Abstract
Antagonism of vascular endothelial growth factor (VEGF) signaling by soluble fms-like tyrosine kinase-1 (sFlt-1) occurs during preeclampsia and is proposed to play an important role in the pathogenesis of preeclampsia. We recently reported that hypertension associated with chronic reductions in uteroplacental perfusion pressure (RUPP) is associated with increased sFlt-1 and decreased free VEGF. Whether restoration of circulating VEGF can restore renal function and chronically decrease arterial pressure associated with placental ischemia remains unknown. We hypothesized that chronic infusion of VEGF121 would attenuate hypertension, increase glomerular filtration rate (GFR), and reverse the endothelial dysfunction associated with chronic RUPP. VEGF121 (at either 90 or 180ug/kg/day) was administered for five days via osmotic mini-pump placed intraperitoneal. Mean arterial pressure (MAP), renal function and tissues were obtained on day 19 of pregnancy from RUPP+VEGF, RUPP and normal pregnant (NP) dams. MAP (mm Hg) was increased in the RUPP (131±3) compared to the NP (102±1) and infusion of VEGF121 resolved the hypertension (105±5). GFR (ml/min) was decreased in the RUPP dams (1.5±0.3), and restored to NP levels (3.1±0.5) by VEGF121 treatment (3.1±0.4). Effective renal plasma flow, decreased by RUPP was also increased by VEGF121 infusion. Relaxation to acetylcholine was enhanced by the VEGF treatment (P<0.05). These data demonstrate that chronic infusion of VEGF121 during late gestation restores GFR and endothelial function, and reduces high blood pressure associated with placental ischemia. The present results suggest VEGF121 may be a candidate molecule for management of preeclampsia and its related complications.
Keywords: preeclampsia, gestation, VEGF, blood pressure, angiogenic
Introduction
Preeclampsia is a major obstetric problem that affects approximately 5% of all pregnancies and is a significant source of maternal and neonatal morbidity and mortality 1. Moreover, the incidence of preeclampsia has seen a 40% increase in recent years2. Although the preeclamptic syndrome has been well characterized and many studies indicate that hypertension, proteinuria, endothelial cell dysfunction and insufficient placentation are key features of this disorder3, 4, the underlying pathophysiological mechanisms of this disorder remain unclear.
Recent studies have established the existence of an imbalance between pro- and anti-angiogenic factors such as vascular endothelial growth factor (VEGF), placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1) in preeclamptic women5-10. Moreover, findings from several clinical studies suggest that alterations in circulating sFlt-1 concentrations may presage the clinical onset of preeclamptic symptoms5, 11-13. Viewed in concert with recent experimental studies in animals, it appears that this dysregulation of angiogenic factors may play a key role in the pathogenesis of preeclampsia9, 10, 14-19.
Although the mechanisms underlying the overexpression of anti-angiogenic factors such as sFlt-1 are currently unresolved, in vitro and in vivo evidence suggest that placental hypoxia/ischemia may initiate this imbalance of angiogenic factors14, 16, 20. Both Gilbert et al. and Makris et al. have shown in vivo that chronic placental ischemia results in elevated circulating levels of sFlt-1 and hypertension14, 16. In addition, previous studies have shown that reduced uterine perfusion pressure (RUPP) is associated with decreased endothelium-dependent vascular relaxation21 and decreased glomerular filtration rate (GFR) 22. In an elegantly designed study reported several years ago, Maynard et al. reported that exogenous administration of sFlt-1 into pregnant rats via adenovirus mediated gene transfer resulted in increased arterial pressure and proteinuria, and decreased plasma free VEGF and PlGF concentrations similar to that observed in the preeclamptic patients10. More recently, Bridges et al. demonstrated that chronic elevations of recombinant sFlt-1 results in endothelial dysfunction that is attenuated by the superoxide dismutase mimetic Tiron 18. Further, recent studies by Li et al. have demonstrated that delivery of recombinant VEGF121, the most soluble of the VEGF isoforms, can rescue the symptoms generated by chronic elevations of sFlt-1 23. Although these findings are intriguing, the mechanisms by which the hypertensive phenotype is rescued remain unclear. Moreover, the efficacy of VEGF121 treatment to improve hypertension and renal function in a model of hypertension during preeclampsia with spontaneously occurring over-expression of sFlt-1 remains unknown.
Thus, the purpose of the present study was to test the hypothesis that chronic infusion of VEGF will attenuate the endothelial dysfunction, impaired renal function and hypertension associated with reduced uterine perfusion pressure (RUPP) in the pregnant rat. To this end, we employed our established model of hypertension associated with placental ischemia in which chronic reductions of uterine perfusion pressure lead to endothelial dysfunction, decreased GFR and hypertension in the pregnant rat.
Methods and Apparatus
Animals
Studies were performed in timed pregnant Sprague-Dawley rats purchased from Harlan Inc. (Indianapolis Ind). Animals were housed in a temperature-controlled room (23°C) with a 12:12 light:dark cycle. All experimental procedures executed in this study were in accordance with National Institutes of Health guidelines for use and care of animals. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Mississippi Medical Center. On day 14 of gestation, rat dams were randomly assigned to one of five experimental groups: 1) normal pregnant (NP) plus vehicle which served as the control group (n=9); 2) RUPP plus vehicle (sterile phosphate buffered saline; PBS) group (n=11); 3) RUPP + VEGF (low dose, 90μg/kg/day; n=8); 4) RUPP + VEGF (high dose, 180 μg/kg/day; n=7) group.
Reduced Uterine Perfusion Pressure (RUPP) Procedure
The RUPP procedure is a well established model for studying the links between placental ischemia and hypertension in the pregnant rat and has been described in detail previously 21, 22, 24-28. In brief, surgical procedures were completed with rats under isoflurane anesthesia (Webster, Sterling, Mass) delivered by an anesthesia apparatus (Vaporizer for Forane Anesthetic, Ohio Medical Products, Madison, Wis). Pregnant rats entering the RUPP groups underwent the following clipping procedure at 14 days of gestational age (dGA). After a midline incision, the lower abdominal aorta was isolated and a silver clip (0.203-mm ID) was placed around the aorta superior to the iliac bifurcation. Silver clips (0.100-mm ID) were also placed on branches of both the right and left ovarian arteries that supply the uterus. Mini-pumps filled with VEGF121 in sterile phosphate buffered saline (PBS) or sterile PBS alone was placed intraperitoneal at the time of the RUPP surgeries and on day 14 of gestation in the NP rats. When the clipping procedure resulted in total reabsorption of the fetuses, rats were excluded from data analyses.
Measurement of Renal Hemodynamics in Conscious Rats With Use of Chronic Protocols
A separate cohort of rats was selected for the determination of renal hemodynamics following chronic VEGF infusion. Renal hemodynamics measurements were determined in conscious pregnant rats on day 19 of gestation. During isoflurane anesthesia, rats were surgically instrumented with venous and arterial catheters of V-3 tubing (SCI, Lake Hayasu City, Ariz) for blood sampling and blood pressure monitoring. The bladder was cannulated with flare-tipped PE 90 tubing for urine collection. All catheters were tunneled to the back of the neck and exteriorized. Animals were allowed to recover for 3 days before renal function measurements. On the day of renal function measurements, the rats were removed from their metabolic cages and placed in modified restraining cages. The venous catheter was connected to an infusion pump that delivered isotonic saline containing [125I]iothalamate (Isotex Diagnostics; 0.05 mCi · kg-1 · min-1) and Para-aminohippuric acid at a fixed rate of 3 mL/h. Arterial pressure was monitored with a pressure transducer (Cobe III Transducer CDX Sema, Birmingham, Ala) for continuous recording. After a 1-hour equilibration period, 2 consecutive 20-minute urine collections were obtained in each rat. Urine volume was determined gravimetrically. GFR and effective renal plasma flow (ERPF) were calculated from concentrations of [125I] and [PAH] in plasma and urine
Measurement of Mean Arterial Pressure (MAP) in Chronically Instrumented Conscious Rats
Animals were instrumented and arterial pressure was determined in both groups of rats at day 19 of gestation as described previously 26. Briefly, on day 17 of gestation rats were instrumented with carotid catheters of V-3 tubing (SCI, Lake Hayasu City, Ariz) while under isoflurane anesthesia (Webster) delivered by an anesthesia apparatus (Vaporizer for Forane Anesthetic). Catheters were tunneled to the back of the neck and exteriorized after implantation. On day 19 of gestation, rat dams were placed in individual restraining cages for arterial pressure measurements using a pressure transducer (Cobe III Transducer CDX Sema, Birmingham, Ala). MAP was recorded continuously for a 2-hour period after 1-hour of stabilization.
Conceptus Measurements
After the measurement of MAP, the dams were placed under isoflourane anesthesia and a ventral midline incision was made to isolate the abdominal aorta for plasma and serum collection. The uterus was exteriorized and the number of viable and resorbed pups was counted and recorded and the pups and placentae were excised, blotted dry and weighed. The heaviest and the lightest placentae were collected from each uterine horn, snap frozen in liquid nitrogen and stored at -80°C until further analyses were performed.
Plasma/Serum Assays and Recombinant Protein
Blood was collected for subsequent assays into Corvac® sterile serum separator tubes (Sherwood Davis, St. Louis, MO) and plasma into BD Vacutainer® EDTA containing tubes. Circulating VEGF and sFlt-1 concentrations were measured using commercial kits available from R&D systems (Minneapolis, MN) as reported previously 14, 18. Recombinant VEGF121 has been previously described 23 and was a gift from Scios, Inc, Fremont, CA.
Vascular Wire Myography Experiments
The non-instrumented carotid was removed and prepared for vessel-reactivity studies in organ-chamber baths, as previously described 18, 29. Carotid arteries were chosen to be consistent with our earlier work 29. Resting tension was adjusted stepwise to reach a final tension of 0.75 g. For studies of vessel relaxation, carotid segments were precontracted with the thromboxane mimetic, U46619 (0.4 μg/mL). After the vessel reached stable tension, concentration responses to acetylcholine (ACh) and sodium nitroprusside (SNP) (10−8 to 10−4 M) were performed to assess endothelial-dependent and smooth muscle-dependent relaxation, respectively. To evaluate viability of vessel segments after the final relaxation curve, maximal contractile responses to U46619 (10−8 to 10−4 M) were also tested.
Statistical Analysis and Calculations
All data are presented as mean ± SEM and statistical significance was accepted when P < 0.05. A Grubb's test was applied to identify statistical outliers. Conceptus data were calculated as mean per pregnancy. sFlt-1:VEGF ratio data were log transformed to obtain normal distributions for subsequent statistical analysis. Comparisons between groups were made with ANOVA followed by post-hoc Newman-Keuls multiple comparison test. Myography data were analyzed by non-linear regression best fit modeling followed by an F test for Log of the half maximal effective concentration (EC50), Hill slope and curve fitting. Statistical calculations were made with GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA USA).
Results
Blood pressure during late gestation
Figure 1 illustrates the effects of RUPP and VEGF121 on MAP during late gestation. VEGF121 infusion reduced MAP in the RUPP rats in a dose dependent manner with the high dose treatment abrogating the placental ischemia induced hypertension.
Figure 1.
Mean arterial pressure (MAP) was increased (P<0.05) in the reduced uterine perfusion pressure (RUPP) compared to normal pregnant+vehicle (NP) rats on day 19 of gestation (term = 21 days). VEGF121 infusion reduced MAP in the RUPP+VEGF121 rats in a dose dependent manner with the high dose treatment abrogating the placental ischemia induced hypertension (P<0.05, RUPP+VEGF121 high dose vs. RUPP).
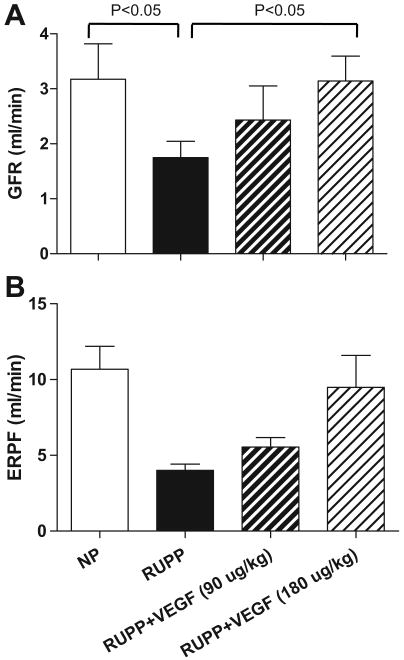
Renal Hemodynamics
Figure 2A illustrates the effects of RUPP and VEGF121 on GFR during late gestation. Similar to the effects of observed with respect to MAP, VEGF121 infusion improved GFR in the RUPP rats in a dose dependent manner with the high dose treatment abrogating the placental ischemia induced decline in renal function. Figure 2B shows that ERPF was restored by high dose VEGF121 infusion.
Figure 2.
Glomerular filtration rate (GFR; Figure 2, panel A) and effective renal plasma flow (ERPF; Figure 2, panel B) was decreased (P<0.05) in the reduced uterine perfusion pressure (RUPP) compared to normal pregnant (NP) rats on day 19 of gestation (term = 21 days). VEGF121 infusion increased GFR (panel A) and ERPF (panel B) in the RUPP+VEGF121 rats in a dose dependent manner with the high dose treatment abrogating the placental ischemia-induced decline in renal function (P<0.05, RUPP+VEGF121 high dose vs. RUPP).
Endothelial function
Because the high dose of chronic infusion of VEGF121 resulted in increased GFR and decreased MAP, we sought to determine if this was due to enhanced vascular endothelial cell function. Figure 3 shows that endothelial dependent vascular relaxation of the carotid artery to ACh was enhanced by chronic infusion of VEGF121 to RUPP rats (Figure 3). The log EC50 for ACh was decreased in the RUPP+VEGF121 compared to RUPP rats (log -6.998 vs. -10.29; P<0.05).
Figure 3.
Endothelial dependent vascular relaxation of the carotid artery (preconstricted to U46619) to acetylcholine (ACh) was enhanced by chronic infusion of VEGF121 (180μg/kg) for five days to reduced uterine perfusion pressure (RUPP) rats when compared to normal pregnant + vehicle control (NP) rats. The log EC50 for ACh was decreased in the RUPP+VEGF121 compared to RUPP rats (log -6.998 vs. -10.29; P<0.05). *Indicates significantly different than RUPP treated group.
Plasma angiogenic factors
As reported previously, RUPP resulted in a decrease of VEGF and an increase of sFlt-1 when compared to NP rats (Figure 4A and B). Infusion of VEGF121 restored circulating VEGF to NP levels (Figure 4A). Interestingly, chronic infusion of VEGF121 decreased plasma sFlt-1 levels to that of NP rats (Figure 4B). While this decrease in plasma sFlt-1 levels may be due to the inability of the sFlt-1 ELISA assay to detect the complexed sFlt-1 bound to VEGF-121, it cannot be ruled out that VEGF121 infusion may negatively regulate sFlt-1 expression.
Figure 4.
Reduced uterine perfusion pressure (RUPP) resulted in a decrease of VEGF and an increase of sFlt-1 when compared to normal pregnant+vehicle (NP) rats at day 19 of pregnancy (Figure 4A and B). Infusion of VEGF121 restored circulating VEGF to NP levels (Figure 4A). Interestingly, chronic infusion of VEGF decreased plasma sFlt-1 levels in the RUPP+VEGF121 group to that of NP rats (Figure 4B).
Conceptus morphometrics
Figure 5A illustrates that RUPP fetuses were smaller than the NP fetuses (P<0.05). Chronic infusion of VEGF121 at 90 μg/kg (low dose) in the RUPP group resulted in fetuses that were not smaller than the NP groups. High dose infusion of VEGF121 (180 μg/kg) into RUPP rats had no effect on fetal growth as fetuses remained smaller (P < 0.05) than those in the NP group. Placental size was not altered by RUPP or VEGF121 infusion in the present study (Figure 5B). Treatment with VEGF121 had no effect on resorption number (data not shown).
Figure 5.
Panel A illustrates that reduced uterine perfusion pressure (RUPP) fetuses were smaller than the normal pregnant+vehicle (NP) fetuses (P<0.05). Chronic infusion of VEGF121 at 90 μg/kg (low dose) in the RUPP+VEGF121 group resulted in fetuses that were not smaller than the NP groups. High dose infusion of VEGF121 (180 μg/kg) into RUPP rats had no effect on fetal growth. Placental size was not altered by RUPP or VEGF121 infusion in the present study (Panel B).
Discussion
The present study reports several novel findings regarding the effects of recombinant VEGF121 infusion in a rat model of preeclampsia whereby sFlt-1 over-expression is generated spontaneously following reduction of utero-placental perfusion pressure. As we have reported previously, RUPP rats have increased arterial pressure, renal impairment and endothelial dysfunction in conjunction with elevated circulating sFlt-1 and decreased plasma VEGF15, 21, 22, 28.
In the present work, we report several novel findings. Foremost, we report that chronic infusion of recombinant VEGF121 results in reductions of arterial pressure in a dose dependent manner. Secondly, we found renal hemodynamic improvement as determined by increased GFR and ERPF. Third, we found that the reduction in AP and restoration of GFR in the high dose VEGF121 infusion group was associated with significant improvements in endothelial function as determined by relaxation responses to ACh. Lastly, we found that five days of VEGF121 infusion negatively regulates sFlt-1 expression in rats with placental ischemia and restores sFlt-1 back to normal pregnant levels by day 19 of pregnancy. Thus, the present study is the first to report on the efficacy of VEGF121 infusion to reduce AP, improve GFR and reduce sFlt-1 levels in a robust, reproducible and well characterized animal model of placental ischemia induced hypertension in which plasma sFlt-1 concentrations are increased due to placental ischemia.
We 14 and others16 have demonstrated placental ischemia results in hypertension that is associated with increases in sFlt-1. In addition, other groups have shown that sFlt-1 over-expression results in hypertension during pregnancy 9, 10, 23. Our results are consistent with those of Li et al. that report VEGF121 reverses the preeclamptic phenotype of the adenovirus-induced sFlt-1 over-expression model23. Moreover, both the present study and that of Li et al.23 show that VEGF121 infusion lowers arterial pressure in a dose dependent manner. Taken together, these data illustrate the importance of sFlt-1 in the pathophysiology of hypertension during preeclampsia. In contrast to the work of Li et al.23, the present study is in a model in which the RUPP animals present with elevated levels of other factors (in addition to sFlt-1) that play important roles in preeclampsia such as tumor necrosis factor-α and soluble endoglin 28, 30-32. Hence, the current findings demonstrate that chronic infusion of VEGF121 lowers blood pressure and improves renal function in the presence of multiple factors involved in the preeclamptic syndrome.
Previous work in our laboratory22 has shown that the hypertension in this model is associated with a decline in renal function. Hence, we set out to determine if the improvement observed in arterial pressure was associated with restoration of GFR in VEGF121 infused rats. Although we found that VEGF121 infusion resulted in dose dependent increases in GFR, only the high dose restored GFR to normal pregnant levels. These data suggest that VEGF antagonism by sFlt-1 play an important role in the decline in renal function and therefore hypertension associated with placental ischemia.
Prior work has shown that RUPP-hypertension is associated with endothelial dysfunction and impaired ACh mediated nitric oxide production and vasorelaxation 21. Moreover, previous studies have provided evidence that VEGF antagonism by sFlt-1 results in decreased endothelial function 10, 18 in both renal microvessels and carotid arteries. In the present investigation, we find that high dose VEGF121 infusion results in significant improvements in ACh mediated vasorelaxation of the carotid artery. Hence, this data suggests one possible mechanism, the restoration of endothelial function in the renal vasculature, for the increased renal function and attenuation of hypertension in this model.
In accord with our previous findings, we found that fetal weight was decreased in the present cohort of RUPP dams15, 21, 22, 28. Although fetal weight was not significantly increased with VEGF121 infusion, the low dose regimen attenuated the growth restriction associated with chronic RUPP. The high dose VEGF121 infusion showed no effect on fetal growth. These findings are similar to the previous report by Li and colleagues, who also reported no significant improvement in fetal growth with the chronic administration of VEGF121 in the sFlt-1 over-expression model. The apparent differential effects observed between the low and high doses of VEGF121 infusion may relate to the extent of arterial pressure change with each dose. The high dose resulted in abrogation of RUPP-induced hypertension, whereas the low dose merely attenuated the hypertension associated with placental ischemia. Thus, the higher pressure in concert with the permeability effects of VEGF121 may have resulted in greater tissue perfusion and augmented fetal growth in the low dose VEGF121 group.
Previous work suggests the primary source of circulating sFlt-1 in preeclamptic humans derives from the uteroplacental unit10, 33, 34 and that excessive placental ischemia plays a role in the dysregulation of sFlt-1 in pregnancy14, 20. Viewed together, these studies suggest that the increase in circulating sFlt-1 observed in preeclampsia may be a consequence of aberrant placental perfusion and that elevated sFlt-1 is likely responsible for the maternal syndrome. Whether there is a pathogenic role for sFlt-1 in early pregnancy remains unknown at the present time. Importantly, our studies suggest that antagonism of sFlt-1 with VEGF121 does appear to ameliorate maternal hypertension without significant adverse effects to the fetus.
It is important to recognize that circulating VEGF levels are much different in the pregnant rat compared to the pregnant woman. Hence, the relative contributions of VEGF compared with placental growth factor (PlGF) may be species specific. Nevertheless, the present studies show that restoration of the angiogenic balance has beneficial effects on blood pressure and renal function in hypertension associated with placental ischemia. Further studies are clearly required to determine the relative contributions of VEGF and PlGF in this and other models.
Perspectives
While it appears clear that anti-angiogenic factors play a role in the pathogenesis of preeclampsia, the manner in which these factors result in the features of the preeclamptic syndrome remain unclear. The present study which relies on data gathered by employing a well characterized and robust animal model of hypertension during pregnancy provides further evidence that antagonism of VEGF plays an important role in the link between placental ischemia, endothelial dysfunction and hypertension in the preeclamptic syndrome. It is difficult to directly extrapolate these findings to preeclampsia in humans largely because of the differences in the duration of hypertension and exposure to toxic factors such as sFlt-1. While the present results suggest that sFlt-1 antagonists, such as VEGF121, may be appropriate candidate molecules for further studies on the management of preeclampsia, careful studies will be required to determine the therapeutic range in women.
Acknowledgments
Sources of Funding: This work was supported in part by National Institutes of Health grants HL38499, HL51971 to J.P.G., and HL90269 to J.S.G. S.A.K is an investigator of the Howard Hughes Medical Institute.
Footnotes
Conflict(s) of Interest/Disclosure(s) Statement: S.A.K. is a co-inventor on patents filed by the Beth Israel Deaconess Medical Center for the diagnosis and therapy of preeclampsia. S.A.K. has served as consultant to Scios, Inc.
References
- 1.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 3.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 4.Redman CW. Current topic: pre-eclampsia and the placenta. Placenta. 1991;12:301–308. doi: 10.1016/0143-4004(91)90339-h. [DOI] [PubMed] [Google Scholar]
- 5.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46:1077–1085. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 6.Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res. 2005;57:1R–7R. doi: 10.1203/01.PDR.0000159567.85157.B7. [DOI] [PubMed] [Google Scholar]
- 7.Karumanchi SA, Bdolah Y. Hypoxia and sFlt-1 in preeclampsia: the “chicken-and-egg” question. Endocrinology. 2004;145:4835–4837. doi: 10.1210/en.2004-1028. [DOI] [PubMed] [Google Scholar]
- 8.Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the Soluble Vascular Endothelial Growth Factor Receptor in Preeclamptic Patients: Pathophysiological Consequences. Journal of Clinical Endocrinology Metabolism. 2003;88:5555–5563. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 9.Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, Anderson GD, Hankins GDV, Saade GR. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. American Journal of Obstetrics and Gynecology. 2007;196:396. doi: 10.1016/j.ajog.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. Journal of Clinical Investigation. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tranquilli AL, Bezzeccheri V, Giannubilo SR, Scagnoli C, Mazzanti L, Garzetti GG. Amniotic vascular endothelial growth factor (VEGF) and nitric oxide (NO) in women with subsequent preeclampsia. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2004;113:17–20. doi: 10.1016/S0301-2115(03)00369-5. [DOI] [PubMed] [Google Scholar]
- 12.Rana S, Karumanchi SA, Levine RJ, Venkatesha S, Rauh-Hain JA, Tamez H, Thadhani R. Sequential Changes in Antiangiogenic Factors in Early Pregnancy and Risk of Developing Preeclampsia. Hypertension. 2007;50:137–142. doi: 10.1161/HYPERTENSIONAHA.107.087700. [DOI] [PubMed] [Google Scholar]
- 13.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert JS, Babcock SA, Granger JP. Hypertension Produced by Reduced Uterine Perfusion in Pregnant Rats Is Associated With Increased Soluble Fms-Like Tyrosine Kinase-1 Expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:H541–H550. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 16.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007;71:977–984. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 17.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 18.Bridges JP, Gilbert JS, Colson D, Gilbert SA, Dukes MP, Ryan MJ, Granger JP. Oxidative Stress Contributes to Soluble Fms-Like Tyrosine Kinase-1 Induced Vascular Dysfunction in Pregnant Rats. Am J Hypertens. 2009;22:564–568. doi: 10.1038/ajh.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert JS, Nijland MJ, Knoblich P. Placental ischemia and cardiovascular dysfunction in preeclampsia and beyond: making the connections. Expert Review of Cardiovascular Therapy. 2008;6:1367–1377. doi: 10.1586/14779072.6.10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nevo O, Soleymanlou N, Wu Y, Xu J, Kingdom J, Many A, Zamudio S, Caniggia I. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1085–R1093. doi: 10.1152/ajpregu.00794.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension. 2000;35:367–372. doi: 10.1161/01.hyp.35.1.367. [DOI] [PubMed] [Google Scholar]
- 22.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37:1191–1195. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Zhang Y, Ying Ma J, Kapoun AM, Shao Q, Kerr I, Lam A, O'Young G, Sannajust F, Stathis P, Schreiner G, Karumanchi SA, Protter AA, Pollitt NS. Recombinant Vascular Endothelial Growth Factor 121 Attenuates Hypertension and Improves Kidney Damage in a Rat Model of Preeclampsia. Hypertension. 2007;50:686–692. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- 24.Alexander BT, Cockrell K, Cline FD, Llinas MT, Sedeek M, Granger JP. Effect of angiotensin II synthesis blockade on the hypertensive response to chronic reductions in uterine perfusion pressure in pregnant rats. Hypertension. 2001;38:742–745. doi: 10.1161/01.hyp.38.3.742. [DOI] [PubMed] [Google Scholar]
- 25.Alexander BT, Llinas MT, Kruckeberg WC, Granger JP. L-arginine attenuates hypertension in pregnant rats with reduced uterine perfusion pressure. Hypertension. 2004;43:832–836. doi: 10.1161/01.HYP.0000119192.32360.a9. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert JS, Dukes M, LaMarca BB, Cockrell K, Babcock SA, Granger JP. Effects of Reduced Uterine Perfusion Pressure on Blood Pressure and Metabolic Factors in Pregnant Rats. American Journal of Hypertension. 2007;20:686–691. doi: 10.1016/j.amjhyper.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med. 2006;122:383–392. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert JS, Gilbert SAB, Arany M, Granger JP. Hypertension Produced by Placental Ischemia in Pregnant Rats Is Associated With Increased Soluble Endoglin Expression. Hypertension. 2008;53:399–403. doi: 10.1161/HYPERTENSIONAHA.108.123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaMarca BD, Chandler DL, Grubbs L, Bain J, Lemore GR, Jr, Granger JP, Ryan MJ. Role of Sex Steroids in Modulating Tumor Necrosis Factor Alpha-Induced Changes in Vascular Function and Blood Pressure. American Journal of Hypertension. 2007;20:1216–1221. doi: 10.1016/j.amjhyper.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension. 2005;46:1022–1025. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 31.LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Current Hypertension Reports. 2007;9:480–485. doi: 10.1007/s11906-007-0088-1. [DOI] [PubMed] [Google Scholar]
- 32.LaMarca BD, Gilbert J, Granger JP. Recent progress toward the understanding of the pathophysiology of hypertension during preeclampsia. Hypertension. 2008;51:982–988. doi: 10.1161/HYPERTENSIONAHA.107.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bujold E, Romero R, Chaiworapongsa T, KIM YM, Kim GJ, Kim MR, Espinoza J, Goncalves L, Edwin S, Mazor M. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. Journal of Maternal-Fetal and Neonatal Medicine. 2005;18:9–16. doi: 10.1080/14767050500202493. [DOI] [PubMed] [Google Scholar]
- 34.Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, Lammoglia R, Charnock-Jones DS. A Vascular Endothelial Growth Factor Antagonist Is Produced by the Human Placenta and Released into the Maternal Circulation. Biol Reprod. 1998;59:1540–1548. doi: 10.1095/biolreprod59.6.1540. [DOI] [PubMed] [Google Scholar]