Abstract
Although neurotrophic factors such as nerve growth factor, basic fibroblast growth factor, brain-derived neurotrophic factor, and neurotrophin 4/5 are elevated after traumatic brain injury (TBI), little is known about the endogenous response of insulin-like growth factor-1 (IGF-1). We evaluated IGF-1, IGF-1 receptor (IGF-1R), and total and phosphorylated Akt (p-Akt), a known downstream mediator of IGF-1 signaling, using ELISA, Western blotting, and immunohistochemistry at 1, 6, 24, 48, and 72 h following 0.5-mm controlled cortical impact brain injury in adult mice. IGF-1 was transiently upregulated in homogenates of injured cortex at 1 h, and cells with increased IGF-1 immunoreactivity were observed in and around the cortical contusion site up to 48 h. IGF-1R and total Akt levels in cortical homogenates were unchanged, although immunohistochemistry revealed regional changes. In contrast, serine p-Akt levels increased significantly in homogenates at 6 h post-injury. Interestingly, delayed increases in vascular IGF-1R, total Akt, and p-Akt immunostaining were observed in and around the cortical contusion. IGF-1 and its downstream mediators were also upregulated in the subcortical white matter. Our findings indicate that moderate TBI results in a brief induction of IGF-1 and its signaling components in the acute post-traumatic period. This may reflect an attempt at endogenous neuroprotection or repair.
Key words: Akt, axonal injury, blood vessels, controlled cortical impact, neurotrophic factor
Introduction
Traumatic brain injury (TBI) is one of the major causes of death and hospitalizations worldwide. Every year nearly 1.4 million suffer a TBI in the United States (http://www.cdc.gov/ncipc/tbi/FactSheets/Facts_About_TBI.pdf). Although much has been learned about the pathophysiology of TBI, and numerous drugs have been tested in clinical trials, no therapeutic approach has proven efficacious in reducing brain damage or behavioral morbidity in brain-injured humans. Many neuroprotective strategies have focused on blocking acute secondary damage and limiting cell death, with less emphasis to date on supplementing endogenous repair and regeneration capabilities. Promoting endogenous repair mechanisms in the adult brain may have great therapeutic potential.
One of the major mechanisms to promote neuronal repair following brain injury is the upregulation and release of endogenous neurotrophic factors (Guan et al., 2003). After TBI, neurotrophic factors such as neurotrophin 4/5, nerve growth factor, basic fibroblast growth factor, and brain-derived neurotrophic factor (BDNF) are upregulated (Conte et al., 2003; DeKosky et al., 1994; Hicks et al., 1999; Royo et al., 2006; Yang et al., 1995; Yang et al., 1996). After weight drop brain injury in rats, insulin-like growth factor-1 (IGF-1) mRNA levels increase in the cortex at 3 days (Sandberg Nordqvist et al., 1996), but to our knowledge no information is available regarding protein levels of this important growth factor in the traumatically injured brain. In humans, TBI results in elevated serum IGF-1 levels for 14 weeks after injury (Wildburger et al., 2001), which may reflect both systemic and central production of IGF-1.
IGF-1 is a potent mitogenic factor that plays an important role in brain growth and development. In the brain, IGF-1 signaling promotes neuronal survival, neurite outgrowth, maturation of oligodendrocytes, and myelination (D'Ercole et al., 1996). IGF-1 expression in the brain is transiently elevated in regions undergoing axonal outgrowth, dendritic maturation, and synaptogenesis (Bondy et al., 1992; D'Ercole et al., 1996; Niblock et al., 2000), suggesting a role for IGF-1 in developmental processes that may be recapitulated after TBI (Emery et al., 2003). Several lines of evidence have demonstrated that IGF-1 also promotes neurogenesis in the developing and adult brain (Aberg et al., 2006; Anderson et al., 2002; Trejo et al., 2001).
Administration of IGF-1 protein reduces neuronal loss and infarct volume and increases glial proliferation in the brain following experimental cerebral ischemia (Cao et al., 2003; Guan et al., 2001; Liu et al., 2001; Loddick et al., 1998), and promotes neuronal survival after spinal cord injury (Hollis et al., 2009; Nakao et al., 2001). In models of brain trauma, IGF-1 administration increased BDNF and NT-3 levels after penetrating injury (Kazanis et al., 2004), and improved motor and cognitive function after fluid percussion injury (Saatman et al., 1997). IGF-1 acts primarily through its receptor, IGF-1 receptor (IGF-1R), which is expressed by neurons, stem cells, and most glial cells (Bondy et al., 1992; Werther et al., 1990). Mice lacking IGF-1R exhibit serious defects in CNS development, consistent with an essential role for IGF-1R in oligodendroglial development and myelination (Zeger et al., 2007). IGF1-R expression is high in the CNS at early developmental stages and declines significantly in adults (Werner et al., 1989). Receptor levels are altered, however, after some forms of brain injury. For example, increased ligand binding to IGF-1R, suggestive of increased numbers of receptors, was observed at 6 h in the rat hippocampus after cerebral ischemia (Bergstedt and Wieloch, 1993). Nevertheless, little is known about IGF-1R expression in response to TBI. Following weight drop injury in rats, no change in IGF-1R mRNA was observed from 1 to 7 d after injury, despite an increase in IGF-1 mRNA (Sandberg Nordqvist et al., 1996).
Cellular responses initiated by IGF-1 are mediated through two major signaling pathways, PI3-kinase/Akt and MAP-kinase (Zheng et al., 2000). A selective role for the serine-threonine protein kinase Akt in IGF-1-induced neuroprotection has been demonstrated following neonatal hypoxic ischemia (Brywe et al., 2005). Phosphorylation of Akt promotes cell survival by inactivating pro-apoptotic proteins such as BAD, GSK-3, and caspase-9 (Zhao et al., 2006). A transient increase in Akt phosphorylation occurs after TBI and cerebral ischemia (Janelidze et al., 2001; Namura et al., 2000; Noshita et al., 2002; Yano et al., 2001; Zhang et al., 2006), and is associated with increased neuronal survival after experimental TBI in rodents (Noshita et al., 2002). The current study was designed to investigate the regional and temporal expression patterns of IGF-1 and of molecules critical for IGF-1 signaling in the mouse brain over the first 72 h following moderate TBI. Because the majority of cell death following controlled cortical impact (CCI) brain injury in the mouse occurs by 3 d post-injury (Saatman et al., 2006), we investigated early IGF-1 changes that might play a role in neuroprotection. Using ELISA or Western blotting in combination with immunohistochemistry, we provide the first semi-quantitative and qualitative descriptions of IGF-1 peptide changes in an experimental model of TBI.
Methods
Animals
Male C57BL/6 mice (20–25 g, Jackson Labs, ME) were housed 4–5/cage in a University of Kentucky Medical Center animal vivarium at a constant temperature (23 ± 2°C) with a 14/10-h light/dark cycle and provided food and water ad libitum. All procedures involving animals were approved by the University of Kentucky's Institutional Animal Care and Use Committee.
Surgery
Controlled cortical impact injury
The CCI injury was performed as previously described (Saatman et al., 2006) with slight modifications. Anesthesia was induced using 3% isoflurane. After securing the head of the animal in a stereotaxic frame (David Kopf Instruments, CA), anesthesia was maintained using 2.5% isoflurane delivered through a nose cone. A midline scalp incision was made and a 5-mm craniotomy was performed lateral to the sagittal suture midway between the bregma and the lambda. A cortical contusion was produced using a pneumatically driven impactor device (Precision System Instruments, KY). After targeting the 3-mm-diameter rounded tip at the surface of the intact dura, the impactor was retracted and set to deliver a 0.5-mm deep impact at a velocity of 3.5 m/sec. These settings produced a moderate brain injury as previously demonstrated using histological and behavioral endpoints (Saatman et al., 2006). The animals were randomly assigned to receive either sham injury (n = 2–4 for each time point), or CCI brain injury (n = 4 for each time point for immunohistochemistry, and n = 6 for each time point for Western blotting and ELISA). After CCI or sham injury, a circular disk made from dental cement was glued over the craniotomy to protect the brain surface, and the incision was sutured. The mice were placed on a Hova-Bator Incubator (model 1583; Randall Burkey Co., TX) to maintain body temperature until they regained consciousness, after which they were returned to their home cages.
Histology
At 1, 6, 24, 48, and 72 h after injury, the animals were deeply anesthetized with sodium pentobarbital (200 mg/kg IP), and transcardially perfused with heparinized saline followed by 4% paraformaldehyde. After 24 h post-fixation in situ, the brain was removed from the skull, post-fixed in 4% paraformaldehyde for 24 h, and cryoprotected by immersion in 30% sucrose. The brains were flash-frozen by immersion in cold isopentene (<−25°C) for 2 min and kept at −80°C until sectioning. Serial coronal 40-μm sections were cut using a sliding microtome (HM 400; Microm, Walldorf, Germany).
Nissl (cresyl violet) staining
For each brain, every tenth section spanning the entire cerebrum was mounted and air-dried onto gelatin-coated slides. The slides were rehydrated through graded ethanol solutions, immersed in water, stained with 0.5% cresyl violet (Acros Organics, NJ), dehydrated with graded ethanol solutions, cleared with xylene, and mounted using Permount (Fisher Scientific, NJ). The slides were viewed under an Olympus AX 80 microscope and imaged using a CCD camera.
Immunohistochemistry
Immunohistochemistry was performed on parallel series of free-floating sections. Initially, the sections were placed in 10 mM citric acid (pH 6.0) at 60°C for antigen retrieval. Subsequently, endogenous peroxidases were quenched using 3% hydrogen peroxide in 50% methanol for 30 min, and non-specific binding sites were blocked using 5% normal horse serum. Following an overnight incubation with primary antibody, using either anti-IGF-1 (Rb polyclonal,1:1000; National Hormone and Peptide Program, CA), anti IGF-1R (chicken polyclonal,1:500; Millipore, CITY?, MA), anti-Akt (rabbit monoclonal, 1:100; Cell Signaling Technology Inc, MA), or anti-p-Akt ser473 (rabbit monoclonal,1:50; Cell Signaling Technology), 1 h of incubation was performed with the appropriate secondary antibodies conjugated with biotin (Jackson Immunoresearch, PA). The sections were then incubated with an avidin-biotin-enzyme complex (Vector Laboratories, CA). Immunoreactivity was visualized using DAB as the chromogen. Omission of primary antibody served as a negative control. The slides were viewed under an Olympus AX 80 microscope and imaged using a CCD camera.
Immunofluorescence co-localization studies
Antigen retrieval was performed as above, and primary antibodies were applied for 2–3 days at 4°C. To determine cellular localization, the sections were incubated in anti-IGF-1 (1:200) in combination with either NeuN (1:500) to label neurons, anti-GFAP (mouse monoclonal, 1:300; Millipore) to label astrocytes, or isolectin B4-FITC (1:200; Vector Laboratories) to label microglia. To verify vascular localization, IGF-1R (1:200; Millipore) was applied in combination with anti-von Willebrand factor (vWF, 1:100, rabbit polyclonal; Dako, CA), while anti-total Akt was co-incubated with FITC-conjugated isolectin B4. To verify axonal localization of total Akt, sections were co-labeled with SMI 32 (mouse monoclonal, 1:200; Covance, CA) to detect dephosphorylated neurofilaments that accumulate in injured axons (Saatman et al., 2003). Biotin-conjugated secondary antibodies were used to bind IGF-1, IGF-1R, and Akt, and Alexa-488- or Alexa-594-conjugated secondary antibodies were used for NeuN, GFAP, and SMI32. After incubation with either streptavidin-488 or streptavidin-594, sections were mounted onto gelatin-coated slides, air dried, and cover-slipped with DAPI Prolong anti-fade mounting medium (Invitrogen, CA). The slides were viewed and imaged on a spinning confocal microscope (Olympus IX-81) equipped with a CCD camera.
Western blotting
At 1, 6, 24, 48, and 72 h after injury, sham-injured or CCI-injured mice were killed using CO2 and immediately decapitated. The brains were blocked coronally at approximately 1.70 mm and −5.2 mm from the bregma, and the entire cortex was rapidly dissected. The ipsilateral and contralateral cortices were separated and placed in chilled lysis buffer (1% Triton, 20 mM Tris-HCl, 150 mM NaCl, 5 mM EGTA, 10 mM EDTA, and 10% glycerol) containing protease inhibitors (Complete Mini™ Protease Inhibitor Cocktail tablet; Pierce Biotechnology, IL) and phosphatase inhibitors (Pierce Biotechnology). The samples were then briefly sonicated and vortexed at 10,000g for 30 min at 4°C, and the supernatants were collected for protein assay. Protein concentrations were determined using a BCA protein assay kit (Pierce Biotechnology). Sixty-microgram protein extracts were electrophoresed on a 6% or 7.5% SDS polyacrylamide gel at 120 V and transferred onto nitrocellulose membranes. The membranes were blocked for 1 h in 5% dry milk and then incubated overnight with primary antibody. The antibodies used were anti-p-Akt ser473 (1:1000), anti-Akt (1:1000), anti-IGF-1R (1:1000), and anti-β-actin (1:5000; Calbiochem Inc., CA). Secondary antibodies were conjugated to an infrared dye (1:8000; IRDye 800CW; Rockland, Gilbertsville, PA). After washing, the membranes were imaged and quantified using a Li-Cor Odyssey Infrared Imaging System (Li-Cor, NE). Sham samples (n = 4 per time point), were run individually and as pooled samples to assess variability in sham injuries within and across time points. Injured samples (n = 6 per time point) were run together on a gel with two shams from the same time point, and the optical density (OD) for each injured sample was normalized to the average OD for shams of that time point. All samples were run in duplicate.
IGF-1 ELISA analysis
Concentrations of IGF-1 in cortical homogenates were measured by ELISA using a kit specific for mouse IGF-1 (OCTEIA® High Sensitivity Mouse IGF-1 ELISA Kit; Immunodiagnostic Systems Inc., AZ) according to the manufacturer's protocol. Briefly, cortical homogenates (25 μL) prepared for the Western blotting study were pretreated for 10 min with a releasing reagent to denature IGF binding proteins. The samples were mixed with a diluent (250 μL) and were aliquotted in duplicate into microtiter strip wells coated with a polyclonal IGF-1 antibody. A monoclonal IGF-1 antibody labeled with biotin was added to the wells and incubated for 2 h at room temperature. Following the incubation, the wells were washed three times prior to addition of TMB enzyme substrate. The samples were then incubated for 30 min, after which 2 N HCl was added to stop the reaction. The resulting yellow acid dye concentration was measured by a fluorescence plate reader at 450 nm with a reference filter of 620 nm. A calibration curve was plotted using the mean absorbance for each calibrator on the y-axis against concentration (0–427 ng/mL) of IGF-1 on the x-axis. IGF-1 values for the samples were read from the calibration curve in nanograms per milliliter.
Statistical analysis
All data are presented as mean ± SEM. For Western blotting studies statistical significance among experimental groups was determined by a nested (duplicate) one-way analysis of variance (ANOVA). A two-way ANOVA (time × injury status) was used for ELISA data with Newman-Keuls post-hoc analysis where applicable using Statistica (StatSoft Inc., OK). For all comparisons p < 0.05 was considered statistically significant.
Results
Time-dependent changes in IGF-1 protein level and immunoreactivity
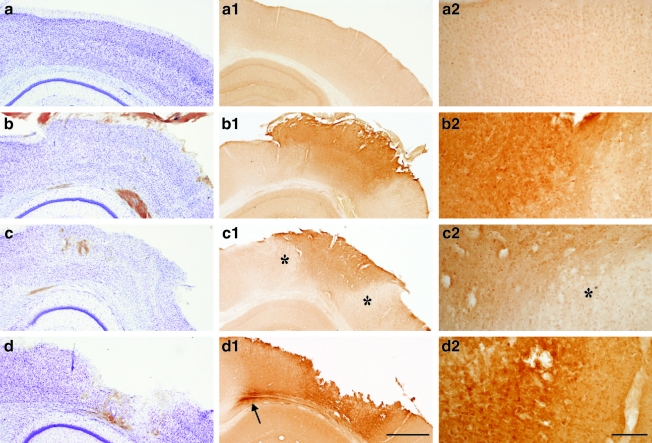
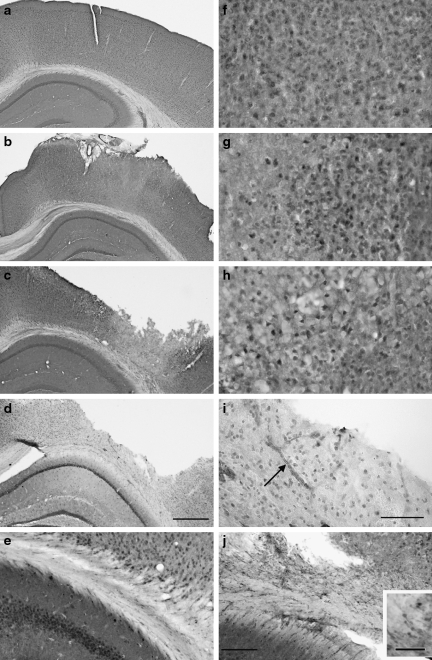
Immunohistochemical analyses revealed temporal changes in cortical and subcortical white matter (SCWM) IGF-1 expression following CCI. In sham-injured animals, modest IGF-1 immunoreactivity was observed with a cytoplasmic distribution in neurons throughout the cortex (Fig. 1a1 and a2), striatum, hippocampus, and thalamus. At 1 h post-injury, IGF-1 labeling was increased in the neuropil and scattered cells within the impact site (Fig. 1b1 and b2). At 6 h post-injury, IGF-1 remained elevated within the central region of the impact site, but was reduced below normal levels near the edges of the impact site (Fig. 1c1 and c2). Compared with sham-injured mice (Fig. 1a), brain-injured mice exhibited no overt loss of Nissl stain at 1 h (Fig. 1b), and only a mild reduction by 6 h (Fig. 1c). Nonetheless, at 1 h and 6 h, the upper layers of the cortex contained numerous pyknotic and shrunken neurons, mainly confined to the area of impact. Neuron death, as evidenced by a loss of Nissl staining, was seen through all cortical layers in the area of impact by 24 h. A cortical cavity developed within the necrotic tissue by 48–72 h (Fig. 1d). At 24 h and 48 h (Fig. 1d1 and d2), IGF-1 expression was elevated in cells surrounding the cortical cavity. Double-labeling experiments with NeuN, GFAP, and isolectin B4 confirmed that most IGF-1-positive cells adjacent to the cortical cavity were neurons, although a small number of astrocytes also expressed IGF-1 (Fig. 2). At 72 h after CCI, IGF-1 labeling in the cortex adjacent to the contusion cavity was equivalent to sham animals (data not shown). In the SCWM subjacent to the cortical contusion, brain injury resulted in a marked increase in IGF-1 starting at 24 h post-injury, with the highest elevation at 48 h (Figs. 1d1 and 3). No notable change in hippocampal IGF-1 immunoreactivity was observed. Tissue incubated with secondary antibody alone (anti-rabbit IgG) did not show any non-specific immunoreactivity.
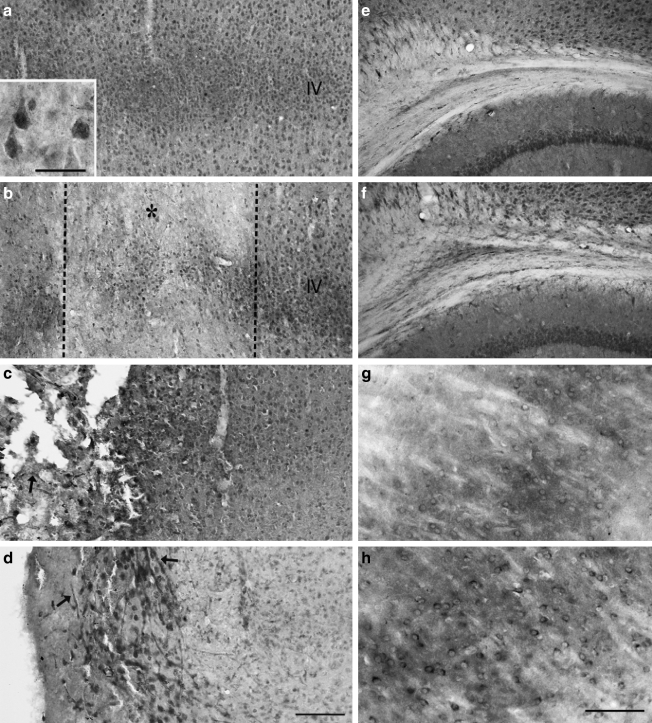
FIG. 1.
Cresyl violet and insulin-like growth factor-1 (IGF-1) immunohistochemical staining of the ipsilateral cortex. (a) Controlled cortical impact (CCI) resulted in no overt cell loss at 1 h (b), mild loss of Nissl staining by 6 h (c), and widespread cell death and cavitation by 48 h (d). Compared to basal levels of IGF-1 expression (a1 and a2), IGF-1 immunoreactivity was increased in the ipsilateral cortex at 1 h (b1 and b2) and 6 h (c1 and c2) after CCI, with a delayed reduction in IGF-1 staining at the contusion borders (denoted by the asterisks). Increased IGF-1 staining was observed in the subcortical white matter (SCWM, arrow), and near the contusion cavity at 48 h after CCI (d1 and d2) (scale bar in d1 = 500 μm, and scale bar in d2 = 50 μm).
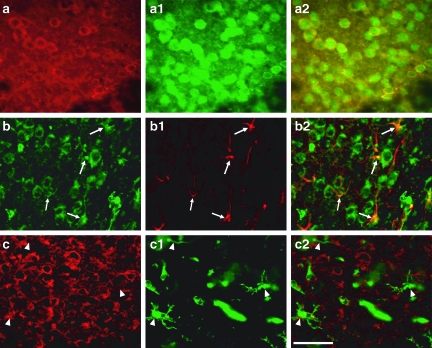
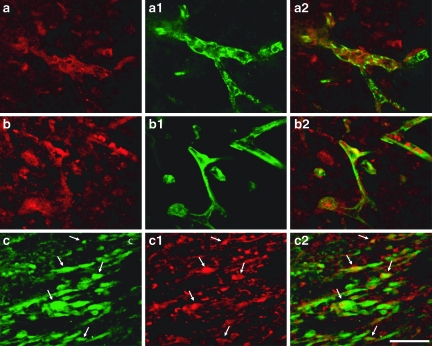
FIG. 2.
Double labeling of insulin-like growth factor-1 (IGF-1) with neuronal and glial markers. Co-localization of IGF-1 (red) and NeuN (green) was observed at the contusion periphery (a, a1, and a2). IGF-1 (green) co-localized with glial fibrillary acid protein (GFAP) (red) labeling at the contusion periphery (b, b1, and b2; arrows). IGF-1 (red) did not co-localize with microglia labeled with fluorescein isothiocyanate (FITC)-conjugated isolectin B4 (c, c1, and c2; arrowheads) (scale bar in c2 = 50 μm).
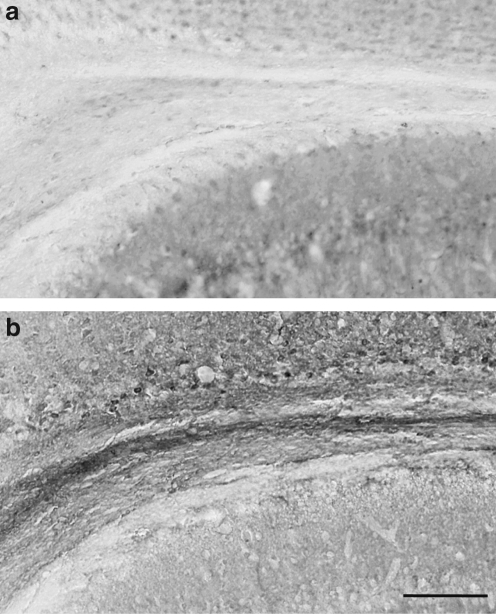
FIG. 3.
Insulin-like growth factor-1 (IGF-1) immunohistochemical staining in the subcortical white matter (SCWM). Compared to sham injury (a), brain injury resulted in a marked increase in IGF-1 immunoreactivity (b, 48 h post-injury) (scale bar in b = 100 μm).
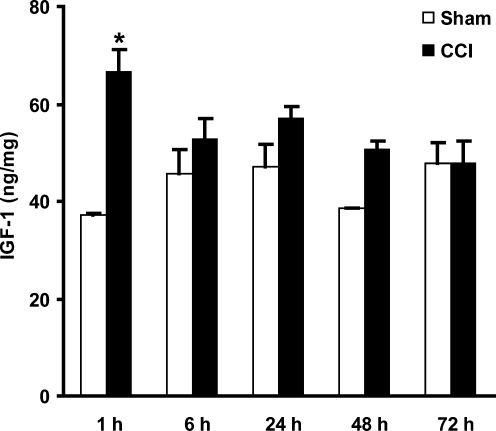
Changes in cortical levels of IGF-1 protein were quantified with a high-sensitivity mouse IGF-1 ELISA. Over the first 72 h post-injury, a transient increase in IGF-1 expression (p = 0.01) was observed at 1 h, with an approximately 80% increase relative to sham animals (Fig. 4).
FIG. 4.
Quantification of cortical insulin-like growth factor-1 (IGF-1) protein levels after controlled cortical impact (CCI) injury. IGF-1 levels measured by enzyme-linked immunosorbent assay (ELISA) were transiently increased 1 h after 0.5-mm CCI (*p < 0.05 compared to sham animals; n = 2 for sham animals, 6 for injured animals, per time point).
Neural and vascular alterations in IGF-1R protein expression
In sham-injured mice, IGF-1R expression was present throughout the brain, including the cortex, hippocampus, and thalamus. Immunolabeling was predominantly cytoplasmic (Fig. 5a). At 1 and 6 h after CCI brain injury, the impact site showed a decrease in cellular staining, although scattered cells, particularly those in cortical layer IV, maintained immunoreactivity. The decrease in receptor staining was most pronounced at the borders of the impact site (Fig. 5b). At later time points (24 and 48 h), cortical tissue adjacent to the contusion cavity showed an elevation in IGF-1R immunolabeling (Fig. 5c). Both cells and blood vessels in the contusion periphery exhibited increased receptor immunoreactivity at 72 h post-injury (Fig. 5d). Vascular expression of IGF-1R was confirmed by double labeling with vWF (Fig. 6a, a1, and a2). IGF-1R labeling in the hilar area of the hippocampus was decreased, more notably in sections caudal to the epicenter, at 1, 6, and 24 h post-CCI, with no overt change at 48 and 72 h (data not shown). A delayed increase in immunoreactivity in the SCWM was observed beginning at 24 h after CCI (Fig. 5e and f ). In the ipsilateral ventral thalamic nuclei, neuronal IGF-1R immunostaining increased in a delayed fashion at 72 h (Fig. 5g and h). Tissue incubated with secondary antibody alone (anti-chicken IgY) did not show any non-specific immunoreactivity.
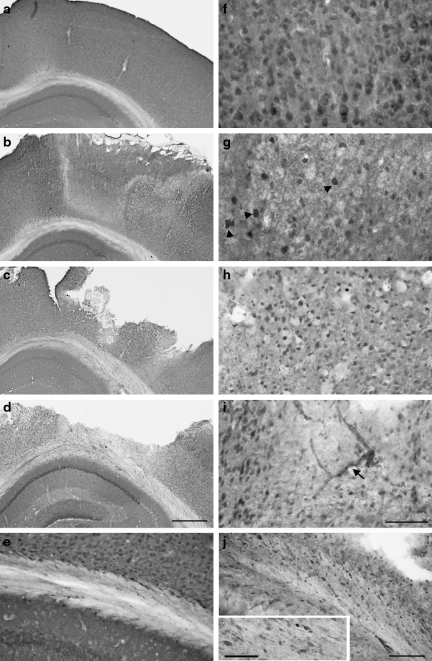
FIG. 5.
Insulin-like growth factor-1 receptor (IGF-1R) immunohistochemical staining of the ipsilateral hemisphere after controlled cortical impact (CCI) brain injury. These images show sham-injured mouse cortex (a), subcortical white matter (SCWM) (e), and thalamus (g). IGF-1R staining in the sham-injured cortex was predominantly in neuron-like cells (inset in a), with slightly higher staining intensity in cortical layer IV (a). At 1 h post-injury, IGF-1R staining was most decreased at the contusion borders (marked by the asterisk; lesion center to the left), but was relatively preserved in layer IV (b). Many intensely-stained cells were seen in the contusion periphery at 48 h (c) and at 72 h (d), in combination with IGF-1R-positive blood vessels (arrows in c and d; lesion cavity is at left). The ipsilateral SCWM exhibited increased IGF-1R staining at 48 h after CCI (f ). In the ventral thalamic nucleus, receptor staining was increased in neurons at 72 h after CCI (h) (scale bar in d = 100 μm [a–f are same magnification]; scale bar in h = 50 μm [g and h are same magnification]; scale bar in inset in a = 20 μm).
FIG. 6.
Vascular and axonal localization of insulin-like growth factor-1 receptor (IGF-1R) and total Akt. IGF-1R (red) was seen to co-localize with von Willebrand factor (green) at 72 h after brain injury (a, a1, and a2). At the same time point total Akt (red) expression co-localized with isolectin B4 (green) (b, b1, and b2). In the subcortical white matter, a subset of total Akt–positive axonal bulbs (green) co-localized with SMI-32 (red) (arrows in c, c1, and c2) (scale bar in c2 = 50 μm).
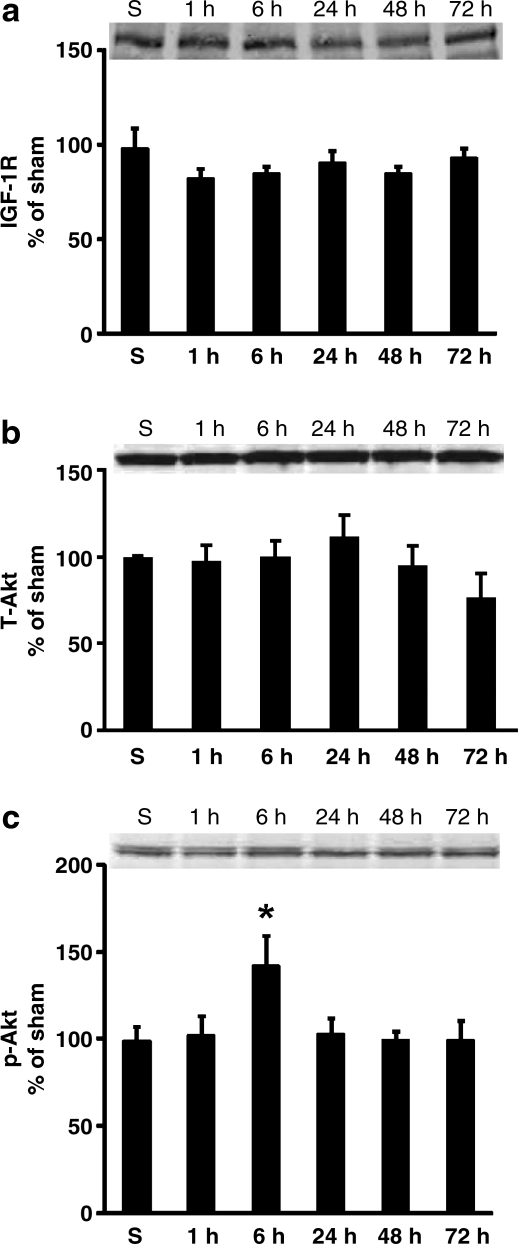
Western blotting was used to quantify IGF-1R protein levels in the ipsilateral cortex after moderate CCI brain injury. At 1, 6, 24, 48, and 72 h after injury, no significant differences in IGF-1R levels were detected among sham-injured and brain-injured animals (Fig. 9a).
FIG. 9.
Quantification of cortical insulin-like growth factor-1 receptor (IGF-1R) (a), total Akt (T-Akt; b), and p-Akt ser (c) protein levels by Western blotting. IGF-1R (a) and total Akt (b) levels were unchanged at 1, 6, 24, 48, and 72 h after 0.5-mm controlled cortical impact injury. However, cortical p-Akt ser increased transiently at 6 h after injury (c; *p < 0.05 compared to sham injury [S]). Relative optical densities of the samples were normalized to sham animals and plotted as percentage change. Insets show blots run with pooled sham (n = 10) and injured samples (n = 6 for each time point). Data are represented as mean + standard error of the mean.
A brief increase in Akt phosphorylation follows transient IGF-1 elevation
In sham-injured brains, antibodies to either total Akt or p-Akt serine produced predominantly neuron-like cellular staining with a cytoplasmic and nuclear distribution (Figs. 7a and f, and 8a and f). A non-uniform reduction in cellular immunoreactivity for total and p-Akt was observed within the impact site and borders at early time points (1, 6, and 24 h) after CCI (Figs. 7b and g, and 8b and g). Although some cortical cells lost Akt immunolabeling, a subset of neuron-like cells within the contusion core exhibited immunoreactivity above the level observed in sham animals, especially for total Akt (Figs. 7g and 8g). At 48 h, tissue adjacent to the lesion showed an increase in cellular total Akt staining (Fig. 7c and h). p-Akt staining at 48 h after CCI was reduced in areas of cell death within the necrotic lesion (Fig. 7c), but was mildly increased in cells adjacent to the lesion (Fig. 8h). Three days following CCI, total and p-Akt immunostaining in neuronal soma and neuropil was greatly reduced at the lesion borders (Figs. 5d and 6d). Interestingly, at 48–72 h, de novo vascular immunoreactivity for total and p-Akt was observed at the lesion edges (Figs. 7i and 8i). Co-labeling with isolectin B4 confirmed the localization of Akt in blood vessels (Fig. 6b, b1, and b2).
FIG. 7.
Total Akt immunohistochemical staining after traumatic brain injury (TBI). Images showing sham-injured mouse cortex (a and f ) and subcortical white matter (SCWM) (e). Non-uniformly decreased cortical labeling was observed concomitantly with increased total Akt immunoreactivity in scattered cells (arrowheads in g) at 1 h after injury (b and g). At 48 h after TBI, many cells bordering the contusion exhibited intense total Akt staining (c and h). Cortical areas bordering the cavity contained Akt-positive cells and blood vessels (arrow in i) 72 h after TBI (d and i). In the SCWM (e and j), axonal swellings and bulbs were clearly labeled with total Akt antibody at 24 h post-injury (j) (scale bar in d = 500 μm [a–d same magnification]; scale bar in i = 50 μm [f–i same magnification]; scale bar in j = 100 μm [e and j same magnification]; scale bar in insert = 50 μm).
FIG. 8.
Immunohistochemical staining for p-Akt ser after controlled cortical impact (CCI). Images showing sham-injured mouse cortex (a and f ) and subcortical white matter (SCWM) (e). Scattered neuron-like cells at the contusion core and neighboring areas exhibited increased cellular staining for p-Akt at 1 h after CCI (b; g, contusion center to the left). At 48 h after CCI, immunoreactivity was reduced in the contused tissue (c), with some cells exhibiting an increase in the peripheral areas of the contusion (h, contusion center to the left). P-Akt-positive cells as well as blood vessels (arrow in i) were observed 72 h after CCI (d and i). Axonal bulbs were detected in the SCWM (j; see also high-magnification insert) at 24 h after CCI (scale bar in d = 500 μm [a–d same magnification]; scale bar in i = 50 μm [f–i same magnification]; scale bar in j = 100 μm [e and j same magnification]; scale bar in insert = 50 μm).
Brain injury resulted in increased staining for total Akt in the SCWM, beginning at 6 h and evident up to 72 h post-injury. The antibody to total Akt also detected traumatic axonal injury in the form of axonal swellings and bulbs by 6 h, but most notably at 24 h, post-injury (Fig. 7j). A subset of these swellings co-localized with a known axonal injury marker, SMI32 (Fig. 6c, c1, and c2). p-Akt staining in the SCWM was also increased; however, axonal bulbs were not as strongly labeled with p-Akt as with total Akt (Fig. 8j). For all time points studied, the dentate hilum exhibited a partial loss in neuronal staining for both total and p-Akt (data not shown). Tissue incubated with secondary antibody alone (anti-rabbit IgG) did not show any non-specific immunoreactivity.
To supplement qualitative immunohistochemical analyses, total Akt and p-Akt protein levels were quantified by Western blotting. This semi-quantitative approach revealed no differences in total Akt among sham or brain-injured cortical samples, at any of the time points studied (Fig. 9b). In contrast, a significant but transient increase in cortical p-Akt ser levels was observed at 6 h following injury (p < 0.05; Fig. 9c).
Discussion
IGF-1 signaling mediated through PI3-kinase/Akt is a major neuronal growth and survival pathway, and has been shown to play a role in neuronal survival in models of ischemic brain injury and spinal cord injury, and in various other types of neuronal insults (Aberg et al., 2006; Brywe et al., 2005; Hung et al., 2007; Kawano et al., 2001; Leinninger et al., 2004; Vincent et al., 2004). Here we demonstrate for the first time acute transient upregulation in IGF-1 protein together with Akt activation after TBI in mice. Following cortical impact, increased IGF-1 expression was noted in and around the impact site and in the subjacent SCWM. Regional increases in total Akt and p-Akt ser labeling may suggest activation of reparative processes within damaged cells. Furthermore, delayed, de novo expression of IGF-1R was observed in blood vessels in the necrotic tissue and along the lesion borders.
In brains of sham-injured mice, we observed mild-intensity neuronal IGF-1 immunostaining. This is consistent with reports in the literature establishing that IGF-1 expression is high during development and declines in adults (Andersson et al., 1988; Rotwein et al., 1988), whereas IGF-1 mRNA is expressed predominantly in neurons and at much lower levels in mature glia (Andersson et al., 1988; Bartlett et al., 1991; D'Ercole et al., 1996).
The majority of studies of IGF-1 expression in CNS injury have evaluated subacute time points (1–7 days), and reported increased IGF-1 protein levels or immunoreactivity (Beilharz et al., 1998; Garcia-Estrada et al., 1992; Gluckman et al., 1992; Hwang et al., 2004; Walter et al., 1997). In contrast, we observed increased IGF-1 immunoreactivity in both neuropil and soma within the cortical impact site in the very acute phase of TBI (1–6 h). Rapid post-traumatic IGF-1 elevation at 1 h was corroborated by quantitative ELISA. At 12 and 24 h after cerebral ischemia in gerbils, increased IGF-1 was confined to neurons in the CA-2/3 and dentate gyrus that survived the ischemic insult, suggesting that acute, sustained IGF-1 elevation may confer neuroprotection (Hwang et al., 2004). An acute post-traumatic IGF-1 increase may therefore reflect a protective or reparative response by injured neurons; however, it is also possible that increased neuropil staining in the current study is due in part to diffusion of IGF-1 from the CSF or blood. Hypoxic-ischemia enhances uptake of IGF-1 from the CSF into the brain parenchyma (Guan et al., 1996), and results in IGF-1 accumulation in blood vessels within 5 h (Beilharz et al., 1998). Blood–brain barrier disruption, which has been demonstrated as early as minutes after CCI brain injury and as late as 7 days post-injury, depending on injury severity (Saatman et al., 2006; Smith et al., 1994), may allow systemic IGF-1 to enter the brain parenchyma. It is important to note that no staining was observed in sections incubated with secondary antibody alone (goat anti-rabbit IgG), ruling out non-specific binding of secondary antibody to extravasated mouse IgG.
Concomitantly with an increase in IGF-1 in the central area of the impact site at 6 h, immunohistochemistry revealed a decrease in the injury periphery. This peripheral, or border, region of the impact site represents an area of high shear strain, and exhibits greater calcium dysregulation, enhanced blood–brain barrier breakdown and astrogliosis, and more pronounced neuronal histopathology compared to the central region of the impact site (Nilsson et al., 1993; Nilsson et al., 1996). More rapid loss of IGF-1 in this vulnerable border zone may promote cell death in this area. Following hypoxic-ischemia in rats, loss of IGF-1 mRNA corresponded closely with the appearance of apoptotic cells (Clawson et al., 1999). It is interesting to note that the border region of the impact site also exhibited the earliest and most marked loss of IGF-1R. At later time points (24–48 h) after CCI, IGF-1 immunostaining was decreased in necrotic tissue within the contusion, reflecting neuronal death, but was increased in the peri-lesional areas. This increase was predominantly in neurons, although some astrocytes also expressed IGF-1. Our findings suggest that IGF-1 expression after trauma follows a unique pattern compared to that seen after cerebral ischemia or penetrating injury, which show increased IGF-1 immunoreactivity associated with microglia or astrocytes (Beilharz et al., 1998; Garcia-Estrada et al., 1992; Gluckman et al., 1992; Hwang et al., 2004; Walter et al., 1997). The role of this trauma-induced elevation in IGF-1 is unclear, but it is feasible that IGF-1 upregulation in surviving neurons may act to limit the progression of cell death, induce progenitor cell differentiation, or promote neurite outgrowth (D'Ercole et al., 1996). IGF-1 quantification by ELISA did not reveal any significant change at 6–72 h after injury, perhaps due to two factors. First, regional increases in IGF-1 may have been countered by decreases in other cells or regions, as described above. Second, probing whole cortical homogenates, including cortical tissue more remote from the impact site, may have reduced sensitivity in detecting changes limited to the impacted area.
Activation of IGF-1R initiates signal transduction pathways that regulate cell cycle, survival, proliferation, and differentiation. In the current study, increased IGF-1 immunolabeling in the contused cortex at 1 and 6 h post-injury was paralleled by a decrease in IGF-1R, which could be due to changes in transcription or translation or to receptor internalization. IGF-1 stimulation has been shown in vitro to result in rapid loss of IGF-1R from the cell surface by internalization (Romanelli et al., 2007). Using immunoblotting, cortical IGF-1R protein levels were unchanged over a 72-h period following CCI in mice. At early time points (1 and 6 h), this might be due to detection of both surface and internalized IGF1-R in the immunoblot, but not the immunohistochemistry assay. At later time points (24 and 48 h), decreased IGF1-R expression due to cell death in the developing necrotic lesion may be offset by the delayed increase observed adjacent to the cortical lesion. Preserved or elevated receptor expression in neurons adjacent to the necrotic tissue may make these cells receptive to increased IGF-1 peptide levels, facilitating pro-survival signaling. In the ipsilateral thalamus, we observed an increase in IGF-1R at 72 h. This delayed increase in thalamic IGF-1R may be in response to axonal injury of thalamo-cortical projections. Axonal injury in the SCWM after mild TBI is reported to cause apoptotic cell death in thalamic neurons (Dikranian et al., 2008).
IGF-1 protects neurons from cell death by binding to IGF-1R and activating the PI3K/Akt pathway (Zhao et al., 2006). PI3K inhibitors or expression of an inactive Akt mutant can suppress the neuroprotective effects of IGF-1, supporting the hypothesis that the survival signal is mediated predominantly through the PI3K/Akt pathway (Burgering and Coffer, 1995; Franke et al., 1995). Activation of Akt by phospholipid binding and phosphorylation at threonine-308 and serine-473 promotes cell survival by activating anti-apoptotic factors like cyclic-AMP-regulating element (CREB), or by inactivating pro-apoptotic targets, including BAD, glycogen synthase kinase-3 (GSK-3), forkhead transcription factors, or caspase-9 (Zhao et al., 2006).
Using immunoblotting, we observed Akt activation 6 h after CCI, subsequent to an elevation in IGF-1 at 1 h. Comparable to our findings, CCI injury in rats resulted in a transient increase in neuronal p-Akt ser and p-Akt thr at 6 h and 72 h, respectively (Zhang et al., 2006). Increased phosphorylation of Akt and Akt substrates has also been reported in brain-injured humans (Zhang et al., 2006). At 1, 6, and 24 h after CCI in mice, immunohistological analysis revealed a heterogeneous response within the contusion site, with some cortical neurons exhibiting decreased Akt immunolabeling, and others increased staining. This varied response could be due to differences in the intensity or progression of cellular damage within the contusion site. Following a more severe CCI injury, Noshita and colleagues (Noshita et al., 2002) reported a decrease in p-Akt ser expression in the contusion core starting as early as 1 h, coupled with a transient increase in peri-lesional areas 4 h after injury. We observed a similar, but more delayed, increase in total Akt and p-Akt ser expression in many cells adjacent to the cortical lesion at 48 h, which followed an upregulation in IGF-1 in this same region at 24–48 h. This delayed increase in IGF-1 and Akt may render protection in those cells, as suggested by histological analyses demonstrating a lack of co-localization of p-Akt and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) in cortical neurons after severe CCI (Noshita et al., 2002).
Following CCI injury in the mouse, blood vessels at the impact periphery exhibited robust expression of IGF-1R at 48–72 h, and total Akt and p-Akt ser at 72 h. IGF-1R is expressed in endothelial cells, and in vitro studies have shown that proliferating endothelial cells express increased numbers of IGF-1R receptors (Chisalita and Arnqvist, 2004). Increased capillary density as well as BrdU-positive endothelial cells were observed in both the cortex and hippocampus up to 48 h following experimental TBI (Morgan et al., 2007). It is interesting to note that vessels in and around the contusion site also stained for p-Akt ser, consistent with an active proliferative response.
Cortical changes in IGF-1 and IGF-1 signaling molecules were accompanied by delayed increases in IGF-1, IGF-1R, total Akt, and p-Akt expression in the SCWM. Increased SCWM IGF-1 could be due in part to increased transport of IGF-1 from the CSF to the injured cortex. After cerebral ischemia, intracerebroventricularly administered IGF-1 is transported through the white matter tracts to the injury site (Guan et al., 2000). Traumatic axonal injury, a well-known pathological feature of TBI, was easily visualized using Akt antibodies. To our knowledge, this is the first report of total Akt and p-Akt ser accumulation in axonal swellings and bulbs following TBI. This accumulation could be due to axonal transport impairment. PI3K/Akt pathway proteins are involved in mediating retrograde transport of neurotrophins by regulating actin polymerization (Reynolds et al., 2000).
In summary, contusive brain injury results in a very early increase in IGF-1 within the impact site, and a delayed increase in nearby cells. Injury-induced IGF-1 may induce cellular changes through the Akt pathway, as increases in p-Akt and/or total Akt were observed with or after the injury-induced elevation in IGF-1. Although IGF-1 upregulation may be part of a neuroprotective response by damaged neurons, the duration and/or magnitude of the endogenous IGF-1 increase is insufficient to prevent massive neuronal loss in the contused cortex following moderate CCI brain injury. IGF-1 administration has been shown to be neuroprotective in models of cerebral ischemia and spinal cord injury (Aberg et al., 2006), and to improve behavioral outcome in TBI (Saatman et al., 1997). Therefore, strategies to either increase the endogenous upregulation of IGF-1 after TBI, or supplement it with exogenous IGF-1, may improve neuronal survival after TBI.
Acknowledgments
We greatly appreciate the technical assistance with photomicroscopy and surgery/brain injury provided by Mary Jennes and Nathan Surles, respectively. This work was supported by National Institutes of Health grants NS045131, NS051220, and NS058484, KSCHIRT 7-20, and a Kentucky Spinal Cord and Brain Injury Research Trust fellowship to S.K.M.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Aberg N.D. Brywe K.G. Isgaard J. Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. ScientificWorldJournal. 2006;6:53–80. doi: 10.1100/tsw.2006.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M.F. Aberg M.A. Nilsson M. Eriksson P.S. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res. Dev. Brain Res. 2002;134:115–122. doi: 10.1016/s0165-3806(02)00277-8. [DOI] [PubMed] [Google Scholar]
- Andersson I.K. Edwall D. Norstedt G. Rozell B. Skottner A. Hansson H.A. Differing expression of insulin-like growth factor I in the developing and in the adult rat cerebellum. Acta Physiol. Scand. 1988;132:167–173. doi: 10.1111/j.1748-1716.1988.tb08314.x. [DOI] [PubMed] [Google Scholar]
- Bartlett W.P. Li X.S. Williams M. Benkovic S. Localization of insulin-like growth factor-1 mRNA in murine central nervous system during postnatal development. Dev. Biol. 1991;147:239–250. doi: 10.1016/s0012-1606(05)80021-1. [DOI] [PubMed] [Google Scholar]
- Beilharz E.J. Russo V.C. Butler G. Baker N.L. Connor B. Sirimanne E.S. Dragunow M. Werther G.A. Gluckman P.D. Williams C.E. Scheepens A. Co-ordinated and cellular specific induction of the components of the IGF/IGFBP axis in the rat brain following hypoxic-ischemic injury. Brain Res. Mol. Brain Res. 1998;59:119–134. doi: 10.1016/s0169-328x(98)00122-3. [DOI] [PubMed] [Google Scholar]
- Bergstedt K. Wieloch T. Changes in insulin-like growth factor 1 receptor density after transient cerebral ischemia in the rat. Lack of protection against ischemic brain damage following injection of insulin-like growth factor 1. J. Cereb. Blood Flow Metab. 1993;13:895–898. doi: 10.1038/jcbfm.1993.112. [DOI] [PubMed] [Google Scholar]
- Bondy C. Werner H. Roberts C.T., Jr. LeRoith D. Cellular pattern of type-I insulin-like growth factor receptor gene expression during maturation of the rat brain: comparison with insulin-like growth factors I and II. Neuroscience. 1992;46:909–923. doi: 10.1016/0306-4522(92)90193-6. [DOI] [PubMed] [Google Scholar]
- Brywe K.G. Mallard C. Gustavsson M. Hedtjarn M. Leverin A.L. Wang X. Blomgren K. Isgaard J. Hagberg H. IGF-I neuroprotection in the immature brain after hypoxia-ischemia, involvement of Akt and GSK3beta? Eur. J. Neurosci. 2005;21:1489–1502. doi: 10.1111/j.1460-9568.2005.03982.x. [DOI] [PubMed] [Google Scholar]
- Burgering B.M. Coffer P.J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- Cao Y. Gunn A.J. Bennet L. Wu D. George S. Gluckman P.D. Shao X.M. Guan J. Insulin-like growth factor (IGF)-1 suppresses oligodendrocyte caspase-3 activation and increases glial proliferation after ischemia in near-term fetal sheep. J. Cereb. Blood Flow Metab. 2003;23:739–747. doi: 10.1097/01.WCB.0000067720.12805.6F. [DOI] [PubMed] [Google Scholar]
- Chisalita S.I. Arnqvist H.J. Insulin-like growth factor I receptors are more abundant than insulin receptors in human micro- and macrovascular endothelial cells. Am. J. Physiol. 2004;286:E896–E901. doi: 10.1152/ajpendo.00327.2003. [DOI] [PubMed] [Google Scholar]
- Clawson T.F. Vannucci S.J. Wang G.M. Seaman L.B. Yang X.L. Lee W.H. Hypoxia-ischemia-induced apoptotic cell death correlates with IGF-I mRNA decrease in neonatal rat brain. Biol. Signals Recept. 1999;8:281–293. doi: 10.1159/000014599. [DOI] [PubMed] [Google Scholar]
- Conte V. Royo C. Shimizu S. Saatman K.E. Watson D. Graham D.I. Stocchetti N. McIntosh T. Neurotrophic factors, pathophysiology, and therapeutic applications in traumatic brain injury. European Journal of Trauma. 2003:335–355. [Google Scholar]
- D'Ercole A.J. Ye P. Calikoglu A.S. Gutierrez-Ospina G. The role of the insulin-like growth factors in the central nervous system. Mol. Neurobiol. 1996;13:227–255. doi: 10.1007/BF02740625. [DOI] [PubMed] [Google Scholar]
- DeKosky S.T. Goss J.R. Miller P.D. Styren S.D. Kochanek P.M. Marion D.W. Upregulation of nerve growth factor following cortical trauma. Exp. Neurol. 1994;130:173–177. doi: 10.1006/exnr.1994.1196. [DOI] [PubMed] [Google Scholar]
- Dikranian K. Cohen R. Mac Donald C. Pan Y. Brakefield D. Bayly P. Parsadanian A. Mild traumatic brain injury to the infant mouse causes robust white matter axonal degeneration which precedes apoptotic death of cortical and thalamic neurons. Exp. Neurol. 2008;211:551–560. doi: 10.1016/j.expneurol.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery D.L. Royo N.C. Fischer I. Saatman K.E. McIntosh T.K. Plasticity following injury to the adult central nervous system: is recapitulation of a developmental state worth promoting? J. Neurotrauma. 2003;20:1271–1292. doi: 10.1089/089771503322686085. [DOI] [PubMed] [Google Scholar]
- Franke T.F. Yang S.I. Chan T.O. Datta K. Kazlauskas A. Morrison D.K. Kaplan D.R. Tsichlis P.N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Estrada J. Garcia-Segura L.M. Torres-Aleman I. Expression of insulin-like growth factor I by astrocytes in response to injury. Brain Res. 1992;592:343–347. doi: 10.1016/0006-8993(92)91695-b. [DOI] [PubMed] [Google Scholar]
- Gluckman P. Klempt N. Guan J. Mallard C. Sirimanne E. Dragunow M. Klempt M. Singh K. Williams C. Nikolics K. A role for IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochem. Biophys. Res. Commun. 1992;182:593–599. doi: 10.1016/0006-291x(92)91774-k. [DOI] [PubMed] [Google Scholar]
- Guan J. Beilharz E.J. Skinner S.J. Williams C.E. Gluckman P.D. Intracerebral transportation and cellular localisation of insulin-like growth factor-1 following central administration to rats with hypoxic-ischemic brain injury. Brain Res. 2000;853:163–173. doi: 10.1016/s0006-8993(99)02030-2. [DOI] [PubMed] [Google Scholar]
- Guan J. Bennet L. Gluckman P.D. Gunn A.J. Insulin-like growth factor-1 and post-ischemic brain injury. Prog. Neurobiol. 2003;70:443–462. doi: 10.1016/j.pneurobio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Guan J. Miller O.T. Waugh K.M. McCarthy D.C. Gluckman P.D. Insulin-like growth factor-1 improves somatosensory function and reduces the extent of cortical infarction and ongoing neuronal loss after hypoxia-ischemia in rats. Neuroscience. 2001;105:299–306. doi: 10.1016/s0306-4522(01)00145-2. [DOI] [PubMed] [Google Scholar]
- Guan J. Skinner S.J. Beilharz E.J. Hua K.M. Hodgkinson S. Gluckman P.D. Williams C.E. The movement of IGF-I into the brain parenchyma after hypoxic-ischaemic injury. Neuroreport. 1996;7:632–636. doi: 10.1097/00001756-199601310-00061. [DOI] [PubMed] [Google Scholar]
- Hicks R.R. Martin V.B. Zhang L. Seroogy K.B. Mild experimental brain injury differentially alters the expression of neurotrophin and neurotrophin receptor mRNAs in the hippocampus. Exp. Neurol. 1999;160:469–478. doi: 10.1006/exnr.1999.7216. [DOI] [PubMed] [Google Scholar]
- Hollis E.R. 2nd, Lu P.Blesch A.Tuszynski M.H.2009IGF-I gene delivery promotes corticospinal neuronal survival but not regeneration after adult CNS injury Exp. Neurol. 21553–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung K.S. Tsai S.H. Lee T.C. Lin J.W. Chang C.K. Chiu W.T. Gene transfer of insulin-like growth factor-I providing neuroprotection after spinal cord injury in rats. J. Neurosurg. Spine. 2007;6:35–46. doi: 10.3171/spi.2007.6.1.35. [DOI] [PubMed] [Google Scholar]
- Hwang I.K. Yoo K.Y. Park S.K. An S.J. Lee J.Y. Choi S.Y. Kang J.H. Kwon Y.G. Kang T.C. Won M.H. Expression and changes of endogenous insulin-like growth factor-1 in neurons and glia in the gerbil hippocampus and dentate gyrus after ischemic insult. Neurochem. Int. 2004;45:149–156. doi: 10.1016/j.neuint.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Janelidze S. Hu B.R. Siesjo P. Siesjo B.K. Alterations of Akt1 (PKBalpha) and p70(S6K) in transient focal ischemia. Neurobiol. Dis. 2001;8:147–154. doi: 10.1006/nbdi.2000.0325. [DOI] [PubMed] [Google Scholar]
- Kawano T. Fukunaga K. Takeuchi Y. Morioka M. Yano S. Hamada J. Ushio Y. Miyamoto E. Neuroprotective effect of sodium orthovanadate on delayed neuronal death after transient forebrain ischemia in gerbil hippocampus. J. Cereb. Blood Flow Metab. 2001;21:1268–1280. doi: 10.1097/00004647-200111000-00003. [DOI] [PubMed] [Google Scholar]
- Kazanis I. Giannakopoulou M. Philippidis H. Stylianopoulou F. Alterations in IGF-I., BDNF and NT-3 levels following experimental brain trauma and the effect of IGF-I administration. Exp. Neurol. 2004;186:221–234. doi: 10.1016/j.expneurol.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Leinninger G.M. Backus C. Uhler M.D. Lentz S.I. Feldman E.L. Phosphatidylinositol 3-kinase and Akt effectors mediate insulin-like growth factor-I neuroprotection in dorsal root ganglia neurons. FASEB J. 2004;18:1544–1546. doi: 10.1096/fj.04-1581fje. [DOI] [PubMed] [Google Scholar]
- Liu X.F. Fawcett J.R. Thorne R.G. Frey W.H. Non-invasive intranasal insulin-like growth factor-I reduces infarct volume and improves neurologic function in rats following middle cerebral artery occlusion. Neurosci. Lett. 2001;308:91–94. doi: 10.1016/s0304-3940(01)01982-6. [DOI] [PubMed] [Google Scholar]
- Loddick S.A. Turnbull A.V. Rothwell N.J. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J. Cereb. Blood Flow Metab. 1998;18:176–179. doi: 10.1097/00004647-199802000-00008. [DOI] [PubMed] [Google Scholar]
- Morgan R. Kreipke C.W. Roberts G. Bagchi M. Rafols J.A. Neovascularization following traumatic brain injury: possible evidence for both angiogenesis and vasculogenesis. Neurol. Res. 2007;29:375–381. doi: 10.1179/016164107X204693. [DOI] [PubMed] [Google Scholar]
- Nakao Y. Otani H. Yamamura T. Hattori R. Osako M. Imamura H. Insulin-like growth factor 1 prevents neuronal cell death and paraplegia in the rabbit model of spinal cord ischemia. J. Thorac. Cardiovasc. Surg. 2001;122:136–143. doi: 10.1067/mtc.2001.114101. [DOI] [PubMed] [Google Scholar]
- Namura S. Nagata I. Kikuchi H. Andreucci M. Alessandrini A. Serine-threonine protein kinase Akt does not mediate ischemic tolerance after global ischemia in the gerbil. J. Cereb. Blood Flow Metab. 2000;20:1301–1305. doi: 10.1097/00004647-200009000-00004. [DOI] [PubMed] [Google Scholar]
- Niblock M.M. Brunso-Bechtold J.K. Riddle D.R. Insulin-like growth factor I stimulates dendritic growth in primary somatosensory cortex. J. Neurosci. 2000;20:4165–4176. doi: 10.1523/JNEUROSCI.20-11-04165.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson P. Hillered L. Olsson Y. Sheardown M.J. Hansen A.J. Regional changes in interstitial K+ and Ca2+ levels following cortical compression contusion trauma in rats. J. Cereb. Blood Flow Metab. 1993;13:183–192. doi: 10.1038/jcbfm.1993.22. [DOI] [PubMed] [Google Scholar]
- Nilsson P. Laursen H. Hillered L. Hansen A.J. Calcium movements in traumatic brain injury: the role of glutamate receptor-operated ion channels. J. Cereb. Blood Flow Metab. 1996;16:262–270. doi: 10.1097/00004647-199603000-00011. [DOI] [PubMed] [Google Scholar]
- Noshita N. Lewen A. Sugawara T. Chan P.H. Akt phosphorylation and neuronal survival after traumatic brain injury in mice. Neurobiol. Dis. 2002;9:294–304. doi: 10.1006/nbdi.2002.0482. [DOI] [PubMed] [Google Scholar]
- Reynolds A.J. Bartlett S.E. Hendry I.A. Molecular mechanisms regulating the retrograde axonal transport of neurotrophins. Brain Res. Brain Res. Rev. 2000;33:169–178. doi: 10.1016/s0165-0173(00)00028-x. [DOI] [PubMed] [Google Scholar]
- Romanelli R.J. LeBeau A.P. Fulmer C.G. Lazzarino D.A. Hochberg A. Wood T.L. Insulin-like growth factor type-I receptor internalization and recycling mediate the sustained phosphorylation of Akt. J. Biol. Chem. 2007;282:22513–22524. doi: 10.1074/jbc.M704309200. [DOI] [PubMed] [Google Scholar]
- Rotwein P. Burgess S.K. Milbrandt J.D. Krause J.E. Differential expression of insulin-like growth factor genes in rat central nervous system. Proc. Natl. Acad. Sci. U.S.A. 1988;85:265–269. doi: 10.1073/pnas.85.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo N.C. Conte V. Saatman K.E. Shimizu S. Belfield C.M. Soltesz K.M. Davis J.E. Fujimoto S.T. McIntosh T.K. Hippocampal vulnerability following traumatic brain injury: a potential role for neurotrophin-4/5 in pyramidal cell neuroprotection. Eur. J. Neurosci. 2006;23:1089–1102. doi: 10.1111/j.1460-9568.2006.04642.x. [DOI] [PubMed] [Google Scholar]
- Saatman K.E. Abai B. Grosvenor A. Vorwerk C.K. Smith D.H. Meaney D.F. Traumatic axonal injury results in biphasic calpain activation and retrograde transport impairment in mice. J. Cereb. Blood Flow Metab. 2003;23:34–42. doi: 10.1097/01.WCB.0000035040.10031.B0. [DOI] [PubMed] [Google Scholar]
- Saatman K.E. Contreras P.C. Smith D.H. Raghupathi R. McDermott K.L. Fernandez S.C. Sanderson K.L. Voddi M. McIntosh T.K. Insulin-like growth factor-1 (IGF-1) improves both neurological motor and cognitive outcome following experimental brain injury. Exp. Neurol. 1997;147:418–427. doi: 10.1006/exnr.1997.6629. [DOI] [PubMed] [Google Scholar]
- Saatman K.E. Feeko K.J. Pape R.L. Raghupathi R. Differential behavioral and histopathological responses to graded cortical impact injury in mice. J. Neurotrauma. 2006;23:1241–1253. doi: 10.1089/neu.2006.23.1241. [DOI] [PubMed] [Google Scholar]
- Sandberg Nordqvist A.C. von Holst H. Holmin S. Sara V.R. Bellander B.M. Schalling M. Increase of insulin-like growth factor (IGF)-1, IGF binding protein-2 and -4 mRNAs following cerebral contusion. Brain Res. Mol. Brain Res. 1996;38:285–293. doi: 10.1016/0169-328x(95)00346-t. [DOI] [PubMed] [Google Scholar]
- Smith S.L. Andrus P.K. Zhang J.R. Hall E.D. Direct measurement of hydroxyl radicals, lipid peroxidation, and blood-brain barrier disruption following unilateral cortical impact head injury in the rat. J. Neurotrauma. 1994;11:393–404. doi: 10.1089/neu.1994.11.393. [DOI] [PubMed] [Google Scholar]
- Trejo J.L. Carro E. Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A.M. Mobley B.C. Hiller A. Feldman E.L. IGF-I prevents glutamate-induced motor neuron programmed cell death. Neurobiol. Dis. 2004;16:407–416. doi: 10.1016/j.nbd.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Walter H.J. Berry M. Hill D.J. Logan A. Spatial and temporal changes in the insulin-like growth factor (IGF) axis indicate autocrine/paracrine actions of IGF-I within wounds of the rat brain. Endocrinology. 1997;138:3024–3034. doi: 10.1210/endo.138.7.5284. [DOI] [PubMed] [Google Scholar]
- Werther G.A. Abate M. Hogg A. Cheesman H. Oldfield B. Hards D. Hudson P. Power B. Freed K. Herington A.C. Localization of insulin-like growth factor-I mRNA in rat brain by in situ hybridization—relationship to IGF-I receptors. Mol. Endocrinol. 1990;4:773–778. doi: 10.1210/mend-4-5-773. [DOI] [PubMed] [Google Scholar]
- Werner H. Woloschak M. Adamo M. Shen-Orr Z. Roberts C.T., Jr. LeRoith D. Developmental regulation of the rat insulin-like growth factor I receptor gene. Proc. Natl. Acad. Sci. U.S.A. 1989;86:7451–7455. doi: 10.1073/pnas.86.19.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildburger R. Zarkovic N. Leb G. Borovic S. Zarkovic K. Tatzber F. Post-traumatic changes in insulin-like growth factor type 1 and growth hormone in patients with bone fractures and traumatic brain injury. Wien. Klin. Wochenschr. 2001;113:119–126. [PubMed] [Google Scholar]
- Yang K. Mu X.S. Xue J.J. Perez-Polo J.R. Hayes R.L. Regional and temporal profiles of c-fos and nerve growth factor mRNA expression in rat brain after lateral cortical impact injury. J. Neurosci. Res. 1995;42:571–578. doi: 10.1002/jnr.490420415. [DOI] [PubMed] [Google Scholar]
- Yang K. Perez-Polo J.R. Mu X.S. Yan H.Q. Xue J.J. Iwamoto Y. Liu J.S. Dixon C.E. Hayes R.L. Increased expression of brain-derived neurotrophic factor but not neurotrophin-3 mRNA in rat brain after cortical impact injury. J. Neurosci. Res. 1996;44:157–164. doi: 10.1002/(SICI)1097-4547(19960415)44:2<157::AID-JNR8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yano S. Morioka M. Fukunaga K. Kawano T. Hara T. Kai Y. Hamada J. Miyamoto E. Ushio Y. Activation of Akt/protein kinase B contributes to induction of ischemic tolerance in the CA1 subfield of gerbil hippocampus. J. Cereb. Blood Flow Metab. 2001;21:351–360. doi: 10.1097/00004647-200104000-00004. [DOI] [PubMed] [Google Scholar]
- Zeger M. Popken G. Zhang J. Xuan S. Lu Q.R. Schwab M.H. Nave K.A. Rowitch D. D'Ercole A.J. Ye P. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia. 2007;55:400–411. doi: 10.1002/glia.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Chen Y. Ikonomovic M.D. Nathaniel P.D. Kochanek P.M. Marion D.W. DeKosky S.T. Jenkins L.W. Clark R.S. Increased phosphorylation of protein kinase B and related substrates after traumatic brain injury in humans and rats. J. Cereb. Blood Flow Metab. 2006;26:915–926. doi: 10.1038/sj.jcbfm.9600238. [DOI] [PubMed] [Google Scholar]
- Zhao H. Sapolsky R.M. Steinberg G.K. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol. Neurobiol. 2006;34:249–270. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]
- Zheng W.H. Kar S. Dore S. Quirion R. Insulin-like growth factor-1 (IGF-1): a neuroprotective trophic factor acting via the Akt kinase pathway. J. Neural Transm. 2000:261–272. doi: 10.1007/978-3-7091-6301-6_17. [DOI] [PubMed] [Google Scholar]