Abstract
Erythropoietin (EPO), essential for erythropoiesis, provides neuroprotection. The EPO receptor (EPOR) is expressed in both neural and non-neural cells in the brain. This study was designed to test the hypothesis that EPO provides beneficial therapeutic effects, even in the absence of the neural EPOR. In this study, EPOR-null mice were rescued with selective EpoR expression driven by the endogenous EpoR promoter in hematopoietic tissue, but not in the neural cells. Anesthetized young adult female EPOR-null and wild-type mice were subjected to traumatic brain injury (TBI) induced by controlled cortical impact. EPO (5000 U/kg) or saline was intraperitoneally administered at 6 h and 3 and 7 days post-injury. Sensorimotor and spatial learning functions were assessed. Expression of EPOR and its downstream signal proteins were evaluated by Western blot analysis. Our data demonstrated that EPO treatment significantly reduced cortical tissue damage and hippocampal cell loss, and improved spatial learning following TBI in both the wild-type and EPOR-null mice. EPO treatment significantly improved sensorimotor functional recovery, with better outcomes in the wild-type mice. EPO treatment upregulated anti-apoptotic proteins (p-Akt and Bcl-XL) in the ipsilateral hippocampus and cortex of the injured wild-type and EPOR-null mice. These data demonstrate that EPO significantly provides neuroprotection following TBI, even in the absence of EPOR in the neural cells, suggesting that its therapeutic benefits may be mediated through vascular protection.
Key words: erythropoietin receptor null, mouse, sensorimotor, spatial learning, traumatic brain injury
Introduction
An estimated 1.4 million people sustain traumatic brain injury (TBI) each year in the United States, and more than 5 million people are coping with disabilities from TBI. The most prevalent and debilitating features in survivors of brain trauma are cognitive deficits and motor dysfunction (Langlois et al., 2006). To date, there is no effective treatment to significantly promote functional recovery after TBI except for routine medical intervention and care (Narayan et al., 2002; Royo et al., 2003). Erythropoietin (EPO) and the EPO receptor (EPOR), essential for erythropoiesis, are also expressed in the nervous system. While EPO and EPOR are only weakly expressed in normal adult brain, expression of EPO and EPOR is greatly increased in response to different types of brain injury (Grasso et al., 2004; Marti 2004). Recent studies show that EPO treatment promotes functional recovery in animal models of TBI (Brines et al., 2000; Grasso et al., 2007; Lu et al., 2005; Xiong et al., 2008a; Yatsiv et al., 2005), brain ischemia (Brines et al., 2000; Chang et al., 2005; Gonzalez et al., 2007; Iwai et al., 2007; Spandou et al., 2005; Wang et al., 2004), and autoimmune encephalomyelitis (Zhang et al., 2005). Inhibition of EPO activity by the administration of soluble EPOR worsens the severity of neuronal injury (Sakanaka et al., 1998), suggesting that endogenous EPO is directly involved in an intrinsic neuronal repair pathway. These findings indicate that the EPO/EPOR system plays an important role in neuroprotection and neurorestoration.
EPOR is expressed not only in neural cells but also in non-neural cells including endothelial cells (NoguChi et al., 2008). Exogenous EPO provides neuroprotection (Brines et al., 2000; Grasso et al., 2007; NoguChi et al., 2008; Wang et al., 2004; Xiong et al., 2008a). Recent studies have demonstrated that EPO treatment is capable of reducing blood–brain barrier (BBB) breakdown (Grasso et al., 2007) and edema (Verdonck et al., 2007) after TBI. These results suggest that multiple pathways may be involved in the mechanism of EPO neuroprotection, including its effects on EPOR in both neural and non-neural cells in the brain. To test our hypothesis that EPO provides neuroprotection following TBI, even in the absence of EPOR in the neural cells, EPOR-null mice in neural cells were used in this study. EPOR-null mice were rescued with selective EPOR expression driven by the endogenous EPOR promoter in hematopoietic tissue but not in the brain. These mice exhibit normal hematopoiesis and erythropoiesis and survive to adulthood (Chen et al., 2007). Our recent study also showed that being EPOR-null in the nervous system worsens sensorimotor functional outcomes in mice after TBI (Xiong et al., 2008b). The aim of this study was to investigate whether EPO treatment would improve histological and functional outcomes after TBI in EPOR-null mice.
Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Henry Ford Health System.
Conditional rescue mice
Conditional rescue mice (C57BL6 background) were provided by Dr. Constance Tom Noguchi at the National Institute of Diabetes & Digestive & Kidney Diseases, National Institutes of Health. The mouse EpoR gene was replaced by the human EpoR gene by substituting exons 1–8 of the endogenous mouse EpoR gene with exons 1–8 of the human EpoR gene. The human EpoR gene was inactivated using a neo-cassette flanked by LoxP sites inserted into intron 6. The heterozygous mice were then cross-bred with transgenic mice expressing Cre recombinase under the direction of the endothelial cell–specific receptor tyrosine kinase Tek (Tie2) promoter/enhancer. Expression of Cre in cells that normally express Tie2, such as embryonic endothelium which gives rise to hematopoietic stem cells, results in recombination of the two LoxP sites and excision of the neo-cassette that restores appropriately regulated expression of the EPOR gene in Cre-expressing cells, and subsequent generations of cells derived from these cells. Mice from resultant litters were screened and crossed to obtain mice homozygous for the disrupted EPOR gene that carry the Tie2-Cre transgene (EPOR-null) (Chen et al., 2007).
Traumatic brain injury model
Young adult female wild-type C57BL/6 mice (Charles River Laboratories, Inc., Wilmington, MA), and female EPOR-null mice were anesthetized intraperitoneally with chloral hydrate, 400 mg/kg body weight. The body weight of the mice ranged from 22–25 g (4–5 months old). Body temperature was maintained at 37°C by using a circulating water heating pad. Each animal was placed in a stereotaxic frame, and TBI was delivered as previously described (Xiong et al., 2007). A 4-mm-diameter craniotomy was performed over the left parietal cortex adjacent to the central suture, midway between the lambda and the bregma. The dura was kept intact over the cortex. Injury was induced by impacting the left cortex (ipsilateral cortex) with a pneumatic piston containing a 2.5-mm-diameter tip at a rate of 4 m/sec and 0.8 mm of compression. Velocity was measured with a linear velocity displacement transducer. A sham group of mice underwent the same craniotomy but were not injured. Young adult female wild-type and EPOR-null mice were randomly divided into three groups: (1) sham group, (2) TBI + saline group, and (3) TBI + EPO group. The number of animals in each group was: 6 (wild-sham), 8 (wild-saline), 8 (wild-EPO), 6 (null-sham), 10 (null-saline), and 10 (null-EPO). In a separate set of experiments, TBI mice were treated with saline or EPO and sacrificed at 6 h, and 1, 4, and 14 days post-injury for Western blot analysis of EPOR and its downstream signal proteins (n = 3/time point per group).
Foot-fault test
To evaluate sensorimotor function, the foot-fault test was carried out prior to TBI and at 1, 4, 7, 14, 21, 28, and 35 days after TBI or sham injury by an investigator blinded to the study groups. The mice were allowed to walk on a grid (12 × 57 cm with 1.3- × -1.3-cm-diameter openings). With each weight-bearing step, a paw might fall or slip between the wires, and if this occurred it was recorded as a foot-fault. A total of 50 steps were recorded for each right forelimb and hindlimb (Baskin et al., 2003; Xiong et al., 2007).
Morris water maze test
To detect spatial learning deficits, a modified version of the Morris water maze (MWM) was used (Choi et al., 2006; Lu et al., 2005; Xiong et al., 2007). All animals were tested during the last 5 days (i.e., from 31–35 days after TBI or sham injury) before sacrifice. Data collection was automated by the HVS Image 2020 Plus Tracking System (US HVS Image, San Diego, CA). For data collection, a white pool (1.2 m in diameter) was divided into four equal quadrants formed by imaging lines. The pool was located in a large test room where there were many clues external to the maze (e.g., pictures and lamps); these were visible from the pool and presumably used by the mice for spatial orientation. The position of the cues remained unchanged throughout the task (Lu et al., 2005). At the start of a trial, the mouse was placed randomly at one of four fixed starting points, facing toward the wall (designated north [N], south [S], east [E], and west [W]), and allowed to swim for 90 sec or until it found the platform within 90 sec. If the animal found the platform, it was allowed to remain on it for 10 sec. If the animal failed to find the platform within 90 sec, it was placed on the platform for 10 sec. Throughout the test period the platform was located in the NE quadrant 1 cm below the water level in a randomly changing position, including locations against the wall, toward the middle of the pool, or off-center, but always within the target quadrant. If the animal was unable to find the platform within 90 sec, the trial was terminated and a maximum score of 90 sec was assigned. If the animal reached the platform within 90 sec, the percentage of time traveled within the NE (correct) quadrant relative to the total amount of time spent swimming before reaching the platform was calculated and employed for statistical analysis. The advantage of this version of the water maze is that each trial or set of trials takes on the key characteristics of a probe trial because the platform is not in a fixed location within the target quadrant (Choi et al., 2006).
Tissue preparation and measurement of lesion volume
At 35 days after TBI, mice were anesthetized intraperitoneally with chloral hydrate, and perfused transcardially first with saline solution, followed by 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS; pH 7.4). The brains were removed and post-fixed in 4% paraformaldehyde at room temperature for 48 h. The brain tissue was cut into seven equally spaced (1 mm) coronal blocks, and processed for paraffin sectioning. A series of adjacent 6-μm-thick sections were cut from each block in the coronal plane and stained with hematoxylin and eosin (H&E). To measure lesion volume the seven brain sections were traced by a microcomputer imaging device (MCID) (Imaging Research, St. Catharine's, Ontario, Canada), as previously described (Chen et al., 2005; Xiong et al., 2007). The indirect lesion area was calculated (i.e., the intact area of the ipsilateral hemisphere was subtracted from the area of the contralateral hemisphere) (Chen et al., 2005), and the lesion volume was presented as a volume percentage of the lesion compared with the contralateral hemisphere. H&E sections from blocks E and F containing hippocampus were used to acquire images of the dentate gyrus (DG) at 20 × magnification.
Western blot analysis
Sham or TBI mice treated with saline or EPO were sacrificed at 6 h, and 1, 4, and 14 days post-injury for Western blot analysis of EPOR and its downstream signal proteins (n = 3/time point per group). Brain tissues from the lesion boundary zone and the hippocampus were collected, washed once in 1 × PBS, and lysed in lysis buffer (20 mM Tris [pH 7.6], 100 mM NaCl, 1% Nonidet P-40, 0.1% SDS, 1% deoxycholic acid, 10% glycerol, 1 mM EDTA, 1 mM NaVO3, 50 mM NaF, and cocktail I of protease inhibitors; Calbiochem, San Diego, CA). After sonication, soluble protein was obtained by centrifugation at 13,000 g for 15 min at 4°C. The protein concentration of each sample was determined by bicinchoninic acid protein assay (Pierce, Rockford, IL). For immunoblotting, equal amounts of cell lysate were subjected to SDS-polyacrylamide gel electrophoresis on Novex Tris-Glycine pre-cast gels (Invitrogen, Carlsbad, CA), and separated proteins were then electrotransferred to polyvinylidene fluoride membranes. The membranes were blocked with 2% I-Block (Applied Biosystems, Foster City, CA) in PBS plus 0.1% Tween 20 for 1 h at room temperature, and then incubated with different primary antibodies overnight at 4°C. We used the following antibodies: anti-EPOR (M20), anti-p-Akt (Ser473), anti-Bcl-XL (H-62), and anti-Actin (I-19) (1:1000; Santa Cruz Biotechnology Inc., Santa Cruz, CA). After washing, the membranes were incubated with HRP-conjugated secondary antibodies (1:2500; Jackson ImmunoResearch Laboratories, West Grove, PA) in blocking buffer for 2 h at room temperature. Specific proteins were visualized using the SuperSignal West Pico chemiluminescence substrate system (Pierce). The intensity of the bands was measured using Scion image analysis (Scion Corporation, Frederick, MD).
Immunofluorescent staining for EPOR-positive neurons
EPOR-positive neurons were identified by double labeling for EPOR and NeuN (marker for neurons). After dehydration, tissue sections were boiled in 10 mM citric acid buffer (pH 6) for 10 min. After washing with PBS, the sections were incubated in 2.4 N HCl at 37°C for 20 min. The sections were incubated with 1% BSA containing 0.3% Triton-X-100 in PBS. The sections were then incubated with mouse anti-NeuN antibody (1:200; Chemicon, Temecula, CA) at 4°C overnight. FITC-conjugated anti-mouse antibody (1:400; Jackson ImmunoResearch) was added to the sections at room temperature for 2 h. The sections were then incubated with rabbit anti-EPOR antibody (1:400; Santa Cruz Biotechnology) at 4°C overnight. The sections were then incubated with Cy3-conjugated anti-rabbit antibody (1:400; Jackson ImmunoResearch) at room temperature for 2 h. Each of the steps was followed by three 5-min rinses in PBS. The tissue sections were mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA).
Cell counting and quantification
Cell counts were performed by observers blinded to the individual status of the animals. Five sections cut from the dorsal DG at 100-μm intervals were analyzed with a microscope at 400 × magnification via the MCID system. All counting was performed on a computer monitor to improve visualization and in one focal plane to avoid oversampling (Zhang et al., 2002). To evaluate the effects of EPO treatment on neuronal damage after TBI, the number of neurons was counted in the hippocampus (DG and CA3) using the MCID system. Counts were averaged and normalized by measuring the linear distance (in millimeters) for each section. This method permits a meaningful comparison of differences between groups. The MCID system was also used to count the total number of EPOR-positive cells (red stained) and NeuN-positive neurons (green stained) in the lesion boundary zone and the hippocampus. The number of EPOR/NeuN-positive cells was expressed in cells/mm2.
Statistical analysis
All data are presented as means with standard deviations (SD). For lesion volume, cell counting, and protein band density, a one-way analysis of variance (ANOVA) followed by post-hoc Student-Newman-Keuls tests were used to compare the difference between groups. Data were analyzed by ANOVA for repeated measurements of functional tests (spatial performance and sensorimotor function). Statistical significance was set at p < 0.05.
Results
Lesion volume
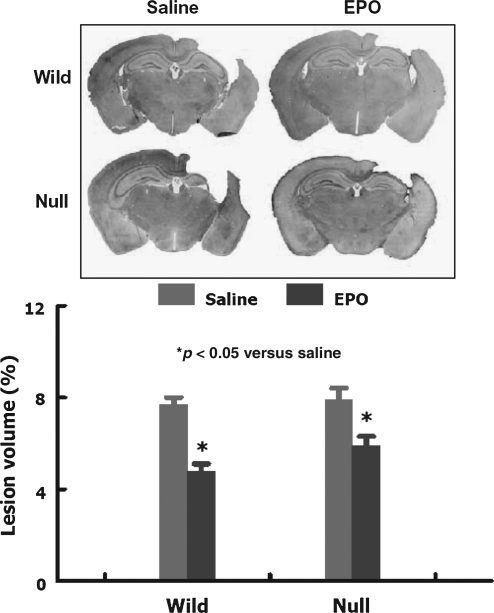
Mice were sacrificed at 35 days post-TBI for lesion volume measurements. TBI caused a similar volume of tissue loss in the ipsilateral hemisphere in both wild-type and EPOR-null mice. EPO treatment similarly significantly reduced lesion volume in the wild-type and EPOR-null mice (Fig. 1).
FIG. 1.
Lesion volume examined 35 days after traumatic brain injury (TBI). TBI caused approximately 8% cortical tissue loss in both wild-type and EPOR-null mice. EPO treatment significantly reduced lesion volume following TBI in both groups. Data represent mean ± SD (n = 8 for the wild-saline and wild-EPO groups; n = 10 for the null-saline and null-EPO groups; EPO, erythropoietin; EPOR, EPO receptor; SD, standard deviation).
Cell loss in the hippocampus
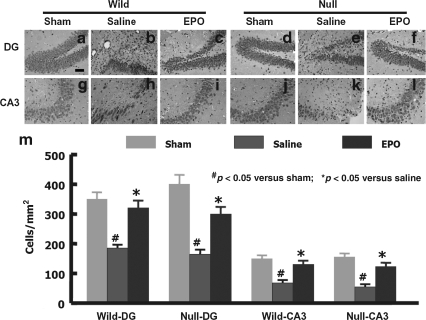
When examined at 35 days post-TBI, the neuron counts in the ipsilateral DG and CA3 region were significantly decreased in the wild-type and EPOR-null mice compared to the sham animals (Fig. 2; p < 0.05). No significant difference was observed in the neuron numbers between wild-type and EPOR-null mice after TBI. However, EPO treatment similarly significantly reduced cell loss in these regions in the injured wild-type and EPOR-null mice compared to saline control mice.
FIG. 2.
Cell density in the dentate gyrus (DG) and CA3 region examined at 35 days after traumatic brain injury (TBI) (hematoxylin and eosin staining). TBI caused significant cell loss in the ipsilateral DG and CA3 region in the wild-type (b and h) and EPOR-null mice (e and k). EPO treatment significantly reduced cell loss in the DG and CA3 region in both groups (c and i, f and l, respectively; scale bar = 50 μm in a–l). (m) The cell numbers in the DG and CA3 region. Data represent mean ± SD (n = 6 for wild-sham mice; n = 8 for wild-saline and wild-EPO mice; n = 6 for null-sham mice; n = 10 for null-saline and null-EPO mice; EPO, erythropoietin; EPOR, EPO receptor; SD, standard deviation).
Spatial learning performance
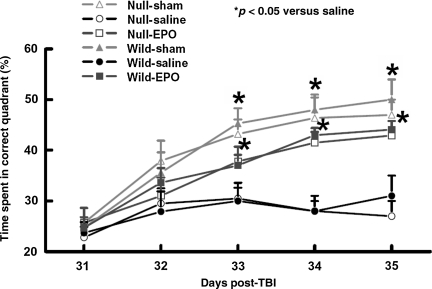
The modified MWM protocol was used to detect spatial learning deficits (Fig. 3). No significant difference in the MWM test was observed between wild-type and EPOR-null mice after sham or TBI. As compared to saline treatment, the time spent in the correct quadrant (northeast) by wild-type and EPOR-null mice treated with EPO significantly increased during the test on days 33–35 (Fig. 3; p < 0.05).
FIG. 3.
Spatial learning function examined at 31–35 days after traumatic brain injury (TBI). TBI caused significantly more spatial learning deficits at days 33–35 post-injury in the wild-type and EPOR-null mice compared to their sham groups (p < 0.05). Compared to the saline group, EPO treatment significantly improved spatial learning performance (time spent in the correct quadrant) at 33, 34, and 35 days after TBI as measured by a recent version of the Morris water maze test. Data represent mean ± SD (n = 6 for wild-sham mice; n = 8 for wild-saline and wild-EPO mice; n = 6 for null-sham mice; n = 10 for null-saline and null-EPO mice; EPO, erythropoietin; EPOR, EPO receptor; SD, standard deviation).
Sensorimotor function
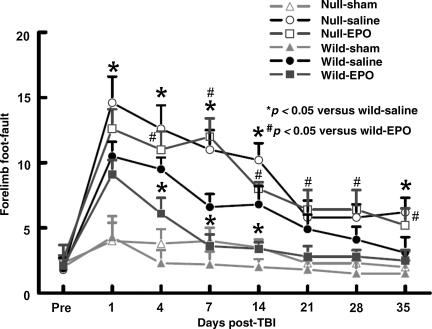
To avoid the unnecessary use of experimental animals, the data from the sham animals in the present study were shared with previously gathered data (Xiong et al., 2008b). The incidence of forelimb foot-faults during baseline (preoperatively) was approximately 4–5% (Fig. 4). The occurrence of right forelimb foot-faults in the injured EPOR-null mice persisted over the 35-day observation time and was significantly higher at 1–14 and 35 days post-injury compared with injured wild-type mice (p < 0.05). EPO treatment significantly reduced the occurrence of right forelimb foot-faults in the injured wild-type mice, but not in the injured EPOR mice.
FIG. 4.
Sensorimotor function (forelimb foot-fault) before and after traumatic brain injury (TBI) or sham injury. “Pre” represents pre-injury levels. TBI impaired sensorimotor performance (contralateral forelimb foot-fault) more in the EPOR-null mice than in the wild-type mice. EPO treatment significantly reduced forelimb foot-faults in the injured wild-type mice (days 4, 7, and 14), but not in the injured EPOR-null mice. Data represent mean ± SD (n = 6 for wild-sham mice; n = 8 for wild-saline and wild-EPO mice; n = 6 for null-sham mice; n = 10 for null-saline and null-EPO mice; EPO, erythropoietin; EPOR, EPO receptor; SD, standard deviation).
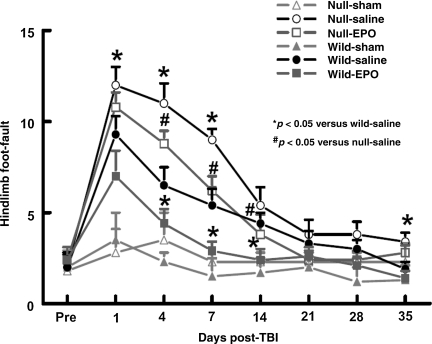
TBI significantly increased the incidence of right hindlimb foot-faults in the injured EPOR-null mice at 1, 4, 7, and 35 days post-injury compared to the injured wild-type mice (Fig. 5; p < 0.05). EPO treatment significantly reduced the incidence of right hindlimb foot-faults in injured wild-type and EPOR-null mice at 4, 7 and 14 days post injury (p < 0.05). However, the incidence of the right hindlimb foot-faults in the EPO-treated injured EPOR-null mice was significantly higher than that of EPO-treated injured wild-type mice at 4–14 days post-injury.
FIG. 5.
Sensorimotor function (hindlimb foot-fault) before and after traumatic brain injury (TBI) or sham injury. “Pre” represents pre-injury levels. TBI impaired sensorimotor performance (contralateral hindlimb foot-fault) more in the EPOR-null mice than in the wild-type mice. EPO treatment significantly reduced forelimb foot-faults in the injured wild-type and EPOR-null mice (days 4, 7, and 14). Data represent mean ± SD (n = 6 for wild-sham mice; n = 8 for wild-saline and wild-EPO mice; n = 6 for null-sham mice; n = 10 for null-saline and null-EPO mice; EPO, erythropoietin; EPOR, EPO receptor; SD, standard deviation).
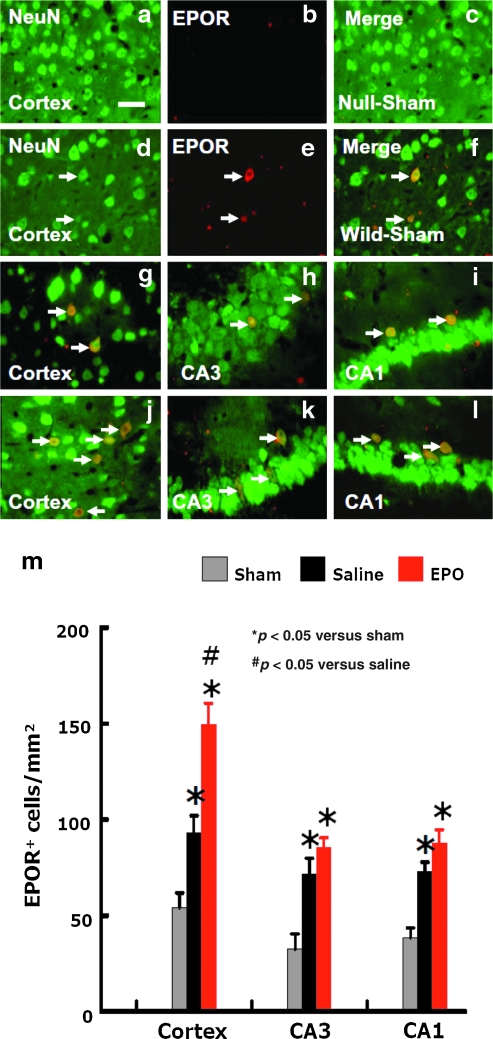
Immunofluorescent staining of EPOR-positive neurons
No EPOR-positive neurons were detected in the sham EPOR-null mice (Fig. 6a–c) and injured EPOR-null mice (data not shown). EPOR expression was detected only in some neurons in the sham and injured wild-type mice (Fig. 6d–i). The number of neurons expressing EPOR in the injured wild-type mice was significantly increased (Fig. 6 g–i and m) compared to sham controls (Fig. 6f). EPO treatment significantly increased the numbers of EPOR-positive neurons in the cortices of the injured wild-type mice, even at day 35 post-injury (Fig. 6 j–l and m; p < 0.05), compared to saline control mice (Fig. 6g–i and m).
FIG. 6.
Double fluorescent staining for EPOR (red) and NeuN (green) to detect the number of neurons expressing EPOR after TBI. No EPOR-positive neurons were detected in the cortex (a, b, and c) of EPOR-null sham mice. Some neurons expressed EPOR in the wild-type mice (d, e, and f). TBI significantly increased the number of neurons expressing EPOR in the cortex (g), CA3 (h), and CA1 (i) regions in the wild-type mice. EPO treatment increased the number of EPOR-positive neurons in the injured cortex (j), CA3 (k), and CA1 (l) of wild-type mice (scale bars = 50 μm in a–l). (m) The number of EPOR-positive neurons. Data represent mean ± SD (n = 6 for wild-sham mice; n = 8 for wild-saline and wild-EPO mice; n = 6 for null-sham mice; n = 10 for null-saline and null-EPO mice; EPO, erythropoietin; EPOR, EPO receptor; SD, standard deviation).
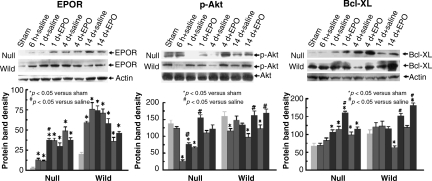
Western blot analysis of EPOR, p-Akt, and Bcl-XL in the hippocampus
No or low levels of EPOR were detected by Western blot analysis in the hippocampi in the sham wild-type or EPOR-null mice (Fig. 7). TBI significantly upregulated EPOR in the hippocampus as early as 6 h post-injury and for at least 14 days in both the wild-type and EPOR-null mice. EPO treatment did not affect EPOR expression in the hippocampus. TBI significantly decreased p-Akt levels in the ipsilateral hippocampi of EPOR-null mice at days 1 and 4 (Fig. 7). Compared to saline treatment, EPO therapy significantly increased p-Akt level at days 1 and 4. Interestingly, in the injured wild-type mice, the hippocampal p-Akt level was significantly reduced as early as 6 h, returned to sham levels at day 1, and was then reduced at days 4 and 14 post-injury. EPO treatment significantly elevated the p-Akt level at days 4 and 14 compared to saline treatment. A very low level of Bcl-XL was detected in the hippocampi in the sham EPOR-null mice, while a higher level of Bcl-XL was found in the hippocampi in the sham wild-type mice (Fig. 7). TBI significantly upregulated Bcl-XL in the ipsilateral hippocampi in the EPOR-null mice from 1–14 days post-injury, but decreased hippocampal Bcl-XL levels were seen at day 4 post-injury in the wild-type mice. Compared to saline treatment, EPO therapy significantly increased hippocampal Bcl-XL levels from days 1–14 in the injured EPOR-null mice, and at days 4 and 14 in the injured wild-type mice.
FIG. 7.
Representative Western blot and densitometry measurement of EPOR, p-Akt, and Bcl-XL in the ipsilateral hippocampus in mice treated with saline or EPO after traumatic brain injury (TBI). Data in the bar graphs are represented as mean ± SD (*p < 0.05 versus sham animals; #p < 0.05 versus saline controls at corresponding time points; n = 3 mice/time point/group; EPO, erythropoietin; EPOR, EPO receptor; SD, standard deviation).
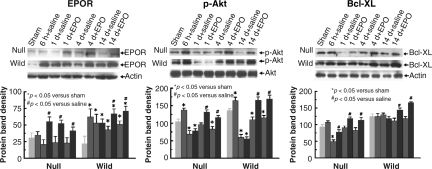
Western blot analysis of EPOR, p-Akt, and Bcl-XL in the cortex
Low levels of EPOR were detected in the cortices in the sham wild-type and EPOR-null mice. TBI significantly upregulated EPOR in the injured cortex as early as 6 h post-injury, and for at least 14 days in the wild-type mice, but not in EPOR-null mice (Fig. 8). EPO treatment significantly increased the cortical EPOR expression in the injured EPOR-null mice from days 1–14, and in the injured wild-type mice at days 4 and 14. TBI significantly increased p-Akt levels as early as 6 h post-injury in the cortices of injured EPOR-null and wild-type mice. However, TBI reduced cortical p-Akt levels from days 1–14 in these two groups (Fig. 8). EPO therapy significantly increased cortical p-Akt levels at days 4 and 14 in the injured wild-type and EPOR mice. A higher level of Bcl-XL was detected in the cortices in sham wild-type mice compared to sham EPOR-null mice. TBI significantly reduced Bcl-XL at 6 h post-injury in the injured cortex in the EPOR-null mice, but not wild-type mice (Fig. 8). Compared to saline treatment, EPO therapy significantly increased cortical Bcl-XL levels from days 1–14 in the injured EPOR-null mice, and at days 4 and 14 in the injured wild-type mice.
FIG. 8.
Representative Western blot and densitometry measurement of EPOR, p-Akt, and Bcl-XL in the ipsilateral cortex in mice treated with saline or EPO after traumatic brain injury (TBI). Data in the bar graphs are represented as mean ± SD (*p < 0.05 versus sham animals; #p < 0.05 versus saline controls at corresponding time points; n = 3 mice/time point/group; EPO, erythropoietin; EPOR, EPO receptor; SD, standard deviation).
Discussion
The main findings of the present study are: (1) TBI significantly upregulated EPOR levels in the hippocampus in both wild-type and EPOR-null mice and in the cortex of wild-type mice, while EPO treatment upregulated the EPOR level in the cortex but not in the hippocampus compared with saline controls; (2) EPO treatment significantly similarly reduced cortical tissue loss and hippocampal cell loss and improved spatial learning in both injured wild-type and EPOR-null mice, while EPO provided better sensorimotor functional recovery in the wild-type mice than in the EPOR-null mice; (3) compared to saline treatment, EPO treatment similarly significantly upregulated anti-apoptotic proteins p-Akt and Bcl-XL in both groups of injured mice at later time points (i.e., days 4 and 14) post-injury. This study for the first time demonstrates that EPO treatment provides significant protection against tissue and cell loss, and also improves sensorimotor and spatial learning functional recovery following TBI, even in the absence of EPOR in the neural cells, suggesting that its neuroprotection may partially be attributable to the beneficial effects of non-neural EPOR-EPO binding.
Our previous study demonstrated that EPOR-null mice did not exhibit higher susceptibility to TBI compared to the wild-type mice except for foot-faults (Xiong et al., 2008b). Although EPORs were null in the brain neural cells, Tie2 would rescue all endothelial cells in the EPOR-null mice (Chen et al., 2007). This led us to address the question of whether EPO provides beneficial therapeutic effects, even in the absence of neural EPOR. EPO is a naturally-occurring cytokine that is most widely recognized for its role in stimulating the maturation, differentiation, and survival of hematopoietic progenitor cells (Naranda et al., 2002; Wojchowski et al., 1999). EPO binds to EPOR. Receptor activation follows after its homodimerization upon EPO binding, which allows autophosphorylation of EPOR-associated Janus-tyrosine kinase-2 (JAK2), and leads to phosphorylation of several downstream signaling pathways, including PI3K/Akt, NF-κB, and STAT5 (Jelkmann, 2000; NoguChi et al., 2008; van der Kooij et al., 2008). EPO-mediated cell survival largely occurs through the activation of these pathways (van der Kooij et al., 2008). While EPO and EPOR are only weakly expressed in normal adult brain, expression of EPO and the EPOR is greatly increased in response to different types of brain injury (Grasso et al., 2004; Marti 2004). EPOR expression is significantly upregulated as early as 1 day and up to 7 days after TBI, whereas EPO is transiently elevated at days 1 and 2 (Liao et al., 2008). The upregulated EPO and EPOR may reflect the brain's survival response to injury. In this study, on Western blot analysis EPOR levels were significantly increased for up to 14 days in the ipsilateral cortex and hippocampus in the injured wild-type mice. Prolonged increased EPOR expression suggests that the endogenous EPO concentration may not be sufficient for cell survival after injury, and increased EPOR provides a platform for treatment with exogenous EPO. This is in agreement with results of recent studies indicating that exogenous EPO provides neuroprotection and neurorestoration in several animal models, including TBI (Cherian et al., 2007; Liao et al., 2008; Lu et al., 2005; Xiong et al., 2008a) and stroke (Wang et al., 2004; Zhang et al., 2006). EPO treatment did not affect EPOR protein levels in the hippocampus, but upregulated EPOR in the cortex in both injured wild-type and EPOR-null mice. Low levels of EPOR in the sham EPOR-null mice and increased EPOR expression in the injured EPOR-null mice must come from non-neural cells. This was confirmed by immunohistochemical staining, which demonstrated that none of the EPOR-positive neurons were found in the brains of sham and injured EPOR-null mice. In contrast, EPOR-positive neurons were detected in the sham wild-type mice, and the number of EPOR-positive neurons increased after TBI. EPO treatment further increased the number of EPOR-positive neurons in the injured cortices of wild-type mice. The effects of EPO treatment on neural EPOR regulation have not been investigated after TBI. However, EPO treatment upregulates the EPOR level in vascular endothelial cells, and confers neurovascular protection after permanent focal cerebral ischemia in adult mice (Li et al., 2007).
This study demonstrates that TBI had different effects (increasing or decreasing) on p-Akt levels in different brain regions (cortex and hippocampus) at different time points in both wild-type and EPOR-null mice. This result is in accordance with results of a previous study, that indicate that the expression of p-Akt may be region- and time-dependent after TBI (Noshita et al., 2002). The expression of p-Akt increased in the hippocampal CA3 region at 1 h in CD1 mice subjected to cortical impact injury (CCI), while the cortical p-Akt level decreased at 1 h, returned to sham levels at 4 h, and then decreased at 24 h post-TBI in the injured cortex (Noshita et al., 2002). In this study the overall effect of TBI consistently decreased brain p-Akt levels at later time points (4 days and 14 days) in both EPOR-null and wild-type mice. Our data indicating that increased EPOR did not translate into increased p-Akt after TBI strongly suggest that brain endogenous EPO formation post-injury is not concordant with its corresponding EPOR, and exogenous EPO may be a useful treatment strategy. Compared to saline controls, EPO treatment significantly increased cortical and hippocampal p-Akt levels in the injured EPOR-null and wild-type mice. p-Akt plays an important role in cell survival and function. After binding of EPO to the EPOR, JAK2 is phosphorylated and induces the phosphatidylinositol 3 kinase (PIK3)/protein kinase B (PKB/Akt) pathway, thereby activating Akt. p-Akt inhibits Bad/Bax functioning, leading to diminished cytochrome c release (NoguChi et al., 2008; van der Kooij et al., 2008; Zhang et al., 2006). Akt-mediated phosphorylation deactivates Bad and inhibits hypoxia-induced apoptosis in neurons (Ruscher et al., 2002). Results of the present study are in agreement with our previous results, indicating that simvastatin treatment significantly increases hippocampal p-Akt levels and improves spatial learning performance in rats following TBI (Wu et al., 2008a,b).
Upon EPO binding to EPOR and JAK2 phosphorylation, NF-κB is released from its inactive NF-κB–IκB complex in the cytosol and translocates to the nucleus. NF-κB induces transcription of anti-apoptotic genes, including Bcl-XL. Bcl-XL binds to Bax, which prevents Bax translocation into mitochondria and inhibits cytochrome c release–induced apoptosis (van der Kooij et al., 2008). The cortical Bcl-XL mRNA level decreased at least for 3 days in rats after TBI induced by lateral fluid percussion and CCI, though Bcl-XL protein levels were not measured in this study (Strauss et al., 2004). In contrast, higher expression of Bcl-XL was observed following severe TBI in humans (Minambres et al., 2008). In this study, the Bcl-XL level in the cortex and hippocampus was evaluated. The expression of Bcl-XL in response to TBI is region- and time-dependent. Bcl-XL protein levels decreased at day 4 in the hippocampus in the injured wild-type mice, while there was no change in the cortical tissue. Bcl-XL protein levels increased from days 1–14 in the hippocampus in the injured EPOR-null mice, while it decreased at day 1 in the cortex. The reason for the disparity in Bcl-XL levels in the different regions after TBI is not known, indicating the complex nature of cell death and survival after TBI. Increased Bcl-XL may help cell survival. Compared to saline controls, EPO treatment upregulated Bcl-XL in the hippocampus and cortex and reduced lesion volume and hippocampal cell loss. This result is in agreement with results of a study that indicated that erythropoietin upregulated Bcl-XL and prevented delayed neuronal death in rats after TBI (Liao et al., 2008). The direct neuroprotective effect of Bcl-XL has been demonstrated by systemic delivery of Bcl-XL protein, which resulted in marked and prolonged neuroprotection against neonatal hypoxic-ischemic brain injury in rats (Yin et al., 2006).
In this study, cell loss in the hippocampus and spatial learning deficits in the EPOR-null mice were comparable to those observed in the wild-type mice after TBI. We theorize that significant cell loss in the hippocampus partially contributes to impairment of spatial learning in the EPOR-null and wild-type mice after TBI. In our present study we found that EPO significantly reduced hippocampal cell loss and concomitantly improved spatial learning performance, which further supports this hypothesis. TBI also impaired sensorimotor function, as revealed by the foot-fault test. The cortical tissue loss and related insults to connected regions after TBI likely contribute to the sensorimotor impairment. We found that EPOR-null mice with TBI showed an increased frequency of foot-faults compared to injured wild-type mice. However, we did not find any significant difference in the contusion volume in the EPOR-null and wild-type mice examined at day 35 post-TBI, suggesting that other factors, such as possible effects of more distal brain regions (Mogensen et al., 2008) and thalamic calcification (Xiong et al., 2008b) may contribute to the increased occurrence of foot-faults in the EPOR-null mice after injury. The beneficial effects of EPO treatment for TBI in EPOR-null mice suggest that EPO may also mediate neuroprotection through mechanisms other than neural EPORs in the brain after injury. Since vascular endothelial EPORs were spared in the EPOR-null mice used in this study, neuroprotection fostered by EPO may be mediated by EPO binding to EPOR in non-neuronal cells, possibly vascular endothelial cells. The endothelial cells are an important component of the BBB, which forms a protective barrier around the brain, and has the important function of maintaining brain homeostasis. BBB breakdown is a significant pathological event that manifests early following TBI (Pun et al., 2009). Accumulating evidence indicates that EPO provides neuroprotection via endothelial cells in addition to its protection of neural cells. EPO promotes survival of endothelial cells through Bcl-XL induction (Zhande and Karsan, 2007). EPO significantly prevents lipopolysaccharide-induced vascular hyporeactivity and endothelial dysfunction in rats (di Villa Bianca et al., 2009). EPO protects endothelial cells and promotes the neurovascular unit repair, which may contribute to its therapeutic benefits after ischemic stroke (Keogh et al., 2007). EPO treatment reduces BBB breakdown after stroke (Chi et al., 2008), and after TBI (Grasso et al., 2007). Restoration of the local blood supply in the injured brain plays a critical role in tissue repair and functional recovery. EPO treatment upregulates the EPOR level in vascular endothelial cells, confers neurovascular protection, and enhances angiogenesis, resulting in local cerebral blood flow recovery in the ischemic brain (Li et al., 2007). In addition, EPO can directly dilate rat middle cerebral arteries via the endothelium (Shafi et al., 2008), and enhanced endothelium-mediated dilation may partially underlie the neuroprotective effects of EPO after brain injury. Several lines of evidence indicate that early administration of EPO provides neuroprotection, while delayed treatment fosters neurorestoration after TBI and stroke. EPO administration within 6 h reduces contusion volume after TBI (Brines et al., 2000; Cherian et al., 2007; Grasso et al., 2007; Hartley et al., 2008; Xiong et al., 2008a; Yatsiv et al., 2005) and stroke (Brines et al., 2000; Gonzalez et al., 2007; Iwai et al., 2007; Wang et al., 2007). Delayed EPO treatment (often 24 h or later post-injury) shows neurorestorative effects, including angiogenesis and neurogenesis (Lu et al., 2005; Wang et al., 2004). EPO-induced vascular protection and angiogenesis may play an important role in neuroprotection and neurorestoration after TBI.
In conclusion, we have demonstrated that EPO significantly provides neuroprotection following TBI, even in the absence of EPOR in the neural cells, suggesting that its therapeutic benefits may be mediated through its vascular protection. EPO treatment reduces contusion volume and cell loss, as well as improves sensorimotor and spatial learning performance after TBI. The beneficial effects of EPO may be mediated through its upregulation of anti-apoptotic proteins (p-Akt and Bcl-XL). In light of the positive outcomes seen with the use of EPO in a small clinical trial (Ehrenreich et al., 2002), and its neuroprotective and neurorestorative benefits seen in pre-clinical studies of stroke and TBI, EPO is a promising agent for the treatment of TBI through its neurovascular protective effects.
Acknowledgments
This work was supported by National Institute of Diabetes & Digestive & Kidney Diseases (NIDDK) Health grants RO1 NS62002 and PO1 NS42345, and NIDDK Intramural Research.
Author Disclosure Statement
No competing financial interests exist.
References
- Baskin Y.K. Dietrich W.D. Green E.J. Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J. Neurosci. Methods. 2003;129:87–93. doi: 10.1016/s0165-0270(03)00212-7. [DOI] [PubMed] [Google Scholar]
- Brines M.L. Ghezzi P. Keenan S. Agnello D. de Lanerolle N.C. Cerami C. Itri L.M. Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.S. Mu D. Wendland M. Sheldon R.A. Vexler Z.S. McQuillen P.S. Ferriero D.M. Erythropoietin improves functional and histological outcome in neonatal stroke. Pediatr. Res. 2005;58:106–111. doi: 10.1203/01.PDR.0000163616.89767.69. [DOI] [PubMed] [Google Scholar]
- Chen J. Zhang C. Jiang H. Li Y. Zhang L. Robin A. Katakowski M. Lu M. Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J. Cereb. Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.Y. Asavaritikrai P. Prchal J.T. Noguchi C.T. Endogenous erythropoietin signaling is required for normal neural progenitor cell proliferation. J. Biol. Chem. 2007;282:25875–25883. doi: 10.1074/jbc.M701988200. [DOI] [PubMed] [Google Scholar]
- Cherian L. Goodman J.C. Robertson C. Neuroprotection with erythropoietin administration following controlled cortical impact injury in rats. J. Pharmacol. Exp. Ther. 2007;322:789–794. doi: 10.1124/jpet.107.119628. [DOI] [PubMed] [Google Scholar]
- Chi O.Z. Hunter C. Liu X. Weiss H.R. Effects of erythropoietin on blood-brain barrier disruption in focal cerebral ischemia. Pharmacology. 2008;82:38–42. doi: 10.1159/000127839. [DOI] [PubMed] [Google Scholar]
- Choi S.H. Woodlee M.T. Hong J.J. Schallert T. A simple modification of the water maze test to enhance daily detection of spatial memory in rats and mice. J. Neurosci. Methods. 2006;156:182–193. doi: 10.1016/j.jneumeth.2006.03.002. [DOI] [PubMed] [Google Scholar]
- di Villa Bianca R. Sorrentino R. Mitidieri E. Marzocco S. Autore G. Thiemermann C. Pinto A. Recombinant human erythropoietin prevents lipopolysaccharide-induced vascular hyporeactivity in the rat. Shock. 2009;31:529–534. doi: 10.1097/SHK.0b013e31818909c0. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H. Hasselblatt M. Dembowski C. Cepek L. Lewczuk P. Stiefel M. Rustenbeck H.H. Breiter N. Jacob S. Knerlich F. Bohn M. Poser W. Ruther E. Kochen M. Gefeller O. Gleiter C. Wessel T.C. De Ryck M. Itri L. Prange H. Cerami A. Brines M. Siren A.L. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol. Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F.F. McQuillen P. Mu D. Chang Y. Wendland M. Vexler Z. Ferriero D.M. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev. Neurosci. 2007;29:321–330. doi: 10.1159/000105473. [DOI] [PubMed] [Google Scholar]
- Grasso G. Sfacteria A. Cerami A. Brines M. Erythropoietin as a tissue-protective cytokine in brain injury: what do we know and where do we go? Neuroscientist. 2004;10:93–98. doi: 10.1177/1073858403259187. [DOI] [PubMed] [Google Scholar]
- Grasso G. Sfacteria A. Meli F. Fodale V. Buemi M. Iacopino D.G. Neuroprotection by erythropoietin administration after experimental traumatic brain injury. Brain Res. 2007;1182:99–105. doi: 10.1016/j.brainres.2007.08.078. [DOI] [PubMed] [Google Scholar]
- Hartley C.E. Varma M. Fischer J.P. Riccardi R. Strauss J.A. Shah S. Zhang S. Yang Z.J. Neuroprotective effects of erythropoietin on acute metabolic and pathological changes in experimentally induced neurotrauma. J. Neurosurg. 2008;109:708–714. doi: 10.3171/JNS/2008/109/10/0708. [DOI] [PubMed] [Google Scholar]
- Iwai M. Cao G. Yin W. Stetler R.A. Liu J. Chen J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38:2795–2803. doi: 10.1161/STROKEAHA.107.483008. [DOI] [PubMed] [Google Scholar]
- Jelkmann W. Use of recombinant human erythropoietin as an antianemic and performance enhancing drug. Curr. Pharm. Biotechnol. 2000;1:11–31. doi: 10.2174/1389201003379068. [DOI] [PubMed] [Google Scholar]
- Keogh C.L. Yu S.P. Wei L. The effect of recombinant human erythropoietin on neurovasculature repair after focal ischemic stroke in neonatal rats. J. Pharmacol. Exp. Ther. 2007;322:521–528. doi: 10.1124/jpet.107.121392. [DOI] [PubMed] [Google Scholar]
- Langlois J.A. Rutland-Brown W. Wald M.M. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Liao Z.B. Zhi X.G. Shi Q.H. He Z.H. Recombinant human erythropoietin administration protects cortical neurons from traumatic brain injury in rats. Eur. J. Neurol. 2008;15:140–149. doi: 10.1111/j.1468-1331.2007.02013.x. [DOI] [PubMed] [Google Scholar]
- Li Y. Lu Z. Keogh C.L. Yu S.P. Wei L. Erythropoietin-induced neurovascular protection, angiogenesis, and cerebral blood flow restoration after focal ischemia in mice. J. Cereb. Blood Flow Metab. 2007;27:1043–1054. doi: 10.1038/sj.jcbfm.9600417. [DOI] [PubMed] [Google Scholar]
- Lu D. Mahmood A. Qu C. Goussev A. Schallert T. Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J. Neurotrauma. 2005;22:1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- Marti H.H. Erythropoietin and the hypoxic brain. J. Exp. Biol. 2004;207:3233–3242. doi: 10.1242/jeb.01049. [DOI] [PubMed] [Google Scholar]
- Minambres E. Ballesteros M.A. Mayorga M. Marin M.J. Munoz P. Figols J. Lopez-Hoyos M. Cerebral apoptosis in severe traumatic brain injury patients: an in vitro, in vivo, and postmortem study. J. Neurotrauma. 2008;25:581–591. doi: 10.1089/neu.2007.0398. [DOI] [PubMed] [Google Scholar]
- Mogensen J. Jensen C. Kingod S.C. Hansen A. Larsen J.A. Mala H. Erythropoietin improves spatial delayed alternation in a T-maze in fimbria-fornix transected rats. Behav. Brain Res. 2008;186:215–221. doi: 10.1016/j.bbr.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Naranda T. Kaufman R.I. Li J. Wong K. Boge A. Hallen D. Fung K.Y. Duncan M.W. Andersen N. Goldstein A. Olsson L. Activation of erythropoietin receptor through a novel extracellular binding site. Endocrinology. 2002;143:2293–2302. doi: 10.1210/endo.143.6.8860. [DOI] [PubMed] [Google Scholar]
- Narayan R.K. Michel M.E. Ansell B. Baethmann A. Biegon A. Bracken M.B. Bullock M.R. Choi S.C. Clifton G.L. Contant C.F. Coplin W.M. Dietrich W.D. Ghajar J. Grady S.M. Grossman R.G. Hall E.D. Heetderks W. Hovda D.A. Jallo J. Katz R.L. Knoller N. Kochanek P.M. Maas A.I. Majde J. Marion D.W. Marmarou A. Marshall L.F. McIntosh T.K. Miller E. Mohberg N. Muizelaar J.P. Pitts L.H. Quinn P. Riesenfeld G. Robertson C.S. Strauss K.I. Teasdale G. Temkin N. Tuma R. Wade C. Walker M.D. Weinrich M. Whyte J. Wilberger J. Young A.B. Yurkewicz L. Clinical trials in head injury. J. Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi C.T. Wang L. Rogers H.M. Teng R. Jia Y. Survival and proliferative roles of erythropoietin beyond the erythroid lineage. Expert Rev. Mol. Med. 2008;10:e36. doi: 10.1017/S1462399408000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noshita N. Lewen A. Sugawara T. Chan P.H. Akt phosphorylation and neuronal survival after traumatic brain injury in mice. Neurobiol. Dis. 2002;9:294–304. doi: 10.1006/nbdi.2002.0482. [DOI] [PubMed] [Google Scholar]
- Pun P.B. Lu J. Moochhala S. Involvement of ROS in BBB dysfunction. Free Radic. Res. 2009;43:348–364. doi: 10.1080/10715760902751902. [DOI] [PubMed] [Google Scholar]
- Royo N.C. Shimizu S. Schouten J.W. Stover J.F. McIntosh T.K. Pharmacology of traumatic brain injury. Curr. Opin. Pharmacol. 2003;3:27–32. doi: 10.1016/s1471-4892(02)00006-1. [DOI] [PubMed] [Google Scholar]
- Ruscher K. Freyer D. Karsch M. Isaev N. Megow D. Sawitzki B. Priller J. Dirnagl U. Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J. Neurosci. 2002;22:10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka M. Wen T.C. Matsuda S. Masuda S. Morishita E. Nagao M. Sasaki R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi N.I. Andresen J. Marrelli S.P. Bryan R.M., Jr. Erythropoietin potentiates EDHF-mediated dilations in rat middle cerebral arteries. J. Neurotrauma. 2008;25:257–265. doi: 10.1089/neu.2007.0347. [DOI] [PubMed] [Google Scholar]
- Spandou E. Papadopoulou Z. Soubasi V. Karkavelas G. Simeonidou C. Pazaiti A. Guiba-Tziampiri O. Erythropoietin prevents long-term sensorimotor deficits and brain injury following neonatal hypoxia-ischemia in rats. Brain Res. 2005;1045:22–30. doi: 10.1016/j.brainres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Strauss K.I. Narayan R.K. Raghupathi R. Common patterns of bcl-2 family gene expression in two traumatic brain injury models. Neurotox. Res. 2004;6:333–342. doi: 10.1007/BF03033444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooij M.A. Groenendaal F. Kavelaars A. Heijnen C.J. van Bel F. Neuroprotective properties and mechanisms of erythropoietin in in vitro and in vivo experimental models for hypoxia/ischemia. Brain Res. Rev. 2008;59:22–33. doi: 10.1016/j.brainresrev.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Verdonck O. Lahrech H. Francony G. Carle O. Farion R. Van de Looij Y. Remy C. Segebarth C. Payen J.F. Erythropoietin protects from post-traumatic edema in the rat brain. J. Cereb. Blood Flow Metab. 2007;27:1369–1376. doi: 10.1038/sj.jcbfm.9600443. [DOI] [PubMed] [Google Scholar]
- Wang L. Zhang Z. Wang Y. Zhang R. Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Wang Y. Zhang Z.G. Rhodes K. Renzi M. Zhang R.L. Kapke A. Lu M. Pool C. Heavner G. Chopp M. Post-ischemic treatment with erythropoietin or carbamylated erythropoietin reduces infarction and improves neurological outcome in a rat model of focal cerebral ischemia. Br. J. Pharmacol. 2007;151:1377–1384. doi: 10.1038/sj.bjp.0707285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojchowski D.M. Gregory R.C. Miller C.P. Pandit A.K. Pircher T.J. Signal transduction in the erythropoietin receptor system. Exp. Cell Res. 1999;253:143–156. doi: 10.1006/excr.1999.4673. [DOI] [PubMed] [Google Scholar]
- Wu H. Lu D. Jiang H. Xiong Y. Qu C. Li B. Mahmood A. Zhou D. Chopp M. Increase in phosphorylation of Akt and its downstream signaling targets and suppression of apoptosis by simvastatin after traumatic brain injury. J. Neurosurg. 2008a;109:691–698. doi: 10.3171/JNS/2008/109/10/0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. Lu D. Jiang H. Xiong Y. Qu C. Li B. Mahmood A. Zhou D. Chopp M. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J. Neurotrauma. 2008b;25:130–139. doi: 10.1089/neu.2007.0369. [DOI] [PubMed] [Google Scholar]
- Xiong Y. Lu D. Qu C. Goussev A. Schallert T. Mahmood A. Chopp M. Effects of erythropoietin on reducing brain damage and improving functional outcome after traumatic brain injury in mice. J. Neurosurg. 2008a;109:510–521. doi: 10.3171/JNS/2008/109/9/0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y. Mahmood A. Lu D. Qu C. Goussev A. Schallert T. Chopp M. Role of gender in outcome after traumatic brain injury and therapeutic effect of erythropoietin in mice. Brain Res. 2007;1185:301–312. doi: 10.1016/j.brainres.2007.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y. Mahmood A. Lu D. Qu C. Kazmi H. Goussev A. Zhang Z.G. Noguchi C.T. Schallert T. Chopp M. Histological and functional outcomes after traumatic brain injury in mice null for the erythropoietin receptor in the central nervous system. Brain Res. 2008b;1230:247–257. doi: 10.1016/j.brainres.2008.06.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsiv I. Grigoriadis N. Simeonidou C. Stahel P.F. Schmidt O.I. Alexandrovitch A.G. Tsenter J. Shohami E. Erythropoietin is neuroprotective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of experimental closed head injury. FASEB. J. 2005;19:1701–1703. doi: 10.1096/fj.05-3907fje. [DOI] [PubMed] [Google Scholar]
- Yin W. Cao G. Johnnides M.J. Signore A.P. Luo Y. Hickey R.W. Chen J. TAT-mediated delivery of Bcl-xL protein is neuroprotective against neonatal hypoxic-ischemic brain injury via inhibition of caspases and AIF. Neurobiol. Dis. 2006;21:358–371. doi: 10.1016/j.nbd.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Zhande R. Karsan A. Erythropoietin promotes survival of primary human endothelial cells through PI3K-dependent, NF-kappaB-independent upregulation of Bcl-xL. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H2467–H2474. doi: 10.1152/ajpheart.00649.2006. [DOI] [PubMed] [Google Scholar]
- Zhang F. Signore A.P. Zhou Z. Wang S. Cao G. Chen J. Erythropoietin protects CA1 neurons against global cerebral ischemia in rat: potential signaling mechanisms. J. Neurosci. Res. 2006;83:1241–1251. doi: 10.1002/jnr.20816. [DOI] [PubMed] [Google Scholar]
- Zhang J. Li Y. Cui Y. Chen J. Lu M. Elias S.B. Chopp M. Erythropoietin treatment improves neurological functional recovery in EAE mice. Brain Res. 2005;1034:34–39. doi: 10.1016/j.brainres.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Zhang R. Wang Y. Zhang L. Zhang Z. Tsang W. Lu M. Zhang L. Chopp M. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33:2675–2680. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]