Abstract
Acute elevation in intracellular calcium ([Ca2+]i) following traumatic brain injury (TBI) can trigger cellular mechanisms leading to neuronal dysfunction and death. The mechanisms underlying these processes are not completely understood, but calcium influx through N-type voltage-gated calcium channels (VGCCs) appears to play a central role. The present study examined the time course of [Ca2+]i flux, glutamate release, and loss of cell viability following injury using an in vitro neuronal–glial cortical cell-culture model of TBI. The effects of N-channel blockade with SNX-185 (e.g. ω-conotoxin TVIA) before or after injury were also examined. Neuronal injury produced a transient elevation in [Ca2+]i, increased glutamate release, and resulted in neuronal and glial death. SNX-185 administered before or immediately after cell injury reduced glutamate release and increased the survival of neurons and astrocytes, whereas delayed treatment did not improve cell survival but significantly facilitated the return of [Ca2+]i to baseline levels. The new findings that N-type VGCCs are critically involved in injury-induced glutamate release and recovery of [Ca2+]i argue for continued investigation of this treatment strategy for the clinical management of TBI. In particular, SNX-185 may represent an effective class of drugs that can significantly protect injured neurons from the secondary insults that commonly occur after TBI.
Key words: in vitro, neuroprotection, N-type VGCC, SNX-185, traumatic brain injury
Introduction
Acute elevation of intracellular neuronal calcium ([Ca2+]i) is a key mediator of cell damage and death following traumatic brain injury (TBI), and in vitro models have advanced our understanding of the magnitude and time course of calcium elevation in injured neurons (Weber et al., 1999). Multiple pathways are likely involved in triggering elevated [Ca2+]i following injury, including depolarization-initiated opening of voltage-gated calcium channels (VGCCs), influx through the membrane pores that form after injury (Slemmer et al., 2002), activation of glutamate receptor-mediated calcium influx pathways (Ahmed et al., 2002; Weber, 2004; Weber et al., 1999), and activation of store-operated/second-messenger-operated calcium channels that respond to depleted intracellular calcium stores (Weber et al., 2001). Injured neurons also experience decreased cellular ATP from ischemia, as well as mitochondrial damage due to calcium accumulation (Ahmed et al., 2000; Schinder et al., 1996; Verweij et al., 2000; Young, 1992), leading to compromised sodium–potassium ATPase activity with resultant cell depolarization (Tavalin et al., 1995, 1997). Coupled with stretch-induced delayed depolarization, changes in membrane potential promote the opening of tetrodotoxin-sensitive sodium channels (Iwata et al., 2004; Wolf et al., 2001b), reversal of sodium–calcium exchangers (Wolf et al., 2001b), and activation of voltage-sensitive calcium channels (Vacher et al., 2008). These findings highlight the complexity of post-traumatic accumulation of [Ca2+]i detected following in vitro mechanical cell injury.
N-type VGCCs play a critical role in many developmental and pathological events in neurons, and have been studied in models of ischemia (Bowersox et al., 1997; Colbourne et al., 1999; Perez-Pinzon et al., 1997), chronic pain (Bowersox et al., 1996; Staats et al., 2004), and TBI (Hellmich et al., 2007; Lee et al., 2004; Samii et al., 1999). Pre-clinical studies carried out in vivo in animal models of TBI report that selective blockade of N-type VGCCs results in decreased calcium accumulation in areas of TBI (Hovda et al., 1994; Samii et al., 1999), preservation of mitochondrial function (Verweij et al., 1997, 2000), and improved behavioral outcome when administered after TBI in rats (Berman et al., 2000) The cellular mechanisms by which N-type VGCC blockers produce these effects have not been systematically examined in an in vitro model of TBI. A clinical trial of systemic, intravenous administration of the N-type VGCC blocker SNX-111 was initiated but not completed (Muizelaar et al., unpublished data), and, as a result, interest in this neuroprotective strategy has waned. In order to revisit N-type VGCCs as potential targets for therapy, a better understanding of their cellular mechanisms of action is needed. Therefore, the present series of experiments examined the effects of the selective N-type VGCC blocker SNX-185 on calcium influx and recovery, injury-induced glutamate release, and neuronal survival using a previously established in vitro stretch-injury model of TBI in mixed neuron–astrocyte cortical cell cultures (Weber et al., 1999).
SNX-185 is a synthetic version of the ω-conotoxin TVIA, and is a highly selective N-type VGCC blocker (Wang et al., 1998) that demonstrates improved bioavailability and more prolonged persistence in brain tissue than SNX-111 (Newcomb et al., 2000). Neuroprotective effects of SNX-185 in TBI have been demonstrated in vivo (Lee et al., 2004) via direct injection into the hippocampus following TBI in rats, but the cellular mechanisms of neuroprotection have not been investigated at the cellular level using in vitro models of TBI. In this report, we studied the effects of SNX-185 on acute calcium elevation, calcium recovery dynamics, and subsequent cell survival following mechanical rapid stretch injury in vitro. A novel in vitro microdialysis procedure was used to characterize glutamate release, as well as the effects of SNX-185 on glutamate release following in vitro cell injury.
Methods
All experiments were performed in accordance with the animal-welfare guidelines set forth in the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, Washington, DC, 1996). All procedures were performed in compliance with a protocol approved by the UC Davis Institutional Animal Care and Use Committee.
Cell culture
Astrocyte cultures were prepared from 1–2-day-old rat pups (Harlan, Indianapolis, IN). Pups were anesthetized with isoflurane and decapitated. The brains were removed, cortices were isolated, cleaned of white matter and meninges, and then sharply minced and incubated for 60 min in Hank's buffered saline solution containing 2 mg/mL papain (Worthington Biochemical Corp., Lakewood, NJ). The resultant cell suspension was strained using mesh strainers (BD Biosciences, San Jose, CA), diluted to 1.5 × 106 cells/mL in astrocyte feed media (Dulbecco's Minimum Essential Medium, DMEM), and supplemented with 10% heat-inactivated fetal calf serum with 2 mM glutamine and 100 U/mL penicillin + 100 μg/mL streptomycin sulfate. Cells were seeded into 75 cm2 flasks (BD Biosciences, San Jose, CA) and cultured to confluence (approximately 2 weeks) with a warm (37°C) astrocyte feed media that was changed every 2–3 days.
Once confluent, flasks were treated with 0.25% trypsin/0.02 EDTA in saline and incubated with 10 mL DMEM for 10 min to resuspend the cells. Cells were then washed and diluted to 1.0 × 106 cells/mL in an astrocyte feed media and seeded onto 25-mm diameter, 1 mL/well, collagen-coated Flexplate wells (Flexcell International Corp., Hillsborough, NC). Astrocyte cultures were grown on Flexplates for 1 week with a warm astrocyte feed media changed every 2–3 days (full volume exchange).
To generate mixed neuronal/astrocyte cultures, a second culture was prepared from cortices of 1–2-day-old rat pups using the dissection techniques described above. The resultant cortical cell suspension was diluted to 1.0 × 106 cells/mL and seeded onto 1-week-old confluent astrocyte cultures in 25-mm diameter Flexplate wells. One day after plating, the cell-culture media was changed to a neuronal feed media. This media consisted of Minimum Essential Media (MEM) supplemented with 5% horse serum, 20 mM glucose (1.20%), 100 U/mL penicillin + 100 μg/mL streptomycin sulfate, and 1% N2 supplement, which consists of 1.0 mM human transferrin, 0.0861 mM recombinant full-chain insulin, 1.0 mM progesterone, 1.0 mM putrescine, and 1.0 mM selenite (Invitrogen Corp., Carlsbad, CA). Each well was also treated with 3 μL 5-fluoro-2-deoxyuridine (FUDR), resulting in ∼0.0175 mM fluorodeoxyuridine and ∼0.0375 mM uridine in the media. FUDR is an antimitotic agent that promotes the development of morphologically complex neurons and inhibits proliferation of non-neuronal cells including reactive astrocytes (Meberg and Miller, 2003; Oorschot, 1989). The use of FUDR, coupled with the addition of N2 neuronal supplement, optimized our yield of morphologically complex neurons with multiple, branching neurites. Four to five days after this media change, half the media (0.5 mL) was very gently aspirated and replaced with 1 mL of warm (37°C) neuronal feed media. The media was again changed 1 week later using gentle aspiration and replacement of only half of the media to minimize osmotic and mechanical insults to the cell cultures. The neurons used for these experiments were 9–15 days in vitro (DIV) and demonstrated complex morphologies under light microscopy (large soma, neurites with >2 degrees branching). There was also the appearance of physical connections with other neurons and glia, expression of the mature neuronal protein NeuN, and the lack of expression of the embryonic neuronal protein nestin.
Calcium imaging
Intracellular calcium ([Ca2+]i) was measured using the ratiometric calcium indicator Fura-2-AM (5 μM) (Chemicon Intl., Temecula, CA). Fura-2-AM was dissolved in DMSO (Sigma Aldridge, St. Louis, MO) and diluted in hepes-buffered saline (pH = 7.4) supplemented with 1% fatty-acid-free bovine serum albumin (Fisher Scientific, Hampton, NH). To load Fura-2 preferentially into neurons and not astrocytes, mixed neuronal–glial cultures were incubated in the loading solution for 60 min at 37°C in the dark. This preferential loading procedure described by Weber and associates (1999) results in high concentrations of Fura-2 in neurons, whereas astrocytes in the co-culture rapidly metabolize and therefore do not retain the dye. After loading, the solution was replaced with Dulbecco's Phosphate-Buffered Saline (0.901 mM CaCl2, 0.493 mM MgCl2–6H2O, 2.67 mM KCl, 1.47 KH2PO4, 137.93 mM NaCl, and 8.06 mM Na2HPO4–7H2O, pH = 7.2–7.4; Invitrogen Corp., Carlsbad, CA) supplemented with 20 mM glucose and imaged at room temperature.
Intracellular imaging was conducted on a high-speed imaging system using a Polychrome IV scanning monochrometer (Till Photonics, Grafelfing, Germany). Light was delivered via fiber optics through the epifluorescence port of a Nikon E600 (Nikon, Melville, NY) microscope coupled to a Nikon Fluor 40× immersion lens (NA 0.75; WD 0.72 mm). The detector was an Orca II-ER CCD digital camera (Hamamatsu USA, Bridgewater, NJ), which was computer controlled by C-Imaging Simple PCI software (Compix, Cranberry Township, PA). Cells were imaged at a rate of one image every 3 sec, and ratios of Fura-2 bound to calcium (340 nm) and free Fura-2 (380 nm) were calculated using the C-Imaging ratioing software. Differential interference contrast and ratioed calcium images were overlaid to achieve a precise map of the calcium dynamics in the field. Between 1–10 neurons and 15–25 astrocytes (which formed an underlying confluent “bed” of cells) were typically observed in the imaged field at a magnification of 400 ×.
All calcium measurements were performed in a field at the center of the well that contained 1–10 morphologically mature neurons. Specific regions of interest (ROIs) were drawn for the nucleus, soma, and proximal extensions of neurons in mixed cultures using the imaging software. The stretch-induced rise and recovery in these subcellular regions did not significantly differ from one another, however, so data analyzed are presented from ROIs drawn to include the neuronal soma. To minimize the effects of residual Fura-2 signal emanating from the astrocyte monolayer, a ROI was drawn over an area of the astrocyte monolayer devoid of neurons and imaged to provide a measure of background fluorescence. This background fluorescence was then subtracted from the neuronal calcium fluorescence. The combination of neuronal specific loading of Fura-2 at 37°C and subtraction of background fluorescence provided relatively specific measurements of intracellular free calcium restricted to neurons following mechanical injury.
Injury model
Mixed neuronal–glial cultures were injured using a Model 94A Cell Injury Controller (Custom Design and Fabrication, Virginia Commonwealth University, Richmond, VA) (Ellis et al., 1995). This apparatus delivered a brief, 50-msec pulse of compressed air into the cell-culture well, which bowed out the 2-mm thick silastic membrane of the Flexplate, resulting in a biaxial stretch injury to the adherent astrocytes and neurons. The pressure of the compressed air pulse was calibrated to produce 5.5, 6.5, or 7.5 mm of vertical displacement corresponding to 31, 38, and 54% membrane stretch respectively, based on previous calibration measurements (Ellis et al., 1995).
After recording stable resting intracellular calcium levels in the ROIs, the Flexplates were quickly removed from the microscope stage, and cells were stretch injured at a mild (i.e. 5.5 mm), moderate (i.e. 6.5 mm), or severe (i.e. 7.5 mm) injury/stretch magnitude. The Flexplates were then immediately returned to the microscope stage, and the identical cells were repositioned under the microscope objective and imaged for intracellular calcium levels. This procedure, from cell injury to return to imaging, required an elapsed time of approximately 30–45 sec. Post-injury calcium levels were measured for 15 min after injury and compared to pre-injury baseline levels.
To quantify changes in intracellular calcium over 24 h following stretch injury, intracellular calcium levels were also measured at 90 min, 3 h, 6 h, or 24 h after injury. This was done by stretch injuring neurons in a neuronal feed media without Fura-2-AM loading, and returning the cultures to an incubator for 90 min, 3 h, 6 h, or 24 h delays until imaging. Cells were then loaded with Fura-2 in DPBS, at one of these three time points described above, and intracellular calcium levels were imaged as above. Since the same neuron(s) could not be followed using this technique, population averages were calculated for each time point to monitor calcium recovery (Weber et al., 1999).
Glutamate experiments
Glutamate-induced changes in [Ca2+]i were measured in Fura-2 loaded neurons treated with 5 mM glutamate (with 25 μM glycine). This experiment was performed in neurons that were pretreated with either SNX-185 (1 μM) or a vehicle control 5 min prior to stretch injury.
Glutamate release into the cell-culture feed media following stretch injury was also measured by microdialysis. Briefly, microdialysis probes (1 mm, CMA/12; CMA/Microdialysis AB, Stockholm, Sweden) were passed through small drill holes in the Flexplate covers and positioned directly above the confluent cell cultures growing on silastic membranes (Fig. 1). The dialysis membrane was completely submerged in the cell-culture media when the Flexplate cover was in place. Injury-induced glutamate release was studied by delivering severe stretch injury to wells pretreated with 1 μM SNX-185 or the vehicle control. These experiments were performed in the presence and absence of the glutamate transporter inhibitor DL-threo-beta-benzyloxy-aspartate (TBOA, 200 μM). Control measurements were also performed in phosphate buffer, in uninjured cultures, and in wells depolarized with 5M KCl.
FIG. 1.
Microdialysis sampling apparatus. Flexcell® culture plates were modified as shown to position three 1-mm microdialysis probes in the feed media directly above the cell cultures.
Dialysis sampling was performed at a rate of 2.5 μL/min to collect three samples of 25 μL from each well (10 min per sample). Microdialysis was initiated 10 min prior to injury and treatment, resulting in one sample before stretch injury and two samples collected at 10 and 20 min after stretch injury. Dialysates were then frozen, and glutamate levels were later analyzed by HPLC as described previously (Zhong et al., 2006).
VGCC blockade
Stock solutions (0.1 mM) of the highly selective N-type VGCC blocker SNX-185 were dissolved in double-distilled water, divided into aliquots, and frozen at −80°C until use. Working solutions were prepared immediately before use. The drug was delivered directly into tissue-culture wells using a micropipette at concentrations calculated to be within the range of those shown to be neuroprotective in a previous in vivo TBI study (Lee et al., 2004). SNX-185 was generously provided by George Miljanich of Elan Pharmaceuticals (San Francisco, CA).
Histopathology
Preliminary studies examined mixed neuronal–glial cell cultures at 6, 9, 12, 15, and 18 DIV to determine optimal cell morphology for the calcium imaging experiments, as well as to establish the presence or absence of specific cellular markers in the mixed neuronal/astrocyte cultures. Specifically, culture wells were examined for the presence of the following characteristics: (1) confluent astrocyte layer, (2) neurite extensions with at least two degrees of branching, (3) cells that expressed NeuN but not nestin as a marker of mature neurons, (4) neurons and astrocytes that expressed N-type VGCCs α1B subunits, and (5) cells that stained with anti-GFAP antibody (astrocytes). These criteria were reached by 9 DIV, and all experiments were subsequently performed on cell cultures between 9 and 15 DIV. The presence of GABAergic (using anti-GAD67 and anti-GABA antibodies) and glutamatergic (using anti-glutamate antibodies) cell populations within the cultures was also examined.
Cultures were fixed at time points of interest in 4% paraformaldehyde at room temperature for 10 min. Cells were then stored in 0.1M PBS at 4°C until staining. For staining, cells underwent 3 × wash in 0.1M PBS and were permeabilized with 0.5% Triton X-100 in PBS for 5 min. Cells were then blocked with 10% horse serum (Invitrogen Corp., Carlsbad, CA) in PBS for 1 h at room temperature prior to incubation in primary antibodies at 4°C overnight. Primary antibodies included mouse anti-nestin (1:1000; Chemicon Intl, Temecula, CA), mouse anti-NeuN (1:500; Chemicon Intl, Temecula, CA), mouse anti-MAP-2 (1:8000; Sigma, St. Louis, MO), rabbit anti-glutamate (1:500; Chemicon Intl, Temecula, CA), mouse anti-GAD67 (1:1000; Chemicon Intl, Temecula, CA), rabbit anti-VGCC-α1B (1:2400; Chemicon Intl, Temecula, CA), and rabbit anti-glial fibrillary acidic protein (1:2000; DAKO, Carpinteria, CA). After 3× wash in PBS, cells were incubated with secondary antibodies at 4°C for 1 h, then washed and transferred to 0.1M PBS for microscopic evaluation. Secondary antibodies included Alexa Fluor® 633-conjuated goat anti-mouse (1:1000; Molecular Probes, Carlsbad, CA), Alexa Fluor 488-conjugated goat anti-mouse (1:1000; Molecular Probes, Carlsbad, CA), and FITC-conjugated swine anti-rabbit (1:500, DAKO, Carpinteria, CA). Wells were visualized on a Zeiss 510 confocal microscope (Carl Zeiss, Inc., Thornwood, NJ) using a 40× immersion objective (total magnification 400 ×), and histologic imaging was performed on cells located near the center of the tissue-culture well.
Cell-survival assessment
Surviving cells were labeled and quantified using carboxyfluorescein diacetate (CFDA), and dead and dying cells were identified using propidium iodide (PrI). Since some damaged neurons can regain their ability to exclude PrI in the first few hours after mechanical injury (Weber et al., 1999), all cell-death assessments were performed 24 h after injury. Wells were treated with 1% CFDA and 1% PrI for 15 min in the neuronal feed media, then washed in 0.1M PBS and transferred to 0.1M PBS supplemented with 20 mM glucose (1.20%). Cell counts were performed on a Nikon E600 microscope equipped with epifluorescence using a 40 × water immersion objective (total magnification 400 ×) in five fields per well. Cells were initially brought into focus, and then, using a motorized stage controller (MS-2000; Applied Scientific Instruments, Eugene, OR), fields were chosen near the well center (approximately 250 μm × 250 μm). The microscope field used for analysis was brought under the objective in a “blinded” fashion by moving the joystick of the motorized stage to a position near the center of the well and then to four other points approximately 1 mm in either direction along the x- and y-axes.
Statistical analysis
Individual Flexplate wells were randomly assigned to an injury condition. Each set of experimental images was saved under a filename based on the date and experiment number, without directly indicating the injury severity or treatment group, so that data analysis was performed in a blinded fashion. Statistical evaluation was performed by analysis of variance using SPSS software version 13.0 (SPSS, Inc., Chicago, IL), followed by appropriate posthoc analyses when indicated. The minimum level for statistical significance was set at p < 0.05. All experiments were performed in a minimum of three culture dish wells per injury and drug-treatment condition.
Results
Immunocytochemistry characterization of cortical neurons in culture
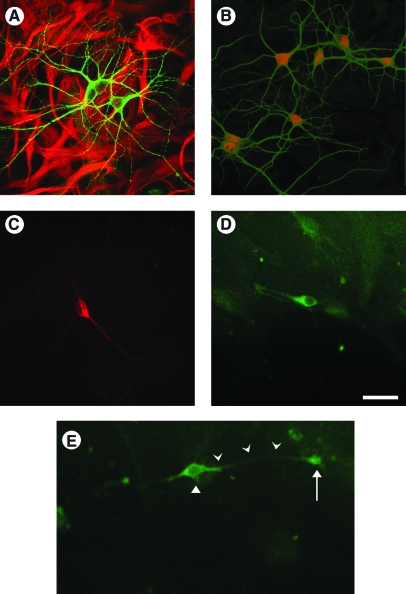
As shown in Figure 2A, mixed neuronal/astrocyte cultures at least 9 DIV demonstrated morphologically complex neurons that stained for MAP2 (green, Alexa Fluor 488) over a confluent bed of astrocytes stained for GFAP (red, Alexa Fluor 633). Neurons also expressed NeuN (red, Fig. 2B), a marker of mature neurons, and did not stain with nestin, a marker of neuronal immaturity. Some neurons also stained with antibodies to glutamate (Fig. 2C) and GAD-67 (Fig. 2D), indicating the presence of glutamatergic and GABAergic populations respectively, in the mixed neuronal/astrocyte cultures by 9 DIV. Neurons grown in mixed neuronal/astrocytes cultures expressed N-type VGCC α1B-subunits on the soma in a perinuclear fashion, along neuronal extensions, and at the terminal ends of large neuronal projections (Fig. 2E). This staining pattern was observed in nearly all neurons in the culture and did not differ among neurons with different morphologies. Astrocytes forming the underlying confluent cell bed also expressed α1B-subunits of N-type VGCCs (data not shown).
FIG. 2.
Histologic characteristics of mixed neuronal–glial cell cultures. Mixed neuronal–glial cultures demonstrated morphologically complex neurons that stained for MAP2 (A, green) overlying a confluent bed of astrocytes that stained for GFAP (A, red). Neurons stained intensely for the mature neuronal marker NeuN (B), showed evidence of both glutamatergic (C) and GABAergic (D) cell populations. Neurons demonstrated N-type VGCCs on the soma in a perinuclear fashion, along cell extensions, and at the terminal end of an extension (D). Images were obtained from cultures at 10–12 DIV. Scale bar = 50 μm.
Injury-induced calcium rise and neuronal injury
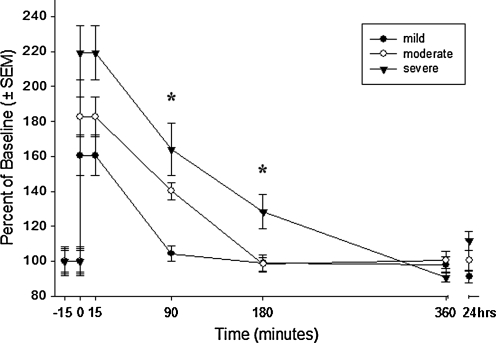
Selective loading of Fura-2 into neurons and not astrocytes allowed for targeted measurements of neuronal intracellular calcium levels. Figure 3 shows intracellular calcium levels ([Ca2+]i), expressed as a percentage of the baseline for up to 24 h following three magnitudes of stretch injury. Stretch injury resulted in a magnitude-dependent rapid rise in [Ca2+]i that slowly returned to the baseline in 90, 180, and 360 min after mild, moderate, and severe injury respectively. Reduced neuronal survival (Fig. 4A), increased neuronal cell death (Fig. 4B), and increased astrocyte cell death (Fig. 4C) were significantly associated with increasing injury severity.
FIG. 3.
Intracellular calcium rise and recovery in neurons. Neurons grown in mixed neuronal–glial cell cultures were loaded with Fura-2 and monitored continuously from 5 min before until 15 min after mild, moderate, or severe injury. Separate wells underwent injury in the feed media, were returned to a cell incubator for recovery, and were loaded with Fura-2 in a delayed fashion to measure calcium levels at 90, 180, 360, and 24 h after injury. Population averages at each time point were calculated to generate calcium recovery plots. Neurons that experienced mild injury demonstrated near-complete calcium recovery at 90 min, while moderate and severely injured neurons fully recovered at 180 and 360 min respectively. Each data point represents the average of 11–32 neurons. *Statistically significant difference at p < 0.05.
FIG. 4.
Cell-survival analysis following stretch injury of cortical neurons and astrocytes. Mixed neuronal/astrocyte cell cultures underwent varying severities of stretch injury and were returned to a cell incubator for 24 h. Cells were then loaded with carboxyfluorescein diacetate to stain surviving cells and propidium iodide to stain non-viable cells, and counted in five high-power fields (hpf, 400 ×) per well. Each bar represents average cell counts from six wells (30-well field counts). Increasing injury severity resulted in a significant reduction in the number of surviving neurons (A, p < 0.0001) and a significant increase in the number of non-viable neurons (B, p < 0.0001). Increasing injury severity also resulted in a significant increase in the number of non-viable astrocytes (C, p < 0.05). All wells were maintained in the cell-culture media before and after injury.
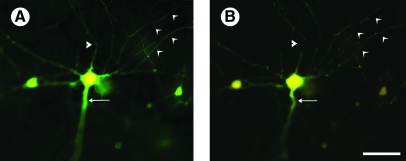
Figure 5 shows a Fura-2-loaded neuron before (Fig. 5A) and 15 min after (Fig. 5B) severe stretch injury, demonstrating the physical transection of neurites, recoil and tortuosity of proximal large neurites, and irregularity of smaller distal neurites following injury. This appearance of neurites is similar to axonal damage following uniaxial stretch described previously by Smith and associates (1999)
FIG. 5.
Effect of severe stretch injury on cellular morphology. Neurons generated in mixed neuronal–glial cortical cultures were loaded with Fura-2 prior to mechanical injury (A). After severe (54%) stretch injury (B), neurons demonstrated physical transection of some cellular extensions (triangular arrowhead), recoil and tortuosity of axonal extensions (arrow), and irregularity of smaller dendritic extensions (concave arrowheads). Images obtained from culture at 11 DIV. Scale bar = 50 μm.
Pre- and post-injury treatment with SNX-185: Calcium measurements
In the first set of experiments, Fura-2-loaded neurons were treated with 100 nM or 1000 nM SNX-185 or the vehicle control 5 min prior to stretch injury (Fig. 6A). The addition of SNX-185 or the vehicle control 5 min before injury did not affect pre-injury resting calcium levels in neurons (data not shown). Vehicle-treated neurons experienced a large and rapid increase in [Ca2+]i that was directly related to injury severity. In contrast, pre-injury treatment with SNX-185 completely prevented the injury-induced calcium rise under all three magnitudes of injury compared to vehicle controls (p < 0.0001). Calcium levels imaged prior to injury and after drug treatment were relatively stable in the absence of injury, and even after injury the elevated [Ca2+]i did not show marked fluctuations during the 15-min period of post-injury imaging (data not shown).
FIG. 6.
Effect of SNX-185 on the injury-induced calcium rise in cortical neurons. Neurons grown in mixed neuronal–glial cell cultures were treated with 100 or 1000 nM SNX-185 5 min prior to (A) or immediately after (B) stretch injury. SNX-185, at either concentration, significantly reduced/prevented an injury-induced cytosolic calcium rise when given prior to or immediately after mild, moderate, or severe stretch injury (p < 0.0001, n = 6). Neurons grown in mixed neuronal–glial cell cultures were treated with 100 nM or 1000 nM SNX-185 5 min after stretch injury (C). Delayed treatment had no significant acute effect on elevated post-injury calcium levels (n = 4–6, p > 0.05).
In the next set of experiments, SNX-185 was delivered immediately after (i.e. <5 sec) stretch injury to Fura-2-loaded neurons (Fig. 6B). As with pretreatment, delivery of SNX-185 immediately after injury at 100 nM and 1000 nM completely prevented injury-induced calcium rise in all injury-severity groups (p < 0.001). Calcium levels prior to injury and after injury-plus-drug treatment were stable during the 15-min post-injury imaging period.
In the third set of experiments (Fig. 6C), SNX-185 was delivered to Fura-2-loaded neurons 5 min after stretch injury. In contrast to pre- and immediate post-injury experiments, delayed delivery of the drug did not significantly alter the rise in post-injury intracellular [Ca2+]i levels during the 15-min monitoring period (Fig. 6C, p > 0.05). As above, calcium levels prior to injury and those reached after injury and after drug treatment were stable and did not fluctuate significantly during the 15-min post-injury imaging period.
Data presented earlier in Figure 3 showed that the increase in [Ca2+]i after stretch injury returned to near baseline levels by 180 min following mild or moderate injury severity, and by 360 min following severe injury. Therefore, we examined the effects of 5 min delayed treatment with SNX-185 on [Ca2+]i levels after injury using similar methods. Since pre-treatment and immediate post-injury treatment almost completely prevented the injury-induced rise of intracellular calcium, only the effects of delayed post-injury treatment with SNX-185 on calcium recovery were investigated in stretch-injured neurons. In addition, because 100 nM and 1000 nM SNX-185 appeared to be approximately equally effective in reducing injury-induced [Ca2+]i elevation, only the 1000 nM concentration was examined for its effects on the recovery of calcium levels. Cells underwent mild, moderate, or severe stretch injury in the cell-culture media, followed 5 min later by treatment with 1000 nM SNX-185. At 90 min after mild injury (Fig. 7A), delayed treatment with 1000 nM SNX-185 did not significantly affect the increase in [Ca2+]i levels (110.61 ± 3.94% [n = 26] vs 104.34 ± 4.20% [n = 32] in untreated neurons [p > 0.05]). However, following moderate injury, neurons treated with SNX-185 5 min following injury (Fig. 7B) demonstrated significantly lower [Ca2+]i at 90 min compared to untreated controls (107.19 ± 4.69% [n = 27] vs 140.17 ± 4.88% [n = 30], p < 0.0001). A similar effect was observed in neurons treated with SNX-185 after severe stretch injury (Fig. 7C). Specifically, intracellular calcium levels at 90 and 180 min were 105.98 ± 3.84 (n = 25) and 99.91 ± 4.34% (n = 28) respectively, while corresponding values in untreated neurons were significantly higher at 163.94 ± 15.05% (n = 11, p < 0.0001) and 128.31 ± 9.69% (n = 32, p < 0.05). Under all injury-severity conditions, [Ca2+]i levels in treated cells 6 and 24 h after stretch injury were approximately at baseline levels similar to those recorded for uninjured control cells (p > 0.05).
FIG. 7.
Effect of delayed SNX-185 treatment on calcium recovery in cortical neurons following stretch injury. Neurons grown in mixed neuronal–glial cell cultures were treated with 1000 nM SNX-185 5 min after mild (A), moderate (B), or severe (C) stretch injury. Delayed treatment significantly enhanced calcium recovery in moderate injury at 90 min (p < 0.0001) and severe injury at 90 and 180 min (p < 0.0001 and <0.05). Each date point represents the average of 8–18 neurons. *Statistically significant difference at p < 0.05.
Post-injury treatment with SNX-185: Cell death/survival
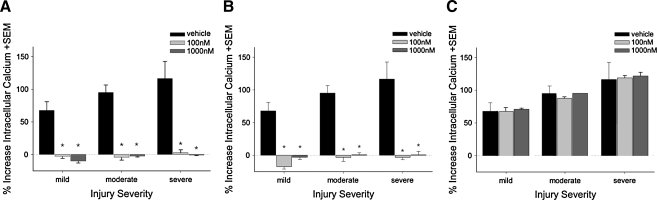
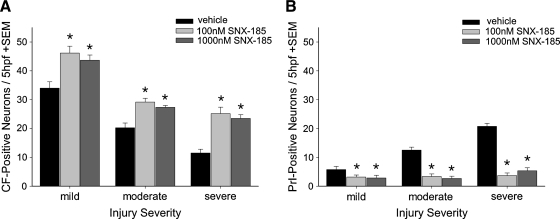
The effects of immediate (i.e. <5 sec) post-stretch-injury treatment with 100 nM or 1000 nM SNX-185 on neuronal survival are shown in Figure 8A,B. Wells treated with 100 nM or 1000 nM SNX-185 immediately after mild, moderate, or severe stretch injury had significantly increased numbers (p < 0.01) of surviving, CFDA-positive neurons (Fig. 8A), and significantly decreased numbers (p < 0.01) of PrI-positive neurons (Fig. 8B) at 24 h after injury compared to vehicle-treated controls. There was no statistical difference between treatment with 100 nM and 1000 nM SNX-185 at any of the three injury severities tested.
FIG. 8.
Effect of immediate post-injury treatment with SNX-185 on neuron survival. Neurons grown in mixed neuronal–glial cell cultures were treated with 100 nM or 1000 nM SNX-185 immediately after mild, moderate, or severe stretch injury and returned to a cell incubator for a 24-h recovery period. Cells were then loaded with carboxyfluorescein diacetate (stains viable cells) and propidium iodide (stains non-viable cells) and visualized with a 400 × upright microscope. Cell counts were performed in five high-power fields (hpf) per well. Immediate post-injury treatment with 100 or 1000 nM SNX-185 significantly improved the number of viable, surviving neurons (A, n = 4–6, p = 0.01) and decreased the number of non-viable, propidium iodide-positive cells (B, n = 4–6, p < 0.05). *Statistically significant difference at p < 0.05.
Treatment with 100 nM or 1000 nM SNX-185 delayed until 5 min post injury had no significant effect on neuronal viability measured with CFDA (Fig. 9A). In contrast, treatment with SNX-185 5 min after mild stretch injury appear to significantly increase (p < 0.05) the number of PrI-positive dying neurons, while treatment with 1000 nM SNX-185 significantly (p < 0.05) reduced cell death under severe injury conditions compared to vehicle-treated neurons. No significant effects of drug treatment were found under conditions of moderate injury severity.
FIG. 9.
Effect of delayed treatment with SNX-185 on neuron survival. Neurons grown in mixed neuronal–glial cell cultures were treated with 100 nM or 1000 nM SNX-185 5 min after mild, moderate, or severe stretch injury and returned to a cell incubator for a 24-h recovery period. Cells were then loaded with carboxyfluorescein diacetate (stains viable cells) and propidium iodide (stains non-viable cells) and visualized with a 400 × upright microscope. Cell counts were performed in five high-power fields (hpf ) per well. Delayed post-injury treatment with 100 or 1000 nM SNX-185 did not significantly affect the number of viable, surviving neurons (A). The number of non-viable, propidium iodide-positive neurons per 5 hpf (B) was significantly increased with delayed 100 (n = 6, p < 0.01) or 1000 nM (n = 6, p < 0.05) SNX-185 treatment following mild injury and significantly decreased when given at 1000 nM 5 min after severe injury (n = 6, p < 0.05). *Statistically significant at p < 0.05.
SNX-185 and glutamate
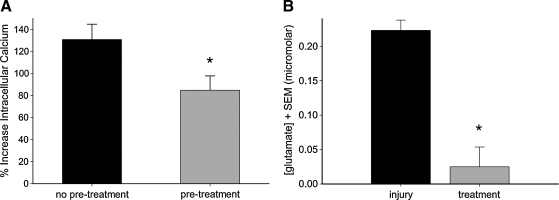
To better understand the effect of SNX-185 on injury-induced calcium elevation, its interaction with glutamate-mediated pathways was explored. In the first set of experiments, glutamate-induced intracellular calcium elevation was investigated in neurons grown in mixed cortical cell cultures. Resting cytosolic calcium levels were monitored for 10 min prior to treatment with 1000 nM SNX-185 or vehicle control. Five minutes later, wells were exposed to 5 mM glutamate (with 25 μM glycine), and the magnitude of the acute calcium rise was measured. The addition of glutamate resulted in a 130.88 ± 13.86% (n = 39) increase in [Ca2+]i levels in neurons. Pretreatment with SNX-185 did not prevent glutamate-induced calcium elevation, but did result in a significant (p < 0.05) reduction to 84.89 ± 12.98% (n = 40) of baseline levels (Fig. 10A).
FIG. 10.
Effect of SNX-185 on glutamate pathways. (A) Neurons grown in mixed neuronal–glial cell cultures were loaded with Fura-2 and exposed to 5 mM glutamate (+25 μM glycine). Pre-treatment with 1000 nM SNX-185 prior to glutamate exposure resulted in a significant decrease in glutamate-induced intracellular calcium rise, but did not completely block glutamate-mediated pathways (n = 39–40, p < 0.05). (B) Microdialysis probes were used to sample from the media directly above the mixed neuronal–glial cell cultures after severe stretch injury. Injury resulted in the release of glutamate into the cell media that that was significantly reduced with 1000 nM SNX-185 pre-treatment (n = 3, p < 0.05). *Statistically significant at p < 0.05.
In the second set of experiments (Fig. 10B), extracellular glutamate levels were measured by microdialysis sampling of the cell-culture media before and after stretch injury with or without SNX-185 treatment. Prior to stretch injury, glutamate concentration in the cell-culture media was below detectable levels. Samples from untreated culture wells collected during the first 10 min after severe stretch injury showed glutamate levels of 0.223 ± 0.015 μM, while wells pretreated with 1000 nM SNX-185 demonstrated glutamate levels of only 0.025 ± 0.028 μM. This difference between glutamate levels in stretch-injured wells that were treated or untreated with SNX-185 was statistically significant (p < 0.05).
Discussion
SNX-185 and calcium dynamics
Stretch injury to cortical neurons resulted in a severity-dependent rapid and large increase in intracellular calcium, consistent with earlier in vitro studies in traumatic biaxial strain-injured cortical neurons (Weber et al., 1999) and injured astrocytes (Rzigalinski et al., 1998). Intracellular calcium levels have also been shown to increase in stretch-injured axons via the opening of VGCCs and reversal of the Na+–Ca2+ exchanger, with the opening of the VGCCs triggered by sodium influx through mechanically sensitive Na+ channels (Wolf et al., 2001a). Fluid shear stress applied to NT2-N cells that resemble primary neurons increased cell-membrane permeability to calcium, resulting in an increase in intracellular calcium (LaPlaca et al., 1997). Although intracellular calcium levels can increase after injury by several mechanisms, the rise in intracellular calcium appears to be an early and rapid event after mechanical cell injury that may be a common pathway leading to cell death following a variety of pathological events, including TBI (Schanne et al., 1979; Siesjo and Bengtsson, 1989; Young, 1996).
The rise in intracellular calcium following stretch injury was transient, with a return to near baseline levels after 90 min with mild injury, and by 360 min with severe injury. The mechanisms underlying the return of intracellular calcium levels to near baseline levels are unknown, and it is possible that even when calcium levels fall, calcium homeostasis remains abnormal for the surviving cells (e.g. neurons). For example, Floyd and associates (2001) reported rapid recovery of intracellular calcium to near basal levels in astrocytes within 15 min of biaxial stretch injury, but found that IP3-mediated cell-signaling pathways remained abnormal for up to 48 h, suggesting an uncoupling of intracellular calcium homeostasis with calcium-signaling pathways.
The injury-induced rise in calcium was blocked by pretreatment or immediate post-injury treatment with SNX-185, while delayed treatment at 5 min did not affect acute calcium elevation but facilitated the return to near baseline levels. This supports the importance of N-type VGCCs in the initial rise in levels of [Ca2+]i following stretch injury, and suggests that continued calcium flux through activated N-type VGCCs contributes to elevated calcium for a prolonged period of time. Blockade of N-type VGCCs, therefore, may prevent both acute calcium elevation, as well as prolonged calcium flux, allowing a more rapid return to pre-injury calcium levels.
The dramatic effect of pre- and immediate post-injury treatment on injury-induced calcium elevation was unexpected, as some calcium rise was expected to occur through non-VGCC-mediated pathways (e.g. glutamate-mediated). N-type channels are found on the soma, dendrites, and axons of neurons throughout the brain (Vacher et al., 2008). They are also highly localized presynaptically, where they likely play an important role in injury-induced glutamate release, which in turn can result in additional AMPA and NMDA-mediated calcium elevation.
Glutamate-induced calcium elevation does not directly require activation of N-type VGCCs, but likely involves their activation as downstream mediators of further depolarization-induced calcium elevation. This explains the observation that glutamate-induced calcium elevation was reduced, but not prevented, by SNX-185. The fact that SNX-185 completely prevented injury-induced calcium elevation, despite its lack of effect on neuronal “sensitivity” to glutamate, suggests that N-type VGCCs are necessary for injury-induced glutamate release to occur. This hypothesis was supported by microdialysis data, which demonstrated a 10-fold decrease in glutamate release when stretch injury was performed in the presence of SNX-185. Although P/Q channels are known to play a role in neurotransmitter release, the findings of the current study suggest that activation of N-type VGCCs is necessary for glutamate release after mechanical injury.
SNX-185 and cell survival
Neuronal injury and death occurred in an injury-severity-dependent manner following stretch injury. The observation that astrocytes also underwent cell injury and death is consistent with recent findings in vivo, suggesting astrocyte death as an early and important event following TBI in rats (Floyd and Lyeth, 2007; Zhao et al., 2003).
SNX-185 applied immediately after stretch injury resulted in significantly higher numbers of viable neurons and significantly lower numbers of dying neurons and astrocytes. Cell death following stretch injury likely results from a dramatic increase in intracellular calcium, which activates proteolytic enzymes such as the calpains, as well as apoptotic pathways (Brophy et al., 2009; Deng et al., 2007; Kovesdi et al., 2007; Wong et al., 2005). High levels of calcium may also disrupt critical calcium signaling pathways such as calcium-dependent protein kinase II (Atkins et al., 2006; Folkerts et al., 2007) and IP-3 (Floyd et al., 2001). These mechanisms would be expected to reduce cell viability, as well as possibly interfere with cellular mechanisms of repair after injury. The ability of SNX-185 to reduce cell death following mechanical stretch injury is therefore likely to be due, at least in part, to its ability to prevent intracellular calcium from rising to pathological levels following cell injury.
Delayed treatment with SNX-185 was ineffective in preventing intracellular calcium rise and subsequent neuronal and astrocyte death, suggesting that once calcium is elevated to levels that activate cell-injury mechanisms, VGCC blockers may no longer be effective. Since N-type VGCCs seem to play a critical role in injury-induced glutamate release, the lack of neuroprotection following delayed treatment may reflect the consequences of prolonged exposure (ie. 5 min) to high levels of glutamate following biaxial strain injury. It is important to note, however, that the in vitro injury model used in these experiments differs from in vivo TBI in several aspects. Cells grown on the silastic membrane bottoms of Flexplates undergo a reproducible biaxial strain injury that is, for the most part, immediate and uniformly distributed across the confluent cell culture (Ellis et al., 1995). In addition, there is little-to-no gradation of the spectrum of injury within a culture well, and a mechanical penumbra of uninjured or partially injured neurons next to severely damaged cells is not produced in this TBI model. In essence, the well bottom represents a focus of injury and does not include a group of adjacently located cells that may experience more delayed, prolonged insults as distal extensions are damaged, synapses are compromised, or local biochemical events occur in the interstitium. This is quite unlike the clinical situation where secondary cell injury and cell-death mechanisms, some of which are calcium-mediated, occur over a period of hours to days. In addition, much of this secondary cell injury in vivo occurs in the penumbra surrounding the brain region of mechanical insult. It is possible, therefore, that the important therapeutic target of delayed SNX-185 administration in vivo might be the penumbra, and not the primary focus of mechanical damage itself, where the rise in intracellular calcium has already occurred and calcium-mediated cytotoxic events have already been initiated. Also, in the intact animal, microvascular injuries (Muellner et al., 2003; Rodriguez-Baeza et al., 2003) and microthrombi (Stein et al., 2004) may cause superimposed ischemia to the injury focus following trauma, which may also require N-type VGCC-dependent glutamate release. Modified in vitro TBI models that include a mechanical penumbra or secondary ischemic insult, therefore, would be useful to understand further the neuroprotective potential of selective N-type VGCC blockade in the clinical management of TBI.
These data suggest that SNX-185 treatment, even when delayed for hours after initial insult, may be effective in preventing or reducing calcium-related secondary injuries that occur clinically over many hours or days after injury. Secondary insults such as ischemia often occur after human TBI (Gennarelli and Graham, 2005), and may also be therapeutic targets for N-type VGCC blockade therapy (Valentino et al., 1993; Bowersox et al., 1993), particularly during the sensitive period by both reducing glutamate release and facilitating recovery of calcium homeostasis. In fact, we have previously shown that delayed treatment with a related N-type VGCC blocker, SNX-111 (Ziconotide), improves the neurological outcome following diffuse axonal injury in rats, including the restoration of mitochondrial function (Verweij et al., 2000). In addition, we recently demonstrated that intracranial injection of SNX-185 into the hippocampus of rats delayed 5 min after lateral fluid percussion injury reduced neuronal injury and loss, and improved motor performance and spatial learning (Lee et al., 2004). Therefore, the results of the present study are consistent with the view that treatment strategies to reduce the rise in intracellular calcium that undoubtedly occurs over an extended period of time following TBI should continue to be explored and developed.
In the last decade, a clinical trial of systemic, intravenous therapy with the N-type VGCC blocker SNX-111 was initiated on patients with severe TBI, but was terminated before clinical benefit could be established (Muizelaar et al., unpublished data). The failure of this trial may not have been due to the therapeutic target or drug, but rather the delivery strategy that was employed. In 2004, the United States Food and Drug Administration approved the intrathecal administration of Ziconotide (Prialt®) for chronic pain, deeming this delivery strategy to be both safe and neurologically efficacious. This clinical application, combined with strong pre-clinical data demonstrating its significant effect on intracellular calcium dynamics and cell survival, support revisiting selective N-type VGCC blockade as a strategy for improving the outcome following severe TBI.
The present study provides a better understanding of calcium homeostasis following TBI, which is vital to understanding mechanisms of cell injury and cell loss following brain injury. The present results indicate that VGCC blockers have three important actions on calcium dynamics following cell injury: significantly reducing the amount of glutamate release following biaxial strain injury, preventing intracellular calcium levels from increasing in injured cells, and facilitating the recovery of elevated intracellular calcium back to near baseline levels. This study provides important insights into the potential “treatment window” for selective N-type VGCC blockers. Although SNX-185 is effective in promoting calcium recovery in neurons that sustained primary injury, it may be most effective in preventing the secondary insults that often occur in the hours, days, and, sometimes, weeks following severe TBI. Future in vitro studies are needed to determine if SNX-185 protects neurons from hypoxia/ischemia, the common causes of secondary injury in patients with TBI. The findings of our study suggest that future clinical trials of selective N-type VGCC blockade should be designed to target delayed primary and secondary insults; a prolonged administration of the drug through targeted, intrathecal delivery could be an effective strategy for neuroprotection.
Conclusion
In conclusion, stretch injury of cultured neurons and astrocytes results in a rapid and large increase in intracellular calcium that reduces cell survival. The blockade of N-type VGCCs with SNX-185 before or immediately after injury prevents the rise in intracellular calcium and decreases cell death, likely due to its ability to dramatically reduce injury-induced glutamate release. Delayed treatment with SNX-185 did not improve cell survival in this in vitro model, suggesting that calcium-dependent cell-injury pathways, once triggered, may be relatively unresponsive to subsequent treatment with calcium channel blockers. However, in vivo, secondary insults, such as hypoxia, ischemia, and biochemical sequelae of adjacently injured cells, may result in neuronal calcium elevations for many hours and days after TBI, and provide rationale targets for selective N-type VGCC blockade.
Acknowledgments
The authors thank Ryan Martin, Kim Katleba, and Dr. Candace Floyd for their invaluable assistance with these experiments, and William T. O'Connor for performing the analysis of the glutamate levels. This research was supported by NIH NS 39090 (RFB), NIH NS 29995 (BGL), and NS 45136 (BGL).
Author Disclosure Statement
No conflicting financial interests exist.
References
- Ahmed S.M. Rzigalinski B.A. Willoughby K.A. Sitterding H.A. Ellis E.F. Stretch-induced injury alters mitochondrial membrane potential and cellular ATP in cultured astrocytes and neurons. J. Neurochem. 2000;74:1951–1960. [PubMed] [Google Scholar]
- Ahmed S.M. Weber J.T. Liang S. Willoughby K.A. Sitterding H.A. Rzigalinski B.A. Ellis E.F. NMDA receptor activation contributes to a portion of the decreased mitochondrial membrane potential and elevated intracellular free calcium in strain-injured neurons. J. Neurotrauma. 2002;19:1619–1629. doi: 10.1089/089771502762300274. [DOI] [PubMed] [Google Scholar]
- Atkins C.M. Chen S. Alonso O.F. Dietrich W.D. Hu B.R. Activation of calcium/calmodulin-dependent protein kinases after traumatic brain injury. J. Cereb. Blood Flow Metab. 2006;26:1507–1518. doi: 10.1038/sj.jcbfm.9600301. [DOI] [PubMed] [Google Scholar]
- Berman R.F. Verweij B.H. Muizelaar J.P. Neurobehavioral protection by the neuronal calcium channel blocker ziconotide in a model of traumatic diffuse brain injury in rats. J. Neurosurg. 2000;93:821–828. doi: 10.3171/jns.2000.93.5.0821. [DOI] [PubMed] [Google Scholar]
- Bowersox S.S. Gadbois T. Singh T. Pettus E.H. Wang Y.X. Luther R.R. Selective N-type neuronal voltage-sensitive clacium channel blocker, SNX-111, produces spinal antinociception in rat models of acute, persistent and neuropathic pain. J. Pharmacol. Exp. Ther. 1996;279:1243–1249. [PubMed] [Google Scholar]
- Bowersox S.S. Singh T. Luther R.R. Selective blockade of N-type voltage-sensitive calcium channels protects against brain injury after transient focal cerebral ischemia in rats. Brain Res. 1997;747:343–347. doi: 10.1016/s0006-8993(96)01325-x. [DOI] [PubMed] [Google Scholar]
- Brophy G.M. Pineda J.A. Papa L. Lewis S.B. Valadka A.B. Hannay H.J. Heaton S.C. Demery J.A. Liu M.C. Tepas J.J. Gabrielli A. Robicsek S. Wang K.K. Robertson C.S. Hayes R.L. alphaII-spectrin breakdown product cerebrospinal fluid exposure metrics suggest differences in cellular injury mechanisms after severe traumatic brain injury. J. Neurotrauma. 2009;26:471–479. doi: 10.1089/neu.2008.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourne F. Li H. Buchan A.M. Clemens J.A. Continuing postischemic neuronal death in CA1: Influence of ischemia duration and cytoprotective doses of NBQX and SNX-111 in rats. Stroke. 1999;30:662–668. doi: 10.1161/01.str.30.3.662. [DOI] [PubMed] [Google Scholar]
- Deng Y. Thompson B.M. Gao X. Hall E.D. Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Exp. Neurol. 2007;205:154–165. doi: 10.1016/j.expneurol.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis E.F. McKinney J.S. Willoughby K.A. Liang S. Povlishock J.T. A new model for rapid stretch-induced injury of cells in culture: Characterization of the model using astrocytes. J. Neurotrauma. 1995;12:325–339. doi: 10.1089/neu.1995.12.325. [DOI] [PubMed] [Google Scholar]
- Floyd C.L. Lyeth B.G. Astroglia: Important mediators of traumatic brain injury. Prog. Brain Res. 2007;161:61–79. doi: 10.1016/S0079-6123(06)61005-4. [DOI] [PubMed] [Google Scholar]
- Floyd C.L. Rzigalinski B.A. Weber J.T. Sitterding H.A. Willoughby K.A. Ellis E.F. Traumatic injury of cultured astrocytes alters inositol (1,4,5)-trisphosphate-mediated signaling. Glia. 2001;33:12–23. doi: 10.1002/1098-1136(20010101)33:1<12::aid-glia1002>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Folkerts M.M. Parks E.A. Dedman J.R. Kaetzel M.A. Lyeth B.G. Berman R.F. Phosphorylation of calcium calmodulin-dependent protein kinase II following lateral fluid percussion brain injury in rats. J. Neurotrauma. 2007;24:638–650. doi: 10.1089/neu.2006.0188. [DOI] [PubMed] [Google Scholar]
- Gennarelli T.A. Graham D.I. Neuropathology. In: Silver J.M., editor; McAllister T.W., editor; Yudofsky S., editor. Textbook of Traumatic Brain Injury. American Psychiatric Publishing; Washington, DC: 2005. pp. 27–50. [Google Scholar]
- Hellmich H.L. Eidson K.A. Capra B.A. Garcia J.M. Boone D.R. Hawkins B.E. Uchida T. Dewitt D.S. Prough D.S. Injured Fluoro-Jade-positive hippocampal neurons contain high levels of zinc after traumatic brain injury. Brain Res. 2007;1127:119–126. doi: 10.1016/j.brainres.2006.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovda D.A. Fu K. Badie H. Samii A. Pinanong P. Becker D.P. Administration of an omega-conopeptide one hour following traumatic brain injury reduces 45calcium accumulation. Acta Neurochir. Suppl. (Wien) 1994;60:521–523. doi: 10.1007/978-3-7091-9334-1_143. [DOI] [PubMed] [Google Scholar]
- Iwata A. Stys P.K. Wolf J.A. Chen X.H. Taylor A.G. Meaney D.F. Smith D.H. Traumatic axonal injury induced proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors. J. Neurosci. 2004;24:4605–4613. doi: 10.1523/JNEUROSCI.0515-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovesdi E. Czeiter E. Tamas A. Reglodi D. Szellar D. Pal J. Bukovics P. Doczi T. Buki A. Rescuing neurons and glia: is inhibition of apoptosis useful? Prog. Brain Res. 2007;161:81–95. doi: 10.1016/S0079-6123(06)61006-6. [DOI] [PubMed] [Google Scholar]
- LaPlaca M.C. Lee V.M. Thibault L.E. An in vitro model of traumatic neuronal injury: Loading rate-dependent changes in acute cytosolic calcium and lactate dehydrogenase release. J. Neurotrauma. 1997;14:355–368. doi: 10.1089/neu.1997.14.355. [DOI] [PubMed] [Google Scholar]
- Lee L.L. Galo E. Lyeth B.G. Muizelaar J.P. Berman R.F. Neuroprotection in the rat lateral fluid percussion model of traumatic brain injury by SNX-185, an N-type voltage-gated calcium channel blocker. Exp. Neurol. 2004;190:70–78. doi: 10.1016/j.expneurol.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Meberg P.J. Miller M.W. Culturing hippocampal and cortical neurons. Methods Cell Biol. 2003;71:111–127. doi: 10.1016/s0091-679x(03)01007-0. [DOI] [PubMed] [Google Scholar]
- Muellner A. Benz M. Kloss C.U. Mautes A. Burggraf D. Hamann G.F. Microvascular basal lamina antigen loss after traumatic brain injury in the rat. J. Neurotrauma. 2003;20:745–754. doi: 10.1089/089771503767869971. [DOI] [PubMed] [Google Scholar]
- Newcomb R. Abbruscato T.J. Singh T. Nadasdi L. Davis T.P. Miljanich G. Bioavailability of Ziconotide in brain: Influx from blood, stability, and diffusion. Peptides. 2000;21:491–501. doi: 10.1016/s0196-9781(00)00175-3. [DOI] [PubMed] [Google Scholar]
- Oorschot D.E. Effect of fluorodeoxyuridine on neurons and non-neuronal cells in cerebral explants. Exp. Brain Res. 1989;78:132–138. doi: 10.1007/BF00230692. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon M.A. Yenari M.A. Sun G.H. Kunis D.M. Steinberg G.K. SNX-111, a novel, presynaptic N-type calcium channel antagonist, is neuroprotective against focal cerebral ischemia in rabbits. J. Neurol. Sci. 1997;153:25–31. doi: 10.1016/s0022-510x(97)00196-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Baeza A. Reina-de la Torre F. Poca A. Marti M. Garnacho A. Morphological features in human cortical brain microvessels after head injury: A three-dimensional and immunocytochemical study. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003;273:583–593. doi: 10.1002/ar.a.10069. [DOI] [PubMed] [Google Scholar]
- Rzigalinski B.A. Weber J.T. Willoughby K.A. Ellis E.F. Intracellular free calcium dynamics in stretch-injured astrocytes. J, Neurochem. 1998;70:2377–2385. doi: 10.1046/j.1471-4159.1998.70062377.x. [DOI] [PubMed] [Google Scholar]
- Samii A. Badie H. Fu K. Luther R.R. Hovda D.A. Effects of an N-type calcium channel antagonist (SNX 111; Ziconotide) on calcium-45 accumulation following fluid-percussion injury. J. Neurotrauma. 1999;16:879–892. doi: 10.1089/neu.1999.16.879. [DOI] [PubMed] [Google Scholar]
- Schanne F.A. Kane A.B. Young E.E. Farber J.L. Calcium dependence of toxic cell death: A final common pathway. Science. 1979;206:700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Schinder A.F. Olson E.C. Spitzer N.C. Montal M. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J. Neurosci. 1996;16:6125–6133. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjo B.K. Bengtsson F. Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression: A unifying hypothesis. J. Cereb. Blood Flow Metab. 1989;9:127–140. doi: 10.1038/jcbfm.1989.20. [DOI] [PubMed] [Google Scholar]
- Slemmer J.E. Matser E.J.T. De Zeeuw C.I. Weber J.T. Repeated mild injury causes cumulative damage to hippocampal cells. Brain. 2002;125:2699–2709. doi: 10.1093/brain/awf271. [DOI] [PubMed] [Google Scholar]
- Smith D.H. Wolf J.A. Lusardi T.A. Lee V.M. Meaney D.F. High tolerance and delayed elastic response of cultured axons to dynamic stretch injury. J. Neurosci. 1999;19:4263–4269. doi: 10.1523/JNEUROSCI.19-11-04263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staats P.S. Yearwood T. Charapata S.G. Presley R.W. Wallace M.S. Byas-Smith M. Fisher R. Bryce D.A. Mangieri E.A. Luther R.R. Mayo M. McGuire D. Ellis D. Intrathecal ziconitide in the treatment of refractory pain in patients with cancer or AIDS: A randomized controlled trial. JAMA. 2004;291:63–70. doi: 10.1001/jama.291.1.63. [DOI] [PubMed] [Google Scholar]
- Stein S.C. Graham D.I. Chen X.H. Smith D.H. Association between intravascular microthrombosis and cerebral ischemia in traumatic brain injury. Neurosurgery. 2004;54:687–691. doi: 10.1227/01.neu.0000108641.98845.88. [DOI] [PubMed] [Google Scholar]
- Tavalin S.J. Ellis E.F. Satin L.S. Mechanical perturbation of cultured cortical neurons reveals a stretch-induced delayed depolarization. J. Neurophysiol. 1995;74:2767–2773. doi: 10.1152/jn.1995.74.6.2767. [DOI] [PubMed] [Google Scholar]
- Tavalin S.J. Ellis E.F. Satin L.S. Inhibition of the electrogenic Na pump underlies delayed depolarization of cortical neurons after mechanical injury or glutamate. J. Neurophysiol. 1997;77:632–638. doi: 10.1152/jn.1997.77.2.632. [DOI] [PubMed] [Google Scholar]
- Vacher H. Mohapatra D.P. Trimmer J.S. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol. Rev. 2008;88:1407–1447. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino K. Newcomb R. Gadbois T. Singh T. Bowersox S.S. Bitner S. Justice A. Yamashiro D. Horrman B.B. Ciaranello R. Miljanich G.P. Ramachandran J. A selective N-type calcium channel antagonist protects against neuronal loss after global cerebral ischemia. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7894–7897. doi: 10.1073/pnas.90.16.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij B.H. Muizelaar J.P. Vinas F.C. Peterson P.L. Xiong Y. Lee C.P. Mitochondrial dysfunction after experimental and human brain injury and its possible reversal with a selective N-type calcium channel antagonist (SNX-111) Neurol. Res. 1997;19:334–339. doi: 10.1080/01616412.1997.11740821. [DOI] [PubMed] [Google Scholar]
- Verweij B.H. Muizelaar J.P. Vinas F.C. Peterson P.L. Xiong Y. Lee C.P. Improvement in mitochondrial dysfunction as a new surrogate efficiency measure for preclinical trials: Dose-response and time-window profiles for administration of the calcium channel blocker Ziconotide in experimental brain injury. J. Neurosurg. 2000;93:829–834. doi: 10.3171/jns.2000.93.5.0829. [DOI] [PubMed] [Google Scholar]
- Wang Y.X. Bezprozvannaya S. Bowersox S.S. Nadasdi L. Miljanich G. Mezo G. Silva D. Tarczy-Hornoch K. Luther R.R. Peripheral versus central potencies of N-type voltage-sensitive calcium channel blockers. Naunyn Schmiedebergs Arch. Pharmacol. 1998;357:159–168. doi: 10.1007/pl00005150. [DOI] [PubMed] [Google Scholar]
- Weber J.T. Calcium homeostasis following traumatic neuronal injury. Curr. Neurovasc. Res. 2004;1:151–171. doi: 10.2174/1567202043480134. [DOI] [PubMed] [Google Scholar]
- Weber J.T. Rzigalinski B.A. Ellis E.F. Traumatic injury of cortical neurons causes changes in intracellular calcium stores and capacitative calcium influx. J. Biol. Chem. 2001;276:1800–1807. doi: 10.1074/jbc.M009209200. [DOI] [PubMed] [Google Scholar]
- Weber J.T. Rzigalinski B.A. Willoughby K.A. Moore S.F. Ellis E.F. Alterations in calcium-mediated signal transduction after traumatic injury of cortical neurons. Cell Calcium. 1999;26:289–299. doi: 10.1054/ceca.1999.0082. [DOI] [PubMed] [Google Scholar]
- Wolf J.A. Stys P.K. Lusardi T.A. Meaney D.F. Smith D.H. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J. Neurosci. 2001a;21:1923–1930. doi: 10.1523/JNEUROSCI.21-06-01923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J.A. Stys P.K. Lusardi T.A. Meaney D.F. Smith D.H. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J. Neurosci. 2001b;21:1923–1930. doi: 10.1523/JNEUROSCI.21-06-01923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J. Hoe N.W. Zhiwei F. Ng I. Apoptosis and traumatic brain injury. Neurocrit. Care. 2005;3:177–182. doi: 10.1385/NCC:3:2:177. [DOI] [PubMed] [Google Scholar]
- Young W. Role of calcium in central nervous system injuries. J. Neurotrauma. 1992;9(Suppl 1):S9–25. [PubMed] [Google Scholar]
- Young W. Death by calcium: A way of life. In: Narayan R.K., editor; Wilberger J.E., editor; Povlishock J.T., editor. Neurotrauma. McGraw-Hill; New York: 1996. pp. 1421–1434. [Google Scholar]
- Zhao X. Ahram A. Berman R.F. Muizelaar J.P. Lyeth B.G. Early loss of astrocytes after experimental traumatic brain injury. Glia. 2003;44:140–152. doi: 10.1002/glia.10283. [DOI] [PubMed] [Google Scholar]
- Zhong C. Zhao X. Van K.C. Bzdega T. Smyth A. Zhou J. Kozikowski A.P. Jiang J. O'Connor W.T. Berman R.F. Neale J.H. Lyeth B.G. NAAG peptidase inhibitor increases dialysate NAAG and reduces glutamate, aspartate and GABA levels in the dorsal hippocampus following fluid percussion injury in the rat. J. Neurochem. 2006;97:1015–1025. doi: 10.1111/j.1471-4159.2006.03786.x. [DOI] [PubMed] [Google Scholar]