Abstract
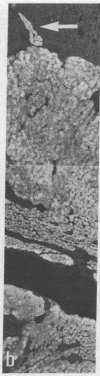
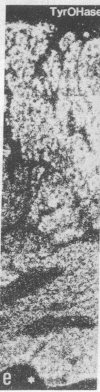
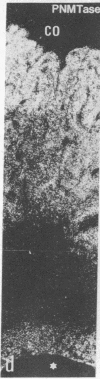
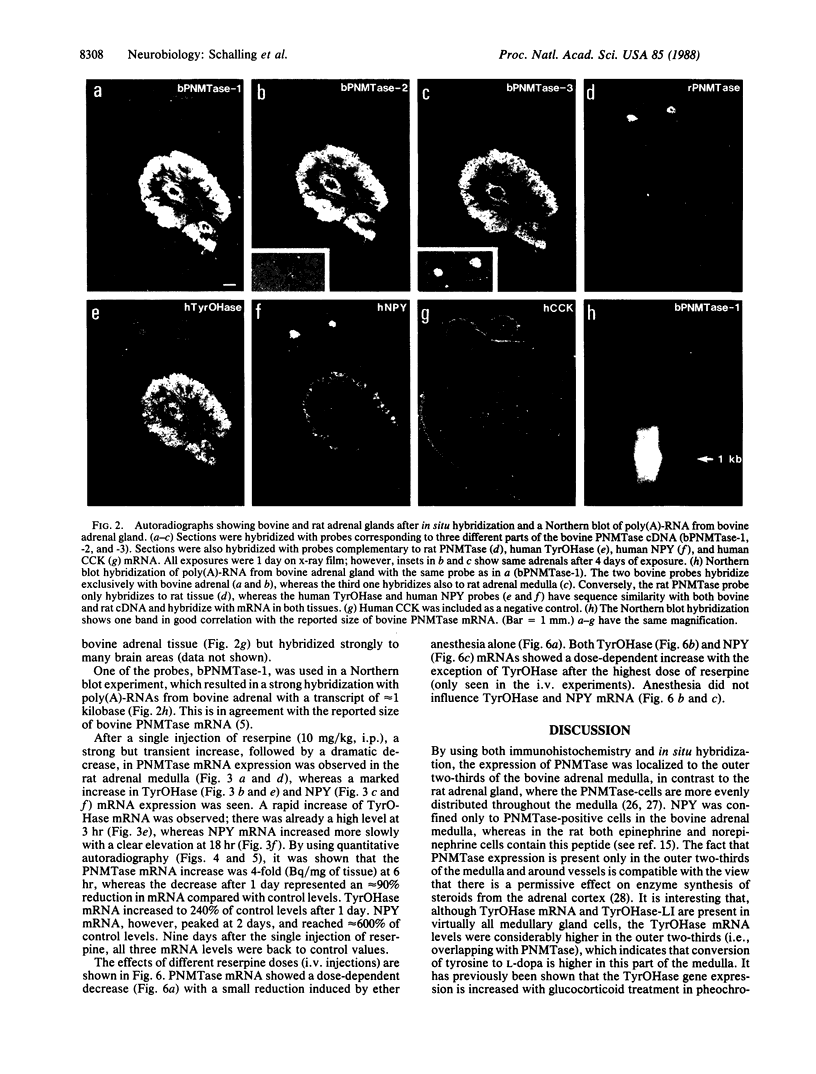
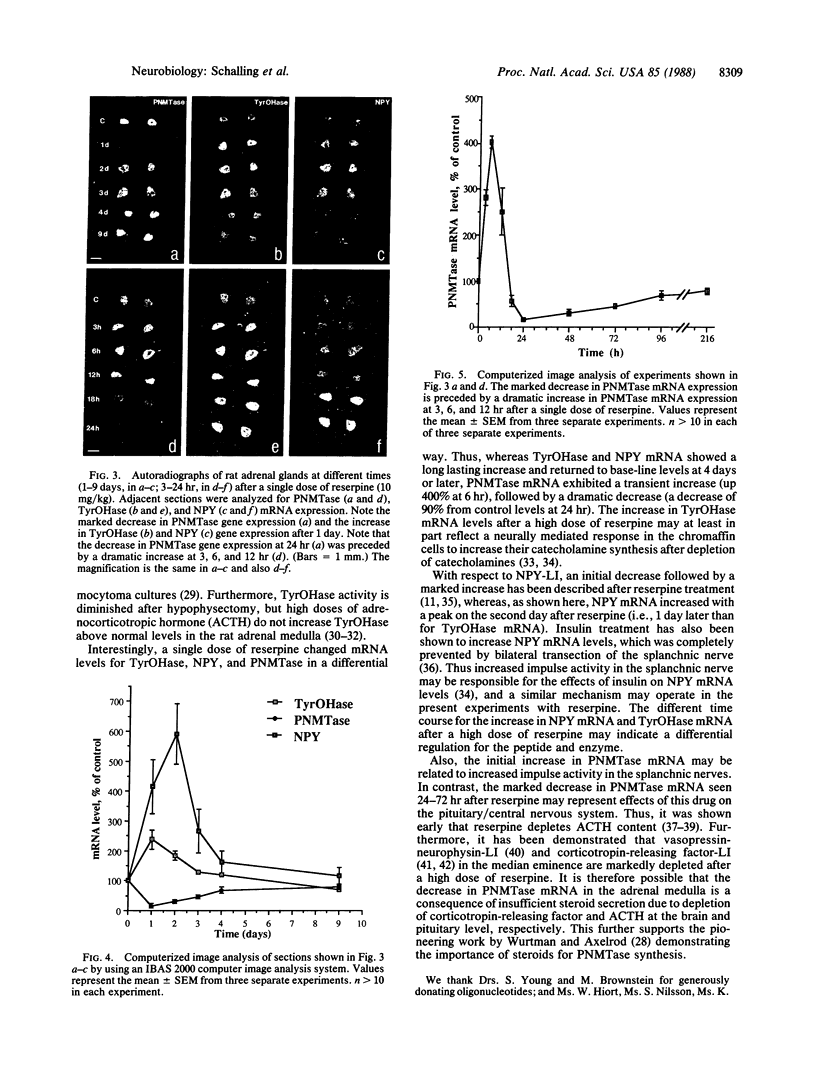
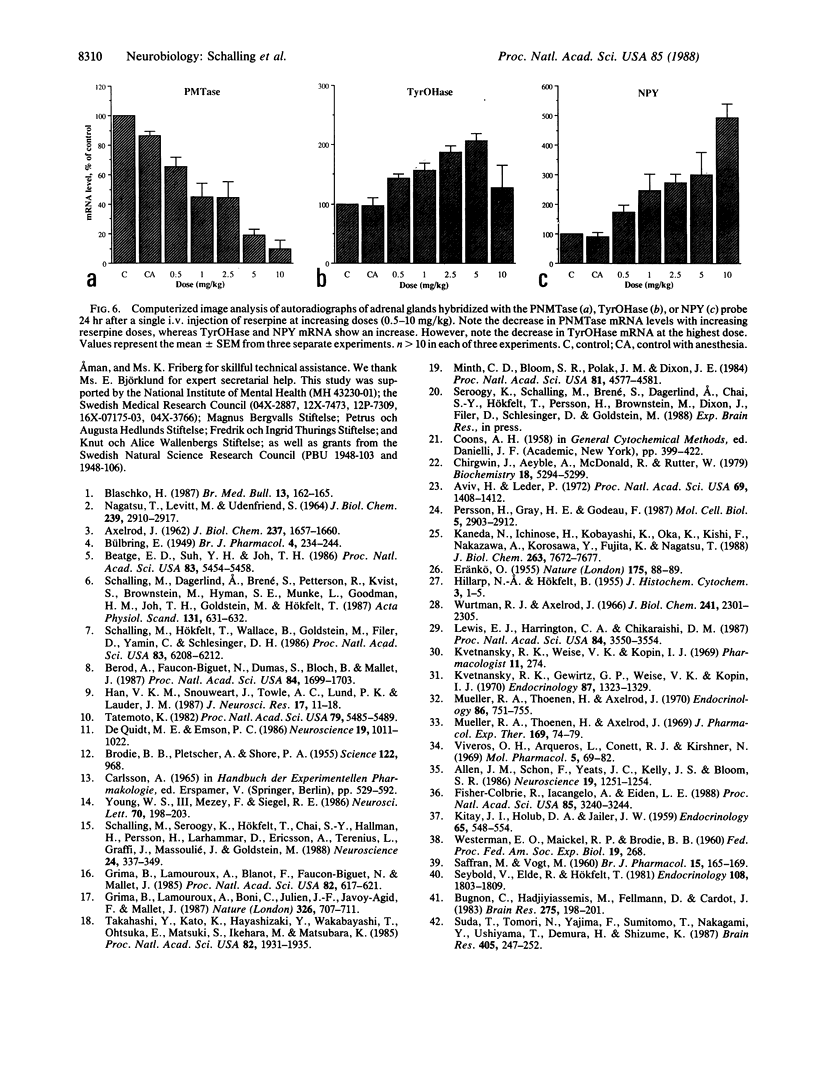
Expression and regulation of the catecholamine-synthesizing enzymes phenylethanolamine N-methyltransferase (PNMTase; S-adenosyl-L-methionine:phenylethanolamine N-methyltransferase, EC 2.1.1.28) and tyrosine hydroxylase [TyrOHase; tyrosine 3-monooxygenase, L-tyrosine, tetrahydropteridine:oxygen oxidoreductase (3-hydroxylating), EC 1.14.16.2] and the coexisting neuropeptide tyrosine (NPY) were studied in rat and bovine adrenal medulla. By using both immunohistochemistry and in situ hybridization, PNMTase- and NPY-positive cells exhibited a close overlap in bovine medulla and were preferentially localized in the outer two-thirds of the medulla. Although TyrOHase and its mRNA were observed in virtually all medullary gland cells, TyrOHase mRNA levels were much higher in the PNMTase- and NPY-positive cells. After administration of the catecholamine-depleting drug reserpine to rats, a brief increase, followed by a dramatic decrease, in the level of PNMTase mRNA was observed in the adrenal medulla. In contrast, mRNA for both TyrOHase and NPY only exhibited an increase, whereby the TyrOHase mRNA peak preceded that of NPY mRNA. Different regulatory mechanisms may thus operate for these three compounds coexisting in the adrenal medulla.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J. Purification and properties of phenylethanolamine-N-methyl transferase. J Biol Chem. 1962 May;237:1657–1660. [PubMed] [Google Scholar]
- Allen J. M., Schon F., Yeats J. C., Kelly J. S., Bloom S. R. Effect of reserpine, phenoxybenzamine and cold stress on the neuropeptide Y content of the rat peripheral nervous system. Neuroscience. 1986 Dec;19(4):1251–1254. doi: 10.1016/0306-4522(86)90139-9. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLASCHKO H. Formation of catechol amines in the animal body. Br Med Bull. 1957 Sep;13(3):162–165. doi: 10.1093/oxfordjournals.bmb.a069606. [DOI] [PubMed] [Google Scholar]
- BRANKO O. Distribution of adrenaline and nonadrenaline in the adrenal medulla. Nature. 1955 Jan 8;175(4445):88–89. doi: 10.1038/175088a0. [DOI] [PubMed] [Google Scholar]
- BRODIE B. B., PLETSCHER A., SHORE P. A. Evidence that serotonin has a role in brain function. Science. 1955 Nov 18;122(3177):968–968. doi: 10.1126/science.122.3177.968. [DOI] [PubMed] [Google Scholar]
- Baetge E. E., Suh Y. H., Joh T. H. Complete nucleotide and deduced amino acid sequence of bovine phenylethanolamine N-methyltransferase: partial amino acid homology with rat tyrosine hydroxylase. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5454–5458. doi: 10.1073/pnas.83.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berod A., Biguet N. F., Dumas S., Bloch B., Mallet J. Modulation of tyrosine hydroxylase gene expression in the central nervous system visualized by in situ hybridization. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1699–1703. doi: 10.1073/pnas.84.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugnon C., Hadjiyiassemis M., Fellmann D., Cardot J. Reserpine-induced depletion of corticoliberin (CRF)-like immunoreactivity in the zona externa of the rat median eminence. Brain Res. 1983 Sep 19;275(1):198–201. doi: 10.1016/0006-8993(83)90438-9. [DOI] [PubMed] [Google Scholar]
- COONS A. H. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Fischer-Colbrie R., Iacangelo A., Eiden L. E. Neural and humoral factors separately regulate neuropeptide Y, enkephalin, and chromogranin A and B mRNA levels in rat adrenal medulla. Proc Natl Acad Sci U S A. 1988 May;85(9):3240–3244. doi: 10.1073/pnas.85.9.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Blanot F., Biguet N. F., Mallet J. Complete coding sequence of rat tyrosine hydroxylase mRNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):617–621. doi: 10.1073/pnas.82.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Boni C., Julien J. F., Javoy-Agid F., Mallet J. A single human gene encoding multiple tyrosine hydroxylases with different predicted functional characteristics. Nature. 1987 Apr 16;326(6114):707–711. doi: 10.1038/326707a0. [DOI] [PubMed] [Google Scholar]
- HILLARP N. A., HOKFELT B. Histochemical demonstration of noradrenaline and adrenaline in the adrenal medulla. J Histochem Cytochem. 1955 Jan;3(1):1–5. doi: 10.1177/3.1.1. [DOI] [PubMed] [Google Scholar]
- Han V. K., Snouweart J., Towle A. C., Lund P. K., Lauder J. M. Cellular localization of tyrosine hydroxylase mRNA and its regulation in the rat adrenal medulla and brain by in situ hybridization with an oligodeoxyribonucleotide probe. J Neurosci Res. 1987;17(1):11–18. doi: 10.1002/jnr.490170103. [DOI] [PubMed] [Google Scholar]
- KITAY J. I., HOLUB D. A., JAILER J. W. "Inhibition" of pituitary ACTH release after administration of reserpine or epinephrine. Endocrinology. 1959 Oct;65:548–554. doi: 10.1210/endo-65-4-548. [DOI] [PubMed] [Google Scholar]
- Kaneda N., Ichinose H., Kobayashi K., Oka K., Kishi F., Nakazawa A., Kurosawa Y., Fujita K., Nagatsu T. Molecular cloning of cDNA and chromosomal assignment of the gene for human phenylethanolamine N-methyltransferase, the enzyme for epinephrine biosynthesis. J Biol Chem. 1988 Jun 5;263(16):7672–7677. [PubMed] [Google Scholar]
- Kvetnansky R., Gewirtz G. P., Weise V. K., Kopin I. J. Effect of hypophysectomy on immobilization-induced elevation of tyrosine hydroxylase and phenylethanolamine-N-methyl transferase in the rat adrenal. Endocrinology. 1970 Dec;87(6):1323–1329. doi: 10.1210/endo-87-6-1323. [DOI] [PubMed] [Google Scholar]
- Lewis E. J., Harrington C. A., Chikaraishi D. M. Transcriptional regulation of the tyrosine hydroxylase gene by glucocorticoid and cyclic AMP. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3550–3554. doi: 10.1073/pnas.84.11.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minth C. D., Bloom S. R., Polak J. M., Dixon J. E. Cloning, characterization, and DNA sequence of a human cDNA encoding neuropeptide tyrosine. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4577–4581. doi: 10.1073/pnas.81.14.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller R. A., Thoenen H., Axelrod J. Effect of pituitary and ACTH on the maintenance of basal tyrosine hydroxylase activity in the rat adrenal gland. Endocrinology. 1970 Apr;86(4):751–755. doi: 10.1210/endo-86-4-751. [DOI] [PubMed] [Google Scholar]
- Mueller R. A., Thoenen H., Axelrod J. Increase in tyrosine hydroxylase activity after reserpine administration. J Pharmacol Exp Ther. 1969 Sep;169(1):74–79. [PubMed] [Google Scholar]
- NAGATSU T., LEVITT M., UDENFRIEND S. TYROSINE HYDROXYLASE. THE INITIAL STEP IN NOREPINEPHRINE BIOSYNTHESIS. J Biol Chem. 1964 Sep;239:2910–2917. [PubMed] [Google Scholar]
- Persson H., Gray H. E., Godeau F. Growth-dependent synthesis of c-myc-encoded proteins: early stimulation by serum factors in synchronized mouse 3T3 cells. Mol Cell Biol. 1985 Nov;5(11):2903–2912. doi: 10.1128/mcb.5.11.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAFFRAN M., VOGT M. Depletion of pituitary corticotrophin by reserpine and by a nitrogen mustard. Br J Pharmacol Chemother. 1960 Mar;15:165–169. doi: 10.1111/j.1476-5381.1960.tb01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalling M., Dagerlind A., Brene S., Petterson R., Kvist S., Brownstein M., Hyman S. E., Mucke L., Goodman H. M., Joh T. H. Localization of mRNA for phenylethanolamine N-methyltransferase (PNMT) using in situ hybridization. Acta Physiol Scand. 1987 Dec;131(4):631–632. doi: 10.1111/j.1748-1716.1987.tb08288.x. [DOI] [PubMed] [Google Scholar]
- Schalling M., Hökfelt T., Wallace B., Goldstein M., Filer D., Yamin C., Schlesinger D. H. Tyrosine 3-hydroxylase in rat brain and adrenal medulla: hybridization histochemistry and immunohistochemistry combined with retrograde tracing. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6208–6212. doi: 10.1073/pnas.83.16.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalling M., Seroogy K., Hökfelt T., Chai S. Y., Hallman H., Persson H., Larhammar D., Ericsson A., Terenius L., Graffi J. Neuropeptide tyrosine in the rat adrenal gland--immunohistochemical and in situ hybridization studies. Neuroscience. 1988 Jan;24(1):337–349. doi: 10.1016/0306-4522(88)90335-1. [DOI] [PubMed] [Google Scholar]
- Seybold V., Elde R., Hökfelt T. Terminals of reserpine-sensitive vasopressin-neurophysin neurons in the external layer of the rat median eminence. Endocrinology. 1981 May;108(5):1803–1809. doi: 10.1210/endo-108-5-1803. [DOI] [PubMed] [Google Scholar]
- Suda T., Tomori N., Yajima F., Sumitomo T., Nakagami Y., Ushiyama T., Demura H., Shizume K. Time course study on the effect of reserpine on hypothalamic immunoreactive CRF levels in rats. Brain Res. 1987 Mar 10;405(2):247–252. doi: 10.1016/0006-8993(87)90294-0. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Kato K., Hayashizaki Y., Wakabayashi T., Ohtsuka E., Matsuki S., Ikehara M., Matsubara K. Molecular cloning of the human cholecystokinin gene by use of a synthetic probe containing deoxyinosine. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1931–1935. doi: 10.1073/pnas.82.7.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K. Neuropeptide Y: complete amino acid sequence of the brain peptide. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5485–5489. doi: 10.1073/pnas.79.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros O. H., Arqueros L., Connett R. J., Kirshner N. Mechanism of secretion from the adrenal medulla. IV. The fate of the storage vesicles following insulin and reserpine administration. Mol Pharmacol. 1969 Jan;5(1):69–82. [PubMed] [Google Scholar]
- Wurtman R. J., Axelrod J. Control of enzymatic synthesis of adrenaline in the adrenal medulla by adrenal cortical steroids. J Biol Chem. 1966 May 25;241(10):2301–2305. [PubMed] [Google Scholar]
- Young W. S., 3rd, Mezey E., Siegel R. E. Quantitative in situ hybridization histochemistry reveals increased levels of corticotropin-releasing factor mRNA after adrenalectomy in rats. Neurosci Lett. 1986 Oct 8;70(2):198–203. doi: 10.1016/0304-3940(86)90463-5. [DOI] [PubMed] [Google Scholar]
- de Quidt M. E., Emson P. C. Neuropeptide Y in the adrenal gland: characterization, distribution and drug effects. Neuroscience. 1986 Nov;19(3):1011–1022. doi: 10.1016/0306-4522(86)90313-1. [DOI] [PubMed] [Google Scholar]