Abstract
Traumatic brain injury (TBI) is a major cause of death and disability. In the United States alone approximately 1.4 million sustain a TBI each year, of which 50,000 people die, and over 200,000 are hospitalized. Despite numerous prior clinical trials no standard pharmacotherapy for the treatment of TBI has been established. Citicoline, a naturally occurring endogenous compound, offers the potential of neuroprotection, neurorecovery, and neurofacilitation to enhance recovery after TBI. Citicoline has a favorable side-effect profile in humans and several meta-analyses suggest a benefit of citicoline treatment in stroke and dementia. COBRIT is a randomized, double-blind, placebo-controlled, multi-center trial of the effects of 90 days of citicoline on functional outcome in patients with complicated mild, moderate, and severe TBI. In all, 1292 patients will be recruited over an estimated 32 months from eight clinical sites with random assignment to citicoline (1000 mg twice a day) or placebo (twice a day), administered enterally or orally. Functional outcomes are assessed at 30, 90, and 180 days after the day of randomization. The primary outcome consists of a set of measures that will be analyzed as a composite measure using a global test procedure at 90 days. The measures comprise the following core battery: the California Verbal Learning Test II; the Controlled Oral Word Association Test; Digit Span; Extended Glasgow Outcome Scale; the Processing Speed Index; Stroop Test part 1 and Stroop Test part 2; and Trail Making Test parts A and B. Secondary outcomes include survival, toxicity, and rate of recovery.
Key words: citicoline, clinical trial, COBRIT, therapy, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability, with an estimated direct cost of 45 billion dollars a year in the United States alone. Every year, approximately 1.4 million people sustain a TBI, of which 50,000 die, and another 235,000 are hospitalized and survive the injury (Sample et al., 2004). As a result, 80,000–90,000 people experience permanent disability associated with TBI (Thurman et al., 1999). Currently, more than 5.3 million Americans, or 3% of the population, live with disabilities resulting from TBI, and virtually no injury is without consequence. This results in an enormous psychosocial burden on patients, their families, and society. The magnitude of this problem has led to numerous clinical trials aimed at improving survival or functional outcome, yet no effective pharmacotherapy has been identified over the past decade. One potential concern has been that many of the agents examined have targeted one portion of the cascade of injury that occurs after TBI. These agents also have a time-limited opportunity to provide neuroprotection and are rarely involved in the neuro-restorative process. A potential agent that could provide both neuroprotection as well as a means to facilitate the recovery process is citicoline. The purpose of this article is to describe the rationale for the choice of citicoline and the design and current state of the Citicoline Brain Injury Treatment (COBRIT) trial. The study is funded by the National Center for Medical Rehabilitation Research (NCMRR) at that National Institute of Child Health and Human Development (NICHD), and is conducted by the Traumatic Brain Injury Clinical Trials (TBI-CT) Network, which consists of eight clinical centers and one data coordinating center.
Methods
Citicoline (also known as CDP-Choline) (Fig. 1) is a naturally occurring endogenous compound. It was originally identified as the key intermediary in the biosynthesis of phosphatidylcholine by Kennedy in 1956 (Kennedy, 1956). Citicoline may have neuroprotective effects through its ability to improve phosphatidylcholine synthesis in the injured brain (Adibhatla and Hatcher, 2002). In addition to its neuroprotective capability, citicoline might potentiate neurorecovery, which has led to its evaluation as a treatment for both stroke and TBI in animal models and in human clinical trials.
FIG. 1.
Molecular structure of citicoline.
A major mechanism of secondary injury in TBI is the formation of reactive oxygen species and lipid peroxidases, which cause significant tissue damage. Animal models of TBI support a key role in oxidative stress (Ikeda and Long, 1990; Kontos et al., 1992). The exogenous administration of citicoline significantly increased levels of glutathione (De La Cruz et al., 2000; Adibhatla et al., 2001; Barrachina et al., 2003), a powerful endogenous antioxidant. Citicoline also attenuates the release of arachidonic acid, cardiolipin, and sphingomyelin (Adibhatla and Hatcher, 2002). Studies in animal models of ischemia and hypoxia have found that citicoline treatment improves free fatty acid concentration, decreases neurological deficits, and improves behavioral performance on learning and memory tasks (Rao et al., 2001).
In animal models, citicoline has been demonstrated to provide for acute neuroprotection, and also to have positive effects in stroke and chronic brain injury. For example, citicoline was found to be neuroprotective in models of uninterrupted occlusion of the basilar artery after subarachnoid hemorrhage (Alkan et al., 2001). Citicoline was associated with increased arterial pressure, smaller infarct volumes, and decreased mortality compared to controls. These results suggest that citicoline provides significant neuroprotection during cerebral ischemia. In addition, recent animal studies (Dixon et al., 1997; Baskaya et al., 2000; Dempsey and Raghavendra Rao, 2003) demonstrated the neuroprotective effect of citicoline in TBI. Citicoline significantly prevented TBI-induced neuronal loss in the hippocampus, decreased cortical contusion volume, and improved neurological recovery. Additionally, there is a dose-dependent attenuation of chronic deficits in motor and spatial performance following citicoline administration. Extracellular levels of acetylcholine, a key mediator of memory processes, are increased (Dixon et al., 1997), suggesting that citicoline enhances cholinergic transmission and may ameliorate chronic functional deficits. A second mechanism that may explain why citicoline improves function in chronically injured animals focuses on observed decreases in dopamine following injury (Yan et al., 2001). In such models, citicoline increased dopamine levels (Ross et al., 2001; Rejdak et al., 2002; Secades and Frontera, 1995), which enhances neurorecovery (Kline et al., 2004).
Human studies in dementia, stroke, and TBI have also showed promise. In a Cochrane meta-analysis (Fioravanti and Yanagi, 2000, 2005), citicoline appeared to improve memory and behavior of patients with “chronic cerebral disturbance” and vascular dementia. Citicoline also has been shown to improve the neuropsychological performance of healthy older people (Babb et al., 2002). Additionally, increases in biomarkers that are representative of citicoline activity, such as phosphodiesters, were observed on proton magnetic resonance spectroscopy and were associated with improvements in verbal memory.
In stroke studies, administration of citicoline was effective early in the post-ischemia recovery process, as demonstrated by improved level of consciousness (Tazaki et al., 1988), and improvements in the modified Rankin score (Clark et al., 2001). Consistent with this observation, MRI data showed a decrease in lesion volume with citicoline compared to placebo in a preliminary trial (Warach et al., 2000). A meta-analysis of four randomized clinical trials of citicoline in stroke (Davalos et al., 2002), suggested that there may a beneficial effect of citicoline as measured by the Rankin scale, a secondary outcome in these trials. A separate meta-analysis in acute and subacute stroke, published in abstract form only, suggested a positive treatment effect of citicoline precursors on rates of death and disability (Ovbiagele et al., 2003).
Randomized clinical trials of citicoline in TBI showed a faster rate of functional recovery from focal motor deficits in patients with severe TBI (Cohadon et al., 1982); improved recall design, a measure of memory (Levin, 1991); a reduction of post-concussional symptoms following mild TBI (Levin, 1991); and reduced inpatient hospital stay and requirement for outpatient follow-up (Calatayud Maldonado et al., 1991). Citicoline has also been shown to enhance cerebral blood flow. Among patients with TBI and very severe memory deficits, hypoperfusion of the inferior left temporal cerebral blood flow normalized after administration of citicoline (Leon-Carrion et al., 2000).
Clinical trials of citicoline in stroke and TBI have shown promise, but have demonstrated efficacy only in secondary outcome measures. The failure to demonstrate efficacy in primary outcome measures may have been due to substantial weaknesses in study design, such as insufficient sample size, inadequate outcome measures, or lack of sensitivity of the outcomes measure chosen (Cohadon et al., 1982; Calatayud Maldonado et al., 1991; Clark et al., 2001). In addition, most of the previous trials used doses substantially lower than those used in this study. These doses may have been inadequate to demonstrate efficacy (Clark et al., 1997, 1999; Warach et al., 2000). A recent trial in intracerebral hemorrhage has suggested potential efficacy at higher doses (Chua, 2007). A more definitive study of outcome after acute and chronic administration in stroke (the international citicoline trial on stroke [ICTUS]) is currently underway. This study also employs higher doses and delivers therapy over 6 weeks (Saver, 2008). Some trials have safely used doses as high as 4000 mg a day, however side effects appear to grow at this level and prior U.S. trials have employed 2000 mg as a maximum dosage. The window of opportunity for neuroprotection and neurofacilitation of citicoline appears to be rather broad. Animal data and human clinical trials have suggested a wide window of opportunity ranging from 6h to over 48h (Alexandrov, 2001). Clinical trials have employed intervention ranges from 8h to 48h. Given that the mechanisms of action impact neurorepair as well as neuroprotection we chose to employ the 24h dosing model to create the widest opportunity to impact outcome. Animal data have used doses as high as 500 mg/kg intraperitoneally in the rodent model to obtain an effect on infarct volume or neurobehavioral function (Adibhatla and Hatcher, 2006). Such a dose in humans would grossly exceed recommended levels. Prior trials have also shown tolerance of the medications for 6 weeks, but with a retreat of outcome differences upon stoppage of the drug (Clarke, 2001). Consequently COBRIT is designed for 90 days of active therapy and measurement of the primary outcome at 90 days with a follow-up measurement at 180 days, 90 days after discontinuing therapy to see if any treatment effect is maintained. Thus the COBRIT trial seeks to provide a well tolerated high dose of citicoline (2000 mg a day) for 3 months in order to maximize the utility of this agents neurorepair mechanisms.
An approach using higher doses of citicoline also seems reasonable because citicoline is generally considered to be a safe compound (Clark et al., 1997). In a large 2001 study Clarke and associates noted no difference in side effects between the placebo and citicoline groups. In other trials, side effects have been noted with only qualitative statements regarding mild diarrhea, leg edema, and back pain, with headache, tinnitus, insomnia, vision problems, and dizziness reported much less frequently (Levin, 1991; Clark et al., 1997; Adibhatla and Hatcher, 2002).
Study overview
The Citicoline Brain Injury Treatment (COBRIT) trial is a two-arm, double-blind, placebo-controlled, phase III, multi-center clinical trial of the effects of citicoline on functional and cognitive outcome in patients with complicated mild, moderate, and severe TBI. Patients are randomly assigned to receive citicoline (1000 mg twice a day) or placebo (twice a day) for 90 days, and are followed for an additional 90 days to see if any observed effect is maintained when the participant is off the drug. At randomization participants are stratified by accrual center and severity of injury. The three strata based on severity of TBI are patients with Glasgow Coma Scale (GCS) scores of 3 to 5; patients with GCS of 6 to 8 with a motor score of 5 or less; and patients with a GCS of 9 or greater or a GCS of 6 to 8 with a motor score of 6. Participants and all clinical sites staff (e.g., treating physicians, study coordinators, study monitors, and nurses) are blinded to the identity of the treatment.
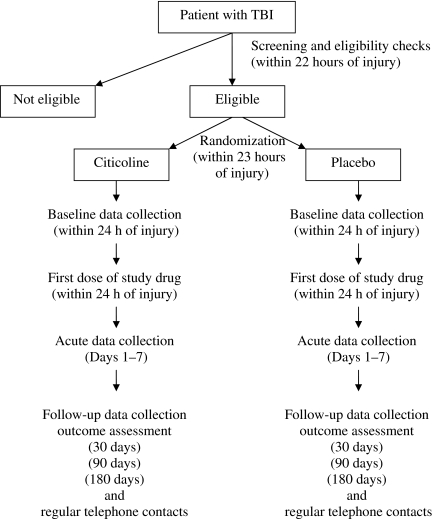
The study has a planned accrual of 1292 eligible patients over approximately 4 years. The anticipated distribution of severe, moderate, and complicated mild TBI patients is 38%, 16%, and 46%, respectively. The primary outcome measure consists of the core battery given 90 days after the day of randomization. The battery consists of a set of functional and cognitive measures. Secondary outcome measures include additional tests for disability, satisfaction with life, and well-being, as well as the core battery assessed at 30 and 180 days after the day of randomization. Figure 2 provides a design schematic of the trial.
FIG. 2.
Design schematic.
Protocol development and external review for COBRIT occurred between 2005 and 2007. Screening and enrollment for the study began in July 2007.
Inclusion and exclusion criteria
The TBI-CT Network carefully considered inclusion and exclusion criteria for this study. The criteria were designed to enroll patients with complicated mild (as defined by computed tomographic imaging), moderate, and severe brain injury, who would be able to receive the drug within 24 h of the injury, and complete the outcome assessments. Table 1 lists the inclusion and exclusion criteria for the trial. Once a patient is considered eligible for the trial, consent is obtained from the patient or the legally authorized representative (LAR), according to the local institutional review board regulations for surrogate consent. To determine if the patient can consent him- or herself, both the Galveston Orientation and Amnesia Test (GOAT) and the mini mental state examination are administered to determine competency. For participants whose LAR originally gave consent, once the participant is determined to be competent, consent for ongoing participation is obtained directly from the participant. A separate consent is obtained for a DNA banking portion of the study. This DNA banking is a totally separate element of the study, wherein DNA samples are obtained and will be stored in a central resource for possible use in the future. Refusal to have DNA samples stored does not affect participation in the COBRIT trial.
Table 1.
COBRIT Inclusion and Exclusion Criteria
|
Inclusion criteria: |
| • Non-penetrating traumatic brain injury |
| • Age 18 (19 in Alabama) to 70 years |
| • GCS criteria: |
| GCS obtained off paralytics: |
| GCS 3–12 with GCS motor score ≤5 |
| GCS 3–12 with GCS motor score =6 and meeting ANY of the following CT criteria: |
| • ≥10 mm total diameter intraparenchymal hemorrhage |
| • Acute extra-axial hematoma thickness ≥5 mm |
| • Contiguous subarachnoid hemorrhage visible on two or more contiguous slices on CT |
| • Intraventricular hemorrhage present on two slices |
| • Midline shift ≥5 mm |
| GCS 13–15 meeting the above CT criteria |
| GCS obtained on paralytics: |
| GCS 3TP (intubated and paralyzed) meeting the above CT criteria |
| • Reasonable expectation of completion of outcomes measures at a network center at 6 months post-injury |
| • Able to swallow oral medication, or if unable to swallow, a gastric tube or PEG are placed by 23 hours after injury |
| • Reasonable expectation of enrollment within 24-h time window |
| • English-speaking |
| Exclusion criteria: |
| • Intubated patients with GCS motor score = 6 and not meeting CT criteria |
| • Bilaterally fixed and dilated pupils |
| • Positive pregnancy test, known pregnancy, or current breast-feeding |
| • Evidence of disease that interferes with outcome assessment |
| • Current acetylcholinesterase inhibitor use |
| • Current citicoline use and unwilling to discontinue |
| • Imminent death or current life-threatening disease |
| • Current enrollment in another study |
| • Prisoner |
PEG, percutaneous endoscopic gastrostomy; GCS, Glasgow Coma Scale; CT computed tomography; COBRIT, Citicoline Brain Injury Treatment trial.
Treatment
Experimental treatment is administered orally or enterally at 1000 mg twice a day for 90 days, with the first dose administered within the first 24 h after injury. Oral or enteral administration is dependent on whether the participant can swallow. Participants who can swallow will receive two 500-mg tablets twice a day. Participants who cannot swallow will receive the tablets of citicoline through a nasogastric tube or percutaneous endoscopic gastrostomy (PEG) tube as 1000 mg via crushed tablets with a 25-mL saline or water flush. If the participant is no longer able to receive the drug by the oral or enteral route at any time during the study, the drug is interrupted until the participant can swallow or receive the drug by the enteral route. Bioavailability data in animals suggest that enteral and intravenous routes are similar (Seccades, 1995; Seccades, 2006). The placebo is formulated as closely as possible in appearance and taste to the active drug to protect the double-blind.
As background treatment, all patients enrolled in the clinical trial are treated according to the guidelines developed by the Brain Trauma Foundation and adapted by the TBI-CT Network. Unique to this study these guidelines include both acute and post-acute care paradigms attempting to standardize interventions as much as is possible.
Baseline and acute data collection
Information to characterize the participant's condition before administration of the experimental treatment is collected as soon as is clinically reasonable. In many instances this will occur after randomization for variables other than baseline CT scan and vital signs. These include participant demographics, medical history, and injury information. In addition, in the first 24 h after randomization, information is collected regarding surgical interventions, more detailed injury information, vital signs, changes in clinical status, and other medical treatments received by the participant. Information regarding alcohol and drug use before the injury will be collected using the Alcohol Use Disorders Identification Test (AUDIT) (Allen et al., 1997), and a modified version of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) (World Health Organization, 2002) during follow-up when the patient is judged mentally competent to provide such information. Additional information such as GCS, concomitant medications, surgical interventions, CT scan, and neuro-worsening are collected daily for days 2–7 of the acute hospitalization.
For those participants for whom a separate consent is obtained for DNA storage a blood sample is collected and stored for determination of possibly interesting genomic links such as Apoe-4 polymorphisms or others that might be identified in the future.
Vital signs are monitored every 12 h during the first 7 days of hospitalization, and at the 30- and 90-day visits. Blood samples are obtained at baseline, day 3, and at the 30- and 90-day visits to measure metabolic, liver, and hematologic function. In addition, adverse events and symptoms are collected at regularly scheduled contacts, and during the 30-, 90-, and 180-day visits.
Outcomes
The primary outcome for COBRIT consists of a set of measures that will be analyzed using a global test procedure at 90 days. The following measures comprise the core battery: the California Verbal Learning Test II (Delis et al., 2000) (CVLT-II); the Controlled Oral Word Association Test (Benton et al., 1994) (COWAT); Digit Span (Wechsler, 1997); Extended Glasgow Outcome Scale (Wilson et al., 1998) (GOS-E); the Processing Speed Index (Wechsler, 1997) (PSI); Stroop Test part 1 and Stroop Test part 2 (Dodrill, 1978); and Trail Making Test parts A and B (Reitan, 1992; Reitan and Wolfson, 1993). The CVLT-II (Delis et al., 2000) is a multifaceted and comprehensive verbal list-learning task that has been studied extensively in the TBI population. The COWAT (Benton et al., 1994) is an executive control measure that assesses verbal initiation and fluency. Digit Span (Wechsler, 1997) is a common measure of working memory. The GOS-E (Wilson et al., 1998) is the successor to the GOS, which is one of the most widely used outcome scales following TBI. The PSI (Wechsler, 1997) is a composite score derived from two subtests of the WAIS-III, specifically the Digit Symbol and Symbol Search subtests. Digit Symbol is a measure of complex attention and psychomotor speed. Symbol Search measures visual working memory. The Stroop Test (Dodrill, 1978) assesses susceptibility to interference, response inhibition, and the ability to shift perceptual set. The Trail Making Test (Reitan, 1992; Reitan and Wolfson, 1993) is a composite index of neuropsychological functioning after TBI. Details concerning the individual scales and tests included in the core battery are in the process of being published (Bagiella Submitted).
Other measures that will be used in secondary analyses include the Brief Symptom Inventory (Derogatis, 1993) (BSI), the Disability Rating Scale (Rappaport et al., 1982) (DRS), the GOAT (Levin et al., 1979), post-traumatic amnesia duration, and the Satisfaction with Life Scale (Diener et al., 1985) (SWLS), at 30, 90, and 180 days. The BSI (Derogatis, 1993) is a self-reported measure of broad psychological status. The DRS (Rappaport et al., 1982) evaluates the cognitive ability to perform activities of daily living, overall level of functioning, and employability. The GOAT (Levin et al., 1979) is an accepted measure of orientation to place, time, and personal information in participants with TBI. Post-traumatic amnesia is defined as the span of time surrounding an injury for which a person has no remembrance of events and measures of severity of injury. The SWLS (Diener et al., 1985) is a brief self-report scale on which an individual rates agreement with five items reflecting general satisfaction with life not related to any particular domain. The core battery measures administered at 30 and 180 days are also secondary outcomes.
Trained researchers administer the measures scheduled at 30, 90, and 180 days. For most participants with severe TBI, the 30-day evaluation takes place during inpatient care. For those discharged from medical care before 30 days, an outpatient appointment is arranged for the first follow-up assessment. The 30-day evaluation requires 90 min for those participants able to fully participate. Every effort is made to conduct the 90-day assessment while the participants are on the study drug. At this assessment, the core battery is performed and secondary measures are ascertained. Another outcome assessment takes place at 180 days following randomization. For those participants who are able to fully participate, these evaluations require 120 min.
Power and sample size
The nine functional and cognitive scales in the core battery are combined in the global test procedure to test the primary null hypothesis that the global odds ratio (OR) equals 1 versus the two-sided alternative that the global OR differs from 1. The logarithm of the global OR appears as the coefficient β in the global logit model:
 |
where Yj is the dichotomized outcome for the jth battery measure (j = 1,…,9), and where T = 1 for citicoline assignment and T = 0 for placebo. The model allows an arbitrary reference probability of a good outcome (Y = 1) for each outcome measure by site and baseline severity (severe, moderate, or complicated mild), but quantifies the treatment effect on each measure as the global OR, equal to exp(β). A global test statistic offers several advantages compared to the multivariate procedures or the multiple testing correction procedures. It offers a method to utilize several important outcome measures, avoids loss of power due to multiple comparisons, and it uses all the available information from all measures (O'Brien, 1984; Pocock et al., 1987; Tang et al., 1993; Tilley et al., 1996). Power for this study was determined based on the following design specifications:
Two-sided type I error fixed at 0.05
85% power
Design alternative effect size for the global: OR = 1.40
Response rate in the control group as indicated in Table 2
Correlations among the nine measures included in the global statistic as indicated in Table 3.
Table 2.
Assumed Proportion with Good Outcome in the Citicoline and Placebo Groups Used in the Power Analysis
| Placebo group | Citicoline group | |
|---|---|---|
| GOS-E | 0.60 | 0.6774 |
| Trails A | 0.51 | 0.5930 |
| Trails B | 0.52 | 0.6026 |
| COWAT | 0.40 | 0.4827 |
| PSI | 0.40 | 0.4827 |
| Stroop 1 | 0.44 | 0.5238 |
| Stroop 2 | 0.50 | 0.5833 |
| CVLT-II | 0.40 | 0.4827 |
| Digit Span | 0.49 | 0.5735 |
GOS-E, Extended Glasgow Outcome Scale; Trails A, Trail Making Test part A; Trails B, Trail Making Test part B; COWAT, Controlled Oral Word Association Test; PSI, Processing Speed Index; Stroop 1, Stroop Test part 1; Stroop 2, Stroop Test part 2; CVLT-II, California Verbal Learning Test II.
Table 3.
Correlation among the Measurements
| Trails A | Trails B | COWAT | PSI | Stroop 1 | Stroop 2 | CVLT-II | Digit Span | |
|---|---|---|---|---|---|---|---|---|
| GOS-E | .57 | .61 | .61 | .57 | .38 | .61 | .60 | .60 |
| Trails A | .60 | .34 | .60 | .42 | .81 | .36 | .32 | |
| Trails B | .46 | .63 | .48 | .75 | .42 | .32 | ||
| COWAT | .66 | .39 | .70 | .30 | .25 | |||
| PSI | .63 | .76 | .60 | .60 | ||||
| Stroop 1 | .51 | .60 | .60 | |||||
| Stroop 2 | .60 | .60 | ||||||
| CVLT-II | .46 |
GOS-E, Extended Glasgow Outcome Scale; Trails A, Trail Making Test part A; Trails B, Trail Making Test part B; COWAT, Controlled Oral Word Association Test; PSI, Processing Speed Index; Stroop 1, Stroop Test part 1; Stroop 2, Stroop Test part 2; CVLT-II, California Verbal Learning Test II.
Estimates of the rates of good response in the control group were obtained from previous studies on TBI patients. Overall response rates were obtained as a weighted average of the specific rates provided for severe, moderate, and complicated mild patients. Correlations among the nine outcome measures were estimated using data from previous TBI studies.
Using the assumptions noted above, it is estimated that 1124 participants will be required to detect a global OR of 1.4 or higher. This effect size corresponds to an absolute improvement of 7.7% for the GOS-E. The anticipated loss to follow-up rate in this study is around 15%. To account for this loss-to-follow-up rate, a total of 1292 eligible participants will be enrolled in the study. The primary analysis will be an intent-to-treat analysis that will include all randomized participants regardless of their compliance with the study treatment or follow-up schedule. All hypothesis testing will be conducted using α = 0.05, two-sided. For continuous variables means and standard deviations will be calculated. For discrete and dichotomous variables we will use contingency tables.
The nine primary COBRIT outcome measures will each be dichotomized to indicate whether the participant had a good or a poor outcome. The GOS-E will be dichotomized as 1–6 for poor outcome and 7–8 for good outcome. All other measures in the battery are continuous. The raw scores will be used to categorize participants into a good-outcome group (if their score is at or above one standard deviation less than the mean for the normal population for that scale), or into a poor-outcome group (if their score is below one standard deviation less than the mean for the normal population for that scale). Participants who die before 90 days will be assigned to the poor-outcome category for each measure.
Additionally, each outcome measure is assigned a code to classify the degree to which a measure has been successfully administered. A completion code indicating that a measure was not given or completed due to CNS limitations will be interpreted as a poor outcome. A code indicating that the measure was not given, not completed, or completed but with apparently invalid results due to non-CNS limitations will be interpreted as missing data. Logistic regression will be used to estimate the global odds ratio using weighted least squares stratified by site and initial severity. This measure and its 95% confidence interval will be used as the primary tool for assessing efficacy of the study treatment.
Secondary outcomes of the study will be:
The rate of recovery of cognitive and functional abilities using the battery of tests administered at 30, 90, and 180 days after the day of randomization
The effect of citicoline on measures of disability, life satisfaction, and psychological well-being at 30, 90, and 180 days after the day of randomization
Treatment differences observed at 90 days after randomization compared to those at 3 months after treatment is discontinued (at 180 days after the day of randomization)
Survival after TBI
Safety profile of citicoline in TBI.
As in all such trials all secondary and teriary analyses can only serve to better understand the reasons for an overall effect on the primary outcome if one is seen, or generate future hypotheses for research. Overall survival will be estimated using Kaplan-Meier curves. To compare the citicoline arm with the placebo arm a stratified log-rank statistic (using the stratification variables from the randomization procedure) will be used. A Cox proportional hazards model with treatment and other important prognostic factors as covariates will be used for the multivariate analysis of the survival times. Risk ratios and their 95% confidence intervals will be computed.
If a participant is unable to be followed through the course of the study, all the information available up to the time of loss to follow-up will be used. If the fraction of missing data is small we expect that such data will have little effect on the final estimates. If the extent of missing data is large compared to the effect size, the multiple imputation procedure proposed by Rubin (1997) and Little (Little and Yau, 1996) will be used.
The organizational structure of COBRIT is similar to that of other large multi-center clinical trials. The study is conducted by the Traumatic Brain Injury Clinical Trials Network, consisting of eight clinical centers and one data coordinating center (DCC), and is funded by the National Center for Medical Rehabilitation Research (NCMRR) at the National Institute of Child Health and Human Development (NICHD). A central DNA repository is maintained at one of the network centers, and an independent central drug distribution center also contributes to the network. This drug distribution center maintains the drug and placebo supplies, and distributes them to the sites as needed based upon randomization rates. In addition, they maintain a web-based randomization system that assigns blinded drug numbers according to the previously described stratum for each new participant.
The main decision-making bodies within the COBRIT trial are the Steering Committee and the Data and Safety Monitoring Committee (DSMC). The Steering Committee provides the overall leadership for the study. It is comprised of the NCMRR/NICHD project scientist and the principal investigators from all the participating clinical centers and the DCC. It is responsible for developing the protocol and the manual of operations, overseeing the conduct of the trial, and reviewing and adapting changes to the protocol during the course of the trial. In addition, it participates in the analysis, interpretation, and publication of study results. The DSMC is appointed by and advisory to the NCMRR/NICHD. The DSMC is responsible for monitoring the trial for evidence of safety or efficacy. Based on the results of the monitoring it recommends changes to the study protocol or performance when they are needed.
Results
As of July 31, 2009, COBRIT has enrolled 800 participants which accounts for 13% of all patients screened. Among those screened, 6% were unable to consent and a LAR was not available to provide consent and 6% refused to participate in the study. The current distribution of severe, moderate and complicated mild TBI is: 29%, 4%, and 67%. The baseline characteristics of the patients randomized to date are presented in Table 4.
Table 4.
Baseline Characteristics of Participants as of April 20, 2009
| Characteristic | Number (percent) |
|---|---|
| Age (n = 683) | |
| 18–30 years old | 246 (36%) |
| >30–45 years old | 162 (24%) |
| >45–60 years old | 185 (27%) |
| >60 years old | 90 (13%) |
| Male (n = 684) | 511 (75%) |
| Race (n = 678) | |
| Caucasian | 549 (81%) |
| African-American | 108 (16%) |
| Asian/Pacific Islander | 11 (2%) |
| Other | 10 (1%) |
| Level of education (n = 656) | |
| Elementary school | 10 (2%) |
| High school | 301 (46%) |
| Technical/vocational/college | 316 (48%) |
| Graduate school | 29 (4%) |
| Glasgow Coma Scale (GCS) (n = 679) | |
| GCS 3–8 with GCS motor ≤5 | 195 (29%) |
| GCS 9–12 with GCS motor ≤5 | 26 (4%) |
| GCS 13-15 or GCS Motor = 6 | 458 (67%) |
| Mechanism of injury (n = 679) | |
| Motor vehicle | 213 (31%) |
| Motorcycle/golf cart/ATV | 109 (16%) |
| Individual struck by vehicle | 38 (6%) |
| Fall from moving object | 60 (9%) |
| Fall from stationary object | 156 (23%) |
| Assault | 66 (10%) |
| Struck on head by object | 15 (2%) |
| Other | 22 (3%) |
ATV, all-terrain vehicle.
The current enrollment is 77% of that expected to date. Based on this enrollment rate, the trial is expected to reach its enrollment goal of 1292 participants by August of 2010.
Discussion
Two important decisions that had to be made in designing COBRIT were the choice of the intervention and the primary outcome. Multiple pharmacologic and other interventions have been tried in TBI, uniformly without success. The network chose citicoline to evaluate because of its neuroprotective, neurorecovery and neurofacilitation properties discussed above as well as the suggestive clinical findings with this agent in the treatment of stroke.
Equally critical was the decision of an appropriate primary endpoint, and how to test for it. Typically, the primary outcome is a parameter of important clinical interest that best represents the mechanism of action of the intervention of interest or a variable on which the treatment may have the greatest effect. To select a primary outcome measure a subgroup of Network investigators including neurosurgeons, neurologists, physiatrists, neuropsychologists, and statisticians, met regularly for more than a year. The goal of the subgroup was to review a set of outcome measures suitable for the TBI population that together would reflect the “global” status of TBI patients. Traumatic brain injury has diffuse cerebral effects which in turn cause an array of impairments and disabilities in functional, physical, emotional, cognitive, and social spheres. It was felt that no single measure could capture the multidimensional nature of TBI outcome and thus it was felt that multiple measures would be necessary to address the breadth of potential deficits and recovery following TBI. Equally important was to select measures that would prove sensitive to the effects of citicoline throughout the spectrum of injury severity. The outcome of their deliberations are the choice of the 9 measures incorporated into the global test procedure.
The choice of several outcome measures inevitably raises the issue of multiple comparisons. While it would be very interesting to determine the effect of the treatment on each individual outcome measure by performing several statistical tests, such a procedure would require controlling the experiment-wise error rate. A global test procedure represents an efficient approach to combine several outcome measures into a unique measure. In recent years a number of different methods have been developed all based on a common assumption that the treatment effect is constant across all measures. To the extent that this assumption is not true, and that there is not an overall effect, but rather one that is seen in only one or more measures, then no efficacy claim can be made, but it may suggest future areas for research and hypothesis generation.
One other important decision was to include the full spectrum of patients from severe to complicated mild. After much discussion in the planning phase, the decision was made to include the complicated mild as they have significant sequelae following the injury and potentially might even benefit more than the severe patients from an effective therapy. Fundamentally there was no a priori reason to assume that citicoline would work on only one end of the injury spectrum. Nonetheless the study is powered only for an overall effect. If a differential effect is seen, then no firm conclusion regarding efficacy can be drawn, but again would suggest areas for future research. Also prior clinical data suggested that sufficient numbers of the severe patients would have recovered enough to be “testable” at 90 days, and conversely that sufficient numbers of uncomplicated mild patients would have enough deficits still present at 90 days to allow a treatment effect to be demonstrated, if one exists.
Finally by addressing several important primary and secondary questions regarding the efficacy of citicoline in the treatment of TBI in a large multi-center randomized trial, the results of the COBRIT trial should provide substantial new clinically important information regarding the possible treatment of acute TBI.
Acknowledgments
The study is supported in part by National Institute Award numbers NICHD U01HD042823, U01HD042738, U01HD042687, U01HD042736, U01HD042678, U01HD042686, U01HD042653, U01HD042689, and U01HD042652, to eight clinical sites and a data coordinating center.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Adibhatla R.M. Hatcher J.F. Citicoline mechanisms and clinical efficacy in cerebral ischemia. J. Neurosci. Res. 2002;70:133–139. doi: 10.1002/jnr.10403. [DOI] [PubMed] [Google Scholar]
- Adibhatla R.M. Hatcher J.F. Dempsey R.J. Effects of citicoline on phospholipid and glutathione levels in transient cerebral ischemia. Stroke. 2001;32:2376–2381. doi: 10.1161/hs1001.096010. [DOI] [PubMed] [Google Scholar]
- Adibhatla R. Hatcher J. Larsen E. Chen X. Sun D. Tsao F. CDP-choline significantly restores phophatuidylcholine levels by differentially affecting phospholipase A2 and CTP: phosphocholinecytidylytransferase after stroke. J Biol Chem. 2006;28:6718–25. doi: 10.1074/jbc.M512112200. [DOI] [PubMed] [Google Scholar]
- Alexandrov A. Citicoline Curr Opinion Invest Drugs. 2001;2:1757–1762. [PubMed] [Google Scholar]
- Alkan T. Kahveci N. Goren B. Korfali E. Ozluk K. Ischemic brain injury caused by interrupted versus uninterrupted occlusion in hypotensive rats with subarachnoid hemorrhage: neuroprotective effects of citicoline. Arch. Physiol. Biochem. 2001;109:161–167. doi: 10.1076/apab.109.2.161.4273. [DOI] [PubMed] [Google Scholar]
- Allen J.P. Litten R.Z. Fertig J.B. Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT) Alcohol. Clin. Exp. Res. 1997;21:613–619. [PubMed] [Google Scholar]
- Babb S.M. Wald L.L. Cohen B.M. Villafuerte R.A. Gruber S.A. Yurgelun-Todd D.A. Renshaw P.F. Chronic citicoline increases phosphodiesters in the brains of healthy older subjects: an in vivo phosphorus magnetic resonance spectroscopy study. Psychopharmacology (Berl.) 2002;161:248–254. doi: 10.1007/s00213-002-1045-y. [DOI] [PubMed] [Google Scholar]
- Bagiella E. Novack T.A. Ansel B. Diaz-Arrastia R. Dikmen S. Hart T. Temkin N. Measuring outcome in TBI treatment trials: Recommendations from the TBI Clinical Trials Network. (Submitted). [DOI] [PMC free article] [PubMed]
- Barrachina M. Dominguez I. Ambrosio S. Secades J. Lozano R. Ferrer I. Neuroprotective effect of citicoline in 6-hydroxydopamine-lesioned rats and in 6-hydroxydopamine-treated SH-SY5Y human neuroblastoma cells. J. Neurol. Sci. 2003;215:105–110. doi: 10.1016/s0022-510x(03)00204-1. [DOI] [PubMed] [Google Scholar]
- Baskaya M.K. Dogan A. Rao A.M. Dempsey R.J. Neuroprotective effects of citicoline on brain edema and blood-brain barrier breakdown after traumatic brain injury. J. Neurosurg. 2000;92:448–452. doi: 10.3171/jns.2000.92.3.0448. [DOI] [PubMed] [Google Scholar]
- Benton A.L. Sivan A.B. Hamsher K.D. Varney N.R. Spreen O. Contributions to Neuropsychological Assessment: A Clinical Manual. Oxford University Press; New York: 1994. [Google Scholar]
- Calatayud Maldonado V. Calatayud Perez J.B. Aso Escario J. Effects of CDP-choline on the recovery of patients with head injury. J. Neurol. Sci. 1991;103(Suppl.):S15–S18. doi: 10.1016/0022-510x(91)90003-p. [DOI] [PubMed] [Google Scholar]
- Chua R.H. Role of intravenous citcoline for supratentorial hemorrhage. Cerebrovasc. Dis. 2007;23(Suppl. 2):73. [Google Scholar]
- Clark W.M. Warach S.J. Pettigrew L.C. Gammans R.E. Sabounjian L.A. A randomized dose-response trial of citicoline in acute ischemic stroke patients. Citicoline Stroke Study Group. Neurology. 1997;49:671–678. doi: 10.1212/wnl.49.3.671. [DOI] [PubMed] [Google Scholar]
- Clark W.M. Wechsler L.R. Sabounjian L.A. Schwiderski U.E. A phase III randomized efficacy trial of 2000 mg citicoline in acute ischemic stroke patients. Neurology. 2001;57:1595–1602. doi: 10.1212/wnl.57.9.1595. [DOI] [PubMed] [Google Scholar]
- Clark W.M. Williams B.J. Selzer K.A. Zweifler R.M. Sabounjian L.A. Gammans R.E. A randomized efficacy trial of citicoline in patients with acute ischemic stroke. Stroke. 1999;30:2592–2597. doi: 10.1161/01.str.30.12.2592. [DOI] [PubMed] [Google Scholar]
- Cohadon F. Richer E. Poletto B. [A precursor of phospholipids in the treatment of severe traumatic comas] Neurochirurgie. 1982;28:287–290. [PubMed] [Google Scholar]
- Davalos A. Castillo J. Alvarez-Sabin J. Secades J.J. Mercadal J. Lopez S. Cobo E. Warach S. Sherman D. Clark W.M. Lozano R. Oral citicoline in acute ischemic stroke: an individual patient data pooling analysis of clinical trials. Stroke. 2002;33:2850–2857. doi: 10.1161/01.str.0000038691.03334.71. [DOI] [PubMed] [Google Scholar]
- De La Cruz J.P. Pavia J. Gonzalez-Correa J.A. Ortiz P. Sanchez de la Cuesta F. Effects of chronic administration of S-adenosyl-L-methionine on brain oxidative stress in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2000;361:47–52. doi: 10.1007/s002109900153. [DOI] [PubMed] [Google Scholar]
- Delis D.C. Kramer J.H. Kaplan E. Ober B.A. California Verbal Learning Test, Second Edition, Adult Version. PsychCorp, A brand of Harcourt Assessment, Inc.; San Antonio: 2000. [Google Scholar]
- Dempsey R.J. Raghavendra Rao V.L. Cytidinediphosphocholine treatment to decrease traumatic brain injury-induced hippocampal neuronal death, cortical contusion volume, and neurological dysfunction in rats. J. Neurosurg. 2003;98:867–873. doi: 10.3171/jns.2003.98.4.0867. [DOI] [PubMed] [Google Scholar]
- Derogatis L.R. Brief Symptom Inventory (BSI) Administration, Scoring And Procedures Manual. NCS Pearson, Inc.; Minneapolis, MN: 1993. [Google Scholar]
- Diener E. Emmons R. Larsen J. Griffin S. The Satisfaction With Life Scale. J. Pers. Assess. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- Dixon C.E. Ma X. Marion D.W. Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J. Neurotrauma. 1997;14:161–169. doi: 10.1089/neu.1997.14.161. [DOI] [PubMed] [Google Scholar]
- Dodrill C.B. A neuropsychological battery for epilepsy. Epilepsia. 1978;19:611–623. doi: 10.1111/j.1528-1157.1978.tb05041.x. [DOI] [PubMed] [Google Scholar]
- Fioravanti M. Yanagi M. Cytidinediphosphocholine (CDP choline) for cognitive and behavioural disturbances associated with chronic cerebral disorders in the elderly. Cochrane Database Syst. Rev. 2000:CD000269. doi: 10.1002/14651858.CD000269. [DOI] [PubMed] [Google Scholar]
- Fioravanti M. Yanagi M. Cytidinediphosphocholine (CDP-choline) for cognitive and behavioural disturbances associated with chronic cerebral disorders in the elderly. Cochrane Database Syst. Rev. 2005:CD000269. doi: 10.1002/14651858.CD000269.pub3. [DOI] [PubMed] [Google Scholar]
- Ikeda Y. Long D.M. The molecular basis of brain injury and brain edema: the role of oxygen free radicals. Neurosurgery. 1990;27:1–11. doi: 10.1097/00006123-199007000-00001. [DOI] [PubMed] [Google Scholar]
- Kennedy E.P. The synthesis of cytidine diphosphate choline, cytidine diphosphate ethanolamine, and related compounds. J. Biol. Chem. 1956;222:185–191. [PubMed] [Google Scholar]
- Kline A.E. Massucci J.L. Ma X. Zafonte R.D. Dixon C.E. Bromocriptine reduces lipid peroxidation and enhances spatial learning and hippocampal neuron survival in a rodent model of focal brain trauma. J. Neurotrauma. 2004;21:1712–1722. doi: 10.1089/neu.2004.21.1712. [DOI] [PubMed] [Google Scholar]
- Kontos C.D. Wei E.P. Williams J.I. Kontos H.A. Povlishock J.T. Cytochemical detection of superoxide in cerebral inflammation and ischemia in vivo. Am. J. Physiol. 1992;263:H1234–H1242. doi: 10.1152/ajpheart.1992.263.4.H1234. [DOI] [PubMed] [Google Scholar]
- Leon-Carrion J. Dominguez-Roldan J.M. Murillo-Cabezas F. del Rosario Dominguez-Morales M. Munoz-Sanchez M.A. The role of citicholine in neuropsychological training after traumatic brain injury. NeuroRehabilitation. 2000;14:33–40. [PubMed] [Google Scholar]
- Levin H.S. O'Donnell V.M. Grossman R.G. The Galveston Orientation and Amnesia Test. A practical scale to assess cognition after head injury. J. Nerv. Ment. Dis. 1979;167:675–684. doi: 10.1097/00005053-197911000-00004. [DOI] [PubMed] [Google Scholar]
- Levin H.S. Treatment of postconcussional symptoms with CDP-choline. J. Neurol. Sci. 1991;103(Suppl.):S39–S42. doi: 10.1016/0022-510x(91)90007-t. [DOI] [PubMed] [Google Scholar]
- Little R. Yau L. Intent-to-treat analysis for longitudinal studies with drop-outs. Biometrics. 1996;52:1324–1333. [PubMed] [Google Scholar]
- O'Brien P.C. Procedures for comparing samples with multiple endpoints. Biometrics. 1984;40:1079–1087. [PubMed] [Google Scholar]
- Ovbiagele B. Kidwell C.S. Starkman S. Saver J.L. Potential role of neuroprotective agents in the treatment of patients with acute ischemic stroke. Curr. Treat. Options Cardiovasc. Med. 2003;5:441–449. doi: 10.1007/s11936-003-0033-9. [DOI] [PubMed] [Google Scholar]
- Pocock S.J. Geller N.L. Tsiatis A.A. The analysis of multiple endpoints in clinical trials. Biometrics. 1987;43:487–498. [PubMed] [Google Scholar]
- Rao A.M. Hatcher J.F. Dempsey R.J. Does CDP-choline modulate phospholipase activities after transient forebrain ischemia? Brain Res. 2001;893:268–272. doi: 10.1016/s0006-8993(00)03280-7. [DOI] [PubMed] [Google Scholar]
- Rappaport M. Hall K.M. Hopkins K. Belleza T. Cope D.N. Disability rating scale for severe head trauma: coma to community. Arch. Phys. Med. Rehabil. 1982;63:118–123. [PubMed] [Google Scholar]
- Reitan R.M. Wolfson D. The Halstead-Reith Neuropsychological Test Battery: Theory and Clinical Interpretation. Neuropsychology Press; Tucson, AZ: 1993. [Google Scholar]
- Reitan R.M. Trail Making Test. Manual for Administration and Scoring. Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- Rejdak R. Toczolowski J. Solski J. Duma D. Grieb P. Citicoline treatment increases retinal dopamine content in rabbits. Ophthalmic Res. 2002;34:146–149. doi: 10.1159/000063658. [DOI] [PubMed] [Google Scholar]
- Ross B.M. Mamalias N. Moszczynska A. Rajput A.H. Kish S.J. Elevated activity of phospholipid biosynthetic enzymes in substantia nigra of patients with Parkinson's disease. Neuroscience. 2001;102:899–904. doi: 10.1016/s0306-4522(00)00501-7. [DOI] [PubMed] [Google Scholar]
- Rubin D. Multiple Imputation for Non-response in Surveys. John Wiley; New York: 1997. [Google Scholar]
- Sample P.L. Johns N. Gabella B. Langlois J. Can traumatic brain injury surveillance systems be used to link individuals with TBI to services? Brain Inj. 2004;18:1177–1189. doi: 10.1080/02699050410001719925. [DOI] [PubMed] [Google Scholar]
- Saver J. Citicoline: update on a promising and widely available agent for neuroprotection and neurorepair. Rev. Neurol. Dis. 2008;5:167–177. [PubMed] [Google Scholar]
- Secades J.J. Frontera G. CDP-choline: pharmacological and clinical review. Methods Find. Exp. Clin. Pharmacol. 1995;17(Suppl. B):1–54. [PubMed] [Google Scholar]
- Tang D.I. Geller N.L. Pocock S.J. On the design and analysis of randomized clinical trials with multiple endpoints. Biometrics. 1993;49:23–30. [PubMed] [Google Scholar]
- Tazaki Y. Sakai F. Otomo E. Kutsuzawa T. Kameyama M. Omae T. Fujishima M. Sakuma A. Treatment of acute cerebral infarction with a choline precursor in a multicenter double-blind placebo-controlled study. Stroke. 1988;19:211–216. doi: 10.1161/01.str.19.2.211. [DOI] [PubMed] [Google Scholar]
- Thurman D.J. Alverson C. Dunn K.A. Guerrero J. Sniezek J.E. Traumatic brain injury in the United States: A public health perspective. J. Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Tilley B.C. Marler J. Geller N.L. Lu M. Legler J. Brott T. Lyden P. Grotta J. Use of a global test for multiple outcomes in stroke trials with application to the National Institute of Neurological Disorders and Stroke t-PA Stroke Trial. Stroke. 1996;27:2136–2142. doi: 10.1161/01.str.27.11.2136. [DOI] [PubMed] [Google Scholar]
- Warach S. Pettigrew L.C. Dashe J.F. Pullicino P. Lefkowitz D.M. Sabounjian L. Harnett K. Schwiderski U. Gammans R. Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Citicoline 010 Investigators. Ann. Neurol. 2000;48:713–722. [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale (WAIS-III) The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- World Health Organization. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97:1183–1194. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- Wilson J.T. Pettigrew L.E. Teasdale G.M. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J. Neurotrauma. 1998;15:573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- Yan H.Q. Kline A.E. Ma X. Hooghe-Peters E.L. Marion D.W. Dixon C.E. Tyrosine hydroxylase, but not dopamine beta-hydroxylase, is increased in rat frontal cortex after traumatic brain injury. Neuroreport. 2001;12:2323–2327. doi: 10.1097/00001756-200108080-00009. [DOI] [PubMed] [Google Scholar]