypic prostate development commences as UGS epithelial cells form outgrowths or buds that penetrate into the surrounding UGS mesenchyme in the ventral, dorsal, and lateral directions caudal to the bladder. In humans, prostate development occurs during the second and third trimester and is complete at the time of birth (Lowsley, 1912; Prins, 1993). This contrasts with the rodent prostate gland, which is rudimentary at birth and undergoes the majority of its development during the first 15 days of life. In the mouse, the initial outgrowth of epithelial buds occurs between fetal days 16.5–17.5 (f16.5–17.5) in a 19 day gestation strain (Sugimura et al., 1986), while in the rat it occurs at f18.5 in a 21 day gestation strain (Hayashi et al., 1991). At birth, the ventral, dorsal, and lateral rodent prostate lobes primarily consist of unbranched, solid elongating buds or ducts and subsequent outgrowth and patterning occur postnatally. During this time, proliferation of epithelial cells occurs primarily at the leading edge of the ducts (i.e. distal tips) (Prins et al., 1992). Branching morphogenesis begins when the elongating UGS epithelial buds contact the prostate mesenchymal pads that are peripheral to the periurethral smooth muscle. At that point, secondary, tertiary, and further branch points are established with continued proximal-to-distal outgrowth and complexity (Timms et al., 1994). Branching patterns are lobe-specific with ventral branching preceding that in the dorsolateral lobes by 3–4 days (Hayashi et al., 1991). Morphogenesis of the entire complex is completed between postnatal days 15 and 30. Final growth and maturation occur at puberty (days 25–40) when circulating androgens levels rise sharply.
Fig. 1.
Rat prostate developmental stages, timeline and morphoregulatory gene expression. The days of fetal and postnatal life are shown at the bottom. Ventral prostate morphology and developmental stages (top) are sequentially aligned to the corresponding days that they appear. Note that cellular differentiation occurs during the later days of branching morphogenesis as indicated by blue-striped lines. Temporal patterns of morphoregulatory genes expression are shown in black-gray-white bars representing relative levels of gene expression as determined by quantitative real-time reverse-transcriptase polymerase chain reaction.
Epithelial and mesenchymal cell differentiation is co-ordinated with branching morphogenesis and occurs in the proximal-to-distal direction (Prins and Birch, 1995; Hayward et al., 1996a, 1996b). Epithelial differentiation from progenitor cells into differentiated basal and luminal cells has been documented in the rat prostate with changing patterns of cytokeratins as well as alterations in androgen receptor (AR) expression, an early marker of epithelial cell differentiation (Prins and Birch, 1995; Hayward et al., 1996b). The process initiates between days 3 and 5 in the rat ventral prostate and ~ 2 days later in the dorsal and lateral lobes. Lumenization of the solid epithelial cords is concomitant with differentiation into basal and luminal cell layers and initiates in the proximal ducts around postnatal day 5, extending to the distal tips by ~ day 12. Between days 10 and 15, functional differentiation commences, as defined by the synthesis of secretory products by differentiated luminal epithelial cells (Prins and Birch, 1995). Concomitant with epithelial differentiation, the prostatic mesenchyme undergoes differentiation postnatally. As UGS epithelial ducts penetrate into the prostate mesenchymal pads, mesenchymal cells condensate around the tip and form a distinctive pattern along the length of the basement membrane. Between days 3 and 5, cells adjacent to the ducts form a periductal layer of smooth muscle cells while interductal cells differentiate into mature fibroblasts (Prins and Birch, 1995; Hayward et al., 1996a). Lying between the basement membrane and the periductal smooth muscle is an extremely thin single cell layer of differentiated fibroblasts. As the proximal ducts branch and grow, the periductal cell layer tapers in the distal direction forming a single layer of smooth muscle cells at the distal tips of the mature prostate (Chang et al., 1999b). As the prostate undergoes branching morphogenesis, a branched vascular bed forms in parallel with neovascularization forming within the prostatic stromal elements and capillary beds extending to the ductal basement membrane (Shabsigh et al., 1999).
Hormonal regulation of prostate development
The determination and initiation of prostatic development in the human and rodent fetus is entirely dependent upon androgens produced by the fetal testes. Surgical or chemical castration (i.e. anti-androgen administration) of rodents during critical periods of fetal life results in inhibition of prostate development (Price, 1936; Jost, 1953; Price and Williams-Ashman, 1961; Cunha, 1973; Lasnitzki and Mizuno, 1977). However, the extent of inhibition depends on the timing of androgen ablation relative to bud initiation. To study this in detail, Cunha employed organ culture of murine UGS explants from male mice which typically initiate prostate budding at f17 in vivo (Cunha, 1973). UGS retrieved from f12–13 mice, before fetal testes production of testosterone at f14, did not produce prostatic buds when cultured for 6–8 days in the absence of androgens. However, UGS explants removed on f14 or f15, i.e. after testosterone production began in vivo, and cultured for 6 days without androgens produced buds in 15% and 53% of the tissues, respectively. By f16, 100% of male UGS explants grew prostatic buds when cultured further in the absence of androgens. Using a similar study design with male rats that normally initiate prostate budding at f18.5, Lasnitzki and Mizuno, (1977) observed comparable results. Explants of male rat UGS removed between f14.5 and f16.5 failed to bud when cultured in the absence of androgen, while UGS removed on f17.5–18.5, that were exposed in vivo to endogenous testosterone, developed prostate buds when cultured without androgens, albeit at half the normal number. The rat explant budding response to androgens in vitro was dose-dependent, and dihydrotestosterone (DHT) showed greater inductive capacity than testosterone. Furthermore, a single day exposure of the f16.5 rat UGS explant to testosterone followed by culture in the absence of androgens was sufficient to drive bud formation, albeit it at a lower number, and continued exposure to testosterone was required to achieve maximal bud number (Lasnitzki and Mizuno, 1977; Takeda et al., 1986). Together these findings indicate that while androgens are essential for prostate determination and for maximal bud number and length during initiation, budding can continue to a large degree in the absence of testosterone due to irreversible commitment of the tissue. Rodent neonatal prostate explant cultures have further shown that while branching morphogenesis can occur in the absence of exogenous androgens, maximal organ growth with full branching as well as complete cellular cytodifferentiation are only realized with the addition of exogenous testosterone (Lipschutz et al., 1997).
In the 1970s, it was determined that the primary androgen responsible for prostatic development is DHT, the reduced metabolite of testosterone (Wilson and Gloyna, 1970). DHT is formed intracellularly in the prostate epithelium by 5α-reductase and has been shown to have higher affinity for the AR as compared with the parent compound, testosterone (Fang et al., 1969). Human males with 5α-reductase deficiency syndrome have complete absence of prostate morphogenesis with normal development of the seminal vesicles and vas deferens which empty into a blind vagina (Sitteri and Wilson, 1974). Similarly, treatment of pregnant female rats with a 5α-reductase inhibitor from f14–22 obliterated the formation of prostatic buds, an effect that could be reversed with concomitant administration of DHT (Wilson and Lasnitzki, 1971). However, more recent studies challenge the absolute requirement of DHT for bud induction after the determination stage because testosterone and non-reducible synthetic androgens were capable of inducing equivalent bud numbers as DHT (Foster and Cunha, 1999).
Androgen action is mediated through interaction with nuclear AR which are members of a superfamily of transcription factors (Liao and Fang, 1969; Committee, 1999). Evidence for the absolute necessity of AR for prostate development comes from the observation of prostatic absence in mice or humans with complete dysfunctional AR (Bardin et al., 1973; Brown, 1995). As shown in Fig. 2, AR are highly expressed in the UGS mesenchyme before and during prostate morphogenesis whereas epithelial AR expression is induced after budding and branching morphogenesis has begun (Shannon and Cunha, 1983; Takeda et al., 1985; Husmann et al., 1991; Prins and Birch, 1995). Classical tissue recombinant studies by Cunha demonstrated that AR in the mesenchyme, and not epithelial AR, are responsible for prostatic morphogenesis (Cunha and Chung, 1981; Cunha et al., 1987). When wild-type murine UGS mesenchyme was recombined with AR-deficient murine UGS epithelium and grafted under the renal capsule, the AR-deficient epithelium underwent androgen-dependent ductal morphogenesis, epithelial proliferation, and columnar cytodifferentiation forming glandular epithelium that resembled normal prostate. On the contrary, when AR-deficient UGS mesenchyme was recombined with wild-type UGS epithelium, vaginal-like differentiation occurred. Although further analysis revealed that epithelial AR are required for expression of secretory proteins in mouse (Donjacour and Cunha, 1993) and rat prostates (Prins and Birch, 1995), epithelial proliferation and cytodifferentiation appear to be largely driven by paracrine factors under mesenchymal AR control. Mesenchymal cell differentiation into periductal smooth muscle in the prostate also requires a signal from the epithelium (Cunha et al., 1992). Because we have shown that AR induction in prostate epithelium begins as early as postnatal days 1–2 (before cytodifferentiation of the epithelium and mesenchyme) (Prins and Birch, 1995), it is possible that androgen-driven epithelial signals contribute to morphogenesis of the prostate by affecting the differentiation of adjacent mesenchymal cells. Recent evidence with AR inactivation restricted to murine prostate epithelial cells confirms the above model and also provides evidence that epithelial AR regulates basal cell proliferation (Simanainen et al., 2007). It is noteworthy that AR expression does not vary along the proximal–distal axis of the developing and adult prostate (Prins et al., 1992; Prins and Birch, 1995), thus differential gene expression along this axis is likely driven by factors other than androgens.
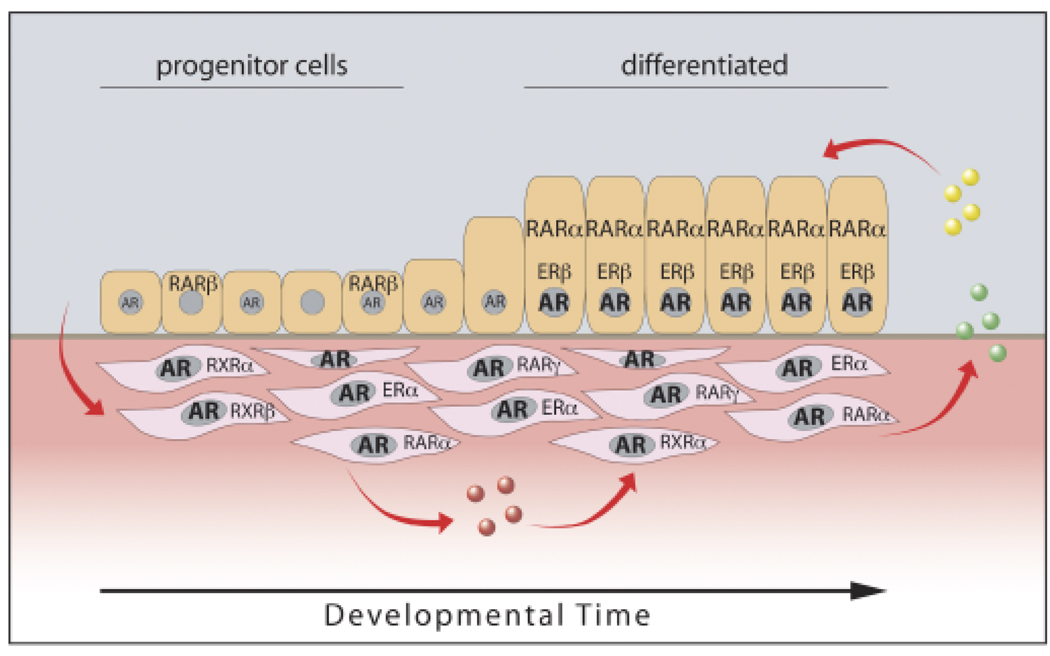
Fig. 2.
Steroid receptor localization and expression over time during rat prostate development. During early perinatal stages, androgen receptor (AR) is highly expressed in mesenchymal cells while epithelial progenitor cells have no or limited AR. In response to circulating androgens (converted to dihydrotestosterone intracellularly), mesenchymal cells produce autocrine (pink circles) and paracrine (green and yellow circles) growth and differentiation factors, which drive epithelial cell proliferation and differentiation. As epithelial cells differentiate, AR is induced to high expression levels. Androgen driven secreted factors from the epithelium are proposed to provide reciprocal signals to the mesenchyme to promote differentiation to smooth muscle cells. Other steroid receptors expressed in a temporal and cell-specific manner include mesenchymal estrogen receptor α (ERα, during early stages), epithelial ERβ (upon differentiation), basal cell retinoic acid receptor β (RARβ), RARα (upon functional differentiation), and periductal mesenchymal RARα, RARγ, RXRα and RXRβ.
There is also clear evidence for a role of other steroids including estrogens and retinoids during prostate development and the readers are referred to recent reviews (Prins et al., 2001; Huang et al., 2004; Prins and Korach, 2008). Studies from our laboratory have identified specific receptors for these steroids during rat prostate morphogenesis, which vary in a time and cell-specific manner (Prins and Birch, 1997; Prins et al., 1998, 2002; Pu et al., 2003). While these transcription factors are not essential for prostate development, we propose that they modulate expression of specific genes that are involved in differentiated function and homeostasis. A schematic summarizing steroid action through specific receptors during prostatic development is shown in Fig. 2.
Developmental genes
Appendicular patterning (proximal-to-distal outgrowth), as seen during continuous branching morphogenesis of glandular structures, is dictated by time-specific and region/cell-specific expression of master regulatory genes that are evolutionarily conserved throughout the animal kingdom. So common are signal pathways across species and between organs of a single species that it is envisioned there is a conserved “morphogenetic code” or common set of rules that is used repeatedly in different combinations to effect formation of separate organs (Hogan, 1999). Although common morphoregulatory genes are expressed by all branched structures, the critical difference is that spatial and temporal combinations of these as well as organ-specific genes give rise to unique structures. Precise coordination of these events implies tight feedback interactions and, for the prostate, androgenic regulation at some level. In this review, we will consider two major categories of morphoregulatory genes involved in prostate development: (1) Nuclear transcription factors that include common and organ-specific homeobox genes and (2) secreted signaling ligands encoded by a small number of conserved multigene families including Hedgehogs, Wnts, Fgfs, and Bmps/Tgfβ/activin (Hogan, 1999). These latter positive and negative regulatory molecules communicate paracrine and autocrine signals between epithelial and mesenchymal cells via their cognate receptors. Importantly, while specific genes may drive cell determination, proliferation, differentiation, or spatial patterning, the interpretation of new signals will always be determined by a cell’s history. In recent years, a marked number of studies in rodent models has permitted formation of a “prostatic morphogenetic code” (Fig. 3). Based on work from our laboratory, we have schematized the temporal expression pattern of several of these key genes over the different stages of rat prostate development (Fig. 1) and these results as well as studies from multiple laboratories will be highlighted below.
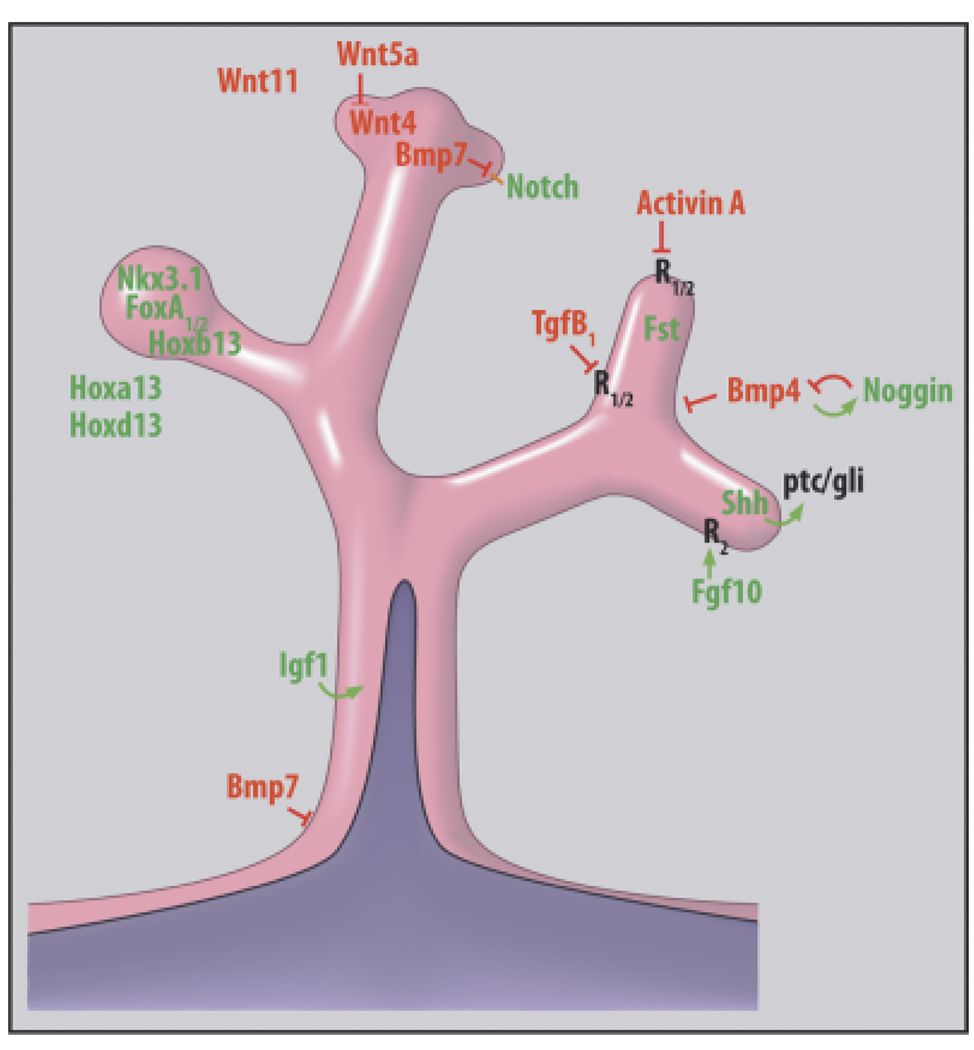
Fig. 3.
A schematized model of an elongating and branching prostate duct showing the localization of multiple transcription factors, secreted morphogens and their respective receptors to form the prostatic morphogenetic code. Factors in green denote stimulatory molecules while factors in red represent inhibitory molecules. Arrows show paracrine stimulatory pathways emanating from the epithelium or mesenchyme in the direction of the secreted morphogen. T-bars denote inhibition as a function of the secreted morphogen. The schematic represents work from the author’s laboratory as well as multiple investigations cited throughout the manuscript.
There are several critical points that must be borne in mind regarding prostate development. The adult prostate gland is a heterogeneous ductal structure with defined proximal, central, and distal regions (Lee et al., 1990). Similarly, during morphogenesis of the prostate, expression of developmental genes, and secretion of paracrine factors is heterogeneous across regions and cell types along the proximal–distal axis. This positional specification must be incorporated into models that describe molecular regulation of prostate development. Frequently, regional expression is most complex at the distal tip and sites of branchpoints where differential and reciprocal signaling is essential for morphogenetic changes. We have recently termed this region the “distal signaling center” similar in nature to distal regions in the limb and lungs (Pu et al., 2003). Regional expression of developmental genes by a subpopulation of cells also results in gradients of secreted morphogens. Complexity is added to this model when interpretation of the morphogen by receiving cells is non-linear due to differing sensitivity thresholds (e.g. presence/absence of cognate receptors). Another level of complexity arises when positive and negative morphogenetic signals as well as their secreted inhibitors overlap in specific regions. Finally, while specific studies typically focus on the nature and role of individual morphoregulatory genes, it is important to appreciate the signaling networks that arise due to cross-regulation in gene expression, a topic that will be discussed at the end of this review.
HOMEOBOX GENES AND TRANSCRIPTION FACTORS
Axis positioning and tissue determination involve expression of specific members of the homeobox gene superfamily (Gehring, 1994). These master regulatory genes encode transcription factors that contain a highly conserved ~ 60 amino acid peptide segment, the DNA-binding Ahomeodomain, which recognizes specific regulatory regions of target genes. Specific homeobox genes have been identified within developing prostate tissue and are thought to, in part, account for prostate determination, budding and morphogenesis. These include members of the Hox gene family (Warot et al., 1997) and the NK gene family (Bieberich et al., 1996; Bhatia-Gaur et al., 1999).
Hox genes
The largest and most extensively studied members of the homeobox superfamily are the Hox genes, which determine patterning in body regions from Drosophila to humans. In mammals, gene duplication has led to four Hox clusters (A, B, C, and D) on separate chromosomes encoding a total of 39 Hox genes (Krumlauf, 1994). Similar genes in the separate clusters are considered paralogs and are largely, although not always, redundant. Expression of these genes, from the 3′ to 5′ end of each cluster, follows a strict pattern of spatial and temporal colinearity during embryogenesis. The 3′ genes designate anterior regions while the 5′ genes encode posterior regions. A generalized model for regional tissue specification is that nested, partially overlapping expression domains of several genes in a Hox cluster determine segment identity. As a rule, the most 5′Hox genes expressed in a given tissue have specification dominance over the more anterior Hox genes that are co-expressed in that tissue.
As the prostate is one of the most posterior organs in the male, the most posterior genes of the Hox clusters are involved in prostate gland identity. Hoxa13 and Hoxd13 have similar expression profiles and patterns in the developing rodent prostates and are believed to have functional redundancy (Podlasek et al., 1997, 1999b). Studies with null mutant mice have shown essential roles for Hoxa13 in prostate growth and Hoxd13 in prostate growth and branching with compound mutants exhibiting severely hypoplastic prostate rudiments despite normal testis (Podlasek et al., 1997, 1999b; Warot et al., 1997). Bushman and colleagues characterized the expression of Hoxa-13 and Hoxd-13 in the mouse prostate and observed that levels are highest during fetal life and decline postnatally (Oefelein et al., 1996; Podlasek et al., 1999b). While expression is strongest in the mesenchyme, epithelial expression is also found at lower levels during fetal and neonatal life. Expression patterns for Hoxa13 and Hoxd13 differ in the rat prostate where levels are lower during the budding and morphogenesis stages and rise to higher expression levels in the adult prostate (Huang et al., 2007). The significance of these differences is unclear.
In contrast to the A and D paralogs, Hoxb13 localizes exclusively to epithelial cells in the murine and rat prostate (Sreenath et al., 1999; Economides and Capecchi, 2003; Huang et al., 2007). Furthermore, levels rapidly rise during the postnatal period as the epithelium differentiates with expression localized to central duct and distal tip epithelial cells (Huang et al., 2007). A clear increasing anterior-to-posterior expression gradient is observed with highest levels in the rat ventral lobe, declining expression in the lateral and dorsal lobes, and minimal detection in the anterior prostate (coagulating gland). It is notable that while Hoxa13 and Hoxd13 are expressed in the seminal vesicles, Hoxb13 is restricted to UGS-derived reproductive tract structures, which suggests that Hoxb13 is important in prostate identity (Huang et al., 2007). Studies with Hoxb13 null mutant mice revealed an essential role in epithelial cell differentiation, because loss of secretory gene production and cell polarity were observed in the ventral lobe (Economides and Capecchi, 2003). This was supported by recent studies from our laboratory in which lentiviral vectors expressing Hoxb13 in undifferentiated rat prostate cells were capable of driving differentiation to a luminal cell phenotype (Huang et al., 2007). Of particular interest, HOXB13 is expressed in normal adult human prostates and in all specimens of prostate cancer where levels are frequently elevated. Based on this ubiquitous HOXB13 expression, it has been suggested that the rodent ventral lobe, typically regarded as having no human homolog, is in fact most representative of the human prostate with regards to Hox gene expression (Edwards et al., 2005).
When examining posterior Hox gene expression in the male accessory glands including the separate rat prostate lobes, we observed that each structure has a unique Hox gene profile, which we propose contributes to the separate lobe branching patterns and functional identity (Huang et al., 2007). This includes the more anterior Hoxa 9, Hoxa 10, and Hoxa 11 genes which are also expressed in the developing rat prostate, although at levels 10-fold lower than the Hox13 genes. [Note: To date, Hoxc13 has not been found in the prostate gland (Takahashi et al., 2004).] Organ culture studies using newborn rat prostate lobes revealed that the posterior Hox genes expressed during prostate development, including Hoxa13, Hoxd13, and Hoxb13, are up-regulated by testosterone (Huang et al., 2007). Interestingly, this was specific to the ventral lobe because lateral lobe Hox genes were not affected by androgens. Furthermore, androgens had limited effects on Hox gene expression in the adult prostate where they only up-regulated Hoxb13 levels. Together, these findings suggest that androgenic regulation of Hox genes may contribute to prostate gland morphogenesis and maintenance of epithelial differentiated status.
Studies on human prostate HOX gene expression have been confined to adult tissues and cells which show expression of all HOX13 paralogs as well as several anterior HOX genes (Miller et al., 2003; Jung et al., 2004b; Takahashi et al., 2004). While there are no reports on the expression or roles for HOX genes during human prostate development, studies on human prostate cancer have identified the potential involvement of HOX gene dysregulation in human prostate cancers (Waltregny et al., 2002; Miller et al., 2003; Jung et al., 2004a; Edwards et al., 2005). Based on these reports, it has been suggested that normal HOX expression is necessary for homeostasis of the human gland.
Nkx3.1
A novel member of the NK homeobox gene family, Nkx3.1, the mammalian homolog of Drosophila NK-3 (bagpipe), was identified in 1996, and its expression in the male reproductive tract was restricted to UGS-derived prostate and bulbourethral gland epithelium (Bieberich et al., 1996; Schiavolino et al., 1997). Importantly, this gene is expressed in the fetal mouse UGS epithelium at bud sites before bud formation suggesting a role for Nkx3.1 in prostate determination (Bieberich et al., 1996; Bhatia-Gaur et al., 1999). Expression of Nkx3.1 continues during ductal outgrowth and branching morphogenesis and is highest at the distal regions of the elongating and branching structures. In the rat prostate lobes, we observed a sharp peak in Nkx3.1 expression between days 6 and 15 as the epithelium undergoes cytodifferentiation with a marked decline to relatively lower steady-state levels thereafter (Prins et al., 2006). Epithelial Nkx3.1 expression is maintained throughout life and is believed to be important for epithelial homeostasis. Null mutant Nkx3.1−/− mice exhibit defective branching patterns, perturbed functional differentiation and adult onset of prostatic intraepithelial neoplasia (PIN) indicating roles for Nkx3.1 in prostate branching morphogenesis and differentiation (Bhatia-Gaur et al., 1999; Schneider et al., 2000; Tanaka et al., 2000; Kim et al., 2002). The importance of Nkx3.1 in maintaining epithelial homeostasis is strongly supported by multiple studies in animal models and humans, which show that Nkx3.1 acts as a tumor suppressor and that loss of expression is involved in prostate carcinogenesis and progression (Bowen et al., 2000; Shen and Abate-Shen, 2003; Bethel et al., 2006; Guan et al., 2007).
Nkx3.1 expression in the adult mouse prostate and the human prostate LNCaP cell line is directly up-regulated by androgens at the transcriptional level (Bieberich et al., 1996; Prescott et al., 1998). We recently demonstrated that androgens strongly and rapidly increase Nkx3.1 expression in the developing rat prostate lobes, which provides another pathway whereby androgens influence prostate development (Pu et al., 2007). However, because epithelial AR expression is absent in the fetal UGS epithelial cells when Nkx3.1 is initially expressed, it is unlikely that prostate development is initiated through direct androgen action on Nkx3.1 gene transcription. Importantly, Nkx3.1 expression during prostate formation has been shown to be strictly dependent on epithelial Shh expression (Schneider et al., 2000). Multiple cis-regulatory elements that mediate distinct expression domains of Nkx3.1 have been identified and key elements important for prostatic expression are contained in a distal 5Kb region located > 7Kb downstream from the coding sequence (Chen et al., 2005). Further deletion analysis is required to identify transcription factors that act through this 5Kb region to regulate prostatic expression during early development.
Fox A1 and A2
Forkhead box genes (Fox), formerly known as hepatocyte nuclear factor or HNF genes, encode a superfamily of winged-helix transcription factors from Drosophila to mammals (Kaestner et al., 2000). Multiple Fox genes have been identified that are specific to endodermal-derived structures and several are involved in organ development (Clevidence et al., 1993). Of these, FoxA1 (formerly Hnf3) localizes to the developing prostate epithelium where it plays an important role in ductal morphogenesis and epithelial cell maturation (Kopachik et al., 1998). FoxA1 expression is observed in rat and mouse f18 UGS epithelial buds and levels increase with prostatic development and are maintained throughout adult life (Kopachik et al., 1998; Gao et al., 2005). Sustained expression of FoxA1 in the rodent and human prostate is required for probasin and PSA expression, respectively, through direct interactions with both FoxA cis-regulatory elements and AR on gene promoters (Gao et al., 2005). In contrast, FoxA2 is only expressed in prostate epithelial cells at the mesenchymal interface during the early budding stage and rapidly declines thereafter (Mirosevich et al., 2004). Null mutant FoxA1−/− mice are neonatal lethal, and Matusik and colleagues determined the prostate phenotype using renal capsule organ rescue and tissue recombination (Gao et al., 2005). At birth and postnatal day 1, prostate rudiments at the budding stage were identical to wild-type prostates indicating that FoxA1 loss does not affect prostate bud initiation. After renal grafting, prostate growth was reduced, lumen formation was incomplete, epithelial cells were disorganized, their differentiation was arrested at the intermediate stage, and they failed to express secretory gene products. Although FoxA1 expression is restricted to epithelial cells, periductal smooth muscle layers were expanded in size in FoxA1−/− rescued prostates, perhaps due to persistent expression of paracrine-acting Shh by the developmentally arrested basal-type epithelial cells. It is noteworthy that expression of several mesenchymal expressed paracrine factors including Fgf10, Fgf7, and Bmp4 were markedly increased in FoxA1−/− rescued prostates indicating indirect regulation of these genes by this epithelial cell transcription factor. Importantly, AR expression was not affected by loss of FoxA1. In all, these detailed studies demonstrate an essential role for FoxA1 in prostate epithelial cell differentiation and continued function in both AR-independent and AR-dependent manners.
Notch1/delta/jagged
The Notch signaling pathway is a highly conserved cell-cell signaling system involved in cell fate specification and patterning in developing tissues (Bolos et al., 2007). It consists of a single-pass transmembrane Notch receptor glycoprotein that interacts with Jagged/Delta membrane proteins on adjacent cells to initiate activation. Activation involves proteolytic cleavage of Notch, releasing the intracellular domain, which translocates to the nucleus where it interacts with transcription factors and regulates gene expression including Hes1, a known downstream target. Nuclear localization of the cytoplasmic domain of Notch1 as well as Hes1 expression is observed in mouse UGS epithelium and in early prostatic buds on f18 indicating its activation at that early stage (Grishina et al., 2005). Delta-like ligand1 (Dll1) is expressed in epithelial cell clusters adjacent to mesenchyme where buds emerge while jagged1 expression localizes to UGS epithelium and proximal mesenchyme at that time (Grishina et al., 2005). Furthermore, Maniac Fringe, which glycosolates Notch1 and potentiates its interaction with Dll1, also localizes to the epithelium of the initial prostatic buds. Together, these localization patterns place an active Notch1 signaling pathway at the sight of bud initiation in the developing prostate suggesting a potential role in that process.
Gao and colleagues have characterized the role of Notch signaling in postnatal murine prostate development. Notch1 is highly expressed in the mouse prostate epithelial compartment at the time of birth, remains high throughout morphogenesis and declines to low expression in adults (Shou et al., 2001). While Notch1 is initially expressed by all progenitor cells, upon cytodifferentiation Notch1 localizes to only basal epithelial cells (Wang et al., 2004). Inhibition of Notch cleavage using secretase inhibitors in a neonatal rat ventral prostate organ culture system markedly reduced branching morphogenesis and interfered with epithelial differentiation (Wang et al., 2006b). After six days of organ culture, the majority of epithelial cells co-expressed basal (CK14) and luminal (CK8) cell cytokeratins rather than distinct cell populations as seen in control cultures. Furthermore, these intermediate cells were highly proliferative. Because null mutant Notch1−/− mice are embryonic lethal, mice homologous for loxP-flanked Notch1 and positive for interferon-inducing Mx-Cre transgene in the prostate epithelium were used to examine phenotypes over time (Wang et al., 2006b). After development was completed (d15–25), deletion of Notch1 was induced and three weeks later, ventral prostates exhibited reduced secretions, enhanced epithelial proliferation, increased epithelial infoldings with occasional bridging and clusters of predifferentiation epithelial cells co-expressing CK8 and CK14. Hyperplastic phenotypes were observed as the animals aged. Together, these findings provide strong support that Notch signaling inhibits expansion of prostatic progenitor cells and facilitates epithelial differentiation during development and that continued pathway activation plays a role in maintaining homeostasis.
To determine gene pathways affected by Notch1 signaling, prostate-specific Notch1 was deleted by crossing loxP-Notch1 mice with Nkx3.1+/−Cre mice and prostate gene expression was examined by microarrays (Wang et al., 2006b). Networks containing c-Fos and c-Jun were the most affected pathway supporting a critical role for Notch1 in cell specification and differentiation. Interestingly, despite known interactions in other developing structures, genes involved in Shh and Wntsignaling pathways were not affected by Notch1 deletion in the prostate.
SECRETED SIGNALING MOLECULES
In addition to developmental determination by homeobox genes and other transcription factors, branching morphogenesis is driven by a complex interplay between epithelial and mesenchymal cells through secretion of paracrine and autocrine factors. While many secreted epithelial–mesenchymal signals have been characterized, a small number of highly conserved signaling molecules have been found to be critical during embryogenesis (Hogan, 1999). In particular, combinations of Hedgehogs, Wnts, Fgfs, and Bmps/Tgfβ/Activins to a large extent control soft tissue development. These positive and negative regulatory molecules are spatially and temporally regulated and communicate signals between cells via their cognate receptors. Below we review members of these key families that are known to be involved in prostate gland development.
Sonic hedgehog (Shh)
Sonic hedgehog (Shh) is a member of the conserved Hedgehog family, which also includes Indian hedgehog (Ihh) and Desert hedgehog (Dhh) and is expressed in developing tissues from Drosophila to mammals. Shh is a secreted glycoprotein produced by epithelial cells at the mesenchymal interface in developing structures where it is involved in determination of cell fate, proliferation and embryonic patterning (see review by Ingham and McMahon, 2001). This secreted morphogen binds to membrane-bound patched (ptc) receptors on adjacent mesenchymal cells and establishes epithelial-mesenchymal cell cross-talk. Liganding of ptc by Shh relieves its inhibition on smoothened (smo) resulting in activation of Gli transcription factors, the downstream effectors. In vertebrates, there are 3 known Gli transcripts; gli1, gli2 and gli3, which have both redundant and unique actions. Importantly, GLI1 and gli2 are transcriptional activators, while gli3 is believed to be a transcriptional repressor (Walterhouse et al., 1999; Meyer and Roelink, 2003), which permits tight regulation of Shh actions. Both short-range and long-range actions of Shh have been described which differ as a function of concentration gradients (Gritli-Linde et al., 2001). Shh is considered to be a master regulatory morphogen because it regulates the expression of other secreted morphogens and homeobox genes in several structures including the prostate (Goyette et al., 2000; Schneider et al., 2000; Haraguchi et al., 2001; Perriton et al., 2002; Chuang and McMahon, 2003; Pu et al., 2004). It also induces ptc and gli1 expression thus establishing an autoregulatory loop (Marigo and Babin, 1996).
Shh is expressed in prostate epithelial cells at the earliest stages of prostate bud induction in the rodent prostate gland and rapidly declines over the next several days as morphogenesis is completed (Podlasek et al., 1999a; Lamm et al., 2002; Berman et al., 2004; Pu et al., 2004). As with other organs, Shh is expressed in a spatially defined manner. During the initial budding phase, Shh has a broad epithelial expression along the ductal length, which rapidly transitions into a distal tip pattern as the ducts elongate and branch (Fig. 5). Of note, expression patterns are heterogeneous at the distal signaling center with foci of high Shh expression at specific sites, which may permit highly localized actions in those cells resulting in differential growth and branchpoint formation (Pu et al., 2004). In developing mouse and rat prostates, ptc expression localizes to the condensed mesenchymal cells adjacent to the elongating epithelial ducts with strongest expression surrounding the distal tips (Fig. 5) (Lamm et al., 2002; Pu et al., 2004). A weaker ptc signal is also found within the epithelial cells in the distal but not central or proximal regions of the branching ducts, which provides an opportunity for autocrine Shh action in the distal epithelium. Gli1, gli2, and gli3 are all expressed in mesenchymal cells at the distal signaling center (Lamm et al., 2002; Pu et al., 2004) with some noteworthy differences. While gli1 and gli2, the transcription activators, are also expressed in periductal mesenchyme along the ductal length, gli3, the transcription repressor, is restricted to the distal tips (Pu et al., 2004). Additionally, gli1 and gli3, but not gli2, are expressed in distal tip epithelial cells immediately adjacent to mesenchyme. Finally, a potential Shh signaling modulator, Scube1, was recently identified as highly expressed in UGM and distal-tip prostate mesenchyme during morphogenesis (Vanpoucke et al., 2008). In total, this complex pattern of transcription activators and repressors may permit differential Shh actions at specific sites in both epithelial and mesenchymal cells during prostate development with the highest Shh signals transmitted at the distal signaling center.
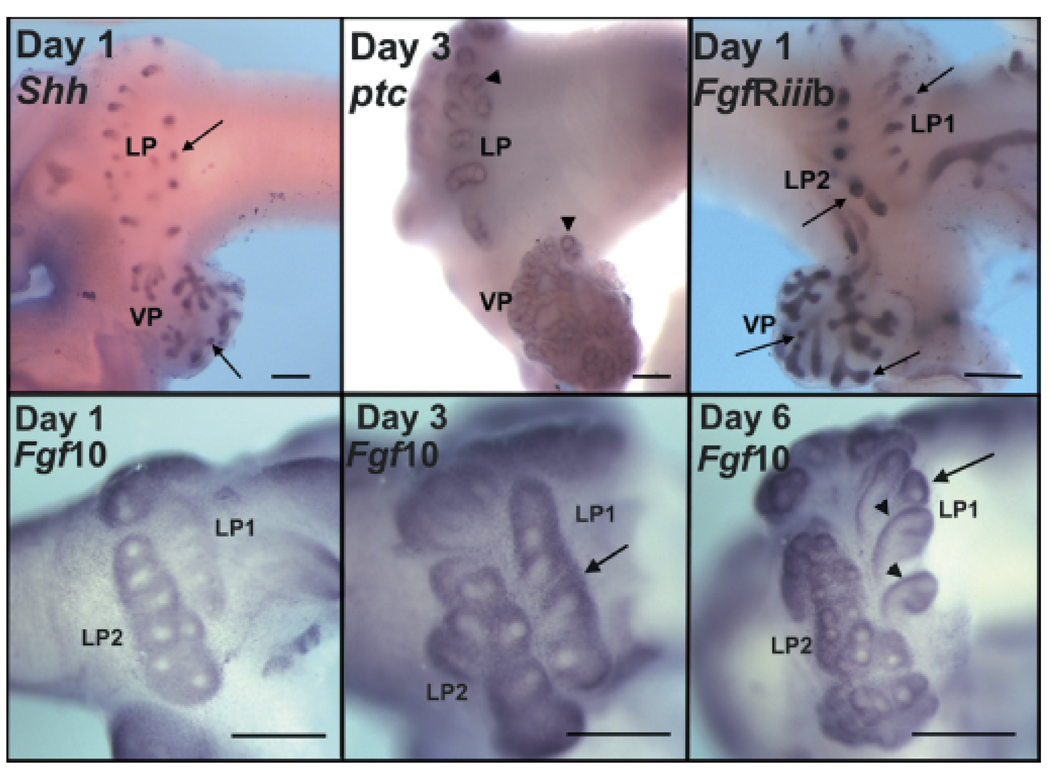
Fig. 5.
Whole mount in situ hybridization of Shh, ptc, FgfR2iiib and Fgf10 expression during rat prostate development. Days and gene are labeled on each image. Top row shows Shh (left), ptc (center) and FgfR2iiib (right) at postnatal days 1 or 3. LP denotes lateral prostate with LP1 and LP2 representing two separate ductal regions and VP denotes the ventral lobe. Arrows point to Shh and FgfR2iiib expression within the epithelium at the distal tips while arrowheads highlight periductal mesenchymal ptc expression at the distal tips. Bottom row shows Fgf10 mRNA in the rat lateral prostate lobe at the ductal elongation stage (day 1 and 3) and at the start of branching morphogenesis (day 6). Arrows point to regions of strongest Fgf10 expression in the distal tip mesenchyme at days 3 and 6. Arrowheads show condensed periductal Fgf10 expression along the ductal length. Bar = 200 µm. (See Huang et al., 2005 for details)
The human fetal prostate expresses SHH with a strong increase in expression between fetal weeks 10 and 13, the time when active morphogenesis is underway (Barnett et al., 2002). Recent analysis has shown the full component of all SHH signaling pathway genes in the human fetal prostate including SMO, PTC1 and GLI1 as well as DHH, which supports an active role for this pathway in human prostate development (Zhu et al., 2007). In addition to the peak between weeks 10 and 16, there was a subsequent decline followed by a second expression peak at week 28 corresponding with elevated proliferation, which indicates a potential active role for SHH during third trimester prostate growth and differentiation.
Specific roles for Shh during prostate development have been examined in rodent models by a number of laboratories using a variety of approaches. Studies using anti-Shh antibodies (Podlasek et al., 1999a) and cyclopamine to block Shh signaling showed that a functional Shh pathway was required for bud initiation and ductal elongation placing Shh as a critical inducer for prostate formation. However, subsequent studies using UGS tissues from null mutant Shh−/− mice were able to demonstrate bud initiation in organ culture (Freestone et al., 2003; Berman et al., 2004) and differentiated prostate formation when grafted in whole or as recombinants of Shh−/− epithelium with wild-type UGS mesenchyme under the renal capsule (Berman et al., 2004). These studies led to the conclusion that Shh is not required for prostate bud induction or elongation. This was more recently challenged by additional examination of the Shh−/− mouse prostate that showed compensatory expression of Ihh, which is not normally expressed in the prostate (Doles et al., 2006). Further, this Ihh was capable of activating the hedgehog signaling pathway in Shh−/− prostates thus indicating that prostatic budding, elongation and differentiation in Shh−/− prostates are possible due to continued hedgehog pathway signaling. Additional studies with Gli−/− mice revealed the requirement of Gli2 for normal budding as well as considerable functional redundancy between these transcription factors (Shaw and Bushman, 2007). In total, the weight of these recent studies leaves the original antibody and inhibitor experiments more reliable in predicting an essential role for hedgehog signaling for prostate initiation and development, although a more definitive assessment will be required for an absolute determination.
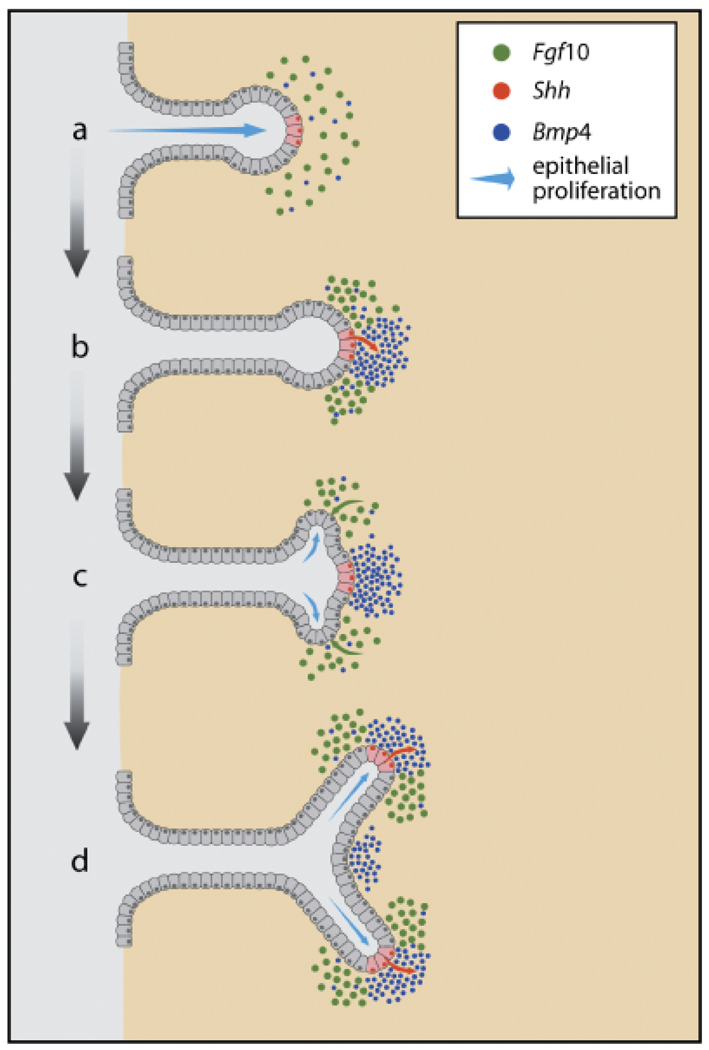
Beyond the issue of Shh’s role in prostate budding, other studies have demonstrated a role for Shh in maintenance of progenitor cells within the prostate for normal ductal patterning (Berman et al., 2004) and for epithelial proliferation, differentiation and branching (Freestone et al., 2003; Pu et al., 2004). Excess Shh added to an organ culture system resulted in reduced ductal growth and branching (Freestone et al., 2003; Wang et al., 2003). However, this was shown to be a function of Fgf10 down-regulation and Bmp4 up-regulation throughout the prostate resulting in growth restraint (Pu et al., 2004). In contrast, localized Shh delivery with microbeads at the distal tips revealed localized Fgf10 down-regulation concomitant with localized growth inhibition. Based on these findings, we propose that focal expression of Shh within the distal signaling center results in localized gradients of growth inhibitory and stimulatory factors that combined, permit differential growth and branching during the branching morphogenesis stage (Fig. 4). This will be discussed further in the section on signaling networks.
Fig. 4.
A proposed model for dichotomous branching of the developing rat prostate ducts as controlled by localized expression and cross-talk of secreted morphoregulatory factors. The distal duct tips express Shh in discreet focal areas (for simplicity, only one shown in red) while the distal mesenchyme expresses Fgf10 (green dots) and Bmp4 (blue dots). As these cells make contact with each other (b), the secreted Shh (red arrow) activates ptc on mesenchymal cells, locally down-regulates Fgf10 (loss of green dots) and up-regulates Bmp4 (blue dots) expression. The focal downregulation of Fgf10 results in lateral subdomains of higher Fgf10 expression adjacent to the Shh foci, which in turn, down-regulates Bmp4 in that region and activates (green arrow) higher epithelial proliferation via epithelial FgfR2iiib (c). The disparate epithelial proliferation rates in the lateral domains results in the sprouting of two buds on each side of the Shh foci (blue arrows) which initiates a branchpoint (d). Further, the elevated Fgf10 in the lateral domains up-regulates Shh and ptc expression (d), which allows repetition of the above steps and results in complex branching patterns (Based on results published by Huang et al., 2005).
While evidence has shown that androgens are capable of up-regulating prostatic Shh expression (Podlasek et al., 1999a; Freestone et al., 2003; Pu et al., 2004), similar Shh levels in male and female f17 UGS tissues and continued expression of Shh following AR blockade cast doubt on its absolute necessity (Freestone et al., 2003; Pu et al., 2007). Furthermore, Shh is expressed in late fetal UGS epithelium and budding prostate ducts when epithelial AR expression is limited or absent. Blockade of Fgf10 signaling in short-term rat prostate organ culture was able to block androgen stimulation of Shh expression indicating that androgen regulation of prostate Shh expression during early development is indirectly mediated through the mesenchyme (Pu et al., 2007). Currently, the factors or signals that induce and drive Shh expression in the UGS epithelium during early prostate gland initiation remain unresolved.
Fibroblast growth factor-10
Fgf10 is a member of the fibroblast growth factor (Fgf) family of secreted morphogens which consists of 23 known members (Ornitz and Itoh, 2001; Raman et al., 2003). Fgfs have a high affinity for heparin and glycosaminoglycans (GAGs) which position them for interaction with membrane associated, tyrosine kinase Fgf receptors (FgfR) on target cells (Uematsu et al., 2000). Developmental studies have shown a critical role for Fgf-10 in initiation and directional outgrowth of buds as well as ductal branching in many branched structures including the prostate gland (Bellusci et al., 1997; Thomson and Cunha, 1999). In the prostate, as in other branched structures, Fgf10 expression is spatially restricted to the distal aspects of the glands where it is believed to function as a chemoattractant for elongating ducts and an inducer of ductal branching through stimulation of epithelial cell proliferation (Lu et al., 1999; Thomson and Cunha, 1999; Donjacour et al., 2003). As previously shown (Huang et al., 2005), the expression pattern of Fgf10 in the mesenchymal pad is broad during early stages of ductal budding and elongation and subsequently condenses tightly around the elongating ducts with strongest expression at the distal tips during active branching morphogenesis (Fig. 5). Identical Fgf10 patterning is observed in the separate rat prostate lobes. There are four Fgf receptors (FgfR) with multiple splice variants that have varying affinities for the Fgf ligands, which adds considerable complexity during development. The splice variant of FgfR2, FgfR2iiib, is the specific transmembrane receptor for Fgf10 as well as for Fgf7 (Finch et al., 1995). It is expressed by prostatic epithelial cells thus establishing an important stromal-epithelial cell paracrine pathway during development. We observed a spatially restricted pattern for FgfR2iiib in the distal tips of elongating rat prostate ducts at day 1 (Fig. 5) with continued distal expression during branching morphogenesis that results in restricted epithelial cell proliferation at these distal sites (Huang et al., 2005).
Studies with null mutant Fgf10−/− mice established an essential role for Fgf10 in prostate initiation and branching morphogenesis, because prostates were rudimentary with limited bud numbers, and growth was severely restricted (Donjacour et al., 2003). Renal grafts of Fgf10−/− prostatic rudiments in intact wild-type hosts showed little growth with limited differentiation. Importantly, while Fgf10 plus testosterone could partially restore prostatic growth of Fgf10−/− rudiments, Fgf10 alone was ineffective suggesting that Fgf10 is essential but not sufficient for prostate bud development and that Fgf10 must interact with other testosteroneinduced genes for prostate formation. Short-term culture of mesenchyme-free, epithelial ducts isolated from newborn rat ventral prostates revealed that while testosterone alone was incapable of initiating ductal branching, this could be induced by Fgf10 alone, which demonstrates that Fgf10 is sufficient for branch point formation (Huang et al., 2005). Furthermore, Fgf-10 induced branching was blocked by a Mek1/2 inhibitor, showing that Fgf10/FgfR2iiib acts through the ras/raf/Mek/Erk1/2-signaling pathway in the developing prostate. This was recently confirmed in organ cultures of f18 murine UGS which showed that the Mek/Erk1/2 pathway was essential for Fgf10 stimulated bud induction and elongation in the presence of testosterone (Kuslak and Marker, 2007). Tissue and cell-specific requirement for FgfR2 in murine prostate morphogenesis was also demonstrated using a Cre-LoxP system to delete FgfR2 in UGS epithelium (Lin et al., 2007). Interestingly, the ventral lobe was more sensitive to epithelial FgfR2 deletion because it was absent in FgfR2cn mice while small dorsal and lateral lobes developed. In those regions, ducts were underdeveloped with impaired branching and epithelial aberrations indicating that the FgfR2 pathway is also necessary for terminal differentiation. Furthermore, androgen dependency for prostatic homeostasis was disturbed in the absence of epithelial FgfR2, which supports a role for FgfR2 in mediating androgenic action in the prostate gland.
Fgf10 was initially described as a prostatic andromedin defined as a paracrine-acting, androgen-regulated, secreted mesenchymal factor that drives proliferation or differentiation of the epithelium (Lu et al., 1999). This was challenged in other studies that failed to show robust androgen regulation of Fgf10 gene expression (Thomson and Cunha, 1999; Thomson, 2001). However, more recent experiments using shorter-term cultures of developing prostates (Pu et al., 2007) and earlier time points (Kuslak and Marker, 2007) have shown that Fgf10 expression in the newborn rat ventral and lateral lobes and the fetal mouse UGS mesenchyme is indeed up-regulated by exogenous androgens. We have also shown that FgfR2iiib is up-regulated in the ventral prostate by testosterone which can further amplify Fgf10-mediated action as a function of androgen levels in the developing tissue. Using an FgfR2 antagonist and a Mek inhibitor, Fgf10 signaling was shown to be essential for testosterone stimulation of epithelial Shh and Hoxb13 expression in the ventral prostate thus establishing that Fgf10 functions as an androgen-regulated paracrine factor that influences epithelial cell gene expression of other morphoregulatory genes (Pu et al., 2007).
Most branched structures expressing Fgf10, including the prostate, also express endogenous regulators of Fgf action to maintain tight regulation of its proliferative signals. This includes the Sprouty proteins that modulate receptor tyrosine kinases including epidermal growth factors (Egfs) and Fgfs. While the role of Sprouty proteins has not been examined for the developing prostate gland to date, it deserves mention that Sprouty 1, 2 and 4 are expressed in the human adult prostate and levels are down-regulated in prostate cancer (Wang et al., 2006a). Of interest, a novel variant of Sprouty1 that represents a fetal isoform is also observed in prostate tumour cells and tissues (Fritzsche et al., 2006) suggesting that dysregulation of developmental Sprouty genes may contribute to abnormal growth with disease. In addition to down-regulation of Fgf10 expression by Shh as described above, stromalTgfβ1 has also been shown to directly suppress Fgf10 expression at the proximal promoter in the developing rat prostate (Tomlinson et al., 2004b). These regulatory networks are further highlighted in the last section.
Bmps/Tgf β/activins
Bone morphogenetic proteins (Bmps) are members of the Tgfβ gene superfamily and, in general act as inhibitors of proliferation during development (Hogan, 1996). Secreted Bmps initiate cell signaling by binding transmembrane Type II receptors (BmpRII or ActRII), which complex with Type I receptors (Alk3 and Alk6) and activate intracellular pathways involving Smads 1, 3 and 5. In the mouse and rat prostate, Bmp-4 is broadly expressed in the UGM and prostate mesenchymal pads before and during bud initiation and levels decline postnatally with expression localized to periductal mesenchyme along the length of the elongating and branching ducts (Lamm et al., 2001; Prins et al., 2006). BmpR1 are expressed by both mesenchymal and epithelial cells in mouse prostate indicating that Bmp actions may be mediated on both cell types during development (Lamm et al., 2001). While targeted disruption of Bmp-4 is embryonic lethal, Bushman and colleagues found that Bmp4+/− heterozygotes possess an increased number of branching tips in the murine ventral prostate indicating that it functions as a prostatic growth inhibitor (Lamm et al., 2001). This conclusion was further supported by organ culture studies with exogenous Bmp4, which prevented ductal budding and outgrowths. Based upon its localization pattern, actions in organ culture systems, increased growth in Bmp4+/− prostates and rapid decline in expression as morphogenesis proceeds, it is believed that Bmp4 restricts ductal outgrowths and that clearing of its expression is required for bud initiation. Continued Bmp4 expression along the ductal length is thought to play an active role in branching morphogenesis by limiting epithelial cell proliferation at restricted sites as modeled in Fig. 4 (Lamm et al., 2001; Pu et al., 2004; Huang et al., 2005; Prins et al., 2006). Because Bmp4 expression is regulated by other prostatic growth factors including up-regulation by Shh and down-regulation by Fgf10, we propose this results in Bmp4 expression gradients at distinct sites that contributes to ductal branching (Pu et al., 2004; Huang et al., 2005). In contrast to secreted growth stimulators, we found that androgens decrease the expression of Bmp4 in the developing prostate and propose that repression of the growth repressors contributes to androgenic regulation of prostate development (Pu et al., 2007).
Bmp7 is another Bmp family member expressed in the prostate gland that plays an inhibitory role in prostate development (Grishina et al., 2005). Expression of Bmp7 has been localized to UGM before bud initiation in the mouse prostate and to epithelial cells during postnatal life (Grishina et al., 2005). In the rat prostate, we observe increasing expression of epithelial Bmp7 between days 1 and 5 with localization restricted to the distal signaling center in the separate lobes (Huang et al., 2005). Bmp7 ligands to BmpRII and Type I receptors Alk2 and Alk6 and activates Smad1. Interestingly, while Alk2, 3 and 6 are expressed in mouse f18 UGS epithelial cells, Alk6 alone is found in newborn proximal mesenchyme while Alk2 and Alk3 are present in the distal mesenchyme which may provide differential responses at these distinct sites (Grishina et al., 2005). To determine a role for Bmp7 during development, null mutant Bmp7−/− mice were analyzed and their prostates exhibited a twofold increase in branching. Furthermore, addition of recombinant Bmp7 to organ cultures inhibited morphogenesis which together indicates that similar to Bmp4, Bmp7 functions to restrict prostate growth during development (Grishina et al., 2005). However, due to expression in distinctly different cell compartments, the mechanisms of growth inhibition are likely to differ. Of particular interest, Notch1 signaling was derepressed in Bmp7 null prostates resulting in widespread Notch activity throughout the epithelium. It is proposed that Bmp7 may restrict ductal branching during prostate development by limiting epithelial domains with Notch1 activity (Grishina et al., 2005).
Like many secreted growth regulators, actions of Bmp4 and, to a lesser degree, Bmp7 are modulated by a secreted endogenous inhibitor termed noggin. Noggin functions by binding to available Bmp ligands in the extracellular regions thus blocking their interactions with transmembrane receptors. A recent study by Bushman and colleagues demonstrated the critical importance of noggin during prostate development using null mutant noggin−/− mice, which showed complete absence of the ventral mesenchymal pad and loss of ventral prostate budding with restricted budding in the dorsolateral regions (Cook et al., 2007). This find reinforces the concept that unopposed Bmp action in the UGM will inhibit prostate formation. Further, organ culture studies revealed that Bmp4 inhibited proliferation of p63+epithelial cells at the distal tips while noggin addition blocked this action. The authors propose that mesenchymal-expressed noggin interacts with secreted Bmp4/7 to create a gradient of Bmp signaling along the ductal axis that restricts and stimulates outgrowth at specific sites.
Tgfβ1 has also been shown to have a growth inhibitory role during prostate gland development. Both TgfβRI and TgfβRII are found in developing prostate stromal and epithelial cells thus permitting Tgfβ action in both cell populations (Chang et al., 1999a). While Tgfβ2 and Tgfβ3 protein localize to rat prostate epithelium upon differentiation, active Tgfβ1 localizes to the postnatal periductal mesenchymal cells as they differentiate into smooth muscle cells (Chang et al., 1999a). Similarly, in mice, high levels of Tgfβ1 mRNA were observed in mesenchyme surrounding areas of active epithelial duct formation (Timme et al., 1994). Organ culture studies with newborn mouse (Tanji et al., 1994) and rat (Itoh et al., 1998; Tomlinson et al., 2004a) prostates demonstrated that exogenous Tgfβ1 inhibited testosterone-induced growth and branching morphogenesis. This may be mediated in part through induction of epithelial p21cip1/waf1, a known downstream gene of prostatic Tgfβ1, which drives epithelial cells into a terminal differentiation pathway, effectively limiting their proliferation (Chang et al., 1999a). It is noteworthy that Tgfβ1 actions varied along the proximal–distal axis with suppression of epithelial and stromal cell proliferation in the proximal ducts yet stimulation of epithelial cell proliferation at the younger and less differentiated distal tips (Tomlinson et al., 2004a). The growth inhibitory actions of Tgfβ1 may also be mediated, in part, by Tgfβ1-induced redistribution of nuclear AR to the cytoplasm in prostate smooth muscle cells effectively suppressing androgen action in those cells (Gerdes et al., 1998). In addition, Tgfβ1 was shown to repress prostatic Fgf10 expression which may further contribute to its growth inhibitory effects (Tomlinson et al., 2004a).
Activins, also members of the Tgfβ gene superfamily, have been shown to influence prostate gland development. Activins are comprised of homo- and heterodimers of βA and βB subunits, which form activins A, AB and B. They have been shown to play important roles in development of multiple structures including the mammary glands (Welt and Crowley, 1998). Studies by Risbridger and colleagues demonstrated a specific role for activins in rat prostate gland development (Cancilla et al., 2001). Activin βA localized to mesenchymal cells surrounding the distal tips of branching ducts, while activin βB was found in some mesenchymal and fibroblastic stromal cells but not in smooth muscle. As the epithelium differentiated into luminal cells, activin βB was strongly expressed. Specific receptors ActRIA and ActRIIA were found throughout the epithelium of developing glands implicating them as direct targets. Addition of exogenous activin A inhibited ductal branching and elongation in newborn rat ventral lobes by limiting epithelial cell proliferation at the distal tips. It also suppressed stromal differentiation of smooth muscle cells towards the distal duct regions. Actions of activins in tissues are counteracted by activin-binding follistatin proteins. Follistatin was expressed throughout the epithelium of developing prostates and maintained into the mature glands. In newborn prostate explants grown in the absence of testosterone, addition of follistatin increased growth and branching. Together these findings support a balanced interaction between activins and follistatins in regulating ductal growth and branching with concentrated action at the distal tips (Cancilla et al., 2001).
Wnt genes and signaling regulators
The Wnt genes encode a large, highly conserved family of secreted glycoproteins that play important roles in controlling tissue patterning, cell fate and proliferation within a broad range of embryonic contexts (Cadigan and Nusse, 1997; Nelson and Nusse, 2004). Wnt genes are the mammalian homologues of the Drosophila polarity gene, wingless. The 19 mammalian Wnt proteins identified to date associate with ECM proteogylcans and bind to frizzled (Fzd) cell surface receptors thus mediating cross-talk between cells (Cadigan and Nusse, 1997). Vertebrate Wnts have been divided into two functional groups by reference to their downstream signaling pathways. In short, the canonical Wnts signal through nuclear β-catenin/TCF-LEF while the noncanonical Wnts function through alternate pathways that include Ca1/PKC or RhoA/JNK (Bejsovec, 2005). While both pathways involve initial liganding to Fzd receptors, the canonical pathway includes recruitment of a coreceptor, LRP5/6 on the cell membrane, while the non-canonical pathway does not involve this molecule. In addition to Wnt ligands, receptors and downstream signaling molecules, the Wnt network also includes a number of extracellular secreted regulators that antagonize Wnt actions. Secreted frizzled-related proteins 1–5 (sFrps) have a cysteine-rich domain similar to Fzd receptor ligand-binding domain and dampen Wnt actions by competitive binding for available Wnts. Wnt inhibitory factors (Wif 1 and 2) also bind to secreted Wnts and block their ability to interact with Fzd receptors on the cell membrane (Hsieh et al., 1999). The dickkopf (Dkk) proteins 1–4 bind to the canonical coreceptor LRP 5/6 and interfere with canonical Wnt signaling specifically (Mao et al., 2001). The large number of secreted inhibitory molecules stresses the critical importance of tight control of Wnt signaling to effect normal development and maintain tissue homeostasis.
Despite a large number of studies that have demonstrated a role for aberrant Wnt signaling during prostate carcinogenesis and progression (Chesire and Isaacs, 2003; Yardy and Brewster, 2005), there is little published work on the role(s) of Wnt genes during prostate development. The numerous functions of Wnt signaling in animal development include crucial morphogenic roles of many, if not most, organs and thus it is expected that this includes the prostate gland. Constitutive expression of stable β-catenin in prostate epithelium of transgenic mice resulted in epithelial hyperplasia and squamous metaplasia by 8 weeks of age with transdifferentiation to epidermal-like cell lineages suggesting that canonical Wnt signaling plays a key role in cell determination, differentiation and proliferation in the prostate (Bierie et al., 2003). A role for β-catenin is also suggested for normal prostate epithelial proliferation because nuclear β-catenin increases in proliferating prostate epithelium of castrated rat prostates following androgen replacement (Chesire et al., 2002). SAGE libraries of adult and developing mouse prostates, UGE and UGM were screened and revealed strong expression of several Wnt gene family members during early development, which was confirmed by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) for Wnt 4, Wnt11, Fzd1, Fzd7, LRP, Lef1 and sFrp2 (Zhang et al., 2006).
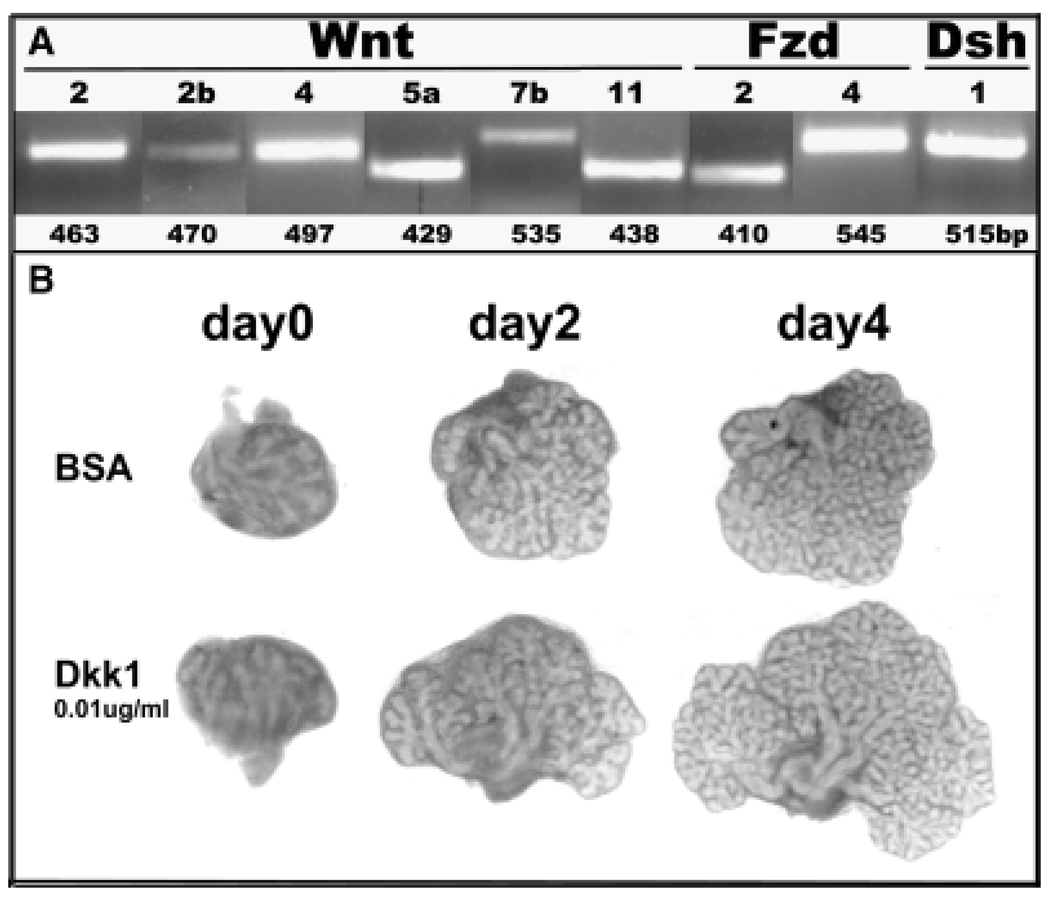
We have screened for expression of Wnt genes and signaling components in the developing rat ventral prostate using DNA arrays and a PCR array and have identified expression of several Wnts, Fzds, Dshs, most intracellular signaling molecules involved in canonical and non-canonical Wnt signaling as well as extracellular regulators (unpublished findings). As shown in Fig. 6A, six Wnt genes were expressed with high signal intensity in Day 3 ventral lobes including three canonical Wnts (Wnt2, Wnt2b and Wnt7b), three non-canonical Wnts (Wnt4, Wnt5a and Wnt11), Fzd2 and 4 and Dsh1. Temporal Wnt gene expression profiles were gathered by qRT-PCR and all, except Wnt7b, showed high expression at birth with levels declining during and after the completion of morphogenesis (Fig. 1, unpublished findings). In contrast, Wnt7b expression was relatively low during early development and expression rapidly increased upon functional cytodifferentiation. Along with spatially restricted expression, these dynamic temporal expression profiles suggest important roles for these morphogens during prostate gland development. Detailed studies with Wnt5a demonstrated that this noncanonical Wnt is a growth and branching repressor during morphogenesis (Huang et al., 2006). Of particular interest, we observed with explant cultures that androgens repress Wnt5a expression in the ventral prostate (Pu et al., 2007), again supporting our proposal that androgens repress the growth repressor genes to drive prostate gland development.
Fig. 6.
Wnt gene expression and action during rat prostate development. A: reverse-transcriptase polymerase chain reaction revealed expression of Wnts 2, 2b, 4, 5a, 7b, and 11 as well as Fzd 2 and 4 and Dsh 1 in the day 3 rat ventral prostate. B: Contralateral rat ventral prostate lobes were collected on the day of birth and cultured in the presence of 10nM testosterone with bovine serum albumin or 0.01 µg/ml Dkk1 for 4 days. This was repeated in 4 separate prostates. The data shows a growth promoting effect of canonical Wnt inhibition by Dkk1 protein.
Expression and functions for the secreted Wnt regulators has also been examined in the developing mouse and rat prostate glands. SFrp1 (Joesting et al., 2005) and sFrp2 (Zhang et al., 2006) were found to be highly expressed in developing mouse prostates using SAGE libraries and Affimetrix DNA arrays, respectfully, which was confirmed by RT-PCR. For both genes, signal was found in the early UGM and prostate mesenchyme, but with bud development, signal was concentrated in the ductal epithelium. Addition of recombinant sFrp1 to rat ventral prostate explant cultures resulted in increased growth over 5 days relative to BSA-treated controls. Similarly, we observed that Dkk1 protein added to newborn rat ventral lobes stimulated growth and branching over a four-day period (Fig. 6B). Because Dkk1 antagonism is specific to canonical signaling, these findings suggest that canonical Wnt signaling may play an inhibitory role with regards to epithelial cell proliferation during development.
Cross-talk between developmental genes
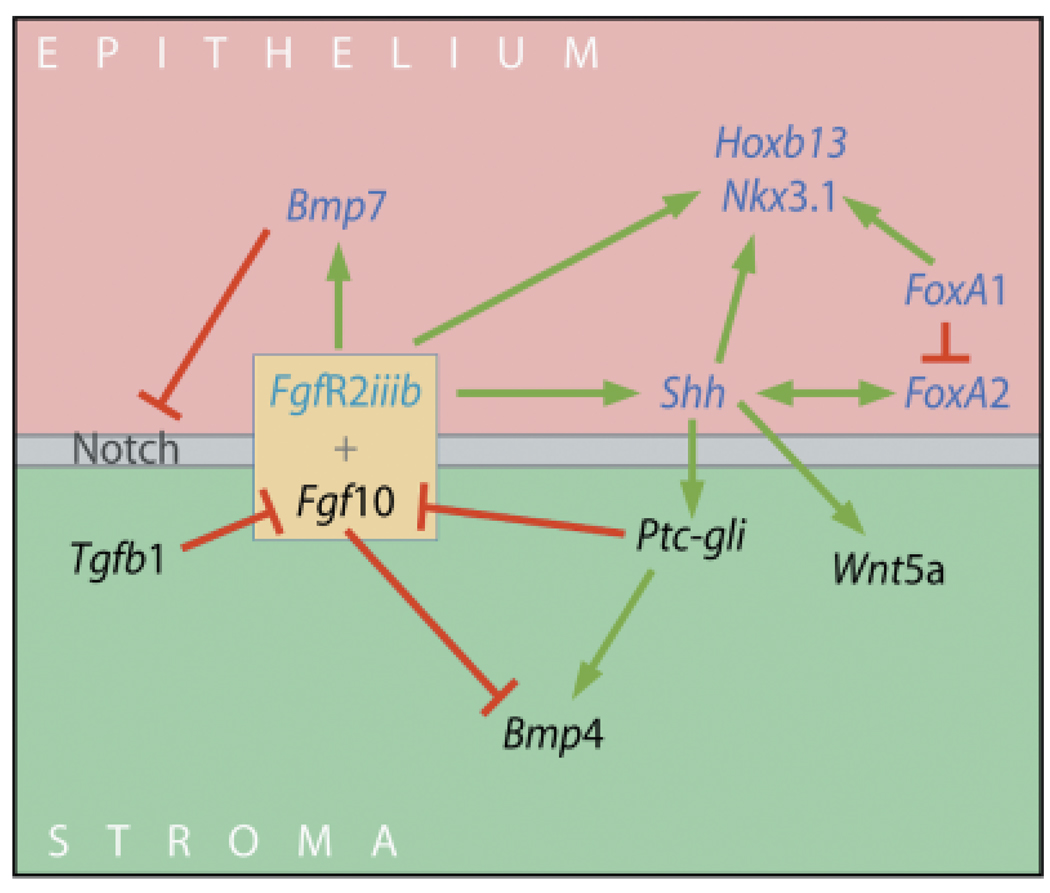
As we have mentioned throughout this review, it is clear there are complex signaling networks in the developing prostate gland that involve cross-regulation of morphoregulatory gene expression. We propose that these gene regulatory networks organize normal prostate development through a temporal series of reciprocal signals and feedback loops that tightly regulate proliferation, differentiation, ductal outgrowth and branchpoint formation. This is schematized in Fig. 7 where we highlight known interactions of developmental genes in the fetal and newborn prostate gland. Androgen action during prostate development includes stimulation or repression of several genes and this action may be potentiated through a resultant cascade of cross-regulatory networks that work together to drive prostate gland development. Mesenchymal Fgf10, acting via epithelial FgfR2iiib, directly up-regulates epithelial Shh expression resulting in up-regulation of mesenchymal ptc and glis which down-regulate mesenchymal Fgf10 expression, thus establishing a negative feedback loop to provide tight control of branching (Pu et al., 2004; Huang et al., 2005). Fgf10 also down-regulates expression of Bmp4, an established restrictor of growth and branching in the prostate gland (Huang et al., 2005). Because Fgf10 and Bmp4 have opposing actions with regards to prostatic ductal outgrowth, localized down-regulation of Bmp4 expression by Fgf10 may contribute to Fgf10’s stimulatory effects. Furthermore, because Shh up-regulates mesenchymal Bmp4 expression at focal sites in prostatic ductal tips (Pu et al., 2004), down-regulation by Fgf10 will contribute to the reciprocal regulation necessary to sculpture the prostatic form. Similar upregulation of mesenchymal Wnt5a by Shh may further contribute to focal growth at the ductal tips (Huang et al., 2006). Both mesenchymal Fgf10 and epithelial Shh stimulate expression of the epithelial homeobox genes, Hoxb13 and Nkx3.1, that drive epithelial differentiation. Shh also has reciprocal stimulatory action with the early epithelial transcription factor FoxA2, which itself is repressed by FoxA1 as ducts elongate (Gao et al., 2005). FoxA1 stimulates Nkx3.1 expression thus in addition to its own role in promoting epithelial differentiation, FoxA1 maintains differentiation by networking with Nkx3.1 (Gao et al., 2005). Fgf10 increases epithelial Bmp7 expression that in turn blocks epithelial Notch1 expression (Grishina et al., 2005), which may serve to enhance proliferation and suppress premature differentiation during the early growth phase. Mesenchymal Tgfβ1, which becomes functional as periductal mesenchyme differentiates into smooth muscle (Chang et al., 1999a), down-regulates Fgf10 expression (Tomlinson et al., 2004a), which will serve to brake prostatic growth as development is completed.
Fig. 7.
A schematic representation of regulatory networks between secreted morphogens and transcription factors in the epithelial and mesenchymal cells at the distal signaling center of the developing prostate gland. Fgf10 (mesenchymal) and FgfR2iiib (epithelial) upregulate (green arrows) epithelial expression of Shh and Bmp7 involved in branching morphogenesis as well as Hoxb13 and Nkx3.1 involved in epithelial differentiation. Shh up-regulates ptc and gli in adjacent mesenchymal cells which down-regulates (red lines) Fgf10 expression thus establishing a negative feedback loop for controlled growth. Shh-ptc-gli also up-regulates the growth inhibitory Wnt5a and Bmp4 molecules in the mesenchyme while Fgf10 down-regulates their expression which further serves to tightly control localized tissue growth. FoxA1 stimulates expression of Nkx3.1 and inhibits FoxA2 expression which has reciprocal up-regulation with Shh. Fgf10/FgfR2iiib up-regulates Notch expression, which drives ductal growth while Bmp7 down-regulates Notch and suppresses regional growth. Tgfβ1 suppresses Fgf10 expression, which may serve as a brake for growth as development nears completion.
Undoubtedly, there are other yet uncharacterized morphoregulatory genes with additional interactions that together contribute to the growth, branching and differentiation of the prostate gland during development. We look forward to learning of these actions in the coming years and predict this will eventually lead to a thorough understanding of the prostate developmental processes. In addition to providing a more complete developmental picture, this information will be of tremendous value towards understanding dysgenesis in growth and differentiation that occurs in benign prostatic hyperplasia and prostate cancer upon aging.
Acknowledgments
The authors gratefully acknowledge the contributions of Drs. William Chang, Carl Woodham, Liwei Huang, Yongbing Pu, David Hepps and Shumyle Alam as well as Ms. Lynn Birch for their research findings that are presented throughout this review. This work was supported in part by NIH grants DK40890, ES12282 (G.S.P.), DK09873 (W.C.), DK09653 (C.W.) and AFUD fellowships to W.C. and L.H.
Contributor Information
Gail S. Prins, Email: gprins@uic.edu, Department of Urology, College of Medicine, University of Illinois at Chicago, Chicago, IL 606012, USA, Tel: +1 312 413 9766, Fax: +1 312 996 1291.
Oliver Putz, Graduate Theological Union, Berkeley, CA, USA.
References
- Bardin CW, Bullock LP, Sherins RJ, Mowszowicz I, Blackburn WR. Androgen metabolism and mechanism of action in male pseudohermaphroditism: a study of testicular feminization. Rec Prog Horm Res. 1973;29:65–109. doi: 10.1016/b978-0-12-571129-6.50006-3. [DOI] [PubMed] [Google Scholar]
- Barnett DH, Huang HY, Wu XR, Laciak R, Shapiro E, Bushman W. The human prostate expresses sonic hedgehog during fetal development. J Urol. 2002;168:2206–2210. doi: 10.1016/S0022-5347(05)64356-X. [DOI] [PubMed] [Google Scholar]
- Bejsovec A. Wnt pathway activation: new relations and locations. Cell. 2005;120:11–14. doi: 10.1016/j.cell.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan B. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–4878. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- Berman DM, Desai N, Wang X, Karhadkar SS, Reynon M, Abate-Shen C, Beachy PA, Shen MM. Roles for hedgehog signaling in androgen production and prostate ductal morphogenesis. Dev Biol. 2004;267:387–398. doi: 10.1016/j.ydbio.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Bethel CR, Faith D, Li X, Guan B, Hicks JL, Lan F, Jenkins RB, Bieberich CJ, De Marzo AM. Decreased NKX3.1 protein expression in focal prostatic atrophy, prostatic intraepithelial neoplasia, and adenocarcinoma: association with gleason score and chromosome 8p deletion. Cancer Res. 2006;66:10683–10690. doi: 10.1158/0008-5472.CAN-06-0963. [DOI] [PubMed] [Google Scholar]
- Bhatia-Gaur R, Donjacour AA, Sciavolino PJ, Kim M, Desai N, Young P, Norton CR, Gridley T, Cardiff RD, Cunha GR, Abate-Shen C, Shen MM. Roles for Nkx3.1 in prostate development and cancer. Gen Dev. 1999;13:966–977. doi: 10.1101/gad.13.8.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich CJ, Fujita K, He WW, Jay G. Prostate-specific and androgen-dependent expression of a novel homeobox gene. J Biol Chem. 1996;271:31779–31782. doi: 10.1074/jbc.271.50.31779. [DOI] [PubMed] [Google Scholar]
- Bierie B, Nozawa M, Renou JP, Shillingford JM, Morgan F, Oka T, Taketo MM, Cardiff R, Miyoshi K, Wagner K, Robinson GW, Huang L. Activation of b-catenin in prostate epithelium induces hyperplasia and squamous transdifferentiation. Oncogene. 2003;22:3875–3887. doi: 10.1038/sj.onc.1206426. [DOI] [PubMed] [Google Scholar]
- Bolos V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocrine Rev. 2007;28:339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- Bowen C, Bubendorf L, Voeller HJ, Slack R, Willi N, Sauter G, Gasser TC, Koivisto P, Lack EE, Kononen J, Kallioniemi OP, Gelmann EP. Loss of NKX3.1 expression in human prostate cancers correlates with tumor progression. Cancer Res. 2000;60:6111–6115. [PubMed] [Google Scholar]
- Brown TR. Human androgen insensitivity syndrome. J Androl. 1995;16:299–303. [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Cancilla B, Jarred RA, Wang H, Mellow SL, Cunha GR, Risbridger GP. Regulation of prostate branching morphogenesis by Activin A and Follistatin. Dev Biol. 2001;237:145–158. doi: 10.1006/dbio.2001.0364. [DOI] [PubMed] [Google Scholar]
- Chang WY, Birch L, Woodham C, Gold LI, Prins GS. Neonatal estrogen exposure alters the transforming growth factor-β signaling system in the developing rat prostate and blocks the transient p21cip1/wafl expression associated with epithelial differentiation. Endocrinology. 1999a;140:2801–2813. doi: 10.1210/endo.140.6.6833. [DOI] [PubMed] [Google Scholar]
- Chang WY, Wilson MJ, Birch L, Prins GS. Neonatal estrogen stimulates proliferation of periductal fibroblasts and alters the extracellular matrix composition in the rat prostate. Endocrinology. 1999b;140:405–415. doi: 10.1210/endo.140.1.6401. [DOI] [PubMed] [Google Scholar]
- Chen H, Mutton LN, Prins GS, Bieberich CJ. Distinct regulatory elements mediate the dynamic expression pattern of Nkx3.1. Dev Dyn. 2005;234:961–973. doi: 10.1002/dvdy.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesire DR, Ewing CM, Gage WR, Isaacs WB. In vitro evidence for complex modes of nuclear beta-catenin signaling during prostate growth and tumorigenesis. Oncogene. 2002;21:2679–2694. doi: 10.1038/sj.onc.1205352. [DOI] [PubMed] [Google Scholar]
- Chesire DR, Isaacs WB. Beta-catenin signaling in prostate cancer: an early perspective. Endocrine Related Cancers. 2003;10:537–560. doi: 10.1677/erc.0.0100537. [DOI] [PubMed] [Google Scholar]
- Chuang P-T, McMahon AP. Branching morphogenesis of the lung: new molecular insights into an old problem. Trends Cell Biol. 2003;13:86–91. doi: 10.1016/s0962-8924(02)00031-4. [DOI] [PubMed] [Google Scholar]
- Clevidence DE, Overdier DG, Tao W, Aian X, Pani L, Lai E, Costa RH. Identification of nine tissue-specific transcription factors of the hepatocyte nuclear factor 3/forkhead DNA-binding-domain family. Proc Natl Acad Sci USA. 1993;90:3948–3952. doi: 10.1073/pnas.90.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee NRN. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97:161–163. doi: 10.1016/s0092-8674(00)80726-6. [DOI] [PubMed] [Google Scholar]
- Cook C, Vezina CM, Allgeier SH, Shaw A, Yu M, Peterson R, Bushman W. Noggin is required for normal lobe patterning and ductal budding in the mouse prostate. Dev Biol. 2007;312:217–230. doi: 10.1016/j.ydbio.2007.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR. The role of androgens in the epithelio-mesenchymal interactions involved in prostatic morphogenesis in embryonic mice. Anat Rec. 1973;175:87–96. doi: 10.1002/ar.1091750108. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Battle E, Young P, Brody J, Donjacour A, Hayashi N, Kinbarra H. Role of epithelial-mesenchymal interactions in the differentiation and spatial organization of visceral smooth muscle. Epithelial Cell Biol. 1992;1:76–83. [PubMed] [Google Scholar]
- Cunha GR, Chung LWK. Stromal-epithelial interactions: I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/Y) mice. J Steroid Biochem. 1981;14:1317–1321. doi: 10.1016/0022-4731(81)90338-1. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, Sugimura Y. The endocrinology and developmental biology of the prostate. Endo Rev. 1987;8:338–363. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- Doles J, Cook C, Shi X, Valosky J, Lipinski R, Bushman W. Functional compensation in hedgehog signaling during mouse prostate development. Dev Biol. 2006;295:13–25. doi: 10.1016/j.ydbio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Donjacour AA, Cunha GR. Assessment of prostatic protein secretion in tissue recombinants made of urogenital sinus mesenchyme and urothelium from normal or androgen-insensitive mice. Endocrinology. 1993;132:2342–2350. doi: 10.1210/endo.132.6.7684975. [DOI] [PubMed] [Google Scholar]
- Donjacour AA, Thomson AA, Cunha G. FGF-10 plays an essential role in the growth of the fetal prostate. Dev Biol. 2003;261:39–54. doi: 10.1016/s0012-1606(03)00250-1. [DOI] [PubMed] [Google Scholar]
- Economides KD, Capecchi MR. Hoxb13 is required for normal differentiation and secretory function of the ventral prostate. Development. 2003;130:2061–2069. doi: 10.1242/dev.00432. [DOI] [PubMed] [Google Scholar]
- Edwards S, Campbell C, Flohr P, Shipley J, Giddings I, Te-Poele R, Dodson A, Foster C, Clark J, Jhavar S, Kovacs G, Cooper CS. Expression analysis onto microarrays of randomly selected cDNA clones highlights HOXB13 as a marker of human prostate cancer. Br J Cancer. 2005;92:376–381. doi: 10.1038/sj.bjc.6602261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Anderson KM, Liao S. Receptor proteins for androgens. J Biochem. 1969;244:6584–6595. [PubMed] [Google Scholar]
- Finch P, Cunha G, Rubin J, Wong J, Ron D. Pattern of keratinocyte growth factor and keratinocyte growth factor receptor expression during mouse fetal development suggests a role in mediating morphogenetic mesenchymal-epithelial interactions. Dev Dyn. 1995;203:223–240. doi: 10.1002/aja.1002030210. [DOI] [PubMed] [Google Scholar]
- Foster BA, Cunha GR. Efficacy of various natural and synthetic androgens to induce ductal branching morphogenesis in the developing anterior rat prostate. Endocrinology. 1999;140:318–328. doi: 10.1210/endo.140.1.6435. [DOI] [PubMed] [Google Scholar]
- Freestone SH, Marker P, Grace OC, Tomlinson DC, Cunha GR, Harnden P, Thomson AA. Sonic hedgehog regulates prostatic growth and epithelial differentiation. Dev Biol. 2003;264:352–362. doi: 10.1016/j.ydbio.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Fritzsche S, Kenzelmann M, Hoffmann MJ, Müller M, Engers R, Gröne HJ, Schulz WA. Concomitant down-regulation of SPRY1 and SPRY2 in prostate carcinoma. Endocrine Related Cancer. 2006;13:839–849. doi: 10.1677/erc.1.01190. [DOI] [PubMed] [Google Scholar]
- Gao N, Ishii K, Mirosevich J, Kuwajima S, Oppenheimer SR, Roberts RL, Jiang M, Yu X, Shappell SB, Caprioli RM, Stoffel M, Hayward SW, Matusik RJ. Forkhead box A1 regulates prostate ductal morphogenesis and promotes epithelial cell maturation. Development. 2005;132:4331–4343. doi: 10.1242/dev.01917. [DOI] [PubMed] [Google Scholar]
- Gehring W. The discovery of the homeobox. In: Duboule D, editor. Guidebook to the homeobox genes. New York: Oxford University press; 1994. pp. 3–10. [Google Scholar]
- Gerdes M, Dang T, Larsen M, Rowley D. Transforming growth factor-b1 induces nuclear to cytoplasmic distribution of androgen receptor and inhibits androgen response in prostate smooth muscle ells. Endocrinology. 1998;139:3569–3577. doi: 10.1210/endo.139.8.6138. [DOI] [PubMed] [Google Scholar]
- Goyette P, Allan D, Peschard P, Chen CF, Wang W, Lohnes D. Regulation of Gli activity by all-trans retinoic acid in mouse keratinocytes. Cancer Res. 2000;60:5386–5389. [PubMed] [Google Scholar]
- Grishina IB, Kinm SY, Ferrara C, Makarenkova HP, Walden PD. BMP7 inhibits branching morphogenesis in the prostate gland and interferes with Notch signaling. Dev Biol. 2005;288:334–347. doi: 10.1016/j.ydbio.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritli-Linde A, Lewis P, McMahon AP, Linde A. The whereabouts of a morphogen: direct evidence for short-and graded long-range activity of hedgehog signaling peptides. Dev Biol. 2001;236:364–386. doi: 10.1006/dbio.2001.0336. [DOI] [PubMed] [Google Scholar]
- Guan B, Pungaliya P, Li X, Uquillas C, Mutton LN, Rubin EH, Bieberich CJ. Ubiquitination by TOPORS regulates the prostate tumor suppressor NKX3.1. J Biol Chem. 2007;283:4834–4840. doi: 10.1074/jbc.M708630200. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, Shiroishi T, Gaffield W, Yamada G. Unique functions of sonic hedgehog signaling during external genitalia development. Development. 2001;128:4241–4250. doi: 10.1242/dev.128.21.4241. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Sugimura Y, Kawamura J, Donjacour AA, Cunha GR. Morphological and functional heterogeneity in the rat prostatic gland. Biol Reprod. 1991;45:308–321. doi: 10.1095/biolreprod45.2.308. [DOI] [PubMed] [Google Scholar]
- Hayward S, Baskin L, Haughney P, Foster B, Bakiya R, Prins G, Cunha G. Stromal development in the ventral prostate, anterior prostate and seminal vesicle of the rat. Acta Anat. 1996a;155:94–103. doi: 10.1159/000147794. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Baskin LS, Haughney PC, Foster BA, Prins GS, Dahiya R, Cunha GR. Epithelial development in the rat ventral prostate, anterior prostate and seminal vesicle. Acta Anat. 1996b;155:81–93. doi: 10.1159/000147793. [DOI] [PubMed] [Google Scholar]
- Hogan BLM. Bone morphogenetic proteins in development. Curr Opin Gen Dev. 1996;6:432–438. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- Hogan BLM. Morphogenesis. Cell. 1999;96:225–233. doi: 10.1016/s0092-8674(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- Huang L, Pu Y, Alam S, Birch L, Prins GS. Estrogenic regulation of signaling pathways and homeobox genes during rat prostate development. J Androl. 2004;25:330–337. doi: 10.1002/j.1939-4640.2004.tb02796.x. [DOI] [PubMed] [Google Scholar]
- Huang L, Pu Y, Alam S, Birch L, Prins GS. The role of Fgf10 signaling in branching morphogenesis and gene expression in the rat prostate gland: lobe-specific supression by neonatal estrogens. Dev Biol. 2005;278:396–414. doi: 10.1016/j.ydbio.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Huang L, Pu Y, Birch L, Belmonte J, Prins GS. Wnt5a is required for prostate morphogenesis. J Androl. 2006;27:85. #115. [Google Scholar]
- Huang L, Pu Y, Birch L, Prins GS. Posterior Hox gene expression and differential androgen regulation in the developing and adult rat prostate lobes. Endocrinology. 2007;148:1235–1245. doi: 10.1210/en.2006-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husmann DA, McPhaul MJ, Wilson JD. Androgen receptor expression in the developing rat prostate is not altered by castration, flutamide, or suppression of the adrenal axis. Endocrinology. 1991;128:1902–1906. doi: 10.1210/endo-128-4-1902. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Review: hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Itoh N, Patel U, Cupp A, Skinner M. Developmental and hormonal regulation of transforming growth factor-β1 (TGF β1), -2, and -3 gene expression in isolated prostatic epithelial and stromal cells: epidermal growth factor and TGFβ interactions. Endocrinology. 1998;139:1378–1388. doi: 10.1210/endo.139.3.5787. [DOI] [PubMed] [Google Scholar]
- Joesting MS, Perrin S, Elenbaas B, Fawell SE, Rubin JS, Franco OE, Hayward SW, Cunha GR, Marker PC. Identification of SFRP1 as a candidate mediator of stromal-to-epithelial signaling in prostate cancer. Cancer Res. 2005;65:10423–10430. doi: 10.1158/0008-5472.CAN-05-0824. [DOI] [PubMed] [Google Scholar]
- Jost A. Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Rec Prog Horm Res. 1953;8:379–418. [Google Scholar]
- Jung C, Kim RS, Lee SJ, Wang C, Jeng MH. HOXB13 homeodomain protein suppresses the growth of prostate cancer cells by the negative regulation of T-cell factor 4. Cancer Res. 2004a;64:3046–3051. doi: 10.1158/0008-5472.can-03-2614. [DOI] [PubMed] [Google Scholar]
- Jung C, Kim RS, Zhang H, Lee SJ, Jeng MH. HOXB13 induces growth suppression of prostate cancer cells as a repressor of hormone-activated androgen receptor signaling. Cancer Res. 2004b;64:9185–9192. doi: 10.1158/0008-5472.CAN-04-1330. [DOI] [PubMed] [Google Scholar]
- Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- Kim MJ, Bhatia-Gaur R, Banach-Petrosky WA, Desai N, Wang Y, Hayward SW, Cunha GR, Cardiff RD, Shen MM, Abate-Shen C. Nkx3.1 mutant mice recapitulate early stages of prostate carcinogenesis. Cancer Res. 2002;62:2999–3004. [PubMed] [Google Scholar]
- Kopachik W, Hayward SW, Cunha GR. Expression of hepatocyte nuclear factor-3α in rat prostate, seminal vesicle, and bladder. Dev Dyn. 1998;211:131–140. doi: 10.1002/(SICI)1097-0177(199802)211:2<131::AID-AJA2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Kuslak SL, Marker PC. Fibroblast growth factor receptor signaling through MEK-ERK is required for prostate bud induction. Differentiation. 2007;75:638–651. doi: 10.1111/j.1432-0436.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- Lamm ML, Catbagan WS, Laciak RJ, Barnett DH, Hebner CM, Gaffield W, Walterhouse D, Iannaccone P, Bushman W. Sonic hedgehog activates mesenchymal Gli1 expression during prostate ductal bud formation. Dev Biol. 2002;249:349–366. doi: 10.1006/dbio.2002.0774. [DOI] [PubMed] [Google Scholar]
- Lamm ML, Podlasek CA, Barnett DH, Lee J, Clemens JQ, Hebner CM, Bushman W. Mesenchymal factor bone morphogenetic protein 4 restricts ductal budding and branching morphogenesis in the developing prostate. Dev Biol. 2001;232:301–314. doi: 10.1006/dbio.2001.0187. [DOI] [PubMed] [Google Scholar]
- Lasnitzki I, Mizuno T. Induction of the rat prostate gland by androgens in organ culture. J Endocrinol. 1977;74:47–55. doi: 10.1677/joe.0.0740047. [DOI] [PubMed] [Google Scholar]
- Lee C, Sensibar JA, Dudek SM, Hiipakka RA, Liao S. Prostatic ductal system in rats: regional variation in morphological and functional activities. Biol Reprod. 1990;43:1079–1086. doi: 10.1095/biolreprod43.6.1079. [DOI] [PubMed] [Google Scholar]
- Liao S, Fang S. Receptor-proteins for androgens and the mode of action of androgens on gene transcription in ventral prostate. Vitamins and Hormones. 1969;27:17–90. doi: 10.1016/s0083-6729(08)61124-3. [DOI] [PubMed] [Google Scholar]
- Lin Y, Liu G, Zhang Y, Hu YP, Yu K, Lin C, McKeehan K, Xuan JW, Ornitz DM, Shen MM, Greenberg N, McKeehan WL, Wang F. Fibroblast growth factor receptor 2 tyrosine kinase is required for prostatic morphogenesis and the acquisition of strict androgen dependency for adult tissue homeostasis. Development. 2007;134:723–734. doi: 10.1242/dev.02765. [DOI] [PubMed] [Google Scholar]
- Lipschutz J, Foster B, Cunha G. Differentiation of rat neonatal ventral prostates grown in a serum-free organ culture system. Prostate. 1997;32:35–42. doi: 10.1002/(sici)1097-0045(19970615)32:1<35::aid-pros5>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Lowsley OS. The development of the human prostate gland with reference to the development of other structures at the neck of the urinary bladder. Am J Anat. 1912;13:299–348. [Google Scholar]
- Lu W, Luo Y, Kan M, McKeehan WL. Fibroblast growth factor-10: a second candidate stromal to epithelial cell andromedin in prostate. J Biol Chem. 1999;274:12827–12834. doi: 10.1074/jbc.274.18.12827. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:423–424. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Marigo V, Babin CJ. Regulation of patched by sonic hedgehog in the developing neural tube. Proc Natl Acad Sci USA. 1996;93:9347–9351. doi: 10.1073/pnas.93.18.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer NP, Roelink H. The amino-terminal region of Gli3 antagonizes the Shh response and acts in dorsoventral fate specification in the developing spinal cord. Dev Biol. 2003;257:343–355. doi: 10.1016/s0012-1606(03)00065-4. [DOI] [PubMed] [Google Scholar]
- Miller G, Miller HL, van Bokhoven A, Lambert J, Werahera P, Schirripa O, Luccia M, Nordeen S. Aberrant HOXC expression accompanies the malignant phenotype in prostate cancer. Cancer Res. 2003;63:5879–5888. [PubMed] [Google Scholar]
- Mirosevich J, Gao N, Matusik RJ. Expression of Foxa transcription factors in the developing an adult Murine prostate. Prostate. 2004;62:339–352. doi: 10.1002/pros.20131. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergance of Wnt, β-catenin and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oefelein M, Chin-Chance C, Bushman W. Expression of the homeotic gene Hox-d13 in the developing and adult mouse prostate. J Urol. 1996;155:342–346. [PubMed] [Google Scholar]
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:1–12. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol. 2002;247:26–46. doi: 10.1006/dbio.2002.0668. [DOI] [PubMed] [Google Scholar]
- Podlasek CA, Barnett DH, Clemens JQ, Bak PM, Bushman W. Prostate development requires sonic hedgehog expressed by the urogenital sinus epithelium. Dev Biol. 1999a;209:28–39. doi: 10.1006/dbio.1999.9229. [DOI] [PubMed] [Google Scholar]
- Podlasek CA, Clemens JQ, Bushman W. Hoxa-13 gene mutation results in abnormal seminal vesicle and prostate development. J Urol. 1999b;161:1655–1661. [PubMed] [Google Scholar]
- Podlasek CA, Duboule D, Bushman W. Male accessory sex organ morphogenesis is altered by loss of function of Hoxd-13. Dev Dyn. 1997;208:454–465. doi: 10.1002/(SICI)1097-0177(199704)208:4<454::AID-AJA2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Prescott J, Blok L, Tindall D. Isolation and androgen regulation of the human homeobox cDNA, NKX3.1. Prostate. 1998;35:71–80. doi: 10.1002/(sici)1097-0045(19980401)35:1<71::aid-pros10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Price D. Normal development of the prostate and seminal vesicles of the rat with a study of experimental postnatal modifications. Am J Anat. 1936;60:79–127. [Google Scholar]
- Price D, Williams-Ashman HG. The accessory reproductive glands of mammals. 3rd edition. Baltimore: Williams & Wilkens Co.; 1961. pp. 366–448. [Google Scholar]
- Prins GS. Development of the prostate. In: Haseltine F, Paulsen C, Wang C, editors. Reproductive issues and the aging male. New York: Embryonic Inc.; 1993. pp. 101–112. [Google Scholar]
- Prins GS, Birch L. The developmental pattern of androgen receptor expression in rat prostate lobes is altered after neonatal exposure to estrogen. Endocrinology. 1995;136:1303–1314. doi: 10.1210/endo.136.3.7867585. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L. Neonatal estrogen exposure upregulates estrogen receptor expression in the developing and adult rat prostate lobes. Endocrinology. 1997;138:1801–1809. doi: 10.1210/endo.138.5.5106. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L, Habermann H, Chang WY, Tebeau C, Putz O, Bieberich C. Influence of neonatal estrogens on rat prostate development. Reprod Fertil Dev. 2001;13:241–252. doi: 10.1071/rd00107. [DOI] [PubMed] [Google Scholar]
- Prins GS, Chang WY, Wang Y, van Breemen RB. Retinoic acid receptors and retinoids are up-regulated in the developing and adult rat prostate by neonatal estrogen exposure. Endocrinology. 2002;143:3628–3640. doi: 10.1210/en.2002-220184. [DOI] [PubMed] [Google Scholar]
- Prins GS, Cooke PS, Birch L, Donjacour AA, Yalcinkaya TM, Siiteri PK, Cunha GR. Androgen receptor expression and 5a-reductase activity along the proximal-distal axis of the rat prostatic duct. Endocrinology. 1992;130:3066–3073. doi: 10.1210/endo.130.5.1572313. [DOI] [PubMed] [Google Scholar]
- Prins GS, Huang L, Birch L, Pu Y. The role of estrogens in normal and abnormal development of the prostate gland. Ann NY Acad Sci. 2006;1089:1–13. doi: 10.1196/annals.1386.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Korach KS. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids. 2008;73:233–244. doi: 10.1016/j.steroids.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Marmer M, Woodham C, Chang WY, Kuiper G, Gustafsson JA, Birch L. Estrogen receptor-β messenger ribonucleic acid ontogeny in the prostate of normal and neonatally estrogenized rats. Endocrinology. 1998;139:874–883. doi: 10.1210/endo.139.3.5827. [DOI] [PubMed] [Google Scholar]
- Pu Y, Deng L, Davies PJP, Prins GS. Retinoic acid metabolizing enzymes, binding proteins and RXRs are differentially expressed in the developing and adult rat prostate lobes and are altered by neonatal estrogens in a lobe-specific manner. The Endocrine Society’s 85th Annual Meeting; of Conference; Philadelphia, PA. 2003. p. 530. Abstract # P3-236. [Google Scholar]
- Pu Y, Huang L, Birch L, Prins GS. Androgen regulation of prostate morphoregulatory gene expression: Fgf10-dependent and -independent pathways. Endocrinology. 2007;148:1697–1706. doi: 10.1210/en.2006-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Y, Huang L, Prins GS. Sonic hedgehog-patchedgli signaling in the developing rat prostate gland: lobe-specific suppression by neonatal estrogens reduces ductal growth and branching. Dev Biol. 2004;273:257–275. doi: 10.1016/j.ydbio.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman R, Venkataraman G, Ernst S, Sasisekharan V, Sasisekharan R. Structural specificty of heparin binding growth factor family of proteins. Proc Natl Acad Sci. 2003;100:2357–2362. doi: 10.1073/pnas.0437842100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavolino PJ, Abrams EW, Yang L, Austenberg LP, Shen MM, Abate-Shen C. Tissue-specific expression of murine Nkx3.1 in the male urogenital system. Dev Dyn. 1997;209:127–138. doi: 10.1002/(SICI)1097-0177(199705)209:1<127::AID-AJA12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Schneider A, Brand T, Zweigerdt R, Arnold H. Targeted disruption of the Nkx3.1 gene in mice results in morphogenetic defects of minor salivary glands: parallels to glandular duct morphogenesis in prostate. Mech Dev. 2000;95:163–174. doi: 10.1016/s0925-4773(00)00355-5. [DOI] [PubMed] [Google Scholar]
- Shabsigh A, Tanji N, D’Agati V, Burchardt T, Burchardt M, Hayek O, Shabsigh R, Buttyan R. Vascular anatomy of the rat ventral prostate. Anat Rec. 1999;256:403–411. doi: 10.1002/(SICI)1097-0185(19991201)256:4<403::AID-AR7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Cunha GR. Autoradiographic localization of androgen binding in the developing mouse prostate. Prostate. 1983;4:367–373. doi: 10.1002/pros.2990040406. [DOI] [PubMed] [Google Scholar]
- Shaw A, Bushman W. Hedgehog signaling in the prostate. J Urol. 2007;177:832–838. doi: 10.1016/j.juro.2006.10.061. [DOI] [PubMed] [Google Scholar]
- Shen MM, Abate-Shen C. Roles of the Nkx3.1 homeobox genes in prostate organogenesis and carcinogenesis. Dev Dyn. 2003;228:767–778. doi: 10.1002/dvdy.10397. [DOI] [PubMed] [Google Scholar]
- Shou J, Ross S, Koeppen H, de Sauvage FJ, Gao WQ. Dynamics of notch expression during murine prostate development and tumorigenesis. Cancer Res. 2001;61:7291–7297. [PubMed] [Google Scholar]
- Simanainen U, Allan CM, Lim P, McPherson S, Jimenez M, Zajac JD, Davey RA, Handelsman DJ. Disruption of prostate epithelial androgen receptor impedes prostate lobe-specific growth and function. Endocrinology. 2007;148:2264–2272. doi: 10.1210/en.2006-1223. [DOI] [PubMed] [Google Scholar]
- Sitteri PK, Wilson JD. Testosterone formation and metabolism during male sexual differentiation in the human embryo. J Clin Endocrinol Metab. 1974;38:113–125. doi: 10.1210/jcem-38-1-113. [DOI] [PubMed] [Google Scholar]
- Sreenath T, Orosz A, Fujita K, Bieberich CJ. Androgen-independent expression of hoxb-13 in the mouse prostate. Prostate. 1999;41:203–207. doi: 10.1002/(sici)1097-0045(19991101)41:3<203::aid-pros8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol Reprod. 1986;34:961–971. doi: 10.1095/biolreprod34.5.961. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Hamada J, Murakawa K, Takada M, Tada M, Nogami I, Hayashi N, Nakamori S, Monden M, Miyamoto M, Katoh H, Moriuchi T. Expression profiles of 39 HOX genes in normal human adult organs and anaplastic thyroid cancer cell lines by quantitative real-time RT-PCR system. Exp Cell Res. 2004;293:144–153. doi: 10.1016/j.yexcr.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Takeda H, Lasnitzki I, Mizuno T. Analysis of prostatic bud induction by brief androgen treatment in the fetal rat urogenital sinus. J Endocrinol. 1986;110:467–470. doi: 10.1677/joe.0.1100467. [DOI] [PubMed] [Google Scholar]
- Takeda H, Mizuno T, Lasnitzki I. Autoradiographic studies of androgen-binding sites in the rat urogenital sinus and postnatal prostate. J Endocrinol. 1985;104:87–92. doi: 10.1677/joe.0.1040087. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Komuro I, Inagaki H, Jenkins NA, Copeland NG, Izumo S. Nkx3.1, a murine homolog of Ddrosophila bagpipe, regulates epithelial ductal branching and proliferation of the prostate and palatine glands. Dev Dyn. 2000;219:248–260. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1054>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- Tanji N, Tsuji M, Terada N, Takeuchi M, Cunha G. Inhibitory effects of transforming growth factor-β 1 on androgen-induced development of neonatal mouse seminal vesicles in vitro. Endocrinology. 1994;134:1155–1162. doi: 10.1210/endo.134.3.8119154. [DOI] [PubMed] [Google Scholar]
- Thomson AA. Role of androgens and fibroblast growth factors in prostatic development. Reproduction. 2001;121:187–195. doi: 10.1530/rep.0.1210187. [DOI] [PubMed] [Google Scholar]
- Thomson AA, Cunha GR. Prostatic growth and development are regulated by FGF10. Development. 1999;126:3693–3701. doi: 10.1242/dev.126.16.3693. [DOI] [PubMed] [Google Scholar]
- Timms BG, Mohs TJ, Didio LJ. Ductal budding and branching patterns in the developing prostate. J Urol. 1994;151:1427–1432. doi: 10.1016/s0022-5347(17)35273-4. [DOI] [PubMed] [Google Scholar]
- Timme TL, Truong LD, Merz VW, Krebs T, Kadmon D, Flanders KC, Park SH, Thompson TC. Mesenchymal-epithelial interactions and transforming growth factor-βexpression during mouse prostate morphogenesis. Endocrinology. 1994;134:1039–1045. doi: 10.1210/endo.134.3.8119140. [DOI] [PubMed] [Google Scholar]
- Tomlinson DC, Freestone SH, Grace OC, Thomson AA. Differential effects of transforming growth factor-β1 on cellular proliferation in the developing prostate. Endocrinology. 2004a;145:4292–4300. doi: 10.1210/en.2004-0526. [DOI] [PubMed] [Google Scholar]
- Tomlinson DC, Gindley JC, Thomson AA. Regulation of Fgf10 gene expression in the prostate: identification of transforming growth factor-β1 and promoter elements. Endocrinology. 2004b;145:1988–1995. doi: 10.1210/en.2003-0842. [DOI] [PubMed] [Google Scholar]
- Uematsu F, Kan M, Wang F, Jang JH, Luo Y, McKeehan WL. Ligand binding properties of binary complexes of heparin and immunoglobulin-like modules of FGF receptor 2. Biochem Biophys Res Commun. 2000;272:830–836. doi: 10.1006/bbrc.2000.2872. [DOI] [PubMed] [Google Scholar]
- Vanpoucke G, Orr B, Grace OC, Chan R, Ashley GR, Williams K, Franco OE, Hayward SW, Thomson AA. Transcriptional profiling of inductive mesenchyme to identify molecules involved in prostate development and disease. Genome Biol. 2008;8:R213.1–R213.14. doi: 10.1186/gb-2007-8-10-r213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterhouse D, Yoon J, Iannaccone P. Developmental pathways: sonic hedgehog-patched-GLI. Envir Health Persp. 1999;107:167–171. doi: 10.1289/ehp.99107167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltregny D, Alami Y, Clausse N, de Leval J, Castronovo V. Overexpression of the homeobox gene HOXC8 in human prostate cancer correlates with loss of tumor differentiation. Prostate. 2002;50:162–169. doi: 10.1002/pros.10045. [DOI] [PubMed] [Google Scholar]
- Wang B, Shou J, Ross S, Koeppen H, de Sauvage FJ, Gao W-Q. Inhibition of epithelial ductal branching in the prostate by sonic hedgehog is indirectly mediated by stromal cells. J Biol Chem. 2003;278:18506–18513. doi: 10.1074/jbc.M300968200. [DOI] [PubMed] [Google Scholar]
- Wang J, Thompson B, Ren C, Ittmann M, Kwabi-Addo B. Sprouty4, a suppressor of tumor cell motility, is down regulated by DNA methylation in human prostate cancer. Prostate. 2006a;66:613–624. doi: 10.1002/pros.20353. [DOI] [PubMed] [Google Scholar]
- Wang XD, Leow CC, Zha J, Tang Z, Modrusan Z, Radtke F, Aguet M, de Sauvage FJ, Gao WQ. Notch signaling is required for normal prostatic epithelial cell proliferation and differentiation. Dev Biol. 2006b;290:66–80. doi: 10.1016/j.ydbio.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Wang XD, Shou J, Wong P, French DM, Gao WQ. Notch1-expressing cells are indispensable for prostatic branching morphogenesis during development and re-growth following castration and androgen replacement. J Biol Chem. 2004;279:24733–24744. doi: 10.1074/jbc.M401602200. [DOI] [PubMed] [Google Scholar]
- Warot X, Fromental-Ramain C, Fraulob V, Chambon P, Dolle P. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development. 1997;124:4781–4791. doi: 10.1242/dev.124.23.4781. [DOI] [PubMed] [Google Scholar]
- Welt CK, Crowley WF. Activin: an endocrine or paracrine agent? Eur J Endocrinol. 1998;139:469–471. doi: 10.1530/eje.0.1390469. [DOI] [PubMed] [Google Scholar]
- Wilson J, Gloyna R. The intranuclear metabolism of testosterone in the accessory organs of reproduction. Rec Prog Horm Res. 1970;26:309–336. doi: 10.1016/b978-0-12-571126-5.50012-1. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Lasnitzki I. Dihydrotestsoterone formation in the fetal tissue of the rabbit and rat. Endocrinology. 1971;89:659. doi: 10.1210/endo-89-3-659. [DOI] [PubMed] [Google Scholar]
- Yardy GW, Brewster SF. Wnt signalling and prostate cancer. Prostate Cancer Prostatic Dis. 2005;8:119–126. doi: 10.1038/sj.pcan.4500794. [DOI] [PubMed] [Google Scholar]
- Zhang TJ, Hoffman BG, Ruiz de Algara T, Helgason CD. SAGE reveals expression of Wnt signalling pathway members during mouse prostate development. Gene Expr Patterns. 2006;6:310–324. doi: 10.1016/j.modgep.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Zhu G, Zhau HE, He H, Zhang L, Shehata B, Wang X, Cerwinka WH, Elmore J, He D. Sonic and desert hedgehog signaling in human fetal prostate development. Prostate. 2007;67:6. doi: 10.1002/pros.20563. [DOI] [PubMed] [Google Scholar]