Abstract
As growth plate chondrocytes mature and hypertrophy, they reorganize their proteoglycan-rich type II collagen extracellular matrix (ECM), involving 1,25(OH)2D3-dependent regulation of matrix metalloproteinases (MMPs). Stromelysin-1 (MMP-3) and 72-kD gelatinase (MMP-2) are found in extracellular matrix vesicles (MVs) and release and activate ECM-bound latent TGF-β1 and TGF-β2, respectively. 1,25(OH)2D3 regulates incorporation of MMP-2 and MMP-3 into MVs and release of these enzymes in the ECM. Plasma membranes (PMs) and MVs contain the 1α,25(OH)2D3 membrane receptor ERp60 (protein disulfide isomerase A3), phospholipase A2 (PLA2), PLA2-activating protein, the nuclear vitamin D receptor and caveolin-1. 1,25(OH)2D3 secreted by chondrocytes binds MV ERp60, activating PLA2. Resulting lysophospholipids destabilize MV membranes, releasing active MMPs. We examined 1,25(OH)2D3-dependent activation of latent TGF-β1 stored in cartilage ECM. Interestingly, TGF-β1 regulates 1,25(OH)2D3 production. 1α,25(OH)2D3 activates PM protein kinase C (PKC)-α via ERp60-dependent PLA2-signaling, lysophospholipid production and phospholipase C-γ. It also regulates distribution of phospholipids and PKC isoforms between MVs and PMs, enriching MVs in PKC-ζ. Direct activation of MV MMP-3 requires ERp60 based on blocking antibodies and PKC based on inhibitor studies. However, treatment of MVs with 1,25(OH)2D3 decreases MV PKC-ζ activity, suggesting more complex feedback mechanisms, potentially involving MV lipid signaling. Our observations indicate that one role of MVs is to provide MMPs at sites distant from the cells. Chondrocytes secrete 1,25(OH)2D3, which acts directly on MV-membranes via ERp60, releasing MMPs. MMP-specific ECM components are hydrolyzed, resulting in release and activation of growth factors that can act back on the cells.
Key Words: 1α,25(OH)2D3; Matrix vesicles; Extracellular matrix; TGF-β1, latent activation; Matrix metalloproteinases; MMP-3
Introduction
Growth plate chondrocytes produce a proteoglycan-rich type II collagen extracellular matrix (ECM). As the cells in the growth plate mature and hypertrophy, there is a massive reorganization of the ECM to accommodate the changes in cell shape and to prepare the matrix for calcification and eventual vascular ingrowth. Concurrently, there is a shift in matrix-processing enzymes from neutral to acidic metalloproteinases [Dean et al., 2001]. In addition, enzyme activities that modulate the size of proteoglycan aggregate, including chondroitinases and ADAMTS, are increased [Cawston and Wilson, 2006].
In vivo studies have shown that activity of matrix processing enzymes in the growth plate is regulated by 1α,25(OH)2D3 [Dean et al., 2001; Lin et al., 2002]. This phenomenon has been confirmed in studies using growth plate chondrocyte cultures [Schmitz et al., 1996b; Maeda et al., 2000]. There are a number of mechanisms by which this occurs. Many of the enzymes are produced as zymogens, and 1α,25(OH)2D3 increases zymogen activation [Schmitz et al., 1996b]. 1α,25(OH)2D3 has been shown to regulate the levels of mineral ions that are required for metalloproteinase activity [Brown et al., 1993]. In addition, 1α,25(OH)2D3 modulates transcription of their mRNA [Schmitz et al., 1996a, b]. Because regulation of matrix metalloproteinase (MMP) activity is critical, chondrocytes produce inhibitors like tissue inhibitor of metalloproteinases (TIMP-1 and TIMP-2) [Dean et al., 1992] and 1α,25(OH)2D3 modulates levels of these inhibitors as well [Dean et al., 2001].
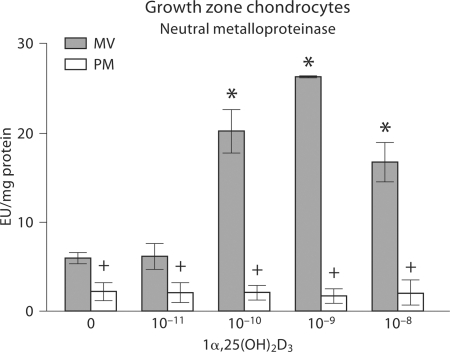
We have shown that growth plate chondrocytes also take advantage of extracellular organelles called matrix vesicles (MVs) to package MMPs and store them in the ECM separate from the matrix proteins [Schmitz et al., 1994; Dean et al., 1996a, b; D'Angelo et al., 2001]. MVs produced by cells in the prehypertrophic and hypertrophic cell zones contain higher levels of acidic metalloproteinases and this is further increased in animals treated with 1α,25(OH)2D3 [Dean et al., 1996a, b]. In addition, 1α,25(OH)2D3 increases activity of neutral MMPs in MVs produced by these cells (fig. 1). Cell culture studies have shown that incorporation of stromelysin-1 (MMP-3) and 72-kD gelatinase (MMP-2) into MVs is regulated by 1α,25(OH)2D3 [Schmitz et al., 1996b]. MMP-2 and MMP-3 are stored in MVs produced by growth zone chondrocytes together with TIMP-1 and TIMP-2, and are released from the MVs in an active form. 1α,25(OH)2D3 reduces MV TIMP levels [Dean et al., 2001], supporting the hypothesis that the MMPs are activated prior to their release in the matrix.
Fig. 1.
Effect of 1α,25(OH)2D3 on neutral metalloproteinase activity in growth plate chondrocyte cultures. Confluent cultures of rat costochondral growth zone cartilage cells were treated with 10–11 to 10–8M 1α,25(OH)2D3 for 24 h. MVs and PMs were isolated and assayed for neutral MMP activity using aggrecan containing polyacrylamide gel beads. Data are means ± SEM for 6 independent cultures and are from 1 of 2 separate experiments, both with comparable results. ∗ p < 0.05, treatment versus control; + p < 0.05, MV versus PM at each concentration of 1α,25(OH)2D3.
Autocrine Regulation of MV MMPs by 1α,25(OH)2D3
Traditionally, 1α,25(OH)2D3 acts via the vitamin D receptor to regulate gene transcription, so genetic modulation of MMP content during MV formation is not an unexpected finding. However, we have also shown that 1α,25(OH)2D3 can act directly on MVs, resulting in release of active MMPs [Dean et al., 1996b]. This indicates that 1α,25(OH)2D3 may act in an autocrine manner.
Like the plasma membrane (PM), MVs contain the 1α,25(OH)2D3 membrane receptor ERp60 [also called protein disulfide isomerase, family A, type 3 (PDIA3), ER60, ERp57, GRP57 and 1,25-MARRS], as well as phospholipase A2 (PLA2), PLA2-activating protein and caveolin-1 (fig. 2), but they do not possess DNA or RNA and therefore do not produce new protein. In chondrocytes, 1α,25(OH)2D3 acts via ERp60 by activating protein kinase C (PKC)-α-dependent signaling through a mechanism that is mediated by PLA2 and requires the presence of PLA2-activating protein [Boyan et al., 2006]. Inhibition of PKC activity with chelerythrine blocks the effects of 1α,25(OH)2D3 on MMP-3 activity in isolated MVs [Maeda et al., 2000], suggesting that a similar mechanism to that which occurs in chondrocytes may be involved, but it is unlikely that this is the case. During MV production, 1α,25(OH)2D3 regulates the differential distribution of PKC isoforms between the PM and the MV membrane, resulting in higher levels of PKC-α in the PM and higher levels of PKC-ζ in the MVs [Sylvia et al., 1996]. However, when isolated PMs or MVs are treated directly with 1α,25(OH)2D3, PKC-α activity is increased in the PM but PKC-ζ activity is decreased in the MVs. This indicates that the direct action of the vitamin D metabolite is differentially distributed, allowing the responses to be specific to each membrane compartment.
Fig. 2.
Presence of caveolin-1 (CAV-1), ERp60 and nuclear vitamin D receptor (nVDR) in rat costochondral growth zone chondrocyte lysates and isolated PMs and MVs. MVs were isolated from trypsin digests of confluent cultures of growth zone chondrocytes. The cell pellet was lysed and PMs were isolated from the lysates by differential centrifugation. Each fraction (PMs, MVs and cell lysates) was separated on SDS-PAGE and then Western blots were probed with antibodies to caveolin-1, vitamin D receptor and ERp60.
In contrast to its effects on PKC isoforms, 1α,25(OH)2D3 stimulates PLA2 activity in both membrane compartments [Schwartz et al., 2005], suggesting an alternate hypothesis. In this hypothesis, activation of PLA2 in the PM is rapidly downregulated, but in the MVs this does not occur and the resulting production of lysophospholipids causes a loss of membrane integrity and eventual release of MMPs into the ECM. That this is receptor mediated is evidenced by the fact that antibodies to ERp60 prevent the release of MV MMPs [Maeda et al., 2000]. Moreover, growth plate chondrocytes produce and secrete 1α,25(OH)2D3 in a regulated manner at levels as high as 10–8M, supporting the hypothesis that it acts in an autocrine manner.
Regulation of TGF-β Activation
Chondrocytes store TGF-β1 in the growth plate ECM as large latent TGF-β1 complexes, which consist of latent TGF-β1-binding protein, latency-associated peptide and latent TGF-β1 [Pedrozo et al., 1998]. Initial studies showed that 1α,25(OH)2D3 metabolites regulate expression of latent TGF-β1-binding protein and latent TGF-β1 and incorporation of latent TGF-β1 in the ECM of chondrocytes [Pedrozo et al., 1999b]. TGF-β1 regulates production of 1,25(OH)2D3 by growth plate chondrocytes [Pedrozo et al., 1999a], suggesting the possibility of a feedback loop modulating TGF-β1 levels in the tissue.
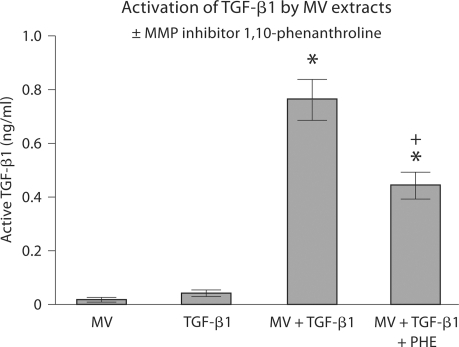
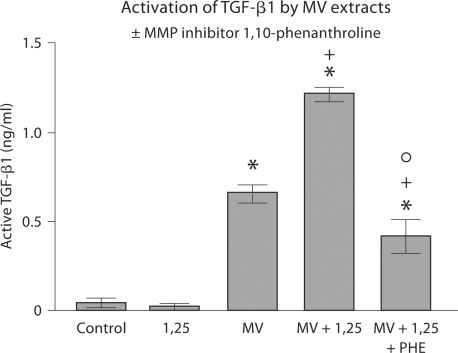
The finding that 1α,25(OH)2D3 increased MMP activity suggested that 1α,25(OH)2D3 might also regulate availability of TGF-β1 by controlling activation of the latent growth factor. Initial experiments on inhibition of TGF-β1 activation by the MMP inhibitor 1,10-phenanthroline showed that MV extracts could activate latent TGF-β1 and that MMPs were responsible for this (fig. 3). A second set of experiments demonstrated that extracts of MVs that had been incubated directly with 1α,25(OH)2D3 were more effective than extracts of untreated MVs (fig. 4). Moreover, the effect of 1α,25(OH)2D3 was via activation of MMPs, based on the reduction in TGF-β1 activation by 1,10-phenanthroline.
Fig. 3.
Activation of latent TGF-β1 by MV extracts. MVs were isolated from confluent cultures of rat costochondral growth zone cartilage cells and assayed for TGF-β1 content by immunoassay (MV). MV extracts were then incubated in DMEM containing 10% fetal bovine serum without exogenous latent TGF-β1 and basal level of TGF-β1 in the reaction mixture was determined (TGF-β1). MV extracts were then incubated with latent TGF-β1 and active TGF-β1 was measured (MV+TGF-β1). Finally, MV extracts were treated with the MMP inhibitor 1,10-phenanthroline (PHE) and then incubated with latent TGF-β1 (MV+TGF-β1+ PHE). Data are means ± SEM for 6 separate MV preparations. ∗ p < 0.05, MV+TGF-β1 versus MV; + p < 0.05, MV+TGF-β1 versus MV+TGF-β1+PHE.
Fig. 4.
Effect of 1α,25(OH)2D3 on activation of latent TGF-β1 by MVs. MVs were isolated from confluent cultures of rat costochondral growth zone cartilage cells and assayed for TGF-β1 content by immunoassay (control). MV extracts were then incubated in DMEM containing 10% fetal bovine serum and 10–8 M 1α,25(OH)2D3 without exogenous latent TGF-β1 and basal level of TGF-β1 in the reaction mixture was determined (1,25). MV extracts were then incubated with latent TGF-β1 and active TGF-β1 was measured (MV). MVs were treated with 1α,25(OH)2D3 and extracts of these MVs were incubated with latent TGF-β1 (MV+1,25). Finally, extracts from MVs treated with 10–8 M 1α,25(OH)2D3 were incubated with latent TGF-β1 in the presence of the MMP inhibitor 1,10 phenanthroline (MV+1,25+PHE). Data are means ± SEM for 6 separate MV preparations. ∗ p < 0.05, MV versus control; + p < 0.05, MV+1,25 versus MV; ° p < 0.05, MV+1,25 versus MV+1,25+PHE.
1α,25(OH)2D3-treated MVs activated latent TGF-β1 and to a lesser extent latent TGF-β2 [Boyan et al., 1994], suggesting that a similar mechanism was involved for both isoforms of this growth factor. Studies in other laboratories had shown that latent TGF-β could be activated by plasmin [Rosenthal et al., 2000] and we were able to show that plasmin could release active TGF-β1 from ECM produced by growth plate chondrocytes [Pedrozo et al., 1999c]. However, MVs do not contain plasmin, indicating that another enzyme was involved. When MV extracts were treated with antibodies to MMP-3, activation of TGF-β1 was blocked [Maeda et al., 2001], indicating that it was responsible for 1α,25(OH)2D3-dependent activation of latent TGF-β1. Others have shown that MMP-2 specifically activates TGF-β2 [Wang et al., 2005, 2006].
Taken together, these studies show that MVs contain enzymes that can result in the release of active TGF-β1 from the ECM produced by growth plate chondrocytes. Moreover, they indicate that 1α,25(OH)2D3 produced by the cells and secreted into the ECM is involved. Additional studies have shown that the effect of 1α,25(OH)2D3 is mediated by 2 pathways, both of which are ERp60 dependent, based on the ability of antibodies to ERp60 to block the effect. One mechanism involves the activation of PLA2, resulting in the formation of lysophospholipids. Lysophospholipids can act directly on latent TGF-β1, resulting in its activation [Gay et al., 2004]. They also function to destabilize the MV membrane, resulting in release of MMP-3 (an activator of TGF-β1). The other mechanism is mediated by PKC, since inhibition of PKC by chelerythrine also prevents 1α,25(OH)2D3-dependent activation of MV MMP-3 [Maeda et al., 2000].
Summary
The studies described above show that 1α,25(OH)2D3 regulates ECM turnover by activating both neutral and acidic matrix metalloproteinases. Part of this effect is via transcriptional regulation of MMP expression and part is via autocrine regulation of MMP release from extracellular MVs. MV MMP-3 specifically regulates activation of TGF-β1 stored in the ECM, providing a feedback loop regulating matrix turnover. Active TGF-β1 regulates 1α,25(OH)2D3 production and its secretion into the ECM. 1α,25(OH)2D3 acting as an autocrine factor acts directly on MVs via ERp60-dependent pathways, increasing local lysophospholipid content by stimulating PLA2 activity and through control of PKC activity.
Acknowledgements
This research was supported by the NIH, NSF EEC 9731643, the Price Gilbert Jr. Foundation and Children's Healthcare of Atlanta. The authors thank Ms. Mimi Fang and Mr. Kevin Wong for their contributions to the data, as well as previous collaborators who have participated in the research summarized in this paper, particularly Drs. Lynda Bonewald, Victor Sylvia and David D. Dean.
Abbreviations used in this paper
- 1α,25(OH)2D3
1,25-dihydroxy vitamin D3
- ECM
extracellular matrix
- MMP
matrix metalloproteinase
- MV
matrix vesicles
- PDIA3
protein disulfide isomerase, family A, type 3
- PKC
protein kinase C
- PLA2
phospholipase A2
- PM
plasma membrane
- TGF
transforming growth factor
- TIMP
tissue inhibitor of metalloproteinase
References
- Boyan B.D., Schwartz Z., Park-Snyder S., Dean D.D., Yang F., Twardzik D., Bonewald L.F. Latent transforming growth factor-β is produced by chondrocytes and activated by extracellular matrix vesicles upon exposure to 1,25-(OH)2D3. J Biol Chem. 1994;269:28374–28381. [PubMed] [Google Scholar]
- Boyan B.D., Wang L., Wong K.L., Jo H., Schwartz Z. Plasma membrane requirements for 1α,25(OH)2D3 dependent PKC signaling in chondrocytes and osteoblasts. Steroids. 2006;71:286–290. doi: 10.1016/j.steroids.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Brown R.A., Kayser M., McLaughlin B., Weiss J.B. Collagenase and gelatinase production by calcifying growth plate chondrocytes. Exp Cell Res. 1993;208:1–9. doi: 10.1006/excr.1993.1216. [DOI] [PubMed] [Google Scholar]
- Cawston T.E., Wilson A.J. Understanding the role of tissue degrading enzymes and their inhibitors in development and disease. Best Pract Res Clin Rheumatol. 2006;20:983–1002. doi: 10.1016/j.berh.2006.06.007. [DOI] [PubMed] [Google Scholar]
- D'Angelo M., Billings P.C., Pacifici M., Leboy P.S., Kirsch T. Authentic matrix vesicles contain active metalloproteases (MMP). a role for matrix vesicle-associated MMP-13 in activation of transforming growth factor-beta. J Biol Chem. 2001;276:11347–11353. doi: 10.1074/jbc.M009725200. [DOI] [PubMed] [Google Scholar]
- Dean D.D., Boyan B.D., Muniz O.E., Howell D.S., Schwartz Z. Vitamin D metabolites regulate matrix vesicle metalloproteinase content in a cell maturation-dependent manner. Calcif Tissue Int. 1996a;59:109–116. doi: 10.1007/s002239900096. [DOI] [PubMed] [Google Scholar]
- Dean D.D., Schwartz Z., Muniz O.E., Carreno M.R., Maeda S., Howell D.S., Boyan B.D. Effect of 1α,25-(OH)2D3 and 24R,25-(OH)2D3 on metalloproteinase activity and cell maturation in growth plate cartilage in vivo. Endocrine. 2001;14:311–323. doi: 10.1385/endo:14:3:311. [DOI] [PubMed] [Google Scholar]
- Dean D.D., Schwartz Z., Muniz O.E., Gomez R., Swain L.D., Howell D.S., Boyan B.D. Matrix vesicles are enriched in metalloproteinases that degrade proteoglycans. Calcif Tissue Int. 1992;50:342–349. doi: 10.1007/BF00301632. [DOI] [PubMed] [Google Scholar]
- Dean D.D., Schwartz Z., Schmitz J.P., Muniz O.E., Lu Y., Calderon F.J., Howell D.S., Boyan B.D. Vitamin D regulation of metalloproteinase activity in matrix vesicles. Connect Tissue Res. 1996b;35:331–336. doi: 10.3109/03008209609029208. [DOI] [PubMed] [Google Scholar]
- Gay I., Schwartz Z., Sylvia V.L., Boyan B.D. Lysophospholipid regulates release and activation of latent TGF-β1 from chondrocyte extracellular matrix. Biochem Biophys Acta. 2004;1684:18–28. doi: 10.1016/j.bbalip.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Lin R., Amizuka N., Sasaki T., Aarts M.M., Ozawa H., Goltzman D., Henderson J.E., White J.H. 1α,25-dihydroxyvitamin D3 promotes vascularization of the chondro-osseous junction by stimulating expression of vascular endothelial growth factor and matrix metalloproteinase 9. J Bone Miner Res. 2002;17:1604–1612. doi: 10.1359/jbmr.2002.17.9.1604. [DOI] [PubMed] [Google Scholar]
- Maeda S., Dean D.D., Gay I., Schwartz Z., Boyan B.D. Activation of latent transforming growth factor β1 by stromelysin 1 in extracts of growth plate chondrocyte-derived matrix vesicles. J Bone Miner Res. 2001;16:1281–1290. doi: 10.1359/jbmr.2001.16.7.1281. [DOI] [PubMed] [Google Scholar]
- Maeda S., Dean D.D., Sylvia V.L., Boyan B.D., Schwartz Z. Metalloproteinase activity in growth plate chondrocyte cultures is regulated by 1,25-(OH)2D3 and 24,25-(OH)2D3 and mediated through protein kinase C. Matrix Biol. 2000;20:87–97. doi: 10.1016/s0945-053x(01)00123-8. [DOI] [PubMed] [Google Scholar]
- Pedrozo H.A., Boyan B.D., Mazock J., Dean D.D., Gomez R., Schwartz Z. TGF-β1 regulates 25-hydroxyvitamin D3 1α- and 24-hydroxylase activity in cultured growth plate chondrocytes in a maturation-dependent manner. Calcif Tissue Int. 1999a;64:50–56. doi: 10.1007/s002239900578. [DOI] [PubMed] [Google Scholar]
- Pedrozo H.A., Schwartz Z., Gomez R., Ornoy A., Xin-Sheng W., Dallas S.L., Bonewald L.F., Dean D.D., Boyan B.D. Growth plate chondrocytes store latent TGF-β1 in their matrix through latent TGFβ binding protein-1. J Cell Physiol. 1998;177:343–354. doi: 10.1002/(SICI)1097-4652(199811)177:2<343::AID-JCP16>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Pedrozo H.A., Schwartz Z., Mokeyev T., Ornoy A., Xin-Sheng W., Bonewald L.F., Dean D.D., Boyan B.D. Vitamin D3 metabolites regulate LTBP1 and latent TGF-β1 expression and latent TGF-β1 incorporation in the extracellular matrix of chondrocytes. J Cell Biochem. 1999b;72:151–165. [PubMed] [Google Scholar]
- Pedrozo H.A., Schwartz Z., Robinson M., Gomez R., Dean D.D., Bonewald L.F., Boyan B.D. Potential mechanisms for the plasmin mediated release and activation of latent TGF-β1 from the extracellular matrix of growth plate chondrocytes. Endocrinology. 1999c;140:5806–5816. doi: 10.1210/endo.140.12.7224. [DOI] [PubMed] [Google Scholar]
- Rosenthal A.K., Gohr C.M., Henry L.A., Le M. Participation of transglutaminase in the activation of latent transforming growth factor β1 in aging articular cartilage. Arthritis Rheum. 2000;43:1729–1733. doi: 10.1002/1529-0131(200008)43:8<1729::AID-ANR8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schmitz J.P., Dean D.D., Calderon F.J., Schwartz Z., Boyan B.D. Matrix vesicles contain active stromelysin-1 regulated by vitamin D3 metabolites. J Bone Miner Res. 1994;9:S379. [Google Scholar]
- Schmitz J.P., Dean D.D., Schwartz Z., Cochran D.L., Grant G.M., Klebe R.J., Nakaya H., Boyan B.D. Chondrocyte cultures express matrix metalloproteinase mRNA and immunoreactive protein: stromelysin-1 and 72kDa gelatinase are localized in extracellular matrix vesicles. J Cell Biochem. 1996a;61:375–391. doi: 10.1002/(SICI)1097-4644(19960601)61:3%3C375::AID-JCB5%3E3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Schmitz J.P., Schwartz Z., Sylvia V.L., Dean D.D., Calderon F., Boyan B.D. Vitamin D3 regulation of stromelysin-1 (MMP-3) in chondrocyte cultures is mediated by protein kinase C. J Cell Physiol. 1996b;168:570–579. doi: 10.1002/(SICI)1097-4652(199609)168:3<570::AID-JCP9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Schwartz Z., Graham E.J., Wang L., Lossdorfer S., Gay I., Johnson-Pais T.L., Carnes D.L., Sylvia V.L., Boyan B.D. Phospholipase A2 activating protein (PLAA) is required for 1α,25(OH)2D3 signaling in growth plate chondrocytes. J Cell Physiol. 2005;203:54–70. doi: 10.1002/jcp.20212. [DOI] [PubMed] [Google Scholar]
- Sylvia V.L., Schwartz Z., Ellis E.B., Helm S.H., Gomez R., Dean D.D., Boyan B.D. Nongenomic regulation of protein kinase C isoforms by the vitamin D metabolites 1α,25-(OH)2D3 and 24R,25-(OH)2D3. J Cell Physiol. 1996;167:380–393. doi: 10.1002/(SICI)1097-4652(199606)167:3<380::AID-JCP2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Wang L., Clutter S., Benincosa J., Fortney J., Gibson L.F. Activation of transforming growth factor-beta1/p38/Smad3 signaling in stromal cells requires reactive oxygen species-mediated MMP-2 activity during bone marrow damage. Stem Cells. 2005;23:1122–1134. doi: 10.1634/stemcells.2004-0354. [DOI] [PubMed] [Google Scholar]
- Wang M., Zhao D., Spinetti G., Zhang J., Jiang L.Q., Pintus G., Monticone R., Lakatta E.G. Matrix metalloproteinase 2 activation of transforming growth factor-β1 (TGF-β1) and TGF-β1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006;26:1503–1509. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]