Abstract
Nitric oxide (NO) containing (nitrergic) interneurons are well-positioned to convey the cortical influences that are crucial for multisensory integration in superior colliculus (SC) output neurons. However, it is not known whether nitrergic interneurons are in this position early in life, and might, therefore, also play a role in the functional maturation of this circuit. In the present study, we investigated the postnatal developmental relationship between these two populations of neurons using B-nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH) histochemistry and SMI-32 immunocytochemistry to label presumptive interneurons and output neurons, respectively. SMI-32 immunostained neurons were proved to mature and retained immature anatomical features until approximately 8 postnatal weeks. In contrast, nitrergic interneurons developed more rapidly. They had achieved their adult-like anatomy by 4 postnatal weeks and were in a position to influence the dendritic elaboration of output neurons. It is this dendritic substrate through which much of the cortico-collicular influence is expressed. Double-labeling experiments showed that the dendritic and axonal processes of nitrergic interneurons already apposed the somata and dendrites of SMI-32 labeled neurons even at the earliest age examined. The results suggest that nitrergic interneurons play a role in refining the cortico-collicular projection patterns that are believed to be essential for SC output neurons to engage in multisensory integration and to support normal orientation responses to cross-modal stimuli.
Keywords: Interneurons, midbrain, cortex, nitric oxide, gaze shifts, orientation
1. Introduction
Multisensory neurons in the cat superior colliculus (SC) not only have the ability to respond to cues from different senses but also to integrate this information and thereby markedly alter their sensitivity to external events. This integrative capacity is of particular value in the initiation and control of orientation to these events (Stein et al., 2004). However, such neurons are not evident in the SC of the newborn animal. Rather, at this time of life, all sensory-responsive SC neurons are unisensory (Stein et al., 1973). Multisensory neurons begin appearing in the SC after the first week of postnatal maturation and increase in frequency thereafter (Stein et al., 1973, Wallace and Stein 1997). Yet these neonatal multisensory neurons differ substantially from those found in the adult. They are not yet able to synthesize cross-modal information to produce an increased response. This integrative capacity appears in some neurons at about 1 month of age, and the incidence of such neurons increases gradually over the next several months, as animals gain experience with cross-modal cues. It is during this time that their inputs from the anterior ectosylvian cortex (AES) become functionally mature (Wallace and Stein 2000; Wallace et al., 2004). These AES inputs, which are essential for SC neurons to engage in multisensory integration (Wallace and Stein, 1994; Jiang et al., 2001; 2002; 2007; Alvarado et al., 2007), are thought to make both monosynaptic and polysynaptic connections with the large, multisensory SC output neurons (Wallace et al., 1993, Meredith et al., 1992; Fuentes-Santamaria et al., 2007). Recently, we have provided anatomical evidence that a crucial component of this cortico-collicular circuit is polysynaptic and that it involves nitric oxide (NO) containing (nitrergic) interneurons (Fuentes-Santamaria et al., 2007).
NO has previously been implicated in the maturational refinement of unisensory visual processes in the superficial layers of the SC (Mize et al., 1996; Cork et al., 2000; Scheiner et al., 2001; Mendez-Otero et al., 2007). As a diffusible gas, NO can spread from the release site to exert both synaptic and nonsynaptic effects, and it can have especially potent effects on immature neural processes in the surrounding region (Kiss and Vizi, 2001; Vizi et al, 2004). It is not known, however, what, if any role NO plays in the maturation of multisensory SC processes, which are restricted to the deep layers of this structure and primarily involve its large output neurons (Stein and Meredith 1993). Nevertheless, the strategic interposition of nitrergic interneurons (and patches of nitrergic fibers) in the AES-SC circuit places it in a particularly effective location to play a similar role in the maturation of deep layer multisensory processes as it does in the maturation of visual processes in superficial layers. Thus, the present study examines the presence and distribution of neuroanatomical markers for nitrergic interneurons and output neurons in the deep SC during the period in which multisensory neurons are developing their functional integrative capabilities. Of particular interest was determining the spatial relationship between these two neuron populations. In order to do so, the large deep layer neurons that send their descending axons to the brainstem and spinal cord (Moschovakis and Karabelas, 1985; Munoz and Guiton, 1991; Guitton and Munoz, 1991; Meredith et al., 2001; Fuentes-Santamaria et al., 2006) were labeled using the neurofilament protein SMI-32. This protein is preferentially expressed in SC output neurons and facilitates detailing their somatic and dendritic morphology (Fuentes-Santamaria et al., 2006). Presumptive nitrergic interneurons were labeled by using either B-nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH) or nitric oxide synthase (NOS).
2. Results
2.1. SMI-32 immunostained output neurons
SMI-32 immunostained neurons were already apparent in the deep SC at 4 postnatal weeks, a time when multisensory integration is just beginning to appear in its constituent neurons (Wallace and Stein, 1997). However, these immunostained neurons were sparsely distributed and concentrated mainly within the SGI and less frequently within the SGP (Figure 1A). SMI-32 immunostaining was observed within the soma as well as proximal dendrites (yellow arrowheads in Figure 1B) and axons (white arrowhead in Figure 1A). Stained secondary dendrites were comparatively sparse and, when present, were truncated so that neurons appeared to have impoverished dendritic fields.
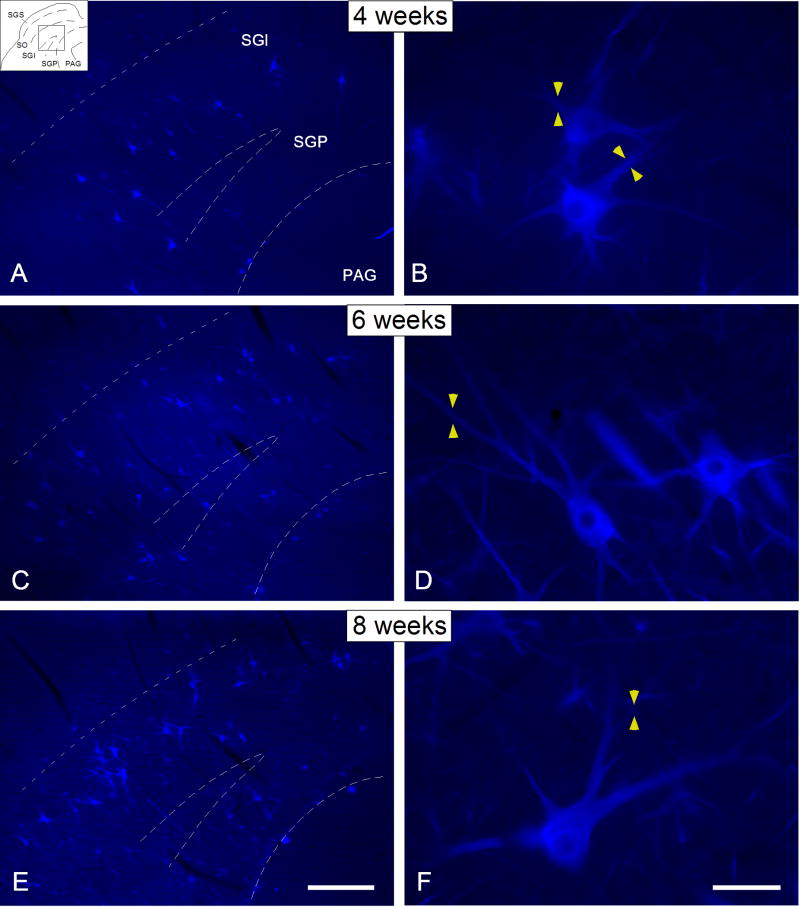
Figure 1.
Digital images illustrating the distribution of SMI-32 immunostaining in the deep SC at 4 (A, B), 6 (C, D) and 8 (E, F) postnatal weeks. SMI-32 immunostained neurons were already apparent in the deep SC at 4 postnatal weeks, although they were sparsely distributed and concentrated mainly within the SGI (Figure 1A). Note that at this age immunostaining was observed within the cell body as well as proximal dendrites (yellow arrowheads in Figure 1B). By 6 postnatal weeks, their distribution extended further into the SGP, and their soma size and dendritic arbors increased in complexity (C, D), reaching a mature appearance by 8 postnatal weeks. Drawing at the top left corner in (A) indicates the location of the tissue magnified in A, C and E. Dashed lines indicate the approximate borders between layers. Scale bar for A, C and E = 250μm; for B, D and F= 25μm.
At 6 postnatal weeks, a similar distribution of SMI-32 immunostaining was noted, albeit with an increase in the density of labeled neurons (Figure 1C). At this time, immunostained neurons were more widely distributed within the SGI and SGP, and had more complex dendritic arbors. Their secondary dendrites were now clearly visible and more elongated (yellow arrowheads in Figure 1D), but tertiary dendrites remained comparatively rare or absent. At the latest age examined (8 weeks), output neurons presented a distribution and morphology very similar to that found in the adult (Figure 1E-F) with large somata and clearly delineated tertiary dendrites (Figure 1E; yellow arrowheads in 1F). Some immunostained neurons were characterized by wide dendritic fields that were either oriented dorsally or radially (Figure 1E, F).
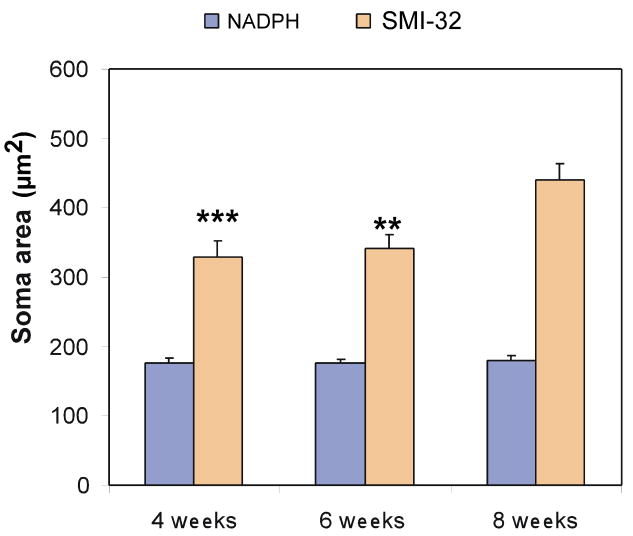
The distribution of soma sizes of SMI-32 immunostained output neurons is shown in Figure 2. Notice that by 4 postnatal weeks, neurons were still relatively small (328.56 ± 22.69μm2; Figure 2). Their mean somatic area increased only slightly at 6 postnatal weeks (341.32 ± 20.08μm2), and reached adult-like levels at 8 postnatal weeks 440.27 ± 23.36μm2) (Figure 2). ANOVA analyses demonstrated a significant effect of age over soma area (F (2, 95) =7.76, p<0.001). Duncan's post-hoc analysis revealed that the soma areas of SMI-32 immunostained output neurons at 8 postnatal weeks were significantly larger that that at either 4 (p<0.001) or 6 (p<0.01) postnatal weeks (there were no differences between 4 and 6 postnatal weeks).
Figure 2.
Bar graphs showing the distribution of soma areas of SMI-32 immunostained neurons and NADPH-stained neurons at 4, 6 and 8 postnatal weeks. Note that the soma areas (μm2) of SMI-32 immunostained output neurons at 8 postnatal weeks are significantly larger than at either 4 (p<0.001) or 6 (p<0.01) postnatal weeks. No differences, however, were found when comparing the soma areas of SMI-32 immunostained output neurons at 4 and 6 postnatal weeks. Conversely, when the soma areas of nitrergic interneurons were examined, no significant differences were observed when comparing the soma areas at any age studied (F (2, 137) =0.98, p>0.05). ** p<0.01; ***p<0.001.
2.2. Distribution of SMI-32 immunostained output neurons relative to NADPH-staining
NADPH histochemistry was used to label intrinsic nitrergic interneurons and their processes as well as afferent nitrergic fibers as described in Methods (see also Fuentes-Santamaria et al., 2007). As previously shown in adults and kittens (Scheiner et al., 2000, 2001; Fuentes-Santamaria et al., 2007), this histochemical marker stains deep layer intrinsic nitrergic neurons with various morphologies. In accordance with a previous study in kittens (Scheiner et al., 2001), nitrergic neurons were present at 4 postnatal weeks of age, when multisensory integration is just appearing in deep layer neurons. Both bipolar and multipolar nitrergic neurons were apparent. Bipolar neurons had dendritic arbors that were oriented either vertically (Figure 3A) or horizontally (Figure 3B), whereas multipolar neurons had characteristic radially-directed arbors (Figure 3C). These nitrergic interneurons showed no substantial morphological changes at later stages (Figure 3D-F and 3G-I for 6 and 8 weeks, respectively) indicating that, in contrast to output neurons, they were adult-like in size and morphology (Fuentes-Santamaria et al., 2007) at, or before, 4 postnatal weeks of age. Similar to the SMI-32 immunostained output neurons, the soma areas of nitrergic interneurons also were evaluated at all the ages studied (Figure 2). ANOVA analysis revealed no significant differences when comparing the soma areas of nitrergic neurons at any age studied (F (2, 137) =0.98, p>0.05).
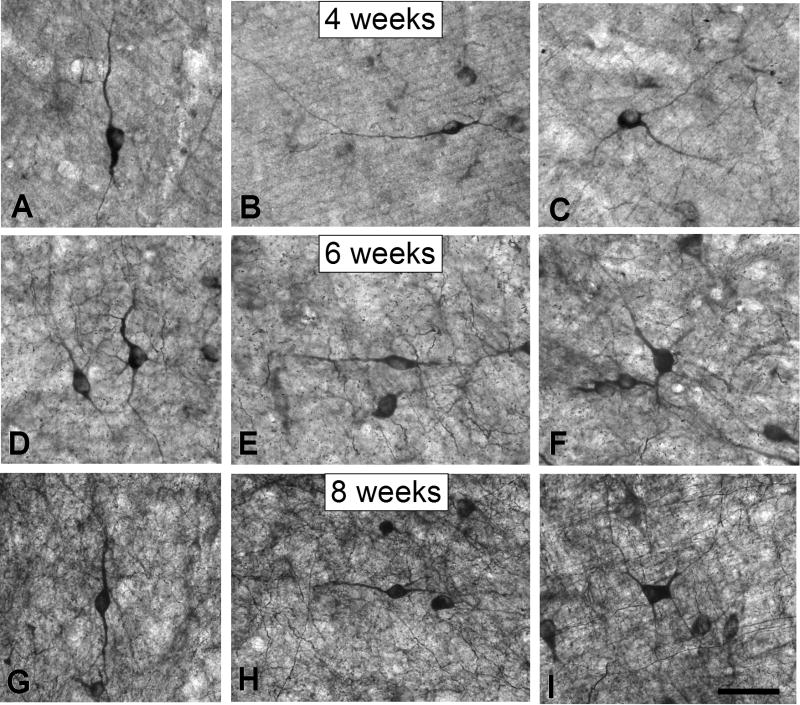
Figure 3.
Digital images show nitrergic neurons in the deep SC at 4 (A-C), 6 (D-F) and 8 (G-I) postnatal weeks. At 4 postnatal weeks, both bipolar and multipolar nitrergic neurons were frequently observed in the SGI and SGP (A-C). Bipolar neurons had dendritic arbors that were oriented either vertically (Figure 2A) or horizontally (Figure 2B), and multipolar neurons had characteristic radially-oriented dendritic arbors (Figure 2C). Note that at the later ages, developing nitrergic neurons presented a similar morphology (D-I) indicating that, in contrast to output neurons, they had already achieved their mature size at or before 4 postnatal weeks of age. Scale bar = 50 μm.
NADPH-staining also was contained within fibers in the SC, although the source and direction of these fibers could not be ascertained with the methods used. These stained fibers were especially evident at midcollicular levels. At 4 postnatal weeks, NADPH-stained fibers in the deep SC were apparent ventrally (arrowheads in Figure 4A) but not dorsally. NADPH-stained fibers were virtually absent from SGI, and only NADPH-stained neurons were observed. The stained fibers in SGP were well-organized, forming a mediolateral band (Figure 4A). A few SMI-32 immunostained neurons were distributed in the ventromedial aspect of this band. At 6 and 8 postnatal weeks, the patches of NADPH-stained fibers (arrowheads in Figure 4B, C; see also asterisk in 4E, G) displayed a mature configuration, appearing as a series of periodic patches that distributed across the mediolateral extent of SGI and SGP. Some of patches in the SGI were interconnected with those in the SGP and PAG via vertically-oriented bundles of stained fibers (Figure 4B, C). This organization resembles that observed in the adult cat (Scheiner et al., 2000). Fibers in these patchy areas displayed both en passant and terminal boutons (data not shown). At 6 and 8 postnatal weeks, but not at 4 postnatal weeks, medium-to-large SMI-32 immunostained neurons (arrows in Figure 4E, G) were located mainly outside these patches (yellow asterisks in Figure 4E, G).
Figure 4.
Digital images illustrate the distribution of output neurons (brown reaction product) relative to NADPH-stained elements (blue reaction product) in the deep SC at 4 (A, D), 6 (B, E) and 8 (C, G) postnatal weeks. At 4 postnatal weeks, NADPH-stained fibers in SGI were virtually absent (A), and only nitrergic interneurons were observed (white arrows in D). In the SGP, however, stained fibers formed a band along the dorsal border of the PAG (arrowheads in A). At 6 (B) and 8 (C) weeks, NADPH-stained fibers were organized as a series of periodic patches similar to that seen in the adult SC. These stained patchy areas (yellow asterisk in E and G) were surrounded by medium-to-large SMI-32 immunostained neurons (white arrows in E and G) and consisted of fibers that had both en passant and terminal boutons (E, G). Drawing at the top left corner in (A) indicates the location of the tissue magnified in A, B and C. Dashed lines in (A) indicate the approximate borders between layers, while the boxes in (A, B and C) show the location of the higher magnification images in D, E and G, respectively. Scale bar for A, B and C= 250μm; for D, E and G= 100μm.
2.3. SC output neurons receive appositions from small nitrergic interneurons early in life
Double-labeling techniques were used to examine the relationship between nitrergic dendrites and nitrergic axonal arbors with the somata and dendrites of SMI-32 immunostained output neurons. At 4 postnatal weeks, NADPH-stained neurons could be seen lying within the dendritic trees of the SMI-32 immunostained neurons (Figure 5A, B). Their dendrites in some cases overlapped, exhibiting close appositions (white arrowheads in Figure 5A, B). In addition, NADPH-stained axons also were present and displayed close associations with the dendrites and somata of SMI-32 immunostained neurons (Fig. 4B, yellow arrowheads). At 6 postnatal weeks (Figure 5C), dendrodendritic relationships were still apparent, but the incidence of close associations between axonal boutons and SMI-32 immunostained neurons showed an increase in parallel with the overall increase in these NADPH-stained fibers. This trend continued, so that by 8 postnatal weeks (Figure 5D) the level of axo-dendritic interaction (yellow arrowheads) had increased to that observed previously in the adult cat SC (Fuentes-Santamaria et al., 2007).
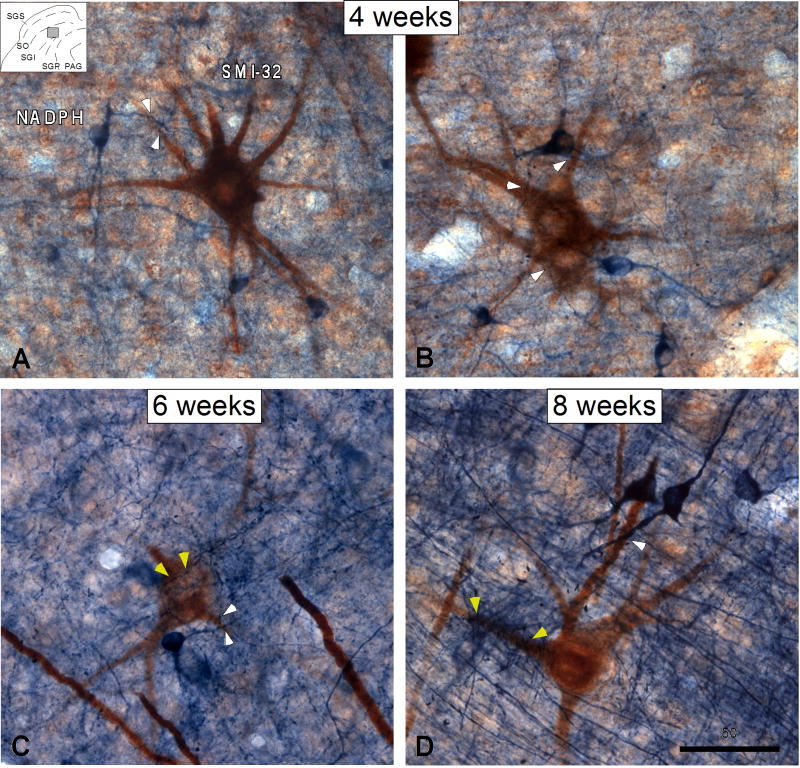
Figure 5.
Digital images show the relationship between NADPH-stained interneurons and SMI-32 immunostained output neurons in the deep SC at 4 (A, B), 6 (C) and 8 (D) postnatal weeks. At all the ages studied, interneuron dendrites often apposed the somata and dendrites of SMI-32 immunostained output neurons (white arrowheads in A-D). Note however, that similar to that in the adult SC, SMI-32 immunostained neurons at 6 and 8 postnatal weeks received numerous appositions from numerous NADPH-stained axons (yellow arrowheads in C-D). Drawing at the top left corner in (A) indicates the approximate location of the tissue magnified in A, B and C. Scale bar = 50μm.
In short, the relationship between NADPH-stained interneurons and SMI-32-stained output neurons is already established at 4 postnatal weeks. Developmental changes during the ensuing 4 weeks are due primarily to increases in the size of the output neuron's dendritic field and the in growth of extrinsic NADPH-stained projections.
3. Discussion
The present study reveals that at 4 postnatal weeks, the laminar distribution of SMI-32 immunostained neurons is restricted largely to the SGI, with soma sizes and dendritic configurations that are comparatively immature. By 6 postnatal weeks, however, their distribution extended further into the SGP, with soma size increasing and dendritic arborization becoming increasingly complex. These changes continue until an adult-like appearance is achieved by 8 postnatal weeks. By contrast, intrinsic nitrergic interneurons have soma sizes and dendritic arbors that are already adult-like by 4 postnatal weeks. However, the density of the NADPH-stained neuropil does not reach its peak intensity until approximately 6 postnatal weeks, a time when the labeled fibers clearly displayed the characteristic patch-like distribution of the adult. This mismatch in the maturation of intrinsic nitrergic neurons and nitrergic fibers suggests the latter are composed primarily of afferents from external nitrergic sources (Beninato and Spencer 1986; Scheiner et al 2000).
Previously, it was shown that fibers from AES cortex often terminate on nitrergic interneurons, whose dendritic and axonal processes, in turn, appose the somata and dendrites of presumptive SC output neurons. AES fibers also directly contact output neurons, albeit to a lesser extent (Fuentes-Santamaria et al., 2007). The present double-labeling observations indicated that this intimate relationship between nitrergic interneurons and SC output neurons is established by 4 postnatal weeks. Thus, not only are the numerous appositions between the dendritic processes of nitrergic interneurons and SMI-32 immunostained elements present at this development time point but also the axonal processes from these interneurons have established appositions with these same output neurons. Preliminary data on the postnatal development of AES cortico-collicular fibers indicate that these have already established contacts with nitrergic interneurons by 4 weeks of age. Taken together, these developmental observations suggest that at, or before, 4 postnatal weeks, nitrergic interneurons are in a position to exert considerable influence over the cortical modulation of SC multisensory neuron properties. The postnatal expression of nitrergic interneurons described here is agreement with a previous study in the kitten describing the development of NO expression in the SC (Scheiner et al., 2001). It is important to emphasize that the presence of “contacts” or “appositions” does not unequivocally imply a synapse. Nevertheless, electron microscopic studies indicate that in more than half of the cases appositions are indeed real synapses (Pilowsky et al., 1992).
Four weeks of age is an important landmark in the maturation of cat SC multisensory integration. Electrophysiological studies have demonstrated that although multisensory neurons are present as early as 2 postnatal weeks, they are immature and incapable of integrating information from different senses until at least two weeks later (Stein et al., 1973; Wallace and Stein, 1997). Although this time point marks the first appearance of some neurons capable of multisensory integration, the population requires approximately 4 additional weeks to achieve its adult-like functional condition (Wallace and Stein, 1997).
The present results indicate that it is not until approximately 8 postnatal weeks that output neurons have nearly completed their growth so that their morphology and laminar distribution resembles that of the adult cat (Fuentes-Santamaria et al., 2006). A similar pattern is found in the dorsal lateral geniculate (dLGN) where putative Y-cells, which also are selectively labeled by SMI-32 (Bickford et al 1998), appear to reach maturity at about this time (Carden et al., 2000a, 2000b). As with the SC, the majority of intrinsic interneuron growth appears to be complete by 4 postnatal weeks (Carden et al., 2000b), suggesting that interneurons may begin influencing output neurons even before these target neurons reach maturity.
Prior studies have demonstrated that influences from the AES are essential for multisensory SC neurons to integrate the information derived from cross-modal stimuli (Wallace and Stein, 1994; Jiang et al., 2001; 2002; Alvarado et al., 2007) in order to control multisensory orientation behavior mediated by the SC (Wilkinson et al., 1996; Jiang et al., 2002; Burnett et al., 2004). Although cortical influences onto output neurons in the adult SC traditionally have been considered to be conveyed via direct monosynaptic inputs (Wallace and Stein, 1993), a disynaptic AES-SC circuit involving nitrergic interneurons recently has been described (Fuentes-Santamaria et al., 2007). Descending cortico-SC afferents terminate on nitrergic interneurons, the dendritic and axonal processes of which appose presumptive SC output neuron labeled with SMI-32. Many of these nitrergic interneurons also coexpress the inhibitory neurotransmitter GABA. This anatomical configuration suggests that nitrergic interneurons could, therefore, convey cortical influences onto SC output neurons disynaptically via nitrergic mechanisms but also by conventional neurotransmitter systems utilizing GABA and presumably other, possibly excitatory, neurotransmitters (Fuentes-Santamaria et al., 2007). In addition, because NO also acts as a retrograde messenger, cortically-mediated NO release from the nitrergic interneurons could influence presynaptic cortico-SC terminals that directly contact output neurons. Given the complexity of this intrinsic SC circuitry, and its relationship with descending cortico-SC afferents, it seems likely, that cortical activation of these early developing nitrergic interneurons during postnatal maturation will have a significant role in crafting the development of multisensory integrative processes that characterize the adult cat SC.
4. Experimental Procedures
4.1. Experimental animals
Data were obtained from 4 (n=3), 6 (n=3) and 8-week-old (n=3) cats. All animal husbandry and experimental procedures were performed in compliance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” (NIH Publications No. 80-23, revised 1996) in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC). Efforts were made to minimize the animals' discomfort and the number of animals used.
4.2. SMI-32 immunocytochemistry
Animals were sedated with ketamine hydrochloride (30 mg/kg, im) and acepromazine maleate (0.05-0.1 mg/kg, im). They then were anesthetized with sodium pentobarbital (100 mg/kg, ip) and perfused transcardially with a 0.9% saline wash followed by a fixative solution of 4% paraformadehyde in 0.1 M phosphate buffer (PB), pH 7.3. The brains were removed, and the brainstem was sectioned at 50μm on a Vibratome in the coronal plane. Sections were rinsed several times in phosphate-buffered saline (PBS) containing 0.2% Triton X-100 (Tx) and, in order to reduce nonspecific binding, were blocked for 1 hour in PBS-Tx (0.2%) containing 10% normal goat serum (NGS). Sections then were incubated overnight at 4° C with neuronal SMI-32 antibody (1: 2500 mouse anti-SMI-32, Sternberger Monoclonals Inc., Jarrettsville, MD) in a solution containing PBS-Tx (0.2%), pH 7.4. After four 15 min rinses in PBS-Tx (0.2%), sections were incubated in anti-mouse conjugated to Alexa 350 (1:200; Molecular Probes, Eugene, OR) for 2 hours at room temperature (RT). Finally, the sections were rinsed in PBS, mounted, coverslipped, and maintained in the refrigerator at 4°C.
4.3. NADPH-d histochemistry and SMI-32 immunocytochemistry: double-labeling
For NADPH-d histochemistry, SC sections were rinsed several times in Tris buffer (pH 7.1) and then incubated in a solution containing 50 ml Tris buffer, 12.5 mg nitroblue tetrazolium, 50 mg NADPH and 0.3% Triton-X at 37°C for 2 hours. For SMI-32 immunocytochemistry, sections were pre-incubated for 1 hour in 10 % NGS and then incubated in SMI-32 monoclonal antibody diluted in PBS-Tx (0.2%) overnight at 4°C. The following day, after several rinses in PBS-Tx (0.2%), sections were incubated in a dilution of biotinylated anti-mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA) for 2 hours at RT. After washing in PBS-Tx (0.2%), the sections were incubated in abidin-biotin peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories) for 1 hour and then reacted with DAB and hydrogen peroxide to produce a brown reaction product.
4.4. Nomenclature
Adjacent cresyl violet stained sections were used to determine laminar boundaries within the SC in accordance with previous terminology (Kanaseki and Sprague, 1974). Briefly, the SC is generally divided into superficial and deep laminae. The deeper layers (an amalgam of the intermediate and deep layers) include the stratum griseum intermediale (SGI), the stratum album intermedium (SAI), the stratum griseum profundum (SGP) and the stratum album profundum (SAP). SC neurons were described in accordance with the following criteria: 1) soma shape, 2) number of dendrites emerging from soma, and 3) dendritic orientation relative to the surface of the structure (Norita, 1980; Cork et al., 2000; Behan et al., 2002). Additionally, in the present study the term “contact or apposition” was considered only when the stained processes (either dendrite or axons) of interneurons and SMI-32 immunostained neuron were in focus in the same focal plane and there was no detectable gap between them (Pilowsky et al., 1992; El Manira et al., 1997; Makeham et al., 2001).
4.5. Measurements of the soma area
The soma area of SMI-32 immunostained neurons as well as NADPH-stained neurons were measured using the public domain image analysis software Scion Image for Windows. Fields of 2.97×104 μm2 were sampled using a 60× objective in every fourth section of the deep SC. Only neurons with a well-defined soma, nucleus and nucleolus were measured.
4.6. Data analysis
Sections were examined with an Olympus BX50 microscope equipped with the fluorescent filter U-MNU (DAPI, blue fluorescence). Images were captured with a Spot RT Slider. Photoshop (Adobe, San Jose, CA) and Canvas (ADC Systems, Victoria, BC, Canada) were used to adjust size, brightness and contrast of publication images. All the data were analyzed using Statistica for Windows, release 7.0 (StatSoft, Inc), and expressed as mean ± standard error with the statistical significance set at the p<0.05 level.
Acknowledgments
We thank N. London for editorial assistance. This work was supported by National Institutes of Health grants NS36916, NS35008, and EY016716; the Wallace Research Foundation; and the Siebert Neuroscience Endowment.
References
- Alvarado JC, Stanford TR, Vaughan JW, Stein BE. Cortex mediates multisensory but not unisensory integration in superior colliculus. J Neurosci. 2007;27(47):12775–12786. doi: 10.1523/JNEUROSCI.3524-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Steinhacker K, Jeffrey-Borger S, Meredith MA. Chemoarchitecture of GABAergic neurons in the ferret superior colliculus. J Comp Neurol. 2002;452(4):334–359. doi: 10.1002/cne.10378. [DOI] [PubMed] [Google Scholar]
- Beninato M, Spencer RF. A cholinergic projection to the rat superior colliculus demonstrated by retrograde transport of horseradish peroxidase and choline acetyltransferase immunohistochemistry. J Comp Neurol. 1986;253(4):525–538. doi: 10.1002/cne.902530409. [DOI] [PubMed] [Google Scholar]
- Bickford ME, Guido W, Godwin DW. Neurofilament proteins in Y-cells of the cat lateral geniculate nucleus: normal expression and alteration with visual deprivation. J Neurosci. 1998;18(16):6549–57. doi: 10.1523/JNEUROSCI.18-16-06549.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett LR, Stein BE, Chaponis D, Wallace MT. Superior colliculus lesions preferentially disrupt multisensory orientation. Neuroscience. 2004;124(3):535–47. doi: 10.1016/j.neuroscience.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Carden WB, Guido W, Ziburkus J, Datskovskaia A, Godwin DW, Bickford ME. A novel means of Y cell identification in the developing lateral geniculate nucleus of the cat. Neurosci Lett. 2000a;295(12):5–8. doi: 10.1016/s0304-3940(00)01581-0. [DOI] [PubMed] [Google Scholar]
- Carden WB, Datskovskaia A, Guido W, Godwin DW, Bickford ME. Development of the cholinergic, nitrergic, and GABAergic innervation of the cat dorsal lateral geniculate nucleus. J Comp Neurol. 2000;418(1):65–80. doi: 10.1002/(sici)1096-9861(20000228)418:1<65::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Chalupa LM, Snider CJ. Topographic specificity in the retinocollicular projection of the developing ferret: an anterograde tracing study. J Comp Neurol. 1998;392(1):35–47. doi: 10.1002/(sici)1096-9861(19980302)392:1<35::aid-cne3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Cork RJ, Calhoun T, Perrone M, Mize RR. Postnatal development of nitric oxide synthase expression in the mouse superior colliculus. J Comp Neurol. 2000;427(4):581–592. [PubMed] [Google Scholar]
- Fuentes-Santamaria V, Stein BE, McHaffie JG. Neurofilament proteins are preferentially expressed in descending output neurons f the cat the superior colliculus: a study using SMI-32. Neuroscience. 2006;138(1):55–68. doi: 10.1016/j.neuroscience.2005.11.045. [DOI] [PubMed] [Google Scholar]
- Fuentes-Santamaria V, Alvarado JC, Stein BE, McHaffie JG. Cortex Contacts both Output Neurons and Nitrergic Interneurons in the Superior Colliculus: Direct and Indirect Routes for Multisensory Integration Cereb. Cortex. 2007 doi: 10.1093/cercor/bhm192. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldi-Guimarães A, Batista CMC, Carneiro K, Tenório F, Cavalcante LA, Mendez-Otero R. A critical survey on nitric oxide synthase expression and nitric oxide function in the retinotectal system. Brain Res Rev. 2007;56(2):403–426. doi: 10.1016/j.brainresrev.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Guitton D, Munoz DP. Control of orienting gaze shifts by the tectoreticulospinal system in the head-free cat. I. Identification, localization, and effects of behavior on sensory responses. J Neurophysiol. 1991;66(5):1605–23. doi: 10.1152/jn.1991.66.5.1605. [DOI] [PubMed] [Google Scholar]
- Jiang W, Wallace MT, Jiang H, Vaughan JW, Stein BE. Cortical areas mediate multisensory integration in superior colliculus neurons. J Neurophysiol. 2001;85(2):506–522. doi: 10.1152/jn.2001.85.2.506. [DOI] [PubMed] [Google Scholar]
- Jiang W, Jiang H, Stein BE. Corticotectal areas facilitate multisensory orientation behavior. J Cogn Neurosci. 2002;14(8):1240–1255. doi: 10.1162/089892902760807230. [DOI] [PubMed] [Google Scholar]
- Jiang W, Jiang H, Rowland BA, Stein BE. Multisensory orientation behavior is disrupted by neonatal cortical ablation. J Neurophysiol. 2006;97(1):557–62. doi: 10.1152/jn.00591.2006. [DOI] [PubMed] [Google Scholar]
- Kanaseki T, Sprague JM. Anatomical organization of pretectal nuclei and tectal laminae in the cat. J Comp Neurol. 1974;158(3):319–337. doi: 10.1002/cne.901580307. [DOI] [PubMed] [Google Scholar]
- Kiss JP, Vizi ES. Nitric oxide: a novel link between synaptic and nonsynaptic transmission. Trends Neurosci. 2001;24(4):211–215. doi: 10.1016/s0166-2236(00)01745-8. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Wallace MT, Stein BE. Visual, auditory and somatosensory convergence in output neurons of the cat superior colliculus: multisensory properties of the tecto-reticulo-spinalprojection. Exp, Brain Res. 1992;88(1):181–186. doi: 10.1007/BF02259139. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Miller LK, Ramoa AS, Clemo HR, Behan M. Organization of the neurons of origin of the descending pathways from the ferret superior colliculus. Neurosci Res. 2001;40(4):301–13. doi: 10.1016/s0168-0102(01)00240-1. [DOI] [PubMed] [Google Scholar]
- Mize RR, Banfro FT, Scheiner CA. Pre- and postnatal expression of amino acid neurotransmitters, calcium binding proteins, and nitric oxide synthase in the developing superior colliculus. Prog Brain Res. 1996;108:313–332. doi: 10.1016/s0079-6123(08)62549-2. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Karabelas AB. Observations on the somatodendritic morphology and axonal trajectory of intracellularly HRP-labeled efferent neurons located in the deeper layers of the superior colliculus of the cat. J Comp Neurol. 1985;239(3):276–308. doi: 10.1002/cne.902390304. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Guitton D. Control of orienting gaze shifts by the tectoreticulospinal system in the head-free cat. II. Sustained discharges during motor preparation and fixation. J Neurophysiol. 1991;66(5):1624–1641. doi: 10.1152/jn.1991.66.5.1624. [DOI] [PubMed] [Google Scholar]
- Norita M. Neurons and synaptic patterns in the deep layers of the superior colliculus of the cat. A Golgi and electron microscopic study. J Comp Neurol. 1980;190(1):29–48. doi: 10.1002/cne.901900104. [DOI] [PubMed] [Google Scholar]
- Scheiner C, Arceneaux R, Guido W, Kratz K, Mize R. Nitric oxide synthase distribution in the cat superior colliculus and co-localization with choline acetyltransferase. J Chem Neuroanat. 2000;18(4):147–159. doi: 10.1016/s0891-0618(00)00037-5. [DOI] [PubMed] [Google Scholar]
- Scheiner CA, Kratz KE, Guido W, Mize RR. Prenatal and postnatal expression of nitric oxide in the developing kitten superior colliculus revealed with NADPH diaphorase histochemistry. Vis Neurosci. 2001;18(1):43–54. doi: 10.1017/s0952523801181046. [DOI] [PubMed] [Google Scholar]
- Stein BE, Labos E, Kruger L. Sequence of changes in properties of neurons of superior colliculus of the kitten during maturation. J Neurophysiol. 1973;36(4):667–79. doi: 10.1152/jn.1973.36.4.667. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. The Merging of the Senses. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- Stein BE, Jiang W, Stanford TR. The Handbook of Multisensory Processes. MIT Press; Cambridge, MA: 2004. Multisensory integration in single neurons of the midbrain; pp. 243–264. [Google Scholar]
- Vizi ES, Kiss JP, Lendvai B. Nonsynaptic communication in the central nervous system. Neurochem Int. 2004;45(4):443–451. doi: 10.1016/j.neuint.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Meredith MA, Stein BE. Converging influences from visual, auditory, and somatosensory cortices onto output neurons of the superior colliculus. J Neurophysiol. 1993;69(6):1797–1809. doi: 10.1152/jn.1993.69.6.1797. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Cross-modal synthesis in the midbrain depends on input from cortex. J Neurophysiol. 1994;71(1):429–432. doi: 10.1152/jn.1994.71.1.429. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Development of multisensory neurons and multisensory integration in cat superior colliculus. J Neurosci. 1997;17(7):2429–2444. doi: 10.1523/JNEUROSCI.17-07-02429.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Onset of cross-modal synthesis in the neonatal superior colliculus is gated by the development of cortical influences. J Neurophysiol. 2000;83(6):3578–3582. doi: 10.1152/jn.2000.83.6.3578. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Perrault TJ, Hairston WD, Stein BE. Visual experience is necessary for the development of multisensory integration. J Neurosci. 2004;24(43):9580–9584. doi: 10.1523/JNEUROSCI.2535-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson LK, Meredith MA, Stein BE. The role of anterior ectosylvian cortex in cross-modality orientation and approach behavior. Exp Brain Res. 1996;112:1–10. doi: 10.1007/BF00227172. [DOI] [PubMed] [Google Scholar]