Abstract
Traumatic spinal cord injury (SCI) leads to permanent neurological deficits, which, in part, is due to the inability of mature axons to regenerate in the mammalian central nervous system (CNS). The oligodendrocyte myelin glycoprotein (OMgp) is one of the myelin-associated inhibitors of neurite outgrowth in the CNS. To date, limited information is available concerning its expression following SCI, possibly due to the lack of a reliable antibody against it. Here we report the generation of a highly specific OMgp polyclonal antibody from the rabbit. Using this antibody, we found that OMgp was almost exclusively expressed in the CNS. Following a moderately contusive SCI using a New York University impactor (10 g rod dropped from a height of 12.5 mm), both OMgp mRNA and protein levels were elevated at 1 and 7 days post-SCI, respectively, and peaked at 28 days compared to those of the sham-operated controls. Spatially, OMgp was expressed throughout the entire rostrocaudal extension of a 10 mm long spinal segment with the highest expression seen at the injury epicenter. OMgp was exclusively localized in neurons and oligodendrocytes in the normal and sham-operated controls with an increased expression found in these cells following SCI. OMgp was not expressed in astrocytes or microglia in all groups. Thus, our study has provided evidence for temporospatial expression and cellular localization of OMgp following SCI and suggested that this molecule may contribute to the overall inhibition of axonal regeneration.
Key words: myelin-associated inhibitor, oligodendrocyte, OMgp, neuron, polyclonal antibody, spinal cord injury
Introduction
The failure of axonal regeneration in the adult mammalian central nervous system (CNS) is thought to be attributed, at least in part, to the gradual decline in the intrinsic growth ability of neurons as the animal matures (Yiu and He, 2006), the presence of reactive astrocytes that form a glial scar at the site of injury, and the presence of myelin-associated growth inhibitors such as Nogo (Chen et al., 2000; Prinjha et al., 2000), myelin-associated glycoprotein (MAG) (Mukhopadhyay et al., 1994), and oligodendrocyte myelin glycoprotein (OMgp) (Mikol and Stefansson, 1988; Wang et al., 2002). As one of these myelin-associated inhibitors, OMgp induces growth cone collapse and neurite growth inhibition (Kottis et al., 2002; Tang et al., 2001) through a common receptor called the Nogo receptor (NgR) and its associated receptor complex (Mi et al., 2004; Park et al., 2005; Shao et al., 2005).
OMgp was characterized as a 120 kDa glycosylphosphatidylinositol (GPI)-linked highly glycosylated molecule isolated from the adult human CNS myelin and cultured ovine oligodendrocytes (Mikol and Stefansson, 1988). The mature OMgp includes five structure domains including a N-terminal region, a COOH sequence, a 32 amino acid (aa)-length cystein-rich (CR) domain containing four cysteins, a highly conserved 172 aa-length leucine-rich repeat (LRR) region, and a serine-threonine rich (S/TR) domain of 197 aa comprising several putative sites for the fixation of O and N carbohydrates (Cross, 1990; Mikol et al., 1990, 1993). Among all the domains, LRR plays an important role in inhibiting neurite outgrowth and cell proliferation. With its eight tandem leucine-rich repeats, OMgp also becomes a member of the leucine-rich repeats protein family (Habib et al., 1998a; Kobe and Kajava, 2001).
As a membrane protein, OMgp is a component of the myelin-axolemmal complex anchored in lipid raft by GPI (Mikol and Stefansson, 1988). Recent research also showed that OMgp is expressed in the node of Ranvier of the CNS and PNS, which maintains the integrity of the node of Ranvier and prevents axons from collateral sprouting (Huang et al., 2005). Cellular expression of OMgp was first defined in oligodendrocytes and was then detected on the surface of large projection neurons such as anterior horn cells of the spinal cord, Purkinje cells of the cerebellum, and pyramidal cells of the hippocampus (Habib et al., 1998b). The OMgp mRNA temporal expression profile is concurrent with CNS myelination (Vourc'h et al., 2003). Although OMgp has been reported to be expressed in the normal CNS, its expression and cellular localization following SCI remain to be investigated. The limited information concerning OMgp expression following SCI may be, at least in part, due to the lack of reliable antibodies that are available for this protein.
To determine the temporospatial expression and cellular localization of OMgp following spinal cord injury (SCI), we first developed and characterized a highly specific OMgp polyclonal antibody. Using this antibody, we investigated OMgp expression in a clinically relevant contusive SCI model and revealed temporospatial elevation and cellular localization of OMgp in the post-traumatic spinal cord of adult rats.
Methods
Construction of recombinant OMgp plasmid, expression cloning, and protein purification
We cloned 6*His-OM gene from AP-5-OM plasmid (a gift from Dr. Zhigang He, Harvard Medical School) by PCR. Then the target gene was reconstructed into a prokaryote expression vector pET-28a (+) (Novagen, Darmstadt, Germany). After confirming by DNA sequence analysis, this recombinant plasmid was transformed into Escherichia coli BL21 and the fusion protein 6*His-OM was induced by IPTG (Sigma, St. Louis, MO) with the tag of 6*His at the beginning of the engineered protein. This fusion protein was purified using Ni-NTA affinity chromatography (Biocolors, Shanghai, China) and confirmed by Western blot analysis. All recombinant plasmids were identified by restriction endonuclease digestion and confirmed by DNA sequencing analysis.
OMgp antibody preparation and identification
Rabbits were immunized with the purified OMgp fusion protein, which was emulsified with an equal volume of Freund's immunoadjuvant to obtain rabbit sera. With ammonium sulfate fractionation, we obtained preliminary purified rabbit-anti-mouse OMgp fusion protein and polyclonal antibody. Using this polyclonal antibody, we examined expression of OMgp in the rat spinal cord, cerebrum, cerebellum, sciatic nerve, cardiac muscle, lung, liver, kidney, and skeletal muscle by Western blot analysis. To confirm the OMgp expression at the mRNA level, we also performed RT-PCR with total length primers (sense 5′-CGA GCT CAT GGA ATA TCA GAT ATT GAA AAT GTC TTC C-3′ and anti-sense 5′-GGG GTA CCT CAG CCT GCC AGC ATG ACC AC-3′). In this study, all proteins were quantified using a BCA Protein Assay Kit (Pierce, Rockford, IL).
Animals
In all, 79 adult female Sprague-Dawley (SD) rats, weighing 200–250 g, were used for this study (Table 1). These included animals used for Western blot analysis (n = 32), quantitative RT-PCR (n = 32), immunohistochemistry (n = 9), and double immunofluorescence (n = 6) studies. SD rats were obtained from the Animal Breeding Center of Chinese Academy of Sciences. All surgical interventions and postoperative care were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publication no. 80–23, 1996) and were approved by the Animal Care Committee of the Use of Laboratory Animals at Shanghai Jiaotong University School of Medicine.
Table 1.
Number of Animals Used in Study
| Groups | Normal | Sham | 4 h | 1 day | 3 day | 7 day | 14 day | 28 day |
|---|---|---|---|---|---|---|---|---|
| WB | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| RT-qPCR | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| IHC | – | 3 | – | – | – | 3 | – | 3 |
| IDL | – | 3 | – | – | – | – | – | 3 |
WB,Western blot; RT-qPCR, real-time quantitative PCR; IHC, immunohistochemistry; IDL, immunofluorescence double labeling.
Spinal cord injury
Impact injury was produced by a weight drop device developed at New York University (Gruner, 1992) and a protocol developed by a multicenter consortium (Basso et al., 1996). In brief, rats were deeply anesthetized with pentobarbital (50mg/kg, i.p.) and a laminectomy was carried out at the T9-T10 level. After the dorsal cord was exposed with dura remaining intact, a weight drop injury was performed on it by using a 10 g rod (2.5 mm in diameter) dropping from a height of 12.5 mm. After the injury, the muscles and skin were closed in layers and the rats were placed in a temperature- and humidity-controlled chamber. Manual bladder emptying was performed three times daily until the urine in the bladder was completely emptied. Those rats receiving T9-T10 laminectomy but no injury were used as sham-operated controls. Normal rats receiving no injury served as non-injury controls. After appropriate survival, rats were sacrificed by an overdose of sodium pentobarbital (80 mg/kg, i.p.), and spinal cord segments containing the injury epicenter (10 mm long) were removed for the following studies.
Western blot analysis
The Western blot analysis followed procedures described previously with modification (Xu et al., 1998; Yan et al., 1999, 2003). Briefly, a 10 mm spinal cord segment containing the injury epicenter was dissected after intracardial perfusion of the rat with 100 mL of saline under anesthesia. The cord segment was homogenized in 0.4 mL of RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 100 μg/mL phenylmethylsulfonyl fluoride [PMSF], 1 μg/mL aprotinin, 1 mM Na3VO, 1 mM EDTA, 4 μg/mL leupeptin [pH 7.5]) and centrifuged at 10,000 g for 10 min at 4°C. The supernatant was removed and centrifuged again. Twenty-five μg of proteins from the supernatant of each sample were loaded onto 10% polyacrylamide gel, separated by SDS-PAGE, and transferred to a nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany) by electrophoresis. The membrane was blocked in TBST buffer (10 mM Tris-HCl, 100 mM NaCl, 2% bovine serum albumin [BSA, Sigma], and 0.05% Tween-20 [pH 7.5]) for 30 min at 30°C. The primary rabbit polyclonal anti-OMgp (1:3000) was added to the membrane for incubation overnight at 4°C. The membrane was washed three times for 5 min with TBST at room temperature (RT), incubated with a secondary horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin (Ig) G antibody (1:10,000; SABC) for 1 h at 37°C, and then washed three times with TBST for 5 min. The Western blot was visualized using the enhanced chemiluminescence (ECL) kit (Pierce) following the manufacturer's instructions. For the negative control, the primary antibody was omitted. The lysate of cerebral cortex neurons cultured in vitro was used as a positive control. Data were presented as mean ± standard deviation of the tested value for each sample. One-way ANOVA was used for statistical comparison of the means with the mean value of the control (sham-operated rats) as a baseline (100%; arbitrary unit). Significant results were followed by Tukey's post hoc tests.
Real-time quantitative PCR
Total RNA from 10 mm long spinal cord (45–50 mg) segments containing the injury epicenter or normal and sham-operated cords obtained from identical regions was extracted with Tri Reagent kit (Molecular Research Center, Cincinnati, OH) according to the manufacturer's instructions. RNA pellets were suspended in 30 μL diethylpyrocarbonate (DEPC)-treated water, followed by an RNase-free DNase step (5 μ DNase I for 45 min at 37°C) to avoid contamination with genomic DNA. The yield and purity of RNA were checked by spectrophotometric determination at 260 and 280 nm. Integrity of RNA was determined by the presence of 28S and 18S ribosomal RNA by electrophoresis of samples through 1.5% agarose gels. Reverse transcription was performed using a reverse transcription system (Promega, Madison, WI) according to the manufacturer's instructions.
The Platinum SYBR Green qPCR SuperMix-UDG kit (Invitrogen, Carlsbad, CA) was used for all PCR reactions. PCR was carried out using 1.0 μL of cDNA, corresponding to 50 ng of total RNA, 3.0 mM MgCl2, and 0.4 μM each primer in a 10 μL final volume. After an initial 2 min, 50°C/2 min, 95°C, 45 cycles of amplification were performed (OMgp/HRPT: 95°C denaturing for 15 s, 59°C annealing for 30 s, 72°C extension for 30 s). The reaction kinetics was represented by an amplification curve in which a region where the fluorescent increased exponentially was observed. The PCR cycle number at which the fluorescent crossed a threshold (CT value) can be related to the amount of starting templates. A comparative threshold cycle (ΔCT) method was used to quantify OMgp mRNAs at different time points. The amount of target mRNAs normalized to an endogenous reference hypoxanthine guanine phosphoribosyl transferase (HPRT) and relative to a calibrator (sham) is given by the equation: XN,C = 2ΔΔCT, where ΔΔCT is the difference between the ΔCT of the sample and the ΔCT of the sham, and ΔCT is the difference between the CT of the OMgp and the CT of the HPRT within the same sample.
The primers used for OMgp reaction were: sense 5′-GTTCAAATGCAAGCCTCACCA-3′ and antisense 5′-TCCGTAAGTTAAGGGTTGTGCTCTG-3′ (Takara, JAPAN); the primers used for HPRT PCR reaction were: sense 5′-CTCATGGACTGATTATGGACAGGAC-3′ and antisense 5′-GCAGGTCAGCAAAGAACTTATAGCC-3′. The specificity of the amplified product was confirmed by melting curve analysis and agarose gel electrophoresis.
Immunohistochemistry
SCI and sham-operated rats were deeply anesthetized by intraperitoneal injections of sodium pentobarbital (80 mg/kg) and perfused with 100 mL of 0.9% saline followed by 400 mL of 4% paraformaldehyde in cold 0.01 M phosphate-buffered saline (PBS, ph 7.4). After perfusion, the spinal cord was carefully removed and a 10 mm long segment containing the injury epicenter was blocked and post-fixed for additional 2 h in the same fixation solution at 4°C. The specimen was transferred to a solution containing 20% sucrose followed by 30% sucrose both in 0.01 M phosphate buffer (pH 7.4) at 4°C, until it was stayed at the bottom of the container. Then, the spinal cord segment was embedded in tissue freezing medium (Tissue-Tek, Elkart, IN), and frozen sections of 15 μm thickness were obtained transversely using a cryostat (Leica CM1900, Bannockburn, IL), and thaw-mounted on gelatin-coated slides. Immunohistochemistry was performed using a Strept Avidin-Biotinylated Peroxidase complex (ABC) kit (Boster, China) according to the manufacturer's recommendation. The spinal cord sections were incubated with primary polyclonal rabbit anti-OMgp (1:1500) antibody for 1 h at 37°C. After reaction, the sections were dehydrated, cleared, and coverslipped. Slides were examined using an Olympus BX60 light microscope. Primary antiserum omission was used as a negative control and preimmune serum was used as a control to confirm the specificity of the antibody.
Immunofluorescence double-labeling method
The immunofluorescence double-labeling (IDL) method was used to identify specific cell types that expressed OMgp in normal and injured spinal cords. In brief, spinal cord segments were embedded in tissue freezing medium, cut horizontally or transversely at 15 μm on a cryostat, and mounted on gelatin-coated slides. After incubation with a blocking buffer (10% normal goat serum, 0.3% Triton x100 in 0.01 M PBS) for 1 h at room temperature, sections reacted with the primary antibody OMgp (1:100) overnight at 4°C. The cell-specific monoclonal antibodies included mouse anti-βIII-tubulin antibody (1:800, Sigma) to identify neurons, mouse anti-glial fibrillary acidic protein antibody (GFAP; 1:200, Sigma) to identify astrocytes, mouse anti-RIP antibody (1:25, a gift from Dr. S.R. Whittemore, University of Louisville, KY) to identify oligodendrocytes, and mouse anti-OX42 antibody (1:200, Chemicon, Temecula, CA) to recognize microglial cells. On the following day, the sections were incubated with rhodamine-conjugated goat anti-rabbit (1:80, Jackson ImmunoResearch Lab, West Grove, PA) and fluoroisothiocyanate-conjugated goat anti-mouse (FITC, 1:100, Jackson ImmunoResearch Lab) antibodies. Sections were washed, mounted, and examined using a Zeiss laser scanning confocal microscope (Carl Zeiss Optical, Inc., Chester, VA). Preimmune serum was used as a control to confirm the specificity of the antibody.
Statistical analysis
All data are presented as mean ± SD. One-way ANOVA with Tukey HSD post hoc t tests were used to determine levels of statistical significance. A p value of < 0.05 was considered statistically significant.
Results
Generation and characterization of the OMgp polyclonal antibody
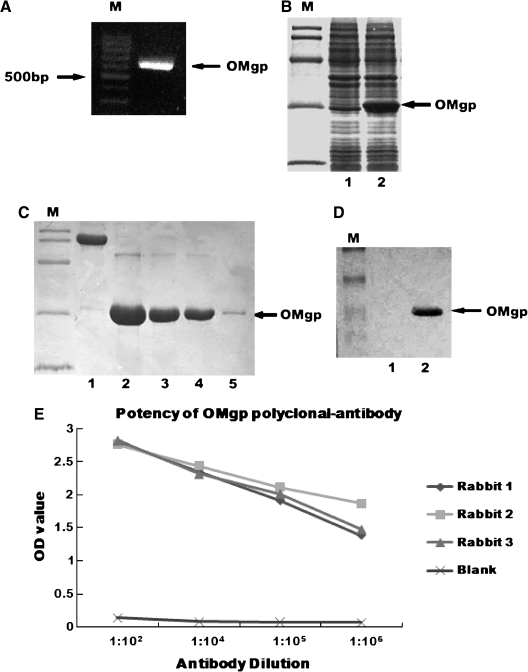
To study the expression pattern and cellular localization of OMgp following SCI, we first generated and characterized the OMgp polyclonal antibody. 6*His-OM fraction was subcloned into the expression plasmid pET-28a (+) (Fig. 1A). IPTG induction of E. coli BL21 resulted in the expression of a recombinant protein with an apparent mass of 28 kDa (Fig. 1B). By using Ni-NTA resins, we purified 3 mg recombinant OMgp (Fig. 1C), which was used to immunize three rabbits. The purified OMgp protein was clearly seen by Western blot analysis (Fig. 1D). ELISA analysis indicated that the potency of this rabbit-anti-OMgp antibody exceeded 1:1,000,000 (Fig. 1E). Thus, we successfully generated a high titer OMgp polyclonal antibody.
FIG. 1.
Expression, purification, and characterization of the OMgp polyclonal antibody. (A) Electrophoresis image of the 6*His-OM fragment (630 bp) by PCR. (B) SDS-PAGE analysis of the recombinant 6*His-OM protein expression. Lane 1, without induction, recombinant OMgp protein was not expressed in the E. coli BL21; lane 2, with IPTG induction, recombinant OMgp protein was expressed in the E. coli BL21. (C) SDS-PAGE image of purified recombinant 6*His-OM. Lane 1, BSA; lanes 2–5, elutional OMgp by turns. (D) Western blot image of purified OMgp protein. Lane 1, negative control; lane 2, anti-His antibody identified purified 6*His-OM protein. (E) All three rabbits receiving OMgp immunization generated high titer OMgp antibody (>1:1,000,000) compared with preimmune serum used as a blank control.
Expression and cellular localization of OMgp in normal rats
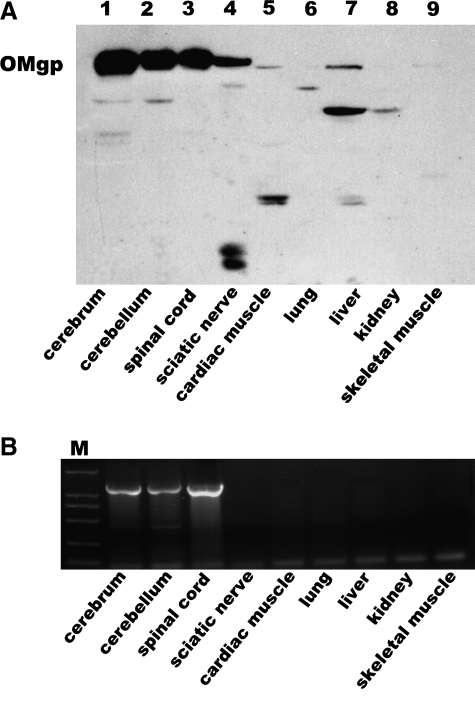
To confirm the specificity of OMgp polyclonal antibody, we examined its expression pattern in several tissues, including neural and non-neural tissues. Western blot analysis showed that the OMgp was highly and almost exclusively expressed in the nervous tissue including the cerebrum, cerebellum, spinal cord, and sciatic nerve (Fig. 2A). Slight expression was found in the cardiac muscle and liver but not in the lung, kidney, or skeletal muscle. Thus, the OMgp antibody is highly specific for the nervous system, particularly for the CNS. RT-PCR showed amplification fraction representing the OMgp cDNA sequence, which agreed largely with the Western blot results. We did not detect the OMgp signal in the sciatic nerve at the mRNA level, indicating that this molecule may have distinct isoforms in the periphery nervous system.
FIG. 2.
Differential expression of OMgp in neural and non-neural tissues. (A) Western blot analysis shows that OMgp (120 kDa) is expressed highly and almost exclusively in the nervous system tissues, with only a slight or no expression in non-neural tissues. (B) RT-PCR results agreed largely with the Western blot result, except for the lack of OMgp signal in the sciatic nerve. Lanes 1, cerebrum; 2, cerebellum; 3, spinal cord; 4, sciatic nerve; 5, cardiac muscle; 6, lung; 7, liver; 8, kidney; 9, skeletal muscle.
To determine whether there was a baseline expression of OMgp in the normal spinal cord and, if so, their cellular localization, we performed IDL of OMgp and several cell-specific markers. We found that OMgp was expressed in neurons (βIII-tubulin-IR; Fig. 3A–C) of the spinal cord gray matter and oligodendrocytes and their processes (RIP-IR; Fig. 3D–F) but was not detected in astrocytes (GFAP-IR; Fig. 3G–I) and microglia (OX-42-IR; Fig. 3J–L). In addition to its likely membrane expression, intracellular OMgp expression was found in both the neurons and oligodendrocytes. Thus, OMgp was expressed in neurons and oligodendrocytes but not in astrocytes and microglia, indicating that the OMgp antibody is highly specific.
FIG. 3.
Cellular localization of OMgp in the normal adult rat spinal cord. Immunofluorescence double labeling shows co-localization of OMgp (A, D, G, J, white arrows) in neurons (βIII-tubulin-IR; B, arrows) and oligodendrocytes (RIP-IR; E, arrows) but not in astrocytes (GFAP-IR; H, yellow arrows) or microglia (OX-42-IR; K, yellow arrows). All can be appreciated in the merged images (C, F, I, L, arrows). (M and N) Preimmune serum control. Scale bar, 50 μm. (Color image is available online at www.liebertpub.com/jon)
Increased expression of OMgp after spinal cord injury
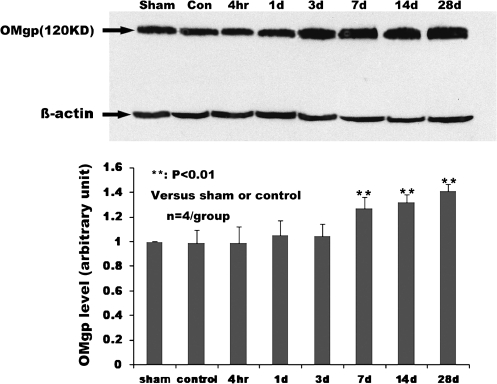
To determine whether SCI induced a change of OMgp expression and, if so, its time course, we examined OMgp expression profiles at both the protein and mRNA levels in the spinal cords up to 28 days post-SCI. Western blot analysis revealed the presence of a basal level expression of OMgp in the normal and sham-operated rat spinal cords (Fig. 4). After SCI, OMgp expression was increased significantly at 7 days post-injury (p < 0.01) and peaked at 28 days (1.4-fold above the baseline level; p < 0.01). To confirm the OMgp expression at the mRNA level, a relative quantitative PCR method was used and the result was normalized to an internal reference gene HPRT. We found a significant increase in OMgp mRNA levels relative to the amount present in sham-operated spinal cord starting as early as 1 day post-injury (Fig. 5D). Melting curve analysis of OMgp (Fig. 5A) and HPRT (Fig. 5B), and the agarose gel electrophoresis analysis of both genes (Fig. 5C), confirmed the specificity of the two amplified products. In addition, a comparative threshold cycle (ΔCT) method was used due to similar amplification efficiencies between target and reference genes. This method showed that the difference in OMgp mRNA levels between the sham-operated and non-injured normal spinal cords was not statistically significant. Our results showed that the OMgp mRNA levels increased over time starting at 1 day post-injury (p < 0.05) and peaking at 28 days (p < 0.01; Fig. 5D). Thus, the tendency of OMgp upregulation at mRNA and protein levels is identical.
FIG. 4.
Time course of OMgp protein expression following SCI. Upper panel, Western blot shows the time course of OMgp expression with β-actin used as an internal control. Lower panel, bar graph indicates the mean ± SD (n = 4/group) for each experimental group. **p < 0.01 compared to non-injured or sham-operated controls.
FIG. 5.
Time course of OMgp mRNA expression following SCI. (A and B) Melting curve analysis showed that the SYBR green I dye detected the OMgp-specific PCR product as a single peak at 82.7°C (A) and the HPRT-specific PCR product as a single peak at 83°C (B). (C) Agarose gel electrophoresis analysis demonstrated that two peaks in A and B correspond to single band of the size predicted 126 and 123 bp, respectively. (D) Expression of OMgp mRNA in the injured spinal cord at 4 h to 28 days after SCI, detected by real-time qPCR and comparative threshold cycle (ΔCT) methods. Bar graphs indicate the mean ± SD (n = 4) for each time point. *p < 0.05; **p < 0.01 compared to the control or sham-operated group.
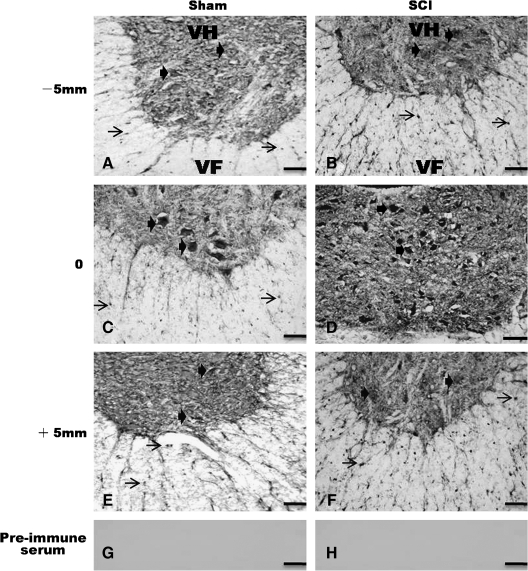
Next, we examined spatial distribution of OMgp expression at 28 days post-SCI, a time point that OMgp was maximally expressed. In a sham-operated control (Fig. 6; left column), OMgp immunoreactivity (IR) was detected at a baseline level in both the gray and white matter. The OMgp-IR cells were morphologically characteristic of neurons in the ventral horn gray matter and glial cells in the ventral white matter (Fig. 6A, C, E). Following SCI, OMgp-IR was markedly increased in both neurons and glial cells of injured spinal cord at 28 days post-injury (Fig. 6; right column) as compared to the sham-operated control. The strongest labeling was found at the lesion epicenter (Fig. 6D) but increased OMgp-IR was seen throughout the entire length (10 mm) of the specimen examined (Fig. 6B and F). In sections where the preimmune serum was used, no labeling was found confirming the specificity of the OMgp antibody (Fig. 6G and H).
FIG 6.
Distribution of OMgp in the spinal cords of sham-operated and spinal cord injured rats. (A, C, E, left column). In sham-operated spinal cords, basal levels of OMgp immunoreactivity (IR) were found in the ventral horn neurons (bold arrows) and white matter glial cells (arrows). (B, D, F, right column) In the spinal cords at 28 days post-injury, a marked increase in OMgp-IR was found in neurons (bold arrows) and glial cells (arrows) at 5mm rostral (−5 mm; B) and caudal (+5 mm; F) to the injury. At the injury epicenter, most labeled cells seemed to derive from cells infiltrated into the lesion site (D). (G and H) Preimmune serum control. VH, ventral horn; VF, ventral funiculus. Scale bar, 50 μm.
Cellular localization of OMgp after spinal cord injury
To determine cell types expressing increased levels of OMgp in the injured spinal cord, we performed immunofluorescence double labeling at 28 days post-injury. For better comparison, all photomicrographs shown in Figure 7 were taken from an area 3–5 mm rostral to the injury epicenter. Coexistence of OMgp and βIII-tubulin, a neuronal marker, was observed in neurons in the spinal cord grey matter (Fig. 7A–C). OMgp was also detected in oligodendrocytes, particularly the myelin segments that they form (RIP-IR; Fig. 7D–F). However, OMgp was not observed in astrocytes (GFAP-IR; Fig. 7G–I) nor in microglial cells (OX-42-IR; Fig. 7J–L). The pattern of OMgp cellular localization after SCI was identical to that observed in the normal spinal cord. Therefore, the increased OMgp expression after SCI was a change mainly in quantity in the same cell populations.
FIG. 7.
Cellular localization of OMgp following SCI. Immunofluorescence double labeling shows localization of OMgp (A, D, G, J, white arrows) in βIII-tubulin immunoreactive (IR) neurons (B, arrows), RIP-IR oligodendrocytes and their myelin (E, arrows) in the injured spinal cord at 28 days post-injury. OMgp was not localized in GFAP-IR astrocytes (H, yellow arrows) nor in OX-42-IR microglia (K, yellow arrows). The co-localization or lack of co-localization of OMgp in specific cell types can be appreciated in the merged images (C, F, I, L, arrows). (M and N) Preimmune serum control. Scale bar, 50 μm. (Color image is available online at www.liebertpub.com/jon)
Discussion
The goal of this study was to determine the expression profile and cellular localization of OMgp following traumatic SCI. Using the antibody developed in our laboratory, we found that OMgp was almost exclusively expressed in the CNS and was localized in neurons and oligodendrocytes but not in astrocytes and microglia. Following SCI, elevated levels of OMgp were observed at and near the site of injury, which persisted for a prolonged period of time. In the injured spinal cord, the cellular localization of OMgp was identical to that of the normal spinal cord except that the labeling intensity in neurons and oligodendrocytes was increased after the injury. Thus, our study has established temporospatial profile and cellular localization of OMgp in a clinically relevant contusive SCI model in adult rats.
OMgp is one of the three well-characterized myelin-associated inhibitors that are implicated in axonal growth inhibition in the adult mammalian CNS (Wang et al., 2002). The other two of this type are Nogo-A (Fournier et al., 2001) and MAG (Domeniconi et al., 2002). These inhibitors generate axonal inhibition through a common Nogo receptor (NgR) that is supplied on neurons (Fournier et al., 2001; Wang et al., 2002). All three inhibitors can induce growth cone collapse and neurite outgrowth inhibition in vitro (Filbin, 2003; McKerracher et al., 1994; Schwab and Caroni, 1988). Blocking NgR in transgenic mice lacking NgR (Kim et al., 2004) with a NgR antagonist peptide NEP1–40 (GrandPre et al., 2002) or a soluble functional blocking NgR fragment NgR(310)ecto-Fc (Li et al., 2004, 2005) enhanced axonal regeneration/sprouting of the corticospinal, rubrospinal, and/or raphespinal tracts and improved behavioral recovery following SCI (GrandPre et al., 2002; Kim et al., 2004; Li et al., 2005). However, a recent study showed that genetic deletion of NgR did not reduce the growth inhibition of axons in vitro or encourage regeneration of CST axons after SCI (Zheng et al., 2005). While the controversy remains to be addressed, the latter study indicates that NgR may not be the only molecule responsible for regenerative failure induced by these myelin inhibitors, and that other binding receptors for these inhibitors may exist. Indeed, paired immunoglobulin-like receptor B (PirB) was recently identified as a functional receptor for the three myelin inhibitors of axonal regeneration (Atwal et al., 2008; Filbin, 2008). The relative contribution of elevated OMgp in mediating growth inhibition following SCI remains to be elucidated.
In the present study, a prolonged expression profile of OMgp has been established following SCI. While the OMgp mRNA expression significantly increased at 1 day post-SCI, a delayed increase of OMgp protein expression was found and reached a significant level at 7 days post-injury. Both mRNA and protein expressions increased steadily up to 28 days, the longest time point that we examined. Since the peak expression was found at 28 days post-injury at the time frame that we studied, it is reasonable to predict that such an increase may last for a longer time period before it returns to a baseline expression level. The prolonged elevation of OMgp observed in this study matches well with the progression of spinal cord secondary injury (Liu et al., 1997), indicating that the OMgp may participate in the complex process of secondary injury. Additionally, the presence of chronically elevated levels of OMgp at the site of SCI indicates that OMgp may be one of the major growth inhibitors that needs to be targeted to promote axonal regeneration. In contrast to our finding, a quick increase followed by a gradual decrease of OMgp mRNA expression up to 7 days was found in rats following a spinal cord complete transection (Guo et al., 2007). Such a difference in the time course of OMgp expression between the two studies may be related to the different injury models and probes used in these studies. It should be noted that increased expression of OMgp mRNA was also found during the postnatal development of several regions of the rat CNS (Vourc'h and Andres, 2004; Vourc'h et al., 2003). OMgp expression increases from birth to the sixth postnatal week, which correlates well with the formation of the CNS myelin (Vourc'h et al., 2003).
While OMgp was found to express almost exclusively in the normal CNS at both the protein and mRNA levels, it was found to express in the PNS (sciatic nerve) only at the protein level. The lack of OMgp mRNA expression in the PNS in contrast to its expression in the CNS may be explained by two possibilities. One possibility is that the transcription and translation of OMgp may not occur simultaneously in the peripheral nerve. A second possibility is that the full lengths of ribonucleotide sequences of OMgp between the CNS and PNS are different.
In terms of cellular localization, we presented evidence that OMgp was expressed specifically in oligodendrocytes and neurons but not in astrocytes or microglia in the normal spinal cord. In oligodendrocytes, OMgp was expressed in both the cell bodies and processes as well as in myelin sheaths. The labeling pattern of OMgp was similar to that of Nogo-A as previously reported (Chen et al., 2000; Huber et al., 2002; Liu et al., 2002). In contrast, MAG was only expressed in oligodendrocytes and their myelin sheaths (Filbin, 1995; McKerracher et al., 1994). Additionally, peripheral Schwann cells and their myelin also expressed MAG (Filbin, 1995; McKerracher et al., 1994). Similar to the normal spinal cord, OMgp was found to express exclusively in neurons and oligodendrocytes after SCI but with increased levels of expression. By referring to in vitro assays about this protein, we postulate that increased expression of OMgp may generate an impediment environment for axonal regeneration in vivo. The neuronal expression of both OMgp and Nogo-A indicates that the two proteins may have functions additional to their neurite growth inhibitory activities. Similar to our finding, OMgp was reported to be localized in oligodendrocytes and neurons at acute stage, 1 day after a spinal cord transection in adult rats (Guo et al., 2007). By employing a double-staining method with our own antibodies and cell-specific markers, our study have provided definitive evidence for the cellular localization of OMgp in both the normal and injured spinal cords.
Although OMgp was localized in neurons and oligodendrocytes, its physiological function remains largely unknown. A recent study reported axonal spouting and functional improvement in OMgp null mice on a mixed 129BL6 genetic background following SCI (Ji et al., 2008). Others reported that the expression of OMgp was reduced in a facial nerve transection model in adult rats (Koyama et al., 2008), suggesting that responses of OMgp to injuries may vary between different injury models. The presence of OMgp in neurons and oligodendrocytes of the normal CNS indicates that this molecule plays physiological roles in specific cell types. The lack of OMgp expression in astrocytes and microglia observed in the present study suggests that these cells are not participating in OMgp-mediated growth inhibition at the site of SCI.
Although it has been shown that OMgp is a strong axonal growth inhibitor in vitro, it is difficult to scale how much OMgp is necessary to affect growth inhibition following SCI. In consequence of the adult spinal cord that received a mechanical trauma, axons are disrupted, surrounding tissues damaged, and secondary lesions initialized (Blight, 2002; Rosenberg and Wrathall, 1997). The characteristic dystrophic growth cones formed by the severed axons were exposed to the damaged glial environment (Silver and Miller, 2004; Tom et al., 2004). Myelin-associated inhibitors from oligodendrocytes and myelin debris can limit axon regrowth during the early phase of injury. In addition to the three myelin-associated inhibitors mentioned above, other inhibitory factors exist in the adult CNS glial environment, which include chondroitin sulphate proteoglycans (CSPG) (Asher et al., 2001; Chau et al., 2004; McKeon et al., 1991; Morgenstern et al., 2002; Rhodes et al., 2003), semaphoring 4D (Sema4D/CD100) (Moreau-Fauvarque et al., 2003), ephrinB3 (Benson et al., 2005), and several EphA molecules (EphA3, EphA4, EphA7) (Willson et al., 2002). Due to the presence of multiple inhibitory molecules at the injury site, comprehending the relative contribution of each of these inhibitors to axonal growth inhibition remains to be investigated. Recent studies showed that myelin-mediated growth inhibition could be reduced by genetic deletion of Nogo (Kim et al., 2003) or other myelin-associated inhibitors (Simonen et al., 2003). Although how individual myelin-associated molecules contribute to the overall myelin inhibition remains to be investigated, it is conceivable that these myelin inhibitors may play intricate roles of inhibition with a distinguished degree of overlap and cross-regulation (Kim et al., 2003; Zheng et al., 2003).
To characterize the expressional profile and cellular localization of OMgp following traumatic SCI, we have developed a highly specific antibody against the OMgp. In pilot studies, we tested a few commercially available antibodies and the results were inconclusive. We therefore developed our own OMgp antibody and confirmed that it is highly specific, assessed by multiple assays. We subcloned the OMgp 660 ribonucleotide sequences from a recombinant plasmid AP-5-OM that encodes cystein-rich and leucine-rich repeat domains of mouse to a prokaryote expression vector pET-28a (+). Since OMgp expressed in rats only have two amino acids different from mice and since the LRR is a highly conserved domain in evolution, the antibody displayed good property in rats. Accordingly, results of OMgp localization in the adult rat CNS using this antibody are highly specific and reliable.
Acknowledgments
We would like to express our gratitude to Dr. Zhigang He (Harvard Medical School, Cambridge, MA) for providing AP-5-OMgp recombinant plasmid. This work was supported by grants from Major State Basic Research Development Program of China (973 project, 2003CB515302), Shanghai Science Development Fund (02JC14014), NIH NINDS (NS36350, NS52290, NS50243), and the Mari Hulman George Fund.
Author Disclosure Statement
No competing financial interests exist.
References
- Asher R.A. Morgenstern D.A. Moon L.D. Fawcett J.W. Chondroitin sulphate proteoglycans: inhibitory components of the glial scar. Prog. Brain Res. 2001;132:611–619. doi: 10.1016/S0079-6123(01)32106-4. [DOI] [PubMed] [Google Scholar]
- Atwal J.K. Pinkston-Gosse J. Syken J. Stawicki S. Wu Y. Shatz C. Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Benson M.D. Romero M.I. Lush M.E. Lu Q.R. Henkemeyer M. Parada L.F. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc. Natl. Acad. Sci. USA. 2005;102:10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blight A.R. Miracles and molecules–progress in spinal cord repair. Nat. Neurosci. 2002;5 Suppl:1051–1054. doi: 10.1038/nn939. [DOI] [PubMed] [Google Scholar]
- Chau C.H. Shum D.K. Li H. Pei J. Lui Y.Y. Wirthlin L. Chan Y.S. Xu X.M. Chondroitinase ABC enhances axonal regrowth through Schwann cell-seeded guidance channels after spinal cord injury. FASEB J. 2004;18:194–196. doi: 10.1096/fj.03-0196fje. [DOI] [PubMed] [Google Scholar]
- Chen M.S. Huber A.B. Van Der Haar M.E. Frank M. Schnell L. Spillmann A.A. Christ R. Schwab M.E. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- Cross G.A. Glycolipid anchoring of plasma membrane proteins. Annu. Rev. Cell Biol. 1990;6:1–39. doi: 10.1146/annurev.cb.06.110190.000245. [DOI] [PubMed] [Google Scholar]
- Domeniconi M. Cao Z. Spencer T. Sivasankaran R. Wang K.C. Nikulina E. Kimura N. Cai H. Deng K. Gao Y. He Z. Filbin M.T. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- Filbin M.T. Myelin-associated glycoprotein: a role in myelination and in the inhibition of axonal regeneration? Curr. Opin. Neurobiol. 1995;5:588–595. doi: 10.1016/0959-4388(95)80063-8. [DOI] [PubMed] [Google Scholar]
- Filbin M.T. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Filbin M.T. PirB, a second receptor for the myelin inhibitors of axonal regeneration Nogo66, MAG, and OMgp: implications for regeneration in vivo. Neuron. 2008;60:740–742. doi: 10.1016/j.neuron.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Fournier A.E. GrandPre T. Strittmatter S.M. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- GrandPre T. Li S. Strittmatter S.M. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- Gruner J.A. A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma. 1992;9:123–128. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- Guo Q. Li S. Su B. Expression of oligodendrocyte myelin glycoprotein and its receptor NgR after the injury of rat central nervous system. Neurosci. Lett. 2007;422:103–108. doi: 10.1016/j.neulet.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Habib A.A. Gulcher J.R. Hognason T. Zheng L. Stefansson K. The OMgp gene, a second growth suppressor within the NF1 gene. Oncogene. 1998a;16:1525–1531. doi: 10.1038/sj.onc.1201683. [DOI] [PubMed] [Google Scholar]
- Habib A.A. Marton L.S. Allwardt B. Gulcher J.R. Mikol D.D. Hognason T. Chattopadhyay N. Stefansson K. Expression of the oligodendrocyte-myelin glycoprotein by neurons in the mouse central nervous system. J. Neurochem. 1998b;70:1704–1711. doi: 10.1046/j.1471-4159.1998.70041704.x. [DOI] [PubMed] [Google Scholar]
- Huang J.K. Phillips G.R. Roth A.D. Pedraza L. Shan W. Belkaid W. Mi S. Fex-Svenningsen A. Florens L. Yates J.R. Colman D.R. Glial membranes at the node of Ranvier prevent neurite outgrowth. Science. 2005;310:1813–1817. doi: 10.1126/science.1118313. [DOI] [PubMed] [Google Scholar]
- Huber A.B. Weinmann O. Brosamle C. Oertle T. Schwab M.E. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J. Neurosci. 2002;22:3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B. Case L.C. Liu K. Shao Z. Lee X. Yang Z. Wang J. Tian T. Shulga-Morskaya S. Scott M. He Z. Relton J.K. Mi S. Assessment of functional recovery and axonal sprouting in oligodendrocyte-myelin glycoprotein (OMgp) null mice after spinal cord injury. Mol. Cell Neurosci. 2008;39:258–267. doi: 10.1016/j.mcn.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-E. Li S. GrandPre T. Qiu D. Strittmatter S.M. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Kim J.E. Liu B.P. Park J.H. Strittmatter S.M. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Kobe B. Kajava A.V. The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol. 2001;11:725–732. doi: 10.1016/s0959-440x(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Kottis V. Thibault P. Mikol D. Xiao Z.-C. Zhang R. Dergham P. Braun P.E. Oligodendrocyte-myelin glycoprotein (OMpg) is an inhibitor of neurite outgrowth. J. Neurochem. 2002;82:1566–1569. doi: 10.1046/j.1471-4159.2002.01146.x. [DOI] [PubMed] [Google Scholar]
- Koyama Y. Fujiwara T. Kubo T. Tomita K. Yano K. Hosokawa K. Tohyama M. Reduction of oligodendrocyte myelin glycoprotein expression following facial nerve transection. J. Chem. Neuroanat. 2008;36:209–215. doi: 10.1016/j.jchemneu.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Li S. Kim J. E. Budel S. Hampton T.G. Strittmatter S.M. Transgenic inhibition of Nogo-66 receptor function allows axonal sprouting and improved locomotion after spinal injury. Mol. Cell Neurosci. 2005;29:26–39. doi: 10.1016/j.mcn.2004.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. Liu B.P. Budel S. Li M. Ji B. Walus L. Li W. Jirik A. Rabacchi S. Choi E. Worley D. Sah D.W. Pepinsky B. Lee D. Relton J. Strittmatter S.M. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J. Neurosci. 2004;24:10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B.P. Fournier A. GrandPre T. Strittmatter S.M. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- Liu X.Z. Xu X.M. Hu R. Du C. Mcdonald J.W. Dong H.X. Wu Y.J. Fan G.S. Jacquin M.F. Hsu C.Y. Choi D.W. Neuronal and glial apoptosis after traumatic spinal cord injury. J. Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon R.J. Schreiber R.C. Rudge J.S. Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L. David S. Jackson D.L. Kottis V. Dunn R.J. Braun P.E. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Mi S. Lee X. Shao Z. Thill G. Ji B. Relton J. Levesque M. Allaire N. Perrin S. Sands B. Crowell T. Cate R.L. McCoy J.M. Pepinsky R.B. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat. Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- Mikol D.D. Gulcher J.R. Stefansson K. The oligodendrocyte-myelin glycoprotein belongs to a distinct family of proteins and contains the HNK-1 carbohydrate. J. Cell Biol. 1990;110:471–479. doi: 10.1083/jcb.110.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikol D.D. Rongnoparut P. Allwardt B.A. Marton L.S. Stefansson K. The oligodendrocyte-myelin glycoprotein of mouse: primary structure and gene structure. Genomics. 1993;17:604–610. doi: 10.1006/geno.1993.1379. [DOI] [PubMed] [Google Scholar]
- Mikol D.D. Stefansson K. A phosphatidylinositol-linked peanut agglutinin-binding glycoprotein in central nervous system myelin and on oligodendrocytes. J. Cell Biol. 1988;106:1273–1279. doi: 10.1083/jcb.106.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau-Fauvarque C. Kumanogoh A. Camand E. Jaillard C. Barbin G. Boquet I. Love C. Jones E. Y. Kikutani H. Lubetzki C. Dusart I. Chedotal A. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J. Neurosci. 2003;23:9229–9239. doi: 10.1523/JNEUROSCI.23-27-09229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern D.A. Asher R.A. Fawcett J.W. Chondroitin sulphate proteoglycans in the CNS injury response. Prog. Brain Res. 2002;137:313–332. doi: 10.1016/s0079-6123(02)37024-9. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay G. Doherty P. Walsh F.S. Crocker P.R. Filbin M.T. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Park J.B. Yiu G. Kaneko S. Wang J. Chang J. He X.L. Garcia K.C. He Z. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45:345–351. doi: 10.1016/j.neuron.2004.12.040. [DOI] [PubMed] [Google Scholar]
- Prinjha R. Moore S.E. Vinson M. Blake S. Morrow R. Christie G. Michalovich D. Simmons D.L. Walsh F.S. Inhibotor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- Rhodes K.E. Moon L.D. Fawcett J.W. Inhibiting cell proliferation during formation of the glial scar: effects on axon regeneration in the CNS. Neuroscience. 2003;120:41–56. doi: 10.1016/s0306-4522(03)00285-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg L.J. Wrathall J.R. Quantitative analysis of acute axonal pathology in experimental spinal cord contusion. J. Neurotrauma. 1997;14:823–838. doi: 10.1089/neu.1997.14.823. [DOI] [PubMed] [Google Scholar]
- Schwab M.E. Caroni P. Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J. Neurosci. 1988;8:2381–2393. doi: 10.1523/JNEUROSCI.08-07-02381.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z. Browning J.L. Lee X. Scott M.L. Shulga-Morskaya S. Allaire N. Thill G. Levesque M. Sah D. McCoy J.M. Murray B. Jung V. Pepinsky R.B. Mi S. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Silver J. Miller J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Simonen M. Pedersen V. Weinmann O. Schnell L. Buss A. Ledermann B. Christ F. Sansig G. Van Der Putten H. Schwab M.E. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron. 2003;38:201–211. doi: 10.1016/s0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- Tang S. Qiu J. Nikulina E. Filbin M.T. Soluble myelin-associated glycoprotein released from damaged white matter inhibits axonal regeneration. Mol. Cell Neurosci. 2001;18:259–269. doi: 10.1006/mcne.2001.1020. [DOI] [PubMed] [Google Scholar]
- Tom V.J. Steinmetz M.P. Miller J.H. Doller C.M. Silver J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J. Neurosci. 2004;24:6531–6539. doi: 10.1523/JNEUROSCI.0994-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vourc'h P. Andres C. Oligodendrocyte myelin glycoprotein (OMgp): evolution, structure and function. Brain Res. Brain Res. Rev. 2004;45:115–124. doi: 10.1016/j.brainresrev.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Vourc'h P. Moreau T. Arbion F. Marouillat-Vedrine S. Muh J.P. Andres C. Oligodendrocyte myelin glycoprotein growth inhibition function requires its conserved leucine-rich repeat domain, not its glycosylphosphatidyl-inositol anchor. J. Neurochem. 2003;85:889–897. doi: 10.1046/j.1471-4159.2003.01764.x. [DOI] [PubMed] [Google Scholar]
- Wang K.C. Koprivica V. Kim J.A. Sivasankaran R. Guo Y. Neve R.L. He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Willson C.A. Irizarry-Ramirez M. Gaskins H.E. Cruz-Orengo L. Figueroa J.D. Whittemore S.R. Miranda J.D. Upregulation of EphA receptor expression in the injured adult rat spinal cord. Cell Transplant. 2002;11:229–239. [PubMed] [Google Scholar]
- Xu J. Fan G. Chen S. Wu Y. Xu X.M. Hsu C.Y. Methylprednisolone inhibition of TNF-a expression and NF-kB activation after spinal cord injury in rats. Mol. Brain Res. 1998;59:135–142. doi: 10.1016/s0169-328x(98)00142-9. [DOI] [PubMed] [Google Scholar]
- Yan P. Liu N. Kim G.-M. Xu J. Xu J. Li Q. Hsu C.Y. Xu X.-M. Expression of the type 1 and type 2 receptors for tumor necrosis factor after traumatic spinal cord injury in adult rats. Exp. Neurol. 2003;183:286–297. doi: 10.1016/s0014-4886(03)00135-3. [DOI] [PubMed] [Google Scholar]
- Yan P. Xu J. Li Q. Chen S. Kim G.-M. Hsu C.Y. Xu X.M. Glucocorticoid receptor expression in the spinal cord after traumatic injury in adult rats. J. Neurosci. 1999;19:9355–9363. doi: 10.1523/JNEUROSCI.19-21-09355.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu G. He Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B. Atwal J. Ho C. Case L. He X.L. Garcia K.C. Steward O. Tessier-Lavigne M. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc. Natl. Acad. Sci. USA. 2005;102:1205–1210. doi: 10.1073/pnas.0409026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B. Ho C. Li S. Keirstead H. Steward O. Tessier-Lavigne M. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]