Abstract
Blast-related traumatic brain injury (bTBI) and post-traumatic stress disorder (PTSD) have been of particular relevance to the military and civilian health care sectors since the onset of the Global War on Terror, and TBI has been called the “signature injury” of this war. Currently there are many questions about the fundamental nature, diagnosis, and long-term consequences of bTBI and its relationship to PTSD. This workshop was organized to consider these questions and focus on how brain imaging techniques may be used to enhance current diagnosis, research, and treatment of bTBI. The general conclusion was that although the study of blast physics in non-biological systems is mature, few data are presently available on key topics such as blast exposure in combat scenarios, the pathological characteristics of human bTBI, and imaging signatures of bTBI. Addressing these gaps is critical to the success of bTBI research. Foremost among our recommendations is that human autopsy and pathoanatomical data from bTBI patients need to be obtained and disseminated to the military and civilian research communities, and advanced neuroimaging used in studies of acute, subacute, and chronic cases, to determine whether there is a distinct pathoanatomical signature that correlates with long-term functional impairment, including PTSD. These data are also critical for the development of animal models to illuminate fundamental mechanisms of bTBI and provide leads for new treatment approaches. Brain imaging will need to play an increasingly important role as gaps in the scientific knowledge of bTBI and PTSD are addressed through increased coordination, cooperation, and data sharing among the academic and military biomedical research communities.
Key words: animal models of blast-related injury, blast physics, blast-related traumatic brain injury, brain imaging, post-traumatic stress disorder
Introduction
Traumatic brain injury (TBI) caused by blast has been of particular relevance to the military and civilian health care sectors since the onset of the Global War on Terrorism (Operation Iraqi Freedom and Operation Enduring Freedom). The news media and many professional articles have referred to blast-related TBI (bTBI) as the “signature injury” of the war.
In order to understand TBI with respect to the military's experience, it is helpful to begin with some definitions and statistics. The Defense and Veterans Brain Injury Center (DVBIC) defines TBI thusly: “A traumatic brain injury (TBI) is a blow or jolt to the head or a penetrating head injury that disrupts the function of the brain.” Not all blows or jolts to the head result in a TBI. The severity of such an injury may range from mild, a brief change in mental status or consciousness, to severe, involving an extended period of unconsciousness or amnesia after the injury. A TBI can result in short- or long-term problems with independent function (DVBIC fact sheet). A TBI is further characterized by the Glasgow Coma Scale (GCS) score, the period of altered consciousness (AoC), the period of loss of consciousness (LOC), and duration of post-traumatic amnesia (PTA), as shown in Table 1.
Table 1.
TBI Grading Scale Based on Neurological Symptoms
| TBI level→ | Mild | Moderate | Severe | Penetrating |
|---|---|---|---|---|
| GCS | 12–15 | 9–12 | 3–8 | Any |
| AoC | ≤ 24 h | > 24 h | > 24 h | Any |
| LoC | 0–30 min | 31 min–24 h | ≥ 24 h | Any |
| PTA | ≤ 24 h | 24 h–7 days | ≥ 7 days | Any |
GCS, Glasgow Coma Scale score; AoC, period of altered consciousness; LOC, period of loss of consciousness; PTA, duration of post-traumatic amnesia.
As of July 31, 2008, the DVBIC had evaluated over 8000 cases of TBI (note that not all cases of TBI are evaluated by the DVBIC). Of those, more than 90% were closed-head injuries, and more than 50% were related to blast as a mechanism. Of the 8000 cases, approximately 87% were mild, 6% were moderate, 3% were severe, and 3% were penetrating (DVBIC data). To complicate matters, troops can be exposed to multiple blasts, and can be exposed and injured but fail to report the exposures or injuries because they do not realize they were affected, do not wish to be perceived as weak, or do not want to let their teams down. Health care providers at every level may not screen for TBI and PTSD, or may overlook mild cases. The development and wide dissemination of improved clinical practice guidelines within the Department of Defense and the Veterans Administration have helped, and continue to help, in these latter cases. The use of the Military Acute Concussion Evaluation (MACE) in the deployed environment has also improved identification of TBI (MACE and clinical practice guidelines are accessible at http://www.dvbic.org/Providers/TBI-Screening.aspx). Many cases of military TBI result from improvised explosive device (IED) attacks against vehicles, and thus victims may be subjected to the direct effects of a blast-wave (primary effects), and its reflections in the confinement of the crew compartment if the hull is ruptured, as well as extreme vehicular accelerations from the blast forces resulting in secondary blast effects of impacts of equipment against the crew, and tertiary blast impacts of the crew against the compartment and one another. The issue of polytrauma is also important, in that nearly all cases of bTBI involve trauma to other parts of the body. This can lead to hypoxia and hypotension that can exacerbate the effects of TBI.
In the summer of 2008 a group of government and civilian physicians and scientists formed an informal working group to develop a workshop to address how imaging technologies could be leveraged to enhance both diagnosis and research on bTBI. One of the first and most obvious questions considered was whether non-impact blast-induced TBI was a unique pathophysiological entity. Blast-related traumatic brain injury, also called “blast induced neurotrauma” (BINT) by Ibolja Cernak and others, has remained an issue of some controversy (Bhattacharjee, 2008). Is non-impact blast-induced TBI the same as BINT, and is that similar to what is seen in high-speed motor vehicle accidents? How might answers to these questions be obtained utilizing the latest in imaging technologies as well as clinically relevant laboratory and computational models? Where and how does post-traumatic stress disorder (PTSD) fit in? Because military TBI cases, particularly moderate and severe injuries, often involve polytrauma, and because PTSD and depression are associated co-morbidities in up to 40% of cases of mild TBI, scientists and clinicians puzzle over what is and what is not blast-related (Hoge et al., 2008). How can we better characterize and diagnose TBI (and, in turn, PTSD and other psychological health pathologies) in the military and civilian sectors? How can we model TBI so that we can improve on the current 0% success rate of TBI therapies translated from animal models to humans? Currently, one group suggests that any mild TBI, regardless of cause, might best be classified as “concussion,” and that “PTSD and depression are important mediators of the relationship between mild traumatic brain injury and physical health problems” (Hoge et al., 2008). In addition, although there have been a number of recent workshops on blast-related TBI, the potential for advanced imaging technology to help answer these important questions and enhance understanding and diagnosis of blast-related TBI has not been adequately addressed. This led to the development of the workshop agenda.
The workshop was organized into four major topics: blast physics, human brain injuries, human brain imaging, and animal models. Several speakers reviewed the current state of the art for each topic, and followed with a roundtable discussion of key questions selected by the planning committee. Priorities for future research in each area were discussed. The presentations and discussions are summarized and lists of these recommendations are presented in this report. The members of the planning committee, invited participants, speakers and their topics, and roundtable participants are listed at the end of this report.
In June 2009 the Journal of Neurotrauma published a seminal contribution to the field of bTBI in the form of an entire issue devoted to this subject that includes examples of CT scans from humans shortly after they were injured by improvised explosive devices, reports on the progress and results in creating new rodent and swine animal models of bTBI, as well as modeling bTBI in vitro, and the current status in the search for biomarkers of bTBI. Readers are referred to this issue (Volume 26, Number 6, June 2009) for more information on these topics. The St. Louis Workshop Report complements these articles with its focus on how modern imaging techniques can be used to enhance research on bTBI, and its presentation of recommendations of future research priorities.
Section 1: Blast Physics
State of the art
It is important to have a basic understanding of the physics of blast insult prior to developing any hypothesis regarding bTBI mechanisms, countermeasures, or treatments. Understanding the processes by which a blast event ultimately inflicts stresses at the cellular and subcellular levels is also a prerequisite to the design of proper animal model testing and interpretation of results.
A blast event as considered here begins with a detonation, the nearly instantaneous combustion of a liquid or solid explosive material resulting in the generation of gaseous products at extremely high pressure and temperature (∼150 k atm/∼2 M psi, ∼3000°K). The gaseous detonation products expand rapidly into the surrounding atmosphere to about 3000-fold their original volume, and are visible as a luminous fireball. Primary fragmentation from the charge casing as well as dirt and ejecta from buried charges will be carried with the fireball expansion and are projected much further than the gaseous products. The rapid expansion of the fireball drives a shockwave into the surrounding air ahead of it. The combined violent expansion of product gases and propagated shockwave constitute the blast flow field (Ritzel workshop lecture and personal communication, 2008).
The most distinctive feature of the air blast wave is the shock front, through which there is a nearly instantaneous change in all gas-dynamic conditions of the air (pressure, density, flow velocity, and temperature). While the air blast wave strength is often characterized exclusively in terms of the peak blast overpressure, it is important to note that this metric will usually refer to the static or side-on pressure above ambient levels, which does not represent the loading condition on a typical target. The static pressure is that pressure which would be sensed by a surface aligned parallel to the blast-wave propagation, and hence does not experience the kinetic energy component of the flow, which may be many-fold higher than the static pressure component. If the same surface were perpendicular to the blast, it would obstruct the flow and be exposed to a much higher pressure of the reflected blast, including both the static and dynamic (kinetic energy) components. The actual stresses and waveform experienced at the cellular level will depend on the transfer function for the target, which is highly geometry- and material-dependent. These distinctions regarding the incident blast flow conditions, imparted loading, and cellular stresses have important implications with regard to the mechanisms for blast injury, as well as the proper simulation of blast in the laboratory, Whereas the static pressure profile is an important component of blast insult, it is by no means the only relevant energy component, particularly for victims within the area of the fireball, where kinetic energy of the flow is dominant (Ritzel workshop lecture and personal communication, 2008).
The blast flow field exhibits energy in various modes in the hydrodynamic domain, including material flow (kinetic energy), static pressure, and internal energy (temperature) (Sternberg and Hurwitz, 1976). Due to the shock front, the frequency content of the incident wave is extremely high; indeed, the rate of the stress rise imparted to tissue followed by rapid relaxation may be of as much concern with regard to cellular damage as stress amplitude (White et al., 1965; Vawter et al., 1978; Viano and Lau, 1988; Doukas et al., 1995; Morrison et al., 2000; Garner et al., 2000). Blast also can propagate energy in the electromagnetic domain, although the power spectrum is highly dependent on the device size and configuration (Fine and Vinci, 1998; Kelly, 1993). Ongoing research is required to understand how these various energy modes interact with biological systems from the global to cellular-level scales, and particularly what respective power spectra may be relevant to tissue damage (Ritzel workshop lecture, 2008; Leung et al., 2008). There are several potential modes for blast energy transfer to the brain, including direct stress-wave coupling through the skull or the soft-tissue portals of the skull (the orbits, sinuses, and external auditory canals), pressurization of intravascular blood from thoracic compression, rapid global compression of the skull plates, propagated stresses from platform acceleration (e.g., energy transmitted through the ground or vehicle structure), as well as stresses from severe global acceleration of the head, which induces shearing actions and diffuse axonal injury. Furthermore, synergistic combinations of the above modes, or other complications such as genetic predisposition to brain injury (Crawford and Cook, personal communication) cannot be ruled out. Blast-induced head accelerations can be far more severe, and blast energy can emanate from widely differing directions than what is experienced in sports-related concussions. There is also speculation that blast-induced head acceleration and effects upon the skull may lower cerebral intravascular and intracranial pressures sufficiently to generate micro-cavitation bubbles that in turn can collapse with pressures exceeding 1000 atm (Stuhmiller, 2008). The proposed “fluid hammer” effect (Young, 1945; Cernak et al., 2001) occurs by means of the blast acting on the thorax and abdomen, forcing blood up into the closed cranial vault and increasing local pressures. This can result in vascular trauma, vasospasm, hypoxia/ischemia, and cerebral hemorrhage. Effects of radio-frequency and infrared energies have also been proposed as areas of study (Armonda et al., 2006; Cernak et al., 2001; Stuhmiller, 2008; Ling, personal communication, 2008).
Although the injury potential of blast has been known since the discovery of explosives, the first analytical approach and taxonomy for blast injury can be credited to Zuckerman (1940), who was referenced by the Germans in their war-time animal studies to quantify “lethality ranges” as a function of charge size (Desaga, 1943; Benzinger 1950). Subsequent to World War II, the nuclear blast threat was the primary driver for concerted research into blast injury and the now well-known works of the Lovelace Foundation in the U.S. (White et al., 1964, 1965; Richmond et al., 1959, 1992). The Swedish were also active in early blast injury research (Clemedson, 1956).
The U.S. Army Medical Research and Materiel Command Military Occupational Medicine Research Program (MOMRP) has been at the forefront of this research in recent times, and has developed an extensive portfolio of blast exposure data in animals and humans over a period of nearly 30 years. A summary of classical blast injury taxonomy as consolidated through the MOMRP program is summarized in Table 2 (Stuhmiller, 2008). However, the origins for these categorizations of blast injury stem from assessment of aerial bombing threats in World War II, and were later extended to the nuclear blast threat. There is no specific identification of the blast injury modes from platform shock acceleration that is more highly relevant to the current issue of IED attacks on personnel in vehicles. Platform shock acceleration (referred to, for example, as “destroyer heel” in World War II) was recognized early as an injury mode distinctive from blast (Clemedson, 1956; Draeger et al., 1945). Furthermore, near-field blast injury modes such as traumatic amputation also do not fit well into Table 2. The classical blast injury taxonomy may need to be revisited, not only in light of current threats including mines, near-field IEDs and thermobaric weapons (Curcio et al., 2002), but also with respect to heretofore largely unexplored injury modes such as bTBI.
Table 2.
Taxonomy of Blast Injury
| Category | Characteristics | Body part affected | Types of injuries |
|---|---|---|---|
| Primary | Unique to high-order explosives Results from the envelopment of the body in the overpressurization wave Body surface and internal organs are rapidly distorted because the body contains highly compressible tissues (air-containing organs) that undergo rapid volume changes |
Gas-filled structures suffer the greatest distortion: upper airways, lungs, gastrointestinal tract, and middle ear Internal distortions of air-containing organs cause distortion of neighboring solid organs (e.g., heart, liver, spleen, and kidneys) Differential loadings within the body, especially the vascular system, can cause transmission of energy to other parts of the body |
Blast lung (pulmonary barotrauma), air emboli introduced across the air–blood boundary of the lung Tympanic membrane rupture, middle ear damage Globe (eye) rupture Laceration of the liver, spleen, and kidneys; abdominal hemorrhage and perforation Contusion to the heart, distortion and rupture of the great vessels Concussion, non-penetrating head injury, blast TBISurges in blood flow and pressure that may lead to other injuries |
| Secondary | Impact on the body from flying debris and bomb fragments | Injury depends on the speed, mass, and shape of the impacting object Any body part may be affected |
Any injury associated with impact of high-speed objects; these modes are not unique to blast; however, blast provides a different way of propelling the objects |
| Penetrating ballistic (fragmentation) or blunt injuries | |||
| Eye penetration (can be occult), and skull fracture | |||
| Tertiary | Whole-body acceleration caused by the blast wind Uneven forces on the body caused by the blast wind |
Depends on the surface condition that the body impacts Primarily head/neck and extremities that can be accelerated relative to the torso Any body part may be affected |
Any injury associated with whole-body motion and impact; these modes are not unique to blast; however, blast provides a different way of accelerating the body Typical injuries that would occur in falls or car crashes; fractures, contusion, and closed- and open-head injuries Traumatic amputation, muscle tears. |
| Quaternary | All explosion-mediated injuries not associated with pressure or wind effects | Any body part may be affected Body surfaces, eyes, respiratory system |
Burns (flash, partial, and full-thickness) |
| High temperatures | Asphyxia | ||
| Toxic gases | Injury or incapacitation from inhaled toxic fire gases | ||
| Collateral | Secondary consequences of trauma | Systemic responses from massive trauma | Not unique to blast |
| Exacerbation or complications of existing conditions | Angina, hyperglycemia, hypotension, hypertension Asthma, chronic obstructive pulmonary disease, or other breathing problems from dust, smoke, or toxic fumes |
Adapted from Stuhmiller, J. (2008). Blast Injury: Translating Research into Operational Medicine, http://www.bordeninstitute.army.mil/published_volumes/blast_injury/blast_injury.pdf.
Blast injury research to date has demonstrated that damage to organs is dependent on the particular stress-wave interaction physics and tissue strength. Air-containing organs (auditory canal, larynx, trachea, lung, and GI tract) appear to be most sensitive to blast, while liver and spleen are intermediate, and kidney, pancreas, and gallbladder appear least sensitive (White, 1961; White et al., 1964; Stuhmiller, 2008). In addition, organs very likely have differing sensitivities to principal and shear stress waves (Cripps and Cooper, 1996), both of which will be imparted as a function of body morphology.
Despite some investigation of blast effects on the CNS with regard to overt physiological damage (e.g., Young, 1945; Clemedson, 1956; Richmond and Jenssen, 1992), the effects of blast on the human brain have remained difficult to assess, particularly with regard to functional or cognitive deficits that may relate to subtle disruption of brain tissues. The complex nature of the blast insult mechanisms in actual injury cases, which are highly dependent on details of the scenario, remains a significant part of the challenge (i.e., defining the blast environment for scientific purposes and identifying the parameters critical to injury). Cooper and associates (1983) and others have highlighted blast injury scenarios in which a civilian victim close to the blast might be spared, but one farther away could be killed instantly. Another significant development for blast victims in the armed services was that personal protective equipment had not been designed for, nor were they particularly effective against, blast effects (primary through quaternary), even in the first Gulf War. Therefore, prior to the current conflict, casualties close enough to a blast to suffer TBI were often killed by the blast outright or by secondary and tertiary effects (Phillips and Richmond, 1990). Improvements in body armor, uniforms, and vehicles, especially over the past decade, have enabled casualties to survive significant blasts, even at very close proximity where they might be engulfed by the fireball, implying peak blast overpressures exceeding 10 bar (1000 kPa or ∼145 psi; 1 bar is atmospheric pressure at sea level), with wave durations under a few milliseconds. While these casualties have survived, they have also incurred multiple traumatic injuries that complicate the clinical picture. Because soldiers are increasingly well protected, they are also at risk for multiple exposures to blast. The cumulative effects of these exposures are just now being recognized and studied. This is not to imply that blast-related TBI was not studied, but rather given the types of exposures and performance of protective equipment, the relative impact of blast-related TBI on casualties and casualty care was less prominent than the effects of blast upon air-filled organs. In the interim, advanced experimental diagnostics (Chavko et al., 2007) and advanced computational simulation methods (computational fluid dynamics and computational material dynamics) are being developed and applied by a wide range of agencies to understand both the details of the blast/human interaction physics, and provide clues as to the key injury mechanisms at the cellular level.
In summary, the basic physics of blast has been studied extensively, particularly since the onset of the nuclear weapons blast threat, and there is now a long history of research into classical blast injury modes within the military medical research community. However, the current research agenda is different in several aspects. Much of the physics expertise is not well known to the biomedical research community, especially those with expertise in neurological disorders. The intensity of the blast exposure is far greater than those we have seen in previous wars, and few data exist regarding human neurological trauma in high-intensity blasts. The fundamental biophysics of blast effects on human brain tissue, as well as whether and how blast can induce symptoms of traumatic brain injury remain significant gaps in our knowledge.
Section 2: Human Blast-Related Traumatic Brain Injury
State of the art
Our current understanding of blast-related TBI is very limited. We know that a large number of military personnel, perhaps up to 300,000 of the 1.6 million who have been deployed, have been exposed to one or more blasts and report symptoms compatible with traumatic brain injury (Tanielian and Jaycox, 2008). These symptoms include alteration in level of consciousness, confusion, and immediate memory loss following injury. Most of these injuries have not been life-threatening, but still may have important adverse effects. At least several hundred individuals have sustained more severe blast-related TBI requiring intensive care unit treatment at neurosurgical centers (Okie, 2005; Armonda et al., 2006; Warden, 2006).
We know that blast events produce many kinds of physical forces that could potentially damage the brain. People exposed to blast injury may be subjected to more than one type of brain injury; for example, in addition to being exposed to a primary blast, there can be accompanying penetrating shrapnel injury and the victim may be violently transported, striking his or her head against a wall or other hard surface (Warden, 2006).
A recent retrospective report described a high incidence of cerebral vasospasm in victims of severe blast-related trauma. Dr. Armonda and colleagues at the National Naval Medical Center in Bethesda used cerebral angiography in 57 severe TBI patients and found that nearly half had evidence of vasospasm (Armonda et al., 2006). Vasospasm was associated with more severe injuries and higher mortality. In a non-randomized fashion, aggressive open surgical and endovascular treatment was associated with improved angiographic and neurological outcomes. Cerebral vasospasm has been reported in association with other mechanisms of TBI, but for methodological reasons the incidence, severity, and response to treatment cannot be compared for blast and non-blast related TBI.
Manifestations of blast-related TBI and PTSD may overlap, and the relationship between these two conditions is complex. Of soldiers with self-reported symptoms consistent with TBI, 27–44% reported concomitant symptoms meeting criteria for PTSD, whereas only 9% of uninjured soldiers and 16% of soldiers with other injuries met criteria for PTSD (Hoge et al., 2008). This survey-based study found that many of the persistent symptoms 3–4 months after return from duty in Iraq could be attributed to concomitant PTSD or depression; after adjusting for PTSD and reported depression, TBI was not associated with most other reported symptoms or poor general health. However, this study was based entirely on self-report, rather than diagnosis by health care professionals, and no brain imaging was performed. This issue is important because treatment of PTSD, while far from satisfactory, is much more advanced than treatment for TBI (Daly et al., 2005; Raskind et al., 2007; Tanielian and Jaycox, 2008). Thus it could be argued that an effective way to reduce the overall burden of disease would be to treat all patients with PTSD using evidence-based approaches in an efficient fashion rather than automatically attributing their symptoms and disabilities to TBI.
Issues Posed and Responses
What more needs to be learned about human blast-related TBI?
Relatively little is known about combat exposure to blast, especially for extremely powerful modern explosives. This presents a fundamental dosimetry problem. Also, relationships between the injuries caused by the primary blast and other simultaneously occurring injuries (“blast plus”) are unclear. Ongoing epidemiological studies may address this question, but the problem of high-quality data capture in a combat setting has not been resolved.
While a substantial number of military personnel have died with blast-related TBI, no autopsy reports from the current conflict have been presented to better understand neuropathology. The issues of severe post-mortem degeneration of the brain in the extreme temperatures of Iraq and Afghanistan, and the sensitivity of requesting an autopsy from next of kin were important issues.
Distinctive characteristics of severe blast-related TBI that have been reported include rapid, malignant cerebral edema, prominent subarachnoid hemorrhage, and a high incidence of cerebral vasospasm (Maas et al., 2008). Milder blast-related TBI has been associated with high rates of PTSD. These features are not restricted to blast-related TBI, and can be seen following other mechanisms of injury as well. There have been no reports of unique symptoms, signs, or deficits defining blast-related TBI, but the issue of ascertainment bias (“you only find what you are looking for”) was raised. This may be a moot point if primary blast is not a major cause of TBI, and most of the injuries are due to other factors such as accompanying projectiles or victims' heads striking a hard surface.
It is difficult to distinguish specific deficits and symptoms following blast exposure to TBI from those of PTSD and other primary mood disorders. As noted above, there are many overlapping symptoms including cognitive dysfunction, mood abnormalities, and sleep disturbances, but clear criteria for each disorder in isolation have not been formulated. The extent to which sequelae such as headaches, movement disorders, and seizures that are supposedly unique to TBI improve or resolve with appropriate treatment of PTSD has not been addressed. Development of new imaging capabilities and identification of biomarkers may provide help in distinguishing TBI and PTSD.
There is no standard way to describe the severity of blast-related TBI. Should we use the designations “mild,” “moderate,” and “severe” based on Glasgow Coma Scale scores, or consider multi-dimensional patho-anatomical or continuous descriptions of injury severity? An NIH-sponsored workshop scientific team and advisory panel published an initial formulation of this problem for conventional TBI (Saatman et al., 2008). Ongoing work will be required to fully address this issue and determine whether the same framework can be used for TBI due to blast.
On the question of genetic factors that might predispose individuals to worse outcomes following TBI, the apolipoprotein E4 allele has been reported to increase the likelihood of adverse outcomes overall (Diaz-Arrastia et al., 2003; Jellinger, 2004; Teasdale et al., 2005; Jordan, 2007), and post-traumatic seizure disorders (Diaz-Arrastia et al., 2003) in non-blast TBI, but similar genetic factors have not been addressed in blast-related TBI.
Many victims of blast-related TBI also have other injuries including burns, traumatic amputations, eye and ear injuries, fractures, muscular contusions, shrapnel injuries, lung injury, and multi-organ failure (DePalma et al., 2005; Okie, 2005). Hypotension and hypoxia are known adverse prognostic signs in TBI (McHugh et al., 2007; Maas et al., 2008), and priority in the treatment of polytrauma patients is given to preventing and reversing systemic cardiorespiratory compromise. Accurate planning of imaging approaches, candidate therapeutics, and rehabilitation strategies will require a clear understanding of the effects of polytrauma.
What are the effects of body armor, stress, sleep deprivation, and repetitive exposure?
Body armor
Due to improved body armor, many military personnel now survive blast-related injuries that would have been fatal in prior conflicts. It has been hypothesized that body armor also potentially diminishes brain damage by reducing thoracic injury with concomitant hydraulic pressure wave transmission to the brain (Leung et al., 2008). Current helmets have been designed to reduce penetrating injury to the brain, but have not been optimized for reduction of primary blast-related injury. Many ongoing experimental projects to address this issue have been initiated by the military.
Stress
Stress consists of multiple factors and is hard to define precisely. Issues raised by the group include the potentially protective effects of low-level, repeated stresses (“pre-conditioning”) (Shein et al., 2007), and the effects of combat-related hypervigilance on the later development of PTSD.
Sleep deprivation
It has been reported anecdotally that active duty military personnel sleep between 5 and 6 h per night on average for up to 1 year. The effects of this sort of sleep deprivation on susceptibility to TBI, recovery from TBI, and development of PTSD have not been reported.
Repetitive exposure
Exposure to more than one blast injury is likely to be the rule rather than the exception for active duty military personnel. Analogously, multiple sports-related concussions are undoubtedly more common than single, isolated injuries among participants in boxing, ice hockey, and American football. Reduced cognitive performance, especially impaired vigilance, may render the injured person less able to avoid additional trauma. Likewise, loss of cognitive reserve may allow what would otherwise have been a sub-threshold injury to cause further neurological decline.
A pragmatic issue regarding the “window of vulnerability” to repeat exposure was raised. This could have important implications for return-to-duty, return-to-work, and return-to-play decisions. In animal studies, two concussive injuries separated by an interval of 3–5 days resulted in behavioral impairments, whereas an identical pair of injuries separated by 7 days did not impair behavioral performance (Longhi et al., 2005; Weber, 2007).
Whether a window of vulnerability exists in humans, its duration, and its underlying mechanisms remain to be determined.
Recommended Research Priorities
Neuropathological studies of blast-related TBI
Pathology has been and remains the cornerstone of modern scientific medicine. Our current advanced understanding of infectious diseases, cancer, coronary artery disease, and many other illnesses are based on pathological characterization.
Performing appropriate neuropathological studies may require radical changes in the way advanced directives with active duty military personnel and their families are handled. For example, pre-deployment discussions regarding autopsies could remove many of the barriers to these critical lines of investigation. If broached appropriately and sensitively, with the explicit goal of understanding what went wrong so that we can better prevent casualties in the future, this could be just as successful as current organ donation programs.
In addition, autopsies on civilian blast injury casualties could be performed. Brain biopsies from surgical decompression and evacuation procedures for severe TBI could be analyzed (Ikonomovic et al., 2004; DeKosky et al., 2007). Cooperative neuropathological studies could be initiated with allies such as Israel, where blast-related TBI is a common ongoing concern in both civilian and military populations (Askenasy, 1989; Peles et al., 2001; Schwartz et al., 2007).
Neuropathological findings must also be correlated with the blast event. This is confounded in the military by the need for operational security. Solving this important problem will require collaboration between military medical, intelligence, and combat arms leadership to identify means of making correlations without jeopardizing strategic or tactical security concerns.
From the imaging perspective, post-mortem scans can be performed, and the field of forensic radiology is rapidly growing (Thali et al., 2007). Direct comparison between imaging characteristics and pathological features could allow refinement and validation of imaging approaches (Mac Donald et al., 2007). Many MRI sequences have similar characteristics in post-mortem tissue as in vivo (Schmierer et al., 2007; Gouw et al., 2008).
Development of standardized assessment tools to assess the severity of blast exposure and the cumulative burden of multiple injuries
These should be simple, reliable, and correlate with clinically relevant outcomes. They should explicitly take into account the potential synergistic effects of repeated injuries in close succession, and include assessments of polytrauma and PTSD-related deficits.
Data sharing and consortia between Department of Defense, Veterans Administration, and civilian academic research groups
This is likely to result in improved scientific rigor, less redundancy of effort, and faster progress than individual groups working in relative isolation. Again, operational security is a concern that needs to be addressed with regard to data sharing.
Pre-deployment neuropsychological testing and imaging for high-risk groups
The cognitive effects of TBI often cannot be resolved using conventional neuropsychological testing, as modest reductions in abilities may still result in performance within the normal range. For example, a person whose verbal memory was in the 75th percentile before injury may perform at the 25th percentile after injury. While such a score may still be in the normal range, the person is significantly impaired relative to pre-injury performance.
Streamlining of human research protection agency/institutional review board regulatory oversight of human studies, especially with regard to military populations
This will substantially reduce the time delay between formulation of important research questions and initiation of data collection. Anecdotal evidence indicates that regulatory hurdles constitute a substantial barrier to new investigators interested in human studies of TBI, and reduce the likelihood that innovative research involving human subjects is performed. On the other hand, the military community can be perceived as at risk because of the rank structure and close-knit relationships. While the military has made great strides in human-subject protection since the mid-20th century, the regulations put in place to protect troops from coercion may make the review process challenging.
Section 3: Human Imaging
State of the art
CT scanning is the current standard-of-care imaging examination for both civilian and military patients with head injuries. CT is capable of rapidly identifying lesions that require urgent management, such as skull fractures, embedded foreign bodies (shrapnel), and hemorrhages in the epidural, subdural, subarachnoid, and intraparenchymal spaces. In the subacute phase of injury, CT identifies those patients with enlarging cerebral contusions with mass effect and/or diffuse cerebral edema requiring decompression. CT is readily available at almost all civilian and military hospitals. There are no current data available about what fraction of blast-TBI victims have had head CTs, nor is it known what, if any, findings can be identified at head CT in blast victims.
However, 85% of patients with mild civilian TBI or concussion with normal GCS scores have a normal head CT (Ghajar and Ivry, 2008). While most of these patients will recover within weeks to months without intervention, an estimated 15% continue to have disabling symptoms for months to years (Alexander, 1995). More recently, MRI has become the technique of choice for identifying brain lesions in patients with mild civilian TBI with normal CT but clinical suspicion for more extensive injury. MRI identifies up to 50% more lesions than CT alone (Lee et al., 2008). Fluid-attenuation inversion recovery (FLAIR) and T2-weighted images have increased sensitivity for non-hemorrhagic contusions and shearing injuries than CT. Using MR-gradient echo T2* or susceptibility weighting imaging techniques, small cerebral hemorrhages can be detected that are not identified by CT (Parizel et al., 2005). However, despite identifying more injuries, it is important to note that conventional MRI findings have had little correlation with long-term outcomes in civilian TBI (Hughes et al., 2004).
PET studies of various types of TBI in civilian patients have greatly contributed to our current understanding of the underlying pathophysiology. Animal models of TBI have found accumulation of cerebral lactate acutely following injury, with this hyperglycolysis followed by metabolic depression that can be monitored using positron emission tomography (PET) measurements of cerebral glucose metabolism and cerebral blood flow (CBF) (Yoshino et al., 1991). These findings have been confirmed in human studies of TBI using PET (Bergsneider et al., 1997). Alterations in human cerebral metabolism following TBI have also been monitored using 13C-labeled glucose mass spectroscopy to monitor glucose and lactate metabolism (Dusick et al., 2007).
Elevated brain lactate concentrations have been identified using proton MR spectroscopy (MRS) in adult TBI (Condon et al., 1998), and in children are associated with poor long-term intellectual and neuropsychological outcomes (Brenner et al., 2003). MRS can also be used to identify underlying cellular injury, with alterations in quantitative N-acetyl-aspartate (NAA) and choline identified in areas of the brain that appeared normal on conventional CT and MRI in patients with civilian TBI (Garnett et al., 2000a, 2000b). In sports-related concussive head injury with mild TBI, alterations in NAA are additionally prolonged by multiple concussive events (Vagnozzi et al., 2008).
More recently, diffusion tensor imaging (DTI) has been used for assessment of white matter tracts. In subjects with mild TBI, the number of lesions identified with DTI is associated with changes in cognitive processing speed (Niogi et al., 2008a, 2008b). Furthermore, lesions identified by DTI can be further assessed in relation to specific axonal pathways using tractography, which has additional promise for predicting cognitive dysfunction in specific domains.
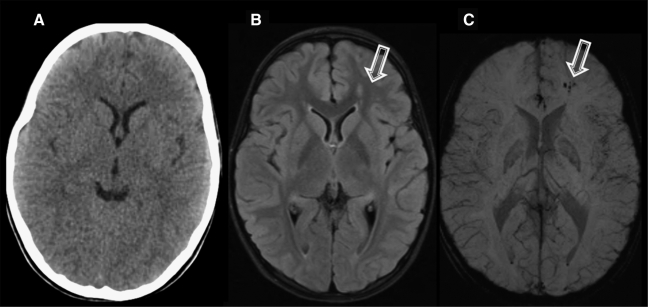
Figure 1 shows examples of several of these brain imaging techniques as applied to civilian patients with TBI.
FIG. 1.
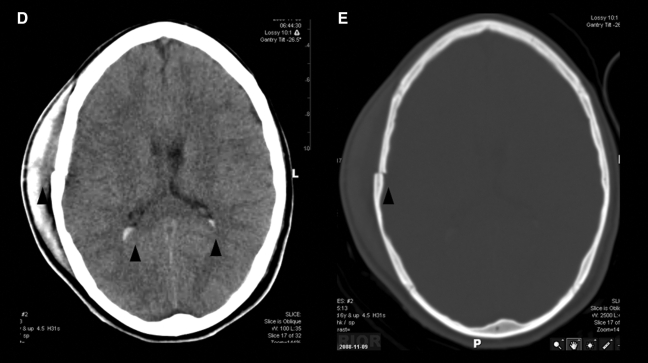
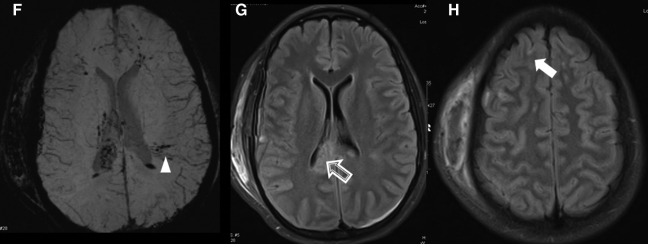
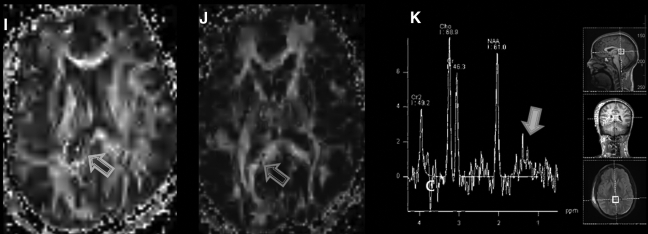
Imaging of civilian TBI. Case 1 (images A–C): A 9-year-old girl was involved in a roll-over all-terrain vehicle accident, with brief loss of consciousness. GCS score upon arrival was 15. Head CT was performed (A), which demonstrated no abnormality. However, FLAIR (B) demonstrated hyperintensities in the left frontal white matter, with corresponding hemorrhage present on the susceptibility-weighted imaging (SWI) sequence (C). Spectroscopy was normal (not shown). Case 2 (images D–K): A 15-year-old girl was an unrestrained passenger in a roll-over motor vehicle accident. She was unconscious and required intubation at the scene of the accident. CT scan (D, windowed for brain parenchyma, and E, windowed for bone) demonstrated multiple findings, including a right scalp hematoma, right parietal bone fracture, and intraventricular hemorrhage (black arrowheads). MRI (images F–K) was performed, which confirmed the CT finding of intraventricular hemorrhage. However, SWI (F) also demonstrated multiple white matter microhemorrhages (white arrowhead). FLAIR (G and H) identified multiple grey- and white-matter lesions, including in the corpus callosum (open arrow) and frontal cortex (closed arrow). In addition, DTI (I and J) and spectroscopy (K) were performed. On DTI there is loss of fractional anisotropy (FA) associated with the injury to the corpus callosum (I, open arrow). This is also demonstrated on the color-encoded FA map, where the normal transverse (red) directionality is lost at the callosal lesion (J, open arrow). Spectroscopy performed of the callosum demonstrated elevation of Choline (Cho) relative to NAA, and an abnormal lactate peak (closed arrow) at 1.3 ppm. Cases courtesy of Jose Pineda, M.D., Washington University, St. Louis, MO.
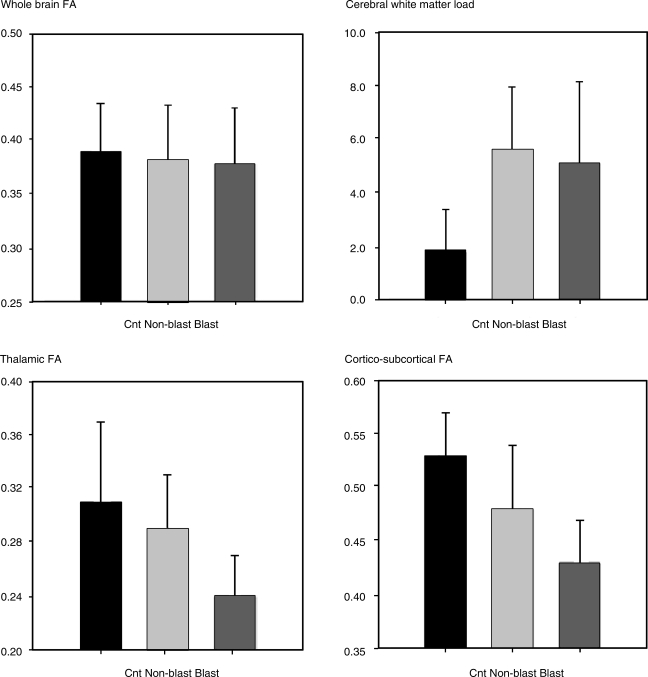
DTI may have particular utility for assessing blast-related brain injury. At the meeting, Deborah Little presented preliminary results from conventional anatomic MRI, DTI, and neurobehavioral testing in five subjects with a history of mild bTBI. All had served in Iraq within the last 4 years and were between the ages of 23 and 28. Subjects were compared to age-, ethnicity-, and gender-matched controls and civilian TBI patients with similar clinical presentations. While whole-brain fractional anisotropy (FA) measurements derived from DTI were similar for all three groups, both the civilian and the military blast-related TBI subjects had loss of white matter integrity as measured by FA and tractography. This approach also showed that blast-TBI subjects had additional reductions in white matter integrity that tended to be preferentially within the thalamus. These preliminary data based on DTI analysis, the first comparing chronic civilian TBI and military bTBI, are shown in Figure 2.
FIG. 2.
DTI analyses from non-blast and blast-TBI patients. Whole-brain and high-resolution DTI data were acquired from five patients with a history and symptoms of mild blast-TBI (Blast) who had served in forward operating units in Iraq within the last 4 years; from five controls (Cnt) who matched these patients for age, education, and ethnicity; and five mild non-blast (Non-blast) TBI patients also matched for age, ethnicity, and gender, who were injured in civilian motor vehicle accidents. Of the bTBI patients, two reported loss of consciousness (LOC) of less than 10 min. Of the civilian non-blast TBI patients, three reported LOC with durations under 15 min. All subjects self-reported post-traumatic amnesia lasting no longer than 24 h. The whole-brain DTI data (B-values: 0, 1000; directions: 27; voxel size: 1.56 × 1.56 × 3 mm3; NEX: 2) were used to calculate whole-brain fractional anisotropy (FA, a measure of white matter structural integrity), and white matter load. White matter load was calculated as the number of cortical white matter tracts that showed reduced FA relative to controls. For whole-brain FA lower numbers reflect greater damage, whereas higher white matter loads reflect higher numbers of damaged tracts. The high-resolution DTI data (B-values: 0, 1000; directions: 27; voxel size: 0.70 × 0.70 × 3 mm3; NEX: 6) were used to assess thalamic integrity and the integrity of fibers running through four thalamic nuclei (the anterior nucleus [AN], the ventral posterior lateral nucleus [VPL], the ventral posterior medial nucleus [VPM], and the lateral geniculate nucleus [LGN]). Although the sample sizes are small, these preliminary data suggest a potential for differential sensitivity of the thalamus in bTBI as represented by these patients.
Recently, there has been intense research in the use of MRI to identify functional connections between cortical brain areas (He et al., 2007). Using the blood oxygen level-dependent technique, functional MRI measures changes in blood flow during task-driven cortical activation. Functional connectivity MRI (fcMRI) uses the same technique to evaluate spontaneous connections between various cortical regions when the brain is at rest. When integrated with DTI, fcMRI has particular promise for defining alterations in brain organization and plasticity, that may be useful in evaluating sequelae of bTBI.
In the current conflict in Iraq, vasospasm has been identified in soldiers with severe war-related neurotrauma. As noted above, of soldiers medically evacuated to the National Naval Medical Center who underwent cerebral angiography between 2003 and 2005, 47% had vasospasm (Armonda et al., 2006). In addition, these patients were evaluated by CT angiography (CTA) and transcranial doppler (TCD). TCD was found to be a helpful screening tool for vasospasm. CTA was useful in those cases without significant streak artifact from shrapnel. The finding of cerebral vasospasm in severe bTBI raises the question of whether there may be a component of vasospasm in the setting of mild or moderate bTBI, with measurable findings of altered vessel caliber by CTA or magnetic resonance angiography (MRA), or of altered CBF, which could be measured by CT or MR perfusion studies or by TCD.
Additional techniques are under development that may be useful for future evaluations of patients with bTBI. These include near-infrared spectroscopy and diffuse optical tomography, which could potentially measure both CBF and resting state connectivity, and could be combined with nuclear medicine techniques (Culver et al., 2008). Pupillometry (Toth and Fletcher, 2005) offers a non-imaging approach to portably assess the autonomic nervous system, and may have applications for medics in the field. Finally, as animal models are developed that are appropriate for bTBI, newer MR techniques and PET ligands should be cross-validated with the animal models to evaluate the suitability of the animal model and imaging approaches to translate into human TBI applications.
Issues Posed and Responses
How can current imaging techniques help?
Based on civilian TBI, non-contrast CT has been most helpful for triaging acutely injured victims and is employed in the military in a similar fashion. Current CT imaging is able to identify moderate and severe injuries (contusions, hematomas, penetrating injuries, and fractures), but cannot distinguish normal from mild blast injury. These CT data are typically not currently available to researchers, nor are they available to physicians following the same patients when they later present to VA hospitals for therapy.
MRI is currently available at military and VA hospitals in Europe and the U.S., and available in extremely limited fashion in Iraq and Afghanistan. Based on civilian TBI data, it is unlikely that the anatomic images of MRI will be predictive of long-term disability, although they will be likely to detect more injury than non-contrast CT alone (Hughes et al., 2004). Additionally, MRI requires careful screening for metallic shrapnel in order to prevent severe injury in the environment of the magnet, which would add additional burden to the doctors and medics. However, based on civilian pediatric TBI data, evaluation with MRS for the presence of lactate may be useful for detection of subtle metabolic derangements in patients with otherwise normal imaging exams.
PET has been the single most useful research tool for evaluating civilian TBI to date, and has greatly added to insight regarding pathophysiology and metabolic changes in the acute and subacute stages of injury. In civilian TBI, PET is most informative in the acute and subacute setting. As this technology requires significant infrastructure for radioisotope production, it is not likely to become useful for field screening. However, measurements of altered glucose and lactate metabolism, CBF, and oxygen extraction correlated between human and animal models using PET have critically guided development of more accessible techniques using CT and MRI. MRI can now be used to measure lactate and oxygen extraction. Both MRI and CT can be used to measure blood flow, blood volume, mean transit time, and to perform non-invasive angiography.
In the chronic phase of injury, both non-blast-related TBI and preliminary data from bTBI suggest that DTI may be extremely helpful for identifying microstructural injury. The preliminary data presented by Deborah Little (Fig. 2) suggest that bTBI may particularly involve the thalamus. Addition of functional connectivity MRI to DTI may give insight into brain dysfunction not otherwise apparent on conventional imaging. The major limitations of these approaches are lack of standardization of acquisition between centers, the need for development of a protocol that could be run on existing military and VA scanners, exclusion of subjects with extensive shrapnel injury, and current lack of baseline data in the military population.
What imaging modality is useful or obtainable for immediate battlefield use?
CT scanning is currently available in military field hospitals and is used to triage blast victims. These data could be analyzed to gain further insight into patterns and mechanisms of blast injury. Given the strong evidence for altered metabolism and hemodynamics in civilian TBI, and the recent report of vasospasm in severe blast TBI, CT angiography and CT perfusion measurements, which add less than 5 min of scan time to a standard head CT, are possible with existing technology and could be employed in a research study.
Portable technologies for the battlefield would include transcranial Doppler to monitor intracerebral hemodynamics. This technology is readily available and is used in many intensive care units (ICUs), including military hospitals, to monitor patients for vasospasm. Portable devices for monitoring pupillometry are similarly employed by ICUs and may prove to be useful in the battlefield. Similarly, near-infrared optical imaging devices are under development that could measure oxygen extraction fraction with 5–15 mm resolution and up to 10 mm deep under the skull. A key issue raised with all of these emerging technologies is balancing research needs, which might guide new therapies and prevention of injury to soldiers, with the existing burdens of gear weight and the need for medical personnel in the field to focus on delivery of life-saving care and movement of personnel out of harm's way as quickly as possible.
What imaging modality is most useful for long-term sequelae of biological change?
There was consensus among the group that in the chronic phase of blast TBI, MRI protocols should include at a minimum structural imaging with DTI, and should likely also include fcMRI. In the outpatient setting, issues such as screening for shrapnel would still need to be performed, but are more feasible than in the acute venue. Additionally, because DTI measurements have been found to vary with age (Head et al., 2004), research would be greatly aided by performing DTI and fcMRI measurements in troops both before and after injury in order to establish appropriate baselines.
How can we build better joint sharing repositories?
The group expressed strong consensus that shared data collection and standardized analysis will be critical for rapid progress to be made in our understanding of blast injury. Key components of the data repository will be standardized protocols, cross-validation of the data between the different sites, and design of protocols to be stable longitudinally. Additionally, it was urged that these protocols be developed to utilize technologies currently available to military and VA sites, or to use technologies that are thought to soon become commercially available. In the current crisis, limiting the research to sophisticated academic centers would be detrimental to rapid progress, and would ultimately impede the ability to translate the results into clinical practice. These issues are particularly critical for MRI, for which there is a tendency for academic medical centers to focus on research using custom-made software for acquisition and processing of data.
Additional features suggested to be critical to the success of a repository were inclusion of pre- and post-deployment data and an ability to include diverse data types (such as serum or psychometric results), in addition to the standard imaging components. In this manner, as biomarkers are discovered they can be quickly correlated with imaging results. Finally, the database will need to include both animal imaging components and histology or autopsy correlates whenever possible.
The military and VA have extensive expertise with teleradiology and computerized medical record systems. This expertise should be incorporated into the development of a shared research repository. Whenever possible, a copy of subjects' military or VA imaging should be anonymized and archived, so that additional acute and subacute data points can be collected for meta-analysis. It would be helpful if pertinent history fields could be identified and prospectively incorporated into the radiology reports so that details of blast exposure could be better documented.
How can data sharing be facilitated while preserving national security?
It is critical that information derived from blast TBI research, designed to develop new strategies for prevention and treatment of injury, not be used by the enemy to develop deadlier weapons. Information linking specific events to the data could be particularly harmful and must be protected. Civilian research has developed sophisticated tools to protect the privacy of research subjects. This expertise must be utilized and incorporated into any shared repository. Furthermore, close collaboration between civilian, VA, and military researchers, physicians, engineers, and special operations forces will be critical to the success of any research program.
Recommended research priorities
Based on civilian TBI, non-contrast CT has been most helpful for triaging acutely injured victims and is currently employed in the military in a similar fashion. Further analysis of these CT data would be extremely helpful to guide the development of animal injury models for blast, and should be made available at whatever point it can be done without risking national security.
Imaging research in the acute and subacute stages of blast injury must be performed in a manner to be minimally burdensome to the troops. Establishment of a “brain team” on the ground to acquire data could particularly facilitate reliable data collection in an unobtrusive manner. Existing imaging technologies of pupillometry, TCD, diffuse optical tomography, CT perfusion, CTA, MR perfusion, MR spectroscopy, and MRA could be implemented on a small scale by this brain team to further evaluate their utility in assessing patients with blast injury. Ultimately the data could be used to determine which soldiers are potentially in a vulnerable metabolic state following blast exposure, and could be used to temporarily remove these soldiers from exposure to repeated blast injury.
-
Of the various imaging modalities applied to civilian TBI, only diffusion tensor MR has been able to identify pathology in the chronic setting, and preliminary data from blast TBI also suggest its utility. A large-scale, multicenter MR research program, including at a minimum DTI, is recommended. In order for this to be successful, it is recommended that there be:
Standardization of the basic acquisition protocol;
Calibration and validation of measurements between various sites;
Measurements of large numbers of soldiers (i.e., entire battalions);
Pre- and post-deployment measurements; and
-
Records kept of blast exposure for these particular soldiers.
In addition, this protocol should have some flexibility built in, so that smaller investigations of existing techniques, such as spectroscopy, can be assessed, and so that findings from animal studies can be rapidly explored as these data become available.
Data-sharing resources should be developed in order to facilitate these research objectives.
Section 4: Animal Models of Brain Blast Injury
State of the art
Animal model studies of blast injury benefit greatly from the extensive literature on closed-head TBI. When studies of TBI began in the 1970s, our knowledge of injury was primarily based on human and animal neuropathology because many of the other techniques now available did not exist. Great strides were made in understanding the cellular and molecular changes seen following TBI, and these discoveries led to pre-clinical studies of drug therapies and other interventions designed to mitigate the effects of TBI.
The use of carefully controlled and homogeneous animal models of TBI across different size scales and brain types contributed essential data to our comprehension of the mechanisms involved in TBI. However, we now understand that TBI is not a homogeneous condition, and that the symptomatic presentation of TBI (e.g., the GCS score) does not capture the full range of anatomic and physiologic changes that can occur in TBI. Since most interventions are based on disrupting or enhancing a specific biochemical mechanism, future clinical efforts need more precise classification of the injury to facilitate effective treatment (Saatman et al., 2008).
Research on the pathophysiology of bTBI is relatively young by comparison, and the first comprehensive studies are just beginning to emerge. Some possible protective mechanisms have been explored, such as injection of hemin, which activates heme-oxygenase-1 and may mitigate oxidative stress. In a trial comparing pre-treatment with IP hemin versus placebo, rats exposed to a 160-kPa blast overpressure had a survival rate of 68% if they received hemin, versus only 35% with placebo (Chavko et al., 2008).
Current animal studies include investigations in both rodent and swine models of bTBI. Large and small blast/shock tubes are used depending on the size of the animal, and the relevant range of exposure pressures for rodents is between 30 and 170 kPa, which can be achieved with laboratory scale air shock tubes. Testing with swine using a large explosively driven blast tube in the field has achieved 1 MPa exposures (Ritzel personal communication, 2008). Exposures at the low end produce a mild injury with no overt immediate trauma, whereas the high end of exposure produces approximately 80% fatalities. Animals are evaluated after exposure (or multiple exposures) for cognitive performance, pathology, changes in gene expression, and other biochemical signatures. There is also great interest in the use of non-invasive methods such as near infrared spectroscopy or pupillometry for post-blast evaluation, and in the use of implanted sensors to measure in-situ forces and strain rates (Chavko et al., 2007). Initial results from these newer studies appear to show that in mild bTBI exposure there may be initial damage to the cortex and hippocampus (as measured by staining for degenerating neurons, peroxidation, and nitration), but that this damage resolves by 8 days post-injury.
Chronic low-level blast exposure is a topic of great interest since it may be very widespread among troops in current military operations. Chronic exposure could also be a far more manageable problem than acute blast injury if the damage mechanisms were fully understood.
One area of great interest in the pathophysiology of blast TBI is the role of the cerebral vasculature. The injured brain has less of an ability to respond to secondary insults because fundamental vascular regulatory mechanisms become damaged, and conversely, hypotension in the aftermath of brain injury puts the brain and the entire organism at greater risk. Recently an evaluation of 18 blast-exposed patients in Operation Iraqi Freedom found that all of the patients presenting with sustained hypotension died, whereas patients without sustained hypotension survived (Nelson et al., 2006). A review of the relevant literature shows a strong association between TBI and cerebrovascular dysfunction in a wide range of animal models (DeWitt and Prough, 2003).
Because many models of blast exposure involve the entire body, researchers studying the cerebral vasculature have devised a different approach to creating blast injury than the existing shock/blast tubes. This device (presentation by Doug DeWitt) uses a small nail gun cartridge, which is readily available in many sizes, allowing variations in the applied force. Studies with this device are just beginning, but a level of simulated blast has been determined where the latency of the righting reflex doubles compared to that in uninjured rats. This level produces no apparent histological damage, and so is being classified as a model of mild bTBI.
The final presentation in this section covered physics and engineering considerations for blast simulation in animal models. Fortunately for the field, the simulation of blast effects is not a new topic, and the computational and measurement tools available are mature and well established. The main challenge is to find—or create—the appropriate model that exists at the intersection of the biological requirements for the animal model and the physical behavior of the blast simulator.
Meaningful and relevant blast simulation should take into account several factors, including the fidelity of the simulation technique combined with the appropriate animal model. First, one must recognize that even simple blast simulations can have complex effects, so investigators will be required to pre-select or possibly design new simulators and select an appropriate animal model, which together re-create the conditions and damage outcomes intended to be studied. Seemingly simple shock-tube devices will produce complex and severe flow conditions that may very likely be injurious although unrelated to blast injury conditions of relevance. In other words, if one believes that the dominant method of injury is the bulk compression of the brain, then the blast device should be designed to create that effect. Just as different animal models are likely to reveal different aspects of bTBI biology, a range of blast devices may be needed to more fully model the complex and heterogeneous exposures seen in combat, and studies are needed to accurately characterize the primary effect on brain tissue that results directly from blast energy waves, as well as from repeated exposures.
Second, effects of size, material properties, and scaling in different species must be addressed. Identical blast overpressure applied to the thorax will have a dramatically different effect on a person than on a rodent because the two skeletons vary greatly in their mechanical properties, such as stiffness, thickness, and rib spacing. Similarly, the effect of whiplash-type injuries will be very different between a human and a swine because the human head is supported by a thin and flexible upright neck, whereas the swine head is not.
Third, devices that create model blasts must be carefully studied and verified using computational simulations and measurements of all the different exposure parameters. A key point here is that all the physical properties should be evaluated (static pressure, dynamic pressure, flow, kinetic energy transfer, heat, and density) because all of these phenomena can create damage, but that damage is not necessarily a relevant simulation of battlefield blast. Such simulations are especially challenging due to the inherent variability of explosive blast parameters in a battlefield setting.
Issues Posed and Responses
How good are current animal models at replicating key aspects of human blast injury? What key aspects of bTBI should we replicate? Do we need to develop new models?
The key for creating successful animal models is that the forces (stress and strain) applied at the tissue and cellular level are the same as those experienced in human blast injury, because it is these forces that drive the biochemical or pathological response. We expect that the responses at the cellular level will be common across species. Along similar lines, there are only a limited number of ways that cells can die, so it would be very surprising if bTBI revealed an entirely new form of apoptosis or axotomy.
The question of creating the proper stresses and strains at the micro level is very challenging. There are important—and uncharacterized—differences between human beings and animal model systems. For example, the thickness, deformability, and openings of the rat skull are very different from those of the human skull. Furthermore, much of the data compiled on material properties of bones, muscles, and nerves, is at lower strain rates than those experienced in blast. All of these variances can introduce potential errors into the animal modeling process.
The current animal models of bTBI are a good start, but are likely to need much more research and development. A major limitation is that the pathophysiology of human blast injury is still unknown, and it is difficult to model the unknown. Moreover, we do not yet know the key damage mechanisms of blast to replicate.
Can imaging assist in developing animal models that more realistically replicate human blast injury? If so, what currently stands in the way?
Imaging may be the major source of a defining pathoanatomical signature of human bTBI. Previous sections of this report have highlighted the lack of knowledge concerning bTBI in humans.
Imaging is also important for animal models, because it enables longitudinal and serial studies that can replace other types of destructive testing and improve understanding of the time course of events following blast injury. At present, every major imaging modality can be found in a system dedicated to small animal research, which should be a great benefit for translational work between animals and humans.
In order for imaging to be most useful, it is important that some standardization occur, especially in the use of more specialized MRI sequences. In addition to differences between vendor interfaces and data formats, the entire description of an imaging sequence can vary greatly between scanners, and this hampers repeatability. Therefore more effort will be needed to demonstrate reproducibility of advanced imaging findings in small animals.
Can animal models be used to validate new imaging modalities? Can animal imaging be used to measure treatment effects?
The link between blast exposure and imaging findings is pathophysiology, and it is clear that animal models are a good means of correlating pathology and physiology, and therefore imaging itself. Animal models will also be useful for validating other assessment tools, such as pupillometry and chemical biomarkers from the central nervous system or peripheral circulation.
In addition, a key advantage of using imaging and animal models together is that some imaging techniques could never be used at the human scale under battlefield conditions. For example, with animal models it may be possible to image immediately after blast exposure and thereby capture data about very short-lived events. Although such studies might have limited translatability, they could be very important for understanding the basic science behind the injury.
In the treatment realm, there are many instances where imaging of animal models has been used to assess treatment. In treatment of stroke, significant translational efforts in both directions established parameters for both injury measurement and application of therapeutic drugs. Although in TBI there is not yet a direct link between effective treatments in animal models and therapies in humans, the value of both animal models and imaging is to understand the mechanisms at work. In fact, imaging may not represent the best clinical end-point to determine the effectiveness of a therapy, but the ability to answer questions like “Is this drug reaching the intended location?” or “Is this drug affecting particular receptors as expected?” is critical.
What new imaging techniques for animals need to be developed?
Much of the current MRI animal imaging is focused on anatomical and diffusion tensor imaging of the brain. Given the importance of the vasculature in the outcome of bTBI, techniques that measure blood flow and perfusion—such as arterial spin labeling and magnetic resonance angiography—should be investigated. This is an area where standardization and reproducibility will be even more important, because such techniques are newer and thus more likely to vary, as they represent the leading edge of the technology. Similarly, efforts to image nuclei other than hydrogen with MRI should be pursued, because measurements of elements such as sodium and phosphorus could provide important new insights into the immediate aftermath of bTBI.
Because axonal injury is likely to have a central role in bTBI, methods that allow co-localization of an imaging signal with axonal injury could be quite powerful. MR spectroscopy will enable us to look at metabolites in this context. DTI will obviously have great utility because of the inherent anisotropy of the healthy axon. PET ligands that interrogate molecules known to be part of axonal injury (e.g., caspase-3) may also have interesting applications.
There is growing evidence that functional connectivity networks are comparable across different species, and that these networks can be observed in different anesthesia states. This represents a good opportunity for developing functional MRI imaging methods that do not depend on active participation or following commands, which would obviously not be suitable for many animal models.
Recommended Research Priorities
Blast simulation devices (and their target animal models) should be validated and calibrated through computational physics simulations and appropriate in-situ measurements
Without well-characterized and validated blast generators, it is impossible to relate the injuries observed in animal models to human blast injury, because the injury mechanism could be completely different.
Human neuropathology after blast injury should be used to guide the development of a range of animal models of blast-related TBI
In addition to using realistic blast exposures, a reasonable criterion for considering such animal models valid would be that they mimic the key neuropathological features of human blast-related TBI.
Designs and use parameters for blast simulation devices and follow-on imaging should be made widely available to the research community
Standardized animal models and injury-producing devices, as well as common and well-understood imaging protocols, should be developed and supported for bTBI. Interdisciplinary and team research should be established with strong collaboration between animal and human studies. Funding agencies can help to facilitate this by supporting consortia and effectively coordinating disparate research efforts.
The data gap between injury mechanism, pathology, and imaging findings in human studies must be addressed
At a minimum, data are needed on the location and characteristics of brain pathology after bTBI. Ideally, these pathological data would be obtained from autopsy in fatal blast injury and correlated with imaging. For non-fatal injuries, imaging in the subacute and chronic time frames will help fill this gap.
Because of the complexity inherent to blast modeling in animals, a comprehensive effort will require multiple animal models at different size scales.
Conclusions
Exposure to blast can cause varying types and degrees of injury. The study of blast physics is mature and supported by many experimental results and computational simulations, although powerful modern explosives have not yet been fully characterized. Similarly, conventional TBI has been extensively studied, but blast TBI is a new research area. Very few data are currently available on key topics such as blast exposure in combat scenarios, pathological characteristics of blast TBI, and imaging signatures of blast TBI. Addressing this data gap is absolutely critical if blast TBI research is to advance. Autopsy and pathoanatomical data need to be obtained, and advanced neuroimaging used in studies of acute, subacute, and chronic cases in order to determine whether there is a distinct pathoanatomical signature that correlates with long-term functional impairment. In addition to facilitating studies of bTBI in humans, these data will enable the development and use of animal models that can illuminate the mechanisms of blast injury and provide leads for new treatment approaches.
Fortunately, the great need for understanding blast TBI is now driving a major research effort. Multidisciplinary teams of clinicians, basic scientists, and engineers are already at work and such efforts should be encouraged. Coordination and cooperation between different academic sites and between military and civilian efforts is imperative, as is the need to develop standards for large-scale trials and data sharing. Many of the tools (particularly imaging methods) developed for conventional TBI can and should be quickly re-purposed for the study of blast TBI. Although the number of unanswered questions on blast TBI may seem overwhelming, an efficient strategy for prioritizing these questions will facilitate the allocation of sufficient scientific, clinical, logistical, and financial resources. We hope that the results of this workshop will guide the translation of these resources into meaningful new knowledge, preventive methods, and durable therapies for blast TBI.
Acknowledgments
The writing team wishes to especially thank Dave Ritzel, who gave two presentations at the workshop, providing the participants with a “blast primer” in the first session, and applying his blast physics expertise to the consideration of animal models in the last session. Dave also carefully reviewed and revised the section “Blast Physics: State of the Art” in this report to assure that the factual information is accurate and the historical context is clear.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect the policy or opinion of the U.S. Army, Department of Defense, or the U.S. government.
Author Disclosure Statement
No conflicting financial interests exist.
Workshop scientific organizing committee
Mark Mintun and Ken Curley (co-chairs), Seong K. Mun, Jean Wrathall, Murray Raskind, David Hovda, John Povlishock, and Joseph Pancrazio.
Presentations
Introduction: Seong K. Mun, Ph.D.
Welcome address: R. Gilbert Jost, M.D.
Opening remarks: Mark Mintun, M.D.
Science and the square deal: Christian Macedonia, M.D., L.T.C.(P.), M.C. U.S.A.
Blast—A Primer: Mr. David Ritzel
Session 1: Human blast-related brain injury presentations
Introduction: Ken Curley, M.D.
What we do and don't know about blast TBI—the clinical and research perspectives: David Moore, M.D., Ph.D.
What do we know regarding the difference between blast and non-blast head trauma?: Thomas Gennarelli, M.D.
Session II: Human blast-related brain injury presentations
Introduction: Ken Curley, M.D.
What we do and don't know about blast TBI—The imaging perspective: James Smirniotopoulos, M.D.
VA experience with blast TBI—Is there a relationship between TBI and PTSD?: Murray Raskind, M.D.
Session II: Human brain imaging session
State-of-the-art imaging options for the investigation of blast-related injury: Mark Mintun, M.D.
PET and neurochemistry in TBI: David A. Hovda, Ph.D.
Diffusion tensor imaging of mild traumatic brain injury—A potential biomarker of neurocognitive outcome?: Pratik Mukherjee, M.D., Ph.D.
DTI and EEG to assess white matter in TBI: Deborah M. Little, Ph.D.
Functional connectivity by MRI—A physiological assay of brain function and new imaging modalities: Maurizio Corbetta, M.D.
Session III: Experimental animal model presentations
Animal studies of TBI: John T. Povlishock, Ph.D.
Pathogenic mechanisms induced by exposure to blast overpressure: Richard M. McCarron, Ph.D.
Blast-induced brain injury and posttraumatic hypotension and hypoxemia: Douglas S. DeWitt, Ph.D.
Modeling native blast injury: David Ritzel
Roundtable members
Regina C. Armstrong, Ph.D., Philip V. Bayly, Ph.D., Timothy B. Bentley, Ph.D., Tammie L.S. Benzinger, M.D., Ph.D., David Brody, M.D., Ph.D., Sylvain Cardin, Ph.D., Joseph P. Culver, Ph.D., Alan I. Faden, M.D., Gary Fiskum, Ph.D., Susan M. Fitzpatrick, Ph.D., Jamshid Ghajar, M.D., Ph.D., F.A.C.S., Rao P. Gullapalli, M.D., Evan D. Kharasch, M.D., Ph.D., Karen Kharasch, Patrick Kochanek, M.D., Markus Lammle, M.D., Michael J. Leggieri, Jr., Lawrence L. Latour, Ph.D., Joseph T. McCabe, Ph.D., Christine L. Mac Donald, Ph.D., Mikulas Chavko, Ph.D., Daniel Marcus, Ph.D., Pratik Mukherjee, M.D., Ph.D., Steve Petersen, Ph.D., Elaine R. Peskind, M.D., Kathy J. Pierce, Ph.D., Jose A. Pineda, M.D., Charles Riedel, M.D., Joshua S Shimony, M.D., Alice Boyd Smith, Lt. Col. U.S.A.F. M.C., Douglas H. Smith, M.D., Victor Sheng-Kwei Song, Ph.D., Barbara B. Sterkel, M.D., Stanislav Svetlov, M.D., Ph.D., Pamela J. VandeVord, Ph.D., Kenneth H. Wong, Ph.D., Jean R. Wrathall, Ph.D., Alan W. Young D.O. P.M.&R., Gregory Zipfel, M.D.
References
- Alexander M. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology. 1995;45:1253–1260. doi: 10.1212/wnl.45.7.1253. [DOI] [PubMed] [Google Scholar]
- Armonda R.A. Bell R.S. Vo A.H. Ling G. DeGraba T.J. Crandall B. Ecklund J. Campbell W.W. Wartime traumatic cerebral vasospasm: recent review of combat casualties. Neurosurgery. 2006;59:1215–1225. doi: 10.1227/01.NEU.0000249190.46033.94. [DOI] [PubMed] [Google Scholar]
- Askenasy J. Association of intracerebral bone fragments and epilepsy in missile head injuries. Acta Neurol. Scand. 1989;79:47–52. doi: 10.1111/j.1600-0404.1989.tb03708.x. [DOI] [PubMed] [Google Scholar]
- Benzinger T. Physiological effects of blast in air and water, Ch. XIV-B, German Aviation Medicine, World War II. Vol. II. U.S. Government Printing Office; Washington, DC: 1950. pp. 1225–1259. [Google Scholar]
- Bergsneider M. Hovda D. Shalmon E. Kelly D. Vespa P. Martin N. Phelps M. McArthur D. Caron M. Kraus J. Becker D. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J. Neurosurg. 1997;86:241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee Y. Neuroscience. Shell shock revisited: solving the puzzle of blast trauma. Science. 2008;319:406–408. doi: 10.1126/science.319.5862.406. [DOI] [PubMed] [Google Scholar]
- Brenner T. Freier M.C. Holshouser B.A. Burley T. Ashwal S. Predicting neuropsychologic outcome after traumatic brain injury in children. Ped. Neurol. 2003;28:104–114. doi: 10.1016/s0887-8994(02)00491-5. [DOI] [PubMed] [Google Scholar]
- Cernak I. Wang Z. Jiang J. Bian X. Savic J. Ultrastructural and functional characteristics of blast injury-induced neurotrauma. J. Trauma. 2001;50:695–706. doi: 10.1097/00005373-200104000-00017. [DOI] [PubMed] [Google Scholar]
- Chavko M. Koller W.A. Prusaczyk W.K. McCarron R.M. Measurement of blast wave by a miniature fiber optic pressure transducer in the rat brain. J. Neurosci. Meth. 2007;159:277–281. doi: 10.1016/j.jneumeth.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Chavko M. Prusaczyk W.K. McCarron R.M. Protection against blast-induced mortality in rats by hemin. J. Trauma. 2008;65:1140–1145. doi: 10.1097/TA.0b013e3181870a8c. [DOI] [PubMed] [Google Scholar]
- Clemedson C.-J. Shockwave transmission to the central nervous system. Acta Physiologica Scand. 1956;37:204–214. doi: 10.1111/j.1748-1716.1956.tb01356.x. [DOI] [PubMed] [Google Scholar]
- Condon B. Oluoch-Olunya D. Hadley D. Teasdale G. Wagstaff A. Early 1H magnetic resonance spectroscopy of acute head injury: four cases. J. Neurotrauma. 1998;15:563–571. doi: 10.1089/neu.1998.15.563. [DOI] [PubMed] [Google Scholar]
- Cooper G. Maynard R. Cross N. Hill J. Casualties from terrorist bombings. J. Trauma. 1983;23:955–967. doi: 10.1097/00005373-198311000-00001. [DOI] [PubMed] [Google Scholar]
- Cripps N.P.J. Cooper G.J. The influence of personal blast protection on the distribution and severity of primary blast gut injury. J. Trauma. 1996;40:S206–S211. doi: 10.1097/00005373-199603001-00045. [DOI] [PubMed] [Google Scholar]
- Culver J. Akers W. Achilefu S. Multimodality molecular imaging with combined optical and SPECT/PET modalities. J. Nucl. Med. 2008;49:169–172. doi: 10.2967/jnumed.107.043331. [DOI] [PubMed] [Google Scholar]
- Curcio C.K. Larriva R.F. Sullivan A. Enhanced blast weapons defensive tactics, techniques, and procedures medical precautions and treatment and threat assessment, CETO Report 004-02, Center for Emerging Threats and Opportunities. Marine Corps Warfighting Laboratory; Quantico, Virginia: 2002. [Google Scholar]
- Daly C.M. Doyle M.E. Radkind M. Raskind E. Daniels C. Clinical case series: the use of prazosin for combat-related recurrent nightmares among Operation Iraqi Freedom combat veterans. Mil. Med. 2005;170:513–515. doi: 10.7205/milmed.170.6.513. [DOI] [PubMed] [Google Scholar]
- DeKosky S.T. Abrahamson E.E. Ciallella J.R. Paljug W.R. Wisniewski S.R. Clark R.S. Ikonomovic M.D. Association of increased cortical soluble abeta42 levels with diffuse plaques after severe brain injury in humans. Arch. Neurol. 2007;64:541–544. doi: 10.1001/archneur.64.4.541. [DOI] [PubMed] [Google Scholar]
- DePalma R.G. Burris D.G. Champion H.R. Hodgson M.J. Blast injuries. N. Engl. J. Med. 2005;352:1335–1342. doi: 10.1056/NEJMra042083. [DOI] [PubMed] [Google Scholar]
- Desaga H. Experimental Investigation of the Blast Effect, Communications from the Domain of Aeromedicine, Aeromedical Research Institute of the German Air Ministry. 1943. Res. Report No. 15.
- DeWitt D.S. Prough D.S. Traumatic cerebral vascular injury: the effects of concussive brain injury on the cerebral vasculature. J. Neurotrauma. 2003;20:795–825. doi: 10.1089/089771503322385755. [DOI] [PubMed] [Google Scholar]
- Diaz-Arrastia R. Gong Y. Fair S. Scott K.D. Garcia M.C. Carlile M.C. Agostini M.A. Van Ness P.C. Increased risk of late posttraumatic seizures associated with inheritance of APOE epsilon4 allele. Arch. Neurol. 2003;60:818–822. doi: 10.1001/archneur.60.6.818. [DOI] [PubMed] [Google Scholar]
- Doukas A.G. McAulliffe D.J. Lee S. Venugopalan V. Flotte T.J. Physical factors involved in stress-wave-induced cell injury: the effect of stress gradient. Ultrasound Med. Biol. 1995;21:961–967. doi: 10.1016/0301-5629(95)00027-o. [DOI] [PubMed] [Google Scholar]
- Draeger R.H. Barr J.S. Dunbar J.Y. Sager W.W. Shelesnyak M.C. A Study of Personnel Injury by ‘Solid Blast’ and the Design and Evaluation of Protective Devices. Report No. 1, Research Project X-517. Naval Medical Research Institute, National Naval Medical Center; Bethesda, Maryland: 1945. [Google Scholar]
- Dusick J.R. Glenn T.C. Lee W.N. Vespa P.M. Kelly D.F. Lee S.M. Hovda D.A. Martin N.A. Increased pentose phosphate pathway flux after clinical traumatic brain injury: a [1,2–13C2]glucose labeling study in humans. J. Cereb. Blood Flow Metab. 2007;27:1593–1602. doi: 10.1038/sj.jcbfm.9600458. [DOI] [PubMed] [Google Scholar]
- Fine J.E. Vinci S.J. Causes of electromagnetic radiation from detonation of conventional explosives: a literature survey, ARL-TR-1690. Army Research Laboratory; Adelphi, Maryland: 1998. [Google Scholar]
- Garner E. Lakes R. Lee T. Swan C. Brand R. Viscoelastic dissipation in compact bone: implications for stress-induced fluid flow in bone. Trans. Amer. Soc. Mech. Eng. 2000;122:166–172. doi: 10.1115/1.429638. [DOI] [PubMed] [Google Scholar]
- Garnett M.R. Blamire A.M. Corkill R.G. Cadoux-Hudson T.A. Rajagopalan B. Styles P. Early proton magnetic resonance spectroscopy in normal-appearing brain correlates with outcome in patients following traumatic brain injury. Brain. 2000a;123:2046–2054. doi: 10.1093/brain/123.10.2046. [DOI] [PubMed] [Google Scholar]
- Garnett M.R. Blamire A.M. Rajagopalan B. Styles P. Cadoux-Hudson T.A. Evidence for cellular damage in normal-appearing white matter correlates with injury severity in patients following traumatic brain injury: A magnetic resonance spectroscopy study. Brain. 2000b;123:1403–1409. doi: 10.1093/brain/123.7.1403. [DOI] [PubMed] [Google Scholar]
- Ghajar J. Ivry R.B. The predictive brain state: timing deficiency in traumatic brain injury? Neurorehab. Neural Repair. 2008;22:217–227. doi: 10.1177/1545968308315600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouw A.A. Seewann A. Vrenken H. van der Flier W.M. Rozemuller J.M. Barkhof F. Scheltens P. Geurts J.J. Heterogeneity of white matter hyperintensities in Alzheimer's disease: post-mortem quantitative MRI and neuropathology. Brain. 2008;131:3286–3298. doi: 10.1093/brain/awn265. [DOI] [PubMed] [Google Scholar]
- Head D. Buckner R.L. Shimony J.S. Williams L.E. Akbudak E. Conturo T.E. McAvoy M. Morris J.C. Snyder A.Z. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb. Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- He B.J. Shulman G.L. Snyder A.Z. Corbetta M. The role of impaired neuronal communication in neurological disorders. Curr. Opin. Neurol. 2007;20:655–660. doi: 10.1097/WCO.0b013e3282f1c720. [DOI] [PubMed] [Google Scholar]
- Hoge C.W. McGurk D. Thomas J.L. Cox A.L. Engel C.C. Castro C.A. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N. Engl. J Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Hughes D.G. Jackson A. Mason D.L. Berry E. Hollis S. Yates D.W. Abnormalities on magnetic resonance imaging seen acutely following mild traumatic brain injury: correlation with neuropsychological tests and delayed recovery. Neuroradiology. 2004;46:550–558. doi: 10.1007/s00234-004-1227-x. [DOI] [PubMed] [Google Scholar]
- Ikonomovic M.D. Uryu K. Abrahamson E.E. Ciallella J.R. Trojanowski J.Q. Lee V.M. Clark R.S. Marion D.W. Wisniewski S.R. DeKosky S.T. Alzheimer's pathology in human temporal cortex surgically excised after severe brain injury. Exp. Neurol. 2004;190:192–203. doi: 10.1016/j.expneurol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Jellinger K.A. Head injury and dementia. Curr. Opin. Neurol. 2004;17:719–723. doi: 10.1097/00019052-200412000-00012. [DOI] [PubMed] [Google Scholar]
- Jordan B.D. Genetic influences on outcome following traumatic brain injury. Neurochem. Res. 2007;32:905–915. doi: 10.1007/s11064-006-9251-3. [DOI] [PubMed] [Google Scholar]
- Kelly B. EMP from Chemical Explosions, Group P-14. Los Alamos National Laboratory; Los Alamos, New Mexico: 1993. [Google Scholar]
- Lee H. Wintermark M. Gean A.D. Ghajar J. Manley G.T. Mukherjee P. Focal lesions in acute mild traumatic brain injury and neurocognitive outcome: CT versus 3T MRI. J. Neurotrauma. 2008;25:1049–1056. doi: 10.1089/neu.2008.0566. [DOI] [PubMed] [Google Scholar]
- Leung L.Y. VandeVord P.J. Dal Cengio A.L. Bir C. Yang K.H. King A.I. Blast related neurotrauma: a review of cellular injury. Mol. Cell Biomech. 2008;5:155–168. [PubMed] [Google Scholar]
- Longhi L. Saatman K.E. Fujimoto S. Raghupathi R. Meaney D.F. Davis J. McMillan B.S.A. Conte V. Laurer H.L. Stein S. Stocchetti N. McIntosh T.K. Temporal window of vulnerability to repetitive experimental concussive brain injury. Neurosurgery. 2005;56:364–374. doi: 10.1227/01.neu.0000149008.73513.44. discussion 364–374. [DOI] [PubMed] [Google Scholar]
- Maas A.I. Stocchetti N. Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- Mac Donald C.L. Dikranian K. Bayly P. Holtzman D. Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J. Neurosci. 2007;27:11869–11876. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh G.S. Engel D.C. Butcher I. Steyerberg E.W. Lu J. Mushkudiani N. Hernandez A.V. Marmarou A. Maas A.I. Murray G.D. Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J. Neurotrauma. 2007;24:287–293. doi: 10.1089/neu.2006.0031. [DOI] [PubMed] [Google Scholar]
- Morrison B. Meaney D.F. Margulies S.S. McIntosh T.K. Dynamic mechanical stretch of organotypic brain slice cultures induces differential genomic expression: relationship to mechanical parameters. J. Biomech. Eng. 2000;122:224–230. doi: 10.1115/1.429650. [DOI] [PubMed] [Google Scholar]
- Nelson T.J. Wall D.B. Stedje-Larsen E.T. Clark R.T. Chambers L.W. Bohman H.R. Predictors of mortality in close proximity blast injuries during Operation Iraqi Freedom. J. Am. Coll. Surg. 2006;202:418–422. doi: 10.1016/j.jamcollsurg.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Niogi S.N. Mukherjee P. Ghajar J. Johnson C.E. Kolster R. Lee H. Suh M. Zimmerman R.D. Manley G.T. Candliss B.D. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008a;131:3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- Niogi S.N. Mukherjee P. Ghajar J. Johnson C. Kolster R.A. Sarkar R. Lee H. Meeker M. Zimmerman R.D. Manley G.T. McCandliss B.D. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. Am. J. Neuroradiol. 2008b;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okie S. Traumatic brain injury in the war zone. N. Engl. J. Med. 2005;352:2043–2047. doi: 10.1056/NEJMp058102. [DOI] [PubMed] [Google Scholar]
- Parizel P.M. Van Goethem J.W. Ozsarlak O. Maes M. Phillips C.D. New developments in the neuroradiological diagnosis of craniocerebral trauma. Europ. Radiol. 2005;15:569–581. doi: 10.1007/s00330-004-2558-z. [DOI] [PubMed] [Google Scholar]
- Peles E. Barell V. Boyko V. Ziv A. Kaplan G. Traumatic brain injury—the National Trauma Registry. Harefuah. 2001;140:381–385. [PubMed] [Google Scholar]
- Phillips Y. Richmond D. Primary blast injury and basic research: a brief history, in: Textbook of Military Medicine: Conventional Warfare: Ballistic, Blast and Burn Injuries. In: Bellamy R.F., editor; Zajtchuck R., editor. Borden Institute; Washington, D.C.: 1990. pp. 221–240. [Google Scholar]
- Raskind M.A. Peskind E.R. Hoff D.J. Hart K.L. Holmes H.A. Warren D. Shofer J. O'Connell J. Taylor F. Gross C. Rohde K. McFall M.E. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol. Psychiat. 2007;61:928–934. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Richmond D.R. Jenssen A. Compendium of the biological effects of complex blast waves. Prepared for the Norwegian Defence Construction Service and HQ Defence Command, Norway Joint Medical Service 1992.
- Richmond D.R. Taborelli R.V. Sherping F. Wetherbe M.B. Sanchez R.T. Goldizen V.C. White C.S. Shock tube studies of the effects of sharp-rising, long-duration overpressures on biological systems. USAEC Technical Report TID-6056, Office of Technical Services, Department of Commerce; Washington D.C: 1959. [Google Scholar]
- Saatman K.E. Duhaime A.C. Bullock R. Maas A.I. Valadka A. Manley G.T. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer K. Wheeler-Kingshott C.A. Boulby P.A. Scaravilli F. Altmann D.R. Barker G.J. Tofts P.S. Miller D.H. Diffusion tensor imaging of post mortem multiple sclerosis brain. Neuroimage. 2007;35:467–477. doi: 10.1016/j.neuroimage.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz I. Tsenter J. Shochina M. Shiri S. Kedary M. Katz-Leurer M. Meiner Z. Rehabilitation outcomes of terror victims with multiple traumas. Arch. Phys. Med. Rehabil. 2007;88:440–448. doi: 10.1016/j.apmr.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Shein N.A. Horowitz M. Shohami E. Heat acclimation: a unique model of physiologically mediated global preconditioning against traumatic brain injury. Prog. Brain Res. 2007;161:353–363. doi: 10.1016/S0079-6123(06)61025-X. [DOI] [PubMed] [Google Scholar]
- Sternberg H.M. Hurwitz H. Calculated spherical shock waves produced by condensed explosives in air and water. Proc. Sixth Int'l. Symp. Detonation. Coronado, California: 1976. [Aug 24–27;]. pp. 528–539. [Google Scholar]
- Stuhmiller J. Blast injury: translating research into operational medicine. In: Santee W., editor; Friedl K., editor. U.S. Army Medical Research and Materiel Command. Ft. Detrick; Maryland: 2008. pp. 1–36. [Google Scholar]
- Tanielian T. Jaycox L.H. Invisible wounds of war: psychological and cognitive injuries, their consequences, and services to assist recovery. RAND. 2008. http://www.rand.org/pubs/monographs/MG720/ http://www.rand.org/pubs/monographs/MG720/
- Teasdale G.M. Murray G.D. Nicoll J.A. The association between APOE epsilon4, age and outcome after head injury: A prospective cohort study. Brain. 2005;128:2556–2561. doi: 10.1093/brain/awh595. [DOI] [PubMed] [Google Scholar]
- Thali M.J. Jackowski C. Oesterhelweg L. Ross S.G. Dirnhofer R. VIRTOPSY—the Swiss virtual autopsy approach. Leg. Med. (Tokyo) 2007;9:100–104. doi: 10.1016/j.legalmed.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Toth C. Fletcher W.A. Autonomic disorders and the eye. J. Neuroophthalmol. 2005;25:1–4. doi: 10.1097/00041327-200503000-00001. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R. Signoretti S. Tavazzi B. Floris R. Ludovici A. Marziali S. Tarascio G. Amorini A.M. Di Pietro V. Delfini R. Lazzarino G. Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athletes—part III. Neurosurgery. 2008;62:1286–1295. doi: 10.1227/01.neu.0000333300.34189.74. discussion 1295–1286. [DOI] [PubMed] [Google Scholar]
- Vawter D.L. Fung Y.C. West J.B. Elasticity of excised dog lung parenchyma. J. Appl. Physiol. 1978;45:261–269. doi: 10.1152/jappl.1978.45.2.261. [DOI] [PubMed] [Google Scholar]
- Viano D.C. Lau I.V. A viscous tolerance criterion for soft tissue injury assessment. J. Biomechanics. 1988;21:387–399. doi: 10.1016/0021-9290(88)90145-5. [DOI] [PubMed] [Google Scholar]
- Warden D. Military TBI during the Iraq and Afghanistan wars. J. Head Trauma Rehabil. 2006;21:398–402. doi: 10.1097/00001199-200609000-00004. [DOI] [PubMed] [Google Scholar]
- Weber J.T. Experimental models of repetitive brain injuries. Prog. Brain Res. 2007;161:253–261. doi: 10.1016/S0079-6123(06)61018-2. [DOI] [PubMed] [Google Scholar]
- White C.S. Biological effects of blast. Technical Progress Report No. DASA 1271, Defense Atomic Support Agency, Department of Defense; Washington D.C: 1961. [Google Scholar]
- White C.S. Bowen I.G. Richmond D.R. A comparative analysis of some of the immediate environmental effects at Hiroshima and Nagasaki. Health Physics. 1964;10:89–150. doi: 10.1097/00004032-196403000-00001. [DOI] [PubMed] [Google Scholar]
- White C.S. Bowen I.G. Richmond D.R. Biological tolerance to air blast and related biomedical criteria, CEX-65.4, Civil Effects Test Operations, U.S. Atomic Energy Commission. 1965. p. 20. [DOI] [PubMed]
- Yoshino A. Hovda D.A. Kawamata T. Katayama Y. Becker D.P. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561:106–119. doi: 10.1016/0006-8993(91)90755-k. [DOI] [PubMed] [Google Scholar]
- Young M. Mechanics of blast injury. War Med. 1945;8:73. [Google Scholar]
- Zuckerman S. Experimental study of blast injuries to the lungs. Lancet. 1940;ii:219–224. [Google Scholar]