Slow wave sleep (SWS) is defined as the sum of stage 3 and stage 4 sleep and is characterized by high-voltage, synchronized electroencephalographic (EEG) waveforms, often measured as slow wave activity (SWA). SWS has been widely hypothesized to be a time of relatively heightened neurophysiologic restoration or recuperation.1,2 This viewpoint is prompted by a number of observations including: (1) enhanced SWA following sleep deprivation in proportion to the duration of prior wakefulness3; (2) reduced SWA during nocturnal sleep following afternoon/evening naps4; (3) a gradual decline in SWA across a night of sleep5; and (4) increased SWS following nights of fragmented sleep.6 Within the two-process model of sleep regulation, heightened SWS/SWA has been viewed as reflecting Process S, the homeostatic component.7 Other authors have proposed that increased SWS/SWA represents ongoing cortical recovery from activities during prior wakefulness.8
A number of agents have been found to increase the time spent in SWS (Table 1). Interestingly, despite the common observation of enhanced SWS, these drugs have several different mechanisms of action. For example, tiagabine is an inhibitor of GAT-1, one of four transporter proteins that promote the reuptake of γ-aminobutyric acid (GABA) into presynaptic terminals and surrounding glial cells. GAT-1 inhibition results in increased synaptic levels of GABA9 and heightened inhibitory activity. In contrast, gaboxadol is an extrasynaptic GABAA receptor agonist that is selective for δ receptors.10 When activated, α4-δ receptors produce a tonic inhibitory conductance, which is thought to result in a more stable inhibitory pattern compared with phasic synaptic inhibition.11 Other drugs have no direct GABAergic effects yet also increase SWS. Examples include α2-δ calcium channel ligands (e.g., gabapentin and pregabalin), serotonin (5HT)2A receptor antagonists (e.g., eplivanserin and ritanserin), and drugs that are active at multiple receptors (e.g., mirtazapine, trazodone, and olanzapine).
Table 1.
Drugs Known to Increase Slow Wave Sleep
| Drug | Mechanism of action | Reference |
|---|---|---|
| Tiagabine | GAT-1 inhibitor | Mathias et al., 200136 |
| Gaboxadol | Selective extrasynaptic GABAA agonist | Deacon et al., 200721 |
| Gabapentin | α2-δ site on voltage-gated calcium ion channels | Bazil et al., 200537 |
| Pregabalin | α2-δ site on voltage-gated calcium ion channels | Hindmarch et al., 200538 |
| GHB | GABAB/GHB agonist | Pardi et al., 200639 |
| Ritanserin | Partially selective 5HT2A receptor antagonist | Dahlitz et al., 199040 |
| Eplivanserin | Antagonist of Serotonin Two A Receptors (ASTAR) | Hindmarch et al., 200822 |
| Mirtazapine | Multiple receptors, including 5HT2 antagonist | Shen et al., 200641 |
| Olanzapine | Multiple receptors, including 5HT2 antagonist | Sharpley et al., 200542 |
| Trazodone | Multiple receptors, including 5HT2 antagonist | Mendelson, 200543 |
GABA, γ-aminobutyric acid; GHB, γ-hydroxybutyrate; 5HT, serotonin.
The availability of drugs that increase SWS has led to research on the behavioral and phenomenologic correlates of pharmacologic SWS enhancement. This article examines the pharmacologic enhancement of SWS with emphasis on two studies12,13 designed to determine whether increasing SWS (with either tiagabine or gaboxadol) reduces the established neurobehavioral and physiologic deficits associated with sleep restriction. In addition, the potential role of SWS enhancement as a novel approach to the treatment of insomnia is considered.
SLOW WAVE SLEEP ENHANCEMENT DURING SLEEP RESTRICTION
Sleep restriction reliably impairs waking neurobehavioral function in healthy adults.14 We therefore proposed that manipulation of SWS in an experimental sleep-restriction paradigm provides a suitable method to test the value of pharmacologically enhanced SWS. Specifically, the predictable responses to sleep restriction may be reduced or prevented if enhancement of SWS with pharmacologic agents increases the restorative value of sleep.
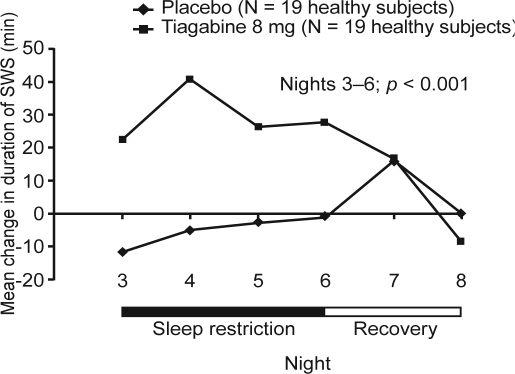
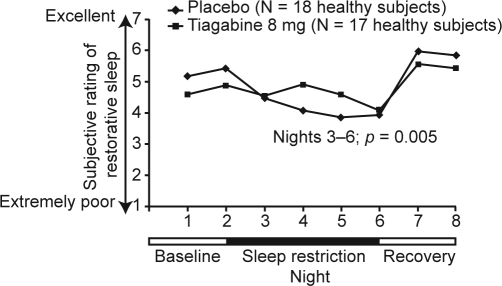
In our first investigation, the impact of enhanced SWS during sleep restriction was assessed using tiagabine in a randomized, double-blind, placebo-controlled, parallel-group study of 38 healthy adults.12 On Nights 1 and 2 (adaptation and baseline), subjects were permitted to sleep for 9 hours, followed by 4 nights (Nights 3–6) during which time in bed was restricted to 5 hours/night. On 2 recovery nights (Nights 7 and 8) subjects spent 12 hours in bed. Subjects were randomly assigned to tiagabine 8 mg or placebo on all sleep-restricted nights (Nights 3–6). Both groups received placebo at baseline and on recovery nights. On Nights 3–6, sleep restriction resulted in the predicted decreases in total sleep time (TST), stage 1 and stage 2 sleep, rapid eye movement (REM) sleep, wake after sleep onset (WASO), number of shifts to wake or stage 1 sleep, and latency to persistent sleep (all p < 0.001 vs baseline), thus validating the study design. Compared with placebo, changes from baseline during sleep restriction in SWS (Figure 1) and for stages 3 and 4 separately were significantly greater in the tiagabine group (all p < 0.001). Tiagabine administration resulted in a mean increase of 29.1 minutes (+40.9%) in SWS, whereas an average of 5.4 fewer minutes (-6.4%) of SWS were seen with placebo (Figure 1). Tiagabine was also shown to improve ratings of the restorative nature of sleep (Figure 2), although no other subjective measure of sleep differed between groups.
Figure 1.
Change from baseline in minutes of slow wave sleep (SWS) in tiagabine- and placebo-treated subjects. The p-value refers to comparison between groups on sleep restriction nights (Nights 3–6). Adapted, with permission, from Walsh et al.12
Figure 2.
Reported restorative nature of sleep in tiagabine- and placebo-treated subjects. The p-value refers to comparison between groups on the sleep restriction nights (Nights 3–6). Adapted, with permission, from Walsh et al.12
Sleep restriction in the placebo group produced the predicted daytime deficit in sustained attention on the Psychomotor Vigilance Task (PVT) and on physiologic sleep tendency assessed using the Multiple Sleep Latency Test (MSLT). The PVT and MSLT were performed at 2-hour intervals between 10.00 and 17.00 h on Days 2, 5, 6, and 7. Subjects in the tiagabine group showed significantly less impairment than the placebo group on the PVT during sleep restriction, characterized by faster mean reaction times and fewer lapses in attention (p < 0.05). In addition, the tiagabine group performed better on the Wisconsin Card Sorting Task (WCST), an assay of executive function involving problem solving and sustained attention. No differences were observed between groups on the MSLT or the Karolinska Sleepiness Scale (KSS), an introspective measure of sleepiness.12 However, subsequent analyses showed that the change from baseline in the MSLT was positively correlated with the change from baseline in SWS for tiagabine (r = 0.52; p < 0.05) (Walsh et al., unpublished data). In addition, the change from baseline in mean afternoon–evening salivary free cortisol levels was increased in response to sleep restriction in the placebo group (+0.031 μg/dL), but not in the tiagabine group (-0.005 μg/dL; p = 0.037 between groups). Enhancement of SWS with tiagabine may have counteracted the loss of hypothalamus–pituitary–adrenal regulation seen with sleep restriction.
The beneficial effects of tiagabine observed in this study for the PVT, WCST, and restorative sleep ratings are unlikely to be due to a direct effect of the drug, which would be expected to impair next-day functioning due to enhanced GABAergic function. Restorative sleep ratings were made only 6 hours after drug administration, at a time when plasma (and presumably brain) drug levels are sustained (tiagabine has an elimination half-life of 7–9 hours). This suggests that tiagabine has an indirect effect, possibly through SWS enhancement. To our knowledge this is the first study to provide evidence supporting the hypothesis that enhancement of SWS can lessen the neurobehavioral and physiologic impact of sleep loss.
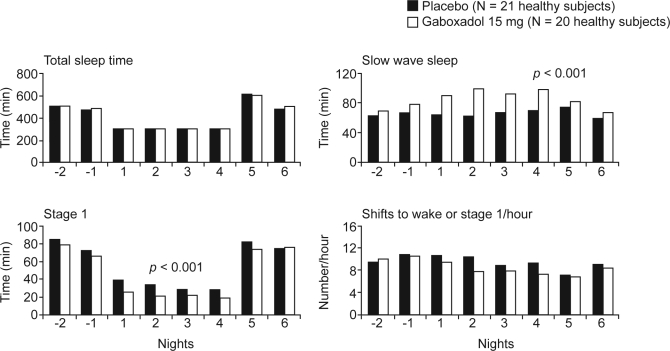
Using a similar sleep-restriction design, we have evaluated the effects of SWS enhancement with gaboxadol 15 mg.13 After baseline assessments, 41 healthy adults received either gaboxadol 15 mg (N = 20; mean ± SD age 31.9 ± 10.2 years) or placebo (N = 21; mean ± SD age 32.0 ± 9.9 years) on four sleep-restriction nights. Figure 3 shows some of the key sleep variables throughout the study. Importantly, TST was essentially identical for the two groups on sleep-restriction nights. The gaboxadol group had significantly more stage 4 sleep and SWS (but not stage 3) compared with the placebo group (both p < 0.001). A mean of 21.8 minutes more SWS was seen with gaboxadol than with placebo on the four sleep-restriction nights. Furthermore, relative to placebo, gaboxadol resulted in small but significant reductions in stage 1 sleep, REM sleep, and shifts to wake or stage 1 sleep (p < 0.05).
Figure 3.
Total sleep time, minutes of slow wave sleep, minutes of stage 1 sleep, and the number of shifts to wake or stage 1 sleep per hour in gaboxadol- and placebo-treated patients. Nights −2 and −1 are baseline, Nights 1–4 are sleep restriction, Nights 5 and 6 are recovery. The p-values refer to comparisons between groups for Nights 1–4. Adapted, with permission, from Walsh et al.13
Sleep restriction in the placebo group resulted in a marked increase in physiologic sleep tendency as rated on the MSLT and on subjective assessments of sleepiness. However, the degree of physiologic sleepiness reported on the MSLT was significantly lower in the gaboxadol group (p < 0.05). Subjects in the gaboxadol group were also more alert (measured subjectively using the KSS), and reported less fatigue and more vigor on the Profile of Moods Scale. Unlike in the tiagabine study discussed above, the PVT showed no significant differences between groups, possibly because the magnitude of impairment from sleep loss in the placebo group was very small—only about one-third of that seen in the tiagabine study. The change in the MSLT assessment of sleepiness was positively correlated with the change in SWS during sleep restriction; the more SWS was enhanced in the study, the more alert was the subject.
Overall, we believe these two studies suggest that pharmacologic enhancement of SWS reduces some of the behavioral, psychologic, and physiologic effects of restricted sleep. Although it is important to remember that sleep restriction is not an experimental model of insomnia, there are other reasons to consider SWS enhancement as a potential treatment for insomnia.
SLOW WAVE SLEEP AND INSOMNIA
Some authors report that SWS is decreased in primary insomnia, whereas other studies have not reported this. One study of patients with insomnia and matched normal sleepers, which included quantitative assessment of the EEG as well as standard polysomnographic measures, will serve to illustrate the possible SWS–insomnia connection.15 Merica and colleagues reported lower SWA levels and increased fast-frequency EEG activity during nonREM (NREM) sleep in patients with insomnia, in addition to more time awake, more stage 1 sleep, and less stage 4 sleep relative to healthy subjects.15 One interpretation of these findings is that the reduced amounts of SWS and SWA in patients with insomnia are associated with subjects' reports of difficulty with sleep onset, sleep maintenance, and nonrestorative sleep.
Other research can also be interpreted to suggest a link between SWS and sleep complaints/insomnia. SWS is substantially decreased in the elderly and in patients with major psychiatric disorders, populations with a high prevalence of insomnia complaints. There also appears to be a strong relationship between SWS and reduced arousability from sleep. When comparing the number of shifts to wakefulness or stage 1 sleep from stages 2, 3, 4, or REM sleep, SWS is associated with far fewer shifts, approximately 20% of the number seen in stage 2 or REM. This is true for healthy adult subjects and for adults of any age with insomnia (Walsh et al., unpublished data). Considering these findings together, it seems reasonable to propose that increasing SWS may be beneficial to those with insomnia.
Several investigations of the use of tiagabine and gaboxadol in patients with primary or transient insomnia have been published recently. The effect of tiagabine (4, 8, 12, and 16 mg) on sleep and next-morning alertness and performance was studied in 58 adult patients with primary insomnia. The increase in SWS was dose-dependent, with a two- to four-fold increase in SWS with the 8 mg through 16 mg doses, compared with placebo (p < 0.001 for tiagabine 8, 12, and 16 mg). Tiagabine also produced a trend toward increased TST and decreased WASO, but had no effect on latency to persistent sleep and little effect on the self-reported ratings of sleep.16
In a separate study of 232 patients with primary insomnia, significantly greater increases in SWS were also seen with tiagabine at doses of 6, 8, and 10 mg compared with placebo (p < 0.01); no significant differences were seen with the 4 mg dose.17 There were concomitantly greater decreases in stage 1 sleep with all doses of tiagabine (p < 0.01); however, no significant differences were observed in WASO, latency to persistent sleep, or TST. There were significant impairments in some self-reported measures of sleep and daytime function, and in psychomotor performance with the 10 mg dose compared with placebo (p < 0.05).
The effect of tiagabine on SWS was confirmed in a study of 207 elderly patients with primary insomnia.18 Significantly greater increases in SWS were found for tiagabine 4, 6, and 8 mg compared with placebo (all p < 0.05), with a corresponding significant decrease in stage 1 sleep (p < 0.05). Tiagabine did not significantly affect WASO, latency to persistent sleep, or TST compared with placebo, although the 6 and 8 mg doses significantly reduced the number of awakenings after sleep onset (NASO) (p < 0.05) and increased the ratio of SWS to stage 1 sleep plus WASO (p < 0.001). However, tiagabine 8 mg significantly decreased subjective TST and the refreshing quality of sleep as well as daily functioning in these elderly patients (p < 0.05).
A dose–response study has been conducted with gaboxadol (5, 10, and 15 mg) in 109 healthy subjects in whom habitual sleep time was advanced by 4 hours to produce transient sleep disruption.19 Zolpidem 10 mg was used as an active control and significantly improved TST, WASO, NASO, and sleep latency compared with placebo (p < 0.05), thus demonstrating assay sensitivity. In addition, sleep improvements with zolpidem were generally better than those reported with any dose of gaboxadol. Gaboxadol produced a dose-related increase in SWS compared with placebo, with the absolute difference from placebo ranging from approximately 5 to 22 minutes. TST was significantly increased by approximately 30 minutes (p < 0.001) and WASO was reduced by approximately 17–20 minutes (p < 0.05) with all doses of gaboxadol relative to placebo. Most subjective sleep measures also improved with gaboxadol relative to placebo.
A dose-related increase in SWS was also reported with gaboxadol compared with placebo in a study of 40 patients with primary insomnia (10 mg: p < 0.01; 20 mg: p < 0.001).20 Zolpidem 10 mg was again used as an active control. Gaboxadol 20 mg significantly reduced WASO (p < 0.01), and both doses of gaboxadol, but not zolpidem, significantly reduced NASO (p < 0.001). Gaboxadol 20 mg and zolpidem significantly increased TST (p < 0.05). However, neither drug had a significant effect on time to sleep onset. There were no reports of next-day residual effects with gaboxadol or zolpidem.
Gaboxadol 15 mg was also shown to enhance SWS in a study of 26 patients with primary insomnia without significantly affecting stage 1, stage 2, or REM sleep.21 Gaboxadol 5 mg and 15 mg significantly improved TST (p < 0.05); WASO also improved, although statistical significance was only achieved with the 5 mg dose. Gaboxadol 15 mg also significantly reduced latency to persistent sleep (p < 0.05). No next-day residual effects were observed with either dose of gaboxadol.
In summary, tiagabine and gaboxadol consistently increase SWS, but generally appear to have an inconsistent and less robust effect on traditional hypnotic efficacy measures than benzodiazepine receptor agonists. One limitation common to these studies is the short duration of treatment (the drug administration period did not exceed 2 nights), and it is possible that drug effects may change with sustained use. Published data for other SWS-enhancing drugs in patients with insomnia are sparse.
The question arises as to whether it is necessary to improve traditional efficacy measures (i.e., latency to persistent sleep or WASO) for a drug to have a role in the management of insomnia or whether manipulation of other aspects of sleep, such as SWS or sleep continuity, may be clinically useful. This may be particularly pertinent if there is an improved safety margin. Although research to date with tiagabine and gaboxadol does not indicate that they have an improved therapeutic margin versus benzodiazepine receptor agonists, drugs with a nonGABAergic mechanism of action may have some safety advantages. For example, in a preliminary report of a Phase I study in healthy subjects, the 5HT2A antagonist eplivanserin (1, 10, and 40 mg) did not impair performance or short-term memory throughout the day when administered in the morning, or the previous evening. Nevertheless, there was a significant increase in the duration of SWS (p = 0.0001) and a corresponding decrease in stage 2 sleep at all doses, irrespective of the time of dosing.22 These data suggest that SWS can be increased by 5HT2A antagonists without producing sedation. In a preliminary report of a Phase II study, eplivanserin 5 mg significantly reduced WASO and NASO in a study of 351 patients with primary insomnia treated for 4 weeks, without residual sedative effects.23
It is also interesting to speculate that enhancement of SWS may benefit patients with insomnia in ways that are not necessarily related to standard efficacy measures. For example, a leading hypothesis related to the pathophysiology of insomnia postulates that general central nervous system (CNS) hyperarousal is involved, as evidenced by elevated whole–body oxygen consumption,24 heart rate,25 cortisol levels,26 cerebral metabolism,27 high-frequency EEG activity,15 and MSLT scores28 compared with healthy controls.
Increasing SWS may have a beneficial effect on these arousal systems. Vgontzas and colleagues have reported that cortisol levels in patients with chronic insomnia were positively correlated with total wake time and negatively correlated with SWS.29 Another study of those with primary insomnia found that efficient overnight consolidation of declarative memory was associated with higher SWS and low cortisol levels. When SWS is decreased, REM sleep may play a partly compensatory role in the consolidation of declarative memory.30 Indeed, evidence from several studies has demonstrated that sleep, most probably SWS, is critical in terms of learning, memory consolidation, and memory retrieval (see article by Walker31 and reference numbers 32-34). Furthermore, enhancement of SWS using electrical stimulation in healthy subjects can enhance sleep-associated consolidation of memory.35
CONCLUSIONS
Our sleep-restriction studies with tiagabine and gaboxadol indicate that pharmacologically increased SWS reduces the negative neurobehavioral and physiologic consequences of sleep loss. We interpret these findings to indicate that the increased SWS is not simply an EEG epiphenomenon, but a physiologically meaningful change in sleep, perhaps similar to the homeostatically regulated changes in sleep following sleep loss.
Although imposed sleep restriction cannot be viewed as a model of insomnia, there are reasons to consider the role of pharmacologic SWS enhancement as a therapy for insomnia. These include evidence that patients with insomnia have reduced SWS and increased fast-frequency EEG activity during sleep, as well as evidence of CNS hyperarousal. In addition, SWS is generally a state of strong sleep continuity, as shown by low rates of shifts to wakefulness or stage 1 sleep. Finally, some SWS-enhancing drugs may have a wide safety margin because their mechanism of action does not involve GABAergic systems. Although research to date with tiagabine and gaboxadol has not yielded promising results, further research with longer-term treatments and with drugs that increase SWS via other mechanisms of action should be instructive in determining the therapeutic value of SWS enhancement for the management of insomnia.
DISCLOSURE STATEMENT
The research studies performed by Dr. Walsh discussed in this article were funded by Cephalon, Lundbeck, and Merck. Dr Walsh has provided consulting services for Actelion, Cephalon, Eli Lilly, Evotec, GlaxoSmithKline, Jazz, Merck, Neurocrine, Neurogen, Pfizer, Respironics, Sanofi-Aventis, Sepracor, Shering-Plough/Organon, Somaxon, Somnus, Takeda, Transcept, and Vanda. Research support has been provided to Dr. Walsh's institution by Actelion, Evotec, Jazz, Merck, Pfizer, Respironics, Sanofi-Aventis, Somaxon, Vanda, and Ventus.
REFERENCES
- 1.Horne J. Human slow wave sleep: a review and appraisal of recent findings, with implications for sleep functions, and psychiatric illness. Experientia. 1992;48:941–54. doi: 10.1007/BF01919141. [DOI] [PubMed] [Google Scholar]
- 2.Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–60. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- 3.Dijk DJ, Brunner DP, Beersma DG, Borbely AA. Electroencephalogram power density and slow wave sleep as a function of prior waking and circadian phase. Sleep. 1990;13:430–40. doi: 10.1093/sleep/13.5.430. [DOI] [PubMed] [Google Scholar]
- 4.Werth E, Dijk DJ, Achermann P, Borbely AA. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am J Physiol. 1996;271:R501–R510. doi: 10.1152/ajpregu.1996.271.3.R501. [DOI] [PubMed] [Google Scholar]
- 5.Borbely AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–95. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet MH. Sleep restoration as a function of periodic awakening, movement, or electroencephalographic change. Sleep. 1987;10:364–73. doi: 10.1093/sleep/10.4.364. [DOI] [PubMed] [Google Scholar]
- 7.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 8.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–50. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Fink-Jensen A, Suzdak PD, Swedberg MD, et al. The gamma-aminobutyric acid (GABA) uptake inhibitor, tiagabine, increases extracellular brain levels of GABA in awake rats. Eur J Pharmacol. 1992;220:197–201. doi: 10.1016/0014-2999(92)90748-s. [DOI] [PubMed] [Google Scholar]
- 10.Winsky-Sommerer R, Vyazovskiy VV, Homanics GE, Tobler I. The EEG effects of THIP (Gaboxadol) on sleep and waking are mediated by the GABA(A)delta-subunit-containing receptors. Eur J Neurosci. 2007;25:1893–9. doi: 10.1111/j.1460-9568.2007.05455.x. [DOI] [PubMed] [Google Scholar]
- 11.Chandra D, Jia F, Liang J, et al. GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci U S A. 2006;103:15230–5. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh JK, Randazzo AC, Stone K, et al. Tiagabine is associated with sustained attention during sleep restriction: evidence for the value of slow-wave sleep enhancement? Sleep. 2006;29:433–43. [PubMed] [Google Scholar]
- 13.Walsh JK, Snyder E, Hall J, et al. Slow wave sleep enhancement with gaboxadol reduces daytime sleepiness during sleep restriction. Sleep. 2008;31:659–72. doi: 10.1093/sleep/31.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 15.Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10:1826–34. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- 16.Walsh JK, Zammit G, Schweitzer PK, Ondrasik J, Roth T. Tiagabine enhances slow wave sleep and sleep maintenance in primary insomnia. Sleep Med. 2006;7:155–61. doi: 10.1016/j.sleep.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Walsh JK, Perlis M, Rosenthal M, et al. Tiagabine increases slow-wave sleep in a dose-dependent fashion without affecting traditional efficacy measures in adults with primary insomnia. J Clin Sleep Med. 2006;2:35–41. [PubMed] [Google Scholar]
- 18.Roth T, Wright KP, Jr, Walsh J. Effect of tiagabine on sleep in elderly subjects with primary insomnia: a randomized, double-blind, placebo-controlled study. Sleep. 2006;29:335–41. doi: 10.1093/sleep/29.3.335. [DOI] [PubMed] [Google Scholar]
- 19.Walsh JK, Deacon S, Dijk DJ, Lundahl J. The selective extrasynaptic GABAA agonist, gaboxadol, improves traditional hypnotic efficacy measures and enhances slow wave activity in a model of transient insomnia. Sleep. 2007;30:593–602. doi: 10.1093/sleep/30.5.593. [DOI] [PubMed] [Google Scholar]
- 20.Lundahl J, Staner L, Staner C, Loft H, Deacon S. Short-term treatment with gaboxadol improves sleep maintenance and enhances slow wave sleep in adult patients with primary insomnia. Psychopharmacology (Berl) 2007;195:139–46. doi: 10.1007/s00213-007-0866-0. [DOI] [PubMed] [Google Scholar]
- 21.Deacon S, Staner L, Staner C, et al. Effect of short-term treatment with gaboxadol on sleep maintenance and initiation in patients with primary insomnia. Sleep. 2007;30:281–7. doi: 10.1093/sleep/30.3.281. [DOI] [PubMed] [Google Scholar]
- 22.Hindmarch I, Saubadu S, Delfolie A, Martinez JM, Pinquier JL. Sleep and psychomotor performance in healthy subjects after morning or evening administration of eplivanserin, a novel sleep compound. J Sleep Res. 2008;17(Suppl.1) Abstract No. P359. [Google Scholar]
- 23.Estivill E, Renault-Djouadi J, Hecquet C. Eplivanserin, a novel sleep compound, reduces night-time awakenings in patients with sleep maintenance insomnia without evidence of residual effects. J Sleep Res. 2008;17(Suppl.1) Abstract No. O60. [Google Scholar]
- 24.Bonnet MH, Arand DL. Insomnia, metabolic rate and sleep restoration. J Intern Med. 2003;254:23–31. doi: 10.1046/j.1365-2796.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 25.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60:610–5. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Rodenbeck A, Huether G, Ruther E, Hajak G. Interactions between evening and nocturnal cortisol secretion and sleep parameters in patients with severe chronic primary insomnia. Neurosci Lett. 2002;324:159–63. doi: 10.1016/s0304-3940(02)00192-1. [DOI] [PubMed] [Google Scholar]
- 27.Nofzinger EA, Nissen C, Germain A, et al. Regional cerebral metabolic correlates of WASO during NREM sleep in insomnia. J Clin Sleep Med. 2006;2:316–22. [PubMed] [Google Scholar]
- 28.Stepanski E, Zorick F, Roehrs T, Roth T. Effects of sleep deprivation on daytime sleepiness in primary insomnia. Sleep. 2000;23:215–9. [PubMed] [Google Scholar]
- 29.Vgontzas AN, Tsigos C, Bixler EO, et al. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res. 1998;45:21–31. doi: 10.1016/s0022-3999(97)00302-4. [DOI] [PubMed] [Google Scholar]
- 30.Backhaus J, Junghanns K, Born J, et al. Impaired declarative memory consolidation during sleep in patients with primary insomnia: Influence of sleep architecture and nocturnal cortisol release. Biol Psychiatry. 2006;60:1324–30. doi: 10.1016/j.biopsych.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 31.Walker M. The role of slow wave sleep in memory processing. J Clin Sleep Med. 2009;5(Supplement):S20–26. [PMC free article] [PubMed] [Google Scholar]
- 32.Buzsáki G. Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res. 1998;7(Suppl 1):17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- 33.Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–9. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 34.Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10:385–92. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- 35.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–3. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 36.Mathias S, Wetter TC, Steiger A, Lancel M. The GABA uptake inhibitor tiagabine promotes slow wave sleep in normal elderly subjects. Neurobiol Aging. 2001;22:247–53. doi: 10.1016/s0197-4580(00)00232-3. [DOI] [PubMed] [Google Scholar]
- 37.Bazil CW, Battista J, Basner RC. Gabapentin improves sleep in the presence of alcohol. J Clin Sleep Med. 2005;1:284–7. [PubMed] [Google Scholar]
- 38.Hindmarch I, Dawson J, Stanley N. A double-blind study in healthy volunteers to assess the effects on sleep of pregabalin compared with alprazolam and placebo. Sleep. 2005;28:187–93. doi: 10.1093/sleep/28.2.187. [DOI] [PubMed] [Google Scholar]
- 39.Pardi D, Black J. gamma-Hydroxybutyrate/sodium oxybate: neurobiology, and impact on sleep and wakefulness. CNS Drugs. 2006;20:993–1018. doi: 10.2165/00023210-200620120-00004. [DOI] [PubMed] [Google Scholar]
- 40.Dahlitz M, Wells P, James R, Idzikowski C, Parkes JD. Treatment of insomnia with ritanserin. Lancet. 1990;336:379. doi: 10.1016/0140-6736(90)91924-y. [DOI] [PubMed] [Google Scholar]
- 41.Shen J, Chung SA, Kayumov L, et al. Polysomnographic and symptomatological analyses of major depressive disorder patients treated with mirtazapine. Can J Psychiatry. 2006;51:27–34. doi: 10.1177/070674370605100106. [DOI] [PubMed] [Google Scholar]
- 42.Sharpley AL, Attenburrow ME, Hafizi S, Cowen PJ. Olanzapine increases slow wave sleep and sleep continuity in SSRI-resistant depressed patients. J Clin Psychiatry. 2005;66:450–4. doi: 10.4088/jcp.v66n0407. [DOI] [PubMed] [Google Scholar]
- 43.Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469–76. doi: 10.4088/jcp.v66n0409. [DOI] [PubMed] [Google Scholar]