Sleep is thought to be important for efficient daytime functioning. Deep nonrapid eye movement (NREM) sleep, also known as slow wave sleep (SWS), is considered to be the most restorative sleep stage and to be associated with sleep quality1,2 and maintenance of sleep.3 However, there is much to be learned about the function of SWS and its effects on other physiologic processes and daytime functioning. This review briefly summarizes some of the characteristics of SWS, how it is measured and regulated, as well as the contribution of SWS to sleep propensity and sleep maintenance.
WHAT IS SWS?
Sleep consists of two distinct phases: rapid eye movement (REM) sleep and NREM sleep, which can be identified using polysomnography. These two sleep phases have distinct sets of associated physiologic changes, neuroanatomic substrates, and neurochemical correlates.
In the young adult, REM sleep and NREM sleep alternate throughout the sleep episode in approximate 80- to 120-minute cycles, with REM sleep accounting for approximately 18–25% of total sleep time.4
According to the classification of Rechtschaffen and Kales,5 NREM sleep is subdivided into four stages: stages 1, 2, 3, and 4. Stages 1 and 2 are often considered “light sleep,” whereas stages 3 and 4 are considered “deep sleep,” and together are called SWS (Figure 1). In the young adult, stages 1 and 2 account for approximately 4–9% and 45–60% of total sleep time, respectively, while normal subjects spend between 10 and 25% of their total sleep time in SWS.4 In the new scoring manual of the American Academy of Sleep Medicine, a distinction between stage 3 and stage 4 sleep is no longer made and SWS is referred to as NREM3 or N3.6 Defining characteristics of NREM sleep on the electroencephalogram (EEG) are: the sleep spindles, with their characteristic “spindle-like” changes in the amplitude of the 12–14 Hz oscillations of the EEG; K complexes, lasting at least 0.5 seconds and consisting of a well-delineated negative sharp wave followed immediately by a positive component; and slow waves or delta waves, with their characteristic slow frequency (< 2 Hz) and high amplitude (> 75 μV). Sleep spindle activity and slow waves vary within NREM sleep and this variation is the basis by which NREM sleep is subdivided into stages 1–4. Although slow waves and sleep spindles are present in stages 2, 3, and 4, spindles are more prevalent in stage 2 sleep, whereas slow waves dominate the EEG during stages 3 and 4.7 The neurophysiologic correlates and mechanisms underlying these EEG phenomena have been elucidated in part.8
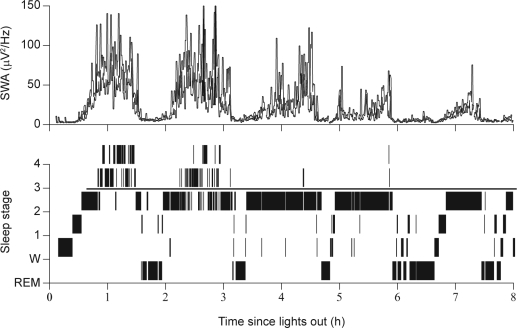
Figure 1.
Slow wave sleep and slow wave activity (SWA) during nocturnal sleep in a healthy 22-year-old male subject (Dijk, unpublished data). REM, rapid eye movement.
SWS is defined on the basis of visual assessment of the frequency, amplitude, and incidence of low-frequency oscillations in the EEG < 2 Hz according to standardized criteria.5,6 The variation in the EEG during NREM sleep can also be quantified by spectral analysis or other automated signal analysis methods, such as period amplitude analysis. Using spectral analysis, variation in the slow and delta oscillations is captured by a measure called slow wave activity (SWA), which represents power density in the 0.75–4.5 Hz range. Variation in sleep spindle activity is captured by power density in the 12–14 Hz or 12–15 Hz range (sigma or spindle frequency activity), but can also be quantified by analysis measures that detect phasic EEG activity.7,9
The dynamics of the visually identified sleep stages and computer detected SWA are illustrated in Figure 1. The time course of SWA clearly illustrates the continuous nature of the NREM sleep process, with little evidence for step-like changes in SWA at the transitions of the sleep stages. Not surprisingly, the maximum levels of SWA are present in stage 4 sleep, which is considered a deeper sleep than stage 3. However, the difference between sleep stages 3 and 4 is minor, and both may be considered delta sleep or SWS, and are called N3 according to the new classification.6 During stage 1 and REM sleep, very little SWA is present, and levels during stage 2 are intermediate between stage 1/REM and SWS. Figure 1 demonstrates very clearly how SWA gradually increases during the course of each NREM episode and then rapidly decreases shortly before the beginning of a REM episode. Moreover, both the rise rate and the maximum level of SWA decline across consecutive NREM episodes. The dynamics of SWA within and across NREM cycles have been described in great detail, and can be simulated using computer modeling.10 The dynamics of SWA are closely related to a number of other EEG measures that are thought to reflect connectivity in cortical networks.11
SWS and SWA are also correlated with variations in endocrine and cardiovascular parameters. For example, the rate of secretion of human growth hormone is greatest during SWS12 and the autonomic nervous system shifts from a sympathetic to a parasympathetic dominance during SWS.13,14 Disruption of SWS can lead to a shift in autonomic balance and associated changes in the response to glucose challenges. In fact, as few as three nights of SWS disruption in humans can lead to a reduction in the ability to respond adequately to a glucose challenge. These changes are reflected in reduced insulin sensitivity and may point to an association between SWS disruption and increased risk of diabetes, possibly mediated through the effects of SWS disruption on the autonomic nervous system.15,16 Thus SWS is not only an EEG phenomenon, or a brain state, but also a state that affects many physiologic systems.
CIRCADIAN REGULATION OF SWS
Circadian rhythmicity is a major determinant of human physiology, endocrinology, and behavior.17 The circadian rhythm of which we are most aware is the sleep–wake cycle, but other major rhythms in physiology and endocrinology include core body temperature, plasma melatonin, and cortisol. These physiologic and behavioral rhythms are all driven by the suprachiasmatic nuclei (SCN) of the hypothalamus. Lesions of this structure abolish rhythmicity of these variables, and lesion studies have also demonstrated that the SCN is involved in the timing of sleep and wakefulness in animals.18,19 Furthermore, a lesion in or near the area homologous to the SCN in humans causes significant disruption to the sleep–wake cycle.20 These data support the role of the SCN in the circadian regulation of sleep. It has now been established that circadian rhythms are generated by transcriptional–translational feedback loops of a core set of “clock” genes.21 These genes are rhythmically expressed in the SCN but also in other brain areas, and in peripheral tissues and leukocytes.22 Variations in these genes are associated with, and can lead to, variation in the timing and structure of sleep.23
Behavioral evidence for the involvement of the circadian timing system in the regulation of sleep was previously obtained in free-running experiments, in which subjects lived in environments free of time cues. Under these conditions, sleep–wake cycles not only desynchronize from the external 24-hour days, but in several cases also from the internal rhythm of body temperature. Detailed analyses of polysomnographically recorded sleep during these spontaneous occurrences of internal desynchrony suggested that sleep duration and sleep structure are determined by the interaction of a circadian oscillator and a sleep–wake oscillator.24–26
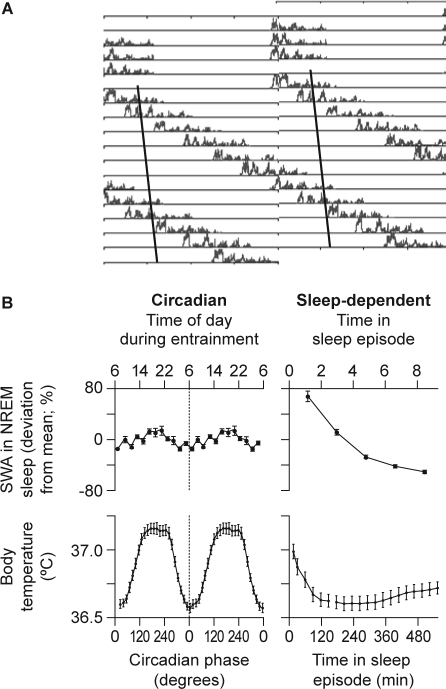
The role of the circadian pacemaker in the regulation of NREM and REM sleep was investigated further using a forced desynchrony protocol in which subjects were scheduled to sleep–wake cycles well outside the circadian range, e.g., 28 or 20 hours.27,28 The circadian pacemakers cannot follow these 28- or 20-hour cycles and hence the sleep–wake cycle desynchronizes from the circadian rhythms of body temperature, melatonin, cortisol, etc. Sleep episodes occur at all phases of the endogenous circadian cycle and SWA declines during all sleep episodes, almost independently of the circadian phase. In these studies, the circadian modulation of SWS and SWA was only minor (Figure 2). The circadian nadir of the rhythm of SWA was located in the early morning hours, when, from a circadian perspective, sleepiness is at its highest. In contrast to SWA, sleep spindle activity showed a marked circadian modulation, with maximum activity coinciding with the melatonin rhythm.27,29,30 These studies have confirmed that REM sleep is also under strong circadian control, with the crest of its rhythm occurring 1–2 hours after the temperature nadir, i.e., close to habitual wake time. These forced desynchrony studies have demonstrated beyond doubt that, whereas sleep spindle activity and REM sleep are determined to a substantial extent by the circadian clock, SWA is primarily determined by time awake, almost independently of the circadian phase. Interestingly, SWA is also virtually independent of another cycle: the menstrual cycle. SWA activity declines in all sleep episodes, during all phases of the menstrual cycle. In contrast, sleep spindle activity is modulated significantly across the phases of the menstrual cycle.31
Figure 2.
A: Time course of slow wave activity (SWA) during sleep episodes from a healthy subject during a forced desynchrony protocol. Data are double plotted, i.e., two consecutive 24-hour periods are plotted next to each other and below each other. The solid line represents the progression of the timing of the nadir of the core body temperature rhythm. Note that SWA declines in all sleep episodes, regardless of when the sleep episodes are initiated relative to the core body temperature nadir.27 B: Estimation of the circadian (left panel) and sleep-dependent (right panel) changes in SWA (upper panel) and core body temperature (lower panel) in 8 healthy subjects living in an environment free from time cues for 33–36 days and scheduled to a 28-hour rest–activity cycle.27 Note that while there is a robust sleep-dependent modulation of SWA, its circadian amplitude is very small, indicating that the SWA is not strongly modulated by circadian phase. NREM, nonrapid eye movement. Reproduced, with permission, from Dijk and Czeisler.27
HOMEOSTATIC REGULATION OF SWS
Homeostatic regulation is the control and maintenance of a stable, constant condition, and involves multiple regulatory mechanisms. Sleep-deprivation studies, which increase sleep pressure, and sleep extension and nap studies, which lead to a reduction in sleep pressure, have been used to investigate whether SWS is homeostatically controlled. This was established early in the modern era of sleep research, in particular through the extensive work by Webb and others, using visual scoring of SWS.32 More recently, this homeostatic process has been investigated in studies using quantitative EEG methods, such as spectral analysis and period-amplitude analysis, and high-density EEG methods, such as brain mapping.33–35
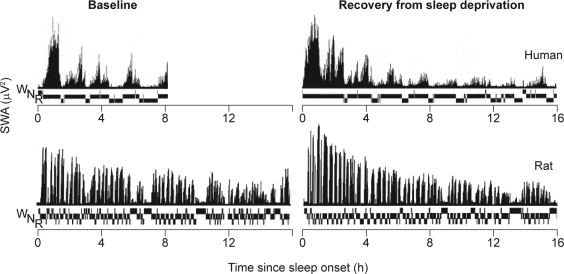
In one such study, nine subjects were kept awake for 36 hours with baseline sleep starting at 11 pm and recovery sleep at 7 pm.36 When recovery sleep is initiated at this circadian phase and subjects are not awakened by the study investigator, very long recovery sleep episodes (> 12 hours) can be observed. Analysis of the dynamics of SWA during the many NREM–REM cycles of recovery sleep revealed that SWA in NREM sleep was initially significantly enhanced compared with baseline, and then reached stable levels during the second part of the sleep episode.36 Also, in these types of experiments, little evidence for circadian control of SWS/SWA was observed (Figure 3).
Figure 3.
Time course of slow wave activity (SWA) and prevailing vigilance state (N, nonrapid eye movement sleep; R, rapid eye movement sleep; W, waking) during baseline sleep and recovery sleep following 36 hours' sleep deprivation in humans36 and 24 hours' sleep deprivation in the rat (Franken, unpublished data). Reproduced, with permission, from Dijk et al36 and Paul Franken.
Homeostatic regulation of SWS has also been demonstrated in a large number of (rodent) species. In the rat, SWA oscillates in synchrony with the NREM–REM cycles, albeit with a shorter periodicity than in humans, reflecting the smaller brain size (Figure 3).37 At baseline, SWA declines during the course of major sleep episodes, which occur during the day. Recovery from sleep deprivation is accompanied by a marked increase in NREM sleep duration and enhancement of SWA, in particular during the initial part of recovery sleep, just as it is in humans (Figure 3). Manipulation of the circadian timing system by shortening or lengthening the photoperiod exerts a predominant effect on the 24-hour sleep pattern, but little effect on 24-hour baseline levels of total sleep time or the response to sleep deprivation. Thus, changes in the duration of the photoperiod and the accompanying changes in circadian organization do not affect the basic process regulating sleep in diurnal rodents38 or humans,39 confirming the robustness of homeostatic control of SWS/SWA.
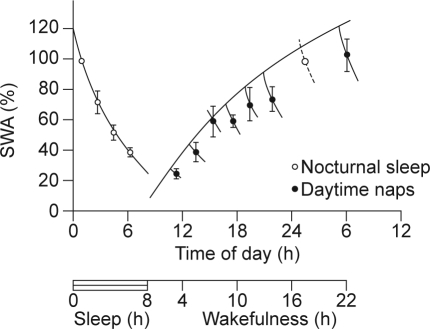
Sleep deprivation and changes in the duration of the photoperiod may be considered an extreme intervention in the study of sleep regulation. In contrast, nap studies provide a more measured approach. Several nap studies have demonstrated that within a physiologic range of wake durations, SWS is regulated very accurately.40–42 In a nap study in healthy female subjects, naps were scheduled at different times of the day, at least 2 days apart, while also maintaining a regular sleep–wake schedule. Analysis of SWA during these naps demonstrated a gradual increase of SWA in the course of the waking day. Thus, whereas during consolidated sleep SWA declines, SWA increases during wakefulness (Figure 4). It is interesting to note that in these studies, the propensity to initiate sleep dissociated from the time course of SWA. Thus SWA increased monotonically with time awake, whereas daytime sleep propensity displayed a more complex pattern, with a local maximum in the afternoon, followed by a decline and a nadir in the wake maintenance zone, shortly before habitual bedtime.
Figure 4.
Slow wave activity (SWA) in nonrapid eye movement (NREM) sleep during baseline sleep and in the first NREM sleep (open circles) and in the first NREM sleep episodes of naps (closed circles) starting at 10, 12, 14, 16, 18, 20, and 22 hours in seven healthy female subjects.33 Data are plotted at the midpoint of NREM–REM cycles. Note that the first value of baseline sleep is replotted at 24 hours. Vertical lines indicate the standard error of the mean. A saturating exponential function was fitted through the extrapolated values at sleep onset. This extrapolation was based on the duration of the NREM–REM cycle of the naps and the time constant of the exponential declining function fitted through the average values of SWA in NREM–REM cycles of baseline sleep. All values are expressed as percentages of the value of slow wave activity in the first NREM–REM cycle of baseline night sleep. Reproduced, with permission, from Dijk.33
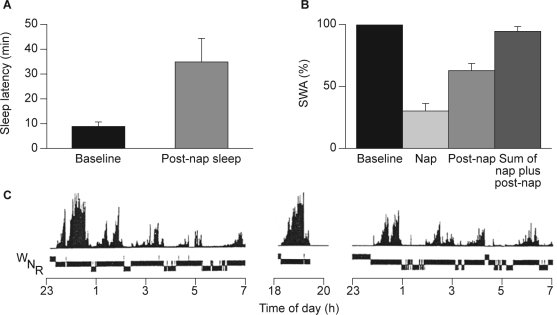
Naps during the day and in particular naps in the late afternoon or early evening led to a reduction in the level of SWS or SWA in the initial part of subsequent nocturnal sleep, and an increase in sleep spindle activity when compared with subjects who did not have a nap (Figure 5).42 This is a consequence of the homeostatic regulation of SWS/SWA: SWA produced during the nap in the early evening reduces the previously increased pressure for SWA, with the duration between the end of the nap and the scheduled beginning of the nocturnal sleep episode being insufficient to restore this pressure for SWA.
Figure 5.
A: Sleep propensity, B: slow wave activity (SWA) and C: time course of SWA and vigilance states (N, nonrapid eye movement sleep; R, rapid eye movement sleep; W, waking) of baseline night, nap and post-nap sleep after a nap in the early evening in a single subject. SWA was expressed as a percentage of total accumulated SWA in baseline sleep. Reproduced, with permission, from Werth et al.42
More recent studies have investigated local aspects of the regulation of SWA in both humans43–45 and rodents.46,47 During baseline sleep, slow waves are most prevalent in frontal areas, and during recovery sleep following sleep deprivation, the frontal cortex is the area of the brain to show the greatest increase in SWA when compared with the central, parietal, and occipital regions.35,43 The greater increase in SWA following sleep deprivation in frontal areas of the brain, together with the predominance of SWA in the frontal areas of the brain, even at baseline, has been interpreted as evidence for a role of SWS in those brain functions that are typically associated with frontal cortices. Further evidence for local regulation of SWS stems from experiments in which particular experiences during wakefulness — leading to activation in particular brain areas — resulted in a local increase in SWA in these areas. For example, extensive somatosensory stimulation of the right hand before sleep led to an increase in delta power in the left hemisphere, in particular over the somatosensory cortex.45 More recently it was shown that the opposite manipulation — immobilization of the arm — leads to a local reduction of SWA in the sensorimotor cortex.48
These studies support the hypothesis that a homeostatic regulatory mechanism, rather than a mechanism based on the circadian rhythm, is the principal mechanism involved in the regulation of SWS and SWA. Moreover, these data also suggest that SWS or the NREM sleep process is regulated in response to local activation of brain areas during wakefulness.
INDIVIDUAL DIFFERENCES IN SWS
Individuals differ markedly with respect to the duration of SWS and level of SWA. To a certain extent, demographic characteristics, including gender and age, influence these inter-individual differences, and genetic factors also appear to play a role.
Gender-Related Differences in SWS/SWA
Gender differences in NREM sleep have been noted for middle-aged and older subjects, with women having more SWS during normal and recovery sleep, and more stage 3 and 4 NREM sleep.49,50 It has been suggested that this difference may be related to the reduction in SWS that occurs with aging, rather than a specific gender difference.50
In a study comparing 13 young men (mean age 23.5 years) and 15 young women (mean age 21.9 years), visual scoring of EEGs revealed that men and women have similar amounts of SWS and REM sleep. However, spectral analysis detected significantly higher power densities during NREM sleep over a wide frequency range (0.25–11.0 Hz) in female subjects compared with male subjects.51 These differences persisted throughout the time course of sleep. The question arises as to whether these differences reflect neuroanatomic differences rather than gender differences in sleep regulatory mechanisms.51 Subsequent studies have shown that women have more SWS and SWA than men, and this sex difference is present in adults of all ages.52,53 The functional significance and correlates of these sex differences in SWS are being investigated, but remain poorly understood.54,55 Understanding differences in SWS between men and women is of interest because many more women complain of sleep problems, such as insomnia. In fact, recent data suggest that the effects of insomnia on SWA differ between men and women.56 Surprisingly, women with primary insomnia had greater low-frequency power (3–5 Hz) across all NREM periods, as well as greater high-frequency power (16–44 Hz) in the first 3 NREM periods, compared with controls (those who slept well). In contrast, men with primary insomnia did not differ from controls with respect to these variables.
Age-Related Reduction in SWS/SWA and Daytime Sleep Propensity
SWS and SWA exhibit major changes across the life span3,57,58 and aging is indeed a very strong predictor of individual differences. In fact, compared with the large number of other sleep parameters, the size of the effect of aging on SWS is one of the greatest.4 SWS reaches a peak during the prepubertal years59 and declines thereafter.58,60 Many of these studies have also shown that the number of nocturnal awakenings increases with age. It should be emphasized that these age-related changes in SWS and sleep continuity are observed even in healthy individuals without sleep complaints and without sleep disorders. The question arises as to whether this reduction in nocturnal sleep quality with aging leads to negative consequences for daytime functioning, e.g., an increase in sleep propensity. Maybe surprisingly, very little evidence for this has emerged. In fact, reviews of the effect of age on sleep propensity, as assessed by the Multiple Sleep Latency Test (MSLT), suggest that daytime sleep propensity declines with aging.61 It was recently shown that older people have lower daytime sleep propensity than young people and that this reduction is even observed when controlled for variation in habitual sleep duration.53,57,62 This age-related reduction in daytime sleep propensity is already observed in middle-aged people and occurs in the presence of statistically significant reductions in total sleep time, SWS, and SWA.57 The age-related reduction in SWS and sleep propensity is observed during all circadian phases.63,64 Thus, in summary, older people, and men in particular, have far less SWS/SWA than young people, and sleep continuity—in particular the continuity of NREM sleep65—is much poorer in older people.
Genes and Individual Differences
Individuals also differ markedly with respect to SWS and SWA even within a narrow age range and within one gender. These differences are robust against manipulations of state variables, e.g., sleep deprivation, and can be considered trait-like.66 In fact, alterations in a number of genes, including those coding for adenosine deaminase67 and the circadian clock gene coding for the PERIOD3 (PER3) protein,68 have now been shown to predict individual differences in SWS. In rodents, other clock genes, which are not only expressed in the SCN but also in other brain areas, have been shown to affect SWS.23 Thus, in contrast to the independent relationship at the behavioral and neuroanatomic levels, SWS/SWA and circadian rhythmicity appear to be related at the molecular level. It remains to be established whether these individual differences in SWS and their genetic predictors are associated with sleep and circadian disorders. The PER3 polymorphism, which predicts differences in SWS, has been shown to be associated with delayed sleep-phase syndrome.69
FUNCTIONAL CORRELATES OF SWS
The large interindividual differences in SWS/SWA, the profound age-related changes in SWS/SWA, and the accurate homeostatic regulation of SWS/SWA raise the question of functional correlates of SWS/SWA. Can differences in SWS/SWA be associated with differences in sleep quality and daytime functioning?
Effect of Reduction of Nocturnal SWS Through Napping on Subsequent Sleep Propensity
By changing sleep pressure, using either nap studies to reduce sleep pressure or sleep deprivation studies to increase sleep pressure, it is possible to affect sleep propensity during the night. For example, in a study of nine healthy subjects, a nap in the early evening led to a marked increase in the latency to sleep onset and a reduction in REM sleep latency during subsequent nocturnal sleep. Thus the latency (mean ± standard error) to stage 1 was increased from 8.8 (± 1.6) minutes to 35.6 (± 8.8) minutes, and the latency to stage 2 from 12.8 (± 2.0) minutes to 45.5 (± 8.8 minutes), while the latency to REM sleep was reduced from 76.5 (± 4.3) minutes to 57.3 (± 7.8) minutes (Figure 5).42 These effects of a nap are so substantial and reliable that post-nap nocturnal sleep has been used as a model for sleep-onset insomnia in a number of studies.70,71
Inter- and Intra-Individual Differences in SWS and Sleep Continuity
SWS and sleep continuity decline across the life span.4 Sleep deprivation enhances SWS and reduces arousals, in particular, micro-arousals.72 The relationship between SWS and sleep continuity was further investigated in a large group of healthy subjects consisting of young (20–30 years; N = 41), middle-aged (40–55 years; N = 31), and elderly (66–83 years; N = 31) subjects. It was observed that SWA and SWS were negatively correlated with the number of awakenings. This correlation persisted even when age was taken into account.3 Overall, these data suggest that interindividual variation in SWS/SWA and sleep continuity are interrelated. This relationship has also been observed in animal studies; the number of brief awakenings is inversely correlated with SWA in rats,73 guinea pigs,74 and mice.75
SWS Disruption on Daytime Functioning
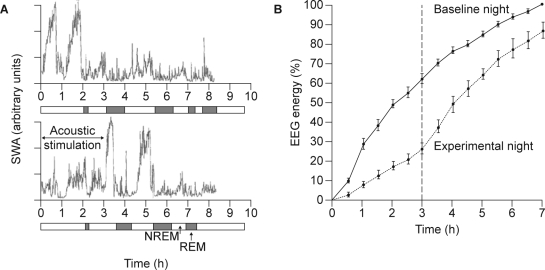
To investigate the functional significance of SWS, experimental approaches are needed. One such approach relies on disruption and reduction of SWS through acoustic stimulation (or mild electrical stimulation in early studies). Such studies have demonstrated that the amount of SWS achieved can be substantially reduced without major changes in NREM and REM time.15,76,77 In other words, the intervention only reduces SWS and, in fact, primarily reduces stage 4 sleep, although the acoustic stimulation will lead to arousals and perhaps to brief awakenings. These experiments have also demonstrated that SWS deprivation results in a SWS rebound during undisturbed subsequent sleep, which may be either within the same sleep episode or during the next sleep episode, in situations where SWS disruption has lasted all night.78,79 For example, in a sleep-disruption study in nine healthy young males, subjects were deprived of SWS using acoustic stimuli (avoiding wakefulness) during the first 3 hours of the third night. The frequencies of delta and theta activity diminished during SWS deprivation. In the hours of sleep following SWS deprivation, both the power densities and the amount of SWS increased compared with the baseline night. This study demonstrates the accurate homeostatic regulation of SWS within a sleep episode (Figure 6).78
Figure 6.
Effect of repeated disruption of sleep on slow wave activity (SWA) in nine healthy subjects. A: Time course of SWA during baseline sleep (top) and during a sleep episode during which SWA was suppressed by acoustic stimulation (bottom).33 B: Accumulation of electroencephalogram (EEG) energy during baseline (solid line) and a sleep episode during which SWA was suppressed by acoustic stimulation (dotted line). Solid vertical lines indicate the standard errors of the means. Dashed vertical line indicates the end of slow wave sleep deprivation during the experimental night. Values are expressed as a % of energy accumulated during the first 7 hours of the baseline night.78 NREM, nonrapid eye movement; REM, rapid eye movement. Reproduced, with permission, from Dijk33 and Dijk et al.78
Similarly, following 2 nights of disrupted sleep with acoustic-stimulated SWS suppression in 10 healthy subjects, the proportion of SWS was shown to increase on the recovery night. It would appear that the SWS deficit on the first night was carried over to the recovery night.79 These findings support the hypothesis that the amount of SWS is more strongly linked to the amount of SWS and SWA that was obtained during the previous sleep periods than to total sleep time, again providing evidence of a memory for SWS in accordance with its homeostatic regulation. These data also imply that SWS deprivation leads to a SWS deficit that is carried over the waking episode, suggesting that this approach can be used to investigate the effects of a SWS deficit on waking function. A number of such studies have been conducted and the results have been equivocal. In an early experiment, Johnson and co-workers compared 3 nights of stage 4 deprivation in 7 young adults and 3 nights of REM deprivation in 7 young adults. Whereas both deprivation procedures led to decreases in a number of measures, including counting performance, significant differences between the two deprivation regimes were not detected and a specific contribution of stage 4 sleep to daytime function was not identified.80 In more recent experiments, effects of SWS deprivation on performance and daytime sleep propensity were reported, but these effects were attributed to the negative effects of the intervention on sleep continuity rather than to SWS specifically.81,82 In view of the close interrelation between SWS and sleep continuity, however, it is debatable whether the effects of SWS and sleep continuity can be separated. Many of these studies used only small sample sizes and did not use spectral analysis to quantify the effects of SWS deprivation.
In one recent experiment, healthy subjects (N = 58) were deprived of SWS for 2 nights, using acoustic stimulation upon appearance of delta waves in the sleep EEG. Measurement of daytime sleep propensity using the MSLT demonstrated a significant increase in daytime sleep propensity after both nights of deprivation compared with the control group who were not deprived of SWS (N = 59).83 In another recent experiment, SWS deprivation was used to investigate its contribution to sleep-dependent improvement on a perceptual learning task in 20 young individuals. The improvement in performance on a texture-discrimination task correlated with SWA, when the control and experimental conditions were combined in those 16 individuals who met a specific initial performance criterion.84 A similar correlation between local SWA and improvement in performance on a visuomotor coordination task was reported using high-density EEG.44
Overall, these studies indicate that daytime sleep propensity is increased by inducing an enhanced pressure for SWS through disruption of night-time SWA, while nocturnal sleep propensity and SWA are reduced following an evening nap during which much of the pressure for SWA is dissipated. These studies, together with the observation that SWS and SWA are associated with sleep continuity (as assessed by the number of awakenings), suggest that enhancement of SWS and SWA may lead to an improvement in sleep continuity3 as well as a reduction in daytime sleep propensity and may contribute to daytime function.
HYPNOTICS
Most hypnotics, in particular the benzodiazepines and the Z-drugs such as zolpidem and zopiclone, reduce SWS and SWA.85 However, a number of compounds that have hypnotic properties can enhance SWS; these include ethanol,86 gamma-hydroxybutyrate/sodium oxybate,87 tiagabine,88 gaboxadol,89 and antagonists of 5HT2 receptors.70 For example, in a direct comparison of seganserin, a 5HT2 antagonist, and temazepam on human sleep stages and EEG power spectra during the night after a nap in the early evening, seganserin was found to produce an increase in SWS and an enhancement of the power density in the delta and theta frequencies during NREM sleep. In contrast, temazepam induced a reduction in the power density in the delta and theta frequencies.70 Thus, 5HT2 antagonism increased SWA and the benzodiazepines had the opposite effect.
Furthermore, eplivanserin, an Antagonist of Serotonin Two A Receptors (ASTAR), when administered to healthy subjects in the morning or in the evening, doubled the duration of SWS and produced a corresponding decrease in stage 2 sleep compared with placebo.90 Spectral analysis of the effects of eplivanserin on the sleep EEG demonstrated that SWA was increased and spindle frequency activity was decreased.91 These data show that antagonism of 5HT2 receptors results in an enhancement of SWS and SWA during sleep, which may lead to an improvement in sleep structure and may be of benefit in patients with primary insomnia.
CONCLUSIONS
SWS is precisely regulated and compensated for. The dominance of SWS in frontal areas associated with higher brain function, or in areas that have been very active during wakefulness, emphasizes the significant role of SWS. The negative correlations between SWA and SWS and measures of sleep continuity in animals and humans suggest that SWS contributes to sleep continuity. Experimental disruption of SWS increases shallow sleep and sleep fragmentation, increases daytime sleep propensity, and may impair daytime function. These data provide a rationale for testing the hypothesis that pharmacologic enhancement of SWS may lead to improvements of sleep maintenance and daytime function in patients with primary insomnia or nonrestorative sleep.
DISCLOSURE STATEMENT
Professor Dijk has received research support from AFOSR, BBSRC, GlaxoSmithKline, H Lundbeck A/S, Merck & Co Inc., Philips Lighting, Organon, Takeda, and the Wellcome Trust. Professor Dijk has served as a consultant for Actelion, Cephalon, GlaxoSmithKline, H Lundbeck A/S, Merck & Co Inc., Pfizer Inc., Philips Lighting, Sanofi-Aventis, and Takeda.
ACKNOWLEDGMENTS
Research reported in this review was conducted in the sleep laboratories at the University of Groningen, the University of Zurich, Harvard Medical School (HMS), and The University of Surrey (UoS). The staff at the Clinical Research Centres at HMS and UoS are gratefully acknowledged. Professor Dijk's recent and current research is supported by the BBSRC, Wellcome Trust, and AFOSR.
REFERENCES
- 1.Bonnet MH. Sleep restoration as a function of periodic awakening, movement, or electroencephalographic change. Sleep. 1987;10:364–73. doi: 10.1093/sleep/10.4.364. [DOI] [PubMed] [Google Scholar]
- 2.Akerstedt T, Hume K, Minors D, Waterhouse J. Good sleep–its timing and physiological sleep characteristics. J Sleep Res. 1997;6:221–9. doi: 10.1111/j.1365-2869.1997.00221.x. [DOI] [PubMed] [Google Scholar]
- 3.Dijk DJ, Groeger J, Deacon S, Stanley N. Association between individual differences in slow wave sleep, slow wave activity and sleep continuity in young, middle-aged and older men and women. Eur Neuropsychopharmacol. 2006;16:S538. [Google Scholar]
- 4.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 5.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington, DC: Public Health Service, US Government Printing Office; 1968. [Google Scholar]
- 6.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, Ill: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 7.Dijk DJ, Hayes B, Czeisler CA. Dynamics of electroencephalographic sleep spindles and slow wave activity in men: effect of sleep deprivation. Brain Res. 1993;626:190–9. doi: 10.1016/0006-8993(93)90579-c. [DOI] [PubMed] [Google Scholar]
- 8.Steriade M. Brain electrical activity and sensory processing during waking and sleep stages. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: W.B. Saunders Co.; 2005. pp. 105–124. [Google Scholar]
- 9.Knoblauch V, Martens W, Wirz-Justice A, Krauchi K, Cajochen C. Regional differences in the circadian modulation of human sleep spindle characteristics. Eur J Neurosci. 2003;18:155–63. doi: 10.1046/j.1460-9568.2003.02729.x. [DOI] [PubMed] [Google Scholar]
- 10.Achermann P, Dijk DJ, Brunner DP, Borbely AA. A model of human sleep homeostasis based on EEG slow-wave activity: quantitative comparison of data and simulations. Brain Res Bull. 1993;31:97–113. doi: 10.1016/0361-9230(93)90016-5. [DOI] [PubMed] [Google Scholar]
- 11.Esser SK, Hill SL, Tononi G. Sleep homeostasis and cortical synchronization: I. Modeling the effects of synaptic strength on sleep slow waves. Sleep. 2007;30:1617–30. doi: 10.1093/sleep/30.12.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Cauter E, Latta F, Nedeltcheva A, et al. Reciprocal interactions between the GH axis and sleep. Growth Horm IGF Res. 2004;14(Suppl A):S10–S17. doi: 10.1016/j.ghir.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Brandenberger G, Ehrhart J, Piquard F, Simon C. Inverse coupling between ultradian oscillations in delta wave activity and heart rate variability during sleep. Clin Neurophysiol. 2001;112:992–6. doi: 10.1016/s1388-2457(01)00507-7. [DOI] [PubMed] [Google Scholar]
- 14.Viola AU, James LM, Archer SN, Dijk DJ. PER3 polymorphism and cardiac autonomic control: effects of sleep debt and circadian phase. Am J Physiol Heart Circ Physiol. 2008;295:H2156–H2163. doi: 10.1152/ajpheart.00662.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dijk DJ. Slow-wave sleep, diabetes, and the sympathetic nervous system. Proc Natl Acad Sci U S A. 2008;105:1107–8. doi: 10.1073/pnas.0711635105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dijk DJ, von Schantz M. Timing and consolidation of human sleep, wakefulness, and performance by a symphony of oscillators. J Biol Rhythms. 2005;20:279–90. doi: 10.1177/0748730405278292. [DOI] [PubMed] [Google Scholar]
- 18.Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993;13:1065–79. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mistlberger RE. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res Brain Res Rev. 2005;49:429–54. doi: 10.1016/j.brainresrev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Cohen RA, Albers HE. Disruption of human circadian and cognitive regulation following a discrete hypothalamic lesion: a case study. Neurology. 1991;41:726–9. doi: 10.1212/wnl.41.5.726. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–75. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Archer SN, Viola AU, Kyriakopoulou V, von Schantz M, Dijk DJ. Inter-individual differences in habitual sleep timing and entrained phase of endogenous circadian rhythms of BMAL1, PER2 and PER3 mRNA in human leukocytes. Sleep. 2008;31:608–17. doi: 10.1093/sleep/31.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franken P, Dijk DJ. A non-circadian role for clock-genes in sleep homeostasis. Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.06723.x. In press. [DOI] [PubMed] [Google Scholar]
- 24.Czeisler CA, Weitzman E, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: its duration and organization depend on its circadian phase. Science. 1980;210:1264–7. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- 25.Zulley J, Wever R, Aschoff J. The dependence of onset and duration of sleep on th circadian rhythm of rectal temperature. Pflugers Arch. 1981;391:314–8. doi: 10.1007/BF00581514. [DOI] [PubMed] [Google Scholar]
- 26.Daan S, Beersma DG, Borbely AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–R183. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 27.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 29.Wei HG, Riel E, Czeisler CA, Dijk DJ. Attenuated amplitude of circadian and sleep-dependent modulation of electroencephalographic sleep spindle characteristics in elderly human subjects. Neurosci Lett. 1999;260:29–32. doi: 10.1016/s0304-3940(98)00851-9. [DOI] [PubMed] [Google Scholar]
- 30.Dijk DJ, Shanahan TL, Duffy JF, Ronda JM, Czeisler CA. Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans. J Physiol. 1997;505:851–8. doi: 10.1111/j.1469-7793.1997.851ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Driver HS, Dijk DJ, Werth E, Biedermann K, Borbely AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81:728–35. doi: 10.1210/jcem.81.2.8636295. [DOI] [PubMed] [Google Scholar]
- 32.Webb WB, Agnew HW. Stage 4 sleep: influence of time course variables. Science. 1971;174:1354–6. doi: 10.1126/science.174.4016.1354. [DOI] [PubMed] [Google Scholar]
- 33.Dijk DJ. EEG slow waves and sleep spindles: windows on the sleeping brain. Behav Brain Res. 1995;69:109–16. doi: 10.1016/0166-4328(95)00007-g. [DOI] [PubMed] [Google Scholar]
- 34.Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–68. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 35.Finelli LA, Borbely AA, Achermann P. Functional topography of the human nonREM sleep electroencephalogram. Eur J Neurosci. 2001;13:2282–90. doi: 10.1046/j.0953-816x.2001.01597.x. [DOI] [PubMed] [Google Scholar]
- 36.Dijk DJ, Brunner DP, Borbely AA. Time course of EEG power density during long sleep in humans. Am J Physiol. 1990;258:R650–R661. doi: 10.1152/ajpregu.1990.258.3.R650. [DOI] [PubMed] [Google Scholar]
- 37.Franken P, Tobler I, Borbely AA. Varying photoperiod in the laboratory rat: profound effect on 24-h sleep pattern but no effect on sleep homeostasis. Am J Physiol. 1995;269:R691–R701. doi: 10.1152/ajpregu.1995.269.3.R691. [DOI] [PubMed] [Google Scholar]
- 38.Dijk DJ, Daan S. Sleep EEG spectral analysis in a diurnal rodent: Eutamias sibiricus. J Comp Physiol. 1989;165:205–15. doi: 10.1007/BF00619195. [DOI] [PubMed] [Google Scholar]
- 39.Fagioli I, Barbato G, Wehr TA. Dynamics of electroencephalographic slow wave activity and body temperature during monophasic and biphasic human sleep. Neurosci Lett. 2001;298:83–6. doi: 10.1016/s0304-3940(00)01686-4. [DOI] [PubMed] [Google Scholar]
- 40.Brunet D, Nish D, MacLean AW, Coulter M, Knowles JB. The time course of ‘process S’: comparison of visually scored slow wave sleep and power spectral analysis. Electroencephalogr Clin Neurophysiol. 1988;70:278–80. doi: 10.1016/0013-4694(88)90089-2. [DOI] [PubMed] [Google Scholar]
- 41.Dijk DJ, Beersma DG, Daan S. EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. J Biol Rhythms. 1987;2:207–19. doi: 10.1177/074873048700200304. [DOI] [PubMed] [Google Scholar]
- 42.Werth E, Dijk DJ, Achermann P, Borbely AA. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am J Physiol. 1996;271:R501–R510. doi: 10.1152/ajpregu.1996.271.3.R501. [DOI] [PubMed] [Google Scholar]
- 43.Cajochen C, Foy R, Dijk DJ. Frontal predominance of a relative increase in sleep delta and theta EEG activity after sleep loss in humans. Sleep Res Online. 1999;2:65–9. [PubMed] [Google Scholar]
- 44.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 45.Kattler H, Dijk DJ, Borbely AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–64. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 46.Vyazovskiy VV, Tobler I. Regional differences in NREM sleep slow-wave activity in mice with congenital callosal dysgenesis. J Sleep Res. 2005;14:299–304. doi: 10.1111/j.1365-2869.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- 47.Vyazovskiy VV, Welker E, Fritschy JM, Tobler I. Regional pattern of metabolic activation is reflected in the sleep EEG after sleep deprivation combined with unilateral whisker stimulation in mice. Eur J Neurosci. 2004;20:1363–70. doi: 10.1111/j.1460-9568.2004.03583.x. [DOI] [PubMed] [Google Scholar]
- 48.Huber R, Ghilardi MF, Massimini M, et al. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–76. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds CF, III, Kupfer DJ, Hoch CC, et al. Sleep deprivation in healthy elderly men and women: effects on mood and on sleep during recovery. Sleep. 1986;9:492–501. doi: 10.1093/sleep/9.4.492. [DOI] [PubMed] [Google Scholar]
- 50.Webb WB. Sleep in older persons: sleep structures of 50- to 60-year-old men and women. J Gerontol. 1982;37:581–6. doi: 10.1093/geronj/37.5.581. [DOI] [PubMed] [Google Scholar]
- 51.Dijk DJ, Beersma DG, Bloem GM. Sex differences in the sleep EEG of young adults: visual scoring and spectral analysis. Sleep. 1989;12:500–7. doi: 10.1093/sleep/12.6.500. [DOI] [PubMed] [Google Scholar]
- 52.Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20-60 years old) Psychophysiology. 2001;38:232–42. [PubMed] [Google Scholar]
- 53.Dijk DJ. Sleep of aging women and men: back to basics. Sleep. 2006;29:12–3. doi: 10.1093/sleep/29.1.12. [DOI] [PubMed] [Google Scholar]
- 54.Latta F, Leproult R, Tasali E, Hofmann E, Van Cauter E. Sex differences in delta and alpha EEG activities in healthy older adults. Sleep. 2005;28:1525–34. doi: 10.1093/sleep/28.12.1525. [DOI] [PubMed] [Google Scholar]
- 55.Latta F, Leproult R, Tasali E, et al. Sex differences in nocturnal growth hormone and prolactin secretion in healthy older adults: relationships with sleep EEG variables. Sleep. 2005;28:1519–24. doi: 10.1093/sleep/28.12.1519. [DOI] [PubMed] [Google Scholar]
- 56.Buysse DJ, Germain A, Hall ML, et al. EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep. 2008;31:1673–82. doi: 10.1093/sleep/31.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dijk DJ, Groeger J, Deacon S, Stanley N. Age-related reduction in daytime sleep propensity. J Sleep Res. 2006;15(Suppl. 1):183. doi: 10.1093/sleep/33.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Landolt HP, Dijk DJ, Achermann P, Borbely AA. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 1996;738:205–12. doi: 10.1016/s0006-8993(96)00770-6. [DOI] [PubMed] [Google Scholar]
- 59.Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–83. [PubMed] [Google Scholar]
- 60.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–8. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 61.Arand D, Bonnet M, Hurwitz T, et al. The clinical use of the MSLT and MWT. Sleep. 2005;28:123–44. doi: 10.1093/sleep/28.1.123. [DOI] [PubMed] [Google Scholar]
- 62.Klerman EB, Dijk DJ. Age-related reduction in the maximal capacity for sleep--implications for insomnia. Curr Biol. 2008;18:1118–23. doi: 10.1016/j.cub.2008.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buysse DJ, Monk TH, Carrier J, Begley A. Circadian patterns of sleep, sleepiness, and performance in older and younger adults. Sleep. 2005;28:1365–76. doi: 10.1093/sleep/28.11.1365. [DOI] [PubMed] [Google Scholar]
- 64.Dijk DJ, Duffy JF, Czeisler CA. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol Int. 2000;17:285–311. doi: 10.1081/cbi-100101049. [DOI] [PubMed] [Google Scholar]
- 65.Dijk DJ, Duffy JF, Czeisler CA. Age-related increase in awakenings: impaired consolidation of nonREM sleep at all circadian phases. Sleep. 2001;24:565–77. doi: 10.1093/sleep/24.5.565. [DOI] [PubMed] [Google Scholar]
- 66.Tucker AM, Dinges DF, Van Dongen HP. Trait interindividual differences in the sleep physiology of healthy young adults. J Sleep Res. 2007;16:170–80. doi: 10.1111/j.1365-2869.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- 67.Retey JV, Adam M, Honegger E, et al. A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proc Natl Acad Sci U S A. 2005;102:15676–81. doi: 10.1073/pnas.0505414102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Viola AU, Archer SN, James LM, et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. 2007;17:613–8. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 69.Archer SN, Robilliard DL, Skene DJ, et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–5. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 70.Dijk DJ, Beersma DG, Daan S, Van den Hoofdakker RH. Effects of seganserin, a 5-HT2 antagonist, and temazepam on human sleep stages and EEG power spectra. Eur J Pharmacol. 1989;171:207–18. doi: 10.1016/0014-2999(89)90109-x. [DOI] [PubMed] [Google Scholar]
- 71.Mathias S, Steiger A, Lancel M. The GABA(A) agonist gaboxadol improves the quality of post-nap sleep. Psychopharmacology (Berl ) 2001;157:299–304. doi: 10.1007/s002130100819. [DOI] [PubMed] [Google Scholar]
- 72.Sforza E, Chapotot F, Pigeau R, Paul PN, Buguet A. Effects of sleep deprivation on spontaneous arousals in humans. Sleep. 2004;27:1068–75. doi: 10.1093/sleep/27.6.1068. [DOI] [PubMed] [Google Scholar]
- 73.Franken P, Dijk DJ, Tobler I, Borbely AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261:R198–R208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- 74.Tobler I, Franken P, Jaggi K. Vigilance states, EEG spectra, and cortical temperature in the guinea pig. Am J Physiol. 1993;264:R1125–R1132. doi: 10.1152/ajpregu.1993.264.6.R1125. [DOI] [PubMed] [Google Scholar]
- 75.Tobler I, Deboer T, Fischer M. Sleep and sleep regulation in normal and prion protein-deficient mice. J Neurosci. 1997;17:1869–79. doi: 10.1523/JNEUROSCI.17-05-01869.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Agnew HWJ, Webb WB, Williams RL. The effects of stage four sleep deprivation. Electroencephalogr Clin Neurophysiol. 1964;17:68–70. doi: 10.1016/0013-4694(64)90011-2. [DOI] [PubMed] [Google Scholar]
- 77.Dijk DJ, Beersma DG. Effects of SWS deprivation on subsequent EEG power density and spontaneous sleep duration. Electroencephalogr Clin Neurophysiol. 1989;72:312–20. doi: 10.1016/0013-4694(89)90067-9. [DOI] [PubMed] [Google Scholar]
- 78.Dijk DJ, Beersma DG, Daan S, Bloem GM, Van den Hoofdakker RH. Quantitative analysis of the effects of slow wave sleep deprivation during the first 3 h of sleep on subsequent EEG power density. Eur Arch Psychiatry Neurol Sci. 1987;236:323–8. doi: 10.1007/BF00377420. [DOI] [PubMed] [Google Scholar]
- 79.Ferrara M, De Gennaro L, Bertini M. Selective slow-wave sleep (SWS) deprivation and SWS rebound: do we need a fixed SWS amount per night? Sleep Res Online. 1999;2:15–9. [PubMed] [Google Scholar]
- 80.Johnson LC, Naitoh P, Moses JM, Lubin A. Interaction of REM deprivation and stage 4 deprivation with total sleep loss: experiment 2. Psychophysiology. 1974;11:147–59. doi: 10.1111/j.1469-8986.1974.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 81.Bonnet MH. Performance and sleepiness following moderate sleep disruption and slow wave sleep deprivation. Physiol Behav. 1986;37:915–8. [PubMed] [Google Scholar]
- 82.Walsh JK, Hartman PG, Schweitzer PK. Slow-wave sleep deprivation and waking function. J Sleep Res. 1994;3:16–25. doi: 10.1111/j.1365-2869.1994.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 83.Dijk DJ, Stanley N, Groeger J, Deacon S. Selective SWS/SWA deprivation is associated with increased daytime sleep propensity in young, middle-aged and older men and women. J Sleep Res. 2006;15(Suppl. 1):250. [Google Scholar]
- 84.Aeschbach D, Cutler AJ, Ronda JM. A role for non-rapid-eye-movement sleep homeostasis in perceptual learning. J Neurosci. 2008;28:2766–72. doi: 10.1523/JNEUROSCI.5548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lancel M. Role of GABAA receptors in the regulation of sleep: initial sleep responses to peripherally administered modulators and agonists. Sleep. 1999;22:33–42. doi: 10.1093/sleep/22.1.33. [DOI] [PubMed] [Google Scholar]
- 86.Dijk DJ, Brunner DP, Aeschbach D, Tobler I, Borbely AA. The effects of ethanol on human sleep EEG power spectra differ from those of benzodiazepine receptor agonists. Neuropsychopharmacology. 1992;7:225–32. [PubMed] [Google Scholar]
- 87.Pardi D, Black J. gamma-Hydroxybutyrate/sodium oxybate: neurobiology, and impact on sleep and wakefulness. CNS Drugs. 2006;20:993–1018. doi: 10.2165/00023210-200620120-00004. [DOI] [PubMed] [Google Scholar]
- 88.Mathias S, Wetter TC, Steiger A, Lancel M. The GABA uptake inhibitor tiagabine promotes slow wave sleep in normal elderly subjects. Neurobiol Aging. 2001;22:247–53. doi: 10.1016/s0197-4580(00)00232-3. [DOI] [PubMed] [Google Scholar]
- 89.Walsh JK, Deacon S, Dijk DJ, Lundahl J. The selective extrasynaptic GABAA agonist, gaboxadol, improves traditional hypnotic efficacy measures and enhances slow wave activity in a model of transient insomnia. Sleep. 2007;30:593–602. doi: 10.1093/sleep/30.5.593. [DOI] [PubMed] [Google Scholar]
- 90.Hindmarch I, Saubadu S, Delfolie A, Martinez JM, Pinquier JL. Sleep and psychomotor performance in healthy subjects after morning or evening administration of eplivanserin, a novel sleep compound. J Sleep Res. 2008;17(Suppl. 1) Abstract No. P359. [Google Scholar]
- 91.Landolt HP, Meier V, Burgess HJ, et al. Serotonin-2 receptors and human sleep: effect of a selective antagonist on EEG power spectra. Neuropsychopharmacology. 1999;21:455–66. doi: 10.1016/S0893-133X(99)00052-4. [DOI] [PubMed] [Google Scholar]