Abstract
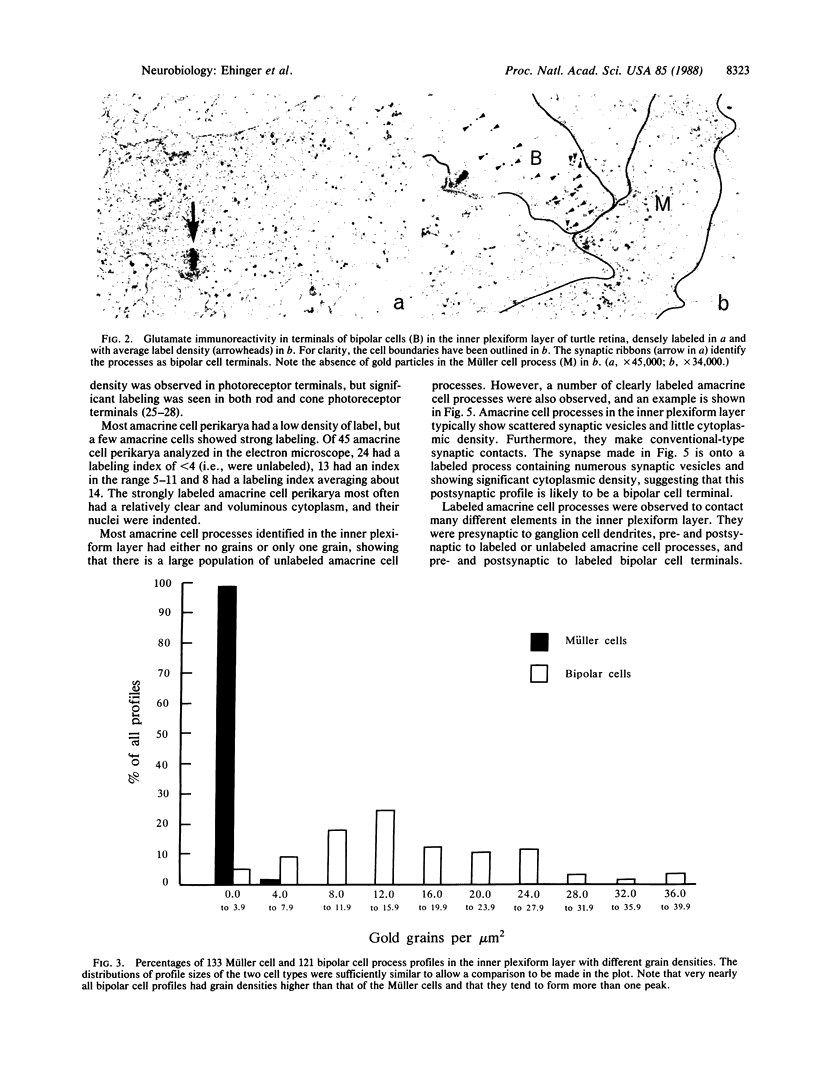
Strong glutamate immunoreactivity was observed by both light and electron microscopy in bipolar cells of the turtle (Pseudemys scripta elegans) retina after postembedding immunohistochemistry. Virtually all bipolar cells showed strong labeling, on average 18 times that of the Müller (glial) cells. The data suggest that both on- and off-center bipolar cells are glutamatergic. Photoreceptors were also labeled, but with a labeling intensity about half that of the bipolar cells. Other types of retinal neurons showed less immunoreactivity, except for a small population of strongly labeled amacrine cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agardh E., Ehinger B., Wu J. Y. GABA and GAD-like immunoreactivity in the primate retina. Histochemistry. 1987;86(5):485–490. doi: 10.1007/BF00500621. [DOI] [PubMed] [Google Scholar]
- Bloomfield S. A., Dowling J. E. Roles of aspartate and glutamate in synaptic transmission in rabbit retina. I. Outer plexiform layer. J Neurophysiol. 1985 Mar;53(3):699–713. doi: 10.1152/jn.1985.53.3.699. [DOI] [PubMed] [Google Scholar]
- Brunken W. J., Witkovsky P., Karten H. J. Retinal neurochemistry of three elasmobranch species: an immunohistochemical approach. J Comp Neurol. 1986 Jan 1;243(1):1–12. doi: 10.1002/cne.902430102. [DOI] [PubMed] [Google Scholar]
- Bruun A., Ehinger B., Sytsma V. M. Neurotransmitter localization in the skate retina. Brain Res. 1984 Mar 19;295(2):233–248. doi: 10.1016/0006-8993(84)90972-7. [DOI] [PubMed] [Google Scholar]
- Dacheux R. F. Connections of the small bipolar cells with the photoreceptors in the turtle. An electron microscope study of Golgi-impregnated, gold-toned retinas. J Comp Neurol. 1982 Feb 10;205(1):55–62. doi: 10.1002/cne.902050106. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Boycott B. B. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966 Nov 15;166(1002):80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. Landolt's club in the amphibian retina: a Golgi and electron microscope study. Invest Ophthalmol. 1966 Oct;5(5):484–496. [PubMed] [Google Scholar]
- Ishida A. T., Fain G. L. D-aspartate potentiates the effects of L-glutamate on horizontal cells in goldfish retina. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5890–5894. doi: 10.1073/pnas.78.9.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Teranishi T., Negishi K. L-Glutamate depolarizes ON-OFF transient type of amacrine cells in the carp retina: an ionophoretic study. Brain Res. 1985 Mar 11;329(1-2):390–394. doi: 10.1016/0006-8993(85)90557-8. [DOI] [PubMed] [Google Scholar]
- Kolb H., Jones J. Light and electron microscopy of the photoreceptors in the retina of the red-eared slider, Pseudemys scripta elegans. J Comp Neurol. 1982 Aug 20;209(4):331–338. doi: 10.1002/cne.902090402. [DOI] [PubMed] [Google Scholar]
- Kolb H., Jones J. Synaptic organization of the outer plexiform layer of the turtle retina: an electron microscope study of serial sections. J Neurocytol. 1984 Aug;13(4):567–591. doi: 10.1007/BF01148080. [DOI] [PubMed] [Google Scholar]
- Lasater E. M., Dowling J. E. Carp horizontal cells in culture respond selectively to L-glutamate and its agonists. Proc Natl Acad Sci U S A. 1982 Feb;79(3):936–940. doi: 10.1073/pnas.79.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz P. D., McReynolds J. S. Synaptic transmission at N-methyl-D-aspartate receptors in the proximal retina of the mudpuppy. J Physiol. 1985 Oct;367:99–115. doi: 10.1113/jphysiol.1985.sp015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland R. H., Mills J. W., Cassidy C. The functions of acetylcholine in the rabbit retina. Proc R Soc Lond B Biol Sci. 1984 Nov 22;223(1230):121–139. doi: 10.1098/rspb.1984.0086. [DOI] [PubMed] [Google Scholar]
- Miller R. F., Dacheux R. F. Synaptic organization and ionic basis of on and off channels in mudpuppy retina. II. Chloride-dependent ganglion cell mechanisms. J Gen Physiol. 1976 Jun;67(6):661–678. doi: 10.1085/jgp.67.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. F., Dacheux R. F. Synaptic organization and ionic basis of on and off channels in mudpuppy retina. III. A model of ganglion cell receptive field organization based on chloride-free experiments. J Gen Physiol. 1976 Jun;67(6):679–690. doi: 10.1085/jgp.67.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. Functional organization of catfish retina. J Neurophysiol. 1977 Jan;40(1):26–43. doi: 10.1152/jn.1977.40.1.26. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T. Relation of spectral types to oil droplets in cones of turtle retina. Science. 1985 Aug 30;229(4716):874–877. doi: 10.1126/science.4023716. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T. Spectral sensitivities of seven morphological types of photoreceptors in the retina of the turtle, Geoclemys reevesii. J Comp Neurol. 1985 Jul 8;237(2):145–154. doi: 10.1002/cne.902370202. [DOI] [PubMed] [Google Scholar]
- Ottersen O. P. Postembedding light- and electron microscopic immunocytochemistry of amino acids: description of a new model system allowing identical conditions for specificity testing and tissue processing. Exp Brain Res. 1987;69(1):167–174. doi: 10.1007/BF00247039. [DOI] [PubMed] [Google Scholar]
- Ottersen O. P., Storm-Mathisen J. Glutamate- and GABA-containing neurons in the mouse and rat brain, as demonstrated with a new immunocytochemical technique. J Comp Neurol. 1984 Nov 1;229(3):374–392. doi: 10.1002/cne.902290308. [DOI] [PubMed] [Google Scholar]
- Ottersen O. P., Storm-Mathisen J., Madsen S., Skumlien S., Strømhaug J. Evaluation of the immunocytochemical method for amino acids. Med Biol. 1986;64(2-3):147–158. [PubMed] [Google Scholar]
- Pourcho R. G., Goebel D. J. Visualization of endogenous glycine in cat retina: an immunocytochemical study with Fab fragments. J Neurosci. 1987 Apr;7(4):1189–1197. doi: 10.1523/JNEUROSCI.07-04-01189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981 Jan 9;211(4478):182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. An excitatory amino acid antagonist blocks cone input to sign-conserving second-order retinal neurons. Science. 1983 Mar 11;219(4589):1230–1232. doi: 10.1126/science.6131536. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. Bipolar cells in the mudpuppy retina use an excitatory amino acid neurotransmitter. Nature. 1983 Jun 9;303(5917):537–538. doi: 10.1038/303537a0. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. The role of excitatory amino acid transmitters in the mudpuppy retina: an analysis with kainic acid and N-methyl aspartate. J Neurosci. 1983 Aug;3(8):1701–1711. doi: 10.1523/JNEUROSCI.03-08-01701.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P., Halasy K., Somogyi J., Storm-Mathisen J., Ottersen O. P. Quantification of immunogold labelling reveals enrichment of glutamate in mossy and parallel fibre terminals in cat cerebellum. Neuroscience. 1986 Dec;19(4):1045–1050. doi: 10.1016/0306-4522(86)90121-1. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Hodgson A. J. Antisera to gamma-aminobutyric acid. III. Demonstration of GABA in Golgi-impregnated neurons and in conventional electron microscopic sections of cat striate cortex. J Histochem Cytochem. 1985 Mar;33(3):249–257. doi: 10.1177/33.3.2579124. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Hodgson A. J., Smith A. D., Nunzi M. G., Gorio A., Wu J. Y. Different populations of GABAergic neurons in the visual cortex and hippocampus of cat contain somatostatin- or cholecystokinin-immunoreactive material. J Neurosci. 1984 Oct;4(10):2590–2603. doi: 10.1523/JNEUROSCI.04-10-02590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm-Mathisen J., Leknes A. K., Bore A. T., Vaaland J. L., Edminson P., Haug F. M., Ottersen O. P. First visualization of glutamate and GABA in neurones by immunocytochemistry. Nature. 1983 Feb 10;301(5900):517–520. doi: 10.1038/301517a0. [DOI] [PubMed] [Google Scholar]
- Weiler R., Schütte M. Morphological and pharmacological analysis of putative serotonergic bipolar and amacrine cells in the retina of a turtle, Pseudemys scripta elegans. Cell Tissue Res. 1985;241(2):373–382. doi: 10.1007/BF00217183. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Eldred W., Karten H. J. Catecholamine- and indoleamine-containing neurons in the turtle retina. J Comp Neurol. 1984 Sep 10;228(2):217–225. doi: 10.1002/cne.902280208. [DOI] [PubMed] [Google Scholar]